Abstract
The role of biological sex in short-term and long-term outcome after traumatic brain injury (TBI) remains controversial. The observation that exogenous female sex steroids (progesterone and estrogen) reduce brain injury coupled with a small number of clinical studies showing smaller injury in women suggest that sex steroids may play a role in outcome from TBI. We used the controlled cortical impact (CCI) model of TBI in mice to test the hypothesis that after CCI, female mice would demonstrate less injury than male mice, related to the protective role of endogenous steroids. Indeed, adult females exhibit histological protection (3.7 ± 0.5 mm3) compared to adult male mice (6.8 ± 0.6 mm3), and females that lacked sex steroids (ovex) showed increased injury compared to intact females. Consistent with histology, sensorimotor deficits measured as reduced contralateral limb use were most pronounced in male mice (31.9 ± 6.9% reduced limb use) compared to a 12.7 ± 3.8% reduction in female mice. Ovex mice exhibited behavioral deficits similar to males (31.5 ± 3.9% reduced limb use). Ovex females demonstrated increased microglial activation relative to intact females in both the peri-injury cortex and the reticular thalamic nucleus. Ovex females also demonstrated increased astrogliosis in comparison to both females and males in the peri-injury cortex. These data indicate that female sex steroids reduce brain sensitivity to TBI and that reduced acute neuroinflammation may contribute to the relative protection observed in females.
Keywords: traumatic brain injury, sex differences, sex steroids, stereology, neuroinflammation
INTRODUCTION
Traumatic brain injury (TBI) remains a major source of morbidity and mortality in the United States. Accidental injuries are the fourth leading cause of death as of 2014 and TBI contributes to 30% of these injury-related deaths(Taylor et al. 2017). Furthermore, nonfatal TBI is associated with higher rates of both psychiatric and medical comorbidities than found in the general population(Dams-O’Connor et al. 2013), resulting in an estimated economic burden of $76.5 billion as of 2010(Finkelstein et al. 2007). TBI outcomes are affected by multifactorial processes, including the heterogeneous nature of the human population, different injury type and severity, as well as timing and character of post-injury clinical care(Renner et al. 2012; Xing et al. 2014). Biological sex may be one of the factors that affects outcome after TBI, although both clinical and experimental studies have produced variable results. While some clinical studies have shown that women have better outcomes than men(Berry et al. 2009; Devitt et al. 2006; Groswasser et al. 1998; Moore et al. 2010), other studies demonstrate no difference (Albrecht et al. 2016; Baum et al. 2016; Coimbra et al. 2003; Davis et al. 2006; Mushkudiani et al. 2007; Ng et al. 2006; Sarajuuri et al. 2005; Slewa-Younan et al. 2004; Slewa-Younan et al. 2008; Tsushima et al. 2009) or worse outcomes in women compared to men(Farace and Alves 2000; Kirkness et al. 2004; Kraus et al. 2000; Ottochian et al. 2009; Ponsford et al. 2008; Scholten et al. 2015; Whelan-Goodinson et al. 2010). In preclinical animal studies, it remains unclear the extent that endogenous female sex hormones play in TBI related injury and outcome(Bramlett and Dietrich 2001; Hall et al. 2005; O’Connor et al. 2003; Roof and Hall 2000a; Roof and Hall 2000b; Semple et al. 2017; Suzuki et al. 2003; Tucker et al. 2017; Wagner et al. 2004; Xiong et al. 2007). Part of the controversy likely lies in the depth and type of injury. Therefore, the current study aims at helping resolve this issue by evaluating the relationship of both sex and endogenous sex steroids on TBI outcomes in the CCI model of TBI.
The role of the sex steroids estrogen and progesterone have been well studied in various models of brain injury including TBI, stroke and global ischemia (for review see (Brotfain et al. 2016; Herson et al. 2009; Stein 2011)), with many studies suggesting that exogenous administration of female sex steroids (estrogen/progesterone) are protective. Models of cerebral ischemia demonstrate a clear sex-specific response, with females showing remarkable protection against ischemic injury relative to males, which is thought to be related to endogenous estrogen levels (for review see (Herson et al. 2009; Hurn and Brass 2003)). In contrast, experimental TBI data related to the role of sex and endogenous sex steroids have provided mixed results. Previous reports have suggested that the immune response plays a crucial role in the progression of TBI(Lozano et al. 2015; Nortje and Menon 2004). For example, experimental TBI rapidly activates glial cells in both acute and chronic stages of TBI(Lozano et al. 2015). Thus, part of the protective function of estrogen and progesterone may arise via modulation of the injury-induced immune response(Bruce-Keller et al. 2000; Cutolo et al. 1995). However, previous reports show that neither sex nor estrogen manipulation affected the activation of microglia in the hippocampus after moderate-severe TBI(Bruce-Keller et al. 2007). In addition, astrocytes are stimulated in the damaged brain(Buffo et al. 2010) and the interaction between microglia and astrocytes in pathology and repair after TBI is complex(Chiu et al. 2016). Therefore, we aim here to study the role that endogenous female sex steroids have in modulating the volume of cortical injury and the regulation of neuroinflammatory response.
MATERIALS AND METHODS
Mice
All procedures were approved by the University of Colorado Animal Care and Use Committee and conform to the National Institutes of Health (NIH) guidelines for the care and use of animals. Experiments were designed to conform to the ARRIVE guidelines(Kilkenny et al. 2010). Blinding and randomization was performed prior to surgery. Adult (8–12 week old) male and female C57BL/6 mice (Charles River, Raleigh, NC) were subjected to CCI as described below. Housing conditions were controlled with a 12:12h light:dark cycle before and concurrent with testing, and all animals received food and water ad libitum.
A total of 76 mice were used for the current study. For those animals that survived to the point of TBI, survival rates were high. Three females and one male died on POD #3–5, resulting in post-injury survival rates as follows: females 84%, males 94%, and ovex 100%.
Controlled Cortical Impact
Anesthesia was induced using 4% inhaled isoflurane, followed by maintenance with 1.5–2.5% inhaled isoflurane. After anesthetic induction, mice were intubated and mechanically ventilated (Minivent, Hugo Sachs Elektronik, March-Hugstetten, Germany) for the remainder of the anesthetic period. Ventilator parameters were standardized, with a tidal volume of 150 µl, a respiratory rate of 150 breaths/minute, and an FiO2 of 30%. A 4 mm circular right craniotomy was completed using a micromotor drill (Stoelting, Wood Dale, IL) centered 2.5 mm anterior to the lambdoid suture and 2.7 mm lateral to the sagittal suture using a stereotactic setup. Following exposure of the dura mater, a 3 mm flat-tipped pneumatic impact device (Impact One Stereotaxic Impactor for CCI, Leica Biosystems, Buffalo Grove, IL) was used to deliver a single, rapid impact using the following standardized parameters: angle 10° to the right of vertical, speed 3 ± 0.02 m/s, duration 500 msec. Depth of impact was varied from 0 mm (sham) to 2 mm (severe). Following impact, hemostasis was achieved, the bone flap replaced, and the skin closed. Each animal was given 1000 µl (sc) normal saline (NS) at the end of surgery. When each animal demonstrated adequate respiratory effort, the animal was extubated and returned to its cage to continue recovery.
