Abstract
Objective
To examine and quantify the potential dose-response relation between intake of total, animal, and plant protein and the risk of mortality from all causes, cardiovascular disease, and cancer.
Design
Systematic review and meta-analysis of prospective cohort studies.
Data sources
PubMed, Scopus, and ISI Web of Science until December 2019, and references of retrieved relevant articles.
Study selection
Prospective cohort studies that reported the risk estimates for all cause, cardiovascular, and cancer mortality in adults aged 18 or older.
Data synthesis
Random effects models were used to calculate pooled effect sizes and 95% confidence intervals for the highest versus lowest categories of protein intake and to incorporate variation between studies. Linear and non-linear dose-response analyses were done to evaluate the dose-response relations between protein intake and mortality.
Results
32 prospective cohort studies were included in the systematic review and 31 in the meta-analysis. During the follow-up period of 3.5 to 32 years, 113 039 deaths (16 429 from cardiovascular disease and 22 303 from cancer) occurred among 715 128 participants. Intake of total protein was associated with a lower risk of all cause mortality (pooled effect size 0.94, 95% confidence interval 0.89 to 0.99, I2=58.4%, P<0.001). Intake of plant protein was significantly associated with a lower risk of all cause mortality (pooled effect size 0.92, 95% confidence interval 0.87 to 0.97, I2=57.5%, P=0.003) and cardiovascular disease mortality (pooled hazard ratio 0.88, 95% confidence interval 0.80 to 0.96, I2=63.7%, P=0.001), but not with cancer mortality. Intake of total and animal protein was not significantly associated with risk of cardiovascular disease and cancer mortality. A dose-response analysis showed a significant inverse dose-response association between intake of plant protein and all cause mortality (P=0.05 for non-linearity). An additional 3% energy from plant proteins a day was associated with a 5% lower risk of death from all causes.
Conclusions
Higher intake of total protein was associated with a lower risk of all cause mortality, and intake of plant protein was associated with a lower risk of all cause and cardiovascular disease mortality. Replacement of foods high in animal protein with plant protein sources could be associated with longevity.
Introduction
Cardiovascular disease and cancer are two leading causes of death, contributing to 26.9 million deaths worldwide in 2016.1 Diet has an important role in these conditions. The optimal macronutrient composition of a diet for supporting longevity remains uncertain,2 3 particularly for protein intake. A global transition towards higher protein diets has occurred in recent decades.4 In addition, adherence to a high protein diet has recently become popular because of its possible effects on weight loss, preservation of muscle mass, and increased strength.5 6
High protein diets have also been linked to improvements in cardiometabolic biomarkers, including blood glucose and blood pressure levels. Increasing evidence suggests that diets rich in protein, particularly protein from plants, significantlydecrease serum concentrations of blood lipids, without any significant effect on concentrations of high density lipoprotein cholesterol and the risk of cardiovascular disease.7 These effects may be related to bioactive peptides and the amino acid composition of plant proteins, but other components in the same foods could also contribute. A significant positive association between animal protein intake and an increased incidence of cardiovascular disease and some cancers has also been reported,8 which could be attributed to the content of high sulfur amino acids in animal proteins.
Findings on the association between total protein intake and longevity are still controversial. Total protein intake was associated with a decreased risk of mortality in some investigations,9 10 but others failed to find such evidence.11 12 The same findings have also been reported for animal or plant proteins.11 13 14 Several studies found that consumption of animal proteins was associated with a higher risk of mortality,15 16 17 whereas others reported no significant association between intake of animal or plant proteins and risk of all cause and cause specific mortality.11 13 18 A recent meta-analysis showed that intake of soy protein was associated with a reduced risk of breast cancer mortality, but it was not associated with all cause and cardiovascular disease mortality.19 No information is available for the strength and shape of a dose-response relation between consumption of proteins and risk of mortality. We conducted a systematic review and dose-response meta-analysis of prospective cohort studies to summarise the association between intake of dietary protein and risk of mortality from all causes, cardiovascular disease, and cancer.
Methods
Findings from this systematic review and meta-analysis were reported based on the preferred reporting items for systematic review and meta-analysis (PRISMA) guideline.20
Search strategy
We conducted a systematic search of all articles published up to 31 December 2019 of online databases, including PubMed/Medline, ISI Web of Science, and Scopus, with no limitation on language or time of publication. Supplementary table 1 provides details of the search terms. To avoid missing any publication, we also checked the reference lists of extracted papers and recent reviews. Unpublished studies were not included because they could have been of lower methodological quality than published studies owing to the absence of peer review.21 Duplicate citations were removed.
Inclusion and exclusion criteria
Published studies were included if they were observational prospective studies conducted on human adults, or studies that reported effect sizes including hazard ratios or relative risks or odds ratios with the corresponding 95% confidence intervals for the association between intake of total protein, animal protein, or plant protein as the exposure of interest and mortality from all causes, cardiovascular disease, total or specific cancers as the outcome of interest. All outcomes were classified based on the World Health Organization’s ICD-10 (international classification of diseases, 10th revision).22 If the same dataset had been published in more than one publication, we included the one with more complete findings or the greatest number of participants.
We excluded letters, comments, reviews, meta-analyses, and ecological studies. We also excluded studies performed on children or adolescents and on patients with chronic kidney disease or who were undergoing haemodialysis, end stage cancer, or critical illness. In addition, studies that considered urine urea nitrogen, as a surrogate index of protein intake, and those that considered individual dietary sources of protein as the exposure, rather than total protein, were excluded. If a study reported the effect sizes for risk of disease and mortality combined, we did not include it in the analysis. Moreover, studies with insufficient data were excluded, as were studies on protein intake from specific sources such as soy or legumes.
Data extraction
Two researchers (SN and OS) conducted data extraction independently and resolved any disagreements in consultation with the principal investigator (AE). From each eligible article we extracted the name of the first author, publication year, study design, location of study, age range and health status at study entry, sex, cohort size, incidence of death, duration of follow-up, exposure, method used for assessment of exposure, comparison categories, and relevant effect sizes of comparison categories together with 95% confidence intervals and confounding variables adjusted for in the statistical analysis. When the data were reported for men and women separately, we considered each part as a distinct study. If an included study reported several risk estimates, we extracted the fully adjusted effect sizes. Numerical estimates were extracted from graphs using Plot Digitizer (http://plotdigitizer.sourceforge.net/).
Risk of bias assessment
Risk of bias was assessed using the non-randomised studies of exposures (ROBINS-E) tool.23 This tool comprises seven domains—bias due to confounding, departure from intended exposures, and missing data, and bias in the selection of participants, classification of exposures, measurement of outcomes, and selection of reported results. Studies were categorised as low risk, moderate risk, serious risk, and critical risk of bias under each domain. Supplementary table 2 presents the results of the risk of bias assessment.
Statistical methods
Odds ratios, relative risks, and hazard ratios (along with 95% confidence intervals) for comparison of the highest versus lowest categories of total, animal, and plant protein intake were used to calculate log odds ratios, relative risks, and hazard ratios with standard errors. A random effects model was used for analyses, in which we calculated both the Q statistic and I2 as indicators of heterogeneity.24 25 26 27 I2 values greater than 50% were considered as significant heterogeneity between studies.21 A random effects model can account for variation between studies, and thus it can provide more conservative results than a fixed effects model.28 29
For studies that reported effect sizes separately for intake of animal and plant protein, we first combined the estimates by using the fixed effects model to obtain an overall estimate and then included the pooled effect size in the meta-analysis. Studies that investigated only cancer or cardiovascular disease mortality in relation to protein intake were also considered in the meta-analysis of all cause mortality. If an estimate was reported for the lowest category of protein intake compared with the highest category, we computed the highest versus lowest estimates using the Orsini method.30 When significant heterogeneity between studies was found, we performed a subgroup analysis to examine possible sources of heterogeneity. These analyses were based on study location, duration of follow-up, sex, dietary assessment tools, health status of study participants, high versus low/middle income countries, single/repeated measurements of protein intake, effect size type, and statistical controlling for confounders (body mass index (BMI), total energy intake, and macronutrients (fat and carbohydrate)). Heterogeneity between subgroups was examined with a fixed effects model.
Publication bias was examined by visual inspection of funnel plots. Formal statistical assessment of funnel plot asymmetry was also done with Egger’s regression asymmetry test and Begg’s test. A trim and fill method was used to detect the effect of probable missing studies on the overall effect. We also conducted a sensitivity analysis using a fixed effects model, in which each prospective cohort study was excluded in turn to examine the influence of that study on the overall estimate.
A method suggested by Greenland31 and Orsini30 was used to compute the trend from the odds ratios, relative risks, or hazard ratios estimates and their respective 95% confidence intervals across categories of protein intake. In this method, the distribution of cases and the odds ratios, relative risks, or hazard ratios with the variance estimates for three or more quantitative categories of exposure were required. We considered the midpoint of dietary protein intake in each category. For studies that reported the protein intake as a range, we estimated the midpoint in each category by calculating the mean of the lower and upper bound. When the highest and lowest categories were open ended, we assumed the length of these open ended intervals to be the same as those of the adjacent intervals.
A two stage, random effects dose-response meta-analysis was applied to examine a possible non-linear association between protein intake and mortality. This meta-analysis was done through modelling of protein intake and restricted cubic splines with three knots at fixed centiles of 10%, 50%, and 90% of the distribution. Based on the Orsini method,30 we calculated restricted cubic spline models by a generalised least squares trend estimation method, which takes into account the correlation within each set of reported odds ratios, relative risks, or hazard ratios. The study specific estimates were then combined by the restricted maximum likelihood method in a multivariate random effects meta-analysis.32 A probability value for non-linearity was estimated by null hypothesis testing, in which the coefficient of the second spline was considered equal to zero. A linear dose-response association between an additional 3% of energy from proteins and mortality was investigated by use of the two stage generalised least squares trend estimation method. Study specific slope lines were first estimated and then these lines were combined to obtain an overall average slope.30 Study specific slope lines were combined by a random effects model. Statistical analyses were conducted using STATA version 14.0. A P value of less than 0.05 was considered significant for all tests, including Cochran’s Q test.
Patient and public involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for design, or implementation of the study. No patients were asked to advise on interpretation or writing up of results. There are no plans to disseminate the results of the research to study participants or the relevant patient community.
