Abstract
Ischemic heart disease is the leading cause of death worldwide. Myocardial infarction results in an irreversible loss of cardiomyocytes with subsequent adverse remodeling and heart failure. Identification of new sources for cardiomyocytes and promoting their formation represent a holy grail of cardiac biology and regenerative medicine. Within the past decade, many types of putative cardiac stem cells (CSCs) have been reported to regenerate the injured myocardium by differentiating into new cardiomyocytes. Some of these CSCs have been “translated” from bench to bed with reported therapeutic effectiveness. However, recent basic research studies on stem cell tracing have begun to question their fundamental biology and mechanisms of action, raising serious concerns over the myogenic potential of CSCs. Here, we review the history of different types of CSCs within the past decade and provide an update of recent cell tracing studies that have challenged the origin and existence of CSCs. In addition to the potential role of CSCs in heart regeneration, proliferation of pre-existing cardiomyocytes has recently gained more attention. This review will also evaluate the methodological and technical aspects of past and current studies on CSCs and cardiomyocyte proliferation, with emphasis on technical strengths, advantages, and potential limitations of research approaches. While our current understanding of cardiomyocyte generation and regeneration continues to evolve, it is important to address the shortcomings and inaccuracies in this field. This is best achieved by embracing technological advancements and improved methods to label single cardiomyocytes/progenitors and accurately investigate their developmental potential and fate/lineage commitment.
Keywords: Cardiac stem cells, cardiomyocyte proliferation, heart regeneration, lineage tracing, myocardial infarction
Introduction
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality worldwide, accounting for an estimated 17.9 million deaths annually1–3. The most common type of CVD, known as ischemic heart disease, is due to plaque buildup within the coronary arteries that supply the myocardium with oxygen and nutrients. Reduction or blockage of blood flow through the coronaries during myocardial infarction (MI) leads to a dramatic and irreversible loss of cardiomyocytes, with estimates placing that loss to upward of a billion cells4. Due to the limited regenerative capacity of the heart, the remaining cardiomyocytes are unable to remuscularize and restore lost cells. Compensatory scarring to replace dead tissue leads to compromised cardiac function and eventually heart failure5, 6. A novel approach to combat heart failure has been to stimulate ‘cardiac stem cells’ (CSCs) to generate new cardiomyocytes or to induce the proliferation of existing cardiomyocytes in order to replace the scar tissue. However, the existence of CSCs has not been convincingly established and the proliferative capacity of cardiomyocytes during development, after birth, and in response to injury remains an area of controversy. The technological breakthroughs of the past decade have allowed for the study of stem cell fate and cardiomyocyte regeneration at an unprecedented resolution. Results from these studies7–9 have led to a paradigm shift of cardiomyocyte generation from endogenous CSCs to the currently accepted model of cardiomyocyte proliferation. Many investigators are exploring different ways to promote the proliferative potential of cardiomyocytes and unlock the cell-cycle arrest of cardiomyocytes in the adult hearts10. In this review, we introduce the rise and fall of stem cell theory for cardiomyocyte generation and discuss the accumulating evidence for cardiomyocyte proliferation. We will present detailed technical aspects of each approach with advantages, potential limitations, and future prospects.
Stem cells for heart repair and regeneration
Cell therapy has been intensely studied over the past two decades as a potential treatment for ischemic heart disease. A wide variety of cells have been evaluated for therapeutic delivery including bone marrow cells, mesenchymal stem cells (MSCs), and endogenous cardiac stem cells11, 12. The initial study of bone marrow cells (BMCs) for regenerating injured myocardium has subsequently led to immense basic and clinical investigation to isolate and deliver c-Kit+ bone marrow cells for treating ischemic diseases13. Early clinical trials showed inconsistent but slight improvement in cardiac function, generating excitement and hope for patients, clinicians, and scientists for treatment of ischemic heart disease14, 15. These earlier studies were often done in smaller Phase I/II cohorts and used more selective post-hoc analyses. Larger and more adequately powered clinical trials as well as meta-analyses subsequently uncovered a minimal or unsustained beneficial effect on heart function16. This raised many concerns over the mechanisms of action by transplanted cells, with controversies about how BMCs can transdifferentiate into cardiomyocytes (Figure 1). With the development of new technologies such as genetic lineage tracing, accumulating experimental evidence begins to question the myogenic potential of previously reported stem cells in vivo.
Figure 1. Timeline of scientific evidence supporting or refuting the notion of myogenic differentiation of stem or progenitor cells.
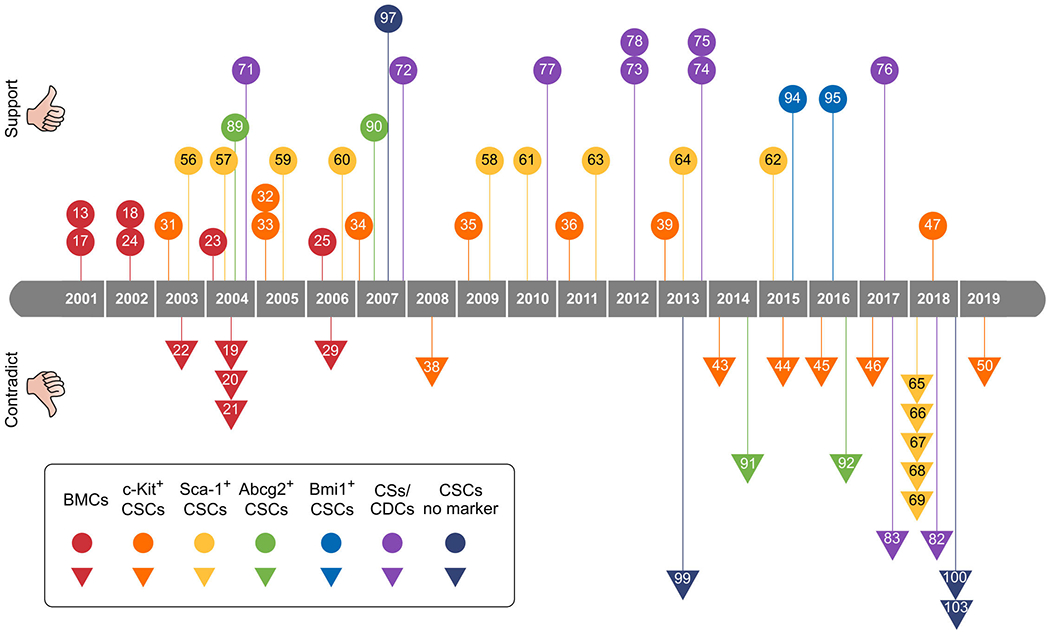
BMCs, Bone marrow-derived cells; CSCs, cardiac stem cells; CSs, cardiospheres; CDCs, cardiospheres-derived cells. Number in the box indicating the reference number of this review.
Bone marrow cells for cardiac regeneration
The adult heart has been considered a terminally differentiated organ with no considerable regenerative capacity. In 2001, Piero Anversa’s lab began to challenge this view by showing that bone marrow cells regenerate injured myocardium13. They isolated c-Kit+ hematopoietic cells from the bone marrow of male GFP mice, and transplanted these cells into the infarcted myocardium of a wild-type female mice13. Using GFP and Y chromosome as identification markers, they found that these transplanted cells in the infarcted female heart expressed cardiomyocyte sarcomere markers (Figure 2A). Additionally, these c-Kit+ hematopoietic cells also differentiated into endothelial and smooth muscle cells13. In a step further, they utilized the known cytokines to mobilize bone marrow cells to migrate to the infarcted myocardium for cardiomyocyte regeneration, which achieved similar beneficial effect as bone marrow transplantation17. This breakthrough report spurred a growing field of research on bone marrow cells for treatment of MI. In 2002, the Anversa group also presented genetic evidence that human bone marrow cells differentiated into cardiomyocytes in the adult stage18 (Figure 1). They examined autopsy specimens of female hearts transplanted to male host and detected 7–10% of cardiomyocytes and vascular cells in the heart that contained the Y chromosome, some of which showed high proliferative ability18. These data demonstrated that in humans, extra-cardiac sources, such as the bone marrow cells, could be mobilized to the heart and differentiate into cardiomyocyte, endothelial cells, and smooth muscle cells. However, co-staining of Y chromosome with a cardiomyocyte marker, as aforementioned, could be severely interfered by non-cardiomyocytes that are interspersed with cardiomyocytes. This would increase the false-positive signals, over-estimating the contribution of bone marrow cells to cardiomyocytes.
Figure 2.
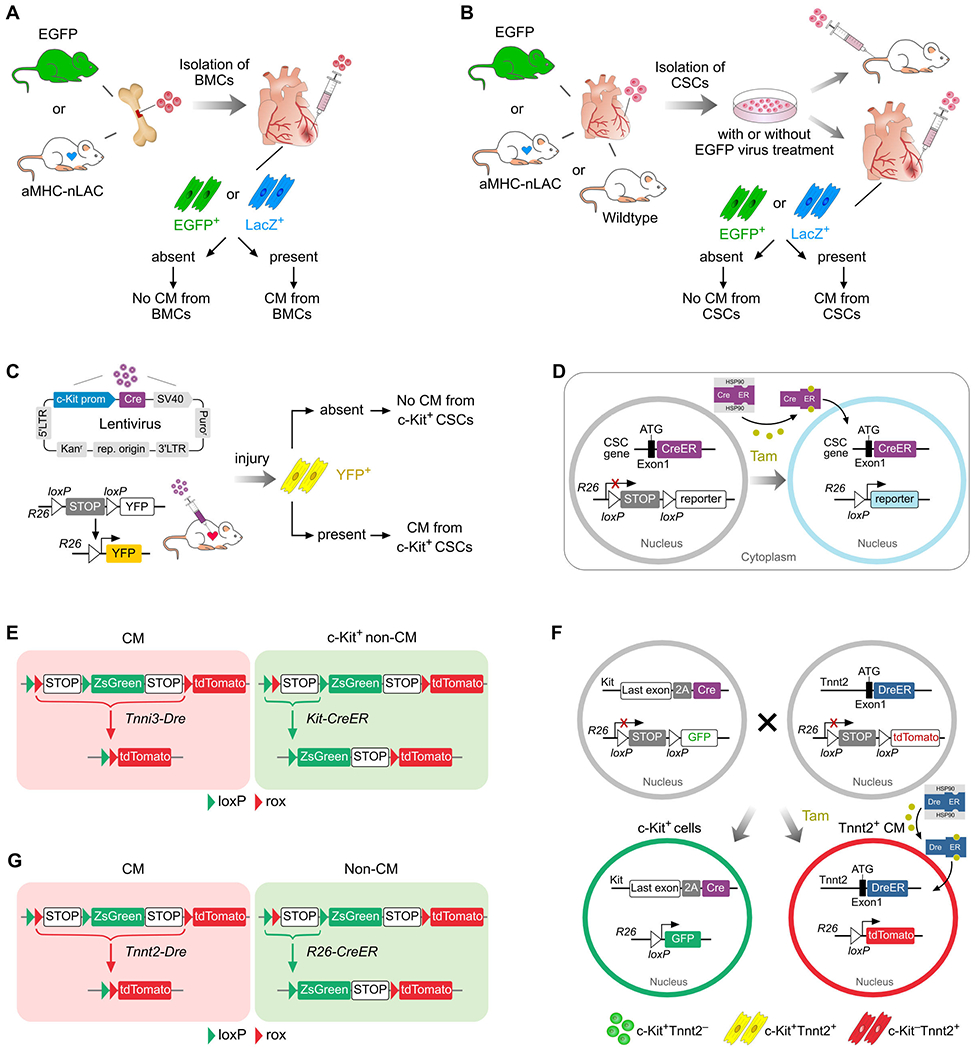
Strategy for studying the myogenic potential of stem cells. A, Transplantation of bone marrow cells (BMCs) into MI heart. B, Isolation of resident CSCs, and subsequent transplantation via circulation or into myocardium of MI heart. C, Injection of lentivirus expressing Cre under c-Kit promoter (prom) into injured myocardium. The Cre-loxP recombination leads to YFP labelling of c-Kit+ cells. D, Genetic lineage tracing of CSCs using knock-in Cre at the ATG of endogenous CSCs gene. E, Dual recombinase-activated lineage tracing (DeaLT) of c-Kit+ non-CM (ZsGreen) could distinguish it from pre-existing CM (tdTomato) that expresses c-Kit by itself. F, Lineage tracing of c-Kit+ cells using Cre knock-in targeted after last exon before 3’ UTR of c-Kit gene maintains c-Kit gene and achieves efficient labelling of c-Kit+ cells. DeaLT distinguishes cell fates of c-Kit+Tnnt2− non-CM (GFP+tdTomato−) from c-Kit+Tnnt2+ CM (GFP+tdTomato+). G, Simultaneous labelling of CM and non-CM by DeaLT. CM, cardiomyocyte; CSCs, cardiac stem cells; R26, Rosa26.