Ovariectomy
Ovariectomy (ovex) surgery was performed 7 days prior to TBI injury. Anesthesia for ovex was induced using 4% inhaled isoflurane, followed by maintenance with 1.5–2.5% inhaled isoflurane. A 1 cm vertical incision was made just lateral to the spine, and following blunt dissection, the ovaries were identified. Fallopian tubes were clamped, followed by excision of the attached ovary. This was repeated on the contralateral side using the same initial skin incision, after which the incision was closed using 7mm clips (Stoelting, Wood Dale, IL).
Behavior
Cylinder test:
We used the cylinder test(Bland et al. 2000) to analyze forelimb use bias because this has been extensively described as a reliable measure of functional deficits following traumatic brain injury. Briefly, each mouse was placed in a transparent cylinder (diameter=9 cm) that was wide enough for the mouse to move freely, and an overhead camera was used to analyze rearing behavior. Limb use was analyzed by calculating the percentage use of the ipsilateral paw placements before and after injury. All experiments and analysis was performed in a blinded manner from video to calculate the percentage of forelimb touches. A total of 10–20 forelimb touches was recorded during the 5 minute test. Baseline paw preference was assessed 1 day prior to surgery and subsequent analysis was made at 7 days after CCI surgery. Because CCI was made to right motor cortex, impairment in left paw use was analyzed and calculated as percent change in left paw use compared to each animal’s own pre-surgery control.
In order to test for generalized motor deficits rather than side-specific, mice completed an open field test as previously described by our group(Allen et al. 2011). Briefly, mice were allowed to habituate to the testing environment, then placed in a 52 cm diameter circular arena. Locomotor activity was calculated by measuring total distance traveled during the 15 minute test session, measured by ANY-maze automated tracking software (Stoelting, Inc., Wood Dale, IL, USA).
Tissue Isolation and Preparation
Following behavioral testing (7 days post-injury), animals were transcardially perfused under isoflurane anesthesia with phosphate-buffered saline for five minutes, followed by five minutes of fixation with 4% paraformaldehyde. Their brains were removed, allowed to post-fix in 4% PFA for 24 hours, and embedded in paraffin, as previously described(Deng et al. 2017; Shimizu et al. 2016). Coronal sections were cut in 6 µm thickness, and every sixth section was mounted in series onto slides for further processing. The interval between serial slide groups was 100 µm.
Quantification of Injury Volume
For quantification of injury volume, one set of serial tissue sections stained with hematoxylin and eosin (H&E) was analyzed using quantitative stereologic analysis to quantify injury volume according to the Cavalieri principle. A Leica photomicroscope (Leica Microsystems, Buffalo Grove, IL) and a StereoInvestigator program (Stereologer 2000, SRC, Tampa, FL) were used for this purpose. For each animal, a total of 8 standardized sections were analyzed to calculate the global injury volume in cubic millimeters. Injured area was traced at 1.25x magnification, and tissue depth was calculated at 40x magnification.
Immunohistochemistry
To analyze astro- and micro-gliosis, one set of serial tissue sections for each animal was used for immunohistochemistry. All slides were batch processed in a single session to avoid potential confounders. Slides were washed with PBS, blocked with 5% normal donkey serum, then incubated for 24 hr with primary antibody at 4°C. Goat anti-GFAP (Santa Cruz Biotechnology, Dallas, Texas) was diluted in blocking solution at 1:200 concentration and rabbit anti-Iba-1 (Wako Pure Chemical Industries, Richmond, VA) was diluted in blocking solution at 1:1000 concentration. After washing with PBS, sections were incubated with appropriate secondary antibodies (Jackson Immuno or Abcam) for 2 hr at room temperature. A single investigator blinded to group acquired all images using a Leica fluorescent microscope and QCapture Pro 7 image capturing software (Q Imaging, Surrey, British Columbia, Canada). After optimizing image parameters for GFAP and Iba-1, all images were acquired using standardized conditions. All digital image processing was performed using an in-house, custom-designed toolbox, imstack, written for MATLAB (Natick, MA). Briefly, each image was digitally processed using the following steps: First, each 12-bit image was converted to the double numeric class by dividing each intensity value by 4095, resulting in intensity values that ranged from 0 to 1. Next, each image was corrected for uneven illumination and background noise by applying a tophat transformation that used a disk-shaped structuring element with a pixel radius of 20. Next, the image was converted into a binary image using a corrected global image threshold value. The initial global threshold value was determined at an earlier stage in which Ostu’s Method was applied to a histogram generated by concatenating all intensity values from all images labeled with the same immunolabel (either GFAP or Iba-1). Once a global threshold was identified, the threshold value was corrected by multiplying its value by a correction factor (e.g. 0.5) to improve signal-to-noise-ratios. The resultant binary images were manually inspected to ensure that images included the maximum number of positively labeled pixels while excluding the maximum number of false positive pixels. Finally, the total number of positive pixels in a given image was determined by summing all of the positive pixels in the binary image.
Statistics
Data in table 1 are presented as mean ± standard deviation because the relative scatter of the data is relevant. All other data are presented as mean ± standard error of the mean (S.E.M.). One-way analysis of variance (ANOVA) was used to calculate differences in injury volume based on depth of injury (figure 1). Two-tailed, one sample t-tests were used to compare each group’s percentage of left paw usage change from baseline in comparison to a theoretical value of zero, as well as each group’s percentage of total distance travelled change from baseline in comparison to a theoretical value of zero. Two-way ANOVAs were used for the remaining analyses, followed by Tukey’s multiple comparisons post-hoc analyses. Statistical significance was considered a p value less than 0.05. All statistical analyses were performed using GraphPad Prism 6 (GraphPad, La Jolla, CA).
Table 1. Physiologic parameters by group.