Results
Literature search
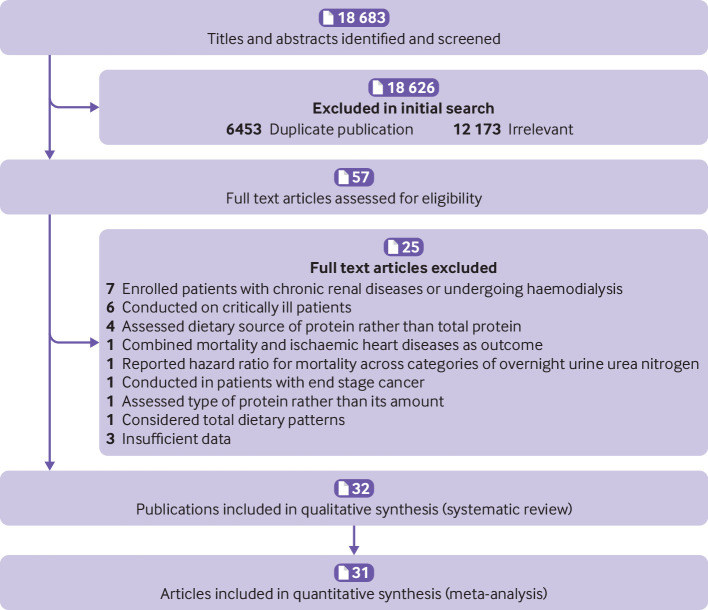
Overall, 18 683 articles were identified in the initial search. After exclusion of duplicate papers and those that did not meet the inclusion criteria, 57 full text articles of potentially relevant studies were identified. After full text review, an additional 25 articles were excluded: seven that enrolled patients with chronic renal diseases or who were undergoing haemodialysis, six that were conducted in the intensive care unit or on critically ill patients, one that was conducted on patients with end stage cancer, and four that reported associations with dietary sources of protein, rather than intake of total protein.34 35 36 37 One article that combined mortality and ischaemic heart disease as the outcome was also excluded.38 Another paper that had considered urine urea nitrogen as a surrogate index of protein intake and reported the hazard ratio for mortality across categories of overnight urine urea nitrogen was excluded.39 One article that had considered total dietary patterns40 and three with insufficient data41 42 43 were also excluded. In one study, the type of protein intake was assessed rather than amount in relation to mortality and was therefore excluded.44
Finally, 32 papers of cohort studies were included in the systematic review,7 9 10 11 12 13 14 15 16 17 18 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 and 31 papers were included in this meta-analysis.7 9 10 11 12 13 14 15 16 17 18 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 63 64 65 Twenty two papers reported effect sizes for all cause mortality,7 9 10 11 12 13 14 15 16 17 18 46 47 49 50 51 52 54 55 59 63 65 17 for cardiovascular disease mortality,9 10 11 13 14 15 16 17 18 47 49 50 51 53 57 58 64 and 14 for cancer mortality.7 9 13 14 15 16 17 18 45 46 48 56 60 61 Of these publications, 26 had reported effect sizes for intake of total protein,7 9 10 12 14 16 17 18 45 46 47 48 49 50 51 52 53 54 55 56 59 60 61 63 64 65 16 for intake of animal protein,7 9 10 12 13 14 15 16 18 45 49 56 58 61 62 64 and 18 for intake of plant protein.7 9 10 11 12 13 14 15 16 18 45 49 56 57 58 61 62 64 Figure 1 shows a flow diagram of study selection.
Fig 1.
Flow diagram of study selection
Of 32 included publications in the systematic review, some studies were done on the same populations. The study by Song et al15 was conducted on the Nurses’ Health Study and the Health Professionals Follow-up Study datasets, and the study by Preis et al64 was conducted on the Health Professionals Follow-up Study dataset. In the current study, the study by Song et al15 was included in the main analysis because it was more complete than the study by Preis et al, but it lacked the required data for the dose-response analysis between intake of total protein and mortality. The study by Preis et al64 had reported such information, however, so was included in the dose-response analysis. The study by Song et al15 was considered in the calculation of total number of participants and cases of mortality.
In addition, two papers (Papanikolaou et al 201913 and Levine et al 201417) were published based on the dataset of the Third National Health and Nutrition Examination Survey (NHANES III). The study by Papanikolaou et al13 was included in the main analysis owing to its comprehensiveness; however, because of lack of required data for the dose-response analysis in that study,13 we also used the study by Levine et al.17
Three additional studies ((Holmes et al 1999 and 2017,55 56 Song et al 20187) were also published, based on the Nurses’ Health Study or Health Professionals Follow-up Study datasets. All three studies were on patients with cancer, who were excluded from the other studies published from these datasets. Therefore these three studies were included. The two studies by Holmes et al55 56 were performed on patients with breast cancer in the Nurses’ Health Study dataset; one had reported the effect size for cancer mortality and the other the effect size for all cause mortality. Therefore both were included. To calculate total number of participants and cases of mortality, one of the duplicate publications (Holmes et al 2017,56 Song et al 2016,15 Papanikolaou et al 201913) was considered.
Characteristics of included studies
Tables 1-3 show the characteristics of the included prospective cohort studies. The number of participants in these studies ranged from 288 to 135 335, with an age range between 19 and 101 years. In total 715 128 participants were included in the 32 publications considered in this systematic review. During the follow-up periods ranging from 3.5 to 32 years, the total number of deaths from all causes was 113 039, from cardiovascular disease was 16 429, and from cancer was 22 303. The sample size from the most comprehensive report was considered when it was published more than once.13 15 56 Three articles included only men,12 61 64 and seven publications included only women.11 14 48 55 56 60 63 Of the remaining studies, three papers had reported hazard ratios for men and women separately.9 52 58 In total, 14 publications described studies in the United States,7 11 13 14 15 17 52 53 55 56 61 62 63 64 17 in non-US countries,9 10 12 16 18 45 46 47 48 49 50 54 57 58 59 60 65 and 1 the populations from 18 different countries.51
Table 1.
Characteristics of included studies for association between protein intake and all cause mortality in adults aged 19 or older
| Author, country | Age* | Sample size | Follow-up (years)† | No of cases | Exposure | Exposure assessment | Health status‡ | Comparison for protein intake | Effect size (95% CI)§ | Adjustment¶ |
|---|---|---|---|---|---|---|---|---|---|---|
| Virtanen 2019, Finland | 42-60 | M 2641 | 22.3 | 1225 | Total protein | Food record | Healthy | >109.1 v <78.5 g/day | HR 1.17 (0.99 to 1.39) | 1, 6, 7, 9, 13, 14, 15, 18, 20, 21, 22, 23, 49, 56, 62 |
| Animal protein | >82.1 v <49.2 g/day | HR 1.13 (0.95 to 1.35) | ||||||||
| Plant protein | >32.2 v <19.6 g/day | HR 0.98 (0.76 to 1.26) | ||||||||
| Hernández-Alonso 2016, Spain | 55-80 | M 3071, W 4145 |
4.8 | 323 | Total protein | FFQ | Unhealthy | ≥18.54 v 15.9-17 %kcal | HR 1.66 (1.13 to 2.43) | 1, 2, 9, 13, 14, 15, 18, 19, 22, 42, 46, 49, 56 |
| Animal protein | ≥13 v 10.4-11.5 %kcal | HR 1.92 (1.31 to 2.82) | ||||||||
| Plant protein | ≥6.26 v 5.27-5.7 %kcal | HR 1.32 (0.88 to 2.00) | ||||||||
| Song 2016, US | 49 | M 46 329, W 85 013 |
26-32 | 36 115 | Animal protein | FFQ | Healthy | >18 v ≤10 %kcal | HR 1.03 (0.98 to 1.08) | 1, 9, 13, 15, 18, 31, 32, 38, 39, 40, 41, 48 |
| Plant protein | >6 v ≤3 %kcal | HR 0.89 (0.84 to 0.96) | ||||||||
| Zaslavsky 2017, US | 65-84 | W 10 034 | 12.4 | 3259 | Total protein | FFQ | Healthy | >75 v <60 g/kg/day | HR 0.74 (0.54 to 1.01) | 1, 6, 9, 13, 14, 15, 63 |
| Dehghan 2017, 18 countries** | 35-70 | M 56 422, W 78 913 |
7.4 | 5796 | Total protein | FFQ | Healthy | ≥18.3 v ≤11.95 %kcal | HR 0.88 (0.77 to 1.00) | 1, 5, 11, 13, 14, 15, 63 |
| Courand 2016, France | 45.1 | M 653, F 475 |
10 | 289 | Total protein | UUN | Unhealthy | >0.93 v <0.70 g/kg/day | HR 0.75 (0.56 to 0.99) | 1, 2, 15, 25, 52, 54, 55, 58 |
| Argos 2013, Bangladesh | 36.9 | M 6535, W 10 709 |
9 | 818 | Total protein | FFQ | Healthy | ≥12.7 v ≤11.4 %kcal | HR 1.07 (0.85 to 1.35) | 1, 2, 9, 12, 14, 15, 19, 42, 44, 63 |
| Kelemen 2005, US | 55-69 | W 29 017 | 15 | 3987 | Total protein | FFQ | Healthy | ≥20.7 v ≤15.2 %kcal | RR 0.99 (0.71 to 1.38) | 1, 9, 13, 14, 15, 20, 21, 22, 23, 48, 51, 56, 63 |
