Abstract
For years, surgeons have explored the relationship between age and surgical outcomes. Over time, it is more widely accepted that frailty and fitness of older patients, rather than their age, should be considered in surgical decision making. The gold standard of frailty assessment is comprehensive geriatric assessment (CGA) which is best performed by geriatricians. In the past decade, Digital Health Technologies that range from electronic solutions for electronic Patient-reported Outcomes to wearables and sensors have emerged. As these solutions are likely to expand and advance in the next years, we will review the history of investigating factors, especially aging-related factors associated with surgical outcomes, and the current supportive data about the potential and challenges of Digital Health Technologies in complementing or replacing some of the components of CGA by 2025.
Keywords: Colorectal, Comprehensive geriatric assessment, Geriatric oncology, Digital health technology
Introduction
For decades, investigators have assessed the relationship between age and surgical outcomes. In 1947, Cutler described the outcomes of 204 patients who underwent urgent surgeries and laid out the solutions for improving the outcome of older patients [1]. He wrote “these older patients are peculiarly susceptible to pneumonia, wound disruptions and sepsis. Many of them succumb, even weeks after the operation, to cerebral-vascular accidents, to coronary occlusions, uremia, anuria, and cardio-vascular-renal collapse”. He continued that while the mortality rate of urgent surgery among older patients was high (44%), it salvaged the life of others who without surgery would have surely succumb to their acute illness. He suggested that surgical teams follow four principles of therapy in order to improve the mortality rate associated with urgent surgery among the aged population; 1) support and protect the patient by every available measure, 2) perform surgery with the least possible delay, 3) perform the simplest needed surgery with the least trauma to relieve the acute condition, and 4) to use every available means to protect patient from postoperative complications and to ensure early detection of such events. In 1970, Jensen and colleagues posed five questions to the researcher community on surgical decision making for older cancer patients in need of elective surgery that are still very relevant [2]. Their posed questions were proper assessment of the likelihood that the older patient survives the surgery, the feasibility and safety of postponing surgery until patient acutely develop symptoms, the optimum surgical resection of the disease and number of surgical sessions needed for optimum surgical resection, and proper assessment and prognosis and life expectancy of patients with or without undergoing surgery.
However, studies on the relationship between age and surgical outcomes have been inconsistent. A study on 217 patients who underwent surgery for colon cancer from 1982 to 1986 showed that perioperative mortality rate among patients younger than age 75 was slightly lower than patients aged 75 or older (3.6% vs. 7.1%). However, none of nine patients aged 90 and older died during the perioperative period [3]. Another study compared the surgical outcomes of 310 patients aged 75 and older with 710 younger patients who underwent colorectal surgeries from 1971 to 1983. While the overall hospital mortality was 4.6%, the rate was 9% for older patients and 3% for younger patients [4]. Another study in 1986, assessed the difference in 3-year survival rate and its cost for 80 patients aged 80 and older vs. 219 younger patients [5].
They found that there was no difference in 3-year survival between two groups, although the hospital length of stay was slightly higher for older patients than younger patients (18 vs. 15 days) and the cost was also slightly higher (~7800 dollars vs. 6400 dollars). Authors concluded that the colon cancer in the very old patients is justifiable and age should not be the reasons for excluding them from surgery. Another study in the same period advanced the field by assessing the relationship between age and comorbid conditions with surgical outcomes of colon cancer patients [6]. They compared the outcomes of 163 patients aged 70 and older with a cohort of younger patients. While they showed that younger patients were more likely to survive the surgery, the survival was mainly attributable to lack of comorbid conditions such as congestive heart failure, and better organ functions. They concluded that an older and younger patient with the same level of organ function and comorbid conditions have the same likelihood of surviving the surgery.
Moving beyond age; the concept of frailty and surgical outcomes
Frailty and surgical outcomes
Aging is associated with an accumulation of physiologic and pathologic deficits. There is a higher prevalence of comorbidities, decline of the functional reserve, and progressive restriction in personal and social resources, which result in a greater vulnerability to important outcomes such as functional decline, institutionalization and falls [7]. There is wide heterogeneity in this process as not all people age in the same way. Hence patients of the same age can fall into different points on a fit to frail spectrum. Frailty is a clinical syndrome of physiologic decline and decreased reserves, with a reduced ability to withstand stressors. Both cancer and cancer therapies act as significant stressors. The inability to withstand stressors in frailty is associated with increased vulnerability to adverse outcomes and intolerance to surgical interventions. Thus, the concept of frailty has gained importance in the provision of health care and the measurement of health outcomes. A large study of 592 patients aged 75 years or older who underwent oncologic abdominopelvic surgery showed that those with preoperative dependency for activities of daily living (ADL) were 2.2-fold more likely to need skilled services after surgery [8].