Values listed are means +/− standard deviation. CCI = controlled cortical impact. Ovex = ovariectomized. N = number of animals in each group. g = grams. POD = post-operative day (post-injury day).
| Group | Male CCI | Female CCI | Ovex CCI | |
|---|---|---|---|---|
| N | 9 | 10 | 11 | |
| Age (weeks) | 9.6 +/− 0.5 | 8.9 +/− 0.9 | 10.5 +/− 1.5 | |
| Weight (g) | Pre-Injury | 24.7 +/− 1.2 | 18.3 +/− 2.6 | 21.2 +/− 1.6 |
| POD #1 | 22.7 +/− 1.7 | 17.1 +/− 1.9 | 20.2 +/− 1.9 | |
| POD #2 | 22.5 +/− 2.2 | 17.9 +/− 2.2 | 21.4 +/− 1.8 | |
| POD #7 | 23.6 +/− 1.4 | 17.7 +/− 3.4 | 21.5 +/− 2.0 | |
| Temperature (℃) | Pre-Injury | 34.6 +/− 0.7 | 35.0 +/− 1.2 | 35.1 +/− 1.7 |
| Time of Injury | 35.7 +/− 0.7 | 36.4 +/− 0.9 | 36.0 +/− 1.5 | |
| Anesthetic End | 36.9 +/− 0.7 | 37.2 +/− 0.9 | 37.4 +/− 0.6 | |
| Duration of Anesthesia (min) | 28.3 +/− 3.5 | 38.3 +/− 5.0 | 33.6 +/− 3.9 | |
| Group | Male Sham | Female Sham | Ovex Sham | |
| N | 6 | 6 | 6 | |
| Age (weeks) | 9.8 +/− 0.2 | 8.3 +/− 1.0 | 10.2 +/− 2.0 | |
| Weight (g) | Pre-Injury | 24.8 +/− 2.8 | 20.3 +/− 1.3 | 20.5 +/− 2.1 |
| POD #1 | 24.1 +/− 2.0 | 18.2 +/− 1.0 | 19.6 +/− 1.5 | |
| POD #2 | 24.7 +/− 0.3 | 18.6 +/− 2.1 | 20.5 +/− 1.4 | |
| POD #7 | 24.4 +/− 2.2 | 19.9 +/− 3.2 | 20.7 +/− 2.1 | |
| Temperature (℃) | Pre-Injury | 34.9 +/− 0.5 | 35.2 +/− 1.3 | 35.5 +/− 1.2 |
| Time of Injury | 35.8 +/− 0.6 | 36.4 +/− 0.9 | 35.9 +/− 0.9 | |
| Anesthetic End | 37.0 +/− 0.3 | 37.0 +/− 0.8 | 37.3 +/− 0.4 | |
| Duration of Anesthesia (min) | 23.3 +/− 2.6 | 27.5 +/− 6.1 | 27.5 +/− 4.2 | |
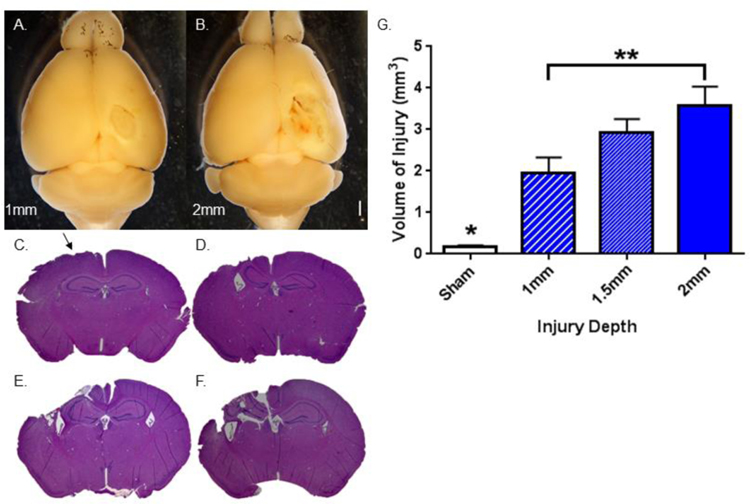
Figure 1. Volume of injury varies with impact depth in male mice.
A–B. Gross examination of the brain 7 days after TBI demonstrates visible increase in injury size as impact depth increases. Calibration bar = 1 mm. Representative images at A. 1 mm impact depth. B. 2 mm impact depth. C–F. Representative H&E-stained coronal sections demonstrate increase in injury volume as impact depth increases. C. Sham. Arrow indicates injury, likely from craniotomy. D. 1mm impact depth. E. 1.5 mm impact depth. F. 2 mm impact depth. G. Stereologic, quantitative analysis of injury volume as impact depth varies. Bars indicate the group mean. Error bars indicate standard error of the mean. All impact depths demonstrate statistically significant injury volume in comparison to sham, analyzed by 1-way ANOVA (*p = 0.01, 0.004, < 0.0001, respectively). Injury volume after 2mm impact depth > injury volume after 1 mm depth (**p = 0.02). n = 5–7 animals per group.
RESULTS
Characterization of Injury Paradigm
Initial studies, focused on characterizing the injury model, subjected male adult mice to graded CCI impact depth, including 0mm (sham), 1mm, 1.5mm, and 2mm. Figures 1A and 1B demonstrate representative images of whole brains isolated from injured animals, with a noticeable difference in gross appearance as impact depth increases. Figures 1C–1F demonstrate representative slices stained with H&E from animals injured at each impact depth. Sham mice showed minimal injury, denoted by the arrow. Mice with 1mm depth of impact had isolated cortical injury (with an intact hippocampus), while mice with 1.5 mm and 2 mm depths of impact revealed apparent injury deep to the cortex, including the hippocampus. Upon stereologic analysis, all impact depths demonstrated a significantly greater volume of injury than sham animals (p = 0.01, 0.004, < 0.0001, respectively, Figure 1G). Injury volume after 2 mm impact depth was also significantly greater than the injury volume calculated after 1 mm impact depth (p = 0.02, Figure 1G). 2 mm impacts were used throughout the remainder of the experiments in order to optimize differences between groups.