| Animal protein | ≥16.1 v ≤10.1 %kcal | RR 0.82 (0.59 to 1.13) | ||||||||
| Plant protein | ≥5.7 v ≤4 %kcal | RR 0.95 (0.82 to 1.10) | ||||||||
| Payette 1999, Canada | 60-94 | M 81, W 207 |
3.5 | 102 | Total protein | Food recall | Healthy | ≥0.8 v <0.8 g/kg/day | RR 1.20 (0.86 to 1.68) | Unadjusted |
| Papanikolaou 2019, US | 19-99 | Both 17 199 | 18 | 4280 | Animal protein | Food recall | Healthy | NR | HR 1.01 (0.99 to 1.02) | Unclear |
| Plant protein | HR 0.99 (0.97 to 1.01) | |||||||||
| Sun 2019, US | 50-79 | W 127 495 | 24 | 35 043 | Plant protein | FFQ | Healthy | NR | HR 0.91 (0.86 to 0.95) | Unclear |
| Levine 2014, US | ≥50 | M 2846, W 3535 |
18 | 2578 | Total protein | Food recall | Healthy | ≥20 v ≤10 %kcal | HR 0.93 (0.74 to 1.19) | 1, 2, 3, 4, 15, 56, 14, 63 |
| Budhathoki 2019, Japan | 40-69 | M 32 201, W 38 495 |
18 | 12 381 | Total protein | FFQ | Healthy | ≥17 v ≤11.82 %kcal | HR 0.99 (0.90 to 1.09) | 1, 6, 9, 13, 14, 15, 16, 17, 18, 20, 21, 22, 23, 31, 32 |
| Animal protein | ≥10.65 v ≤4.95 %kcal | HR 0.98 (0.88 to 1.08) | ||||||||
| Plant protein | ≥8.1 v ≤5.32 %kcal | HR 0.87 (0.78 to 0.96) | ||||||||
| Halbesma 2009, Netherlands | 20-75 | Both 8461 | 6.4 | 443 | Total protein | UUN | Healthy | ≥1.4 v 1.11-1.25 g/kg/day | HR 1.03 (0.73 to 1.43) | 1, 2, 59 |
| Holmes 1999, US | 54 | W 1982 | 13 | 378 | Total protein | FFQ | Unhealthy | ≥81.5 v ≤60.9 g/day | RR 0.65 (0.47 to 0.88) | 1, 8, 9, 14, 50, 51, 35, 48, 61 |
| Mendonça 2019, UK | ≥85 | M 289, W 428 |
5 | 457 | Total protein | Food recall | Healthy | ≥0.8 v ˂0.8 g/kg/day | HR 0.91 (0.73 to 1.14) | 1, 2, 14, 56, 63 |
| Chan 2019, China | ≥65 | M 1480 | 13.8 | 594 | Total protein | FFQ | Healthy | ≥20 v ≤12.85 %kcal | HR 0.77 (0.58 to 1.01) | 1, 7, 9, 14, 15, 18, 37, 39, 40, 41, 56 |
| Animal protein | ≥12.8 v ≤5.85 %kcal | HR 0.79 (0.60 to 1.03) | ||||||||
| Plant protein | ≥9.3 v ≤5.1 %kcal | HR 1.08 (0.82 to 1.43) | ||||||||
| Chan 2019, China | ≥65 | W 1540 | 13.8 | 369 | Total protein | FFQ | Healthy | ≥19.8 v ≤12.85 %kcal | HR 0.67 (0.46 to 0.96) | 1, 7, 9, 14, 15, 18, 37, 39, 40, 41, 56 |
| Animal protein | ≥11.9 v ≤5.3 %kcal | HR 0.84 (0.58 to 1.20) | ||||||||
| Plant protein | ≥9.9 v ≤5.8 %kcal | HR 0.65 (0.45 to 0.92) | ||||||||
| Dwyer 1994, US | 65-74 | W 1341 | 14.5 | 48 | Total protein | Food recall | Healthy | Per 15 g increase | RR 1.04 (0.96 to 1.11) | 1 |
| Dwyer 1994, US | 65-74 | M 1231 | 14.5 | 71 | Total protein | Food recall | Healthy | Per 15 g increase | RR 1.00 (0.95 to 1.06) | 1 |
| Song 2018, US | 30-75 | M 623, W 919 |
9 | 817 | Total protein | FFQ | Unhealthy | Per 3.2 % increase | HR 1.10 (1.03 to 1.70) | 1, 2, 9, 13, 14, 15, 18, 31, 32, 41, 49, 60 |
| Animal protein | Per 3.4 % increase | HR 1.12 (1.04 to 1.20) | ||||||||
| Plant protein | Per 1.4 % increase | HR 0.94 (0.85 to 1.03) | ||||||||
| Bates 2010, UK | ≥65 | M 548, F 552 | 13 | 749 | Total protein | Food record | M per 17 g increase, F per 14 g increase | HR 0.86 (0.77 to 0.97) | 1, 2 | |
| Campmans-Kuijpers 2015, European countries | 57.5 | M 2255, W 1827 |
9.4 | 787 | Total protein | FFQ | Unhealthy | Per 5% increase | HR 1.00 (0.88 to 1.10) | 1, 9, 14, 15, 18, 30, 41, 45, 49, 63 |
| Animal protein | Per 5% increase | HR 1.01 (0.90 to 1.10) | ||||||||
| Plant protein | Per 5% increase | HR 0.55 (0.32 to 0.90) | ||||||||
| Tharrey 2018, US-Canada | ≥25 | Both 81 377 | 9.4 | 2276 | Animal protein | FFQ | Healthy | Per 18 g increase | HR 1.12 (1.05 to 1.19) | 1, 2, 3, 6, 7, 9, 13, 15, 18, 20, 22, 25, 33, 36, 47, 63 |
| Plant protein | Per 18 g increase | HR 0.95 (0.89 to 1.02) |
1 kcal=4.18 kJ=0.00418 MJ.
FFQ=food frequency questionnaire; HR=hazard ratio; M=men; NR=not reported; OR=odds ratio; RR=risk ratio, UUN=urine urea nitrogen; W=women.
Presented as mean or range.
Number of years that individuals were followed up in the prospective cohort studies.
People with comorbidities were considered as unhealthy.
These effect sizes are for comparison of the highest and the lowest categories.
Adjustments: age (1), sex (2), race (3), waist circumference (4), urban or rural location (5), occupation status (6), marital status (7), menopausal status (8), body mass index (9), weight change (10), waist to hip ratio (11), height (12), physical activity (13), total energy (14), smoking (15), intake of green tea (16), coffee (17), alcohol (18), total fat (19), saturated fat (20), monounsaturated fat (21), polyunsaturated fat (22), trans fat (23), cholesterol (24), sodium (25), potassium (26), animal fat (27), vegetable fat (28), methionine (29), total protein (30), animal protein (31), plant protein (32), folate (33), magnesium (34), calcium (35) vitamin A, C, E, B6, and B12 (36), total grains (37), whole grains (38), fruit (39), vegetable (40), fibre (41), carbohydrates (42) amino acids (43), water arsenic concentration (44), healthy diet (45), glycaemic index (46), type of diet in the vegetarian spectrum (47), use of supplements (48), drug treatment (49), oral contraceptive (50), postmenopausal hormone (51), serum lipids (52), glucose intolerance (53), systolic blood pressure (54), estimated glomerular filtration rate (55), chronic diseases at baseline and follow-up (56), number of frailty criteria (57), fasting glucose (58), cardiovascular risk factors (59), cancer stage (60), tumour size (61), duration of diabetes (62), education (63), social class (64).
Canada, Sweden, United Arab Emirates, Argentina, Brazil, Chile, China, Colombia, Iran, Malaysia, occupied Palestinian territory, Poland, South Africa, Turkey, Bangladesh, India, Pakistan, and Zimbabwe.
Table 2.
Characteristics of included studies for the association between protein intake and cardiovascular disease mortality in adults aged over 18
| Author, country | Age* | Sample size | Follow-up (years)† | No of cases | Exposure | Exposure assessment | Health status‡ | Comparison for protein intake | Effect size (95% CI)§ | Adjustment¶ |
|---|---|---|---|---|---|---|---|---|---|---|
| Hernández-Alonso 2016, Spain | 55-80 | M 3071, W 4145 |
4.8 | 81 | Total protein | FFQ | Unhealthy | ≥18.54 v 15.9-17 %kcal | HR 2.10 (0.93 to 4.75) | 1, 2, 9, 13, 14, 15, 18, 19, 22, 42, 46, 49, 56 |
| Animal protein | ≥13 v 10.4-11.5 %kcal | HR 2.98 (1.36 to 6.51) | ||||||||
| Plant protein | ≥6.26 v 5.27-5.7 %kcal | HR 0.78 (0.32 to 1.88) | ||||||||
| Song 2016, US | 49 | M 46 329, W 85 013 |
26-32 | 8851 | Animal protein | FFQ | Healthy | >18 v ≤10 %kcal | HR 1.09 (0.99 to 1.20) | 1, 9, 13, 15, 18, 31, 32, 38, 39, 40, 41, 48 |
| Plant protein | >6 v ≤3 %kcal | HR 0.88 (0.80 to 0.97) | ||||||||
| Dehghan 2017, 18 countries** | 35-70 | M 56 422, W 78 913 |
7.4 | 1649 | Total protein | FFQ | Healthy | ≥18.3 v ≤11.95 %kcal | HR 0.90 (0.71 to 1.15) | 1, 5, 11, 13, 14, 15, 63 |
| Courand 2016, France | 45.1 | M 653, W 475 |
10 | 202 | Total protein | FFQ | Unhealthy | >0.93 v <0.70 g/kg/day | HR 0.76 (0.54 to 1.06) | 1, 2, 15, 25, 52, 54, 58 |
| Kelemen 2005, US | 55-69 | W 29 017 | 15 | 739 | Total protein | FFQ | Healthy | ≥20.7 v ≤15.2 %kcal | RR 0.84 (0.39 to 1.79) | 1, 9, 13, 14, 15, 20, 21, 22, 23, 48, 51, 56, 63 |
| Animal protein | ≥16.1 v ≤10.1 %kcal | RR 0.88 (0.42 to 1.86) | ||||||||
| Plant protein | ≥5.7 v ≤4 %kcal | RR 0.70 (0.49 to 0.99) | ||||||||
| Sauvaget 2004, Japan | 35-89 | M 1436, W 2295 |
14 | 60 | Total protein | Food record | Healthy | ≥79.5 v ≤57.5 g/day | HR 0.42 (0.20 to 0.85) | 1, 2, 9, 15, 18, 56, 60 |