Two major frailty models have been described in the literature [9].
The frailty phenotype defines frailty as a distinct clinical syndrome meeting three or more of five phenotypic criteria: weakness, slowness, low level of physical activity, self-reported exhaustion, and unintentional weight loss.
The frailty index defines frailty as cumulative deficits identified in a comprehensive geriatric assessment (CGA).
Several tools exist for estimating frailty. Variations of the Fried Criteria [7] or instruments based on CGA [10], including the Frailty Index, are used to assess frailty in many studies. Fagard et al. [11] conducted a systematic review analyzing five articles, involving four studies and 486 participants (≥65years) who underwent elective colorectal cancer surgery. Regardless of varying definitions of frailty and postoperative outcomes, the frail patients had less favorable outcomes in all the studies. Compared to the non-frail group, the frail group had a higher risk of developing moderate to severe postoperative complications, had longer hospital stays, higher readmission rates, and decreased long-term survival rates.
Investigators have developed a modified frailty index (m-FI) that can be applied to the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) dataset, with the higher score representing greater frailty [12]. Using this index, many studies have shown the relationship between frailty and surgical outcomes. A study of 6551 women who underwent gynecologic cancer surgery showed that while the rate of grade IV or V complications was 2% in fit patients (m-FI score = 0), the rate was 24.4% among patients with an m-FI score ≥ 4 [13]. There are however limitations to the use of m-FI in clinical practice and decision making, particularly because the index can only be calculated retrospectively.
Geriatricians and geriatric oncologists argue that the gold standard of assessing frailty is through CGA, which is a multidimensional evaluation of older adults that includes assessment of their functional activity, cognitive and nutritional status, social support, polypharmacy, and comorbid conditions [14]. Through this assessment, impairments and factors that lead to frailty in patients can be identified, and modifications and interventions can be implemented in promptly. Studies looking at the relationship between frailty through CGA and surgical outcomes in older patients, and specifically in older patients with cancer, are emerging. A systematic review from 2016 [15] analyzed 23 studies on postoperative outcomes in older surgical patients (mean age ranged from 75 to 87) undergoing cardiac, oncological, general, vascular and hip fracture surgeries. It showed strong evidence that frailty in older-old (75—85 years) and oldest-old (over 85 years) surgical patients predicts postoperative mortality, complications, and prolonged length of stay. A study of 178 patients aged 70 years or older who underwent rectal surgery in Norway showed that 62% of frail patients experienced severe complications, compared with 33% of fit patients [16]. There has also been interest in whether certain components of the CGA are more predictive of postoperative outcomes. A systematic review from 2015 reviewed six studies and looked at individual components of the CGA as predictors of adverse outcomes among geriatric patients undergoing major oncologic surgery [17]. Deficiencies in instrumental activities of daily living, activities of daily living, fatigue, cognition, frailty, and cognitive impairment were associated with increased 30-day postoperative complications. Additionally, frailty, deficiencies in instrumental activities of daily living, and depression predicted discharge to a nonhome institution. In this systematic review, age was not found to be a predictor of either overall or major complications. It is clear from current literature that frailty status rather than chronologic age should be used in surgical decision making and postoperative outcome measurements in geriatric oncology.
Novel technologies to assess functional and frailty status of older patients
Over the past decades, technology, especially Digital Health technology has expanded rapidly. Digital Health technology includes a wide variety of web-based and wireless solutions that range from video conferencing solution to wearable devices [18]. In its broadest form, the Digital Health Technologies are formed under the umbrella of the Internet of Things or IoT [19]. Dr. Fallahzadeh and colleagues have depicted a framework on the interaction between various digital technologies with each other and with the healthcare provider [20]. (Fig. 1) These technologies provide unique opportunities for surgical teams to have a more robust assessment of older cancer patients, but they are also not without challenges. In this section, we will review some of the more commonly used digital health technologies in the past decade.
Fig. 1.