Sex differences in injury volume
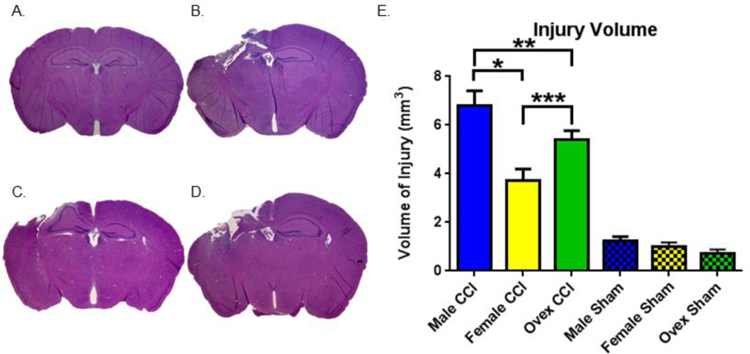
To test the hypothesis that female mice are relatively resistant to brain injury compared to male mice, we performed stereologic analysis of CCI-induced injury 7 days after injury. Figure 2A–2D are representative tissue sections stained with H&E from each animal group. Sham animals demonstrated minimal cortical injury likely associated with craniectomy (quantified in Figure 2E). All injury groups demonstrated marked injury, with noted tissue injury deep to the cortex (including the corpus callosum and hippocampus). Male mice demonstrated the highest volume of injury; 6.8 mm3 ± 0.6 (n=9). In contrast, female mice had significantly smaller injury volume compared to males; 3.7 mm3 ± 0.5 (n=10, P<0.05 compared to male). Interestingly, the removal of endogenous female sex steroids using ovex surgery resulted in an intermediate injury volume; 5.4 mm3 ± 0.4 (n=10, P<0.05 compared to both intact females and males). All injury groups demonstrated a higher volume of injury than sham groups (p < 0.0001), and there was no difference between sham groups (p = 0.7 – 0.9). These data indicate a sex difference in response to injury, in part mediated by endogenous female sex steroids. No differences in age, weight or duration of anesthesia were observed between groups (Table 1).
Figure 2. Volume of injury differs between groups.
A.–D. Representative H&E-stained coronal sections demonstrating variable injury by animal group. A. Sham. B. Male. C. Female. D. Ovariectomized female (ovex). E. Stereologic, quantitative analysis of injury volume by group. Bars indicate the group mean. Error bars indicate standard error of the mean. Injury volume for male CCI > female CCI, *p < 0.0001. Injury volume for male CCI > ovex CCI, **p = 0.048. Injury volume for ovex CCI > female CCI, ***p = 0.01. Sham group differences nonsignificant. n = 9–11 for CCI groups, n = 6 for sham groups.
Sex differences in sensorimotor impairments
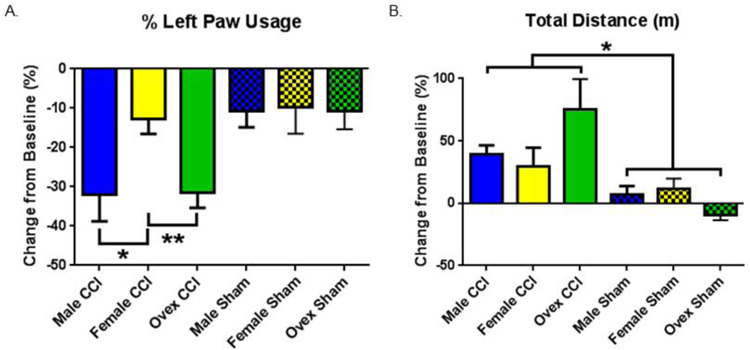
To analyze the functional correlate of CCI-induced injury, we performed the cylinder task to assess limb use asymmetry as a measure of motor deficits. Figure 3A demonstrates the difference in left paw usage between the post-injury and baseline values. All injury groups demonstrated a significant decrease in the percentage of left paw usage post-injury, consistent with right-sided cortical injury. Consistent with our histological observations, male animals exhibited the greatest degree of motor deficits, reducing their left paw usage by a mean of 31.9% ± 6.9 (n=9, P < 0.05 compared to sham). Remarkably, females did not exhibit impairment in left paw usage, reducing by 12.7% ± 3.8 (p > 0.5 compared to sham). Importantly, ovex female mice demonstrated significant motor deficits; exhibiting reduced left paw usage by a mean of 31.5% ± 3.9 (P < 0.05 compared to sham and P < 0.05 compared to intact females). Differences between groups, when analyzed by 2-way ANOVA, demonstrated a greater reduction in left paw usage in males and ovex in comparison to females (p = 0.02 and 0.01, respectively), demonstrating a larger lateral motor deficit in males and ovex animals.
Figure 3. Behavioral test outcomes differ between groups.
Each animal’s pre-injury value serves as its own baseline. Graphs depict post-injury change from baseline. Bars indicate the group mean. Error bars indicate standard error of the mean. A. Percent left paw usage. Male CCI > Female CCI, *p = 0.02. Ovariectomized female (ovex) CCI > Female, **p = 0.01. Male CCI vs Ovex CCI nonsignificant. Sham group differences nonsignificant. B. Distance travelled in meters (m). Injury > Sham, *p = 0.003. n = 9–11 for CCI groups, n = 6 for sham groups.
We made the surprising observation that CCI resulted in increased locomotor activity (Figure 3B). Comparing groups using 2-way ANOVA, injured animals increased their total distance traveled post-injury significantly compared to uninjured sham animals (P < 0.05), with no differences among groups observed (Post-hoc Tukey’s for multiple comparisons). These results indicate that a global motor deficit does not explain differences in the change in percentage of left paw usage.
Glial reactivity following CCI
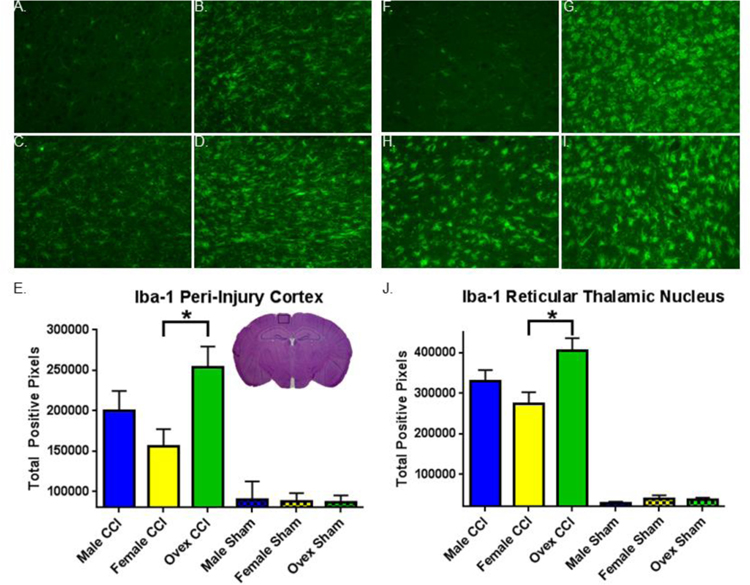
To assess the contribution of microgliosis to CCI-induced injury we used immunolabeling for Iba-1, a macrophage/microglia specific calcium binding protein. Figure 4A–4D are representative photomicrographs of Iba-1 immunofluorescence in the peri-injury cortex of each animal group, and Figure 4E–4I are representative sections in the reticular thalamic nucleus. We performed an unbiased computer-aided analysis of Iba-1 staining using a custom-designed toolbox in MATLAB to compare the degree of Iba-1 infiltration following injury in these areas. Figure 4E and 4J represent the mean number of Iba-1 positive pixels per image in each group in the peri-injury cortex and reticular thalamic nucleus, respectively. In both regions, sham animals had very low levels of Iba-1 staining, with a marked increase in all groups following injury (p < 0.0001 in both areas). Male mice exhibited slight increase in Iba-1 staining compared to female mice, which was not significant (P=0.2). In contrast, removal of sex steroids caused a significant increase in Iba-1 immunoreactivity compared to intact females (P < 0.05 in both thalamus and cortex ; Figure 4E,F).
