Abstract
BACKGROUND:
Neonatal intensive care practices have resulted in marked improvements in the survival of premature infants; however, they remain at significant risk for adverse neurodevelopmental outcomes. The impact of current nutritional practices on brain development following early extra-uterine exposure in premature infants is not well known.
METHODS:
We performed a systematic review to investigate nutritional effects on postnatal brain development in healthy term and prematurely born infants utilizing advanced magnetic resonance imaging tools.
RESULTS:
Systematic screen yielded 595 studies for appraisal. Of these, 22 total studies were selected for inclusion in the review, with findings summarized in a qualitative, descriptive fashion.
CONCLUSION:
Fat and energy intake are associated with improved brain volume and development in premature infants. While breast milk intake and long-chain polyunsaturated fatty acid supplementation has been proven beneficial in term infants, the impact in preterm infants is less well understood.
INTRODUCTION
Premature infants are born at a time of rapid brain development marked by complex and precisely programmed neurodevelopmental events, rendering the preterm brain vulnerable to a host of insults and leading to a high incidence of lifelong neurocognitive, behavioral, and motor impairments.1,2 Aberrant sensory experiences, toxic stress with systemic inflammation, and early alterations in the infant microbiome have all been associated with altered neurodevelopment.3–5 These early exposures are exacerbated by the abrupt cessation of placental nutritive and neuroendocrine support, culminating in an increased risk for altered neurodevelopment. Current nutritional practices are unable to mimic the exponential nutrient accretion that normally occurs during the third trimester of pregnancy, leading to a high rate of postnatal growth failure in preterm infants.6 Given that poor postnatal growth is associated with worse neurologic outcomes, it is imperative to identify key nutritional interventions in the early postnatal period to support optimal neurodevelopment and prevent long-term neurodevelopmental impairment.7,8
Magnetic resonance imaging (MRI) is the imaging modality of choice to evaluate neonatal brain development. Recent studies demonstrate that leading-edge quantitative MRI techniques such as volumetric segmentation, diffusion tensor imaging (DTI), resting-state and functional MRI, and proton magnetic resonance spectroscopy (1H MRS) may serve as important early biomarkers of functional neurodevelopmental outcomes in premature infants.9–12 The aim of this systematic review is to appraise emerging research investigating nutritional effects on neonatal brain development utilizing advanced MRI tools and highlight nutritional interventions that have been recently identified as beneficial to neonatal brain health. To accomplish this, we will provide an overview of existing literature in healthy term infants followed by emerging data on the role of optimized nutritional management for supporting early postnatal brain development in the premature infant. Finally, we will explore the future role of advanced MRI tools in identifying neonatal nutritional practices that best support the developing brain and optimize neurodevelopmental outcomes across the lifespan.
METHODS
Prior to initiation, this systematic review protocol was registered with PROSPERO (https://www.crd.york.ac.uk/PROSPERO), registration number CRD42019119577. Our aim was to capture all studies evaluating the impact of neonatal nutritional interventions on brain development as measured by MRI. The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist was utilized for this review.13
Search strategy
Eligibility criteria and methods of analysis were determined a priori, and a medical librarian with systematic review experience (S.K.) developed all searches. The search strategy was restricted to English and included terms relating to or describing neonates or prematurity, nutritional interventions, and MRI techniques, including Medical Subject Headings (MeSH terms) (Fig. 1). As we were reviewing relatively new interventions, there were no date or study-type restrictions so as to ensure capture of every applicable study. We performed an extensive search of the electronic databases PubMed, CINAHL, and Scopus (up to 15 January 2019). Search strategies and results were tracked using an Excel workbook designed specifically for systematic reviews.14 The reference lists of studies selected for inclusion were also manually reviewed to identify any potential additional studies not captured by the electronic database search.
Fig. 1.
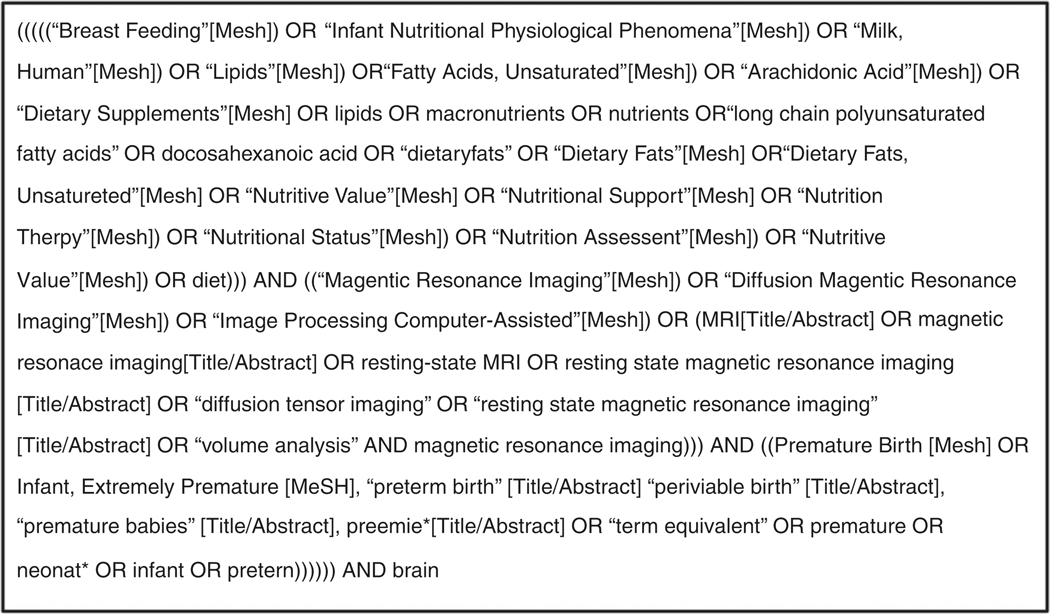
Search strategy utilized for electronic database search
Article selection and exclusion criteria
Prior to screening the electronic database search results, a Cohen’s κ test of inter-rater reliability was performed, wherein two screeners evaluated a random subset of eligible studies to ensure standardization of inclusion/exclusion criteria (S.K. and K.M.O.). The full set of titles and abstracts retrieved using the search strategy was then screened by one main review author (K.M.O.); if there was any question as to the eligibility of a study, it was recommended for full-text review. Nutritional interventions performed in the neonatal period (defined as the first 28 days life) in both term and preterm infants were eligible for inclusion. Studies out of the scope of the review question were excluded, including animal studies, those without a nutritional intervention or one performed outside of the neonatal period, those in which no brain MRI was obtained, or those exclusively investigating a genetic or metabolic disorder (Fig. 2). The full text of potentially eligible studies was then assessed for inclusion in the review using the same inclusion/exclusion criteria.
