Abstract
Riboswitches are widespread RNA motifs that regulate gene expression in response to fluctuating metabolite concentrations. Known primarily from bacteria, riboswitches couple specific ligand binding and changes in RNA structure to mRNA expression in cis. Crystal structures of the ligand-binding domains of most of the phylogenetically widespread classes of riboswitches, each specific to a particular metabolite or ion, are now available. Thus, the bound states—one end point—have been thoroughly characterized, but the unbound states have been more elusive. Consequently, how the unbound, sensing riboswitch refolds into ligand-binding induced output state is less clear. The ligand recognition mechanisms of riboswitches are diverse, but we find that they share a common structural strategy in positioning their binding sites at the point of the RNA three-dimensional fold where the residues farthest from one another in sequence meet. We review how riboswitch folds adhere to this fundamental strategy and propose future research directions to understand and harness their ability to specifically control gene expression.
Keywords: riboswitch, RNA structure, RNA folding, contact order
INTRODUCTION
In the early 1980s, the discovery of catalytic RNAs (59; 94) put to rest the perception of RNA as a passive player in the control of cellular fate and greatly stimulated the search for RNAs with activities previously thought to be exclusive to proteins. A decade later, Ellington and Szostak demonstrated that a pool of RNA molecules of random sequence could be subjected to selection in vitro to obtain “aptamers” capable of specific binding to small molecules (42), thus further expanding the known capacity of RNA. Using in vitro evolution methods (42; 151; 184), researchers selected RNA aptamers specific for biologically relevant molecules, such as adenosine triphosphate (ATP) (156), theophylline (71), and cobalamin (111). These two concepts—RNA as catalyst and RNA as a specific, selectable interactor—led to the creation of fused aptamer-ribozyme RNAs that require small-molecule binding for cleavage of the phosphodiester backbone (131; 175), in vitro proof of ligand-responsive RNA catalytic activity. In mammalian cells, insertion of a dye-binding aptamer into the 5′ untranslated region (UTR) of a reporter gene allowed for controllable expression of a reporter (194). At that time, the evidence for natural small molecule-responsive RNA gene regulators in vivo was limited to T-box leader sequences, which had been shown to regulate transcription of amino acid-related operons in response to aminoacylated tRNA levels (57). Genetic evidence had suggested a direct interaction between T-box sequences and their ligands, but the involvement of other factors (i.e., proteins) had not been excluded at that time (reviewed recently in (209)). It is noteworthy that the preliminary work by Henkin and colleagues also led to the discovery of “S-box” sequences regulating methionine and cysteine biosynthesis (58), later shown to be riboswitches as well (43; 203). In fact, many regulatory sequences, later shown to be riboswitches, had been identified in phenotypic screens and presumed to be binding sites for protein regulators (87; 113), or shown to regulate transcription or translation (120; 126; 127). Naturally existing RNA aptamers were hypothesized much later (51; 172).
In 2002, the discovery of metabolite-sensing regulatory RNAs responsive to thiamine pyrophosphate (TPP) and flavin mononucleotide (FMN) provided evidence of multiple classes of riboswitches functional both in vivo and in vitro (121; 200). These findings led to the reevaluation of many operons with unknown regulatory mechanisms and an explosion in the discovery of riboswitches (reviewed in (20; 160)). As riboswitches are widespread in bacteria and archaea and function largely without proteins (albeit in the context of the transcription and translation machinery), it has been suggested that these ancient RNA motifs played a role as gene regulators in the RNA world or represent vestigial domains of ribozymes from that era (19; 21). Hypothetically, the bubbling muck or deep-ocean hydrothermal vent that spawned our RNA-based replicative forbearers would have been ripe with riboswitches, ribozymes, and other RNA-based operators.
Riboswitches are sequence elements generally found in the untranslated regions of bacterial mRNAs, where they regulate gene expression in cis by responding to the levels of a specific ligand (Figure 1), usually metabolically associated with the regulated gene. However, there are exceptions as riboswitches occasionally act in trans (33; 110; 118) and occasionally appear in eukaryotes (173) and archaea (9; 173). Riboswitches are formally comprised of two structural domains—a highly conserved aptamer domain responsible for binding specifically to the ligand and a less conserved expression domain or expression platform (200) responsible for changing gene expression. Across loci and organisms, riboswitches with homologous aptamer domains are often paired with different expression platforms, suggesting that after genes initially acquire riboswitches, the aptamer domain sequences remain conserved while the expression platforms sequences vary over time to best fit the regulatory need. Molecular communication between the two domains transduces specific ligand binding to modulation of genetic output. For example, TPP riboswitches are activated by TPP but not closely related thiamine (121; 201), and FMN riboswitches are activated by FMN but not closely related flavin (121). Ligand binding leads to a change in gene expression by altering or stabilizing the RNA secondary structure and by preventing an alternative structure from forming (Figure 1). This occurs most commonly by altering the mRNA transcript length through transcription termination or by affecting ribosome access to the Shine-Dalgarno sequence for initiating translation, but other mechanisms exist (33; 95; 99; 118). Since the original reports of the TPP and FMN classes of riboswitches, many more classes of riboswitches have been discovered and have been shown to respond to specific ligands (reviewed in (13; 20; 123; 133)), ranging in size from ions to complex molecules like cobalamin and tRNAs. Apart from the riboswitches with identified ligands, many putative, uncharacterized “orphan” riboswitches (10) have been predicted from sequence alignments and covariation analysis (11; 27; 191), as well as genome-wide transcriptome studies (31; 132; 164; 177). Thus, a family portrait of riboswitches is far from complete, with many members’ functions still to be determined.
Figure 1.
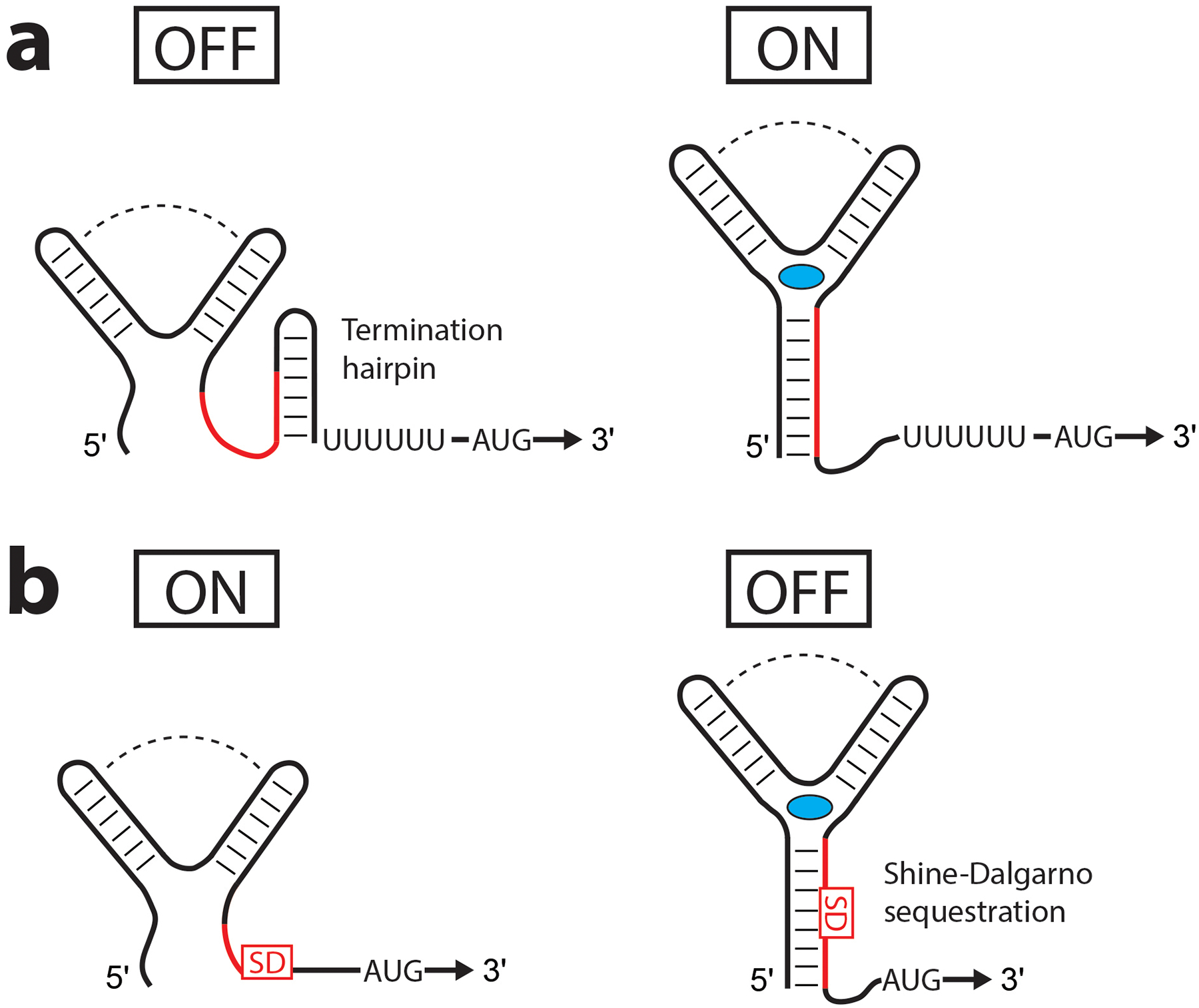
Common mechanisms of transcriptional and translational control. The major mechanisms that riboswitches use to change gene expression are through changes in RNA secondary structure affecting transcription termination by formation of an intrinsic termination hairpin (a) or translation initiation by sequestration of the Shine-Dalgarno (SD) site (b). The ligand is indicated by a blue oval, and the RNA sequence (the ‘switch’ sequence) in red base pairs in alternative secondary structures in the ON and OFF states.
Riboswitches hold great potential as antibiotic targets and modular tools for synthetic biology; however, neither of these goals have been fully realized. Many critical bacterial pathways are controlled by riboswitches (e.g., purine metabolism and amino acid metabolism), and some antibiotics naturally target riboswitches (17; 100). Ongoing efforts to develop antimicrobials for riboswitches are promising (34; 64; 114). Due to their modular nature, synthetic riboswitches have attracted use as ligand-responsive gene regulators in many settings, including mammalian cells (5; 66; 105; 199) and viruses (85; 86), but synthetic riboswitches typically require high (~mM) concentrations of ligand for activation (15; 35; 179). At these concentrations, in the case of theophylline, for example, non-specific effects on gene expression have been observed in cell lines under the control of synthetic riboswitches (85), making their improvement highly desirable.
AN OVERVIEW OF RIBOSWITCH MECHANISM
Riboswitches control gene expression by mechanisms common to bacteria. In bacteria, gene regulation occurs through the use of DNA-binding transcription activators and repressors (e.g., sigma factors, lac repressor), which dictate which genes are transcribed. After transcription initiation, genes are regulated either through transcriptional or translational control, both of which ultimately regulate translation initiation and are the most common ways transcripts are regulated, riboswitch-containing or not. Bacterial transcription terminates via two pathways, which are influenced by mRNA structure (reviewed in (36; 141; 206)). The first pathway, Rho-dependent termination occurs by recruitment of the Rho protein, which binds to unstructured, C-rich Rho-utilization sites and moves in a 5′-to-3′ direction along the mRNA, eventually encountering RNA polymerase and terminating transcription. Rho-independent termination, or intrinsic transcription termination, utilizes a stable RNA hairpin followed by a U-rich sequence that, upon folding, causes RNA polymerase to stall and disassociate. Termination hairpins are predictable and have served as guideposts for discovering riboswitches (11; 31), which often use this regulatory mechanism. The intrinsic termination hairpin occurs upstream of or coincident with the Shine-Dalgarno sequence and translation start site such that terminated transcripts lack the downstream coding sequence (Figure 1a). A second layer of regulation, translational control exists in some fully transcribed transcripts, as well. In bacteria, initiation of translation requires binding of the ribosome to the Shine-Dalgarno sequence, and ribosome access is hindered if the Shine-Dalgarno sequence is base paired (Figure 1b).
In some cases, transcribed RNAs are further regulated by metabolite-responsive RNA-binding proteins as for genes involved in biosynthesis of tryptophan or histidine (55; 98). Classic studies of these operons led to our initial understanding of metabolite-controlled transcription termination (reviewed in (206)). The purpose of these systems is to rapidly regulate protein expression in response to amino acid availability. Each pathway uses a protein that both specifically recognizes the metabolite and binds the mRNA encoding the metabolite-related genes, often coupled to the formation of intrinsic termination hairpin or the availability of the Shine-Dalgarno sequence for ribosome binding.
Widespread transcriptional and translational control mechanisms
Riboswitches preclude the need for a metabolite-responsive protein by directly binding the metabolite using specific RNA structures that promote transcriptional or translational control using transcription termination hairpins or Shine-Dalgarno sequestration, respectively (Figure 1). Riboswitches can either activate (“ON” switch) or repress (“OFF” switch) gene expression in the ligand-bound state. Often presented as ON/OFF switches for the sake of simplicity (as in Figure 1), the body of experimental evidence suggests riboswitches use a wide range of control regimes. In many cases, relatively small fractional changes differentiate the active and inactive states (8; 46; 76; 121; 178; 193; 197; 201). The simplistic view of riboswitch function concerns the regulatory end points (Figure 1) when a better model is that a collection of partially folded (and partially transcribed) states decide the outcome (13; 36; 205). Although many alternative forms of control will be discussed below, for a given class of riboswitches, primarily transcriptional and/or translational control are used. For TPP riboswitches, for example, the preferential use of one type of control shows an organismal bias, with some phyla of bacteria (e.g., Firmicutes) preferring transcriptional control while others (e.g., Proteobacteria) prefer translational control (10).
Operons under the control of multiple riboswitches allow for two-input regulation by either two of the same class of “tandem” riboswitches (2; 115; 152; 174; 193; 213) or two different classes of riboswitches (174). In principle, two-input regulation could require one or both ligands for repression, one or both for activation, or a mixture in which one ligand represses and one activates. Along these lines, synthetic systems have been designed to include varieties of riboswitches using transcriptional and translational control (15; 44) as well as alternative forms of control, such as ribozyme-catalyzed cleavage (199) and ribosomal frameshifting (66; 105; 208).