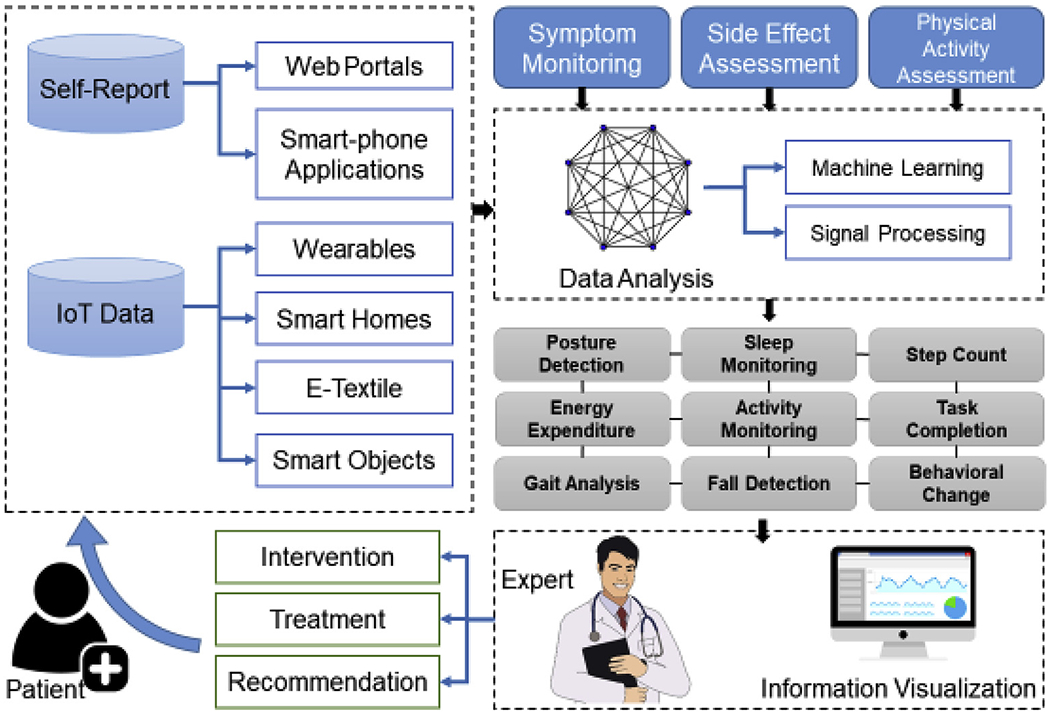
Framework of interaction between digital health technologies and healthcare providers within the umbrella of internet of things.
Electronic patient reported outcomes
Traditionally, solutions for Patient-Reported Outcomes (ePRO) have been used for research purposes [21]. However, a seminal study by Basch and colleagues showed the utility of ePROs in the routine care of cancer patients [22]. In this study, 766 patients who were receiving chemotherapy were randomized to either routine care or reporting their symptoms in and between clinic appointments via a web-based solution. Subsequently, the electronically generated report of their symptoms was presented to the medical oncology team and interventions would have been conducted to improve upon the identified symptoms. The intervention led to improvement in the overall survival of patients (31.2 months vs. 26 months, p = 0.03) compared to the patients in routine care. Before showing the improvement in overall survival of patients, the authors showed the improvement in Health-related Quality of Life of patients in the intervention arm compared to control arm (34% vs. 18%), and less likelihood of Emergency Room visit (34% vs. 41%) [23]. While the study is very promising in opening a new field in the assessment and management of cancer patients, applying the same solution in the care of older cancer patients could be challenging. The study participants had a median age of 61, which is somewhat younger than the median age of many newly diagnosed patients with cancer. Participants who did not have any computer-related experience were older (67 vs. 60) and were more likely to less educated compared to those who had some computer experience. Nonetheless, over time, the use of ePROs for both research and routine care has increased [24]. And as a result, calls have been made to test the feasibility of ePROs in postsurgical monitoring of patients [25]. In a study of 977 patients who underwent various surgical procedures, patients were randomized to either their symptoms on postoperative day seven and 14 via a mobile application, and receive a phone call from a registered nurse if needed, or the routine postoperative care management. Like Basch study, the enrolled patients were relatively young with an average age of 45. At the end of the study, patients in the intervention arm experienced an improvement in seven out of 24 assessed symptoms compared to the control arm [26]. Another study assessed the feasibility of implementing ePRO in a multicenter clinical trial. In this study, 500 patients with locally advanced rectal cancer completed ePRO during the neoadjuvant treatment and postoperatively. With a median age of 56 years, 43% of the studied population had high school education or less. The completion rate of assessment ranged from 92% in the preoperative period to 71% in the postoperative period [27].