Fig. 2.
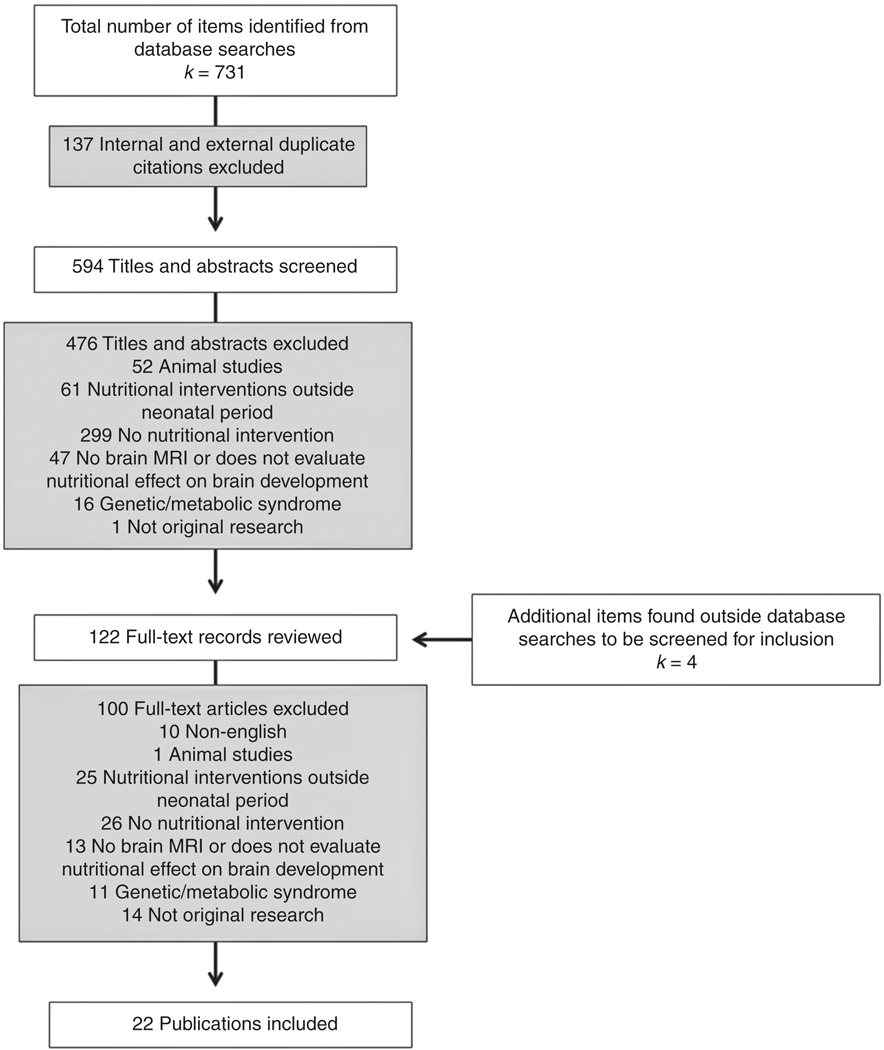
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) diagram of results of electronic database search, title, and abstract screening, as well as full-text review. Ineligible studies excluded
Data extraction and risk of bias assessment
Data were extracted from all selected studies using a standardized form that contained categories for the reporting of bibliographic information, study design characteristics, sample size, specific nutritional interventions, MRI techniques, and study outcomes/findings. Studies were appraised for risk of bias using either the Newcastle-Ottawa Tool for nonrandomized studies or Cochrane Risk of Bias (RoB 2.0) tool for randomized controlled trials.15–17 Due to the expected heterogeneity of specific interventions and MRI outcomes assessed across study designs, a descriptive synthesis of study findings was performed.
RESULTS
Electronic search of PubMed, CINAHL, and Scopus databases yielded 594 studies for review. Cohen’s κ screening of 66 studies for inter-rater reliability was performed by two screeners, with a result of 0.8 (raw agreement 0.9). Following initial screen, 476 abstracts were excluded. Full-text records of 118 studies were reviewed, 18 of which were deemed eligible for systematic review. Four additional studies were discovered upon manual inspection of reference lists of included studies, yielding 22 total studies for inclusion in the systematic review, including 6 randomized controlled trials, 15 cohort studies, and 1 cross-sectional study; 4 pairs of articles followed the same neonatal population or cohort at different time-points or evaluating different outcomes. Characteristics and results of each study are summarized in Table 1.
Table 1.
