Abstract
Introduction
Gastric cancer is the fourth most common cancer in the world. By the time the patients are diagnosed with stage IV gastric cancer, many patients already have distant metastases. There is no unified systemic treatment plan in existence. The use of gastrectomy is ambiguous in patients with stage IV gastric cancer. The objective of this study was to evaluate the beneficial outcome of gastrectomy in patients with stage IV gastric cancer.
Methods
Clinical information of patients with gastric cancer from 2000 to 2010 in the Surveillance, Epidemiology, and End Results database were extracted and analysed. The risk factors for stage IV gastric cancer were also analysed.
Results
We observed that the median survival time for patients after surgery was greater than that for patients not treated surgically. The five-year survival rate for chemotherapy patients was higher than that of non-chemotherapeutic patients. Patients who receive both chemotherapy and surgery could achieve a more significant survival benefit. The risks following gastrectomy (partial, subtotal, hemi-) were lower than those of other surgical procedures, which provided guidance on the choice of surgical method. The numbers of regional lymph node metastasis were found to be related to prognosis.
Conclusions
In patients with stage IV gastric cancer, gastrectomy (partial, subtotal or hemi) should be selected when surgery is necessary. The number of regional lymph node metastasis could be considered as a prognostic factor for patients with stage IV gastric cancer and lymph node dissection could reduce the risk of patients undergoing surgery.
Keywords: Gastric cancer, Stage IV, Surgery, Chemotherapy, Lymph node, Prognosis
Introduction
Gastric cancer is the fourth most common cause of cancer-related deaths worldwide.1 According to the statistical report released by the National Central Cancer Registry of China, the incidence of gastric cancer in China is 30/100,000, ranking second in all types of tumours.2 Electronic gastroscopes can detect gastric cancer early, and can greatly improve the survival rate for these patients. However, in some developing countries and underdeveloped regions, many patients do not have regular electronic gastroscopy for economic reasons. Patients are diagnosed after complications such as bleeding, obstruction and perforation. These patients already have distant metastases when they are diagnosed with gastric cancer. According to the Union for International Cancer Control/American Joint Committee on Cancer (UICC/AJCC) staging system, tumours with distant metastasis (M1) are classified as a stage IV. The treatment options for stage IV gastric cancer are still not well determined. The current treatment options include palliative gastrectomy, chemotherapy, targeted therapy, radiotherapy, gastric stent or bypass surgery. Currently, chemotherapy is the main treatment option, but five-year overall survival rate rarely exceeds 5% of treated patients. The median survival time is about 13–16 months, which is also not as expected.3–6
At present, there is no unified systemic treatment plan for stage IV gastric cancer, and the effect of gastrectomy on longevity is still unclear.7 Some scholars believe that surgery has no significant effect on asymptomatic patients with stage IV gastric cancer. However, some studies suggest that gastrectomy can improve quality of life, reduce complications, and that survival time in patients with stage IV gastric cancer can be prolonged after surgical resection.8–11 At the same time, age differences, race, sex and other factors may have an influence on the outcome of surgery and chemotherapy in these patients. These factors may also influence the prognosis of stage IV gastric cancer.
The Surveillance, Epidemiology, and End Results (SEER) database is supported by the Surveillance Research Program, which collects cancer case data from the United States. The SEER database contains data such as age, sex, race and treatment method. It has a large sample quantity and strong statistical performance, which gives the research based on this database a high clinical reference value. We can thus obtain a large number of reliable clinical data from the SEER database.
To investigate whether there is a significant difference in the prognosis of patients with stage IV gastric cancer with different factors, we analysed the extracted data for gastric cancer patients from 2000 to 2010.
Materials and methods
We used SEER*Stat statistical software to retrieve information on patients with gastric cancer from 2000 to 2010. According to calculations using X-Tile software, the patients were divided into young (≤ 49 years), middle-aged (50–73 years) and elderly (≥ 74 years) groups.