Rarer mechanisms of riboswitch control
Beyond common forms of transcriptional and translational control, riboswitches are controlled by several alternative mechanisms. For example, adenine riboswitches respond to both ligand concentration and temperature, relevant to the pathogen Vibrio vulnificus which lives both in humans and in a colder marine environment (144). In the eukaryotic TPP riboswitches from A. thaliana (173), transcripts are alternatively spliced in the presence of TPP by sequestering a splice acceptor site, which appears to regulate RNA decay (101). In addition to controlling translation initiation, the E. coli lysC lysine riboswitch exposes RNase E cleavage sites in its lysine-bound (OFF) state to independently control lysC mRNA decay (25). In contrast to transcriptional switches, which do not change states after the choice of termination, translational switches can operate in bursts of protein production followed by inactive periods as long as the transcript is not degraded (54; 150). Thus, in the translational control regime, additional control by decay as in the lysC riboswitch more abruptly halts translation from longer lived transcripts, provided that the decay enzyme or complex is active. A similar mechanism appears to be occurring in some c-di-GMP riboswitches, which undergo two layers of control depending on the c-di-GMP concentration (C. Waters and coworkers, personal communication). At modest c-di-GMP concentrations, the riboswitch represses translation, and the mRNA is degraded, but the transcript is stabilized under high c-di-GMP concentrations to prevent RNase degradation.
A more general question is whether the terminated transcripts of riboswitches perform other functions before decay. Two possible answers are found in riboswitches from the human pathogen Listeria monocytogenes. First, S-adenosylmethionine (SAM) riboswitches regulate expression of methionine and cysteine metabolism, including the SreA protein (110). When SAM is abundant, SreA is transcriptionally repressed by the SAM-bound riboswitch, thereby producing a truncated transcript. However, this transcript then associates in trans with the transcript of the virulence factor PrfA and downregulates its expression (110). The PrfA transcript also contains a thermosensor, adding an additional control parameter (110). A second example of control in trans, the ethanolamine utilization (eut) operon contains a cobalamin riboswitch that produces a transcript encoding for binding sites to the EutV protein, which is sequestered from binding to other sites in the eut operon (33; 118). In the presence of cobalamin, transcription termination produces a shorter transcript that lacks the binding sites for EutV, which can then upregulate eut expression.
RIBOSWITCH APTAMER DOMAINS AND THE LIGANDS THEY RECOGNIZE
Though riboswitches may regulate gene expression through any of the mechanisms described above, ligand specificity is achieved by their aptamer domains. Riboswitch ligands broadly include ions and metabolites, such as amino acids, second messengers, and enzyme cofactors. The strategies of recognizing each type of ligands can differ wildly and unexpectedly in that seemingly related molecules—such as cyclic-diguanosine monophosphate (c-di-GMP) and cyclic-diadenosine monophosphate (c-di-AMP)—interact with their riboswitches via completely unrelated mechanisms and tertiary folds.
Bacteria use multiple unrelated classes of riboswitches for sensing amino acid availability for lysine, glycine, and glutamine. On one hand, lysine, glycine, and glutamine are each recognized directly by their respective riboswitch classes (Table 1), which are structurally distinct RNAs that have evolved specificity to their respective ligands (24; 68; 149; 158). On the other hand, the T-box riboswitches sense amino acid availability by binding to specific tRNAs, which exist in pools of charged (i.e., aminoacylated) and uncharged forms. For their use in translation, specific tRNAs and their corresponding cognate amino acids are recognized and covalently linked by aminoacyl-tRNA synthetases (69). Thus, T-box recognition of specific tRNAs relies on the specificity between aminoacyl-tRNA synthetases and their cognate tRNAs, allowing the anticodon triplet of each specifically sensed tRNA (e.g., tRNALys, tRNAGly, and tRNAGln) to serve as a proxy for specifying their respective cognate amino acid. This mechanism avoids the apparent evolutionary difficulty in discriminating against amino acids of similar shapes and sizes (e.g., tyrosine and phenylalanine). As T-box riboswitches have been reviewed recently (56; 63; 209), they will not be extensively discussed.
Table 1.
Relationship between the ligand binding pocket and the longest intramolecular interaction in riboswitches of known structure
| Namea | PK helixb | P1 helixc | Longest RNA-RNA-ligand interactiond | Longest RNA pairing interactione | Relation through foldf | PDB IDg |
|---|---|---|---|---|---|---|
| purineh | No | Yes | A21-U75 | P1: [G15,A21]-[U75,C81] | 3′ pair of P1 | 4FE5 |
| deoxyguanosine | No | Yes | A30-U81 | P1: [G22,A30]-[U81,C89] | 3′ pair of P1 | 3SKI |
| c-di-AMP | Yes | No | G5-C108 | P1: [U4,C7]-[G106,A109] | Internal pair of P1 | 4QK8 |
| c-di-GMP (Class I)i | No | Yes | G4-C88 | P1: [C1,G4]-[C88,G92] | 3′ pair of P1 | 3IWN |
| c-di-GMP (Class II) | No | Yes | A13·A74 | P1: [G7,G12]-[C75,U81] | 3′ pair of P1 | 3Q3Z |
| ZTP | Yes | No | G17-C69 | PK: [G17,G21]-[C65,C69] | 5′ pair of PK | 5BTP |
| preQ1 (Class I) | Yes | No | U7·A30 | PK: [C8,G11]-[C31,A34] | Stacks on PK | 3FU2 |
| preQ1 (Class II)j | Yes | No | U31·(A71-U40) | PK: [U35,U40]-[A71,G76] | 3′ pair of PK | 4JF2 |
| preQ1 (Class III)j | Yes | No | U8·(A85-U16) | PK: [G11,U16]-[A85,C90] | 3′ pair of PK | 4RZD |
| SAM (Class I)k | No | Yes | A6-U88 | P1: [G1,C8]-[G86,C93] | Internal pair of P1 | 2GIS |
| SAM (Class II) | Yes | No | A19·A47 | PK: [C15,U18]-[A48,G51] | Stacks on PK | 2QWY |
| SAM (Class III) | No | Yes | C6-G48 | P1: [G1,C6]-[G48,U53] | 3′ pair of P1 | 3E5C |
| SAH | Yes | No | G7-C32 | P1: [G8,C13]-[G48,C53] | Adjacent sequence to PK | 3NPQ |
| glutamine | No | Yes | C1-G59 | P1: [C1,G5]-[C55,G59] | 5′ pair of P1 | 5DDP |
| lysine | Yes | Yes | G11·G163 | P1: [G1,A10]-[G165,C174] | Stacks on P1 | 3DIL |
| Mg2+ | No | Yes | U167·A101-Pi-3′ | P1: [G15,C21]-[G168,C174] | Stacks on P1 | 2QBZ |
| Mn2+ | No | Yes | A95·G8-Pi-3′ | P1: [A3-G7]-[C96,U100] | Stacks on P1 | 4Y1I |
| Ni2+/Co2+ | No | Yes | N7-A14·A89 and C16-G88-N7l | P1: [G6,G13]-[C90,C97] | A14·A89 stacks on P1, C16-G88 stacks on A14·A89 | 4RUM |
| F− | Yes | No | 5′-Pi-G8-C47 and 5′-Pi-G42-C13 | PK: [G8,C13]-[G42,C47] | 5′ pair of PK and 3′ pair of PK | 3VRS |
| glucosamine-6-phosphate | Yes | No | C2-G64 | P1: [C2,C5]-[G61,G64] | 5′ pair of PK | 2Z75 |
| glycine (intraaptamer)m | No | Yes | 5′-Pi-A36 | P1: [G1,G9]-[A63,U71] | Stacks on P1 | 3P49 |
| glycine (interaptamer) | No | No | γ: U46·A137 | α: A136·(G8-C64) β: A43·(G156-C82) | Adjacent sequence | 3P49 |
| cobalamin (env8)n | Yes | Yes | C11-G70 | P1: [G1,A6]-[U78,C83] | Distal, separated by 5 layers | 4FRN |
| cobalamin (Tte)n | Yes | Yes | G48-C166 | P1: [A6,U17]-[G168,U178] | Distal, separated by 2 layers | 4GMA |
| TPP | No | Yes | G14·A47 | P1: [G4,U9]-[A85,C90] | Distal, separated by 6 layers | 3K0J |
| THF | Yes | Yes | A8·G78 | P1: [G1,A5]-[G84,C88] | Distal, separated by 3–4 layers | 4LVV |
| FMN | No | Yes | C31-G84 | P1: [G1,C8]-[G105,C112] | Distal, separated by 3–4 layers | 3F2Q |
Riboswitch name abbreviations are: cyclic diadenosine monophosphate (c-di-AMP), cyclic diguanosine monophosphate (c-di-GMP), 5-aminoimidazole-4-carboxyamide riboside 5′-triphosphate (ZTP), 7-aminomethyl-7-deazaguanine (preQ1), S-adenosylmethionine (SAM), S-adenosylhomocysteine (SAH), thiamine pyrophosphate (TPP), tetrahydrofolate (THF), flavin mononucleotide (FMN).
The presence of a pseudoknot (PK) (136) in the riboswitch is indicated.
The presence of a first paired helical element (P1) connecting the 5′ and 3′ ends of the riboswitch aptamer domain is indicated.
The longest range base pairing interaction that also interacts with the riboswitch ligand. Residue numbers are as found in the referenced PDB models.
The longest range pairing interaction in the riboswitch fold. Paired helical elements are referred to by residue range [a,b]-[c,d] where the 5′ sequence of the helix spans residues a to b and the 3′ sequence spans residues c to d.
The relationship in tertiary structure between the longest range pairing interaction and the longest range pairing interaction that also interacts with the ligand.
Protein Data Bank codes for accessing structural models to which the sequences correspond.
The purine riboswitches recognizing adenine and guanine (13) are structurally similar and typify a single class of riboswitches.
The cyclic-AMP-GMP riboswitch (148) is included in the class I c-di-GMP riboswitches due to their structural similarity.
PreQ1 class III riboswitches (103) are structurally related to class II riboswitches (102) in sharing a similar ligand binding site.
The SAM class IV riboswitches (137; 183) are related to SAM class I riboswitches by sequence and structure.
Interactions between the N7 atoms of purine residues and bound Co2+ ions are described for the two Co2+ ions closest to helix P1.
Owing to symmetry, both aptamer domains contain pseudosymmetric ligand-P1 interactions. In aptamer domain 2, the long-range interaction occurs between the phosphate of A111, which stacks on the P1 helix consisting of residues [C80,G85]-[A153,G158].
Structures of two different cobalamin riboswitches, one from environmental sample 8 (env8) and one from Thermoanaerobacter tengcongensis (Tte), were determined by the Batey lab (74).
Metabolite riboswitches
The majority of metabolite riboswitches recognize ligands associated with purine metabolism (Table 1) that are predicted to be cofactors from long ago (192; 195). In metabolite riboswitches, ligand specificity is largely achieved through hydrogen bonds from RNA bases and the sugar-phosphate backbone, π-π stacking interactions from either nucleobases or ribose sugars, and bound cations to accommodate negatively charged functional groups. A comprehensive catalog of each structurally characterized riboswitch ligand-binding pocket will not be presented here as many excellent reviews have covered this subject (13; 104; 133; 157; 161).
Riboswitches specific to the purines adenine and guanine are nearly identical structurally, and specificity is determined by a residue interacting with the Watson-Crick face of the ligand (13; 14; 88; 163). Purine riboswitches bind their ligands at a pocket formed by a three-helix junction (e.g., Figure 1), which positions the purine nucleobase adjacent to and stacking on the helical region bringing the 5′ and 3′ ends of the RNA together. Deoxyguanosine is recognized by a structurally similar riboswitch that contains additional mutations to accommodate the ribose moiety and discriminate against the 2′-hydroxyl group present in guanosine (39; 89). While the cyclic dinucleotides c-di-AMP and c-di-GMP are sensed by different classes of riboswitches, cyclic AMP-GMP is sensed by riboswitches structurally similar to one of the two distinct classes of c-di-GMP riboswitches, which recognize c-di-GMP via unrelated mechanisms (96; 165; 166).
Multiple structurally distinct classes of riboswitches have been discovered for the modified purine 7-aminomethyl-7-deazaguanine (preQ1) (92; 102; 119; 153) and the ubiquitous purine-like cofactor SAM (52; 112; 122; 203). For example, individual classes of SAM riboswitches show little sequence homology (139), which has been interpreted as independent evolution of aptamers (116). A separate class of riboswitches have also evolved specificity to the closely related metabolite S-adenosylhomocysteine (SAH) (189), which SAM riboswitches discriminate against (reviewed in (12; 188)). Despite using multiple classes of structurally distinct folds (116), preQ1 riboswitches always contain pseudoknots (136) close to the ligand pocket. The preQ1 class I riboswitches are the smallest natural riboswitch aptamers studied to date (92).
At least two routes are available for riboswitch sensing of folate, a metabolite required for two steps in purine synthesis. In addition to riboswitches specific to tetrahydrofolate (THF) (3), a class of riboswitches bind to the purine biosynthetic intermediate 5-aminoimidazole-4-carboxyamide riboside 5′-monophosphate (ZMP) or the triphosphorylated form (ZTP), which build up when either folate levels are low or when the enzyme that converts ZMP to inosine is limiting (18; 90). Despite their shared control folate biosynthetic genes, the two classes of riboswitches are structurally distinct.
Aside from THF and SAM, riboswitches recognize many coenzymes, which often contain purine-derived aromatic rings. Both FMN (121; 201) and molybdenum cofactor (MoCo) (143) are coenzymes synthesized from GTP. While structural studies of MoCo riboswitches have not been reported, possibly due to the instability of MoCo, FMN riboswitches have been investigated in detail (6; 100; 159; 186; 197). The coenzyme cobalamin contains a purine-like 5,6-dimethylbenzimidazole moiety and an adenosine moiety in one form (adenosylcobalamin), and part of the coenzyme thiamine pyrophosphate (TPP) is synthesized from a purine biosynthetic intermediate (aminoimidazole ribotide) (32). The cobalamin riboswitches exist in two classes of riboswitches (Table 1) with considerable variation in the lengths of helices (74). The only class of riboswitches found in all three domains of life thus far (95; 173; 176), TPP riboswitches in Arabidopsis thaliana regulate the thiC gene, which commits the purine biosynthetic intermediate 5-aminoimidazole ribotide to thiamine biosynthesis.