In the geriatric surgical oncology setting, a study on electronic Rapid Fitness Assessment (eRFA), a web-based geriatric assessment, showed that among 636 patients with median age of 80, the time to complete the assessment was 11 min, and only 13% of patients needed someone else to complete the assessment for them. The authors assessed the satisfaction of 50 patients with the eRFA, and 88% expressed that answering the questions via a web-based solution was easy [28]. In another study assessed the implementation of the eRFA as a point of care in the thoracic surgery clinics. Out of 83 eligible patients, 65 patients with the median age of 71 completed the eRFA. Among these, 75% of patients were able to complete the assessment on their own and 25% needed partial or complete assistance with completing the assessment [29]. Due to rapid advances in technology, development of more patient-friendly web-based solutions to collect data, and the increase in the aging population with more familiarity with the technology, it is very plausible that by 2025, the majority of geriatric assessment be performed using web-based platforms.
Wearables and senors
Proper assessment of perioperative functional status is critical in surgical decision making and functional recovery of older cancer patients. Traditionally, the assessment of the functional status of cancer patients is by Karnofsky Performance Status (KPS) [30] or Eastern Cooperative Oncology Group — Performance Status (ECOG-PS) [31]. However, these assessments are prone to biases and may have a high degree of interobserver variability [32]. As a result, a more objective method of assessing the functional status of cancer patients is needed. Wearable activity tracker devices, while still being mainly used as a wellness device, can aid in assessing the functional status of cancer patients. Most of the devices can now assess the number of daily steps accurately in the general population [32]. However, assessing the number of steps among older patients with very slow gait speed or those who use assistive devices such as walker is still challenging [33]. Nonetheless, in addition to assessing the number of daily steps, these devices may act as a motivator to increase the activity level. A systematic review of 26 studies with a total of 2767 participants showed that those who wore activity trackers increased their physical activity by almost 27% over their baseline [34]. These devices have also started to emerge as a monitoring tool for postoperative recovery. A study on 20 patients who underwent major abdominal cancer surgery who wore activity trackers 3—7 days before surgery, during hospitalization and up to two weeks after hospital discharge showed that adherence to wearing activity tracker was high (88% before surgery and 83% after surgery). Patients number of daily steps on day 7 after surgery was only 19% of preoperative activity level and correlated with comprehensive complication index [35]. With the recent increased interest in optimizing the functional activity of patients before surgery through prehabilitation programs, these devices might help monitor older patients’ adherence to such programs [36]. Data is also emerging on the correlation between the data from the wearable activity trackers and frailty. In a study on 125 older adults (fit: 44, pre-frail:60, frail:21), gait speed and the number of steps per day, both measured by the wearable trackers, had the strongest correlation with pre-frailty status. Moreover, the stride length was the most sensitive test to discriminate between the three frailty categories [37]. In another study, a sensor was attached to the upper arm of participants and they were asked to flex their muscles as fast and as forcefully as possible for 20 s. Prefrail and frail patients were able to flex the biceps much slower than fit patients (29% less in prefrail and 59% less in frail patients) [38]. Such sensor has also been shown to be useful in predicting the frailty of older patients hospitalized after fall-related injuries [39]. Another system is Real-time tracking systems (RTLS). These systems allow the monitoring of patients’ movement within the healthcare system (e.g., hospital) following hospitalization and/or surgery [40]. Wake Forest Baptist Health used RTLS to track the location of patients after major abdominal surgeries in ten patients. The RTLS was able to identify all of the movement of these patients through different locations correctly [41]. Healthcare institutions experience a high incidence of geriatric events following major cancer surgery among older patients which prolongs hospitalization, incurs higher cost, and increases the mortality of these patients [42]. As a result, systems such as RTLS might help detect and manage these events earlier in the postoperative setting.
Conclusion
For decades, surgical teams were concerned about the outcomes of older patients and methods of improving such outcomes. Frailty assessment of older patients plays a crucial role in differentiating older patients who may do well with surgery from those who may not. The gold standard of assessing frailty is CGA. However, as Digital Health Technologies are emerging, there are opportunities for easier and more reliable assessment of older patients. It is foreseeable that by 2025, at least portions of traditional CGA be replaced by the data collected from these solutions.
Acknowledgements
The project was supported, in part, by the Beatriz and Samuel Seaver Foundation, the Memorial Sloan Kettering Cancer and Aging Program, the NIH/NCI Cancer Center Support Grant P30 CA008748. The manufacture of the device used in this study did not have any role in the design, conduct, and final analysis of the data. Any opinions, findings, conclusions, or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the funding organizations.