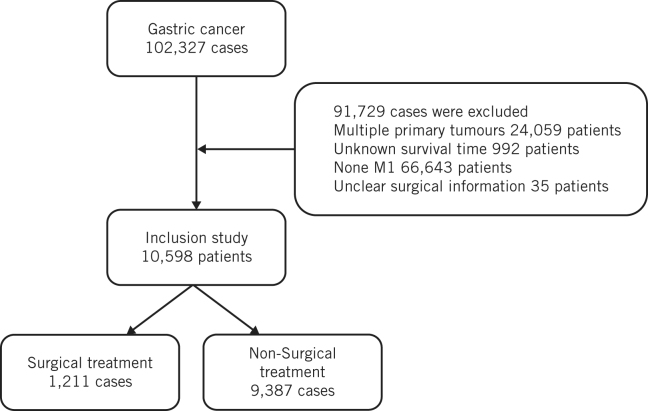
In accordance with the SEER Program Code Manual (third edition), patients were grouped into surgical and non-surgical treatment groups. The non-surgical treatment group includes local tumour destruction, local tumour excision, gastrectomy, near-total or total gastrectomy, gastrectomy with removal of other organs. In accordance with the AJCC staging system, patients were divided into T0, T1, T2, T3, T4 and TX groups, and N0, N1, N2, N3 and NX groups. Based on the treatment of lymph nodes, patients were divided into lymph node resection, lymph node biopsy, untreated and unknown groups. Based on chemotherapy status, patients were divided into chemotherapy and non-chemotherapeutic treatment groups. Based on radiotherapy status, patients were divided into radiotherapy, non-radiotherapy and unknown groups (Fig 1).
Figure 1.
Patient selection criteria.
The value of surgical resection in patients with stage IV gastric cancer remains controversial. In this study, patients were divided into surgery and non-surgery groups according to surgical treatment information, to explore the risk factors of stage IV gastric cancer and the influence of surgery on the prognosis of patients with stage IV gastric cancer.
In accordance with the International Classification of Diseases for Oncology third revision code and behaviour recode, we included information on patients with gastric malignancy. We excluded patients with multiple primary tumours, unclear diagnosis and incomplete clinical information.
Statistical analysis
Clinical information was screened using MS Excel. SPSS version 25.0 statistical software was used to analyse clinical data. The chi-square test (univariate analysis) was used for risk factor analysis. Survival analysis was performed using the Kaplan–Meier method (log-rank test) and Cox proportional hazard model. A p-value of less than 0.05 was considered statistically significant.
Results
Clinical characteristics
The study included 10,598 patients, including 1,211 (11.4%) in the surgical treatment group and 9,387 (88.6%) in the non-surgical treatment group (Table 1). There were 1,762 (16.2%) young patients, 6,093 (57.5%) middle-age patients and 525 (5.0%) elderly patients. A total of 3,822 (36.1%) were female and 6,776 (63.9%) male patients. Of the total, 7,571 (71.4%) were white and 1,456 (13.7%) black. There were 2,284 (21.6%) T4 and 3,886 (36.7%) N0 cases. Most (88.2%) of the patients in this study had no lymph node biopsy or resection but 6,131 (57.9%) patients received chemotherapy.
Table 1.
Patient demographics and clinical characteristics.