Summary of included studies
| Study | Type | Population | Nutritional intervention | MRI outcome | Results |
|---|---|---|---|---|---|
| Breast milk | |||||
| Belfort et al.23 | Cohort | GA <30 weeks, BW < 1250 g, no congenital anomalies (n= 180) | BM intake in first 28 days of life (days >50% BM, mean daily intake) | Total and regional brain volumes at TEA and 7 years (n = 160 at TEA, n = 108 at 7 years) |
TEA and BM: ↑ deep nuclear GM and hippocampal volumes 7 years and BM: No associations |
| Deoni et al.18 | Cross-sectional | GA 37–42 weeks, healthy and typically developing (n = 133) |
Feed type: Breastfed (≥3 months) vs. formula or both Breastfeeding duration: <12 months vs. >15 months |
WM microstructure using mcDESPOT myelin water fraction (VFM) at age 10 months-4 years |
Breastfed vs. formula (2.2–4 years): ↑ VFM (BM vs. formula: right premotor cortex and frontal lobe, CST; BM vs. both: premotor cortices, left optic radiation, right prefontal cortex and parietal lobe) Breastfeeding duration: ↑VFM (Broca’s area, parietal lobe, secondary somatosensory cortex; right auditory cortex and frontal lobe; left optic radiation, premotor, and primary somatosensory cortex) |
| Isaacs et al.24 | Cohort | GA ≤30 weeks, neurologically normal (n = 50) | %BM (of total dietary intake) | Total and regional brain volumes at 13–19 years | %>BM: ↑ Total and WM volume Sex-specific: ↑ Total and WM volume in males |
| Lu by et al.22 | Cohort | Full-term, IQ >75 (n = 148) | Breastfed (for >30) days vs. non-breastfed | Total regional brain volumes at 9–14 years | ↑ GM (both cortical and subcortical) and total brain volume |
| Kafouri et al.20 | Cohort | Full-term, no cardiac, neurologic, or psychiatric disease (n = 571) | Duration of exclusive breast feeding | Regional cortical thickness at 12–18 years | ↑ Cortical thickness in superior and inferior parietal lobe |
| Ou et al.21 | Cohort | Full-term, BW 5–95%ile, no neurologic/psychiatric history (n = 56) | Breastfed vs. formula-fed in first 12 months | WM microstructure using DTI (FA) at 8 years |
Predominant breastfeeding vs. formula (sex-specific): ↑ FA in males (left SLF, cingulum, corpus callosum, and posterior crossing fibers) Excusive breastfeeding vs. formula (sex-specific): ↑ FA in males (left SLF, corona radiata, external capsule, PLIC, posterior crossing fibers, and 8/9 regions of interest) |
| Ou et al.19 | Cohort | Full-term, BW 5–95%ile, no medical/psychiatric history (n = 42) | Breastfed vs. formula-fed in first 12 months | GM Volume using VBM and fMRI at 8 years |
BM and GM volume: ↑ Left parietal and temporal lobe volumes BM and fMRI: ↑ Activation for perception (temporal right frontal lobe) and language (left temporal and occipital lobes) |
| Pogribna et al.25 | Cohort | G A ≤29 weeks, BW ≤ 1000 g, no congenital CNS anomalies (n = 75) | Duration of BM intake | WM microstructure using DTI (FA and MD) at TEA | ↑ FA in corpus callosum |
| Vasu et al.26 | Cohort | G A <32 weeks (control GA 37–42 weeks), clinically well (n = 61) |
BM (EBM/DBM) and macronutrient intake until 34 weeks PMA | Total brain volume and cerebral arterial vessel tortuosity (CAVT) score (via MR angiography) at TEA | BM and total brain volume: No association BM and CAVT: Borderline ↑ overall CAVT (p = 0.05) Macronutrient and total brain volume, CAVT score: No association |
| LCPUFA supplementation | |||||
| Almaas et al.30 | RCT | BW <1500 g, no congenital anomalies or severe IVH (n = 81) | BM (EBM/DBM) ± high-dose LCPUFA (DHA 32 mg/AA 31 mg total) | Total and regional brain volumes and cortical surface reconstruction (volume, surface area, thickness) at 8 years |
Volumes: No difference between groups Cortical surface: No difference between groups |
| Almaas et al.29 | RCT | BW <1500 g, no congenital anomalies or severe IVH (n = 82) | BM (EBM/DBM) ± high-dose LCPUFA (DHA 32 mg/AA 31 mg total) | WM microstructure using DTI (FA, MD, AD, RD) at 8 years | Borderline ↑ FA in corpus callosum (p = 0.08) |
| Lepping et al.31 | RCT | GA 37–42 weeks, healthy (n = 42) | Formula ± LCPUFA (DHA/AA) in 4 DHA groups: control, 0.32%, 0.64%, 0.96% | GM and WM volume using VBM, fMRI, rsMRI, and 1H MRS at 9 years |
fMRI and LCPUFA: ↑ Activation parietal lobe, anterior cingulate cortex, and cerebellum rsMRI and LCPUFA: ↑ Prefrontal-parietal cortex connectivity (dorsal attention network) VBM and LCPUFA: ↑ WM volume anterior cingulate cortex and R parietal lobe 1H MRS and LCPUFA: ↑ N-acetylaspartate and myo-inositol |
| Tarn et al.33 | Cohort | G A <32 weeks, no congenital anomalies, syndromes, or infections | Early and near-term red blood cell DHA, AA, EPA, and LA levels | WM microstructure using DTI (FA, MD, AD, RD) and brain injury (IVH, WM injury, cerebellar hemorrhage) on early and near-term MRI |
Early DHA and brain injury: |IVH incidence/severity Early DHA and DTI: ↓MD in PLIC and optic radiations Early LA and DTI: ↑ MD in optic radiations |