Figure 4. Iba-1 immunohistochemistry varies by group.
A.–D. Representative images of Iba-1 immunofluorescent staining in peri-injury cortex (CTX). A. Sham CTX. B. Male CTX. C. Female CTX. D. Ovariectomized female (ovex) CTX. E. Quantification of total positive Iba-1 pixels / image in the peri-injury cortex. Bars indicate the group mean. Error bars indicate standard error of the mean. Injury > sham, p< 0.0001. Ovex CCI > female CCI, *p = 0.01. F.–I. Representative images of Iba-1 immunofluorescent staining in the reticular thalamic nucleus (RTN). F. Sham RTN. G. Male RTN. H. Female RTN. I. Ovex RTN. J. Quantification of total positive Iba-1 pixels / image in the RTN. Injury > sham, p< 0.0001. Ovex CCI > female CCI, *p = 0.0002.
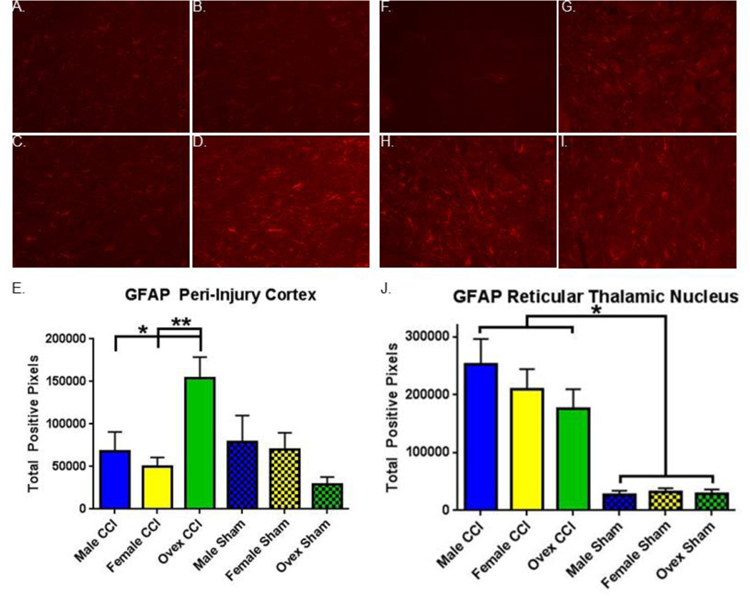
To assess the effect of CCI and sex on astrogliosis we immunolabeled for GFAP. Figure 5A–5D are representative images of GFAP immunofluorescence in the peri-injury cortex of each animal group, and Figure 5E–5I are representative sections in the reticular thalamic nucleus. Using MALAB to quantitate the total number of positive pixels, we found statistically higher numbers of positive pixels in the injured animals than sham animals in both the peri-injury cortex and reticular thalamic nucleus (Figure 5E, P < 0.05 and Figure 5J, P < 0.05, respectively). In the peri-injury cortex (Figure 5E), ovariectomized females had higher levels of GFAP than both males and females (p = 0.01 and p = 0.002, respectively). In the reticular thalamic nucleus (Figure 5J), there were no group differences other than sham versus injury.
Figure 5. Glial fibrillary acidic protein (GFAP) immunohistochemistry varies by group.
A.–D. Representative images of GFAP immunofluorescent staining in peri-injury cortex (CTX). A. Sham CTX. B. Male CTX. C. Female CTX. D. Ovariectomized female (ovex) CTX. E. Quantification of total positive GFAP pixels / image in the peri-injury cortex. Bars indicate the group mean. Error bars indicate standard error of the mean. Injury > sham, p = 0.04. Ovex CCI > male CCI, *p = 0.01. Ovex CCI > female CCI, **p = 0.002. F.–I. Representative images of GFAP immunofluorescent staining in the reticular thalamic nucleus (RTN). F. Sham RTN. G. Male RTN. H. Female RTN. I. Ovex RTN. J. Quantification of total positive GFAP pixels / image in the RTN. Injury > sham, p< 0.0001.
DISCUSSION
Our study shows that moderate to severe traumatic brain injury results in acute sex-specific outcomes and neuroinflammatory responses. We observed that female mice suffer less injury following experimental TBI compared to male mice, in both histological injury and acute motor dysfunction. Removal of endogenous female sex steroids results in worse histological and functional outcomes, implicating hormonal influences in endogenous protection following TBI. Further, our study identifies a complex interplay in acute neuroinflammatory responses in peri-injury brain regions that likely mediate the observed sex-specific outcomes.
A growing number of studies have examined the role of ovarian hormones in neuroprotection after TBI, though experimental data demonstrating sex differences using CCI are limited. Here, we find that after a moderate/severe CCI traumatic injury, the injury seen in females with ovex is equivalent to the injuries seen in male mice and greater than the injury seen in intact female mice. Our behavioral data show results corresponding to the severity of injury in regard to female sex hormones and contralateral paw usage. Interestingly, two prior studies using a mouse CCI model reported no differences in injury size in male and female mice(Bruce-Keller et al. 2007; Hall et al. 2005), however, these studies used shallower impacts (less severe TBI) compared to the current study. This point may be particularly important as we show injuries which are significantly larger with deeper impact and our data suggest that loss of female hormones due to ovex may only worsen injury with more severe trauma, as previous studies showed ovex had no effect on injury volume with milder injury(Bruce-Keller et al. 2007). Consistent with this hypothesis, a recent study reports that there was no sex effect on lesion size 30 days after a 1.0 mm deep CCI, but did find that females had smaller lesions than males after a 2.0 mm deep CCI (Tucker et al. 2016). Further, ovex in a moderate fluid percussion model of TBI resulted in larger lesions in female mice compared to intact females, and were similar to injury in male rats (Bramlett and Dietrich 2001) consistent with the current study. In addition, our data is in agreement with prior studies demonstrating the protective capacity of the female sex steroids estrogen (17β-estradiol) and progesterone (P4). In particular, acute and chronic administration of P4 provides robust neuroprotection following TBI (for review see (Stein 2011)). However, the role of endogenous P4 remains understudied, particularly in light of recent failed clinical trials in patients suffering TBI who were administered P4 during the acute phase of recovery.