| Animal protein | ≥43.5 v ≤25.5 g/day | HR 0.45 (0.23 to 0.89) | ||||||||
| Plant protein | ≥39.5 v ≤28.5 g/day | HR 1.12 (0.57 to 2.21) | ||||||||
| Nagata 2015, Japan | 35-101 | M 13 355 | 16 | 328 | Total protein | FFQ | Healthy | NR | HR 1.01 (0.73 to 1.40) | 1, 7, 9, 13, 14, 15, 18, 20, 21, 22, 23, 25, 30, 41 |
| Animal protein | HR 1.01 (0.74 to 1.38) | |||||||||
| Plant protein | HR 1.14 (0.72 to1.80) | |||||||||
| Nagata 2015, Japan | 35-101 | W 15 724 | 16 | 349 | Total protein | FFQ | Healthy | NR | HR 1.01 (0.74 to 1.38) | 1, 7, 8, 9, 13, 14, 15, 18, 20, 21, 22, 23, 25, 30, 41 |
| Animal protein | HR 1.26 (0.81 to 1.96) | |||||||||
| Plant protein | HR 0.81 (0.52 to 1.26) | |||||||||
| Papanikolaou 2019, US | 19-99 | Both 17 199 | 18 | - | Animal protein | Food recall | Healthy | NR | HR 1.01 (0.99 to 1.02) | Unclear |
| Plant protein | HR 1.00 (0.97 to 1.02) | |||||||||
| Sun 2019, US | 50-79 | W 127 495 | 24 | - | Total protein | FFQ | Healthy | NR | HR 0.87 (0.79 to 0.95) | Unclear |
| Kurihara 2019, Japan] | ≥30 | M 3224, W 4520 |
13.9 | 354 | Plant protein | Food record | Healthy | ≥7.9 v ≤6.6 %kcal | HR 0.86 (0.75 to 0.99) | 1, 2, 9, 15, 18, 25, 26, 27, 31, 41 |
| Levine 2014, US | ≥50 | M 2846, W 3535 |
18 | 1193 | Total protein | Food recall | Healthy | ≥20 v ≤10 %kcal | HR 0.88 (0.63 to 1.22) | 1, 2, 3, 4, 15, 56, 14, 63 |
| Budhathoki 2019, Japan | 40-69 | M 32 201, W 38 495 |
18 | 3025 | Total protein | FFQ | Healthy | ≥17 v ≤11.82 %kcal | HR 0.97 (0.80 to 1.18) | 1, 6, 9, 13, 14, 15, 16, 17, 18, 20, 21, 22, 23, 31, 32 |
| Animal protein | ≥10.65 v ≤4.95 %kcal | HR 0.97 (0.79 to 1.19) | ||||||||
| Plant protein | ≥8.1 v ≤5.32 %kcal | HR 0.73 (0.59 to 0.91) | ||||||||
| Preis 2010, US | 40-75 | M 43 960 | 18 | 1155 | Total protein | FFQ | Healthy | ≥21.15 v ≤15.65 %kcal | RR 1.05 (0.85 to 1.30) | 1, 9, 13, 14, 15, 18, 20, 21, 22, 23, 33, 34, 41, 46, 48, 56 |
| Animal protein | ≥16.3 v ≤10.4 %kcal | RR 1.10 (0.88 to 1.37) | ||||||||
| Plant protein | ≥6 v ≤4 %kcal | RR 0.66 (0.49 to 0.88) | ||||||||
| Chan 2019, China | ≥65 | M 1480 | 13.8 | 117 | Total protein | FFQ | Healthy | ≥20 v ≤12.85 %kcal | HR 0.75 (0.41 to 1.39) | 1, 7, 9, 14, 15, 18, 37, 39, 40, 41, 56 |
| Animal protein | ≥12.8 v ≤5.85 %kcal | HR 0.75 (0.41 to 1.39) | ||||||||
| Plant protein | ≥9.3 v ≤5.1 %kcal | HR 0.92 (0.47 to 1.77) | ||||||||
| Chan 2019, China | ≥65 | W 1540 | 13.8 | 117 | Total protein | FFQ | Healthy | ≥19.8 v ≤12.85 %kcal | HR 0.78 (0.38 to 1.62) | 1, 7, 9, 14, 15, 18, 37, 39, 40, 41, 56 |
| Animal protein | ≥11.9 v ≤5.3 %kcal | HR 0.93 (0.42 to 2.03) | ||||||||
| Plant protein | ≥9.9 v ≤5.8 %kcal | HR 0.71 (0.35 to 1.45) | ||||||||
| Bates 2010, UK | ≥65 | M 548, W 552 | 13 | 199 | Total protein | Food record | Healthy | M per 17 g increase, F per 14 g increase | HR 0.79 (0.67 to 0.94) | 1, 2 |
| Campans-Kuijpers 2015, European countries | 57.5 | M 2255, W 1827 |
9.4 | 266 | Total protein | FFQ | Unhealthy | Per 5% increase | HR 1.00 (0.82 to 1.23) | 1, 9, 14, 15, 18, 30, 41, 45, 49, 63 |
| Animal protein | Per 5% increase | HR 1.02 (0.83 to 1.25) | ||||||||
| Plant protein | Per 5% increase | HR 0.81 (0.33 to 1.99) | ||||||||
| Esrey 1996, US | 30-59 | M 2071, W 1854 |
12 | 52 | Total protein | Food recall | Healthy | Per 1% increase | RR 1.01 (0.95 to 1.07) | 1, 2, 9, 15, 52, 53, 54 |
| Esrey 1996, US | 60-79 | M 282, W 339 |
12 | 40 | Total protein | Food recall | Healthy | Per 1% increase | RR 1.00 (0.93 to 1.08) | 1, 2, 9, 15, 52, 53, 54 |
1 kcal=4.18 kJ=0.00418 MJ.
FFQ=food frequency questionnaire; HR=hazard ratio; M=men; NR=not reported; OR=odds ratio; RR=risk ratio, UUN=urine urea nitrogen; W=women.
Presented as mean or range.
Number of years that individuals were followed up in the prospective cohort studies.
People with comorbidities were considered as unhealthy.
These effect sizes are for comparison of the highest and the lowest categories.
Adjustments: age (1), sex (2), race (3), waist circumference (4), urban or rural location (5), occupation status (6), marital status (7), menopausal status (8), BMI (9), weight change (10), waist to hip ratio (11), height (12), physical activity (13), total energy (14), smoking (15), intake of green tea (16), coffee (17), alcohol (18), total fat (19), saturated fat (20), monounsaturated fat (21), polyunsaturated fat (22), trans fat (23), cholesterol (24), sodium (25), potassium (26), animal fat (27), vegetable fat (28), methionine (29), total protein (30), animal protein (31), plant protein (32), folate (33), magnesium (34), calcium (35) vitamin A, C, E, B6, and B12 (36), total grains (37), whole grains (38), fruit (39), vegetable (40), fibre (41), carbohydrates (42) amino acids (43), water arsenic concentration (44), healthy diet (45), glycaemic index (46), type of diet in the vegetarian spectrum (47), use of supplements (48), drug treatment (49), oral contraceptive (50), postmenopausal hormone (51), serum lipids (52), glucose intolerance (53), systolic blood pressure (54), estimated glomerular filtration rate (55), chronic diseases at baseline and follow-up (56), number of frailty criteria (57), fasting glucose (58), cardiovascular risk factors (59), cancer stage (60), tumour size (61), duration of diabetes (62), education (63), social class (64).
Canada, Sweden, United Arab Emirates, Argentina, Brazil, Chile, China, Colombia, Iran, Malaysia, occupied Palestinian territory, Poland, South Africa, Turkey, Bangladesh, India, Pakistan, and Zimbabwe.
Table 3.
Characteristics of included studies for the association between protein intake and cancer mortality in adults aged >18 years
| Author, country | Age* | Sample size | Follow-up (years)† | No of cases | Exposure | Exposure assessment | Health status‡ | Comparison for protein intake | Effect size (95% CI)§ | Adjustment¶ |
|---|---|---|---|---|---|---|---|---|---|---|
| Hernández-Alonso 2015, Spain | 55-80 | M 3071, W 4145 |
4.8 | 130 | Total protein | FFQ | Unhealthy | ≥18.54 v 15.9-17 %kcal | HR 1.44 (0.80 to 2.59) | 1, 2, 9, 13, 14, 15, 18, 19, 22, 42, 46, 49, 56 |
| Animal protein | ≥13 v 10.4-11.5 %kcal | HR 1.81 (1.00 to 3.31) | ||||||||
| Plant protein | ≥6.26 v 5.27-5.7 %kcal | HR 1.39 (0.70 to 2.75) | ||||||||
| Song 2016, US | 49 | M 46 329, W 85 013 |
26-32 | 13 159 | Animal protein | FFQ | Healthy | >18 v ≤10 %kcal | HR 1.02 (0.94 to 1.11) | 1, 9, 13, 15, 18, 31, 32, 38, 39, 40, 41, 48 |
| Plant protein | >6 v ≤3 %kcal | HR 0.92 (0.82 to 1.03) | ||||||||
| Argos 2013, Bangladesh | 36.9 | M 6535, W 10 709 |
9 | 135 | Total protein | FFQ | Healthy | ≥12.7 v ≤11.4 %kcal | HR 1.79 (0.99 to 3.25) | 1, 2, 9, 12, 14, 15, 19, 42, 44, 63 |
| Kelemen 2005, US | 55-69 | W 29 017 | 15 | 1676 | Total protein | FFQ | Healthy | ≥20.7 v ≤15.2 %kcal | RR 1.07 (0.64 to 1.79) | 1, 9, 13, 14, 15, 20, 21, 22, 23, 48, 51, 56, 63 |
| Animal protein | ≥16.1 v ≤10.1 %kcal | RR 0.77 (0.47 to 1.27) | ||||||||
| Plant protein | ≥5.7 v ≤4 %kcal | RR 1.04 (0.83 to 1.32) | ||||||||
| Smit 2007, US | 35-79 | M 9777 | 12 | 167 | Total protein | Food recall | Healthy | ≥104 v ≤61 g/day | OR 1.32 (0.81 to 2.17) | 1, 5, 9, 13, 14, 15, 63 |
| Animal protein | ≥41 v ≤13 g/day | OR 1.01 (0.52 to 1.96) | ||||||||
| Plant protein | ≥32 v ≤16 g/day | OR 1.19 (0.66 to 2.13) | ||||||||
| Papanikolaou 2019, US | 19-99 | Both 17 199 | 18 | - | Animal protein | Food recall | Healthy | NR | HR 1.00 (0.98 to 1.03) | Unclear |
| Plant protein | HR 1.01 (0.97 to 1.05) | |||||||||