| Characteristic | Surgery | Non-surgery | p-value | ||
| (n) | (%) | (n) | (%) | ||
| Age: | |||||
| Young (≤ 49 years) | 252 | 20.8 | 1510 | 16.1 | < 0.001 |
| Middle age (50–73 years) | 686 | 56.6 | 5407 | 57.6 | |
| Elderly (≥ 74 years) | 273 | 22.5 | 252 | 20.8 | |
| Sex: | |||||
| Female | 520 | 42.9 | 3302 | 35.2 | < 0.001 |
| Male | 691 | 57.1 | 6085 | 64.8 | |
| Race: | |||||
| Black | 181 | 14.9 | 1275 | 13.6 | < 0.001 |
| White | 799 | 66.0 | 6772 | 72.1 | |
| Other | 225 | 18.6 | 1292 | 13.8 | |
| Unknown | 252 | 20.8 | 252 | 20.8 | |
| T stage: | |||||
| T0 | 2 | 0.2 | 52 | 0.6 | < 0.001 |
| T1 | 76 | 6.3 | 1656 | 17.6 | |
| T2 | 62 | 5.1 | 460 | 4.9 | |
| T3 | 357 | 29.5 | 1104 | 11.8 | |
| T4 | 630 | 52.0 | 1654 | 17.6 | |
| TX | 84 | 6.9 | 4461 | 47.5 | |
| N stage: | |||||
| N0 | 333 | 27.5 | 3553 | 37.9 | < 0.001 |
| N1 | 265 | 21.9 | 3324 | 35.4 | |
| N2 | 194 | 16.0 | 357 | 3.8 | |
| N3 | 375 | 31.0 | 242 | 2.6 | |
| Unknown | 44 | 3.6 | 1911 | 20.4 | |
| Lymph node: | |||||
| Resection | 908 | 75.0 | 90 | 1.0 | < 0.001 |
| Biopsy | 10 | 0.8 | 155 | 1.7 | |
| None | 281 | 23.2 | 9070 | 96.6 | |
| Unknown | 12 | 1.0 | 72 | 0.8 | |
| Chemotherapy: | |||||
| Yes | 739 | 61.0 | 5392 | 57.4 | 0.017 |
| No | 472 | 39.0 | 3995 | 42.6 | |
| Radiotherapy: | |||||
| Yes | 177 | 14.6 | 1452 | 15.5 | 0.024 |
| No | 1 | 0.1 | 67 | 0.7 | |
| Unknown | 1033 | 85.3 | 7868 | 83.8 | |
Analysis of prognostic factors in stage IV gastric cancer
The prognostic factors for stage IV gastric cancer by COX model are shown in Table 2. The risk for elderly (≥ 74 years) patients (hazard ratio, HR, 1.195; p <0.05) was higher than for the other two groups. According to the UICC/AJCC staging system, the tumour infiltration depth was not sensitive to predict the prognosis of patients (p>0.05), while hazard ratio increased in patients with regional lymph node metastasis. The patients treated with surgery (HR 0.489; p <0.05) and chemotherapy (p <0.05) presented a significantly lower risk. The hazard ratio in the lymph node resection and radiotherapy group was not statistically significant (p>0.05).
Table 2.
Analysis of prognostic factors of gastric cancer.
| Characteristic | Hazard ratio | p-value |
| Age: | < 0.001 | |
| Young (≤ 49 years) | 1 | – |
| Middle age (50–73 years) | 0.997 | 0.921 |
| Elderly (≥ 74 years) | 1.195 | < 0.001 |
| Sex: | 0.176 | |
| Female | 1 | – |
| Male | 1.031 | – |
| Race: | < 0.001 | |
| Black | 1 | – |
| White | 0.999 | 0.973 |
| Other | 0.921 | 0.044 |
| Unknown | 0.428 | < 0.001 |
| T stage: | < 0.001 | |
| T0 | 1 | – |
| T1 | 0.896 | 0.473 |
| T2 | 0.672 | 0.012 |
| T3 | 0.739 | 0.051 |
| T4 | 0.923 | 0.603 |
| TX | 0.881 | 0.403 |
| N stage: | < 0.001 | |
| N0 | 1 | – |
| N1 | 1.054 | 0.046 |
| N2 | 1.138 | 0.017 |
| N3 | 1.311 | < 0.001 |
| Unknown | 1.172 | < 0.001 |
| Surgery: | < 0.001 | |
| No | 1 | – |
| Yes | 0.489 | – |
| Lymph node: | 0.061 | |
| Resection | 1 | – |
| Biopsy | 1.197 | 0.098 |
| None | 1.179 | 0.015 |
| Unknown | 1.325 | 0.030 |
| Chemotherapy: | < 0.001 | |
| Yes | 1 | – |
| No | 2.878 | – |
| Radiotherapy: | 0.417 | |
| Yes | 1 | – |
| No | 1.192 | 0.186 |
| Unknown | 1.009 | 0.778 |
Survival analysis of surgical and non-surgical groups
The median survival time calculated by MS Excel was four months. As shown in Table 3, using the Kaplan–Meier method and the log-rank test, sex, race and other clinical characteristics were considered to perform the survival analysis on surgery and non-surgery. Our results showed that the median survival time of patients after surgery was greater than that of non-surgical patients in different groups. The survival rate of the chemotherapeutic patients was higher than that of the non-chemotherapy patients (overall survival rate 13.6% vs 10.6%; p <0.05), and the survival time was significantly prolonged after chemotherapy (median 16 months vs 4 months; p <0.05). Considering two factors, chemotherapy and surgery, our data show that patients received chemotherapy and surgery at the same time had the longest survival time (median 16 months), while patients who did not receive chemotherapy and surgery had the worst prognosis (median 1 month).