Neuroinflammation is a well-established component of both acute and chronic injury following TBI (Chiu et al. 2016). The initiation of inflammatory responses following TBI involves activation of glia, both astrocytes and microglia, which then release various factors that recruit the inflammatory response. It has been suggested that females may fare better after TBI due to reduced cerebral edema and increased release of anti-inflammatory cytokines, which can be attributed to varying levels of estrogen and progesterone (Maghool et al. 2013; Roof et al. 1993; Villapol et al. 2017). Consistent with this hypothesis, our data show that TBI induces microgliosis in both cortex and reticular thalamic nucleus and that there appears to be a sex and hormone effect. Therefore, endogenous sex steroids may play a role in limiting glial activation, as ovariectomy allowed a massive inflammatory response after TBI. In contrast, one study reports that TBI induces increased microglial reactivity in the ipsilateral hippocampus that is not altered by gender, ovex, or estrogen treatment (Bruce-Keller et al. 2007). However, this previous study used Mac-1 to stain for microglia, (Bruce-Keller et al. 2007) whereas we use Iba-1. A recent study showed a similar increase in Iba-1 positive cells following penetrating cortical injury (Acaz-Fonseca et al. 2015). It is worth noting that number of microglia is not always the most reliable indicator of inflammatory response, as microglia exist in various activation states (pro/anti-inflammatory). Interestingly, we observed equivalent levels of morphologically activated, pro-inflammatory microglia (spherical) at the site of injury in all groups (data not shown). Therefore, we believe that analysis of numbers of Iba-1 positive microglia is a reliable surrogate of inflammatory status in the injured brain. Nonetheless, future studies are needed to address the activation status of microglia (morphology and cytokine release profile) following TBI in male, female and ovx females. The astrocyte response is somewhat more complex. We find that in the cortex, there is no difference between males and intact females in either sham or TBI groups. However, surprisingly we observed markedly greater astrogliosis in ovex females following TBI. Further, while TBI increased astrogliosis in the reticular thalamic nucleus, there is no effect of sex or ovariectomy. While the implications of this response is unclear and warrants further investigation, together these data indicate that female sex steroids reduce brain sensitivity to TBI and that reduced acute neuroinflammation may contribute to the relative protection observed in females.
The cortical injury seen after severe (2mm) TBI and the effect of sex and endogenous sex steroids (ovex) has on this injury corresponds to the impairment we observed in the sensorimoror function tested by left paw usage. A recent report on sex differences in behavior found that several neurobehavioral tasks reliably detect TBI-induced dysfunction, however very few behavioral paradigms demonstrated an influence of sex on TBI outcomes. Interestingly, measurements of cognition were among the most reliable sex-specific responses to TBI detects.(Tucker et al. 2017) Relevant to the current study, the prior study did not assess contralateral paw usage, a reliable sensorimotor task that proved effective in our hands at observing sex-specific responses to TBI. In light of significant sex differences observed in cortical injury and inflammatory response, our sensorimotor functional measurements are not surprising. Less clear is how the injury/neuroinflammation to the reticular thalamic nucleus plays a role in these functional impairments. The ipsilateral reticular nucleus of the thalamus degenerates following TBI(Huusko and Pitkanen 2014) and as discussed above, we observed significant microgliosis and astrocytosis in the ipsilateral reticular nucleus 7 days after injury. The thalamic reticular thalamus receives input from the cerebral cortex and the Globus Pallidus, which likely plays a part in disinhibition of thalamic neurons and, thus, is essential for the initiation of movement (Parent and Hazrati 1995). Further, the recipients of reticular thalamic nucleus neurons are thalamic projection neurons (also called relay neurons) that are integral to processes involved in motor control, memory and arousal (Halassa and Acsady 2016). Therefore, it remains unknown how the impact of increased microglia and astrocytes affect neural-glial and glial-glial interaction in this part of the thalamus. Further, aside from the motor impairments suggested by the data here, the impact of native estrogen and progesterone on memory and cognition after TBI merits further study.
CONCLUSIONS
A wealth of data indicates that the majority of morbidity and mortality following TBI occur in males. Preclinical animal models of TBI have tried to determine the factors related to this male predominance, and our data combined with those of others suggest that endogenous female sex hormones likely play an important role, particularly in more severe injuries. Removal of ovaries prior to TBI appears to worsen the volume of injury, reduce contralateral motor function and increase neuroinflammatory activation in important motor areas of the brain. Further exploration of the role that endogenous male and female hormones play in the neuropathology of TBI is necessary given the variability in outcomes of hormonal alterations and TBI in pre-clinical models. Further, investigations of response to injury may be useful in optimizing gender-specific expectations following injury.
ACKNOWLEGEMENTS
We would like to acknowledge Frank Strnad, who completed a portion of the animal perfusions post-injury. We would also like to acknowledge Myriam Moreno Garcia who managed the animal colonies. Funding provided by National Institutes of Health, R01NS046072 (RJT) and R01NS092645 (PSH).