| Levine 2014, US | ≥50 | M 2846, W 3535 |
18 | 630 | Total protein | Food recall | Healthy | ≥20 v ≤10 %kcal | HR 0.89 (0.56 to 1.44) | 1, 2, 3, 4, 15, 56, 14, 63 |
| Budhathoki 2019, Japan | 40-69 | M 32 201, W 38 495 |
18 | 5055 | Total protein | FFQ | Healthy | ≥17 v ≤11.82 %kcal | HR 1.00 (0.86 to 1.16) | 1, 6, 9, 13, 14, 15, 16, 17, 18, 20, 21, 22, 23, 31, 32 |
| Animal protein | ≥10.65 v ≤4.95 %kcal | HR 0.97 (0.83 to 1.14) | ||||||||
| Plant protein | ≥8.1 v ≤5.32 %kcal | HR 1.04 (0.88 to 1.23) | ||||||||
| Holmes 2017, US | 30-55 | W 6348 | 29 | 919 | Total protein | FFQ | Unhealthy | ≥84.6 v ≤66.7 g/day | RR 0.95 (0.77 to 1.17) | 1, 8, 9, 13, 14, 15, 10, 18, 49, 50, 51, 59, 60 |
| Animal protein | ≥64.6 v ≤46.8 g/day | RR 0.85 (0.68 to 1.05) | ||||||||
| Plant protein | ≥23.3 v ≤16 g/day | RR 1.09 (0.87 to 1.37) | ||||||||
| Borugian 2004, Canada | 19-75 | W 603 | 10 | 112 | Total protein | FFQ | Unhealthy | ≥83 v ≤52 g/day | RR 0.40 (0.20 to 0.80) | 1, 14, 59 |
| Rohan 1993, Australia | 20-74 | W 412 | 5.5 | 112 | Total protein | FFQ | Unhealthy | ≥103 v ≤59 g/day | HR 0.74 (0.34 to 1.66) | 1, 9, 14 |
| Chan 2019, China | ≥65< | M 1480 | 13.8 | 216 | Total protein | FFQ | Healthy | ≥20 v ≤12.85 %kcal | HR 0.66 (0.41 to 1.07) | 1, 7, 9, 14, 15, 18, 37, 39, 40, 41, 56 |
| Animal protein | ≥12.8 v ≤5.85 %kcal | HR 0.70 (0.44 to 1.12) | ||||||||
| Plant protein | ≥9.3 v ≤5.1 %kcal | HR 1.27 (0.80 to 2.03) | ||||||||
| Chan 2019, China | ≥65 | W 1540 | 13.8 | 120 | Total protein | FFQ | Healthy | ≥19.8 v ≤12.85 %kcal | HR 0.59 (0.31 to 1.13) | 1, 7, 9, 14, 15, 18, 37, 39, 40, 41, 56 |
| Animal protein | ≥11.9 v ≤5.3 %kcal | HR 0.76 (0.41 to 1.42) | ||||||||
| Plant protein | ≥9.9 v ≤5.8 %kcal | HR 0.57 (0.31 to 1.02) | ||||||||
| Song 2018, US | 30-75 | M 623, W 919 |
9 | 185 | Total protein | FFQ | Unhealthy | Per 3.2% increase | HR 1.07 (0.94 to 1.23) | 1, 2, 9, 13, 14, 15, 18, 31, 32, 41, 49, 60 |
| Animal protein | Per 3.4% increase | HR 1.13 (0.98 to 1.30) | ||||||||
| Plant protein | Per 1.4% increase | HR 0.77 (0.62 to 0.95) | ||||||||
| Palli 2000, Italy | NR | M 239 W 143 |
11 | 317 | Total protein | FFQ | Unhealthy | Unclear (T3 v T1) | HR 1.00 (0.75 to 1.32) | 1, 2, 14, 64 |
| Animal protein | Unclear (T3 v T1) | HR 1.09 (0.83 to 1.44) | ||||||||
| Plant protein | Unclear (T3 v T1) | HR 0.82 (0.62 to 1.09) |
1 kcal=4.18 kJ=0.00418 MJ.
FFQ=food frequency questionnaire; HR=hazard ratio; M=male; NR=not reported; OR=odds ratio; RR=risk ratio, UUN=urine urea nitrogen; W=women.
Presented as mean or range.
Number of years that individuals were followed up in the prospective cohort studies.
People with comorbidities were considered as unhealthy.
These effect sizes are for comparison of the highest and the lowest categories.
Adjustments: age (1), sex (2), race (3), waist circumference (4), urban or rural location (5), occupation status (6), marital status (7), menopausal status (8), BMI (9), weight change (10), waist to hip ratio (11), height (12), physical activity (13), total energy (14), smoking (15), intake of green tea (16), coffee (17), alcohol (18), total fat (19), saturated fat (20), monounsaturated fat (21), polyunsaturated fat (22), trans fat (23), cholesterol (24), sodium (25), potassium (26), animal fat (27), vegetable fat (28), methionine (29), total protein (30), animal protein (31), plant protein (32), folate (33), magnesium (34), calcium (35) vitamin A, C, E, B6, and B12 (36), total grains (37), whole grains (38), fruit (39), vegetable (40), fibre (41), carbohydrates (42) amino acids (43), water arsenic concentration (44), healthy diet (45), glycaemic index (46), type of diet in the vegetarian spectrum (47), use of supplements (48), drug treatment (49), oral contraceptive (50), postmenopausal hormone (51), serum lipids (52), glucose intolerance (53), systolic blood pressure (54), estimated glomerular filtration rate (55), chronic diseases at baseline and follow-up (56), number of frailty criteria (57), fasting glucose (58), cardiovascular risk factors (59), cancer stage (60), tumour size (61), duration of diabetes (62), education (63), social class (64).
Canada, Sweden, United Arab Emirates, Argentina, Brazil, Chile, China, Colombia, Iran, Malaysia, occupied Palestinian territory, Poland, South Africa, Turkey, Bangladesh, India, Pakistan, and Zimbabwe.
To examine protein intake, 11 publications had used dietary records or recalls10 12 13 17 47 52 53 57 59 61 65 and 19 had used a food frequency questionnaire.7 9 11 14 15 16 18 45 46 48 49 51 55 56 58 60 62 63 64 In the studies by Halbesma et al54 and Courand et al,50 intake of total protein was estimated with the use of overnight urine urea nitrogen. In total, 31 publications used baseline data of protein intake in their analysis (single measurement), whereas one article considered the average protein intake throughout the follow-up (repeated measurements) as the main exposure.15 All studies except for one60 adjusted the associations for age.
Most cohorts controlled for some conventional risk factors, including BMI (n=24), smoking (n=22), and alcohol consumption (n=14). Others also adjusted for physical activity (n=14), energy intake (n=25), other dietary variables (n=14), and macronutrients (fat or carbohydrate; n=12). Based on the ROBINS-E tool, 15 articles had a low risk of bias in all components (supplementary table 2).7 11 12 13 14 15 16 17 18 46 51 55 62 63 64 Nine papers provided effect sizes for mortality from cardiovascular disease and cancer, without reporting any effect size for all cause mortality.10 45 48 53 56 57 58 60 61 The reported effect sizes in these studies were combined and the overall effect size was considered in the meta-analysis of all cause mortality.
Systematic review
Of 29 articles on the association between intake of total protein and all cause mortality, six reported an inverse association,9 10 47 48 54 55 one showed a positive association,7 and the others reported no significant association.12-18 45 46 49-53 56 58-61 63-65 For the association between intake of animal protein and all cause mortality, two studies showed an inverse association10 16 and the others indicated no significant association.7 9 12-15 18 45 49 58 61 62 64 Moreover, seven publications showed an inverse association between intake of plant protein and all cause mortality.9 11 16 18 49 57 64 For cardiovascular disease mortality, two studies reported a protective association with intake of total protein,10 47 one study with animal protein,10 and six articles with plant protein.11 14 15 18 57 64 One study indicated an inverse association between intake of total protein and cancer mortality.48 One study also showed an inverse association between intake of plant protein and cancer mortality.7
Meta-analysis on protein intake and all cause mortality
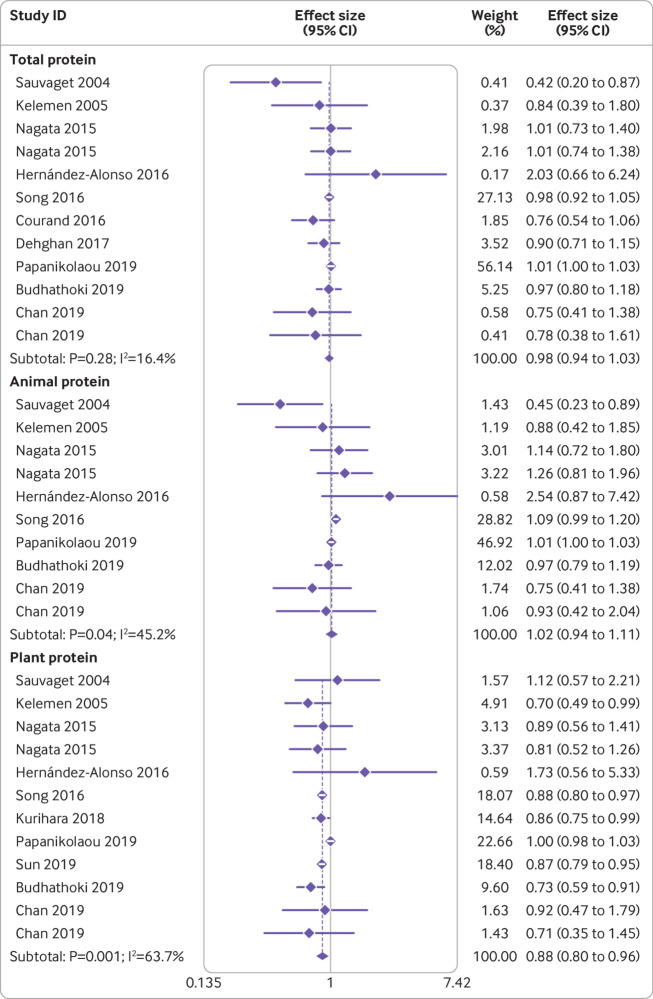
Of 29 papers on intake of total protein and all cause mortality, 21 presented sufficient data for comparison of the highest versus lowest categories of total protein intake.9 10 12 13 14 15 16 18 45 46 48 50 51 54 55 58 59 60 61 63 65 Of 480 304 participants included in these articles, 72 261 died. The summary effect size for all cause mortality comparing the highest and lowest intakes of total protein was 0.94 (95% confidence interval 0.89 to 0.99, P=0.02), indicating a significant inverse association between total protein intake and all cause mortality (fig 2). Significant heterogeneity was seen between studies (I2=58.4%, P<0.001).