In 2004, work from Loren Field and Robert Robbins’ labs independently reported that c-Kit+ bone marrow cells do not differentiate into cardiomyocytes to promote cardiac regeneration, but instead, adopt a hematopoietic fate19, 20. Both labs utilized the same transplantation strategy as Anversa’s lab and transplanted genetic tagged cells from transgenic reporter mice (Figure 2A). They did not find convincing evidence supporting myogenic differentiation of transplanted cells19, 20. While hematopoietic cells from the bone marrow did not differentiate into cardiomyocytes, it was later reported that a small proportion could fuse with cardiomyocytes at a very low rate21, 22. The promising reports of adult BMC plasticity to generate cardiomyocytes led to a rush of clinical trials14, 23–25. A number of pilot clinical trials showed variable outcome in terms of efficacy, with most unable to reproduce the initial favorable outcome in animal studies26, 27. It is important to note that considerable heterogeneity exists in the specific bone marrow cells used for the pre-clinical and clinical studies in this field, with differences in the cell isolation, storage, and enrichment processes28. Nonetheless, no study has yet demonstrated a robust generation of engraftable cardiomyocytes after transplantation of BMCs. Subsequent research has shown that the bone marrow-derived cells may secrete angiogenic factors to promote neovascularization of the injured heart after MI29, which provides reasonable explanation for mechanisms of action for cell transplantation-based therapy. In addition to the paracrine effect, bone marrow cell therapy also causes local inflammation with regional accumulation of distinct macrophage subtypes, which may improve heart function after cardiac injury30.
Resident c-Kit+ cardiac stem cells for cardiomyocyte contribution
While the myogenic potential of bone marrow cells has been refuted, Anversa group also proposed that the adult heart contains resident stem cells, c-Kit+ CSCs31. Through self-renewal and multipotency assays, they demonstrated that isolated c-Kit+ CSCs are multipotent and could expand in vitro at a clonal level. Their work demonstrated that single c-Kit+ CSC could differentiate into multiple cell lineages including cardiomyocytes, endothelial cells, and smooth muscle cells. When they injected GFP labeled c-Kit+ CSCs into the border region of infarcted myocardium, they detected new cardiomyocytes that were differentiated from these transplanted cells in vivo31 (Figure 2B). Alternatively, c-Kit+ cells delivered to the coronary arteries could also promote recovery of injured heart through cardiomyocyte differentiation32 (Figure 2B). The existence of resident CSCs was reported to be evolutionally conserved, as c-Kit+ cells from adult rat, mouse, dog, and human hearts have the ability to regenerate cardiomyocytes33–35 (Figure 1). Moreover, a clinical trial led by Bolli et al. reported that c-Kit+ CSCs isolated from atrial appendages of patients undergoing surgical revascularization and transplanted into patients with chronic ischemic heart disease led to significantly improved heart function with short-term safety36. One caveat of these studies is that most conclusions largely depended on immunostaining, in which artifacts or false-positives may arise. Additionally, cell purification and culture may alter the property of the cells or spur the myogenic potential in vivo. Independent studies from different groups showed that transplanted c-Kit+ CSCs into the infarcted adult mouse hearts failed to undergo cardiomyogenic differentiation37, 38 (Figure 2B). Studies over human heart samples suggested these c-Kit+ cells were mast cells37, 38.
While the experiments above mainly used a cell transplantation assay, a report in 2013 led by Torella and colleagues utilized a lentiviral system in which a small portion of the Kit gene promoter was used to drive Cre expression to presumably label c-Kit+ CSCs in vivo without cell transplantation39. However, this approach is not necessarily reflective of in vivo genetic lineage tracing studies. By injecting the lentivirus that expresses Cre under the control of c-Kit promoter into the myocardium of Rosa26-YFP reporter mice, they showed presence of c-Kit-expressing cells throughout the myocardium (Figure 2C). After isoproterenol-induced heart injury, a substantial number (~10%) of newly formed cardiomyocytes were expressing YFP39. Functionally, ablation of these c-Kit+ CSCs resulted in severe cardiomyopathy with significant hypertrophy of the spared cardiomyocytes39. These data indicated that endogenous c-Kit+ CSCs are both necessary and sufficient for heart repair and regeneration. A limitation of this study worth noting is that the c-Kit promoter40 may not be able to reliably recapitulate the endogenous c-Kit regulatory elements. There is possibility that cardiomyocytes may uptake the lentivirus and subsequent YFP expression would lead to inaccurate assessment of the contribution of non-myocyte c-Kit+ CSCs to newly formed cardiomyocytes. The specificity of a lentiviral approach to trace c-Kit+ cells remains uncertain41, 42.
To resolve the c-Kit+ CSCs conundrum, van Berlo et al. were the first to generate a transgenic c-Kit mouse for lineage tracing studies by employing a knock-in strategy for Cre expression under the endogenous c-Kit gene promoter43. Cre-mediated recombination results in excision of a stop codon that leads to permanent expression of a constitutively active fluorescent reporter, e.g. GFP, in c-Kit-expressing cells and their progeny, even if they differentiate into cardiomyocytes (Figure 2D)43. Fate-mapping of c-Kit+ cells revealed very few labeled cardiomyocytes, and from those, many were a result of cell-fusion rather than differentiation43, indicating that this level of contribution is unlikely to be physiologically significant for cardiac repair, contradicting previous c-Kit studies (Figure 1). Subsequent studies from two other independent laboratories using similar Cre knock-in strategies showed findings consistent with van Berlo, and concluded that c-Kit+ CSCs contribute minimally, if any, to cardiomyocytes during homeostasis and after injury44, 45. Additionally, these studies demonstrated that c-Kit was also expressed in a subset of cardiomyocytes, which suggests that lineage tracing of c-Kit+ cells may be confounded by the presence of a subset of c-Kit+ cardiomyocytes. A dual recombinase-activated lineage tracing (DeaLT) was later developed to distinctly label c-Kit+ non-cardiomyocytes from those c-Kit+ cardiomyocytes (Figure 2E), which showed that c-Kit+ non-cardiomyocytes do not contribute to any new cardiomyocytes46.
In 2018, Vicinanza et al. contended that previous fate mapping results based on Kit-Cre knock-in lines have inherent technical limitations (Figure 1). First, they reasoned that c-Kit Cre tools are not sensitive enough to track all c-Kit+ cells, especially c-Kitlow cells47. It was argued that those unlabeled c-Kitlow cells could be more likely to generate new cardiomyocytes47. Secondly, previous gene knock-in targeting strategies unavoidably interrupted the endogenous allele43–45, leading to haploinsufficiency of c-Kit expression, which was reported to regulate CSCs function and survival of cardiomyocytes48, 49. To address these two caveats, two new c-Kit knock-in alleles, Kit-IRES-Cre and Kit-2A-Cre were generated, such that efficient labeling could be achieved while preserving the endogenous Kit gene50. Combined with the DeaLT system, these new Cre-labeled c-Kit+ non-myocytes again demonstrated that no new cardiomyocytes were generated from a c-Kit lineage in the adult heart (Figure 2F). Taken together, these studies utilizing more advanced technologies do not provide scientific evidence supporting the existence of c-Kit+ CSCs8.
Sca-1+ cardiac progenitor cells for cardiomyocyte regeneration
In addition to c-Kit, Sca-1 (stem cell antigen-1) is also considered as a cardiomyocyte stem cell marker and has been studied intensively in the last decade (Figure 1). Sca-1, also known as Ly-6a, is a member of Ly-6 gene family expressed in bone marrow stem cells51. Sca-1 has also been reported as a stem or progenitor cell marker in other organs such as mammary gland, prostate, lung, and liver52–55. In the adult heart, Schneider’s group started the adventure of Sca-1+ stem cells, and found its differentiation into cardiomyocytes with sarcomere structure by performing in vitro cell culture assays56. Another study showed the in vitro potential of Sca-1+ cells from adult hearts to differentiate into mature cardiomyocytes, osteocytes, and adipocytes57, supporting its multipotency. Furthermore, transplantation of Sca-1+ cells isolated from aMHC-Cre-nLAC mice into R26-reporter mice showed in vivo differentiated into cardiomyocytes56. Further study revealed that transplantation of sheets of clonally expanded Sca1+ cells ameliorates cardiac function after MI through cardiomyocyte differentiation in addition to paracrine mechanisms58.
Several studies have highlighted the heterogeneity of Sca-1+ cells residing in the adult heart. Pfister et al. reported that Sca-1+ cells consisted of CD31+ and CD31− populations, the latter of which have the capacity to differentiate into cardiomyocytes59. Subsequent studies confirmed that Sca-1+CD31− population increased after MI and transplantation of these cells significantly improved heart function through pro-angiogenesis effect60, 61. Additional study further defined these populations into four using PDGFRa expression, with the PDGFRa+CD31−Sca-1+ population containing stem cell properties of multiple cardiac lineages62. A different study showed that adult Sca-1+ cells contained CSH1 and CSH2 subpopulations, with myogenic capacity residing within the CSH2 cells63. Taken together, these studies documented the myogenic potential of Sca-1+ cells (Figure 1). However, most of these studies used cell culture or transplantation assays (Figure 2B), which do not address whether endogenous Sca-1+ cells are capable of differentiating into cardiomyocytes in vivo.
To directly address the in vivo myogenic potential of Sca-1+ cells, Uchida investigated Sca-1 lineage tracing using tet-off system to trace Sca-1+ cells in tissue homeostasis and after injury (Figure 1). Their fate-tracing tools showed that the labeled Sca-1+ cells contributed to cardiomyocytes in aging heart and after pressure overload injury64. However, recent studies from 5 independent labs showed that Sca-1+ cells do not generate new cardiomyocytes65–69 (Figure 1). Specifically, transplantation of Sca-1+ cells into the infarcted myocardium did not induce their differentiation into new cardiomyocytes (Figure 2B)69. Genetic lineage tracing based on Sca-1 knock-in Cre lines (Figure 2D) showed that Sca-1+ cells mainly adopted an endothelial cell fate, but not cardiomyocyte, during homeostasis or after injury65–68. Therefore, the beneficial effect from Sca-1+ cell transplantation is unlikely to be due to direct cardiomyocyte differentiation, but more from angiogenesis and paracrine effects. These studies also highlighted the improvement in mouse genetics for lineage tracing and fate mapping that could lead to a change in interpretation of plasticity of stem cells70.