Controlling amino acid metabolism is a minor theme among metabolite riboswitches (161), which include T-box riboswitches in addition to individual riboswitch classes specific to lysine, glycine, and glutamine. The lysine riboswitch recognizes lysine at a pocket created by a five-helix junction that nearly envelops the entire ligand and prevents accommodation of bulkier side chains (50; 158). In contrast, the glutamine riboswitch, which possesses the weakest affinity of all riboswitches (apparent dissociation constant, KD ~ 0.6 mM), essentially binds glutamine in a very open pocket of a two-helix junction, which is likely responsible for the weak affinity (149). The first example of a tandem riboswitch to be structurally determined (24; 68), glycine riboswitches often exist in a configuration of two pseudosymmetrical aptamer domains followed by a single expression platform. Each three-helix aptamer domain binds to a single glycine molecule, and the two domains associate through several interactions (24; 155). Glutamine riboswitches are also sometimes present in tandem arrangements of two and three glutamine aptamers (2), and whether the repeated elements associate is an open question. In all three amino acid riboswitches, the carboxylate moieties of the ligands bind to a metal ion (K+ in lysine, Mg2+ in glutamine and glycine).
An outlier both categorically and functionally, the glmS riboswitch/ribozyme catalyzes self-cleavage in the presence of glucosamine-6-phosphate to regulate expression of genes associated with cell wall synthesis (11; 202). In this riboswitch, the glucosamine-6-phosphate molecule acts as a cofactor—the amine group providing the general acid—to stimulate catalysis rather than to stabilize folding (91; 93). This is in contrast to other self-cleaving ribozymes, which use a nucleobase functional group as the general acid to catalyze cleavage of the phosphodiester backbone (reviewed in (45; 73)).
Ion riboswitches
Recognition of small ions by riboswitches is a tricky matter, owing to the fact that RNAs require ions to fold (16; 38; 107). The ZTP riboswitch (147; 182), glycine and glutamine riboswitches (68; 106; 149), and TPP riboswitch (176) also require Mg2+ for ligand binding. Indeed, riboswitches are known to partially compact with Mg2+ and further compact in the presence of ligand (108; 210). In the four known classes of ion riboswitches—magnesium (Mg2+), manganese (Mn2+), nickel/cobalt (Ni2+/Co2+), and fluoride (F−)—the controlled operons encode for proteins associated with metal homeostasis, such as a metal ion transporters or efflux pumps (9; 28; 29; 48). The riboswitches collectively bind with affinities in the low to mid μM range, near to levels of toxicity if the corresponding metal homeostasis operons are knocked out (4; 9; 28; 190).
The Mg2+, Mn2+, and F− riboswitches achieve ligand specificity largely through phosphate interactions, which locally contort the sugar-phosphate backbone (30; 139; 146). Backbone interactions are mediated by Mg2+ ions in the case of the F− riboswitch, which recognizes a cluster of three Mg2+ ions surrounding a single F− ion at a junction formed by a pseudoknot (146). In addition to phosphate interactions, the Mn2+ riboswitch presents N7 of a nearby by adenine to bind Mn2+ (139) to discriminate against the more abundant ion Mg2+, which prefers hard ligands like oxygen. The same types of soft purine N7-ion interactions are observed in the Ni2+/Co2+ riboswitch, which uses two N7-Co2+ interactions per bound Co2+, likely discriminating against ions that prefer harder contacts (48).
RELATIONSHIP BETWEEN RNA FOLD AND LIGAND RECOGNITION BY APTAMER DOMAINS
Although riboswitches recognize ions, amino acids, and complex metabolites by greatly differing mechanisms, their overall structural organization follows common principles (Table 1). From 5′ end to 3′ end, the aptamer domain precedes the expression platform. This arrangement is essential if the rate of ligand-assisted RNA folding is similar to the rate of the genetic decision, referred to as kinetic control (196; 197; 211). Otherwise, the genetic decision, or the RNA structure folding into and dictating the decision, would precede the sensing of the metabolite imbalance by the aptamer domain. For thermodynamically controlled riboswitches, in which an equilibrium could be reached prior to the regulatory decision (211), the expression platform should be able to precede the aptamer domain. To our knowledge, there are no examples of bacterial riboswitches where this is the case, though synthetic theophylline-responsive hammerhead ribozymes can contain the aptamer domain within the 3′ half of the ribozyme, which is the expression platform (199).
Another common feature of riboswitches, the ligand binding pocket is almost always situated adjacent to the RNA structure that undergoes alternative folding, referred to as the switch or switching helix, switching sequence, or transmitter. More specifically, riboswitches can be divided into two groups based on the proximity of the ligand to the longest range base pairing interactions—that is, the longest contacts or the contacting residues farthest away from one another in sequence. These relationships are presented for all structurally distinct riboswitches in Table 1. In the first group (“proximal” riboswitches), the longest contacts also directly interact with the ligands. In the second group (“distal” riboswitches), which only includes four riboswitch classes, the longest contacts are separated from the ligand, typically by layers of bases interacting through π-π stacking. In both cases, the longest contacts in the RNA tertiary fold are linked to the ligand, but the relationship can be obscured for distal riboswitches.
Proximal riboswitches with ligand-P1 interactions
Proximal riboswitches interact with their ligands through either the first paired region (P1) connecting the 5′ and 3′ ends of the RNAs or a pseudoknot helix (PK) formed by a distant loop or single-stranded region pairing with an internal loop or bulge (Table 1). The separation of riboswitches into P1 and PK types is based on whether or not the residues participating in the longest contacts are part of the P1 helix or the PK helix. Several P1-type riboswitches have pseudoknots which are shorter range than the P1 helix (e.g., c-di-AMP riboswitch, cobalamin riboswitch, lysine riboswitch) (Table 1). The importance of the P1 helix for riboswitch function has been recently discussed (1; 133); for many riboswitches, the P1 helix is also the switch helix, alternatively paired in the final ligand-bound and ligand-free states. This point is crucial: the vast majority or riboswitch crystal structures solved to date are in the ligand-bound state in which the switch helix is folded (i.e., right side of Figure 1).
For P1 riboswitches (Figure 2), the simplest fold is exemplified by SAM class III riboswitches (Figure 2a), which accommodate the SAM ligand stacking directly above the P1 helix at the center of a three-helix junction with a Y-shaped fold (112). In purine riboswitches, the ligand also interacts directly with the P1 helix, which is more disordered in the absence of ligand (22; 23; 128; 129). Purine and deoxyguanosine riboswitches (14; 39; 163) and both classes of c-di-GMP riboswitches (96; 148; 165–167) use the same overall topology, differing from the SAM class III fold in that the non-P1 helices of the riboswitch interact. Both classes of c-di-GMP riboswitches bind a single c-di-GMP molecule at the crux of a three-helix junction. However, specific contacts above and below the guanines bases of the c-di-GMP molecule are different (166). An even greater contrast is made to c-di-AMP riboswitches, which bind to two copies of c-di-AMP using a pseudosymmetric fold (49; 75; 77; 145) that sandwiches the c-di-AMP molecules between a pair of three-helix junctions.
Figure 2.
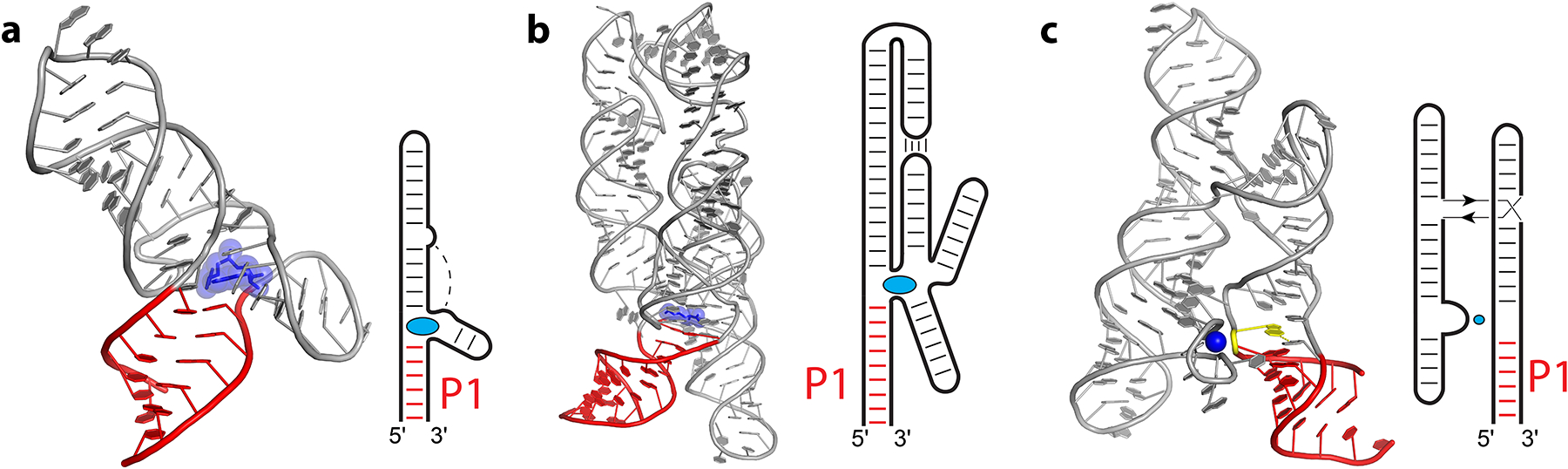
Representative structures of proximal P1-type riboswitches. Cartoon representations and secondary structure diagrams are shown for the SAM class III riboswitch (a) from Enterococcus faecalis (112), lysine riboswitch (b) from Thermotoga maritima (158), and Mn2+ riboswitch (c) from Lactococcus lactis (138). Direction interaction between ligands (blue) and first paired region connecting the 5′ and 3′ ends (P1, red) are emphasized for all three riboswitches. The metal-facilitated stacking interaction of G10 (yellow) on the G9·A95 pair of P1 in the Mn2+ riboswitch is also shown, with other ions omitted for clarity. In the simplified secondary structure diagrams, thin lines and arrows indicate chain connectivity.
The lysine riboswitch also situates its ligand directly above the switch helix (Figure 2b) in a junction that is the meeting point of five helices packed together via a kissing loop interaction (50; 158). In this case, lysine contacts a purine-purine pair of the longest contact helix P1, a non-Watson-Crick pair commonly observed at this position in other riboswitches. The Mn2+ riboswitch also uses the P1 architecture but at a junction created between P1 and a distant Mn2+-binding loop (138), and a purine-purine pair caps the P1 helix near the ligand (Figure 2c). However, a key difference between the ion riboswitches and metabolite riboswitches is that Mn2+ does not directly stack on the P1 helix but instead interacts with the phosphate of the residue that stacks on P1 (138). The Mg2+ riboswitch follows a similar pattern, with one Mg2+ bound to the phosphate of a noncanonical base pair at the end of the P1 helix (30). In contrast, in the Ni2+/Co2+ riboswitch, a Co2+ ion interacts with the N7 of the noncanonical purine-purine pair that stacks on the P1 helix (48).
Other proximal riboswitches interacting with the P1 helix include c-di-AMP (49; 75; 145), SAM class I (122), and glutamine riboswitches (149) (Table 1). Aside from the Y-shaped regularity among some riboswitches, the overall folds of others differ considerably despite all linking the ligand binding pocket to the P1 helix. These differences are greatest across SAM riboswitches, which have both P1-type and PK-type folds. For example, SAM class I riboswitches bind SAM sandwiched between two helices near a four-helix junction (122), and SAM class II riboswitches utilize an entirely unrelated double pseudoknot fold (52), in which SAM binds in the RNA major groove and completes a pseudo-base pairing interaction in a gap of a helical element (52).
Proximal riboswitches with ligand-pseudoknot interactions
In the second group of proximal riboswitches, the longest contacts are in the PK helix, and in all of these cases, the ligand interacts with the PK helix. For example, in the preQ1 class II riboswitch (102), preQ1 stacks directly on the PK helix formed by pairing between the 3′ end of the RNA and an internal loop (Figure 3a). In the absence of preQ1, the PK helix is more dynamic (78; 169). In class I preQ1 riboswitches, preQ1 interacts with the pseudoknot, which is more dynamic in the absence of preQ1, as well (79; 80).
Figure 3.