Fig 2.
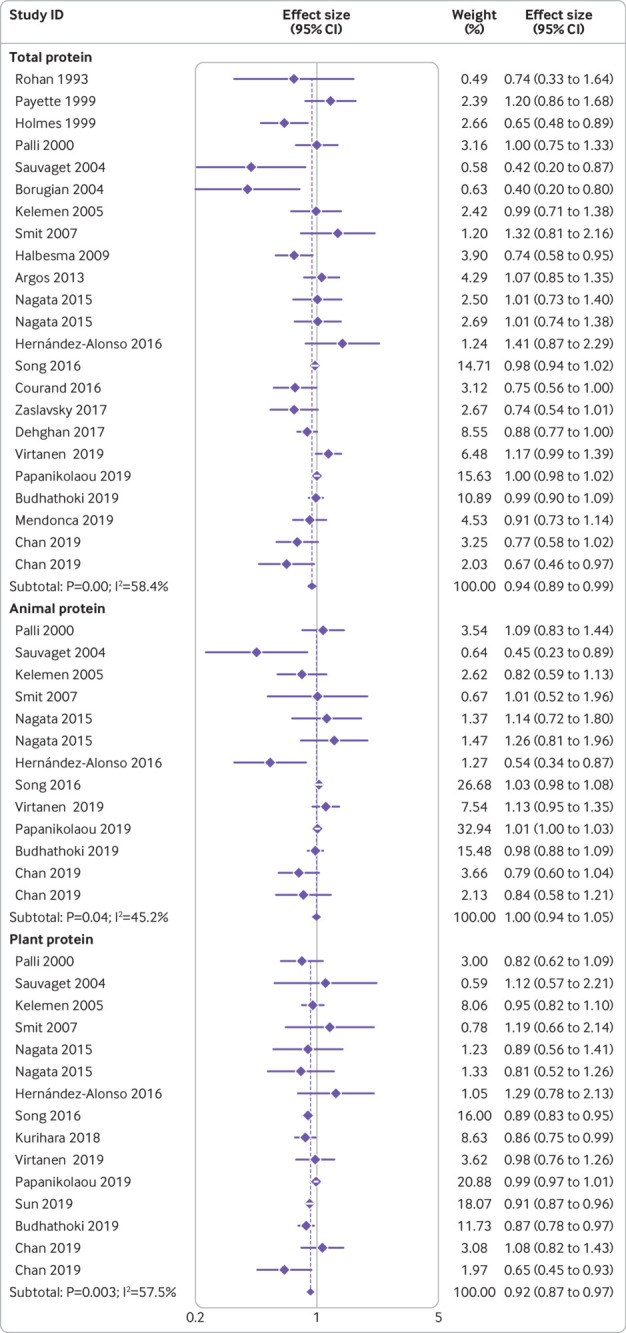
Forest plot for association between protein intake and risk of all cause mortality in adults aged 19 or older, expressed as comparison between highest and lowest categories of protein intake. Diamonds represent pooled estimates from random effects analysis
When the association between consumption of animal protein and all cause mortality was examined in 11 publications,10 11 13 14 15 16 17 19 56 58 61 including a total of 304 100 participants and 60 495 deaths, no significant association was found (pooled effect size comparing highest and lowest intakes was 1.00, 95% confidence interval 0.94 to 1.05, P=0.86), with moderate heterogeneity among the studies (I2=45.2%, P=0.04; fig 2). Consumption of plant protein, however, which was examined in 13 articles10 11 12 13 14 15 16 17 19 56 57 58 61 with a total of 439 339 participants and 95 892 deaths, was inversely associated with all cause mortality (pooled effect size comparing the highest and lowest intakes was 0.92, 0.87 to 0.97, P=0.002), with significant heterogeneity among the studies (I2=57.5%, P=0.003; fig 2).
Meta-analysis on protein intake and cardiovascular disease mortality
Ten publications9 10 13 14 15 16 18 50 51 58 examined the association between intake of total protein and risk of cardiovascular disease mortality. These studies included a total of 427 005 participants and 15 518 deaths. The summary effect size for cardiovascular disease mortality, comparing the highest and lowest protein intakes, was 0.98 (95% confidence interval 0.94 to 1.03, P=0.51), indicating no clear significant association between total protein intake and cardiovascular disease mortality (fig 3). No significant heterogeneity was seen among the studies (I2=16.4%, P=0.28).
Fig 3.
Forest plot for association between protein intake and risk of cardiovascular disease mortality in adults aged 19 or older, expressed as comparison between highest and lowest categories of protein intake. Diamonds represent pooled estimates from random effects analysis
The association between consumption of animal protein and cardiovascular disease mortality was examined in eight papers,9 10 13 14 15 16 18 58 which included 290 542 participants and 13 667 deaths. No significant association was found (pooled effect size comparing the highest and lowest intakes was 1.02, 95% confidence interval 0.94 to 1.11, P=0.56), with no significant heterogeneity among the studies (I2=31.7%, P=0.16; fig 3). For plant protein consumption, however, which was examined in 10 articles9 10 11 13 14 15 16 18 57 58 with a total of 425 781 participants and 14 021 deaths, an inverse association was found with cardiovascular disease (pooled effect size comparing the highest and lowest intakes was 0.88, 0.80 to 0.96, P=0.003; fig 3). No significant heterogeneity was found between studies (I2=63.7%, P=0.001).
Meta-analysis on protein intake and cancer mortality
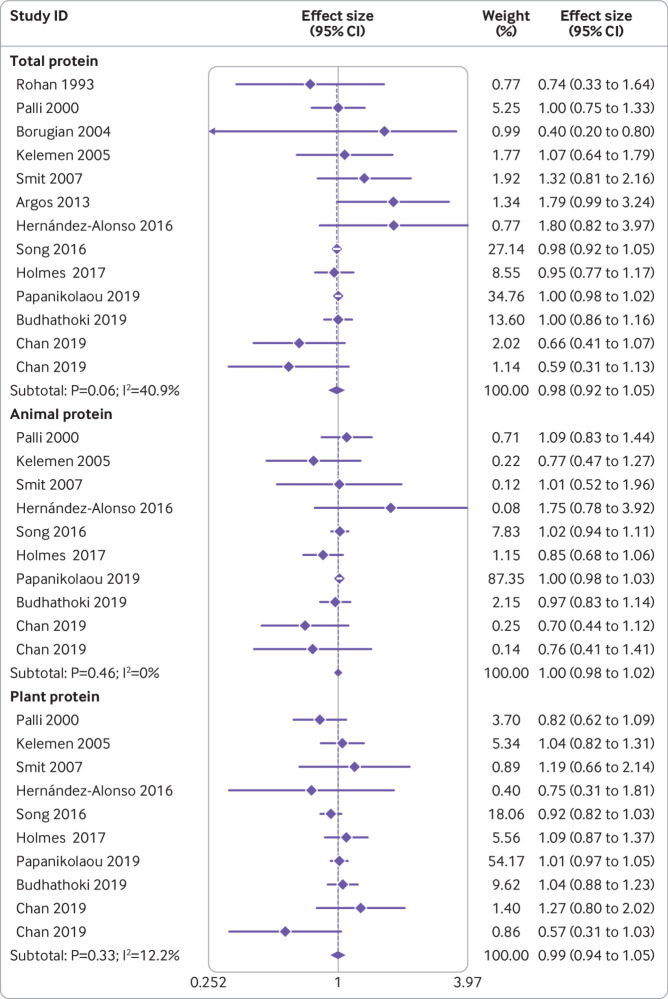
Twelve papers,9 13 14 15 16 18 45 46 48 56 60 61 with a total of 292 629 participants and 22 118 deaths, examined the association between intake of total protein and cancer mortality. The summary effect size for cancer mortality comparing the highest and lowest protein intakes was 0.98 (95% confidence interval 0.92 to 1.05, P=0.63), indicating no clear association; however, evidence of moderate heterogeneity was found between studies (I2=40.9%, P=0.06; fig 4). The same findings were obtained for animal protein consumption and cancer mortality based on nine publications9 13 14 15 16 18 45 56 61 with a total of 274 370 participants and 21 759 deaths (pooled effect size comparing the highest and lowest protein intakes was 1.00, 95% confidence interval 0.98 to 1.02, P=0.88), with no significant heterogeneity among the studies (I2=0%, P=0.46; fig 4). This was also the case for plant protein consumption, which was examined in nine articles9 13 14 15 16 18 45 56 61 with a total of 274 370 participants and 21 759 deaths (pooled effect size comparing the highest and lowest protein intakes was 0.99, 0.94 to 1.05, P=0.68). Moreover, no significant heterogeneity among the studies was found in this case (I2=12.2%; P=0.33; fig 4).
Fig 4.