Cardiospheres and cardiosphere-derived stem cells
Cardiospheres was first termed based on the observation that isolated undifferentiated cells grew as self-adherent clusters from subcultures of postnatal atrial or ventricular heart tissues71. These cardiospheres were thought to possess properties of adult cardiac stem cells such as long-term self-renewal and ability to differentiate into cardiomyocytes after transplantation into infarcted hearts (Figure 1). Since cardiospheres could be readily isolated from human heart biopsy specimens and expanded ex vivo, they offered a potential therapeutic strategy to regenerate the damaged myocardium. When endomyocardial samples were plated on tissue culture dishes, it was noted that cardiosphere-derived cells (CDCs) sprouted from the core. Co-culturing of CDCs with neonatal rat cardiomyocytes resulted in differentiation of CDCs into cardiomyocytes based on gene and protein expression analysis. When CDCs were injected into the infarcted myocardium of mouse, rat, or pig, it was reported that the transplanted cells generated cardiomyocytes and vascular endothelial cells, promoting the recovery of heart function72–76. However, considering the limited number of differentiated cardiomyocytes, it was hypothesized that most of the beneficial effects might be derived from the CDCs’ paracrine effects after transplantation77, which provided a variety of growth factors to prevent apoptosis and promote angiogenesis. The initial human clinical trial (CADUCEUS) investigated intracoronary infusion of autologous CDCs obtained through endomyocardial biopsy in patients with acute MI. This trial showed that CDCs treatment reduced scar size by MRI, increased muscle contractility and wall thickness, albeit no significant improvements in the end diastolic or systolic volumes of the left ventricle and the ejection fraction78, 79. Due to small sample size and a skewed distribution on the data for statistical analysis, the results from these clinical trials need to be more carefully analyzed and cautiously interpreted80.
Despite support from many studies regarding the beneficial effects of CDCs in promoting heart function (Figure 1), there are many others that have challenged the efficacy of CDCs in cardiac repair. Several studies failed to show the beneficial effects of CDCs in reducing scar size and improving ejection fraction81–83. It has also been suggested that the beneficial effects of CDCs are not likely through differentiation of cardiomyocytes. Recent studies have demonstrated that exosomes from CDCs promoted angiogenesis, cardiomyocyte survival and proliferation84, which are sufficient to explain the therapeutic effects of CDCs. Additional mechanisms of action may include the direct cell-cell contact between CDCs and cardiomyocytes that may promote their survival and proliferation85.
Abcg2+ side population cells for cardiac regeneration
Side population cells were initially defined as a cell fraction with the ability to extrude Hoechst33342 dye and later regarded as a stem cell population86, 87 identifiable by surface marker Abcg2, also known as Bcrp188. Abcg2+ cells were observed in the adult heart, with studies showing their differentiation into cardiomyocytes in vitro (Figure 1)89. Injection of side population cells into the rats undergoing heart injury showed their recruitment to the injured regions and ability to differentiate into cardiomyocytes, endothelial cells, and smooth muscle cells, which suggested that they may be endogenous cardiac stem cells (Figure 2B)90. However, genetic lineage tracing based on Abcg2-CreER demonstrated that Abcg2+ cells was only able to differentiate into the multiple cardiac cell lineages during the embryonic stages, but that this ability was lost in adulthood91, 92 (Figure 2D). The fusion of Abcg2+ cells with preexisting cardiomyocytes was likely to stimulate cardiomyocyte cell cycle entry and proliferation, rather than these cells directly differentiating into cardiomyocytes93. Therefore, genetic fate mapping studies have refuted the cardiac stem cell property of adult endogenous Abcg2+ side population and their myogenic regenerative potential in vivo.
Bmi1+ cardiomyocyte stem cells
Bmi1+ cells have been reported to represent another endogenous cardiac progenitors94, 95, which was supported by genetic lineage tracing studies using Bmi1-CreER;R26-YFP transgenic mice (Figure 2D). YFP labeled, Bmi1+ cells could be induced to differentiate into vascular endothelial cells and smooth muscle cells, as well as cardiomyocytes if co-cultured with neonatal rat cardiomyocytes. Additionally, Bmi1+ cells contributed to new cardiomyocytes in this inducible CreER mouse model detected 1 year after tamoxifen labeling, indicating stem cell contribution during homeostasis94. Another study showed that after MI, contribution of Bmi1+ cells to cardiomyocytes significantly increased, with de novo cardiomyocytes generation reaching to 13.8% after MI compared to 4.7% in age-paired non-infarcted hearts95. Such exceedingly high levels of de novo cardiomyocytes formation contradicts the consensus in the cardiomyocyte regeneration field, as DNA synthesis detected in cardiomyocytes is an order of magnitude lower than this level7–9. Notably, these studies reported that Bmi1+ is a sub-population of Sca-1+ cells. Since Sca-1 lineage tracing studies have documented their inability to generate new cardiomyocytes65–68, it is less likely that its sub-population (Bmi1+ cells) could then generate cardiomyocytes. It is more likely that the Bmi1-Cre used in the previous studies94, 95 may also label few cardiomyocytes, which represents a similar caveat as labeling of c-Kit+ cardiomyocytes45, 46. Given the perceived lack of rigor in the cardiac stem cell field, such extraordinary findings need to be re-evaluated and independently verified.
Other CSCs with unknown markers: exist or not?
Since many of the previously reported CSCs such as c-Kit, Sca-1, and side population may not be authentic stem cells for cardiomyocytes, it raises an enduring question as to whether cardiac stem cells actually exist in the adult heart. Conventional Cre-loxP mediated genetic lineage tracing is technically challenging to address this question, as Cre needs to be driven by a known stem cell promoter96. Hsieh et al. devised an ingenious ‘pulse-chase’ strategy of genetically labeling pre-existing cardiomyocytes and assessing the dilution of cardiomyocyte percentage during aging and after injury97. They showed that the percentage of labeled cardiomyocytes during aging remained stable, indicating that stem or precursor cells did not refresh at a high rate. However, after MI or pressure overload injury, there was a decrease in the percentage of labeled cardiomyocytes, suggestive of an increased renewal rate from stem cells97. Subsequent advancement in technology employing multi-isotope imaging mass spectrometry (MIMS) analysis using 15N isotope incorporation98 supported cardiomyocyte proliferation and excluded a substantial contribution from stem cells in cardiomyocyte renewal in the uninjured heart99. Whether any single cardiomyocyte is generated from CSCs in the adult heart remains technically challenging to answer.
As conventional Cre-loxP strategy (Figure 2D) for tracing CSCs relies on the CSCs gene or marker, the promoters remain unknown for putative CSCs which have not been discovered yet. To circumvent this difficulty, an alternative strategy of addressing CSCs have been proposed. Since CSCs are, by definition, undifferentiated cells, they are not mature cardiomyocytes nor do they express organized sarcomeric structures. To address the existence of CSCs, the challenging question could be addressed by testing whether non-myocytes in the heart contain CSCs that may differentiate into new cardiomyocytes after heart injury (Figure 2G). To examine this, the dual recombinase-activated lineage tracing (DeaLT) system46 was utilized to simultaneously and exclusively label pre-existing cardiomyocytes and non-myocytes within the same heart (Figure 2G). Fate mapping results showed that non-myocytes contribute to new cardiomyocytes in fetal but not adult heart100,101, which was subsequently supported by a lineage tracing study of proliferating cells using Ki67 that shows no evidence for the trans-differentiation of other cell types toward cardiomyocytes102. The above studies, without relying on particular stem cell markers, demonstrate that the adult heart lacks an endogenous stem cell for cardiomyocyte regeneration103. These above supporting or conflicting the myogenic potential of cardiac stem cells in this review are listed in Figure 3.
Figure 3. Studies supporting or conflicting the cardiomyocyte differentiation of stem cells.

BMCs, bone marrow cells; CSCs, cardiac stem cells; CDCs, cardiosphere derived cells; DeaLT, dual recombinase activated lineage tracing. Red dot, transplantation; green dot, cell culture; blue dot, endogenous cells or lineage tracing approach.
Cardiomyocyte proliferation and heart regeneration
The potential of endogenous CSCs for cardiac regeneration has been disappointing since recent studies suggest that adult mammalian heart does not harbor CSCs for cardiomyocyte regeneration. How can we now rectify the observation of the low level of cardiomyocyte turnover? Considering that the endogenous source for generating new cardiomyocytes seem to derived from self-duplication, more focus should be directed toward this process, of which is known to occur in hearts of many non-mammalian regenerative species. To explore this, an essential basis for finding new approaches in promoting endogenous cardiomyocyte regeneration is to correctly detect and accurately quantify their proliferation by the state-of-the-art technologies.
Cardiomyocyte proliferation quantified by different methods
Cell proliferation marker staining
The mammalian heart has long been viewed as a post-mitotic organ with little regenerative capacity. It was previously thought that the total number of cardiomyocytes is established shortly after birth and there is hardly any proliferating cardiomyocyte with aging. However, in the past several years, the quiescent nature of the mammalian heart has been challenged with several reports supporting the generation of new cardiomyocytes after birth. The earliest reports of heart regeneration came from post-mortem histological specimen from newborns who died of diphtheria104, 105. To explore whether there is any evidence of cardiomyocyte proliferation in adult human heart, Beltrami et al. examined heart tissues of patients who died from MI, and detected about 4% and 1% of cardiomyocytes that expressed cell proliferation marker Ki67 in the infarct border zone and remote region, respectively106. Considering that this Ki67 staining on heart sections reflects only a snapshot of cardiomyocyte proliferation on the day of death (per day), their results, if stands correctly, indicate that, in total, an extremely high level of cardiomyocyte proliferation occurs in a year. However, this contradicts the 2017 consensus statement based on extensive recent experimentation that cardiomyocytes turnover is current estimated at 0.5% to 2% per year in both human and mouse hearts7–9.
Staining for cell proliferation markers, such as Ki67, pH-H3, PCNA, or Aurora B, have been widely used to study cardiomyocyte proliferation (Figure 4A)107, 108. However, given that staining for these markers represents only a snapshot of cell proliferation and considering the extremely low level of cardiomyocyte proliferation, these studies are unable to reveal the bulk number of cardiomyocyte proliferation in a time window. Additionally, these studies rely on indirect assays of cell division, which are challenging to interpret in the setting of cardiomyocyte polyploidy as well as potential DNA repair upon injury. Furthermore, these proliferation markers are unable to discriminate between karyokinesis and cytokinesis. Another caveat for marker staining is that the proliferation signals from non-myocytes would significantly interfere the unambiguous observation of authentic cardiomyocyte proliferation in a tissue section, where all cells are squeezed and entangled with each other, creating potential for false positive data.
Figure 4. Cardiomyocyte proliferation detected by different approaches.

A, Immunostaining for antibodies against cell proliferation markers such as Ki67 or Aurora B (Aurkb). Arrow (left panel), Ki67+ cardiomyocytes; arrow (right panel), Aurora B expression between dividing cardiomyocytes. B, Illustration showing isotope incorporation (such as carbon-14 or nitrogen-15) or nucleotide analog incorporation (BrdU, EdU). C, A schematic of the MADM system for detection of GFP+ cells and RFP+ cells (arrowheads) after G2-X segregation (Seg.). GFP+RFP+ cells (yellow, arrow) could be results of either cell division after G2-Z segregation or no cell division. D, A schematic showing working principle of rainbow reporter for labelling of individual cells with different fluorescent reporters. Rainbow mice carry a cassette of 4 fluorescent genes, inserted in the Rosa26 locus under the control of CAG promotor. Upon Cre mediated recombination, the default GFP expression is replaced by random expression of one of three other fluorescent genes: mCherry, mOrange, and mCerulean. Immunofluorescent image of heart sections shows mOrange+ and mCherry+ clones (magnified in boxes) in the heart. E, Illustration showing genetic lineage tracing of proliferation marker gene Ki67 using CreER-loxP recombination. Arrowheads, tdTomato+ cardiomyocytes that have expressed Ki67 during tracing period.