| van Wezel-Meijler et al32 | RCT | GA <34 weeks, BW < 1750 g, no CNS abnormalities or severe ROP (n = 42) | Formula ± LCPUFA supplementation (DHA 15 mg/AA 31 mg per 100 mL) until 6 months | Global and visual myelination score using T1/T2 images at 3 and 12 months |
3 months and LCPUFA: No difference between groups 12 months and LCPUFA: No difference between groups |
| Macronutrient and energy | |||||
| Beauport et al.34 | Cohort | G A <30 weeks, no congenital anomalies or severe IVH (n = 51) | Cumulative macronutrient and energy intake in first 2 weeks of life | Brain maturation and injury using semi-quantitative |
Total score: ↓ Severity with ↑ fat and energy intake Kidokoro score (grouped by severity) at TEA GM score: ↓ Severity with ↑ fat, carbohydrate, protein, and energy intake |
| Coviello et al.28 | Cohort | GA <31 weeks, no congenital anomalies or CNS infection (n = 131) | Cumulative macronutrient, energy, and BM intake in first 28 days of life | Total and regional brain volumes and WM microstructure using DTI (FA) at TEA |
Cumulative (fat/energy) and enteral (protein/fat/energy) intake and volume: ↑ Total and regional brain volumes (cerebellum, BG/thalamus) PN and volume: ↓ Cerebellum, BG/thalamus, cortical GM, and total brain volumes Cumulative (fat/energy) and enteral (protein/fat/energy) intake and DTI: ↑ FA in PLIC PN and DTI: ↑ FA in PLIC BM andDTI (sex-specific): ↑ FA in left PLIC in males |
| Hansen-Pupp et al.35 | Cohort | GA <31 weeks, no major anomalies (n = 51) | Daily protein and energy intake until 35 weeks PMA | Total and regional brain volumes at TEA | No association with any volume |
| Isaacs et al.36 | Cohort | GA ≤30 weeks, neurologically normal (n = 76) | Standard feeds (donor BM or term formula) vs. high-nutrient (↑ protein/energy) formula | Total and regional brain volumes at 13–19 years | High-nutrient (vs. standard): t caudate volume Sex-specific: t caudate volume in males |
| Paviotti et al.37 | Cohort | BW <1500 g, no congenital anomalies or severe IVH (n = 42) | Mean macronutrient and energy intake until discharge | Total brain and cerebellar volume at TEA | No association with total brain or cerebellar volume |
| Schneider et al.27 | Cohort | GA <30 weeks, no congenital anomalies or severe IVH (n = 49) | Cumulative macronutrient, energy, and BM intake in first 2 weeks of life | Total and regional brain volume and WM microstructure using DTI (FA) at 3 time-points (1st week of life, 3 weeks of life or 34–35 weeks, TEA) |
Lipid/energy and volume: ↑ BG and total brain volume (greater enteral effect) Lipid/energy and DTI: ↑ FA (corona radiata, thalamic radiations, SLF, CST) Protein and volume: ↑Total brain volume Enteral protein and DTI: ↑ FA (corona radiata, thalamic radiations, CST) BM and DTI: ↑ FA (corona radiata, thalamic radiations, SLF) |
| Strom men et a I.38 | RCT | BW <1500 g, no congenital anomalies (n = 50) | High-nutrient (↑ protein, fat, LCPUFA, vitamin A) vs. control until 52 weeks PMA or 5.5 kg | WM microstructure using DTI (MD) at TEA (n = 25 with DTI) | ↓ MD in SLF, borderline for CST (p = 0.05) |
| Tan et al. (2008)39 | RCT | GA ≤29 weeks, <7 days of life, no congenital anomalies (n = 65) |
Hyperalimentation: Enteral and parenteral (↑ macronutrient and calories) vs. standard nutrition until 34 weeks PMA Intake/deficit: Energy and protein for first 28 days of life |
Total and regional brain volumes and WM maturation using T2 relaxation times at TEA |
Hyperalimentation and olumes: No significant difference between group WM maturation (fronto-parietal): No significant difference between groups Energy intake in first 28 days: ↑Total brain volume Energy deficit in first 28 days: ↓Total brain volume |
BW birth weight, GA gestational age, PMA postmenstrual age, TEA term-equivalent age, IVH intraventricular hemorrhage, DTI diffusion tensor imaging, FA fractional anisotropy, MD mean diffusivity, AD axial diffusivity, RD radial diffusivity, fMRI functional MRI, rsMRI resting-state MRI, 1H MRS proton magnetic resonance spectroscopy, VBM voxel-based morphometry, WM white matter, GM gray matter, CST corticospinal tract, SLF superior longitudinal fasciculus, PLIC posterior limb of internal capsule, BG basal ganglia, BM breast milk, LCPUFA long-chain polyunsaturated fatty acids, DHA docosahexanoic acid, AA arachidonic acid, LA linoleic acid, EPA eicosapentaenoic acid, PN parenteral nutrition, RCT randomized controlled trial
All six randomized controlled trials demonstrated a low risk of bias based on assessment with the Cochrane Risk of Bias Tool. Newcastle-Ottawa scores for all 15 cohort studies were consistent with good methodological quality (3–4 stars in selection domain, 1–2 stars in comparability domain, and 2–3 stars in outcome/exposure domain). The one cross-sectional study by Deoni et al.18 was evaluated using a modified Newcastle-Ottawa Score with a result corresponding to moderate methodological quality (11/16 points).