Table 3.
Survival analysis of surgery and non-surgery groups.
| Characteristic | Surgery | Non-surgery | p-value | ||
| 5-year survival rate | Median (months) | 5-year survival rate | Median (months) | ||
| Age: | |||||
| Young (≤ 49 years) | 0.138 | 15 | 0.026 | 6 | < 0.001 |
| Middle age (50–73 years) | 0.123 | 12 | 0.027 | 5 | < 0.001 |
| Elderly (≥ 74 years) | 0.115 | 8 | 0.006 | 2 | < 0.001 |
| Sex: | |||||
| Female | 0.103 | 12 | 0.017 | 4 | < 0.001 |
| Male | 0.14 | 12 | 0.025 | 4 | < 0.001 |
| Race: | |||||
| Black | 0.152 | 14 | 0.014 | 4 | <0.001 |
| White | 0.123 | 11 | 0.021 | 4 | < 0.001 |
| Other | 0.129 | 13 | 0.031 | 4 | < 0.001 |
| Unknown | 0.5 | 12 | 0.441 | 13 | 0.350 |
| T stage: | |||||
| T0 | – | 22 | 0.02 | 2 | 0.135 |
| T1 | 0.197 | 14 | 0.02 | 4 | < 0.001 |
| T2 | 0.356 | 21 | 0.037 | 7 | < 0.001 |
| T3 | 0.178 | 15 | 0.038 | 7 | < 0.001 |
| T4 | 0.077 | 11 | 0.025 | 4 | < 0.001 |
| TX | 0.049 | 10 | 0.014 | 4 | < 0.001 |
| N stage: | |||||
| N0 | 0.291 | 17 | 0.026 | 4 | < 0.001 |
| N1 | 0.116 | 12 | 0.024 | 5 | < 0.001 |
| N2 | 0.078 | 12 | 0.035 | 6 | < 0.001 |
| N3 | 0.035 | 9 | 0.034 | 6 | < 0.001 |
| Unknown | 0 | 7 | 0.010 | 3 | 0.006 |
| Lymph node: | |||||
| Resection | 0.115 | 12 | 0.060 | 8 | 0.003 |
| Biopsy | 0.0375 | 11 | 0.018 | 6 | 0.043 |
| None | 0.111 | 10 | 0.022 | 4 | < 0.001 |
| Unknown | 0 | 7 | 0 | 2 | 0.146 |
| Chemotherapy: | |||||
| Yes | 0.136 | 16 | 0.029 | 8 | < 0.001 |
| No | 0.106 | 4 | 0.012 | 1 | < 0.001 |
| Radiotherapy: | |||||
| Yes | 0.083 | 14 | 0.176 | 6 | <0.001 |
| No | 0 | 2 | 0.05 | 1 | 0.975 |
| Unknown | 0.139 | 11 | 0.021 | 4 | <0.001 |
Analysis of risk factors for prognosis in various groups
In this study, patients were divided into surgery and non-surgery groups. The Cox model analysis of the clinical data showed that patients in the the elderly group were found to be at higher risk (five-year survival rate 0.6%; median two months) in the non-surgical group, but there was no significant difference in hazard ratio among patients of different ages who underwent surgery (p >0.05). The risk of patients with stage IV gastric cancer was associated with regional lymph node metastasis (p <0.001). The regional lymph node metastasis as an independent risk factor for predicting the prognosis of patients with stage IV gastric cancer. The risk of stage IV gastric cancer increases with regional lymph node metastasis. Chemotherapy and lymph node resection can reduce the risks for patients (Table 4).