Isotope incorporation
In 2009, Bergman et al. took advantage of the integration of carbon-14 (14C), generated by nuclear bomb tests during the Cold War, into the genomic DNA of human cardiomyocytes109, and performed retrospective birth dating analysis of 14C in cardiomyocyte DNA, which serves as a date mark for when a cell was born (Figure 4B). Their study revealed that about 50% of cardiomyocytes renew during a normal life span, with a 1% annual turnover rate at the age of 25 and 0.45% at the age of 75109. Subsequent studies revealed that endothelial cells and fibroblasts have much faster turnover rates than cardiomyocytes110.
The newly developed technology using MIMS98 allows one to view and measure stable isotope incorporation with submicrometre resolution on the tissue section, which provides a technical basis for studying in situ cardiomyocyte proliferation using isotype incorporation. Taking advantage of 15N isotype labeling using MIMS, Senyo et al. detected an increase in 15N:14N ratio above the natural ratio, indicating DNA duplication in a subset of cardiomyocytes (Figure 4B). Based on MIMS analysis and quantification, about 4.4% of cardiomyocytes in a mouse undergo DNA replication in a year99. DNA replication may not necessarily mean complete cell cycle or cell division, as cardiomyocytes may contain multiple nuclei (polyploidy) during their cell cycle. Of note, about 17% of 15N+ cardiomyocytes were single nucleus and diploid, indicating that these cells resulted from complete cell cycle with cytokinesis, yielding truly new cardiomyocytes99. Quantification analysis showed generation of new cardiomyocytes at a yearly rate of 0.76% (4.4% x 17%) in the adult mouse hearts. These above studies based on isotype incorporation assay consistently documented low, discrete rate of cardiomyocyte turnover in the adult mammalian heart, providing valuable quantitative information on cardiomyocyte proliferation.
Mosaic analysis with double markers and Rainbow reporters
Considering that cardiomyocytes may have multiple nuclei in the adult stage, it is difficult to distinguish newly generated cardiomyocytes that have undergone cytokinesis from those that only completed karyokinesis or incomplete cell cycle events using isotype incorporation or proliferation marker staining techniques. To distinguish these events of cell division, Zong et al. took advantage of inter-chromosome recombination and developed an alternative strategy to distinctly label daughter cells after cytokinesis111. The mosaic analysis with double markers (MADM) strategy utilizes two reciprocally chimeric reporter genes that are knocked into the same location on homologous chromosomes, each containing the N terminus of one reporter and the C terminus of the other reporter interrupted by a loxP-containing intron (Figure 4C). After DNA replication in dividing cells (at the G2 phase), induced inter-chromosome recombination by Cre-loxP creates functional reporter gene expression, e.g. GFP and RFP on two chromosomes111. This system enables asymmetric labeling of daughter cells to precisely determine precursor-progeny lineages in various developmental studies112–114. Ali et al. utilized MADM system to investigate postnatal cardiomyogenesis, and showed limited, life-long, symmetric division of cardiomyocytes as a rare event in the first month of life in mice and very seldom in the adult stage and that MI did not increase the rate of cardiomyocyte division above the basal level115 (Figure 4C). While single-labeled cells by MADM are the result of bona fide cell division and is an attractive feature for clonal analysis, the inefficiency of inter-chromosome recombination in MADM underestimates the gross number of cell division in the adult hearts.
Subsequently, a stochastic four-color reporter system (Rainbow) was used to retrospectively identify the source of new cardiomyocytes during early mouse development and after injury116. Through titration of tamoxifen, this system permits labeling of a small number of cells and their progeny with a distinct fluorescent protein, allowing retrospective tracing of cellular expansion through easily identifiable clones in vivo (Figure 4D). Clonal analysis of cardiomyocytes at various timepoints during development showed that a distinct subpopulation of cardiomyocytes may have the potential for proliferation after birth116. These studies provide a valuable means for measuring cardiomyocyte division in mammalian heart, which could be iterated and improved in the future to enhance the efficiency of measuring in situ cardiomyocyte proliferation.
Genetic lineage tracing using inducible Cre driven by Ki67
In addition to the cell division analysis at single cell resolution by MADM and Rainbow, recent studies have utilized cell proliferation markers to genetically lineage trace proliferating cardiomyocytes in vivo. Clevers et al. took advantage of the widely used cell proliferation marker, Ki67, to study cell proliferation in the intestine, brain, and heart102, 117, 118. Distinct from previous studies that utilized marker staining, Clevers used Ki67 based inducible Cre to genetically label proliferating cells with a permanent genetic marker (Figure 4E). Kretzschmar et al. reported that cycling cardiomyocytes were observed shortly after birth, but hardly, if any, in the homeostatic or injured adult myocardium102. Most of the cycling cells after injury were non-cardiomyocytes, especially fibroblasts that contribute to fibrosis and which do not transdifferentiate into cardiomyocytes after injury102. Quantitatively, only 11 out of an estimated 8 million cardiomyocytes analyzed expressed Ki67 during adult homeostasis within an 18-month time window, reinforcing the notion that adult cardiomyocytes rarely proliferate. However, this number of cell division is much lower than those quantified by other approaches99, 109. We reasoned that CreER mouse systems rely on tamoxifen induction, and the continuous induction of CreER activity at high efficiency over a large time window (e.g. months) could be technically challenging to capture those proliferating cardiomyocytes. Since Ki67 inducible Cre only traced the proliferating cardiomyocytes during the period of tamoxifen administration, it is difficult to trace cardiomyocyte proliferation once tamoxifen levels decline over time. New genetic approaches that could seamlessly record proliferating cardiomyocytes over a long time window is needed for future genetic tracing studies of cardiomyocyte proliferation.
Technical caveat and room for improvement
While the above studies using isotype incorporation, marker staining, or genetic fate mapping approaches consistently revealed the limited self-renewal capacity of cardiomyocytes, there are obvious discrepancies in the magnitude of cardiomyocyte generation measured from these different methods. Isotype incorporation measures DNA synthesis events, which may capture cells that have undergone karyokinesis, but may not necessarily denote complete cell division. Quantification on the single-nuclear cardiomyocytes would strongly indicate their cell division. Genetic methods based on MADM provides strong evidence for cell division by single color cardiomyocytes. However, some cell division could also exhibit double-labeled color or no color, which could not be distinguished from current MADM strategy. Additionally, the efficiency of inducible Cre for inter-chromosome recombination is too low to target the majority of cardiomyocytes (although an attractive feature for clonal analysis studies), hence a limitation for accurate quantification of the bulk number of cardiomyocyte division. For proliferation marker-driven inducible CreER, almost no proliferating cardiomyocytes were observed in adults, and the reported number is several orders of magnitude lower than isotype incorporation methods. The inducible CreER may encounter difficulty in continuously tracing most cardiomyocyte proliferation for a prolonged time window. It is crucial to explore new methods that may combine the advantages of the above approaches, simultaneously measuring the generic cell cycle (from DNA synthesis to cell division) and also distinguishing cardiomyocytes that have completed cytokinesis from those that have undergone karyokinesis.
Polyploidy and cardiomyocyte proliferation
While the adult mammalian heart has a limited regenerative ability, teleost fish and urodele amphibians are able to survive after heart apical resection through robust cardiomyocyte proliferation119, 120. One notable difference between zebrafish and mammalian cardiomyocytes is the DNA copy number or nuclei number. Fish cardiomyocytes are single nucleated with two sets of homologous chromosomes (diploid), while most adult mouse cardiomyocytes have more than two sets of chromosomes (polyploid), either enclosed within a single nucleus or separated into multiple nuclei10. Of note, even in the mouse species, different mouse strains may have different percentages of mononuclear cardiomyocytes, which are highly correlated with cardiomyocyte proliferation and heart function after MI121. After surveying 120 inbred mouse strains, Patterson et al. identified Tnni3k as one gene that influences variation in the composition of mononuclear diploid cardiomyocytes121. This interesting study demonstrated that intrinsic heart regeneration is not limited nor uniform among individuals, but rather is a variable trait dependent on complex genetic background121. Recent elegant genetic study in zebrafish provided direct causal-effect relationship between polyploidization and cell proliferation122. Zebrafish cardiomyocytes were susceptible to polyploidization upon cytokinesis inhibition mediated by dominant-negative Ect2, and these polyploidy cardiomyocytes failed to regenerate myocardium after heart injury122, suggesting that cardiomyocyte polyploidization may be a barrier to heart regeneration. Subsequent studies revealed that thyroid hormone signaling regulates cardiomyocyte polyploidization and also the regenerative ability of adult hearts123. It would be worthwhile to explore underlying molecular pathways that promote these rare diploid cardiomyocytes in mammals, or to find new sources other than pre-existing cardiomyocytes for cardiac repair and regeneration.
Promotion of pre-existing cardiomyocyte proliferation
Many approaches have been reported to stimulate cardiomyocyte proliferation. In this review, we provide a general overview of each approach, as detailed information are available in recent excellent reviews15, 124–127. One obvious approach is to stimulate cardiomyocyte mitotic division through overexpression of cell cycle genes128. Cyclin D1 promotes cardiomyocyte DNA synthesis129, and Cyclin A2 induces cardiomyocyte mitosis130. Their forced expression in the heart enhanced cardiac function after myocardial infarction in both mouse131 and pig models132. Recent studies combined 4 cell-cycle regulators (CDK1, CDK4, cyclin B1 and D1) to efficiently induce cardiomyocyte division, which significantly improved cardiac function after MI133. In contrast, deletion of negative cell-cycle regulator Meis1 was shown to be sufficient for reactivation of cardiomyocyte mitosis in the adult heart134. In addition to cell-cycle genes, the Hippo pathway was recently reported to regulate cell proliferation and organ size135, 136. Recent studies have shown a critical role of the Hippo tumor-suppressor pathway which controls organ size by coordinating the regulation of cell proliferation and apoptosis. Knockout of Salv or over-expression of YAP significantly increased cardiomyocyte proliferation, reduced fibrosis and improved heart function after MI137–139. Additionally, Neuregulin1/ErbB4 and ErbB2, and epicardial derived growth factors such as follistatin-like 1, have been demonstrated to trigger cardiomyocyte proliferation and promote mammalian heart regeneration107, 140, 141. In addition to these pathways, microRNAs have been implicated in regulating cardiomyocyte division during normal aging or in response to injury. Several studies have shown that miR-590, miR-199a, miR-99/100 can promote cardiomyocyte proliferation and improve heart function after MI both in mouse and pig models142–144. Within the context of neonatal heart regeneration, transient fibroblast senescence was found to induce cardiomyocyte proliferation through senescence-associated secretory phenotype factors and also through p53-mediated pathways145, 146. Interestingly, certain physiological conditions such as exercise, pregnancy, and hypoxia have also been suggested to induce cardiomyocyte proliferation through various mechanisms147–151, which merit further investigations to understand their role in promoting heart regeneration after injury.
Future Directions
Due to the lack of strong evidence supporting the existence of resident CSCs, efforts are now focused on how to mobilize and promote those few cardiomyocytes that have potential to proliferate in mammalian hearts. While endogenous cardiomyocytes can proliferate at a low frequency, methods that promote cardiomyocyte proliferation may be a vital research field in the future. The aforementioned pathways could be manipulated, optimized, and synergized to achieve better functional improvement after cardiac injury. In addition to inducing cardiomyocyte proliferation, other alternative approaches could be considered (Figure 5). In situ reprogramming of fibroblasts to cardiomyocytes by over-expression of specific transcriptional factors represents a promising direction for future study152, 153. Recent studies have also suggested that small molecules can induce reprogramming of fibroblasts to cardiomyocytes in vivo, which offers a dual pharmaceutical approach to regenerate cardiomyocytes and also reduce scar formation154. Additionally, human embryonic stem cells or induced pluripotent stem cell-derived cardiac progenitors and cardiomyocytes have been successfully transplanted in large animal models155, 156. These studies confirmed the survival of transplanted cells within the host myocardium and an improvement in cardiac function after injury. Even without direct cardiomyocyte regeneration, methods that promote neovascularization such as transplantation of MSCs that secrete angiogenic factors, or modulate inflammation after injury157, may be an alternative approach to improve survival of myocardium, reduce scar formation, and ameliorate inflammation after MI. Furthermore, dual stem cell therapy, such as human iPS cell-derived cardiomyocytes and MSCs, or human ES cell-derived epicardium and cardiomyocytes, synergistically improves cardiac function and augment vascularization in the injured myocardium158, 159. Recent advances in engineered epicardial patch containing growth factors107 or multiple cardiac cell types160, 161 improved heart function and neovascularization after MI. Additionally, treatment of modified RNA of VEGFA lead to augmented vascular regeneration, partly through cell fate switch of epicardial progenitor cells162, 163. With the fallacy of endogenous putative CSCs, it is important to invest efforts and resources toward more promising directions as mentioned above to attain the ultimate goal of heart repair and regeneration.