Footnotes
Author Disclosure Statement:
None
REFERENCES
- Acaz-Fonseca E, Duran JC, Carrero P, Garcia-Segura LM, Arevalo MA (2015) Sex differences in glia reactivity after cortical brain injury Glia 10.1002/glia.22867 [DOI] [PubMed]
- Albrecht JS, McCunn M, Stein DM, Simoni-Wastila L, Smith GS (2016) Sex differences in mortality following isolated traumatic brain injury among older adults The journal of trauma and acute care surgery 81:486–492 10.1097/TA.0000000000001118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D et al. (2011) SK2 channels are neuroprotective for ischemia-induced neuronal cell death J Cereb Blood Flow Metab 31:2302–2312 10.1038/jcbfm.2011.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum J, Entezami P, Shah K, Medhkour A (2016) Predictors of Outcomes in Traumatic Brain Injury World neurosurgery 90:525–529 10.1016/j.wneu.2015.12.012 [DOI] [PubMed] [Google Scholar]
- Berry C, Ley EJ, Tillou A, Cryer G, Margulies DR, Salim A (2009) The effect of gender on patients with moderate to severe head injuries The Journal of trauma 67:950–953 10.1097/TA.0b013e3181ba3354 [DOI] [PubMed] [Google Scholar]
- Bland ST, Schallert T, Strong R, Aronowski J, Grotta JC, Feeney DM (2000) Early exclusive use of the affected forelimb after moderate transient focal ischemia in rats : functional and anatomic outcome Stroke 31:1144–1152 [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD (2001) Neuropathological protection after traumatic brain injury in intact female rats versus males or ovariectomized females Journal of neurotrauma 18:891–900 10.1089/089771501750451811 [DOI] [PubMed] [Google Scholar]
- Brotfain E, Gruenbaum SE, Boyko M, Kutz R, Zlotnik A, Klein M (2016) Neuroprotection by Estrogen and Progesterone in Traumatic Brain Injury and Spinal Cord Injury Current neuropharmacology 14:641–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ et al. (2007) Gender and estrogen manipulation do not affect traumatic brain injury in mice Journal of neurotrauma 24:203–215 10.1089/neu.2006.0163 [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Keeling JL, Keller JN, Huang FF, Camondola S, Mattson MP (2000) Antiinflammatory effects of estrogen on microglial activation Endocrinology 141:3646–3656 10.1210/endo.141.10.7693 [DOI] [PubMed] [Google Scholar]
- Buffo A, Rolando C, Ceruti S (2010) Astrocytes in the damaged brain: molecular and cellular insights into their reactive response and healing potential Biochemical pharmacology 79:77–89 10.1016/j.bcp.2009.09.014 [DOI] [PubMed] [Google Scholar]
- Chiu CC, Liao YE, Yang LY, Wang JY, Tweedie D, Karnati HK, Greig NH (2016) Neuroinflammation in animal models of traumatic brain injury Journal of neuroscience methods 272:38–49 10.1016/j.jneumeth.2016.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coimbra R, Hoyt DB, Potenza BM, Fortlage D, Hollingsworth-Fridlund P (2003) Does sexual dimorphism influence outcome of traumatic brain injury patients? The answer is no! The Journal of trauma 54:689–700 10.1097/01.TA.0000058314.31655.5F [DOI] [PubMed] [Google Scholar]
- Cutolo M, Sulli A, Seriolo B, Accardo S, Masi AT (1995) Estrogens, the immune response and autoimmunity Clinical and experimental rheumatology 13:217–226 [PubMed] [Google Scholar]
- Dams-O’Connor K et al. (2013) The impact of previous traumatic brain injury on health and functioning: a TRACK-TBI study Journal of neurotrauma 30:2014–2020 10.1089/neu.2013.3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DP et al. (2006) Traumatic brain injury outcomes in pre- and post- menopausal females versus age-matched males Journal of neurotrauma 23:140–148 10.1089/neu.2006.23.140 [DOI] [PubMed] [Google Scholar]
- Deng G et al. (2017) Autonomous CaMKII Activity as a Drug Target for Histological and Functional Neuroprotection after Resuscitation from Cardiac Arrest Cell reports 18:1109–1117 10.1016/j.celrep.2017.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devitt R, Colantonio A, Dawson D, Teare G, Ratcliff G, Chase S (2006) Prediction of long-term occupational performance outcomes for adults after moderate to severe traumatic brain injury Disability and rehabilitation 28:547–559 10.1080/00222930500219258 [DOI] [PubMed] [Google Scholar]
- Farace E, Alves WM (2000) Do women fare worse: a metaanalysis of gender differences in traumatic brain injury outcome Journal of neurosurgery 93:539–545 10.3171/jns.2000.93.4.0539 [DOI] [PubMed] [Google Scholar]
- Finkelstein EA, Corso PS, miller TR (2007) Incidence and economic burden of injuries in the United States J Epidemiol Community Health 61:926–927 [Google Scholar]
- Groswasser Z, Cohen M, Keren O (1998) Female TBI patients recover better than males Brain injury : [BI] 12:805–808 [DOI] [PubMed] [Google Scholar]
- Halassa MM, Acsady L (2016) Thalamic Inhibition: Diverse Sources, Diverse Scales Trends in neurosciences 39:680–693 10.1016/j.tins.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED, Gibson TR, Pavel KM (2005) Lack of a gender difference in posttraumatic neurodegeneration in the mouse controlled cortical impact injury model Journal of neurotrauma 22:669–679 10.1089/neu.2005.22.669 [DOI] [PubMed] [Google Scholar]
- Herson PS, Koerner IP, Hurn PD (2009) Sex, sex steroids, and brain injury SeminReprodMed 27:229–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurn PD, Brass LM (2003) Estrogen and stroke: a balanced analysis Stroke 34:338–341 [DOI] [PubMed] [Google Scholar]
- Huusko N, Pitkanen A (2014) Parvalbumin immunoreactivity and expression of GABAA receptor subunits in the thalamus after experimental TBI Neuroscience 267:30–45 10.1016/j.neuroscience.2014.02.026 [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research PLoS biology 8:e1000412 10.1371/journal.pbio.1000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkness CJ, Burr RL, Mitchell PH, Newell DW (2004) Is there a sex difference in the course following traumatic brain injury? Biological research for nursing 5:299–310 10.1177/1099800404263050 [DOI] [PubMed] [Google Scholar]
- Kraus JF, Peek-Asa C, McArthur D (2000) The independent effect of gender on outcomes following traumatic brain injury: a preliminary investigation Neurosurgical focus 8:e5. [DOI] [PubMed] [Google Scholar]
- Lozano D, Gonzales-Portillo GS, Acosta S, de la Pena I, Tajiri N, Kaneko Y, Borlongan CV (2015) Neuroinflammatory responses to traumatic brain injury: etiology, clinical consequences, and therapeutic opportunities Neuropsychiatric disease and treatment 11:97–106 10.2147/NDT.S65815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghool F, Khaksari M, Siahposht Khachki A (2013) Differences in brain edema and intracranial pressure following traumatic brain injury across the estrous cycle: involvement of female sex steroid hormones Brain research 1497:61–72 10.1016/j.brainres.2012.12.014 [DOI] [PubMed] [Google Scholar]