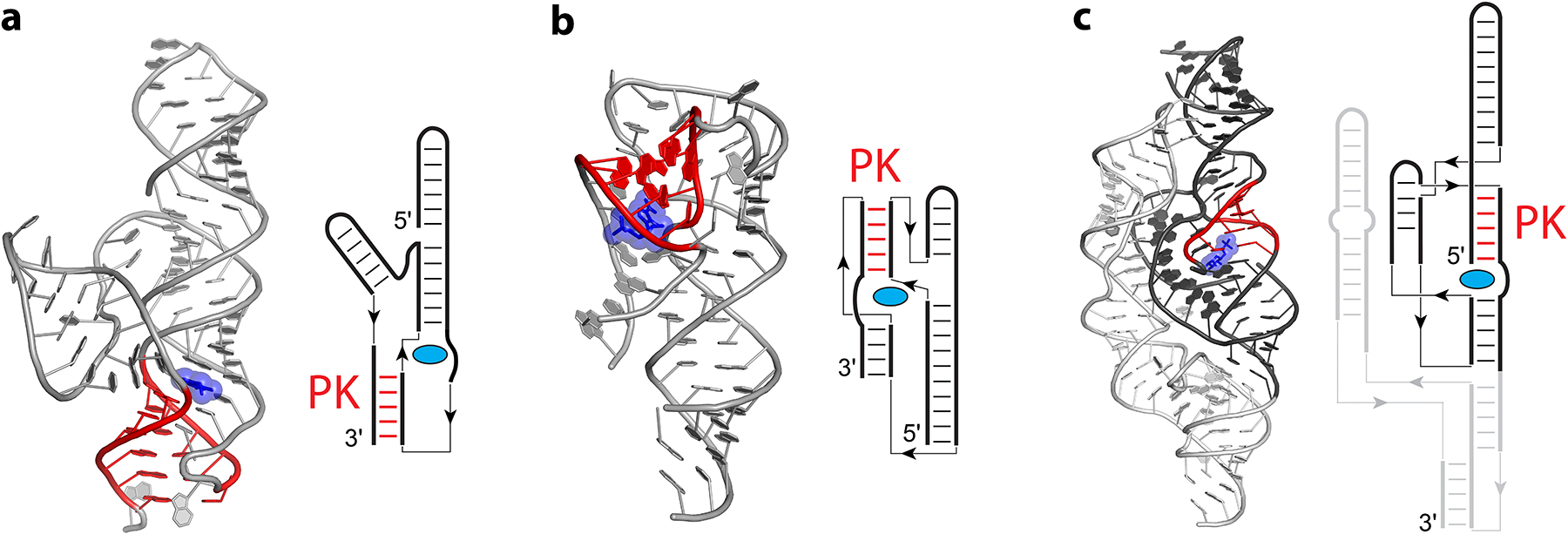
Representative structures of proximal PK-type riboswitches. Cartoon representations and secondary structure diagrams are shown for the preQ1 class II riboswitch (a) from Lactobacillus rhamnosus (102), ZTP riboswitch (b) from Fusobacterium ulcerans (76), and glmS riboswitch/ribozyme (c) from Thermoanaerobacter tengcongensis (93). Direction interaction between ligands (blue) and pseudoknot helices (PK, red) are emphasized for all three riboswitches. The central and peripheral domains of the glmS ribozyme (c) are indicated by dark grey and light grey, respectively. In the simplified secondary structure diagrams, thin lines and arrows indicate chain connectivity.
In ZTP riboswitches, the overall fold of the RNA is divided into two halves connected by a variable linker, the length of which tunes the riboswitch’s affinity (76). A pseudoknot joins the riboswitch’s halves and creates the binding pocket specific for ZMP via a specific phosphate-Mg2+-phosphate interface (76; 147; 182). In the ZTP riboswitch, a similar interaction occurs, as ZMP stacks directly on the PK helix (76; 147; 182), which is instead formed by a loop at the 3′ end pairing with an internal bulge (Figure 3b). Mutation of the base pair stacking on ZMP completely abolishes ZMP binding (76; 147).
The overall fold of the glmS ribozyme-riboswitch contains a central domain of four helices, three of which are coaxial, surrounded by a peripheral domain that, while enhancing, is not required for function (93; 198). Setting aside the difference in functional output, the central domain recognizes glucosamine-6-phosphate in a manner consistent with other riboswitch aptamer domains. Glucosamine-6-phosphate is surrounded by stacking interactions above and below its ribose moiety, Mg2+ ions near the ligand phosphate moiety, and numerous hydrogen bonds to the functional groups of the ribose ring. Despite the complex architecture of the glmS ribozyme (93), the core domain of the ribozyme situates glucosamine-6-phosphate directly adjacent to the PK helix (Figure 3c), which is necessary for ribozyme activity (198). For other PK riboswitches, such as the preQ1 classes 1 and 3 (92; 103), SAM class II (52), SAH (40), and F− riboswitches (146), direct interaction with the PK helix also occurs (Table 1).
Owing to the small size of glycine, the glycine riboswitch functions both like a metabolite riboswitch and like an ion riboswitch (Figure 4). Glycine is bound in a pocket surrounded by nucleotides as a metabolite would be, but the longest contacts are mediated by the glycine-bound Mg2+ ion. This ion contacts the phosphates of the nucleotides involved in or adjacent to the longest contacts (24; 68), both within a single aptamer (Figure 4a) and between the two aptamers (Figure 4b). Within each aptamer domain, glycine interacts with the longest contacts via the Mg2+ ion, which coordinates to the phosphate of a base that stacks on P1 (Figure 4a). Between the aptamer domains, there are two types of long-range interactions mediated by the bound glycine and Mg2+ ion. One of these interactions (referred to as γ) is a modestly conserved single pair through the center of the two glycine domains, and the other interaction (referred to as β) is a highly conserved series of A-minor interactions (136) that link the glycine binding pocket to the switch helix contained within aptamer domain 2 (115). Owing to pseudosymmetry, the latter interaction occurs twice between the two aptamer domains (α and β) (Figure 4b). Topologically, the long-range interaction is similar to a pseudoknot, but instead of forming Watson-Crick base pairs to a distant single-stranded region, the A-rich loop forms A-minor interactions with a distant helix. Glycine riboswitches also exist single aptamer configurations, in which one of the two aptamers are replaced with a “ghost aptamer” domain that does not bind glycine (154). In glycine riboswitches lacking the second domain, interaction β is conserved between the first aptamer and the ghost aptamer (154). In glycine riboswitches lacking the first domain, however, β is absent, and glycine binding is proposed to stabilize the switch helix (Figure 4a).
Figure 4.
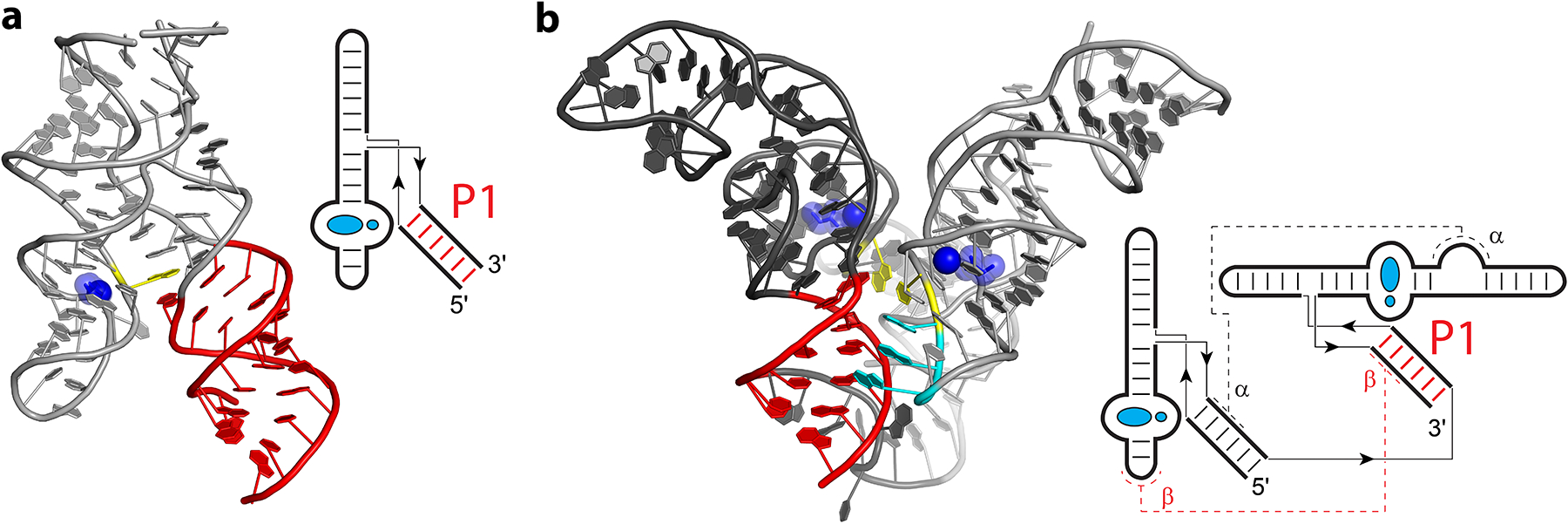
Intra- and inter-aptamer interactions of the Fusobacterium nucleatum glycine riboswitch (24). Cartoon representations and secondary structure diagrams are shown for the glycine riboswitch aptamer domain 1 and both aptamers. (a) Inter-aptamer interactions between the glycine-Mg2+ ligands (blue) and P1 helix (red), as well as the Mg2+-facilitated stacking interaction of A36 (yellow) on G9·A63, are shown for the isolated aptamer domain 1. (b) Intra-aptamer interactions between the aptamer domain 1 (light grey) and aptamer domain 2 (dark grey) are shown, emphasizing the longest range RNA-RNA interactions γ (yellow) and β (cyan and red). Interaction β occurs between A-rich loop residues A43, A44, and A45 (cyan) near the glycine of aptamer domain 1 and the P1 helix of aptamer domain 2 (red). In the simplified secondary structure diagrams, thin lines and arrows indicate chain connectivity.
Distal riboswitches
From the proximal riboswitches discussed above, a clear trend emerges in that the ligand is situated adjacent to the RNA residues that participate in the longest contacts in the RNA structure. This is clearly the most commonly evolved solution for riboswitches. In the distal riboswitches, which are literally and figuratively outliers, the ligand is separated by multiple stacks of residues from the longest contacts (Figure 5). The overall secondary structures include P1 helices for all although the folds can be quite complex. In each distal riboswitch, the switch helix contains the longest contacts in the RNA, referred to as the P1 helix, which connects the 5′ and 3′ ends of the aptamer domain.
Figure 5.
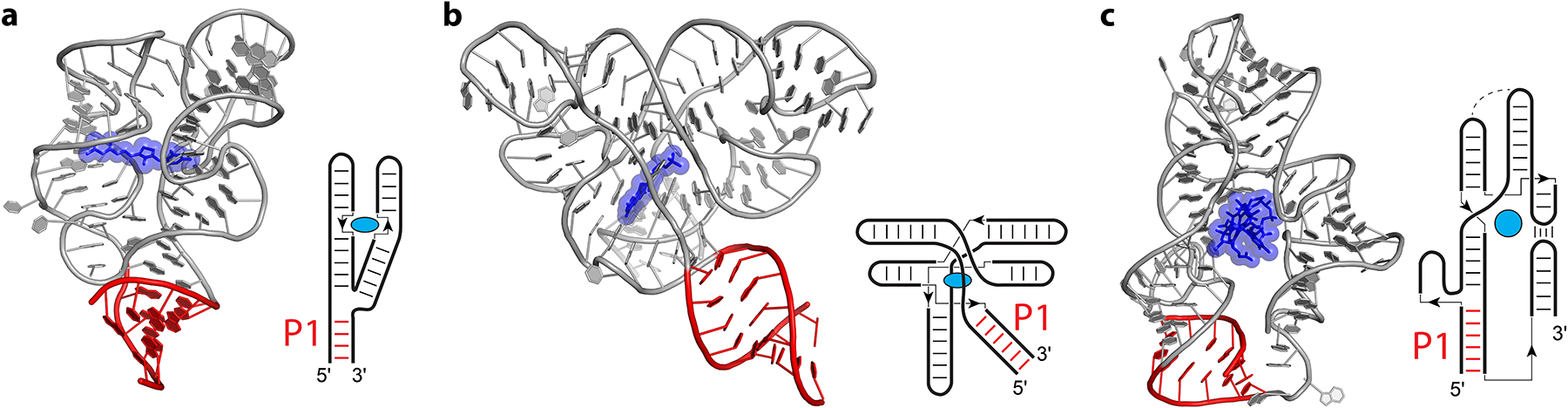
Distal riboswitches. Cartoon representations and secondary structure diagrams are shown for the TPP riboswitch (a) from Escherichia coli (97), the FMN riboswitch (b) from Fusobacterium nucleatum (159), and the cobalamin riboswitch (c) from an environmental sample (env8) (74). Ligands (blue) and the first paired region connecting the 5′ and 3′ ends (P1, red) are indicated. In the simplified secondary structure diagrams, thin lines and arrows indicate chain connectivity.
For the TPP riboswitch (Figure 5a), which has an overall Y-shaped architecture similar to the purine riboswitches and other P1 riboswitches (41; 162; 176), the ligand is the farthest removed from the switch helix. The location of TPP is ~15–20 Å away from the junction where proximal riboswitches bind their ligands (Figure 5a). Rather than binding directly to the P1 helix, each half of TPP (pyrophosphate and pyrimidine moieties) interacts with one helical element of the riboswitch, which is stabilized by TPP and additional interactions between the two helices likely not present in the absence of TPP (6; 60; 97; 176). Resistance mutations to the TPP riboswitch-targeting antibiotic pyrithiamine map to the junction connecting the switch helix to the TPP binding site (95; 176). From single molecule experiments, the P1 helix of the TPP riboswitch was observed to be highly dynamic, more stable in the presence of Mg2+ than in the absence, and yet more stable in the presence of Mg2+ and TPP (60).
In the FMN riboswitch, which has one of the most unique structures (159) of riboswitches solved to date, the ligand is 3–4 layers of nucleotides away from the switch helix, 13–14 Å away from the equivalent ligand binding site in proximal P1-type riboswitches (Figure 5b). The pseudosymmetric fold of this RNA creates a pocket that surrounds the ligand from all sides, providing stacking interactions that are communicated from the binding pocket to the switch helix. Resistance mutations to the antibiotic roseoflavin and recently developed small molecule ribocil map to residues important for tertiary contacts of the RNA fold and ligand binding (64; 65; 100) and are even farther away from the switch helix than the ligand (Figure 5b).
The structures of two classes of cobalamin riboswitches have been solved from an environmental sample (env8) and Thermoanaerobacter tengcongensis (74). In both, the overall shape of the ligand is accommodated in a large pocket that is separated from helix P1 by a second coaxially stacked helix (Figure 5c). Consequently, the ligand is separated from the longest contacts by ~19 Å for env8 and ~7 Å in T. tengcongensis.
In the THF riboswitch (Figure 6a), further discussed below, up to two ligands were observed binding to the RNA in crystals (67; 181), but only one of them appears to be important for gene control while the other is important for folding (180). THF is recognized by a fold consisting of a long helical element that bends back on itself to form a pseudoknot (67; 181). One THF binding site is formed by the pocket at the pseudoknot and believed to be the primary site for gene control (180). A second binding site is formed at the riboswitch’s “elbow”, where it bends back on itself, more than 30 Å from the P1 helix. The relevant ligand is bound at a pseudoknot junction that interacts with P1, the switch helix, which is separated from the ligand by 3–4 layers of stacking interactions about 12 Å away from the location where the ligand binding site would be in proximal P1-type riboswitches (Figure 6a). A number of antifolate drugs and other compounds can bind to the THF riboswitch—some even more tightly than THF—and they are often weaker transcription regulators (180).