References
- [1].Cutler CW Jr. Urgent surgery in the aged. Ann Surg 1947;126(5):763. [PubMed] [Google Scholar]
- [2].Jensen H, Nielsen J, Balslev I. Carcinoma of the colon in old age. Ann Surg 1970;171(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bader TF. Colorectal cancer in patients older than 75 years of age. Dis Colon Rectum 1986;29(11):728–32. [DOI] [PubMed] [Google Scholar]
- [4].Payne JE, Chapuis PH, Pheils MT. Surgery for large bowel cancer in people aged 75 years and older. Dis Colon Rectum 1986;29(11):733–7. [DOI] [PubMed] [Google Scholar]
- [5].Hobler KE. Colon surgery for cancer in the very elderly. Cost and 3-year survival. Ann Surg 1986;203(2):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Greenburg AG, Saik RP, Pridham D. Influence of age on mortality of colon surgery. Am J Surg 1985;150(1):65–70. [DOI] [PubMed] [Google Scholar]
- [7].Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56(3):M146–56. [DOI] [PubMed] [Google Scholar]
- [8].Alexander K, Shahrokni A, Korc-Grodzicki B. Post surgical skilled care utilization at hospital discharge in older cancer patients. J Geriatr Oncol 2014;5: S46–7. [Google Scholar]
- [9].Chen X, Mao G, Leng SX. Frailty syndrome: an overview. Clin Interv Aging 2014;9:433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Extermann M, Aapro M, Bernabei R, Cohen HJ, Droz J-P, Lichtman S, et al. Use of comprehensive geriatric assessment in older cancer patients:: Recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit Rev Oncol Hematol 2005;55(3):241–52. [DOI] [PubMed] [Google Scholar]
- [11].Fagard K, Leonard S, Deschodt M, Devriendt E, Wolthuis A, Prenen H, et al. The impact of frailty on postoperative outcomes in individuals aged 65 and over undergoing elective surgery for colorectal cancer: A systematic review. J Geriatr Oncol 2016;7(6):479–91. [DOI] [PubMed] [Google Scholar]
- [12].Tsiouris A, Hammoud ZT, Velanovich V, Hodari A, Borgi J, Rubinfeld I. A modified frailty index to assess morbidity and mortality after lobectomy. J Surg Res 2013;183(1):40–6. [DOI] [PubMed] [Google Scholar]
- [13].Uppal S, Igwe E, Rice LW, Spencer RJ, Rose SL. Frailty index predicts severe complications in gynecologic oncology patients. Gynecol Oncol 2015;137(1): 98–101. [DOI] [PubMed] [Google Scholar]
- [14].VanderWalde N, Jagsi R, Dotan E, Baumgartner J, Browner IS, Burhenn P, et al. NCCN Guidelines Insights: Older Adult Oncology, Version 2.2016. J Natl Compr Cancer Netw 2016;14(11):1357–70. [DOI] [PubMed] [Google Scholar]
- [15].Lin H-S, Watts JN, Peel NM, Hubbard RE. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr 2016;16(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kristjansson SR, Nesbakken A, Jordhøy MS, Skovlund E, Audisio RA, Johannessen H-O, et al. Comprehensive geriatric assessment can predict complications in elderly patients after elective surgery for colorectal cancer: A prospective observational cohort study. Crit Rev Oncol Hematol 2010;76(3): 208–17. [DOI] [PubMed] [Google Scholar]
- [17].Feng MA, McMillan DT, Crowell K, Muss H, Nielsen ME, Smith AB. Geriatric assessment in surgical oncology: A systematic review. J Surg Res 2015;193(1): 265–72. Pmc4267910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Santos A, Macedo J, Costa A, Nicolau MJJPT. Internet of things and smart objects for M-health monitoring and control 2014;16:1351–60. [Google Scholar]
- [19].Dimitrov DVJHir. Medical internet of things and big data in healthcare 2016;22:156–63 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fallahzadeh R, Rokni SA, Ghasemzadeh H, Soto-Perez-de-Celis E, Shahrokni AJJcci. Digital health for geriatric oncology 2018;2:1–12. [DOI] [PubMed] [Google Scholar]
- [21].Trotti A, Colevas AD, Setser A, Basch EJJoco. Patient-reported outcomes and the evolution of adverse event reporting in oncology 2007;25(32):5121–7. [DOI] [PubMed] [Google Scholar]