DISCUSSION
Breast milk intake
Available MRI studies performed in full-term infants suggest that the neurodevelopmental benefits of breast milk seen in this population may be mediated through improved structural brain development.18–21 Deoni et al.18 utilized brain MRI to measure myelin water fraction (VFM), a marker of white matter microstructural development, in a cross-sectional study comparing formula and breastfed infants evaluated at toddler age. Breastfed infants demonstrated increased VFM in several brain regions involved in vision, language, and higher-order cognition, correlating with improved visual and receptive language scores. When comparing brain volumes in school-aged children, Ou et al.19 found that breast milk versus formula was associated with larger gray matter volume in the parietal and temporal lobes. Breastfed children in this cohort also demonstrated increased activation of the frontal, temporal, and occipital lobes on functional MRI during tasks of language and visual perception, which correlated to better task performance compared to formula-fed infants. Luby et al.22 found a similar relationship between breastfeeding and improved gray matter volume on long-term follow-up in school-aged children that correlated with higher IQ scores.
Breastfeeding duration also plays an important role in the brain development of healthy term infants. In the Deoni et al.18 study, infants who breastfed for an extended duration (>15 months) demonstrated increased VFM in regions involved in vision, language, and motor control by toddler age, with associated improved gross and fine motor, receptive and expressive language, and visual reception scores. Beneficial effects of breastfeeding persisted into adolescence in a study by Kafouri et al.,20 with longer duration of breastfeeding associated with increased cortical thickness of the parietal lobe and greater full-scale IQ scores.
These benefits of breastfeeding on improved brain structure and function in full-term infants cannot be directly extrapolated to the immature preterm brain, given the significantly greater risk for neurodevelopmental impairment and oro-motor immaturity that often precludes direct breastfeeding. Additionally, preterm infants have significantly greater nutrient and energy requirements compared to their healthy term counterparts, warranting fortification of breast milk feeds in attempts to increase macro- and micronutrient content.6 However, recent MRI studies suggest that breast milk intake also has beneficial effects on the developing preterm brain.23–27 Specifically, increased breast milk intake has been associated with improved measures of white matter microstructure using DTI at term-equivalent age (TEA), including greater fractional anisotropy (FA) in several important developmental regions such as the corpus callosum, corona radiata, thalamic radiations, and superior longitudinal fasciculus.25,27 The contributory effect of breast milk on cerebral volumes in preterm infants has been less consistent. Vasu et al.26 found no significant association between breast milk intake and total brain volume in preterm infants receiving fortified maternal or donor breast milk. Early expressed maternal breast milk intake in the first 28 days of life was related to increased hippocampal and deep nuclear gray matter volume at TEA in a study by Belfort et al.23 By 7 years of age the association between breast milk and hippocampal volume in this cohort was smaller and not sustained after adjusting for covariates, but breast milk-fed infants still demonstrated improved neurodevelopmental outcomes in verbal and performance IQ, full-scale IQ, math computation, and working memory. Another long-term follow-up study by Isaacs et al.24 in adolescents born preterm demonstrated increased white matter and total brain volume in breast milk-fed infants, corresponding to superior verbal and full-scale IQ scores.
MRI studies of both term and preterm infants have found interesting sex-specific differences related to breast milk intake with preferential benefit seen in males. Ou et al.21 performed MRI studies in breast milk-fed term infants at school age, and found greater FA suggestive of improved microstructural development in the white matter tracts of the superior longitudinal fasciculus, cingulum, corpus callosum, corona radiata, posterior limb of internal capsule (PLIC), external capsule, and posterior crossing fibers in males only on DTI analysis. Coviello et al.28 also noted a sex-specific positive effect of breast milk in preterm infants, with only males demonstrating greater FA in the PLIC on DTI by TEA. On subgroup analysis by gender, Isaacs et al.24 also found that the association between breast milk intake and brain volumes in preterm infants remained significant for males only.
Taken together, existing studies of breastfed term infants demonstrated superior gray matter volumes and improved white matter microstructural development corresponding to improved neurodevelopmental outcomes through adolescence.18–22 However, none of these studies were randomized controlled trials and infants were all exclusively breastfed, making it nearly impossible to disentangle any potential confounding effect of the maternal–infant bond on the observed benefits. Results from preterm studies have been less consistent, but should also be interpreted with caution as these were all relatively small cohort studies with the exception of Belfort et al.23 Additionally, the enrollment periods for these studies ranged from 1982 to 2010 and did not necessarily reflect the most current nutritional practices, including the now widespread use of pasteurized donor breast milk and breast milk fortification.23–26 Despite these limitations, the existing literature suggests greater regional and total brain volumes and improved white matter microstructural development in breast milk-fed preterm infants, with associated improved developmental outcomes.23–26 Further research is needed in contemporary cohorts to better delineate and solidify these findings. The preferential benefits of breast milk seen in males provides an example of how advanced MRI techniques highlight differences that might aid in future individualized optimization of nutritional practices.