Forest plot for association between protein intake and risk of cancer mortality in adults aged 19 or older, expressed as comparison between highest and lowest categories of protein intake. Diamonds represent pooled estimates from random effects analysis
Linear and non-linear dose-response analysis
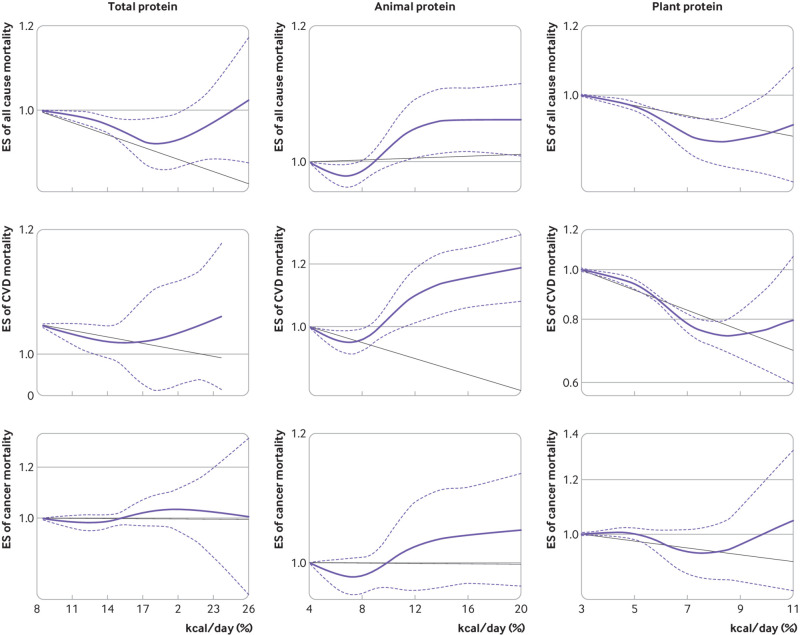
Eight 9 10 16 17 18 46 51 64 of 21 publications on the association between total protein intake and all cause mortality were included in the dose-response analysis (fig 5). No significant non-linear association was found (P=0.40 for non-linearity). Furthermore, linear dose-response meta-analysis showed no significant association between total protein intake and all cause mortality by an additional 3% of energy from protein a day (pooled effect size 0.99, 0.97 to 1.00, P=0.10; supplementary fig 1). Combining data from five 10 15 16 18 of 11 papers in the dose-response analysis of animal protein intake and all cause mortality, no significant non-linear association was seen (P=0.54 for non-linearity; fig 5). Moreover, the linear association between an increase of 3% of energy from animal proteins a day and all cause mortality was not significant (pooled effect size 0.99, 0.96 to 1.02, P=0.61; supplementary fig 1). In the dose-response analysis of plant protein intake and all cause mortality, based on six articles9 10 15 16 18 57 of 13 publications, a significant non-linear association was found (P=0.05 for non-linearity; fig 5). Based on linear dose-response analysis, an additional 3% of energy from plant proteins a day was associated with a 5% lower risk of death from all causes (pooled effect size 0.95, 95 0.93 to 0.98, P<0.001; supplementary fig 1).
Fig 5.
Non-linear dose-response association of intakes of total, animal, and plant protein (based on percentage of kcal/day (1 kcal=4.18 kJ=0.00418 MJ) with risk of mortality from all causes, cardiovascular disease (CVD), and cancer in adults aged 19 or older. Dietary intake of protein was modelled with restricted cubic splines in a multivariate random effects dose-response model. Black line indicates the linear model; solid purple line indicates the spline model; dashed lines represent 95% confidence intervals. ES=effect size
Non-linear dose-response analysis of seven of 10 papers9 10 16 17 18 51 64 showed no significant association between intake of total protein and cardiovascular disease mortality (P=0.07; fig 5). Findings from a linear dose response meta-analysis showed no significant association between total protein intake and cardiovascular disease mortality (pooled effect size 0.98, 0.97 to 1.00, P=0.08; supplementary fig 2). No significant non-linear association was found between animal protein intake and cardiovascular disease mortality based on five publications9 10 15 16 18 (P=0.37 for non-linearity; fig 5). As with non-linear dose-response meta-analysis, linear dose-response analysis showed no significant association between animal protein intake and cardiovascular disease mortality based on an additional 3% of energy from animal proteins a day (pooled effect size 0.98, 0.94 to 1.02, P=0.32; supplementary fig 2). An inverse association between plant protein intake and cardiovascular disease mortality was found in the non-linear dose-response analysis based on six articles9 10 15 16 18 57 (P<0.001 for non-linearity; fig 5). Linear dose-response analysis showed no significant association between an additional 3% of energy from plant protein intake and cardiovascular disease mortality (pooled effect size 0.96, 95 0.89 to 1.04, P=0.30; supplementary fig 2).
Of 13 papers on the association between intake of total protein and cancer mortality, five9 16 17 18 46 were included in the non-linear dose-response analysis. No significant association was found between intake of total protein and cancer mortality (P=0.84; fig 5). This was also the case for animal protein (P=0.93) and plant protein intakes (P=0.52) based on four papers9 15 16 18 (fig 5). Linear dose-response analysis showed that an additional 3% of energy from total protein intake (pooled effect size 0.98, 0.94 to 1.03, P=0.39), animal protein intake (0.99, 0.96 to 1.02, P=0.50), and plant protein intake (0.94, 0.85 to 1.03, P=0.19) was not associated with cancer mortality (supplementary fig 3).
Subgroup and sensitivity analyses, and publication bias
To test the robustness of the findings and investigate possible sources of heterogeneity between studies, subgroup analyses were conducted. These analyses were performed based on predefined criteria, including study location, duration of follow-up, sex, dietary assessment tools, health status of study participants, high versus low or middle income countries, single or repeated measurements of protein intake, effect size type, and statistical controlling for confounders (BMI, total energy intake, and macronutrients (fat and carbohydrate)). Supplementary table 3 presents findings for the different subgroups.
A significant inverse association was seen between total protein intake and all cause mortality in women, in studies that used a food frequency questionnaire for assessment of total protein intake, among those studies that did not control for total energy intake and macronutrients intake, those with a follow up duration of less than 15 years, and those that were performed on people with comorbidities. For cardiovascular disease mortality, a significant inverse association with total protein intake was seen in studies that did not control for total energy intake and macronutrients intake, and among those with a follow-up of less than 15 years.
For animal protein intake, a significant inverse association was seen with all cause mortality in studies with a follow up duration of less than 15 years. In addition, inverse associations between animal protein intake and mortality from cardiovascular disease were observed in studies that did not control for macronutrients intake.
Plant protein intake was inversely associated with all cause mortality in both men and women, in studies that were performed in US and non-US countries, in studies with a follow-up of more than 15 years and less than 15 years, in studies that applied a food frequency questionnaire for dietary assessment, among studies that controlled their analysis for energy and macronutrients intakes and BMI, in studies that were done on individuals without comorbidities, in studies performed in high income countries, and in studies that reported a hazard ratio for their analysis. The same findings were also seen between plant protein intake and cardiovascular disease mortality, but this association was not significant in men and women in studies that were performed in the US and those with a follow-up of more than 15 years.
Findings from the sensitivity analysis using a fixed effects model showed that exclusion of the studies by Song et al,15 Kurihara et al,57 Budhathoki et al,18 and Sun et al11 resulted in a change in the significant inverse association between plant protein intake and cardiovascular disease mortality to a marginally significant inverse association. Sensitivity analysis for the other associations examined showed that exclusion of any single study from the analysis did not appreciably alter the pooled effect sizes. No missing studies were imputed in regions of the contour enhanced funnel plots. No publication bias was found based on Begg’s rank correlation test. For the association between total protein intake and mortality from all causes and from cardiovascular diseases, and between plant protein intake and mortality from cardiovascular diseases, Egger’s linear regression test indicated possible publication bias. Application of the trim and fill method, however, did not result in a change in the average effect size, further suggesting that the results were not affected by publication bias.
Discussion
In this systematic review and meta-analysis, we found a significant inverse association between intake of total protein and all cause mortality; no clear significant association was seen between total or animal protein intake and cardiovascular disease and cancer mortality. Intake of plant protein was associated with a lower risk of all cause and cardiovascular disease mortality. The inverse associations between plant protein intake and mortality from all causes and cardiovascular disease remained significant in studies that controlled for energy, BMI, and macronutrients intake, and in studies with follow-up of less than 15 years, and those that applied a food frequency questionnaire for dietary assessment.
Comparison with other studies
We systematically and quantitatively summarised earlier investigations on the association between intake of total, animal, and plant proteins and mortality. A recent systematic review and meta-analysis showed that consumption of soy protein was significantly associated with a decreased risk of mortality from breast cancer, but it was not associated with mortality from all causes and cardiovascular disease.19 In addition, high intake of legumes, grains, and nuts as major sources of plant proteins was associated with a lower risk of all cause and cardiovascular disease mortality.66 67 Long term observational studies indicated that high consumption of total and animal proteins was associated with an increased risk of cancer and diabetes.17 68 69 Substitution of non-meat proteins for meat proteins has been favourably associated with fasting insulin levels and reduced insulin resistance.70 Consumption of low carbohydrate, high protein, and fat diets was not associated with increased risk of coronary heart disease in women. When vegetable sources of fat and protein were chosen, however, these diets were associated with a lower risk of coronary heart disease.71 Overall, all available studies support the beneficial effects of plant proteins on human health.
In this meta-analysis, no significant association was seen between animal protein intake and mortality. Unlike our findings, one meta-analysis found that each reduction of three servings of processed meat in a week was associated with a small reduction in the risk of overall cancer mortality over a lifetime.72 In addition, fish consumption was associated with a lower risk of all cause mortality among high consumers than among those with the lowest intake.73 Thus lack of a significant association between animal protein intake and mortality in our meta-analysis could be due to combining protein from different animal sources, including poultry, eggs, and dairy foods. Also, the discrepant associations of animal meat and animal protein intake. Here, we compared our findings with a previous meta-analysis72 on animal meat. In that meta-analysis the exposure variable was meat as a food group, whereas our exposure variable was protein as a nutrient. Animal meat contains fat, sodium, iron, and B vitamins in addition to protein so that those nutrients could affect the risk of mortality differently, whereas animal protein is protein only from animal sources. Therefore, findings for animal meat and animal protein could be different. with mortality explained by the fat content of meat. Some studies investigating the association of animal protein intake and mortality have controlled their analysis for fat intake.12 15 16 18 58 62 64 In addition, different methods used in the processing and cooking of meats might provide further explanation for the discrepancy.
In the interpretation of our findings, it must be considered that humans do not consume single macronutrients, such as proteins. Dietary intake of other nutrients and biologically active factors in foods containing protein could also account for the association between protein intake and mortality. In addition, when the contribution of a single nutrient is assessed as a disease risk, the interaction between nutrients in the gut should be taken into account. Some studies included in this meta-analysis had controlled for the confounding effects of other macronutrients (fat or carbohydrates).7 12 14 15 16 17 18 46 57 58 62 64 When we confined the analysis to studies that had made these adjustments, the inverse association of plant protein with all cause and cardiovascular disease mortality changed little, whereas the inverse association between intake of total protein and all cause mortality became non-significant. Therefore, dietary fat intake is not likely to account for the protective association between plant protein intake and mortality. Consumption of animal and plant proteins could be a marker of broader dietary intake patterns—or even of social class, an important independent predictor of many health outcomes. Our findings must be interpreted in this context, and future investigations should consider whether intake of animal and plant proteins is a marker of overall dietary patterns or of social class.