Figure 6.
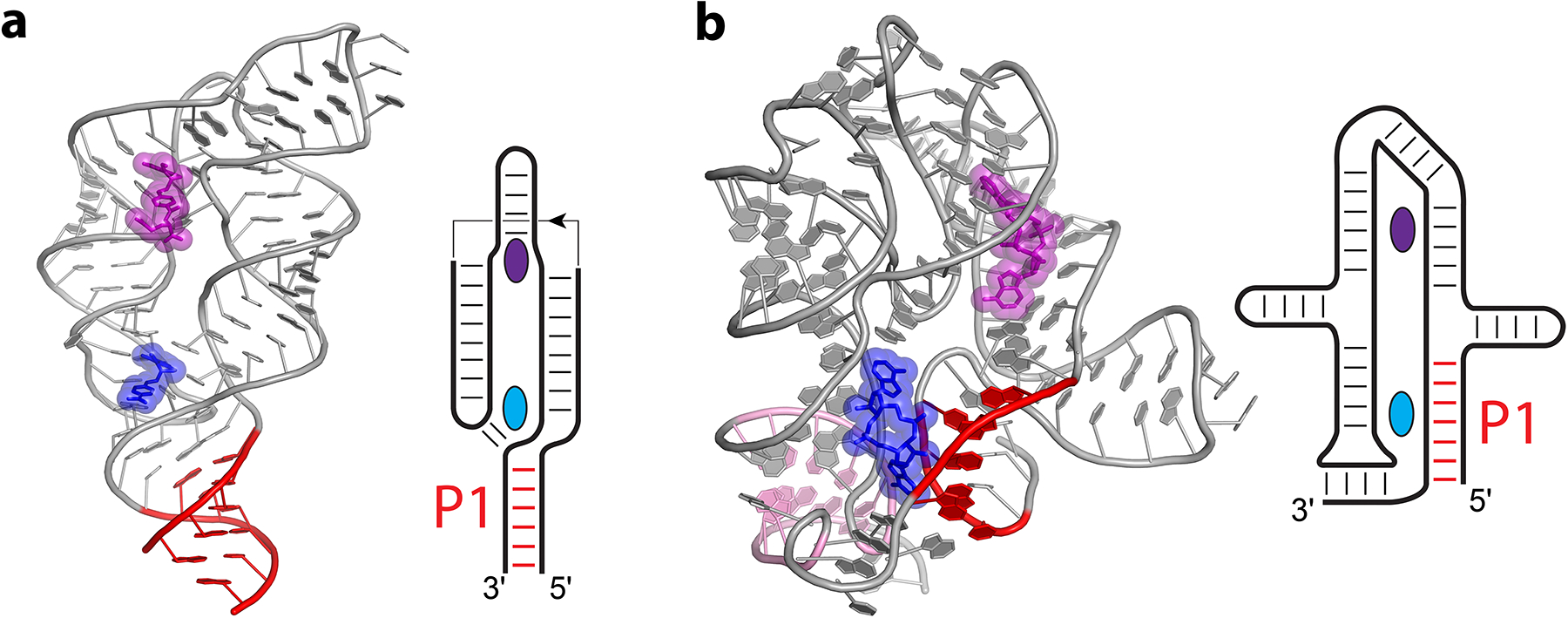
Importance of proximal ligands in riboswitches binding multiple ligands. Cartoon representations and secondary structure diagrams are shown for the THF riboswitch (a) from Streptococcus mutans (181) and c-di-AMP riboswitch (b) from Thermoanaerobacter pseudethanolicus (49). Proximal ligands (blue), distal ligands (purple), and the first paired region connecting the 5′ and 3′ ends (P1, red) are indicated. For the c-di-AMP riboswitch, the pseudoknot is also indicated (pink). In secondary structure diagrams, thin lines and arrows indicate chain connectivity.
Function and prediction
Aside from providing a general framework for viewing riboswitch folds, part of the utility in discussing this trend is in prediction. Notably, the c-di-AMP (49; 75; 145), THF (181), and glycine riboswitches (24; 68; 115) bind to two ligands, raising the question if one is more important than the other for riboswitch activation. In these riboswitches, one ligand interacts more closely with the longest contacts than the other—that is, one ligand is relatively proximal, and the other is relatively distal. The THF riboswitch contains a long-range pseudoknot that sits near one ligand while the second ligand is farther removed (Figure 6a). In this case, the ligand closer to the pseudoknot is required for gene control while the second less conserved binding site is less relevant to biological function (180). For glycine riboswitches (Figure 4), both ligands play a role, with interactions promoting dimerization being important for glycine binding while interactions stabilizing the switch helix being more important for gene control (155). The c-di-AMP riboswitch also contains a long-range pseudoknot closer to the proximal ligand than the distal ligand, but the longest contacts are in the P1 helix (Figure 6b), which directly interacts with the proximal ligand. Notably, the switch helix is the pseudoknot in this riboswitch, and a loop near the pseudoknot helix interacts with the proximal ligand (49; 145). For the c-di-AMP riboswitch, there is evidence that the distal site is required to observe binding at both sites (49; 117); however, the relevance to gene control has yet to be established. The cooperativity observed in the c-di-AMP riboswitch complicates the dissection of interactions important for binding in one pocket versus additional changes to the second pocket (i.e., allostery).
Multiple ions are described in models of the Mg2+, Mn2+, and Ni2+/Co2+ riboswitches. In each case, the ions of interest—as judged by anomalous scattering in crystal structures (48; 138; 140), chemical probing and in-line probing (30; 48; 124; 140; 187), and mutational analysis (48)—are also the ions adjacent to the longest contacts. In the case of the Mg2+ riboswitch, which associates with at least a dozen Mg2+ ions, the ion closest to the P1 helix is the Mg2+ ion involved in the most inner-sphere phosphate contacts and, correspondingly, is the site most sensitive to phosphorothioate nucleotide analog interference (187). Though only two Mn2+ ions were observed in the Mn2+ riboswitch crystal structure solved in the presence of Mn2+, nearby Mg2+ ions could be replaced by Mn2+ at high enough concentrations in crystals (138). In particular, one Mg2+ ion adjacent to the critical Mn2+ is coordinated to five backbone phosphates, which is highly unusual (212), and three of the same phosphates also coordinate to the critical Mn2+. Thus, the ion is predicted to play a role in folding to allow for Mn2+ sensing (138), which would be consistent with its proximity to the longest contacts in the RNA.
For P1 riboswitches, the P1 helices are not only the longest range paired regions of the RNA but also the final transcribed elements in the riboswitch aptamer domain as the 3′ switch sequence marks the end of the aptamer domain (Figure 2). Only upon transcription and folding of P1 would the entire binding pocket be formed. This relationship has been discussed previously for some P1 riboswitches (1) and was observed among early riboswitch structures, which contained P1 helices (14; 122; 176). The same is true for some of the PK riboswitches, in which the 3′ end of the pseudoknot is transcribed after other helical elements, as exemplified by the preQ1 class II riboswitch (Figure 3a). In contrast, in the ZMP riboswitch (Figure 3b), fluoride riboswitch, and SAM class II riboswitch, the 3′ half of a helical element is transcribed after the pseudoknot. The glmS ribozyme contains a peripheral domain 3′ to the pseudoknot as well (Figure 3c), raising the possibility that the ligand binding site can form partially prior to completion of transcription. This is important from a mechanistic point of view. In kinetically controlled riboswitches, ligand binding competes with riboswitch refolding during transcription (196; 197; 211), so having a preformed site or faster folding site would favor the ligand (and in the case of the glmS riboswitch, allow for self-cleavage). In thermodynamically controlled switches, transient folding and unfolding is expected to tune output to match ligand concentration (26; 61; 150; 169), so faster folding would be less relevant.
Folding and unfolding of the longest range base pairing interaction leads to the greatest possible change in the RNA structure. For riboswitches transitioning from a bound to an unbound state, the 3′ end either unfolds or alternatively pairs with an adjacent sequence, reducing the length of the longest contacts (e.g., Figure 1a). More generally, the distance between interacting residues in a macromolecule is described by contact order (135; 204). For proteins, high relative contact order (average contact order over total sequence) is associated with slower folding rates (70; 135), but for RNA, this correlation has not been observed (168). Riboswitches exist in a state with higher contact order in the presence of ligand and a lower contact order state in the absence, and the ligand binding site is generally adjacent to the regions containing the longest contacts to control contact order (Table 1).
ABSENT LIGANDS, FLUOROGENIC APTAMERS, AND FUTURE INVESTIGATIONS INTO RIBOSWITCH MECHANISMS
Despite the structural studies reviewed above characterizing many classes of riboswitches, substantial evidence suggests that at least an order of magnitude more unique classes of riboswitches remain (20; 31; 191). If one assumes that all of the most common, essential metabolites were once recognized by RNA (53; 195), then where are specific domains for other ions, amino acids, metabolites, and cofactors? Do non-proteinaceous solutions for regulating trp, his, and pyr operons still exist? Tryptophan and histidine are aromatic, planar molecules that, in their free forms, resemble other riboswitch ligands, and the lack of evidence for pyrimidine riboswitches is surprising given the dominant role that nucleotide binding plays in riboswitches as a whole. There is also the notable absence of the cofactors nicotinamide adenine dinucleotide, coenzyme A, and pyridoxal phosphate from known riboswitch ligands (20).
Aside from expanding our understanding of ancient gene control mechanisms, unearthing the RNA-based solutions for sensing these metabolites will provide new tools. An exciting direction that riboswitch-related research has recently taken makes use of the fluorescent RNA-chromophore complexes, such as Spinach (130) and Mango (37), and the aptamer domains of natural riboswitches to create biosensors that fluoresce when bound to both the riboswitch ligand and the dye molecule (81–84; 109; 125; 148). Sensing metabolite concentrations in living cells via a noninvasive fluorescent output enables researchers to answer many important questions about each individual ligand for which a biosensor has been designed. This approach will greatly benefit from development of the next generation of fluorescent RNAs to be better folded and more specific to their fluorescent dyes (207). As natural aptamers are typically superior to synthetic ones, improved methods to search libraries of orphan riboswitches for specific ligands of interest could be doubly beneficial.
Perhaps the greatest challenge to understanding how riboswitches function is the lack of structural data of riboswitches in ligand-free states, which has been reviewed (62; 104). Ligand-free riboswitch structures have been solved (68; 72; 138; 149; 158; 171), and these structures are the ligand-less aptamer domains in a bound-like state, typically the same RNA sequence that crystallized in the ligand-bound state. Riboswitch sequences are often chosen for biophysical and structural study based on their conformational homogeneity. The judicious choice of RNA termini is the difference between observing binding or not among species of ZTP riboswitches (76; 90), which biases the types of RNAs that are studied. Thus, RNAs often lack an extended 3′ end containing the expression platform. Already the more challenging state to study crystallographically, ligand-less aptamer domains are often more flexible than their ligand-bound forms, as judged by in-line and chemical probing (142; 170; 185), small-angle X-ray scattering (210), and many other methods (47; 103). Some riboswitches appear largely pre-folded in the unbound state (6; 76; 158; 159; 210), suggesting that for these riboswitches, the ligand-sensing state is more closely related structurally to the bound state. For the lysine riboswitch, the empty binding pocket is seemingly inaccessible (50; 158), and for the SAM class I and preQ1 riboswitches, the binding pockets are blocked by nucleotides as the RNAs have refolded (72; 171). Breathing must occur to allow for binding in these cases. Ion riboswitches in the ligand-free state are often difficult to interpret because at high concentrations, other ions occupy the same binding sites (138; 140). In the Mn2+ riboswitch, for example, the binding pocket is disordered in the absence of Mn2+ although the overall fold is maintained presumably due to Mg2+ and Sr2+ ions (138). Whether or not this overall foldedness is representative of the ligand-free state remains to be determined.
Connecting the dots between the partially transcribed riboswitch and the genetically determined states (Figure 1) would ideally include structural information on a nucleotide-by-nucleotide basis monitored during transcription. Characterizing RNA folding landscapes (7; 36; 60; 134) through the use of complementary biophysical tools seeks to address this question. Terminated riboswitches still bound to RNA polymerase, decay factors, or other factors are also relatively unexplored states. Perhaps with their inclusion, atomic resolution structural information of undiscovered states may be achievable in the future.
ACKNOWLEDGMENTS
We are grateful to M. Chen, N. Demeshkina, C. Fagan, T. Numata, Lj. Sjekloca, and R. Trachman III for helpful discussions, and to M. C. Hammond, K. Ruff, and C. Waters for discussing unpublished data. This review was supported in part by the intramural program of the NHLBI, NIH, and a Lenfant Biomedical Fellowship to C.P.J.