LCPUFA supplementation
Long-chain polyunsaturated fatty acids (LCPUFAs) such as docosahexanoic acid (DHA) and arachadonic acid (AA) play a significant role in brain development and their presence in breast milk has been postulated to mediate some of breast milk’s neurodevelopmental benefits; however, their supplementation in the infant diet has yielded mixed results on MRI studies.29–32 A recent study by Lepping et al.31 of term infants randomized to receive standard formula versus supplementation with AA and varying concentrations of DHA (0.32%, 0.64%, or 0.96%) demonstrated improved MRI findings on long-term follow-up in LCPUFA-supplemented groups. At 9 years of age, children who received supplementation with 0.64% DHA demonstrated greater cortical connectivity on resting-state MRI between prefrontal and parietal areas of the dorsal attention network.31 LCPUFA-supplemented children also demonstrated increased activation in the anterior cingulate cortex, parietal regions, and cerebellum while performing tasks of inhibition, with significantly greater white matter volume in the anterior cingulate cortex using voxel-based morphometry. Proton magnetic resonance spectroscopy also revealed significantly greater myo-inositol and N-acetylaspartate levels in LCPUFA-supplemented groups, which are important markers of neuronal cell integrity and signal transduction.31
Tam et al.33 highlighted the relationship between early DHA levels and brain development in preterm infants, finding a lower incidence and severity of intraventricular hemorrhage on early postnatal MRI with increasing red blood cell DHA levels in a cohort of predominantly breast milk-fed infants.33 Additionally, higher DHA levels were associated with decreased mean diffusivity (MD) in the PLIC and optic radiations on early DTI studies, suggestive of improved white matter development, as well as improved language and motor outcomes by 30–36 months of age.33 Despite these positive associations between DHA levels and brain development, MRI studies of preterm infants receiving specific LCPUFA supplementation have demonstrated less promising results. van Wezel-Meijler et al.32 supplemented formula with a relatively low concentration of LCPUFA (DHA and AA) until 6 months of age, with no significant effect on global myelination as measured by serial T1 and T2-weighted MRI scans obtained at 3 and 12 months of age. With higher-dose DHA supplementation, Almaas et al.29,30 found a borderline positive association with FA on DTI of the corpus callosum, potentially signifying greater white matter organization, but no difference in brain volumes on MRI or neurodevelopmental outcomes by 8 years of age.
These randomized controlled trials utilizing advanced MRI techniques demonstrated a greater effect of LCPUFA supplementation on term versus preterm infants.29–32 It should be noted that enrollment for all these studies ranged from 1993 to 2005, potentially limiting the generalizability to contemporary neonates. Additionally, the less striking effect of LCPUFA supplementation seen in preterm infants could be related to study design, with a potential dose and duration-dependent effect of LCPUFA supplementation for which the threshold may have not been reached at the doses administered during these trials. Further research is warranted to better evaluate these questions.29,30,32 The study by van Wezel-Meijer et al.32 also introduces the interesting concept of performing serial brain MRIs as a potential method of evaluating nutritional interventions over time.
Macronutrient and energy intake
Studies of neonatal macronutrient intake have primarily focused on the preterm population, and have reported a consistent positive relationship between cumulative fat and energy intake and preterm brain development by TEA.27,28,34–39 Schneider et al.27 confirmed the importance early nutrition by demonstrating that greater lipid and energy intake in the first 2 weeks of life were associated with increased FA on DTI in several essential pathways including the corona radiata, thalamic radiations, and superior longitudinal fasciculus. Greater lipid and energy intake also correlated with larger basal nuclei and total brain volumes, which were associated with higher psychomotor development index scores at 18 months corrected age. In a separate evaluation of preterm infants from this same cohort, term-equivalent MRIs were evaluated using a semi-quantitative brain injury and maturation scoring system for gray and white matter known as the Kidokoro score.40 Higher fat and energy intake in the first 2 weeks of life were associated with improved Kidokoro scores, especially in the gray matter.34 Coviello et al.28 showed that greater cumulative fat and energy intake through the first month of life were associated with improved preterm brain growth and microarchitecture, reflected by increased FA in the PLIC on DTI and increased total and regional brain volumes in the cerebellum, basal ganglia, and thalamus at TEA. Tan et al.39 similarly found larger total brain volume with increased energy intake in the first month of life. These findings are in agreement with those of Strommen et al.,38 who showed decreased MD in the superior longitudinal fasciculi on DTI in preterm infants receiving a regimen of enhanced protein, fat, and energy supply, suggestive of improved white matter organization by TEA in this region involved in behavior and language. In contrast to these findings of cumulative nutrient intake, studies of mean nutrient intake during neonatal intensive care unit stay did not find any association between mean lipid and energy intake and cerebral volumes by TEA.26,35,37
Interestingly, although current nutritional practices emphasize the importance of early protein administration to prevent negative nitrogen balance and mimic in utero nutrient accretion, protein was less consistently associated with preterm brain development in comparison to lipid and energy intake.27,28,34 Cumulative protein intake was associated with improved gray matter but not total brain Kidokoro scores in Beauport et al.34 Schneider et al.27 found a positive association between cumulative protein intake and head growth, as well as total but not regional brain volumes. Conversely, the authors found no relationship between total protein intake and white matter microstructure measured by DTI. However, greater enteral protein intake predicted higher FA in the corona radiata, thalamic radiations, and corticospinal tracts.27 Coviello et al.28 also noted a contribution from enteral but not total cumulative protein intake on brain development, which was associated with increased basal ganglia, thalami, and total brain volumes and greater FA in the PLIC on DTI. As with average lipid and energy intake, mean protein intake did not correlate with cerebral volumes at TEA.26,35,37
In the above studies of early nutritional intake in preterm infants, enteral nutrition had the most significant contribution to brain development on MRI.27,28 Confirming the long-term importance of early enteral feeds, Isaacs et al.36 found that preterm infants fed a high-nutrient diet demonstrated significantly greater caudate volumes and verbal IQ scores in adolescence. In contrast, Coviello et al.28 demonstrated that a longer duration of parenteral nutrition in the first month of life correlated with lower volumes in the cerebellum, basal ganglia and thalami, cortical gray matter, and total brain, as well as a negative association with FA measured in the PLIC on DTI. These negative findings relating to parenteral nutrition should be interpreted with caution, due to potential confounding given that parenteral nutrition is often required by the sickest neonates and may be a marker of other systemic factors that are detrimental to brain development.