Figure 5.
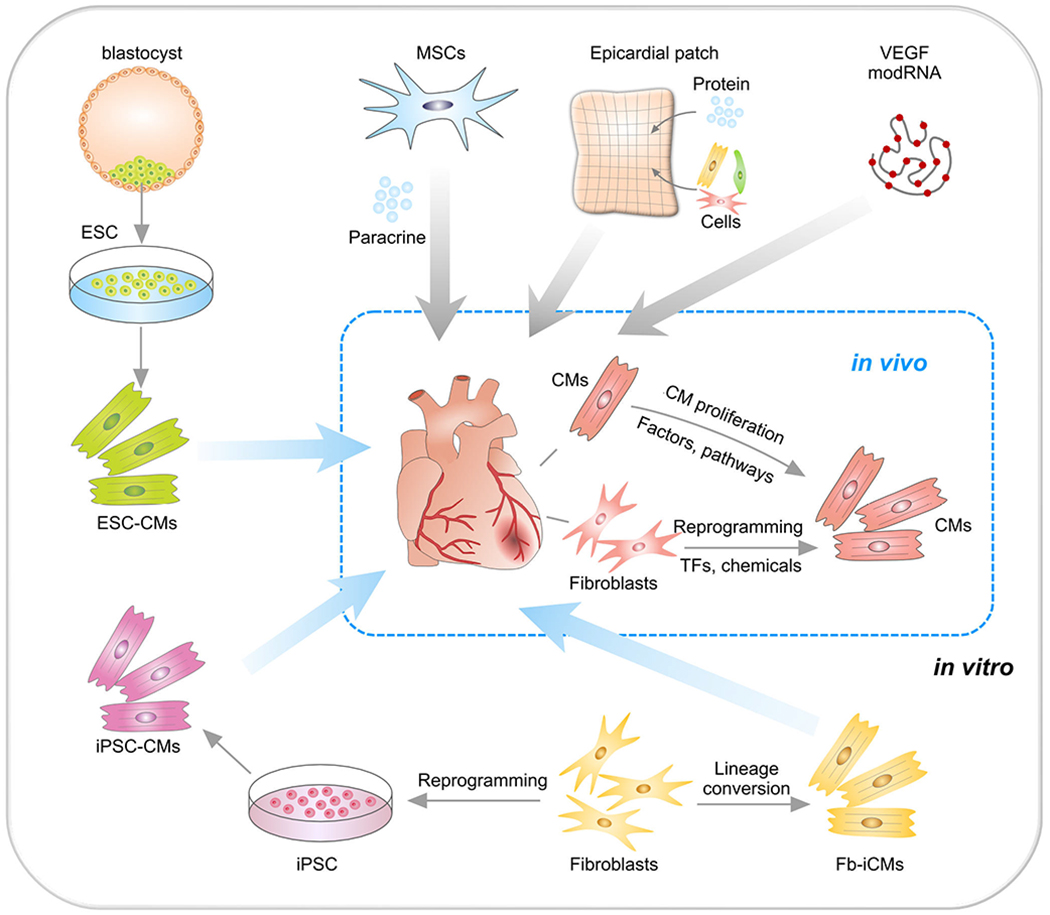
Strategies for treating cardiac repair and regeneration. Transplantation of embryonic stem cell (ESC)-derived cardiomyocytes (CMs) or induced pluripotent stem cells (iPSC)-derived CMs into MI heart. CMs could also be reprogrammed from fibroblasts (Fb) in vitro or by transcriptional factors (TF) or chemicals in vivo. Transplantation of mesenchymal stem cells (MSCs) promote neovascularization and cardiomyocyte survival through paracrine mechanism. Epicardial patch containing growth factors or cardiac cells restore heart function after MI. Administration of modified RNA (modRNA) of paracrine factors promote heart function and drive heart progenitor cell fate. CMs could be promoted to proliferation by regulation of factors or signaling pathways.
Supplementary Material
Acknowledgments
Funding Sources:
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB19000000, XDA16010507,), National Key Research & Development Program of China (2019YFA0110400, 2019YFA0802000), and National Science Foundation of China (31730112, 31625019, 91849202, 81761138040, 31922032, 31701292, 81872241). Reza Ardehali was supported in part by the NIH DP2 (HL127728) and the American Heart Association Innovative Project Award (18IPA34170309).
Nonstandard Abbreviations and Acronyms
- Abcg2
ATP binding cassette subfamily G member 2
- aMHC
alpha myosin heavy chain
- Aurora B
Aurora kinase B
- BMC
Bone marrow cell
- Bmi1
BMI1 proto-oncogene, polycomb ring finger
- CD31
PECAM1, platelet and endothelial cell adhesion molecule 1
- CDK
Cyclin dependent kinase
- Ect2
Epithelial cell transforming 2
- ErbB2
Erb-b2 receptor tyrosine kinase 2
- ErbB4
Erb-b2 receptor tyrosine kinase 4
- iPS
Induced pluripotent stem cell
- IRES
Internal ribosome entry site
- Ly-6a
Lymphocyte antigen 6 complex, locus A
- MIMS
Multi-isotope imaging mass spectrometry
- MRI
Magnetic Resonance Imaging
- PCNA
Proliferating cell nuclear antigen
- PDGFRa
Platelet derived growth factor receptor alpha
- pH-H3
Phosphorylated histone H3
- Salv
Salvador family WW domain containing 1
- TNNI3
Troponin I3, cardiac type
- Tnni3k
TNNI3 interacting kinase
- TNNT2
Troponin T2
- VEGFA
Vascular endothelial growth factor A
- YAP
Yes1 associated transcriptional regulator
Footnotes
Disclosures:
None
References:
- 1.Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med. 2012;366:54–63. doi: 10.1056/NEJMra1112570 [DOI] [PubMed] [Google Scholar]
- 2.Braunwald E The war against heart failure: the Lancet lecture. Lancet. 2015;385:812–824. doi: 10.1016/S0140-6736(14)61889-4 [DOI] [PubMed] [Google Scholar]
- 3.Organization WH. https://www.who.int/health-topics/cardiovascular-diseases/#tab=tab_1.
- 4.Reinecke H, Minami E, Zhu WZ, Laflamme MA. Cardiogenic differentiation and transdifferentiation of progenitor cells. Circ Res. 2008;103:1058–1071. doi: 10.1161/CIRCRESAHA.108.180588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Velagaleti RS, Pencina MJ, Murabito JM, Wang TJ, Parikh NI, D’Agostino RB, Levy D, Kannel WB, Vasan RS. Long-term trends in the incidence of heart failure after myocardial infarction. Circulation. 2008;118:2057–2062. doi: 10.1161/CIRCULATIONAHA.108.784215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ezekowitz JA, Kaul P, Bakal JA, Armstrong PW, Welsh RC, McAlister FA. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J Am Coll Cardiol. 2009;53:13–20. doi: 10.1016/j.jacc.2008.08.067 [DOI] [PubMed] [Google Scholar]
- 7.Eschenhagen T, Bolli R, Braun T, Field LJ, Fleischmann BK, Frisén J, Giacca M, Hare JM, Houser S, Lee RT et al. Cardiomyocyte Regeneration: A Consensus Statement. Circulation. 2017;136:680–686. doi: 10.1161/CIRCULATIONAHA.117.029343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai CL, Molkentin JD. The Elusive Progenitor Cell in Cardiac Regeneration: Slip Slidin’ Away. Circ Res. 2017;120:400–406. doi: 10.1161/CIRCRESAHA.116.309710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vagnozzi RJ, Molkentin JD, Houser SR. New Myocyte Formation in the Adult Heart: Endogenous Sources and Therapeutic Implications. Circ Res. 2018;123:159–176. doi: 10.1161/CIRCRESAHA.118.311208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tzahor E, Poss KD. Cardiac regeneration strategies: Staying young at heart. Science. 2017;356:1035–1039. doi: 10.1126/science.aam5894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kloner RA. Current state of clinical translation of cardioprotective agents for acute myocardial infarction. Circ Res. 2013;113:451–463. doi: 10.1161/CIRCRESAHA.112.300627 [DOI] [PubMed] [Google Scholar]
- 12.Nguyen PK, Rhee JW, Wu JC. Adult Stem Cell Therapy and Heart Failure, 2000 to 2016: A Systematic Review. JAMA Cardiol 2016;1:831–841. doi: 10.1001/jamacardio.2016.2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587 [DOI] [PubMed] [Google Scholar]
- 14.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0 [DOI] [PubMed] [Google Scholar]
- 15.Chien KR, Frisén J, Fritsche-Danielson R, Melton DA, Murry CE, Weissman IL. Regenerating the field of cardiovascular cell therapy. Nat Biotechnol. 2019;37:232–237. doi: 10.1038/s41587-019-0042-1 [DOI] [PubMed] [Google Scholar]
- 16.Nowbar AN, Mielewczik M, Karavassilis M, Dehbi HM, Shun-Shin MJ, Jones S, Howard JP, Cole GD, Francis DP, DAMASCENE WG. Discrepancies in autologous bone marrow stem cell trials and enhancement of ejection fraction (DAMASCENE): weighted regression and meta-analysis. BMJ. 2014;348:g2688. doi: 10.1136/bmj.g2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344–10349. doi: 10.1073/pnas.181177898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quaini F, Urbanek K, Beltrami AP, Finato N, Beltrami CA, Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081 [DOI] [PubMed] [Google Scholar]
- 19.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446 [DOI] [PubMed] [Google Scholar]
- 20.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460 [DOI] [PubMed] [Google Scholar]
- 21.Nygren JM, Jovinge S, Breitbach M, Sawen P, Roll W, Hescheler J, Taneera J, Fleischmann BK, Jacobsen SE. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494–501. doi: 10.1038/nm1040 [DOI] [PubMed] [Google Scholar]
- 22.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069 [DOI] [PubMed] [Google Scholar]
- 23.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9 [DOI] [PubMed] [Google Scholar]
- 24.Strauer BE, Brehm M, Zeus T, Köstering M, Hernandez A, Sorg RV, Kögler G, Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c [DOI] [PubMed] [Google Scholar]
- 25.Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779 [DOI] [PubMed] [Google Scholar]
- 26.Traverse JH, Henry TD, Pepine CJ, Willerson JT, Zhao DX, Ellis SG, Forder JR, Anderson RD, Hatzopoulos AK, Penn MS et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. JAMA. 2012;308:2380–2389. doi: 10.1001/jama.2012.28726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sürder D, Manka R, Moccetti T, Lo Cicero V, Emmert MY, Klersy C, Soncin S, Turchetto L, Radrizzani M, Zuber M et al. Effect of Bone Marrow-Derived Mononuclear Cell Treatment, Early or Late After Acute Myocardial Infarction: Twelve Months CMR and Long-Term Clinical Results. Circ Res. 2016;119:481–490. doi: 10.1161/CIRCRESAHA.116.308639 [DOI] [PubMed] [Google Scholar]
- 28.Banerjee MN, Bolli R, Hare JM. Clinical Studies of Cell Therapy in Cardiovascular Medicine: Recent Developments and Future Directions. Circ Res. 2018;123:266–287. doi: 10.1161/CIRCRESAHA.118.311217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A, Li RK. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–1877. doi: 10.1172/JCI27019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vagnozzi RJ, Maillet M, Sargent MA, Khalil H, Johansen AK, Schwanekamp JA, York AJ, Huang V, Nahrendorf M, Sadayappan S et al. An acute immune response underlies the benefit of cardiac stem-cell therapy. Nature. 2020;577:405–409. doi: 10.1038/s41586-019-1802-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/S0092-8674(03)00687-1 [DOI] [PubMed] [Google Scholar]
- 32.Dawn B, Stein AB, Urbanek K, Rota M, Whang B, Rastaldo R, Torella D, Tang XL, Rezazadeh A, Kajstura J et al. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci U S A. 2005;102:3766–3771. doi: 10.1073/pnas.0405957102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linke A, Müller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, Cascapera S, Böhm M, Quaini F et al. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci U S A. 2005;102:8966–8971. doi: 10.1073/pnas.0502678102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N et al. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosoda T, D’Amario D, Cabral-Da-Silva MC, Zheng H, Padin-Iruegas ME, Ogorek B, Ferreira-Martins J, Yasuzawa-Amano S, Amano K, Ide-Iwata N et al. Clonality of mouse and human cardiomyogenesis in vivo. Proc Natl Acad Sci U S A. 2009;106:17169–17174. doi: 10.1073/pnas.0903089106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Zaruba MM, Soonpaa M, Reuter S, Field LJ. Cardiomyogenic potential of C-kit(+)-expressing cells derived from neonatal and adult mouse hearts. Circulation. 2010;121:1992–2000. doi: 10.1161/CIRCULATIONAHA.109.909093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pouly J, Bruneval P, Mandet C, Proksch S, Peyrard S, Amrein C, Bousseaux V, Guillemain R, Deloche A, Fabiani JN et al. Cardiac stem cells in the real world. J Thorac Cardiovasc Surg. 2008;135:673–678. doi: 10.1016/j.jtcvs.2007.10.024 [DOI] [PubMed] [Google Scholar]
- 39.Ellison GM, Vicinanza C, Smith AJ, Aquila I, Leone A, Waring CD, Henning BJ, Stirparo GG, Papait R, Scarfo M et al. Adult c-kit(pos) Cardiac Stem Cells Are Necessary and Sufficient for Functional Cardiac Regeneration and Repair. Cell. 2013;154:827–842. doi: 10.1016/j.cell.2013.07.039 [DOI] [PubMed] [Google Scholar]