LITERATURE CITED
- 1.Aboul-ela F, Huang W, Abd Elrahman M, Boyapati V, Li P. 2015. Linking aptamer-ligand binding and expression platform folding in riboswitches: prospects for mechanistic modeling and design. Wiley interdisciplinary reviews. RNA 6:631–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ames TD, Breaker RR. 2011. Bacterial aptamers that selectively bind glutamine. RNA biology 8:82–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ames TD, Rodionov DA, Weinberg Z, Breaker RR. 2010. A eubacterial riboswitch class that senses the coenzyme tetrahydrofolate. Chemistry & biology 17:681–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anton A, Grosse C, Reissmann J, Pribyl T, Nies DH. 1999. CzcD is a heavy metal ion transporter involved in regulation of heavy metal resistance in Ralstonia sp. strain CH34. Journal of bacteriology 181:6876–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Auslander S, Stucheli P, Rehm C, Auslander D, Hartig JS, Fussenegger M. 2014. A general design strategy for protein-responsive riboswitches in mammalian cells. Nature methods 11:1154–60 [DOI] [PubMed] [Google Scholar]
- 6.Baird NJ, Ferré-D’Amaré AR. 2010. Idiosyncratically tuned switching behavior of riboswitch aptamer domains revealed by comparative small-angle X-ray scattering analysis. Rna 16:598–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baird NJ, Inglese J, Ferré-D’Amaré AR. 2015. Rapid RNA-ligand interaction analysis through high-information content conformational and stability landscapes. Nature communications 6:8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baird NJ, Kulshina N, Ferré-D’Amaré AR. 2010. Riboswitch function: flipping the switch or tuning the dimmer? RNA biology 7:328–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker JL, Sudarsan N, Weinberg Z, Roth A, Stockbridge RB, Breaker RR. 2012. Widespread genetic switches and toxicity resistance proteins for fluoride. Science 335:233–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrick JE, Breaker RR. 2007. The distributions, mechanisms, and structures of metabolite-binding riboswitches. Genome biology 8:R239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrick JE, Corbino KA, Winkler WC, Nahvi A, Mandal M, et al. 2004. New RNA motifs suggest an expanded scope for riboswitches in bacterial genetic control. Proceedings of the National Academy of Sciences of the United States of America 101:6421–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batey RT. 2011. Recognition of S-adenosylmethionine by riboswitches. Wiley interdisciplinary reviews. RNA 2:299–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batey RT. 2012. Structure and mechanism of purine-binding riboswitches. Quarterly reviews of biophysics 45:345–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batey RT, Gilbert SD, Montange RK. 2004. Structure of a natural guanine-responsive riboswitch complexed with the metabolite hypoxanthine. Nature 432:411–5 [DOI] [PubMed] [Google Scholar]
- 15.Berens C, Suess B. 2015. Riboswitch engineering - making the all-important second and third steps. Current opinion in biotechnology 31:10–5 [DOI] [PubMed] [Google Scholar]
- 16.Bloomfield VA, Crothers DM, Tinoco I. 2000. Nucleic acids : structures, properties, and functions. Sausalito, Calif.: University Science Books; x, 794 p. pp. [Google Scholar]
- 17.Blount KF, Breaker RR. 2006. Riboswitches as antibacterial drug targets. Nature biotechnology 24:1558–64 [DOI] [PubMed] [Google Scholar]
- 18.Bochner BR, Ames BN. 1982. ZTP (5-amino 4-imidazole carboxamide riboside 5’-triphosphate): a proposed alarmone for 10-formyl-tetrahydrofolate deficiency. Cell 29:929–37 [DOI] [PubMed] [Google Scholar]
- 19.Breaker RR. 2006. Riboswitches and the RNA World In The RNA world : the nature of modern RNA suggests a prebiotic RNA world, ed. Gesteland RF, Cech T, Atkins JF:89–107. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press; Number of 89–107 pp. [Google Scholar]
- 20.Breaker RR. 2011. Prospects for riboswitch discovery and analysis. Molecular cell 43:867–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breaker RR. 2012. Riboswitches and the RNA world. Cold Spring Harbor perspectives in biology 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brenner MD, Scanlan MS, Nahas MK, Ha T, Silverman SK. 2010. Multivector fluorescence analysis of the xpt guanine riboswitch aptamer domain and the conformational role of guanine. Biochemistry 49:1596–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buck J, Furtig B, Noeske J, Wohnert J, Schwalbe H. 2007. Time-resolved NMR methods resolving ligand-induced RNA folding at atomic resolution. Proceedings of the National Academy of Sciences of the United States of America 104:15699–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butler EB, Xiong Y, Wang J, Strobel SA. 2011. Structural basis of cooperative ligand binding by the glycine riboswitch. Chemistry & biology 18:293–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caron MP, Bastet L, Lussier A, Simoneau-Roy M, Masse E, Lafontaine DA. 2012. Dual-acting riboswitch control of translation initiation and mRNA decay. Proceedings of the National Academy of Sciences of the United States of America 109:E3444–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen B, Zuo X, Wang YX, Dayie TK. 2012. Multiple conformations of SAM-II riboswitch detected with SAXS and NMR spectroscopy. Nucleic acids research 40:3117–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corbino KA, Barrick JE, Lim J, Welz R, Tucker BJ, et al. 2005. Evidence for a second class of S-adenosylmethionine riboswitches and other regulatory RNA motifs in alpha-proteobacteria. Genome biology 6:R70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cromie MJ, Shi Y, Latifi T, Groisman EA. 2006. An RNA sensor for intracellular Mg(2+). Cell 125:71–84 [DOI] [PubMed] [Google Scholar]
- 29.Dambach M, Sandoval M, Updegrove TB, Anantharaman V, Aravind L, et al. 2015. The ubiquitous yybP-ykoY riboswitch is a manganese-responsive regulatory element. Molecular cell 57:1099–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dann CE 3rd, Wakeman CA, Sieling CL, Baker SC, Irnov I, Winkler WC. 2007. Structure and mechanism of a metal-sensing regulatory RNA. Cell 130:878–92 [DOI] [PubMed] [Google Scholar]
- 31.Dar D, Shamir M, Mellin JR, Koutero M, Stern-Ginossar N, et al. 2016. Term-seq reveals abundant ribo-regulation of antibiotics resistance in bacteria. Science 352:aad9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.David S, Estramareix B. 1997. Sugars and nucleotides and the biosynthesis of thiamine. Advances in carbohydrate chemistry and biochemistry 52:267–309 [DOI] [PubMed] [Google Scholar]
- 33.DebRoy S, Gebbie M, Ramesh A, Goodson JR, Cruz MR, et al. 2014. Riboswitches. A riboswitch-containing sRNA controls gene expression by sequestration of a response regulator. Science 345:937–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deigan KE, Ferré-D’Amaré AR. 2011. Riboswitches: discovery of drugs that target bacterial gene-regulatory RNAs. Accounts of chemical research 44:1329–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desai SK, Gallivan JP. 2004. Genetic screens and selections for small molecules based on a synthetic riboswitch that activates protein translation. Journal of the American Chemical Society 126:13247–54 [DOI] [PubMed] [Google Scholar]
- 36.Dethoff EA, Chugh J, Mustoe AM, Al-Hashimi HM. 2012. Functional complexity and regulation through RNA dynamics. Nature 482:322–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dolgosheina EV, Jeng SC, Panchapakesan SS, Cojocaru R, Chen PS, et al. 2014. RNA mango aptamer-fluorophore: a bright, high-affinity complex for RNA labeling and tracking. ACS chemical biology 9:2412–20 [DOI] [PubMed] [Google Scholar]
- 38.Draper DE, Grilley D, Soto AM. 2005. Ions and RNA folding. Annual review of biophysics and biomolecular structure 34:221–43 [DOI] [PubMed] [Google Scholar]
- 39.Edwards AL, Batey RT. 2009. A structural basis for the recognition of 2’-deoxyguanosine by the purine riboswitch. Journal of molecular biology 385:938–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edwards AL, Reyes FE, Heroux A, Batey RT. 2010. Structural basis for recognition of S-adenosylhomocysteine by riboswitches. Rna 16:2144–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edwards TE, Ferré-D’Amaré AR. 2006. Crystal structures of the thi-box riboswitch bound to thiamine pyrophosphate analogs reveal adaptive RNA-small molecule recognition. Structure 14:1459–68 [DOI] [PubMed] [Google Scholar]
- 42.Ellington AD, Szostak JW. 1990. In vitro selection of RNA molecules that bind specific ligands. Nature 346:818–22 [DOI] [PubMed] [Google Scholar]
- 43.Epshtein V, Mironov AS, Nudler E. 2003. The riboswitch-mediated control of sulfur metabolism in bacteria. Proceedings of the National Academy of Sciences of the United States of America 100:5052–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Espah Borujeni A, Mishler DM, Wang J, Huso W, Salis HM. 2016. Automated physics-based design of synthetic riboswitches from diverse RNA aptamers. Nucleic acids research 44:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferré-D’Amaré AR, Scott WG. 2010. Small self-cleaving ribozymes. Cold Spring Harbor perspectives in biology 2:a003574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fiegland LR, Garst AD, Batey RT, Nesbitt DJ. 2012. Single-molecule studies of the lysine riboswitch reveal effector-dependent conformational dynamics of the aptamer domain. Biochemistry 51:9223–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Furtig B, Nozinovic S, Reining A, Schwalbe H. 2015. Multiple conformational states of riboswitches fine-tune gene regulation. Current opinion in structural biology 30:112–24 [DOI] [PubMed] [Google Scholar]
- 48.Furukawa K, Ramesh A, Zhou Z, Weinberg Z, Vallery T, et al. 2015. Bacterial riboswitches cooperatively bind Ni(2+) or Co(2+) ions and control expression of heavy metal transporters. Molecular cell 57:1088–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao A, Serganov A. 2014. Structural insights into recognition of c-di-AMP by the ydaO riboswitch. Nature chemical biology 10:787–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garst AD, Heroux A, Rambo RP, Batey RT. 2008. Crystal structure of the lysine riboswitch regulatory mRNA element. The Journal of biological chemistry 283:22347–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gelfand MS, Mironov AA, Jomantas J, Kozlov YI, Perumov DA. 1999. A conserved RNA structure element involved in the regulation of bacterial riboflavin synthesis genes. Trends in genetics : TIG 15:439–42 [DOI] [PubMed] [Google Scholar]
- 52.Gilbert SD, Rambo RP, Van Tyne D, Batey RT. 2008. Structure of the SAM-II riboswitch bound to S-adenosylmethionine. Nature structural & molecular biology 15:177–82 [DOI] [PubMed] [Google Scholar]
- 53.Gilbert W. 1986. The RNA world. Nature 319:618 [Google Scholar]
- 54.Golding I, Paulsson J, Zawilski SM, Cox EC. 2005. Real-time kinetics of gene activity in individual bacteria. Cell 123:1025–36 [DOI] [PubMed] [Google Scholar]
- 55.Gollnick P, Babitzke P, Antson A, Yanofsky C. 2005. Complexity in regulation of tryptophan biosynthesis in Bacillus subtilis. Annual review of genetics 39:47–68 [DOI] [PubMed] [Google Scholar]
- 56.Grigg JC, Ke A. 2013. Sequence, structure, and stacking: specifics of tRNA anchoring to the T box riboswitch. RNA biology 10:1761–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grundy FJ, Henkin TM. 1993. tRNA as a positive regulator of transcription antitermination in B. subtilis. Cell 74:475–82 [DOI] [PubMed] [Google Scholar]
- 58.Grundy FJ, Henkin TM. 1998. The S box regulon: a new global transcription termination control system for methionine and cysteine biosynthesis genes in gram-positive bacteria. Molecular microbiology 30:737–49 [DOI] [PubMed] [Google Scholar]
- 59.Guerrier-Takada C, Gardiner K, Marsh T, Pace N, Altman S. 1983. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell 35:849–57 [DOI] [PubMed] [Google Scholar]
- 60.Haller A, Altman RB, Souliere MF, Blanchard SC, Micura R. 2013. Folding and ligand recognition of the TPP riboswitch aptamer at single-molecule resolution. Proceedings of the National Academy of Sciences of the United States of America 110:4188–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haller A, Rieder U, Aigner M, Blanchard SC, Micura R. 2011. Conformational capture of the SAM-II riboswitch. Nature chemical biology 7:393–400 [DOI] [PubMed] [Google Scholar]
- 62.Haller A, Souliere MF, Micura R. 2011. The dynamic nature of RNA as key to understanding riboswitch mechanisms. Accounts of chemical research 44:1339–48 [DOI] [PubMed] [Google Scholar]
- 63.Henkin TM. 2014. The T box riboswitch: A novel regulatory RNA that utilizes tRNA as its ligand. Biochimica et biophysica acta 1839:959–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Howe JA, Wang H, Fischmann TO, Balibar CJ, Xiao L, et al. 2015. Selective small-molecule inhibition of an RNA structural element. Nature 526:672–7 [DOI] [PubMed] [Google Scholar]
- 65.Howe JA, Xiao L, Fischmann TO, Wang H, Tang H, et al. 2016. Atomic Resolution Mechanistic Studies of Ribocil: A Highly Selective Unnatural Ligand Mimic of the E. coli FMN Riboswitch. RNA biology:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hsu HT, Lin YH, Chang KY. 2014. Synergetic regulation of translational reading-frame switch by ligand-responsive RNAs in mammalian cells. Nucleic acids research 42:14070–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang L, Ishibe-Murakami S, Patel DJ, Serganov A. 2011. Long-range pseudoknot interactions dictate the regulatory response in the tetrahydrofolate riboswitch. Proceedings of the National Academy of Sciences of the United States of America 108:14801–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang L, Serganov A, Patel DJ. 2010. Structural insights into ligand recognition by a sensing domain of the cooperative glycine riboswitch. Molecular cell 40:774–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ibba M, Francklyn C, Cusack S. 2005. The aminoacyl-tRNA synthetases. Georgetown, Tex., U.S.A.: Landes Bioscience; : Eurekah.com. 420 p. pp. [Google Scholar]
- 70.Ivankov DN, Garbuzynskiy SO, Alm E, Plaxco KW, Baker D, Finkelstein AV. 2003. Contact order revisited: influence of protein size on the folding rate. Protein science : a publication of the Protein Society 12:2057–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jenison RD, Gill SC, Pardi A, Polisky B. 1994. High-resolution molecular discrimination by RNA. Science 263:1425–9 [DOI] [PubMed] [Google Scholar]