- [22].Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 2017;318(2):197–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Basch E, Deal AM, Kris MG, Scher HI, Hudis CA, Sabbatini P, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol 2016;34(6):557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jensen RE, Snyder CF, Abernethy AP, Basch E, Potosky AL, Roberts AC, et al. Review of electronic patient-reported outcomes systems used in cancer clinical care. J Oncol Pract 2013;10(4):e215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Smith AB, Basch EJJoop. Role of patient-reported outcomes in postsurgical monitoring in oncology 2017;13:535–8 (8). [DOI] [PubMed] [Google Scholar]
- [26].Jaensson M, Dahlberg K, Eriksson M, Nilsson UJBBJoA. Evaluation of post-operative recovery in day surgery patients using a mobile phone application: a multicentre randomized trial 2017;119:1030–8 (5). [DOI] [PubMed] [Google Scholar]
- [27].Basch E, Dueck AC, Rogak LJ, Mitchell SA, Minasian LM, Denicoff AM, et al. Feasibility of implementing the patient-reported outcomes version of the common terminology criteria for adverse events in a multicenter trial. NCCTG N1048 2018;36(31):3120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shahrokni A, Tin A, Downey RJ, Strong V, Mahmoudzadeh S, Boparai MK, et al. Electronic rapid fitness assessment: A novel tool for preoperative evaluation of the geriatric oncology patient. J Natl Compr Canc Netw 2017;15(2):172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Downey RJ, Korc-Grodzicki B, Weber R, Vickers AJ, Jones DR, Bains MS, et al. Assessing the clinical feasibility of implementing a novel assessment of frailty: the electronic rapid fitness assessment in diverse thoracic surgery clinics. Am Soc Clin Oncol 2017. [Google Scholar]
- [30].Schag CC, Heinrich RL, Ganz P. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol 1984;2(3):187–93. [DOI] [PubMed] [Google Scholar]
- [31].Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol 1998;16(4):1582–7. [DOI] [PubMed] [Google Scholar]
- [32].Kelly CM, Shahrokni AJJoo. Moving beyond Karnofsky and ECOG performance status assessments with new technologies, 2016; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Alinia P, Cain C, Fallahzadeh R, Shahrokni A, Cook D, Ghasemzadeh H. How Accurate Is Your Activity Tracker? A Comparative Study of Step Counts in Low-Intensity Physical Activities. JMIR mHealth and uHealth 2017;5(8):e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, et al. Using pedometers to increase physical activity and improve health: a systematic review 2007;298(19):2296–304. [DOI] [PubMed] [Google Scholar]
- [35].Sun V, Dumitra S, Ruel N, Lee B, Melstrom L, Melstrom K, et al. Wireless monitoring program of patient-centered outcomes and recovery before and after major abdominal cancer surgery 2017;152:852–9 (9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rumer KK, Saraswathula A, Melcher MLJCoiot. Prehabilitation in our most frail surgical patients: are wearable fitness devices the next frontier? 2016;21: 188–93 (2). [DOI] [PubMed] [Google Scholar]
- [37].Schwenk M, Mohler J, Wendel C, Fain M, Taylor-Piliae R, Najafi BJG. Wearable sensor-based in-home assessment of gait, balance, and physical activity for discrimination of frailty status: baseline results of the Arizona frailty cohort study 2015;61:258–67 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Toosizadeh N, Mohler J, Najafi BJJotAGS. Assessing upper extremity motion: an innovative method to identify frailty 2015;63:1181–6 (6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Toosizadeh N, Joseph B, Heusser MR, Jokar TO, Mohler J, Phelan HA, et al. Assessing upper-extremity motion: an innovative, objective method to identify frailty in older bed-bound trauma patients 2016;223:240–8 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Maged K, GJIjohg Berry. Real-time locating systems (RTLS) in healthcare: a condensed primer 2012;117:56 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dorrell RD, Vermillion SA, CJJSe Clark. Feasibility of real-time location systems in monitoring recovery after major abdominal surgery 2017;31:5457–62 (12). [DOI] [PubMed] [Google Scholar]
- [42].Tan H-J, Saliba D, Kwan L, Moore AA, Litwin MSJJoCO. Burden of geriatric events among older adults undergoing major cancer surgery 2016;34:1231 (11). [DOI] [PMC free article] [PubMed] [Google Scholar]