Table 4.
Analysis of prognostic risk factors in different groups.
| Characteristic | Surgery | Non-surgery | ||
| Hazard ratio | p-value | Hazard ratio | p-value | |
| Age: | ||||
| Young (≤ 49 years) | 1 | < 0.001 | 1 | < 0.001 |
| Middle age (50–73 years) | 1.043 | 0.641 | 1.001 | 0.983 |
| Elderly (≥ 74 years) | 1.218 | 0.069 | 1.199 | < 0.001 |
| Sex: | 0.512 | 0.148 | ||
| Female | 1 | 1 | ||
| Male | 1.048 | 1.035 | ||
| Race: | ||||
| Black | 1 | 0.331 | 1 | 0.108 |
| White | 1.099 | 0.348 | 0.992 | 0.814 |
| Other | 0.934 | 0.584 | 0.920 | 0.055 |
| Unknown | 0.001 | 0.927 | 0.727 | 0.401 |
| T stage: | ||||
| T0 | 1 | < 0.001 | 1 | < 0.001 |
| T1 | 0.672 | 0.695 | 0.909 | 0.538 |
| T2 | 0.414 | 0.387 | 0.711 | 0.035 |
| T3 | 0.589 | 0.600 | 0.765 | 0.088 |
| T4 | 0.792 | 0.817 | 0.931 | 0.647 |
| TX | 0.837 | 0.861 | 0.887 | 0.433 |
| N stage: | ||||
| N0 | 1 | < 0.001 | 1 | 0.002 |
| N1 | 1.776 | < 0.001 | 1.026 | 0.339 |
| N2 | 2.085 | < 0.001 | 1.050 | 0.444 |
| N3 | 2.421 | < 0.001 | 1.106 | 0.180 |
| Unknown | 1.947 | 0.002 | 1.134 | < 0.001 |
| Lymph node: | ||||
| Resection | 1 | 0.002 | 1 | 0.108 |
| Biopsy | 1.161 | 0.720 | 1.381 | 0.029 |
| None | 1.530 | < 0.001 | 1.315 | 0.020 |
| Unknown | 1.618 | 0.181 | 1.413 | 0.041 |
| Chemotherapy: | < 0.001 | < 0.001 | ||
| Yes | 1 | 1 | ||
| No | 1.990 | 2.999 | ||
| Radiotherapy: | ||||
| Yes | 1 | 0.595 | 1 | 0.431 |
| No | 2.736 | 0.321 | 1.175 | 0.229 |
| Unknown | 0.988 | 0.902 | 1.024 | 0.466 |
Analysis of prognostic risk factors in surgery group
As shown in Table 5, the patients were divided into six groups according to SEER Program Coding and Staging Manual, including six different treatment methods. By Cox model analysis, it can be seen that the lowest hazard ratio was found in the partial, subtotal, hemi group (HR 0.055, p< 0.001) and was also significantly decreased in other groups apart from the local tumour destruction group (p = 0.075). Thus, in addition to local tumour destruction, all other surgical methods can reduce patient‘s hazard ratio and prolong patient survival compared with patients in the non-surgery group (p < 0.001).
Table 5.