- Moore DW, Ashman TA, Cantor JB, Krinick RJ, Spielman LA (2010) Does gender influence cognitive outcome after traumatic brain injury? Neuropsychological rehabilitation 20:340–354 10.1080/09602010903250928 [DOI] [PubMed] [Google Scholar]
- Mushkudiani NA et al. (2007) Prognostic value of demographic characteristics in traumatic brain injury: results from the IMPACT study Journal of neurotrauma 24:259–269 10.1089/neu.2006.0028 [DOI] [PubMed] [Google Scholar]
- Ng I, Lee KK, Lim JH, Wong HB, Yan XY (2006) Investigating gender differences in outcome following severe traumatic brain injury in a predominantly Asian population British journal of neurosurgery 20:73–78 10.1080/02688690600682259 [DOI] [PubMed] [Google Scholar]
- Nortje J, Menon DK (2004) Traumatic brain injury: physiology, mechanisms, and outcome Current opinion in neurology 17:711–718 [DOI] [PubMed] [Google Scholar]
- O’Connor CA, Cernak I, Vink R (2003) Interaction between anesthesia, gender, and functional outcome task following diffuse traumatic brain injury in rats Journal of neurotrauma 20:533–541 10.1089/089771503767168465 [DOI] [PubMed] [Google Scholar]
- Ottochian M, Salim A, Berry C, Chan LS, Wilson MT, Margulies DR (2009) Severe traumatic brain injury: is there a gender difference in mortality? American journal of surgery 197:155–158 10.1016/j.amjsurg.2008.09.008 [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN (1995) Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop Brain research Brain research reviews 20:91–127 [DOI] [PubMed] [Google Scholar]
- Ponsford JL et al. (2008) Gender differences in outcome in patients with hypotension and severe traumatic brain injury Injury 39:67–76 10.1016/j.injury.2007.08.028 [DOI] [PubMed] [Google Scholar]
- Renner C et al. (2012) The influence of gender on the injury severity, course and outcome of traumatic brain injury Brain injury : [BI] 26:1360–1371 10.3109/02699052.2012.667592 [DOI] [PubMed] [Google Scholar]
- Roof RL, Duvdevani R, Stein DG (1993) Gender influences outcome of brain injury: progesterone plays a protective role Brain Res 607:333–336 [DOI] [PubMed] [Google Scholar]
- Roof RL, Hall ED (2000a) Estrogen-related gender difference in survival rate and cortical blood flow after impact-acceleration head injury in rats Journal of neurotrauma 17:1155–1169 10.1089/neu.2000.17.1155 [DOI] [PubMed] [Google Scholar]
- Roof RL, Hall ED (2000b) Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone JNeurotrauma 17:367–388 [DOI] [PubMed] [Google Scholar]
- Sarajuuri JM, Kaipio ML, Koskinen SK, Niemela MR, Servo AR, Vilkki JS (2005) Outcome of a comprehensive neurorehabilitation program for patients with traumatic brain injury Archives of physical medicine and rehabilitation 86:2296–2302 10.1016/j.apmr.2005.06.018 [DOI] [PubMed] [Google Scholar]
- Scholten AC, Haagsma JA, Andriessen TM, Vos PE, Steyerberg EW, van Beeck EF, Polinder S (2015) Health-related quality of life after mild, moderate and severe traumatic brain injury: patterns and predictors of suboptimal functioning during the first year after injury Injury 46:616–624 10.1016/j.injury.2014.10.064 [DOI] [PubMed] [Google Scholar]
- Semple BD, Dixit S, Shultz SR, Boon WC, O’Brien TJ (2017) Sex-dependent changes in neuronal morphology and psychosocial behaviors after pediatric brain injury Behavioural brain research 319:48–62 10.1016/j.bbr.2016.10.045 [DOI] [PubMed] [Google Scholar]
- Shimizu K, Quillinan N, Orfila JE, Herson PS (2016) Sirtuin-2 mediates male specific neuronal injury following experimental cardiac arrest through activation of TRPM2 ion channels Experimental neurology 275 Pt 1:78–83 10.1016/j.expneurol.2015.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slewa-Younan S, Green AM, Baguley IJ, Gurka JA, Marosszeky JE (2004) Sex differences in injury severity and outcome measures after traumatic brain injury Archives of physical medicine and rehabilitation 85:376–379 [DOI] [PubMed] [Google Scholar]
- Slewa-Younan S, van den Berg S, Baguley IJ, Nott M, Cameron ID (2008) Towards an understanding of sex differences in functional outcome following moderate to severe traumatic brain injury: a systematic review Journal of neurology, neurosurgery, and psychiatry 79:1197–1201 10.1136/jnnp.2008.147983 [DOI] [PubMed] [Google Scholar]
- Stein DG (2011) Is progesterone a worthy candidate as a novel therapy for traumatic brain injury? Dialogues in clinical neuroscience 13:352–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Bramlett HM, Dietrich WD (2003) The importance of gender on the beneficial effects of posttraumatic hypothermia Experimental neurology 184:1017–1026 10.1016/S0014-4886(03)00389-3 [DOI] [PubMed] [Google Scholar]
- Taylor CA, Bell JM, Breiding MJ, Xu L (2017) Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths - United States, 2007 and 2013 MMWR Surveill Summ 66:1–16 10.15585/mmwr.ss6609a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsushima WT, Lum M, Geling O (2009) Sex differences in the long-term neuropsychological outcome of mild traumatic brain injury Brain injury : [BI] 23:809–814 10.1080/02699050903200530 [DOI] [PubMed] [Google Scholar]
- Tucker LB, Burke JF, Fu AH, McCabe JT (2017) Neuropsychiatric Symptom Modeling in Male and Female C57BL/6J Mice after Experimental Traumatic Brain Injury Journal of neurotrauma 34:890–905 10.1089/neu.2016.4508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker LB, Fu AH, McCabe JT (2016) Performance of Male and Female C57BL/6J Mice on Motor and Cognitive Tasks Commonly Used in Pre-Clinical Traumatic Brain Injury Research Journal of neurotrauma 33:880–894 10.1089/neu.2015.3977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villapol S, Loane DJ, Burns MP (2017) Sexual dimorphism in the inflammatory response to traumatic brain injury Glia 65:1423–1438 10.1002/glia.23171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AK et al. (2004) Evaluation of estrous cycle stage and gender on behavioral outcome after experimental traumatic brain injury Brain research 998:113–121 [DOI] [PubMed] [Google Scholar]
- Whelan-Goodinson R, Ponsford JL, Schonberger M, Johnston L (2010) Predictors of psychiatric disorders following traumatic brain injury The Journal of head trauma rehabilitation 25:320–329 10.1097/HTR.0b013e3181c8f8e7 [DOI] [PubMed] [Google Scholar]
- Xing G, Carlton J, Jiang X, Wen J, Jia M, Li H (2014) Differential Expression of Brain Cannabinoid Receptors between Repeatedly Stressed Males and Females may Play a Role in Age and Gender-Related Difference in Traumatic Brain Injury: Implications from Animal Studies Frontiers in neurology 5:161 10.3389/fneur.2014.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Lu D, Qu C, Goussev A, Schallert T, Chopp M (2007) Role of gender in outcome after traumatic brain injury and therapeutic effect of erythropoietin in mice Brain research 1185:301–312 10.1016/j.brainres.2007.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]