- 72.Jenkins JL, Krucinska J, McCarty RM, Bandarian V, Wedekind JE. 2011. Comparison of a preQ1 riboswitch aptamer in metabolite-bound and free states with implications for gene regulation. The Journal of biological chemistry 286:24626–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jimenez RM, Polanco JA, Luptak A. 2015. Chemistry and Biology of Self-Cleaving Ribozymes. Trends in biochemical sciences 40:648–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnson JE Jr., Reyes FE, Polaski JT, Batey RT. 2012. B12 cofactors directly stabilize an mRNA regulatory switch. Nature 492:133–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jones CP, Ferré-D’Amaré AR. 2014. Crystal structure of a c-di-AMP riboswitch reveals an internally pseudo-dimeric RNA. The EMBO journal [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jones CP, Ferré-D’Amaré AR. 2015. Recognition of the bacterial alarmone ZMP through long-distance association of two RNA subdomains. Nature structural & molecular biology 22:679–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jones CP, Ferré-D’Amaré AR. 2015. RNA quaternary structure and global symmetry. Trends in biochemical sciences 40:211–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kang M, Eichhorn CD, Feigon J. 2014. Structural determinants for ligand capture by a class II preQ1 riboswitch. Proceedings of the National Academy of Sciences of the United States of America 111:E663–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kang M, Peterson R, Feigon J. 2009. Structural Insights into riboswitch control of the biosynthesis of queuosine, a modified nucleotide found in the anticodon of tRNA. Molecular cell 33:784–90 [DOI] [PubMed] [Google Scholar]
- 80.Kang M, Peterson R, Feigon J. 2010. Structural Insights into riboswitch control of the biosynthesis of queuosine, a modified nucleotide found in the anticodon of tRNA. Molecular cell 39:653–5 [DOI] [PubMed] [Google Scholar]
- 81.Kellenberger CA, Hallberg ZF, Hammond MC. 2015. Live Cell Imaging Using Riboswitch-Spinach tRNA Fusions as Metabolite-Sensing Fluorescent Biosensors. Methods in molecular biology 1316:87–103 [DOI] [PubMed] [Google Scholar]
- 82.Kellenberger CA, Hammond MC. 2015. In vitro analysis of riboswitch-Spinach aptamer fusions as metabolite-sensing fluorescent biosensors. Methods in enzymology 550:147–72 [DOI] [PubMed] [Google Scholar]
- 83.Kellenberger CA, Wilson SC, Sales-Lee J, Hammond MC. 2013. RNA-based fluorescent biosensors for live cell imaging of second messengers cyclic di-GMP and cyclic AMP-GMP. Journal of the American Chemical Society 135:4906–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ketterer S, Gladis L, Kozica A, Meier M. 2016. Engineering and characterization of fluorogenic glycine riboswitches. Nucleic acids research 44:5983–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ketzer P, Haas SF, Engelhardt S, Hartig JS, Nettelbeck DM. 2012. Synthetic riboswitches for external regulation of genes transferred by replication-deficient and oncolytic adenoviruses. Nucleic acids research 40:e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ketzer P, Kaufmann JK, Engelhardt S, Bossow S, von Kalle C, et al. 2014. Artificial riboswitches for gene expression and replication control of DNA and RNA viruses. Proceedings of the National Academy of Sciences of the United States of America 111:E554–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kil YV, Mironov VN, Gorishin I, Kreneva RA, Perumov DA. 1992. Riboflavin operon of Bacillus subtilis: unusual symmetric arrangement of the regulatory region. Molecular & general genetics : MGG 233:483–6 [DOI] [PubMed] [Google Scholar]
- 88.Kim JN, Breaker RR. 2008. Purine sensing by riboswitches. Biology of the cell / under the auspices of the European Cell Biology Organization 100:1–11 [DOI] [PubMed] [Google Scholar]
- 89.Kim JN, Roth A, Breaker RR. 2007. Guanine riboswitch variants from Mesoplasma florum selectively recognize 2’-deoxyguanosine. Proceedings of the National Academy of Sciences of the United States of America 104:16092–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim PB, Nelson JW, Breaker RR. 2015. An ancient riboswitch class in bacteria regulates purine biosynthesis and one-carbon metabolism. Molecular cell 57:317–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Klein DJ, Been MD, Ferré-D’Amaré AR. 2007. Essential role of an active-site guanine in glmS ribozyme catalysis. Journal of the American Chemical Society 129:14858–9 [DOI] [PubMed] [Google Scholar]
- 92.Klein DJ, Edwards TE, Ferré-D’Amaré AR. 2009. Cocrystal structure of a class I preQ1 riboswitch reveals a pseudoknot recognizing an essential hypermodified nucleobase. Nature structural & molecular biology 16:343–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Klein DJ, Ferré-D’Amaré AR. 2006. Structural basis of glmS ribozyme activation by glucosamine-6-phosphate. Science 313:1752–6 [DOI] [PubMed] [Google Scholar]
- 94.Kruger K, Grabowski PJ, Zaug AJ, Sands J, Gottschling DE, Cech TR. 1982. Self-splicing RNA: autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell 31:147–57 [DOI] [PubMed] [Google Scholar]
- 95.Kubodera T, Watanabe M, Yoshiuchi K, Yamashita N, Nishimura A, et al. 2003. Thiamine-regulated gene expression of Aspergillus oryzae thiA requires splicing of the intron containing a riboswitch-like domain in the 5’-UTR. FEBS letters 555:516–20 [DOI] [PubMed] [Google Scholar]
- 96.Kulshina N, Baird NJ, Ferré-D’Amaré AR. 2009. Recognition of the bacterial second messenger cyclic diguanylate by its cognate riboswitch. Nature structural & molecular biology 16:1212–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kulshina N, Edwards TE, Ferré-D’Amaré AR. 2010. Thermodynamic analysis of ligand binding and ligand binding-induced tertiary structure formation by the thiamine pyrophosphate riboswitch. Rna 16:186–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kumar PK, Kumarevel T, Mizuno H. 2006. Structural basis of HutP-mediated transcription anti-termination. Current opinion in structural biology 16:18–26 [DOI] [PubMed] [Google Scholar]
- 99.Lee ER, Baker JL, Weinberg Z, Sudarsan N, Breaker RR. 2010. An allosteric self-splicing ribozyme triggered by a bacterial second messenger. Science 329:845–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee ER, Blount KF, Breaker RR. 2009. Roseoflavin is a natural antibacterial compound that binds to FMN riboswitches and regulates gene expression. RNA biology 6:187–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li S, Breaker RR. 2013. Eukaryotic TPP riboswitch regulation of alternative splicing involving long-distance base pairing. Nucleic acids research 41:3022–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liberman JA, Salim M, Krucinska J, Wedekind JE. 2013. Structure of a class II preQ1 riboswitch reveals ligand recognition by a new fold. Nature chemical biology 9:353–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liberman JA, Suddala KC, Aytenfisu A, Chan D, Belashov IA, et al. 2015. Structural analysis of a class III preQ1 riboswitch reveals an aptamer distant from a ribosome-binding site regulated by fast dynamics. Proceedings of the National Academy of Sciences of the United States of America 112:E3485–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liberman JA, Wedekind JE. 2012. Riboswitch structure in the ligand-free state. Wiley interdisciplinary reviews. RNA 3:369–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lin YH, Chang KY. 2016. Rational design of a synthetic mammalian riboswitch as a ligand-responsive −1 ribosomal frame-shifting stimulator. Nucleic acids research [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lipfert J, Das R, Chu VB, Kudaravalli M, Boyd N, et al. 2007. Structural transitions and thermodynamics of a glycine-dependent riboswitch from Vibrio cholerae. Journal of molecular biology 365:1393–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lipfert J, Doniach S, Das R, Herschlag D. 2014. Understanding nucleic acid-ion interactions. Annual review of biochemistry 83:813–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lipfert J, Herschlag D, Doniach S. 2009. Riboswitch conformations revealed by small-angle X-ray scattering. Methods in molecular biology 540:141–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Litke JL, You M, Jaffrey SR. 2016. Developing Fluorogenic Riboswitches for Imaging Metabolite Concentration Dynamics in Bacterial Cells. Methods in enzymology 572:315–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Loh E, Dussurget O, Gripenland J, Vaitkevicius K, Tiensuu T, et al. 2009. A trans-acting riboswitch controls expression of the virulence regulator PrfA in Listeria monocytogenes. Cell 139:770–9 [DOI] [PubMed] [Google Scholar]
- 111.Lorsch JR, Szostak JW. 1994. In vitro selection of RNA aptamers specific for cyanocobalamin. Biochemistry 33:973–82 [DOI] [PubMed] [Google Scholar]
- 112.Lu C, Smith AM, Fuchs RT, Ding F, Rajashankar K, et al. 2008. Crystal structures of the SAM-III/S(MK) riboswitch reveal the SAM-dependent translation inhibition mechanism. Nature structural & molecular biology 15:1076–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lundrigan MD, Koster W, Kadner RJ. 1991. Transcribed sequences of the Escherichia coli btuB gene control its expression and regulation by vitamin B12. Proceedings of the National Academy of Sciences of the United States of America 88:1479–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lunse CE, Schuller A, Mayer G. 2014. The promise of riboswitches as potential antibacterial drug targets. International journal of medical microbiology : IJMM 304:79–92 [DOI] [PubMed] [Google Scholar]
- 115.Mandal M, Lee M, Barrick JE, Weinberg Z, Emilsson GM, et al. 2004. A glycine-dependent riboswitch that uses cooperative binding to control gene expression. Science 306:275–9 [DOI] [PubMed] [Google Scholar]
- 116.McCown PJ, Liang JJ, Weinberg Z, Breaker RR. 2014. Structural, functional, and taxonomic diversity of three preQ1 riboswitch classes. Chemistry & biology 21:880–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Meehan RE, Torgerson CD, Gaffney BL, Jones RA, Strobel SA. 2016. Nuclease-Resistant c-di-AMP Derivatives That Differentially Recognize RNA and Protein Receptors. Biochemistry 55:837–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mellin JR, Koutero M, Dar D, Nahori MA, Sorek R, Cossart P. 2014. Riboswitches. Sequestration of a two-component response regulator by a riboswitch-regulated noncoding RNA. Science 345:940–3 [DOI] [PubMed] [Google Scholar]
- 119.Meyer MM, Roth A, Chervin SM, Garcia GA, Breaker RR. 2008. Confirmation of a second natural preQ1 aptamer class in Streptococcaceae bacteria. Rna 14:685–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miranda-Rios J, Navarro M, Soberon M. 2001. A conserved RNA structure (thi box) is involved in regulation of thiamin biosynthetic gene expression in bacteria. Proceedings of the National Academy of Sciences of the United States of America 98:9736–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mironov AS, Gusarov I, Rafikov R, Lopez LE, Shatalin K, et al. 2002. Sensing small molecules by nascent RNA: a mechanism to control transcription in bacteria. Cell 111:747–56 [DOI] [PubMed] [Google Scholar]
- 122.Montange RK, Batey RT. 2006. Structure of the S-adenosylmethionine riboswitch regulatory mRNA element. Nature 441:1172–5 [DOI] [PubMed] [Google Scholar]
- 123.Montange RK, Batey RT. 2008. Riboswitches: emerging themes in RNA structure and function. Annual review of biophysics 37:117–33 [DOI] [PubMed] [Google Scholar]
- 124.Montange RK, Mondragon E, van Tyne D, Garst AD, Ceres P, Batey RT. 2010. Discrimination between closely related cellular metabolites by the SAM-I riboswitch. Journal of molecular biology 396:761–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nakayama S, Luo Y, Zhou J, Dayie TK, Sintim HO. 2012. Nanomolar fluorescent detection of c-di-GMP using a modular aptamer strategy. Chemical communications 48:9059–61 [DOI] [PubMed] [Google Scholar]
- 126.Nou X, Kadner RJ. 1998. Coupled changes in translation and transcription during cobalamin-dependent regulation of btuB expression in Escherichia coli. Journal of bacteriology 180:6719–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nou X, Kadner RJ. 2000. Adenosylcobalamin inhibits ribosome binding to btuB RNA. Proceedings of the National Academy of Sciences of the United States of America 97:7190–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nozinovic S, Reining A, Kim YB, Noeske J, Schlepckow K, et al. 2014. The importance of helix P1 stability for structural pre-organization and ligand binding affinity of the adenine riboswitch aptamer domain. RNA biology 11:655–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ottink OM, Rampersad SM, Tessari M, Zaman GJ, Heus HA, Wijmenga SS. 2007. Ligand-induced folding of the guanine-sensing riboswitch is controlled by a combined predetermined induced fit mechanism. Rna 13:2202–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Paige JS, Wu KY, Jaffrey SR. 2011. RNA mimics of green fluorescent protein. Science 333:642–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Peracchi A, Beigelman L, Usman N, Herschlag D. 1996. Rescue of abasic hammerhead ribozymes by exogenous addition of specific bases. Proceedings of the National Academy of Sciences of the United States of America 93:11522–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Perkins TT, Kingsley RA, Fookes MC, Gardner PP, James KD, et al. 2009. A strand-specific RNA-Seq analysis of the transcriptome of the typhoid bacillus Salmonella typhi. PLoS genetics 5:e1000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Peselis A, Serganov A. 2014. Themes and variations in riboswitch structure and function. Biochimica et biophysica acta [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pitt JN, Ferré-D’Amaré AR. 2010. Rapid construction of empirical RNA fitness landscapes. Science 330:376–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Plaxco KW, Simons KT, Baker D. 1998. Contact order, transition state placement and the refolding rates of single domain proteins. Journal of molecular biology 277:985–94 [DOI] [PubMed] [Google Scholar]
- 136.Pleij CW, Rietveld K, Bosch L. 1985. A new principle of RNA folding based on pseudoknotting. Nucleic acids research 13:1717–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Poiata E, Meyer MM, Ames TD, Breaker RR. 2009. A variant riboswitch aptamer class for S-adenosylmethionine common in marine bacteria. Rna 15:2046–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Price IR, Gaballa A, Ding F, Helmann JD, Ke A. 2015. Mn(2+)-sensing mechanisms of yybP-ykoY orphan riboswitches. Molecular cell 57:1110–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Price IR, Grigg JC, Ke A. 2014. Common themes and differences in SAM recognition among SAM riboswitches. Biochimica et biophysica acta 1839:931–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ramesh A, Wakeman CA, Winkler WC. 2011. Insights into metalloregulation by M-box riboswitch RNAs via structural analysis of manganese-bound complexes. Journal of molecular biology 407:556–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ray-Soni A, Bellecourt MJ, Landick R. 2016. Mechanisms of Bacterial Transcription Termination: All Good Things Must End. Annual review of biochemistry 85:319–47 [DOI] [PubMed] [Google Scholar]