- 40.Cairns LA, Moroni E, Levantini E, Giorgetti A, Klinger FG, Ronzoni S, Tatangelo L, Tiveron C, De Felici M, Dolci S et al. Kit regulatory elements required for expression in developing hematopoietic and germ cell lineages. Blood. 2003;102:3954–3962. doi: 10.1182/blood-2003-04-1296 [DOI] [PubMed] [Google Scholar]
- 41.Davis J, Maillet M, Miano JM, Molkentin JD. Lost in Transgenesis: A User’s Guide for Genetically Manipulating the Mouse in Cardiac Research. Circ Res. 2012;111:761–777. doi: 10.1161/CIRCRESAHA.111.262717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molkentin JD, Houser SR. Are Resident c-Kit+ Cardiac Stem Cells Really All That Are Needed to Mend a Broken Heart? Circ Res. 2013;113:1037–1039. doi: 10.1161/CIRCRESAHA.113.302564 [DOI] [PubMed] [Google Scholar]
- 43.van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marban E, Molkentin JD. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509:337–341. doi: 10.1038/nature13309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sultana N, Zhang L, Yan J, Chen J, Cai W, Razzaque S, Jeong D, Sheng W, Bu L, Xu M et al. Resident c-kit(+) cells in the heart are not cardiac stem cells. Nat Commun. 2015;6:8701. doi: 10.1038/ncomms9701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Q, Yang R, Huang X, Zhang H, He L, Zhang L, Tian X, Nie Y, Hu S, Yan Y et al. Genetic lineage tracing identifies in situ Kit-expressing cardiomyocytes. Cell Res. 2016;26:119–130. doi: 10.1038/cr.2015.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He L, Li Y, Li Y, Pu W, Huang X, Tian X, Wang Y, Zhang H, Liu Q, Zhang L et al. Enhancing the precision of genetic lineage tracing using dual recombinases. Nat Med. 2017;23:1488–1498. doi: 10.1038/nm.4437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vicinanza C, Aquila I, Cianflone E, Scalise M, Marino F, Mancuso T, Fumagalli F, Giovannone ED, Cristiano F, Iaccino E et al. KitCre knock-in mice fail to fate-map cardiac stem cells. Nature. 2018;555:E1–E5. doi: 10.1038/nature25771 [DOI] [PubMed] [Google Scholar]
- 48.Gude NA, Firouzi F, Broughton KM, Ilves K, Nguyen KP, Payne CR, Sacchi V, Monsanto MM, Casillas AR, Khalafalla FG et al. Cardiac c-Kit Biology Revealed by Inducible Transgenesis. Circ Res. 2018;123:57–72. doi: 10.1161/CIRCRESAHA.117.311828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou B, Wu SM. Reassessment of c-Kit in Cardiac Cells: A Complex Interplay Between Expression, Fate, and Function. Circ Res. 2018;123:9–11. doi: 10.1161/CIRCRESAHA.118.313215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He L, Han M, Zhang Z, Li Y, Huang X, Liu X, Pu W, Zhao H, Wang Q, Nie Y et al. Reassessment of c-Kit Cells for Cardiomyocyte Contribution in Adult Heart. Circulation. 2019;140:164–166. doi: 10.1161/CIRCULATIONAHA.119.039909 [DOI] [PubMed] [Google Scholar]
- 51.Acar M, Kocherlakota KS, Murphy MM, Peyer JG, Oguro H, Inra CN, Jaiyeola C, Zhao Z, Luby-Phelps K, Morrison SJ. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature. 2015;526:126–130. doi: 10.1038/nature15250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Welm BE, Tepera SB, Venezia T, Graubert TA, Rosen JM, Goodell MA. Sca-1(pos) cells in the mouse mammary gland represent an enriched progenitor cell population. Dev Biol. 2002;245:42–56. doi: 10.1006/dbio.2002.0625 [DOI] [PubMed] [Google Scholar]
- 53.Xin L, Lawson DA, Witte ON. The Sca-1 cell surface marker enriches for a prostate-regenerating cell subpopulation that can initiate prostate tumorigenesis. Proc Natl Acad Sci U S A. 2005;102:6942–6947. doi: 10.1073/pnas.0502320102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McQualter JL, Brouard N, Williams B, Baird BN, Sims-Lucas S, Yuen K, Nilsson SK, Simmons PJ, Bertoncello I. Endogenous fibroblastic progenitor cells in the adult mouse lung are highly enriched in the sca-1 positive cell fraction. Stem Cells. 2009;27:623–633. doi: 10.1634/stemcells.2008-0866 [DOI] [PubMed] [Google Scholar]
- 55.Clayton E, Forbes SJ. The isolation and in vitro expansion of hepatic Sca-1 progenitor cells. Biochem Biophys Res Commun. 2009;381:549–553. doi: 10.1016/j.bbrc.2009.02.079 [DOI] [PubMed] [Google Scholar]
- 56.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsuura K, Nagai T, Nishigaki N, Oyama T, Nishi J, Wada H, Sano M, Toko H, Akazawa H, Sato T et al. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2004;279:11384–11391. doi: 10.1074/jbc.M310822200 [DOI] [PubMed] [Google Scholar]
- 58.Matsuura K, Honda A, Nagai T, Fukushima N, Iwanaga K, Tokunaga M, Shimizu T, Okano T, Kasanuki H, Hagiwara N et al. Transplantation of cardiac progenitor cells ameliorates cardiac dysfunction after myocardial infarction in mice. J Clin Invest. 2009;119:2204–2217. doi: 10.1172/JCI37456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, Colucci WS, Liao R. CD31− but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res. 2005;97:52–61. doi: 10.1161/01.RES.0000173297.53793.fa [DOI] [PubMed] [Google Scholar]
- 60.Wang X, Hu Q, Nakamura Y, Lee J, Zhang G, From AH, Zhang J. The role of the sca-1+/CD31− cardiac progenitor cell population in postinfarction left ventricular remodeling. Stem Cells. 2006;24:1779–1788. doi: 10.1634/stemcells.2005-0386 [DOI] [PubMed] [Google Scholar]
- 61.Liang SX, Tan TY, Gaudry L, Chong B. Differentiation and migration of Sca1+/CD31− cardiac side population cells in a murine myocardial ischemic model. Int J Cardiol 2010;138:40–49. doi: 10.1016/j.ijcard.2008.08.032 [DOI] [PubMed] [Google Scholar]
- 62.Noseda M, Harada M, McSweeney S, Leja T, Belian E, Stuckey DJ, Abreu Paiva MS, Habib J, Macaulay I, de Smith AJ et al. PDGFRalpha demarcates the cardiogenic clonogenic Sca1(+) stem/progenitor cell in adult murine myocardium. Nat Commun. 2015;6:6930. doi: 10.1038/ncomms7930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takamiya M, Haider KH, Ashraf M. Identification and characterization of a novel multipotent sub-population of Sca-1⁺ cardiac progenitor cells for myocardial regeneration. PLoS One. 2011;6:e25265. doi: 10.1371/journal.pone.0025265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uchida S, De Gaspari P, Kostin S, Jenniches K, Kilic A, Izumiya Y, Shiojima I, grosse K, Karsten, Renz H, Walsh K et al. Sca1-Derived Cells Are a Source of Myocardial Renewal in the Murine Adult Heart. Stem Cell Reports. 2013;1:397–410. doi: 10.1016/j.stemcr.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vagnozzi RJ, Sargent MA, Lin SJ, Palpant NJ, Murry CE, Molkentin JD. Genetic Lineage Tracing of Sca-1+ Cells Reveals Endothelial but Not Myogenic Contribution to the Murine Heart. Circulation. 2018;138:2931–2939. doi: 10.1161/CIRCULATIONAHA.118.035210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang L, Sultana N, Yan J, Yang F, Chen F, Chepurko E, Yang FC, Du Q, Zangi L, Xu M et al. Cardiac Sca-1+ Cells Are Not Intrinsic Stem Cells for Myocardial Development, Renewal and Repair. Circulation. 2018;138:2919–2930. doi: 10.1161/CIRCULATIONAHA.118.035200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Neidig LE, Weinberger F, Palpant NJ, Mignone J, Martinson AM, Sorensen DW, Bender I, Nemoto N, Reinecke H, Pabon L et al. Evidence for Minimal Cardiogenic Potential of Stem Cell Antigen 1-Positive Cells in the Adult Mouse Heart. Circulation. 2018;138:2960–2962. doi: 10.1161/CIRCULATIONAHA.118.035273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tang J, Li Y, Huang X, He L, Zhang L, Wang H, Yu W, Pu W, Tian X, Nie Y et al. Fate Mapping of Sca1 Cardiac Progenitor Cells in the Adult Mouse Heart. Circulation. 2018;138:2967–2969. doi: 10.1161/CIRCULATIONAHA.118.036210 [DOI] [PubMed] [Google Scholar]
- 69.Soonpaa MH, Lafontant PJ, Reuter S, Scherschel JA, Srour EF, Zaruba MM, Rubart-von der Lohe M, Field LJ. Absence of Cardiomyocyte Differentiation Following Transplantation of Adult Cardiac-Resident Sca-1. Circulation. 2018;138:2963–2966. doi: 10.1161/CIRCULATIONAHA.118.035391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee RT. Adult Cardiac Stem Cell Concept and the Process of Science. Circulation. 2018;138:2940–2942. doi: 10.1161/CIRCULATIONAHA.118.036407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51 [DOI] [PubMed] [Google Scholar]
- 72.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marbán E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209 [DOI] [PubMed] [Google Scholar]
- 73.Malliaras K, Li TS, Luthringer D, Terrovitis J, Cheng K, Chakravarty T, Galang G, Zhang Y, Schoenhoff F, Van Eyk J et al. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation. 2012;125:100–112. doi: 10.1161/CIRCULATIONAHA.111.042598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tseliou E, Pollan S, Malliaras K, Terrovitis J, Sun B, Galang G, Marbán L, Luthringer D, Marbán E. Allogeneic cardiospheres safely boost cardiac function and attenuate adverse remodeling after myocardial infarction in immunologically mismatched rat strains. J Am Coll Cardiol. 2013;61:1108–1119. doi: 10.1016/j.jacc.2012.10.052 [DOI] [PubMed] [Google Scholar]
- 75.Malliaras K, Smith RR, Kanazawa H, Yee K, Seinfeld J, Tseliou E, Dawkins JF, Kreke M, Cheng K, Luthringer D et al. Validation of contrast-enhanced magnetic resonance imaging to monitor regenerative efficacy after cell therapy in a porcine model of convalescent myocardial infarction. Circulation. 2013;128:2764–2775. doi: 10.1161/CIRCULATIONAHA.113.002863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grigorian-Shamagian L, Liu W, Fereydooni S, Middleton RC, Valle J, Cho JH, Marbán E. Cardiac and systemic rejuvenation after cardiosphere-derived cell therapy in senescent rats. Eur Heart J. 2017;38:2957–2967. doi: 10.1093/eurheartj/ehx454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chimenti I, Smith RR, Li TS, Gerstenblith G, Messina E, Giacomello A, Marban E. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–980. doi: 10.1161/CIRCRESAHA.109.210682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marbán L, Mendizabal A, Johnston PV et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lim GB. Stem cells: Myocardial regeneration after infarction-promising phase I trial results. Nat Rev Cardiol. 2012;9:187. doi: 10.1038/nrcardio.2012.25 [DOI] [PubMed] [Google Scholar]