In several of these studies, improved weight gain was associated with greater total and regional brain volumes by TEA.27–29 In the trial by Tan et al.39 of hyperalimentation versus standard feeding in preterm infants, anthropometric measures of weight, length, and head circumference at 36 weeks postmenstrual age correlated significantly with cortical and total brain volumes. However, larger head size did not always signify improved brain growth, with Paviotti et al.37 reporting no correlation between head circumference and brain volumes.
These MRI studies in preterm neonates emphasize the neurodevelopmental importance of optimizing early nutrition, especially initiation and advancement of enteral feeds. Enteral feeds demonstrated the greatest positive influence on brain development, with parenteral nutrition and energy deficits demonstrating a negative impact. All were cohort studies with relatively small sample sizes, but nevertheless consistently demonstrated an association between greater cumulative fat and energy intake and improved white matter microstructure, as well as total and regional brain volumes.27,28,34,36,38 These results also suggest that cumulative macronutrient intake, rather than average, is the most important determinant for improved brain development.26,35,37,39
Summary and future directions
Despite significant advances in early postnatal care of preterm infants, short- and long-term neurodevelopmental impairments remain prevalent and wide-ranging, even in the absence of overt brain injury.1,2 This systematic review highlights the importance of meeting basic nutrient and energy needs to sustain the exponential brain growth of the ex utero preterm infant. Studies of healthy term infants utilizing advanced MRI techniques provide evidence for improved structural brain development, functional activation, and neurodevelopmental outcomes through adolescence in breastfed infants and those with dietary supplementation of LCPUFAs.18–22,31 Preterm MRI studies demonstrate less robust relationships between breast milk and LCPUFA supplementation and improved preterm brain development, but suggest improved global and regional brain growth, white matter organization, and functional neurodevelopmental outcomes with increasing breast milk intake.23–25,29 Additionally, preterm studies of macronutrient intake underscore the importance of early enteral feeding and lipid intake, which has been less well studied compared to other macronutrients.27,28,34,36,38,39
However, this review also highlights the profound gaps in measuring and assessing the impact of early postnatal nutrition on neonatal brain neurodevelopment. It is difficult to rectify the positive impact of breast milk and fat intake on preterm brain development with the equivocal results of dietary supplementation with LCPUFAs, as LCPUFAs are thought to be an important component of breast milk and mediator of brain development.23–25,27–30,34 Further study is required to determine whether this discrepancy is simply related to suboptimal dosing and study design in previous studies, or rather suggests that other components in breast milk aside from LCPUFAs might play a more significant role in preterm brain development.
A significant proportion of published studies reported on cross-sectional neuroimaging and behavioral outcomes throughout the lifespan, reflecting outdated nutritional practices.18,19,21–24,29–32,36,39 Alongside the increased awareness of the multitude of benefits from human breast milk feeds for premature infants, there are an equal number of unanswered questions. What is the role of donor milk on premature brain development? How does the pasteurization process impact the potential immunologic benefits, and are they systemic or brain specific? Future work will require longitudinal, real-time assessment of preterm brain development as these nutritional practices are ever-evolving, with equal emphasis on macro- and micro-nutrient intake, along with neuropeptide, neurohormone, and neurosteroid intake to better mimic both the nutritional and neuroendocrine functions of the placenta for the ex utero fetus.23,34,35
As might be expected with early studies of novel, evolving MRI techniques, the majority of existing studies are small cohort studies.19,21,24–27,34–37 Moreover, those studies with more extensive longitudinal follow-up represent outdated nutritional practices.23,24,32,36 Conversely, recent studies that have described more current nutritional practices have yet to report long-term neurodevelopmental follow-up and correlate with MRI findings.27,28,34,37,38 The majority of studies also excluded infants with significant brain injury, thereby excluding an especially vulnerable group that might benefit the most from nutritional optimization and limiting the generalizability of current data in the highest-risk group of premature infants.18–25,27–30,32,34,36,37 Indeed, Beauport et al.34 reported improved early nutrition was associated with less dysmaturation and injury by TEA.
Despite the short-term follow-up of more recent studies utilizing advanced, quantitative MRI techniques such as volumetric brain segmentation and DTI in preterm infants, they serve to emphasize the necessity of optimizing early nutrition and enteral feeding in the first months of life, highlighting that important changes can be measured by TEA in preterm infants receiving differential nutritional interventions.27,28,34,38,39 Future large, randomized controlled trials are needed to further elucidate the optimal timing, type, and duration of targeted nutritional interventions to augment their effectiveness on neurodevelopment leveraging advanced, multimodal MRI tools, and high-fidelity neuropsychological outcome measures.