- 142.Regulski EE, Breaker RR. 2008. In-line probing analysis of riboswitches. Methods in molecular biology 419:53–67 [DOI] [PubMed] [Google Scholar]
- 143.Regulski EE, Moy RH, Weinberg Z, Barrick JE, Yao Z, et al. 2008. A widespread riboswitch candidate that controls bacterial genes involved in molybdenum cofactor and tungsten cofactor metabolism. Molecular microbiology 68:918–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Reining A, Nozinovic S, Schlepckow K, Buhr F, Furtig B, Schwalbe H. 2013. Three-state mechanism couples ligand and temperature sensing in riboswitches. Nature 499:355–9 [DOI] [PubMed] [Google Scholar]
- 145.Ren A, Patel DJ. 2014. c-di-AMP binds the ydaO riboswitch in two pseudo-symmetry-related pockets. Nature chemical biology 10:780–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ren A, Rajashankar KR, Patel DJ. 2012. Fluoride ion encapsulation by Mg2+ ions and phosphates in a fluoride riboswitch. Nature 486:85–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ren A, Rajashankar KR, Patel DJ. 2015. Global RNA Fold and Molecular Recognition for a pfl Riboswitch Bound to ZMP, a Master Regulator of One-Carbon Metabolism. Structure [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ren A, Wang XC, Kellenberger CA, Rajashankar KR, Jones RA, et al. 2015. Structural Basis for Molecular Discrimination by a 3’,3’-cGAMP Sensing Riboswitch. Cell reports 11:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ren A, Xue Y, Peselis A, Serganov A, Al-Hashimi HM, Patel DJ. 2015. Structural and Dynamic Basis for Low-Affinity, High-Selectivity Binding of L-Glutamine by the Glutamine Riboswitch. Cell reports 13:1800–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rinaldi AJ, Lund PE, Blanco MR, Walter NG. 2016. The Shine-Dalgarno sequence of riboswitch-regulated single mRNAs shows ligand-dependent accessibility bursts. Nature communications 7:8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Robertson DL, Joyce GF. 1990. Selection in vitro of an RNA enzyme that specifically cleaves single-stranded DNA. Nature 344:467–8 [DOI] [PubMed] [Google Scholar]
- 152.Rodionov DA, Dubchak I, Arkin A, Alm E, Gelfand MS. 2004. Reconstruction of regulatory and metabolic pathways in metal-reducing delta-proteobacteria. Genome biology 5:R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Roth A, Winkler WC, Regulski EE, Lee BW, Lim J, et al. 2007. A riboswitch selective for the queuosine precursor preQ1 contains an unusually small aptamer domain. Nature structural & molecular biology 14:308–17 [DOI] [PubMed] [Google Scholar]
- 154.Ruff KM, Muhammad A, McCown PJ, Breaker RR, Strobel SA. 2016. Singlet glycine riboswitches bind ligand as well as tandem riboswitches. Rna [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ruff KM, Strobel SA. 2014. Ligand binding by the tandem glycine riboswitch depends on aptamer dimerization but not double ligand occupancy. Rna 20:1775–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sassanfar M, Szostak JW. 1993. An RNA motif that binds ATP. Nature 364:550–3 [DOI] [PubMed] [Google Scholar]
- 157.Serganov A. 2009. The long and the short of riboswitches. Current opinion in structural biology 19:251–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Serganov A, Huang L, Patel DJ. 2008. Structural insights into amino acid binding and gene control by a lysine riboswitch. Nature 455:1263–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Serganov A, Huang L, Patel DJ. 2009. Coenzyme recognition and gene regulation by a flavin mononucleotide riboswitch. Nature 458:233–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Serganov A, Nudler E. 2013. A decade of riboswitches. Cell 152:17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Serganov A, Patel DJ. 2009. Amino acid recognition and gene regulation by riboswitches. Biochimica et biophysica acta 1789:592–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Serganov A, Polonskaia A, Phan AT, Breaker RR, Patel DJ. 2006. Structural basis for gene regulation by a thiamine pyrophosphate-sensing riboswitch. Nature 441:1167–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Serganov A, Yuan YR, Pikovskaya O, Polonskaia A, Malinina L, et al. 2004. Structural basis for discriminative regulation of gene expression by adenine- and guanine-sensing mRNAs. Chemistry & biology 11:1729–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sharma CM, Hoffmann S, Darfeuille F, Reignier J, Findeiss S, et al. 2010. The primary transcriptome of the major human pathogen Helicobacter pylori. Nature 464:250–5 [DOI] [PubMed] [Google Scholar]
- 165.Smith KD, Lipchock SV, Ames TD, Wang J, Breaker RR, Strobel SA. 2009. Structural basis of ligand binding by a c-di-GMP riboswitch. Nature structural & molecular biology 16:1218–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Smith KD, Shanahan CA, Moore EL, Simon AC, Strobel SA. 2011. Structural basis of differential ligand recognition by two classes of bis-(3’−5’)-cyclic dimeric guanosine monophosphate-binding riboswitches. Proceedings of the National Academy of Sciences of the United States of America 108:7757–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Smith KD, Strobel SA. 2011. Interactions of the c-di-GMP riboswitch with its second messenger ligand. Biochemical Society transactions 39:647–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sosnick TR, Pan T. 2004. Reduced contact order and RNA folding rates. Journal of molecular biology 342:1359–65 [DOI] [PubMed] [Google Scholar]
- 169.Souliere MF, Altman RB, Schwarz V, Haller A, Blanchard SC, Micura R. 2013. Tuning a riboswitch response through structural extension of a pseudoknot. Proceedings of the National Academy of Sciences of the United States of America 110:E3256–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Steen KA, Malhotra A, Weeks KM. 2010. Selective 2’-hydroxyl acylation analyzed by protection from exoribonuclease. Journal of the American Chemical Society 132:9940–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Stoddard CD, Montange RK, Hennelly SP, Rambo RP, Sanbonmatsu KY, Batey RT. 2010. Free state conformational sampling of the SAM-I riboswitch aptamer domain. Structure 18:787–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Stormo GD, Ji Y. 2001. Do mRNAs act as direct sensors of small molecules to control their expression? Proceedings of the National Academy of Sciences of the United States of America 98:9465–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sudarsan N, Barrick JE, Breaker RR. 2003. Metabolite-binding RNA domains are present in the genes of eukaryotes. Rna 9:644–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sudarsan N, Hammond MC, Block KF, Welz R, Barrick JE, et al. 2006. Tandem riboswitch architectures exhibit complex gene control functions. Science 314:300–4 [DOI] [PubMed] [Google Scholar]
- 175.Tang J, Breaker RR. 1997. Rational design of allosteric ribozymes. Chemistry & biology 4:453–9 [DOI] [PubMed] [Google Scholar]
- 176.Thore S, Leibundgut M, Ban N. 2006. Structure of the eukaryotic thiamine pyrophosphate riboswitch with its regulatory ligand. Science 312:1208–11 [DOI] [PubMed] [Google Scholar]
- 177.Toledo-Arana A, Dussurget O, Nikitas G, Sesto N, Guet-Revillet H, et al. 2009. The Listeria transcriptional landscape from saprophytism to virulence. Nature 459:950–6 [DOI] [PubMed] [Google Scholar]
- 178.Tomsic J, McDaniel BA, Grundy FJ, Henkin TM. 2008. Natural variability in S-adenosylmethionine (SAM)-dependent riboswitches: S-box elements in bacillus subtilis exhibit differential sensitivity to SAM In vivo and in vitro. Journal of bacteriology 190:823–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Topp S, Reynoso CM, Seeliger JC, Goldlust IS, Desai SK, et al. 2010. Synthetic riboswitches that induce gene expression in diverse bacterial species. Applied and environmental microbiology 76:7881–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Trausch JJ, Batey RT. 2014. A disconnect between high-affinity binding and efficient regulation by antifolates and purines in the tetrahydrofolate riboswitch. Chemistry & biology 21:205–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Trausch JJ, Ceres P, Reyes FE, Batey RT. 2011. The structure of a tetrahydrofolate-sensing riboswitch reveals two ligand binding sites in a single aptamer. Structure 19:1413–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Trausch JJ, Marcano-Velazquez JG, Matyjasik MM, Batey RT. 2015. Metal Ion-Mediated Nucleobase Recognition by the ZTP Riboswitch. Chemistry & biology [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Trausch JJ, Xu Z, Edwards AL, Reyes FE, Ross PE, et al. 2014. Structural basis for diversity in the SAM clan of riboswitches. Proceedings of the National Academy of Sciences of the United States of America 111:6624–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tuerk C, Gold L. 1990. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249:505–10 [DOI] [PubMed] [Google Scholar]
- 185.Tyrrell J, McGinnis JL, Weeks KM, Pielak GJ. 2013. The cellular environment stabilizes adenine riboswitch RNA structure. Biochemistry 52:8777–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Vicens Q, Mondragon E, Batey RT. 2011. Molecular sensing by the aptamer domain of the FMN riboswitch: a general model for ligand binding by conformational selection. Nucleic acids research 39:8586–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wakeman CA, Ramesh A, Winkler WC. 2009. Multiple metal-binding cores are required for metalloregulation by M-box riboswitch RNAs. Journal of molecular biology 392:723–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang JX, Breaker RR. 2008. Riboswitches that sense S-adenosylmethionine and S-adenosylhomocysteine. Biochemistry and cell biology = Biochimie et biologie cellulaire 86:157–68 [DOI] [PubMed] [Google Scholar]
- 189.Wang JX, Lee ER, Morales DR, Lim J, Breaker RR. 2008. Riboswitches that sense S-adenosylhomocysteine and activate genes involved in coenzyme recycling. Molecular cell 29:691–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Waters LS, Sandoval M, Storz G. 2011. The Escherichia coli MntR miniregulon includes genes encoding a small protein and an efflux pump required for manganese homeostasis. Journal of bacteriology 193:5887–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Weinberg Z, Wang JX, Bogue J, Yang J, Corbino K, et al. 2010. Comparative genomics reveals 104 candidate structured RNAs from bacteria, archaea, and their metagenomes. Genome biology 11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Weiss MC, Sousa FL, Mrnjavac N, Neukirchen S, Roettger M, et al. 2016. The physiology and habitat of the last universal common ancestor. Nature microbiology 1 [DOI] [PubMed] [Google Scholar]
- 193.Welz R, Breaker RR. 2007. Ligand binding and gene control characteristics of tandem riboswitches in Bacillus anthracis. Rna 13:573–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Werstuck G, Green MR. 1998. Controlling gene expression in living cells through small molecule-RNA interactions. Science 282:296–8 [DOI] [PubMed] [Google Scholar]
- 195.White HB 3rd. 1976. Coenzymes as fossils of an earlier metabolic state. Journal of molecular evolution 7:101–4 [DOI] [PubMed] [Google Scholar]
- 196.Wickiser JK, Cheah MT, Breaker RR, Crothers DM. 2005. The kinetics of ligand binding by an adenine-sensing riboswitch. Biochemistry 44:13404–14 [DOI] [PubMed] [Google Scholar]
- 197.Wickiser JK, Winkler WC, Breaker RR, Crothers DM. 2005. The speed of RNA transcription and metabolite binding kinetics operate an FMN riboswitch. Molecular cell 18:49–60 [DOI] [PubMed] [Google Scholar]
- 198.Wilkinson SR, Been MD. 2005. A pseudoknot in the 3’ non-core region of the glmS ribozyme enhances self-cleavage activity. Rna 11:1788–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Win MN, Smolke CD. 2008. Higher-order cellular information processing with synthetic RNA devices. Science 322:456–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Winkler W, Nahvi A, Breaker RR. 2002. Thiamine derivatives bind messenger RNAs directly to regulate bacterial gene expression. Nature 419:952–6 [DOI] [PubMed] [Google Scholar]
- 201.Winkler WC, Cohen-Chalamish S, Breaker RR. 2002. An mRNA structure that controls gene expression by binding FMN. Proceedings of the National Academy of Sciences of the United States of America 99:15908–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Winkler WC, Nahvi A, Roth A, Collins JA, Breaker RR. 2004. Control of gene expression by a natural metabolite-responsive ribozyme. Nature 428:281–6 [DOI] [PubMed] [Google Scholar]
- 203.Winkler WC, Nahvi A, Sudarsan N, Barrick JE, Breaker RR. 2003. An mRNA structure that controls gene expression by binding S-adenosylmethionine. Nature structural biology 10:701–7 [DOI] [PubMed] [Google Scholar]
- 204.Woodson SA. 2002. Folding mechanisms of group I ribozymes: role of stability and contact order. Biochemical Society transactions 30:1166–9 [DOI] [PubMed] [Google Scholar]
- 205.Woodson SA. 2010. Compact intermediates in RNA folding. Annual review of biophysics 39:61–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yanofsky C. 2000. Transcription attenuation: once viewed as a novel regulatory strategy. Journal of bacteriology 182:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.You M, Jaffrey SR. 2015. Structure and Mechanism of RNA Mimics of Green Fluorescent Protein. Annual review of biophysics 44:187–206 [DOI] [PubMed] [Google Scholar]
- 208.Yu CH, Luo J, Iwata-Reuyl D, Olsthoorn RC. 2013. Exploiting preQ(1) riboswitches to regulate ribosomal frameshifting. ACS chemical biology 8:733–40 [DOI] [PubMed] [Google Scholar]
- 209.Zhang J, Ferré-D’Amaré AR. 2015. Structure and mechanism of the T-box riboswitches. Wiley interdisciplinary reviews. RNA 6:419–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhang J, Jones CP, Ferré-D’Amaré AR. 2014. Global analysis of riboswitches by small-angle X-ray scattering and calorimetry. Biochimica et biophysica acta [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhang J, Lau MW, Ferré-D’Amaré AR. 2010. Ribozymes and riboswitches: modulation of RNA function by small molecules. Biochemistry 49:9123–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zheng H, Shabalin IG, Handing KB, Bujnicki JM, Minor W. 2015. Magnesium-binding architectures in RNA crystal structures: validation, binding preferences, classification and motif detection. Nucleic acids research 43:3789–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhou H, Zheng C, Su J, Chen B, Fu Y, et al. 2016. Characterization of a natural triple-tandem c-di-GMP riboswitch and application of the riboswitch-based dual-fluorescence reporter. Scientific reports 6:20871. [DOI] [PMC free article] [PubMed] [Google Scholar]