- 80.Fogel J, Znamensky V. Cardiosphere-derived cells for heart regeneration. Lancet. 2012;379:2426. doi: 10.1016/S0140-6736(12)61063-0 [DOI] [PubMed] [Google Scholar]
- 81.Redgrave RE, Tual-Chalot S, Davison BJ, Singh E, Hall D, Amirrasouli MM, Gilchrist D, Medvinsky A, Arthur HM. Cardiosphere-Derived Cells Require Endoglin for Paracrine-Mediated Angiogenesis. Stem Cell Reports. 2017;8:1287–1298. doi: 10.1016/j.stemcr.2017.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao ZA, Han X, Lei W, Li J, Yang Z, Wu J, Yao M, Lu XA, He L, Chen Y et al. Lack of Cardiac Improvement After Cardiosphere-Derived Cell Transplantation in Aging Mouse Hearts. Circ Res. 2018;123:266–287. doi: 10.1161/CIRCRESAHA.118.313005 [DOI] [PubMed] [Google Scholar]
- 83.Kasai-Brunswick TH, Costa AR, Barbosa RA, Farjun B, Mesquita FC, Silva Dos Santos D, Ramos IP, Suhett G, Brasil GV, Cunha ST et al. Cardiosphere-derived cells do not improve cardiac function in rats with cardiac failure. Stem Cell Res Ther. 2017;8:36. doi: 10.1186/s13287-017-0481-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ibrahim A-E, Cheng K, Marbán E. Exosomes as Critical Agents of Cardiac Regeneration Triggered by Cell Therapy. Stem Cell Reports. 2014;2:606–619. doi: 10.1016/j.stemcr.2014.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xie Y, Ibrahim A, Cheng K, Wu Z, Liang W, Malliaras K, Sun B, Liu W, Shen D, Cheol Cho H et al. Importance of Cell-Cell Contact in the Therapeutic Benefits of Cardiosphere-Derived Cells. STEM CELLS. 2014;32:2397–2406. doi: 10.1002/stem.1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919 [DOI] [PubMed] [Google Scholar]
- 88.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028 [DOI] [PubMed] [Google Scholar]
- 89.Martin CM, Meeson AP, Robertson SM, Hawke TJ, Richardson JA, Bates S, Goetsch SC, Gallardo TD, Garry DJ. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2004;265:262–275. doi: 10.1016/j.ydbio.2003.09.028 [DOI] [PubMed] [Google Scholar]
- 90.Oyama T, Nagai T, Wada H, Naito AT, Matsuura K, Iwanaga K, Takahashi T, Goto M, Mikami Y, Yasuda N et al. Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. J Cell Biol. 2007;176:329–341. doi: 10.1083/jcb.200603014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maher TJ, Ren Y, Li Q, Braunlin E, Garry MG, Sorrentino BP, Martin CM. ATP-binding cassette transporter Abcg2 lineage contributes to the cardiac vasculature after oxidative stress. Am J Physiol Heart Circ Physiol. 2014;306:H1610–8. doi: 10.1152/ajpheart.00638.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Doyle MJ, Maher TJ, Li Q, Garry MG, Sorrentino BP, Martin CM. Abcg2-Labeled Cells Contribute to Different Cell Populations in the Embryonic and Adult Heart. Stem Cells Dev. 2016;25:277–284. doi: 10.1089/scd.2015.0272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yellamilli A, Ren Y, McElmurry RT, Lambert JP, Gross P, Mohsin S, Houser SR, Elrod JW, Tolar J, Garry DJ et al. Abcg2-expressing side population cells contribute to cardiomyocyte renewal through fusion. FASEB J. 2020. February 25 [Epub ahead of print]. doi: 10.1096/fj.201902105R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Valiente-Alandi I, Albo-Castellanos C, Herrero D, Arza E, Garcia-Gomez M, Segovia JC, Capecchi M, Bernad A. Cardiac Bmi1(+) cells contribute to myocardial renewal in the murine adult heart. Stem Cell Res Ther. 2015;6:205. doi: 10.1186/s13287-015-0196-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Valiente-Alandi I, Albo-Castellanos C, Herrero D, Sanchez I, Bernad A. Bmi1 (+) cardiac progenitor cells contribute to myocardial repair following acute injury. Stem Cell Res Ther. 2016;7:100. doi: 10.1186/s13287-016-0355-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tian X, Pu WT, Zhou B. Cellular Origin and Developmental Program of Coronary Angiogenesis. Circ Res. 2015;116:515–530. doi: 10.1161/CIRCRESAHA.116.305097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–974. doi: 10.1038/nm1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Steinhauser ML, Bailey AP, Senyo SE, Guillermier C, Perlstein TS, Gould AP, Lee RT, Lechene CP. Multi-isotope imaging mass spectrometry quantifies stem cell division and metabolism. Nature. 2012;481:516–519. doi: 10.1038/nature10734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436. doi: 10.1038/nature11682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li Y, He L, Huang X, Issa Bhaloo S, Zhao H, Zhang S, Pu W, Tian X, Li Y, Liu Q et al. Genetic Lineage Tracing of Non-Myocyte Population by Dual Recombinases. Circulation. 2018;138:793–805. doi: 10.1161/CIRCULATIONAHA.118.034250 [DOI] [PubMed] [Google Scholar]
- 101.Li Y, Lv Z, He L, Huang X, Zhang S, Zhao H, Pu W, Li Y, Yu W, Zhang L et al. Genetic Tracing Identifies Early Segregation of the Cardiomyocyte and Nonmyocyte Lineages. Circ Res. 2019;125:343–355. doi: 10.1161/circresaha.119.315280 [DOI] [PubMed] [Google Scholar]
- 102.Kretzschmar K, Post Y, Bannier-Hélaouët M, Mattiotti A, Drost J, Basak O, Li VSW, van den Born M, Gunst QD, Versteeg D et al. Profiling proliferative cells and their progeny in damaged murine hearts. Proc Natl Acad Sci U S A. 2018;115:E12245–E12254. doi: 10.1073/pnas.1805829115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maliken BD, Molkentin JD. Undeniable Evidence That the Adult Mammalian Heart Lacks an Endogenous Regenerative Stem Cell. Circulation. 2018;138:806–808. doi: 10.1161/CIRCULATIONAHA.118.035186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Polezhaev LV, Kolchin SP, Solntseva GN. [Stimulation of regeneration of the heart muscle in the course of diphtherial myocarditis]. Dokl Akad Nauk SSSR. 1965;164:949–952. doi: no DOI [PubMed] [Google Scholar]
- 105.Sadokova IE. [Changes in nucleic acid content in the myocardium damaged by diphtheria and stimulation of restoratove processes]. Dokl Akad Nauk SSSR. 1967;177:246–248. doi: no DOI [PubMed] [Google Scholar]
- 106.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303 [DOI] [PubMed] [Google Scholar]
- 107.Wei K, Serpooshan V, Hurtado C, Diez-Cunado M, Zhao M, Maruyama S, Zhu W, Fajardo G, Noseda M, Nakamura K et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature. 2015;525:479–485. doi: 10.1038/nature15372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yu W, Huang X, Tian X, Zhang H, He L, Wang Y, Nie Y, Hu S, Lin Z, Zhou B et al. GATA4 regulates Fgf16 to promote heart repair after injury. Development. 2016;143:936–949. doi: 10.1242/dev.130971 [DOI] [PubMed] [Google Scholar]
- 109.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, Dos Remedios C et al. Dynamics of Cell Generation and Turnover in the Human Heart. Cell. 2015;161:1566–1575. doi: 10.1016/j.cell.2015.05.026 [DOI] [PubMed] [Google Scholar]
- 111.Zong H, Espinosa JS, Su HH, Muzumdar MD, Luo L. Mosaic analysis with double markers in mice. Cell. 2005;121:479–492. doi: 10.1016/j.cell.2005.02.012 [DOI] [PubMed] [Google Scholar]
- 112.Volz KS, Jacobs AH, Chen HI, Poduri A, McKay AS, Riordan DP, Kofler N, Kitajewski J, Weissman I, Red-Horse K. Pericytes are progenitors for coronary artery smooth muscle. Elife. 2015;4:10.7554/eLife.10036. doi: 10.7554/eLife.10036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Riccio P, Cebrian C, Zong H, Hippenmeyer S, Costantini F. Ret and Etv4 Promote Directed Movements of Progenitor Cells during Renal Branching Morphogenesis. PLoS Biol. 2016;14:e1002382. doi: 10.1371/journal.pbio.1002382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu C, Sage JC, Miller MR, Verhaak RG, Hippenmeyer S, Vogel H, Foreman O, Bronson RT, Nishiyama A, Luo L et al. Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell. 2011;146:209–221. doi: 10.1016/j.cell.2011.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ali SR, Hippenmeyer S, Saadat LV, Luo L, Weissman IL, Ardehali R. Existing cardiomyocytes generate cardiomyocytes at a low rate after birth in mice. Proc Natl Acad Sci U S A. 2014;111:8850–8855. doi: 10.1073/pnas.1408233111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sereti K-I, Nguyen NB, Kamran P, Zhao P, Ranjbarvaziri S, Park S, Sabri S, Engel JL, Sung K, Kulkarni RP et al. Analysis of cardiomyocyte clonal expansion during mouse heart development and injury. Nat Commun. 2018;9:98. doi: 10.1038/s41467-018-02891-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Basak O, van de Born M, Korving J, Beumer J, van der Elst S, van Es JH, Clevers H. Mapping early fate determination in Lgr5+ crypt stem cells using a novel Ki67-RFP allele. EMBO J. 2014;33:2057–2068. doi: 10.15252/embj.201488017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Basak O, Krieger TG, Muraro MJ, Wiebrands K, Stange DE, Frias-Aldeguer J, Rivron NC, van de Wetering M, van Es JH, van Oudenaarden A et al. Troy+ brain stem cells cycle through quiescence and regulate their number by sensing niche occupancy. Proc Natl Acad Sci U S A. 2018;115:E610–E619. doi: 10.1073/pnas.1715911114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857 [DOI] [PubMed] [Google Scholar]