Footnotes
ADDITIONAL INFORMATION
Competing interests: The contents of this article are solely the responsibility of the authors and do not reflect the official policy or position of the US Air Force, Department of Defense, or the US Government. The authors declare no competing interests.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
REFERENCES
- 1.Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB & Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics 124, 717–728 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Sammallahti S. et al. Nutrition after preterm birth and adult neurocognitive outcomes. PLoS ONE 12, e0185632 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker SM Biological and neurodevelopmental implications of neonatal pain.Clin. Perinatol. 40, 471–491 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Patra A, Huang H, Bauer JA & Giannone PJ Neurological consequences of systemic inflammation in the premature neonate. Neural Regen. Res 12, 890–896 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu J. & Claud EC Connection between gut microbiome and brain development in preterm infants. Dev. Psychobiol 15, 739–751 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franz AR et al. Intrauterine, early neonatal, and postdischarge growth and neurodevelopmental outcome at 5.4 years in extremely preterm infants after intensive neonatal nutritional support. Pediatrics 123, e101–e109 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Schneider N. & Garcia-Rodenas CL Early nutritional interventions for brain and cognitive development in preterm infants: A review of the literature. Nutrients 9, E187 (2017).28241501 [Google Scholar]
- 8.Keunen K, van Elburg RM, van Bel F. & Benders MJ Impact of nutrition on brain development and its neuroprotective implications following preterm birth. Pediatr. Res 77, 148–155 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson PJ, Cheong JL & Thompson DK The predictive validity of neonatal MRI for neurodevelopmental outcome in very preterm children. Semin. Perinatol 39, 147–158 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Rose J. et al. Neonatal brain microstructure correlates of neurodevelopment and gait in preterm children 18–22 mo of age: an MRI and DTI study. Pediatr. Res 78, 700–708 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Woodward LJ, Clark CA, Bora S. & Inder TE Neonatal white matter abnormalities an important predictor of neurocognitive outcome for very preterm children. PLoS ONE 7, e51879 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kendall GS et al. White matter NAA/cho and cho/cr ratios at MR spectroscopy are predictive of motor outcome in preterm infants. Radiology 271, 230–238 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J. & Altman DG Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J. Surg 8, 336–341 (2010). [DOI] [PubMed] [Google Scholar]
- 14.VonVille HM Excel Workbooks for Systematic Reviews (2018). https://shwca.se/Excel-SR-workbooks-guides (2018). [Google Scholar]
- 15.Wells GA et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality f Nonrandomised Studies in Meta-analyses (2019). http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 16.Higgins JT et al. A revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst. Rev 10, 29–31 (Suppl. 1) (2016). [Google Scholar]
- 17.Hillen MA, Medendorp NM, Daams JG & Smets EMA Patient-driven second opinions in oncology: a systematic review. Oncologist 22, 1197–1211 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deoni SC et al. Breastfeeding and early white matter development: a cross-sectional study. Neuroimage 82, 77–86 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ou X. et al. Voxel-based morphometry and fMRI revealed differences in brain gray matter in breastfed and milk formula-fed children. Am. J. Neuroradiol 37, 713–719 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kafouri S. et al. Breastfeeding and brain structure in adolescence. Int. J. Epidemiol 42, 150–159 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Ou X. et al. Sex-specific association between infant diet and white matter integrity in 8-y-old children. Pediatr. Res 76, 535–543 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Luby JL, Belden AC, Whalen D, Harms MP & Barch DM Breastfeeding and childhood IQ: the mediating role of gray matter volume. J. Am. Acad. Child Adolesc. Psychiatry 55, 367–375 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belfort MB et al. Breast milk feeding, brain development, and neurocognitive outcomes: a 7-year longitudinal study in infants born at less than 30 weeks’ gestation. J. Pediatr 177, 133–139.e1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isaacs EB et al. Impact of breast milk on intelligence quotient, brain size, and white matter development. Pediatr. Res 67, 357–362 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pogribna U. et al. Perinatal clinical antecedents of white matter microstructural abnormalities on diffusion tensor imaging in extremely preterm infants. PLoS ONE 8, e72974 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vasu V. et al. Preterm nutritional intake and MRI phenotype at term age: a prospective observational study. BMJ Open 4, e005390 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneider J. et al. Nutrient intake in the first two weeks of life and brain growth in preterm neonates. Pediatrics 141, e20172169 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Coviello C. et al. Effects of early nutrition and growth on brain volumes, white matter microstructure, and neurodevelopmental outcome in preterm newborns. Pediatr. Res 83, 102–110 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Almaas AN et al. Diffusion tensor imaging and behavior in premature infants at8 years of age, a randomized controlled trial with long-chain polyunsaturated fatty acids. Early Hum. Dev 95, 41–46 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Almaas AN et al. Long-chain polyunsaturated fatty acids and cognition in VLBW infants at 8 years: an RCT. Pediatrics 135, 972–980 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Lepping RJ et al. Long-chain polyunsaturated fatty acid supplementation in the first year of life affects brain function, structure, and metabolism at age nine years. Dev. Psychobiol 61, 5–16 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Wezel-Meijler G. et al. Dietary supplementation of long-chain polyunsaturated fatty acids in preterm infants: efects on cerebral maturation. Acta Paediatr. 91, 942–950 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Tam EW et al. Early postnatal docosahexaenoic acid levels and improved preterm brain development. Pediatr. Res 79, 723–730 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beauport L. et al. Impact of early nutritional intake on preterm brain: a magnetic resonance imaging study. J. Pediatr 181, 29–36.e1 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Hansen-Pupp I. et al. Postnatal decrease in circulating insulin-like growth factor-Iand low brain volumes in very preterm infants. J. Clin. Endocrinol. Metab 96, 1129–1135 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Isaacs EB et al. The effect of early human diet on caudate volumes and IQ. Pediatr. Res 63, 308–314 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Paviotti G. et al. Higher growth, fat and fat-free masses correlate with larger cerebellar volumes in preterm infants at term. Acta Paediatr. 106, 918–925 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Strommen K. et al. Enhanced nutrient supply to very low birth weight infants is associated with improved white matter maturation and head growth. Neonatology 107, 68–75 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Tan M, Abernethy L. & Cooke R. Improving head growth in preterm infants—a randomised controlled trial II: MRI and developmental outcomes in the first year. Arch. Dis. Child Fetal Neonatal Ed 93, F342–F346 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Kidokoro H, Neil JJ & Inder TE New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. Am. J. Neuroradiol 34, 2208–2214 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]