Analysis of prognostic risk factors in surgery group.
| Hazard ratio | p-values | |
| No surgery | 1 | < 0.001 |
| Local tumour destruction | 0.103 | 0.075 |
| Gastrectomy: | ||
| Partial, subtotal, hemi | 0.055 | < 0.001 |
| Near total or total | 0.097 | < 0.001 |
| With removal of a portion of oesophagus | 0.105 | < 0.001 |
| With resection of other organs | 0.079 | < 0.001 |
Discussion
There is no systematic treatment for stage IV gastric cancer; currently, chemotherapy is considered the main treatment option. Prognosis is poor because of the heterogeneity of the cancer. Surgical treatment remains controversial in the clinical practice. we divided patients into two groups, according to UICC/AJCC staging, to investigate the effect of surgical treatment in stage IV gastric cancer. This study contains data for 10,598 patients in the SEER database from 2000 to 2010. Median survival time was observed to be four months. According to the analysis of age, sex, race and other factors in stage IV gastric cancer, we found that elderly patients were found to be at a higher risk among the non-surgical group, while there was no significant difference in hazard ratio among patients of different age groups who went through surgery.
Tumour infiltration depth was not sensitive enough in predicting patient prognosis. Patients treated with surgery and chemotherapy presented a significantly lower risk. By comparing the effects of different surgical approaches on prognosis, in addition to local tumour destruction, all other surgical methods can reduce patient’s hazard ratio and prolong patient survival compared with non-surgical treatments. The risk of stage IV gastric cancer increased with the number of regional lymph node metastases, and lymph node resection improved the prognosis of stage IV gastric cancer patients. Surgical treatment, chemotherapy and regional lymph node metastasis were independent risk factors. Patients who received chemotherapy and surgery at the same time had the longest survival time, while patients who did not receive chemotherapy or surgery had the worst prognosis.
Reasonable surgical selection can therefore improve the prognosis and prolong the survival time for patients with stage IV gastric cancer. However, for patients who cannot undergo surgery, conversion therapy can be tried. Conversion therapy is the conversion of an unresectable tumour into a resectable tumour and the first surgery for unresectable stage IV cancer of the stomach was reported by Nakajima in 1997.12
Based on the biological behaviour of the tumour and combined with clinical experience, Yoshida classified gastric cancer stage IV according to the difficulty of tumour resection and conversion therapy. This classification guided the treatment options for different levels of stage IV gastric cancer through conversion treatment or removal of the tumour as a possible way to prolong the survival time of patients.13 At the same time, Yoshida et al have confirmed that patients with stage IV gastric cancer may benefit from surgery after receiving induction chemotherapy.12 Paolo Morgagnia et al also pointed out that transformation surgery can also prolong the survival time of stage IV gastric cancer patients.14 R0 resection is associated with long-term survival during the conversion surgery, and the survival time for patients with R0 resection is also better than for patients receiving chemotherapy. It is considered the most important prognostic factor in patients with advanced gastric cancer.5,14,15 Non-curative resection also often leads to higher survival rates than chemotherapy alone. 13 These conclusions are consistent with current conversion therapy. Palliative surgery and palliative chemotherapy can prolong the survival time of patients with stage IV gastric cancer who cannot tolerate surgery.13,16
This study has some limitations. First, although the SEER database can provide us with detailed and accurate clinical information, we found that it does not currently include the scope of specific lymph node dissection, nor can we obtain specific treatment plans for patients. Second, according to clinical experience, it can be concluded that the general fitness and comorbid conditions in patients with gastric cancer who are able to tolerate surgery are better than those who cannot tolerate surgery. We cannot exclude this point through data analysis, but we believe that under the premise of being able to tolerate surgery, reasonable surgical method selection is beneficial to prolong the survival time of patients. Last, in the REGATTA trial, it was confirmed that there was no significant survival advantage for patients with stage IV gastric cancer who received surgery plus chemotherapy versus chemotherapy alone.17 However, in the REGATTA trial, palliative gastrectomy was performed only in the non-surgical group during haemorrhage and obstruction, and the remaining non-surgical patients had no urgent surgical needs. This study found a survival advantage for surgical patients compared with non-surgical patients, but the SEER database also cannot determine the patient’s comorbidity situation and symptoms (perforation, obstruction and bleeding), which may cause bias. However, when we further explored the risk of different surgical procedures in patients in the surgical group, we found that the risk of gastrectomy (partial, subtotal, hemi) was lower than that of other surgical procedures, which provided guidance on the choice of surgical methods.