- 120.Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY, Poss KD. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464:601–605. doi: 10.1038/nature08804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Patterson M, Barske L, Van Handel B, Rau CD, Gan P, Sharma A, Parikh S, Denholtz M, Huang Y, Yamaguchi Y et al. Frequency of mononuclear diploid cardiomyocytes underlies natural variation in heart regeneration. Nat Genet. 2017;49:1346–1353. doi: 10.1038/ng.3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.González-Rosa JM, Sharpe M, Field D, Soonpaa MH, Field LJ, Burns CE, Burns CG. Myocardial Polyploidization Creates a Barrier to Heart Regeneration in Zebrafish. Dev Cell. 2018;44:433–446.e7. doi: 10.1016/j.devcel.2018.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hirose K, Payumo AY, Cutie S, Hoang A, Zhang H, Guyot R, Lunn D, Bigley RB, Yu H, Wang J et al. Evidence for hormonal control of heart regenerative capacity during endothermy acquisition. Science. 2019;364:184–188. doi: 10.1126/science.aar2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ptaszek LM, Mansour M, Ruskin JN, Chien KR. Towards regenerative therapy for cardiac disease. Lancet. 2012;379:933–942. doi: 10.1016/S0140-6736(12)60075-0 [DOI] [PubMed] [Google Scholar]
- 125.Garbern JC, Lee RT. Cardiac stem cell therapy and the promise of heart regeneration. Cell Stem Cell. 2013;12:689–698. doi: 10.1016/j.stem.2013.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lin Z, Pu WT. Strategies for Cardiac Regeneration and Repair. Sci Transl Med 2014;6:239rv1. doi: 10.1126/scitranslmed.3006681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Foglia MJ, Poss KD. Building and re-building the heart by cardiomyocyte proliferation. Development. 2016;143:729–740. doi: 10.1242/dev.132910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pasumarthi KB, Field LJ. Cardiomyocyte cell cycle regulation. Circ Res. 2002;90:1044–1054. doi: 10.1161/01.res.0000020201.44772.67 [DOI] [PubMed] [Google Scholar]
- 129.Soonpaa MH, Koh GY, Pajak L, Jing S, Wang H, Franklin MT, Kim KK, Field LJ. Cyclin D1 overexpression promotes cardiomyocyte DNA synthesis and multinucleation in transgenic mice. J Clin Invest. 1997;99:2644–2654. doi: 10.1172/JCI119453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chaudhry HW, Dashoush NH, Tang H, Zhang L, Wang X, Wu EX, Wolgemuth DJ. Cyclin A2 mediates cardiomyocyte mitosis in the postmitotic myocardium. J Biol Chem. 2004;279:35858–35866. doi: 10.1074/jbc.M404975200 [DOI] [PubMed] [Google Scholar]
- 131.Cheng RK, Asai T, Tang H, Dashoush NH, Kara RJ, Costa KD, Naka Y, Wu EX, Wolgemuth DJ, Chaudhry HW. Cyclin A2 induces cardiac regeneration after myocardial infarction and prevents heart failure. Circ Res. 2007;100:1741–1748. doi: 10.1161/CIRCRESAHA.107.153544 [DOI] [PubMed] [Google Scholar]
- 132.Shapiro SD, Ranjan AK, Kawase Y, Cheng RK, Kara RJ, Bhattacharya R, Guzman-Martinez G, Sanz J, Garcia MJ, Chaudhry HW. Cyclin A2 induces cardiac regeneration after myocardial infarction through cytokinesis of adult cardiomyocytes. Sci Transl Med 2014;6:224ra27. doi: 10.1126/scitranslmed.3007668 [DOI] [PubMed] [Google Scholar]
- 133.Mohamed TMA, Ang YS, Radzinsky E, Zhou P, Huang Y, Elfenbein A, Foley A, Magnitsky S, Srivastava D. Regulation of Cell Cycle to Stimulate Adult Cardiomyocyte Proliferation and Cardiac Regeneration. Cell. 2018;173:104–116.e12. doi: 10.1016/j.cell.2018.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mahmoud AI, Kocabas F, Muralidhar SA, Kimura W, Koura AS, Thet S, Porrello ER, Sadek HA. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature. 2013;497:249–253. doi: 10.1038/nature12054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, Martin JF. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–461. doi: 10.1126/science.1199010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci U S A. 2013;110:13839–13844. doi: 10.1073/pnas.1313192110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lin Z, von Gise A, Zhou P, Gu F, Ma Q, Jiang J, Yau AL, Buck JN, Gouin KA, van Gorp PR et al. Cardiac-specific YAP activation improves cardiac function and survival in an experimental murine MI model. Circ Res. 2014;115:354–363. doi: 10.1161/CIRCRESAHA.115.303632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Leach JP, Heallen T, Zhang M, Rahmani M, Morikawa Y, Hill MC, Segura A, Willerson JT, Martin JF. Hippo pathway deficiency reverses systolic heart failure after infarction. Nature. 2017;550:260–264. doi: 10.1038/nature24045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060 [DOI] [PubMed] [Google Scholar]
- 141.D’Uva G, Aharonov A, Lauriola M, Kain D, Yahalom-Ronen Y, Carvalho S, Weisinger K, Bassat E, Rajchman D, Yifa O et al. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat Cell Biol. 2015;17:627–638. doi: 10.1038/ncb3149 [DOI] [PubMed] [Google Scholar]
- 142.Eulalio A, Mano M, Ferro MD, Zentilin L, Sinagra G, Zacchigna S, Giacca M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492:376–381. doi: 10.1038/nature11739 [DOI] [PubMed] [Google Scholar]
- 143.Gabisonia K, Prosdocimo G, Aquaro GD, Carlucci L, Zentilin L, Secco I, Ali H, Braga L, Gorgodze N, Bernini F et al. MicroRNA therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs. Nature. 2019;569:418–422. doi: 10.1038/s41586-019-1191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Aguirre A, Montserrat N, Zacchigna S, Nivet E, Hishida T, Krause MN, Kurian L, Ocampo A, Vazquez-Ferrer E, Rodriguez-Esteban C et al. In vivo activation of a conserved microRNA program induces mammalian heart regeneration. Cell Stem Cell. 2014;15:589–604. doi: 10.1016/j.stem.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Feng T, Meng J, Kou S, Jiang Z, Huang X, Lu Z, Zhao H, Lau LF, Zhou B, Zhang H. CCN1-Induced Cellular Senescence Promotes Heart Regeneration. Circulation. 2019;139:2495–2498. doi: 10.1161/CIRCULATIONAHA.119.039530 [DOI] [PubMed] [Google Scholar]
- 146.Sarig R, Rimmer R, Bassat E, Zhang L, Umansky KB, Lendengolts D, Perlmoter G, Yaniv K, Tzahor E. Transient p53-Mediated Regenerative Senescence in the Injured Heart. Circulation. 2019;139:2491–2494. doi: 10.1161/CIRCULATIONAHA.119.040125 [DOI] [PubMed] [Google Scholar]
- 147.Boström P, Mann N, Wu J, Quintero PA, Plovie ER, Panáková D, Gupta RK, Xiao C, MacRae CA, Rosenzweig A et al. C/EBPβ controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell. 2010;143:1072–1083. doi: 10.1016/j.cell.2010.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vujic A, Lerchenmüller C, Wu TD, Guillermier C, Rabolli CP, Gonzalez E, Senyo SE, Liu X, Guerquin-Kern JL, Steinhauser ML et al. Exercise induces new cardiomyocyte generation in the adult mammalian heart. Nat Commun. 2018;9:1659. doi: 10.1038/s41467-018-04083-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zacchigna S, Martinelli V, Moimas S, Colliva A, Anzini M, Nordio A, Costa A, Rehman M, Vodret S, Pierro C et al. Paracrine effect of regulatory T cells promotes cardiomyocyte proliferation during pregnancy and after myocardial infarction. Nat Commun. 2018;9:2432. doi: 10.1038/s41467-018-04908-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kimura W, Xiao F, Canseco DC, Muralidhar S, Thet S, Zhang HM, Abdulrahman Y, Chen R, Garcia JA, Shelton JM et al. Hypoxia fate mapping identifies cycling cardiomyocytes in the adult heart. Nature. 2015;523:226–230. doi: 10.1038/nature14582 [DOI] [PubMed] [Google Scholar]
- 151.Nakada Y, Canseco DC, Thet S, Abdisalaam S, Asaithamby A, Santos CX, Shah AM, Zhang H, Faber JE, Kinter MT et al. Hypoxia induces heart regeneration in adult mice. Nature. 2017;541:222–227. doi: 10.1038/nature20173 [DOI] [PubMed] [Google Scholar]
- 152.Song K, Nam Y-J, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–598. doi: 10.1038/nature11044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Huang C, Tu W, Fu Y, Wang J, Xie X. Chemical-induced cardiac reprogramming in vivo. Cell Res. 2018;28:686–689. doi: 10.1038/s41422-018-0036-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Palpant NJ, Gantz JA et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shiba Y, Fernandes S, Zhu W-Z, Filice D, Muskheli V, Kim J, Palpant NJ, Gantz J, Moyes KW, Reinecke H et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489:322–325. doi: 10.1038/nature11317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yan W, Abu-El-Rub E, Saravanan S, Kirshenbaum LA, Arora RC, Dhingra S. Inflammation in myocardial injury: mesenchymal stem cells as potential immunomodulators. Am J Physiol Heart Circ Physiol. 2019;317:H213–H225. doi: 10.1152/ajpheart.00065.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Park SJ, Kim RY, Park BW, Lee S, Choi SW, Park JH, Choi JJ, Kim SW, Jang J, Cho DW et al. Dual stem cell therapy synergistically improves cardiac function and vascular regeneration following myocardial infarction. Nat Commun. 2019;10:3123. doi: 10.1038/s41467-019-11091-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bargehr J, Ong LP, Colzani M, Davaapil H, Hofsteen P, Bhandari S, Gambardella L, Le Novère N, Iyer D, Sampaziotis F et al. Epicardial cells derived from human embryonic stem cells augment cardiomyocyte-driven heart regeneration. Nat Biotechnol. 2019;37:895–906. doi: 10.1038/s41587-019-0197-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen CH, Wei HJ, Lin WW, Chiu I, Hwang SM, Wang CC, Lee WY, Chang Y, Sung HW. Porous tissue grafts sandwiched with multilayered mesenchymal stromal cell sheets induce tissue regeneration for cardiac repair. Cardiovasc Res. 2008;80:88–95. doi: 10.1093/cvr/cvn149 [DOI] [PubMed] [Google Scholar]
- 161.Ye L, Chang YH, Xiong Q, Zhang P, Zhang L, Somasundaram P, Lepley M, Swingen C, Su L, Wendel JS et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell. 2014;15:750–761. doi: 10.1016/j.stem.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zangi L, Lui KO, von Gise A, Ma Q, Ebina W, Ptaszek LM, Spater D, Xu H, Tabebordbar M, Gorbatov R et al. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat Biotechnol. 2013;31:898–907. doi: 10.1038/nbt.2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chien KR, Zangi L, Lui KO. Synthetic Chemically Modified mRNA (modRNA): Toward a New Technology Platform for Cardiovascular Biology and Medicine. Cold Spring Harbor Perspectives in Medicine. 2015;5:a014035–a014035. doi: 10.1101/cshperspect.a014035 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