Conclusion
In conclusion, in patients with stage IV gastric cancer, gastrectomy (partial, subtotal, hemi) should be selected when surgery is necessary. The number of regional lymph node metastasis could be considered as a prognostic factor for patients with stage IV gastric cancer and lymph node dissection could reduce the risks of patients undergoing surgery.
Funding
Sponsored by the National Key Research and Development Program of China (2017YFC1308602) and the National Natural Science Foundation of China (81472321& 81703055).
References
- 1.Zhao AJ, Quin YY, Sun H et al. . Screening for gastric cancer with magnetically controlled capsule gastroscopy in asymptomatic individuals. Gastrointest Endosc 2018; : 466–474. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Li H, Sun KX et al. . [Report of cancer incidence and mortality in China, 2014]. Zhonghua Zhong Liu Za Zhi 2018; : 241–246. [DOI] [PubMed] [Google Scholar]
- 3.Chang YR, Han DS, Kong SH et al. . The value of palliative gastrectomy in gastric cancer with distant metastasis. Ann Surg Oncol 2012; : 1231–1239. [DOI] [PubMed] [Google Scholar]
- 4.Saito M, Kiyozaki H, Takata O et al. . Treatment of stage IV gastric cancer with induction chemotherapy using S-1 and cisplatin followed by curative resection in selected patients. World J Surg Oncol 2014; : 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamaguchi K, Yoshida K, Tanahashi T et al. . The long-term survival of stage IV gastric cancer patients with conversion therapy. Gastric Cancer 2018; : 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee CM, Choi IK, Kim JH et al. . Is noncurative gastrectomy always a beneficial strategy for stage IV gastric cancer? Ann Surg Treat Res 2017; : 23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park JH, Yang JY, Park YH, Lee WA. The long-term prognostic difference between gastrectomy with and without preoperative chemotherapy in patients with clinical stage IV gastric cancer. Asian J Surg 2019; : 922–929. [DOI] [PubMed] [Google Scholar]
- 8.Müsri FY, Mutlu H, Karaağaç M et al. . Primary tumor resection and survival in patients with stage iv gastric cancer. J Gastric Cancer 2016; : 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamada N, Akai A, Nomura Y et al. . The impact and optimal indication of non-curative gastric resection for stage IV advanced gastric cancer diagnosed during surgery: 10 years of experience at a single institute. World J Surg Oncol 2016; : 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Einama T, Abe H, Shichi S et al. . Long-term survival and prognosis associated with conversion surgery in patients with metastatic gastric cancer. Mol Clin Oncol 2017; : 163–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim KH, Lee KW, Baek SK et al. . Survival benefit of gastrectomy. Gastric Cancer 2011; : 130–138. [DOI] [PubMed] [Google Scholar]
- 12.Nakajima T, Ota K, Ishihara S et al. . Combined intensive chemotherapy and radical surgery for incurable gastric cancer. Ann Surg Oncol 1997; : 203–208. [DOI] [PubMed] [Google Scholar]
- 13.Zurleni T, Gjoni E, Altomare M et al. . Conversion surgery for gastric cancer patients: a review. World J Gastrointest Oncol 2018; : 398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida K, Yamaguchi K, Okumura N et al. . Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification. Gastric Cancer 2016; : 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgagni P, Solaini L, Framarini M et al. . Conversion surgery for gastric cancer: A cohort study from a western center. Int J Surg 2018; : 360–365. [DOI] [PubMed] [Google Scholar]
- 16.Solaini L, Ministrini S, Bencovenga M et al. . Conversion gastrectomy for stage IV unresectable gastric cancer: a GIRCG retrospective cohort study. Gastric Cancer 2019; : 1285–1293. [DOI] [PubMed] [Google Scholar]
- 17.Fujitani K, Yang HK, Mizusawa J et al. . Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol 2016; : 309–318. [DOI] [PubMed] [Google Scholar]