Mechanisms
In this study, intake of plant protein was inversely associated with mortality from all causes and cardiovascular disease. The same finding was also seen for intake of total protein and all cause mortality. Given that plant protein is part of total protein, the observed inverse association for intake of total protein seems to be related to its plant protein component. The mechanisms through which plant proteins could affect human health are not well known. Whereas consumption of animal protein was associated with increased concentrations of insulin-like growth factor 1, dietary intake of plant proteins was not associated with raised levels.74 75 Increased levels of insulin-like growth factor 1 have been linked to an increased risk of age related diseases, such as cancers.76 77 In addition, dietary plant proteins were associated with favourable changes in blood pressure, waist circumference, body weight, and body composition, which might help to lower the risk of several chronic diseases, including cardiovascular disease and type 2 diabetes.78 Intake of animal protein, independent of body weight, was associated with hypercholesterolaemia, whereas consumption of plant proteins was associated with low levels of plasma cholesterol.79 80 81
Bacterial fermentation of plant proteins in the gut could help to lower the production of potentially toxic and carcinogenic metabolites, such as ammonia, amines, phenols, tryptophan metabolites, and sulfides.82 Bioactive peptides derived from plant proteins could also have beneficial health promoting properties. These proteins and peptides have antioxidative, anti-inflammatory, antihypertensive, and antimicrobial activities.83 84 85 Anorectic peptides have been shown to exert their antiobesogenic activity through decreasing food intake.86 Earlier studies have indicated that bioactive peptides could reduce blood cholesterol levels.87 Furthermore, the different associations between animal or plant proteins and mortality risk could be due to the differences in amino acid composition. Plant proteins contain lower amounts of lysine and histidine amino acids than animal proteins; high intake of these amino acids has been shown to increase secretion of lipoproteins containing apo B.88 Therefore, intake of plant proteins could be associated with protection against cardiovascular diseases through this mechanism. In addition to amino acids, plant proteins are rich in non-essential amino acids such as arginine and pyruvate precursors, which in turn can lead to upregulation of glucagon and downregulation of insulin secretion.89 The action of glucagon on hepatocytes is mediated through increased cyclic adenosine monophosphate concentrations, which downregulate the synthesis of required enzymes for de novo lipogenesis and upregulate the low density lipoprotein receptors and production of insulin-like growth factor 1 antagonist.89
Strengths and weaknesses of this study
This meta-analysis has several strengths. Firstly, the large number of participants and deaths included allowed us to quantitatively assess the association of protein intake and risk of mortality, thus making it more powerful than any single study. Secondly, a dose-response analysis was conducted to evaluate the linear and non-linear associations. Thirdly, because all the studies included were prospective, the influence of recall and selection bias is negligible. In addition, we considered subtypes of total protein intake, including animal and plant proteins. These data provide a comprehensive insight into the association between intake of dietary protein and risk of mortality based on the current evidence.
This study has some limitations, most of which are common to observational studies and meta-analyses. Residual or unmeasured confounding factors could have affected the magnitude of the association between protein intake and mortality. Although most studies had controlled for potential confounders, some did not take into account dietary consumption of other nutrients and others did not consider total energy intake and BMI as covariates. Lack of controlling for other nutrients, such as amount and type of dietary fat, which is present in most food sources of protein, could affect the independent association of protein intake with mortality. In addition, some studies in this review did not report sufficient information to be included in the dose-response meta-analysis.Also, different methods for dietary assessment, including food frequency questionnaires, dietary recall, and records, were used in the included cohorts, and the units of protein intake varied in different studies. Measurement errors in dietary assessment are inevitable and would have tended to underestimate the associations with protein intake. In addition, our conclusions about animal protein intake could have less generalisability to low or middle income economics, in which diets are carbohydrate-rich and consumption of animal sources is low.
Conclusions, policy implications, and future research
We found that high intake of total proteins was associated with a lower risk of mortality from all causes. Intake of plant protein was also associated with a lower risk of mortality from all causes and cardiovascular diseases, which is consistent with its beneficial effects on cardiometabolic risk factors, including blood lipid and lipoprotein profiles, blood pressure, and glycaemic regulation. These findings have important public health implications as intake of plant protein can be increased relatively easily by replacing animal protein and could have a large effect on longevity. Also, an additional 3% of energy from plant proteins a day was associated with a 5% lower risk of death from all causes. Our findings therefore strongly support the existing dietary recommendations to increase consumption of plant proteins in the general population. Extrapolation of these findings to the worldwide population should be done cautiously because most studies included in the meta-analysis are from Western nations and few studies have been reported from other countries. Therefore, further studies are required. Additional studies should also focus on the mechanisms through which dietary protein affect mortality.
What is already known on this topic
Consumption of high protein diets has been suggested to control body weight and improve cardiometabolic abnormalities
Regular consumption of red meat and high intake of animal proteins have been linked to several health problems
Data on the association between different types of proteins and mortality are conflicting
What this study adds
High intake of total protein is associated with a lower risk of mortality from all causes
Intake of plant protein is associated with a lower risk of mortality from all causes and from cardiovascular diseases, and an additional 3% of energy from plant proteins a day is associated with a 5% lower risk of death from all causes
These findings support current dietary recommendations to increase consumption of plant proteins in the general population
Web extra.
Extra material supplied by authors
Web appendix: Supplementary material
Contributors: OS and SN contributed to the literature search, data extraction, and data analysis. AE contributed to study conception, manuscript drafting, and data analysis. WCW contributed to study conception, manuscript drafting, data analysis, and approving the final manuscript. All authors acknowledge full responsibility for the analyses and interpretation of the report. AE is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: The meta-analysis was funded by the Research Council of School of Nutritional Sciences and Dietetics of Tehran University of Medical Sciences, Tehran, Iran (grant No 46459). The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/coi_disclosure.pdf and declare: support from the Research Council of School of Nutritional Sciences and Dietetics of Tehran University of Medical Sciences, Tehran, Iran for the submitted work; no financial relationships with any organisation that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
Data sharing: No additional data available.
The corresponding author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported. No important aspects of the study have been omitted and any discrepancies from the study as planned have been disclosed.
Dissemination to participants and related patient and public communities: No patients were involved in setting the research question or the outcome measures, nor were they involved in the design and implementation of the study. We plan to disseminate these findings to participants in our annual newsletter and to the general public in a press release.
References
- 1. World Health Organization NCD mortality and morbidity. World Health Organisation, 2016. [Google Scholar]
- 2. Simpson SJ, Raubenheimer D. Perspective: tricks of the trade. Nature 2014;508:S66. 10.1038/508S66a [DOI] [PubMed] [Google Scholar]
- 3. Solon-Biet SM, McMahon AC, Ballard JWO, et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab 2014;19:418-30. 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shan Z, Rehm CD, Rogers G, et al. Trends in dietary carbohydrate, protein, and fat intake and diet quality among US adults, 1999-2016. JAMA 2019;322:1178-87. 10.1001/jama.2019.13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Westerterp-Plantenga MS, Lemmens SG, Westerterp KR. Dietary protein - its role in satiety, energetics, weight loss and health. Br J Nutr 2012;108(Suppl 2):S105-12. 10.1017/S0007114512002589. [DOI] [PubMed] [Google Scholar]
- 6. Leidy HJ, Clifton PM, Astrup A, et al. The role of protein in weight loss and maintenance. Am J Clin Nutr 2015;101:1320S-9S. 10.3945/ajcn.114.084038. [DOI] [PubMed] [Google Scholar]
- 7. Song M, Wu K, Meyerhardt JA, et al. Low-carbohydrate diet score and macronutrient intake in relation to survival after colorectal cancer diagnosis. JNCI Cancer Spectr 2018;2:pky077. 10.1093/jncics/pky077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Delimaris I. Adverse effects associated with protein intake above the recommended dietary allowance for adults. ISRN Nutr 2013;2013:126929. 10.5402/2013/126929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chan R, Leung J, Woo J. High protein intake is associated with lower risk of all-cause mortality in community-dwelling Chinese older men and women. J Nutr Health Aging 2019;23:987-96. 10.1007/s12603-019-1263-1. [DOI] [PubMed] [Google Scholar]
- 10. Sauvaget C, Nagano J, Hayashi M, Yamada M. Animal protein, animal fat, and cholesterol intakes and risk of cerebral infarction mortality in the adult health study. Stroke 2004;35:1531-7. 10.1161/01.STR.0000130426.52064.09. [DOI] [PubMed] [Google Scholar]
- 11. Sun Y, Liu B, Snetselaar L, et al. Association of major dietary protein sources with all-cause and cause-specific mortality: the Women’s Health Initiative (FS03-08-19). Curr Dev Nutr 2019;3:nzz046-FS03 10.1093/cdn/nzz046.FS03-08-19. [DOI] [Google Scholar]
- 12. Virtanen HEK, Voutilainen S, Koskinen TT, et al. Dietary proteins and protein sources and risk of death: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr 2019;109:1462-71. 10.1093/ajcn/nqz025. [DOI] [PubMed] [Google Scholar]
- 13. Papanikolaou Y, Fulgoni V., III Animal and plant protein usual intakes are not associated with mortality in US adults (P18-037-19). Curr Dev Nutr 2019;3:nzz039-P18 10.1093/cdn/nzz039.P18-037-19. [DOI] [Google Scholar]
- 14. Kelemen LE, Kushi LH, Jacobs DR, Jr, Cerhan JR. Associations of dietary protein with disease and mortality in a prospective study of postmenopausal women. Am J Epidemiol 2005;161:239-49. 10.1093/aje/kwi038. [DOI] [PubMed] [Google Scholar]
- 15. Song M, Fung TT, Hu FB, et al. Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Intern Med 2016;176:1453-63. 10.1001/jamainternmed.2016.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hernández-Alonso P, Salas-Salvadó J, Ruiz-Canela M, et al. High dietary protein intake is associated with an increased body weight and total death risk. Clin Nutr 2016;35:496-506. 10.1016/j.clnu.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 17. Levine ME, Suarez JA, Brandhorst S, et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab 2014;19:407-17. 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Budhathoki S, Sawada N, Iwasaki M, et al. Japan Public Health Center–based Prospective Study Group Association of animal and plant protein intake with all-cause and cause-specific mortality in a Japanese cohort. JAMA Intern Med 2019;179:1509-18. 10.1001/jamainternmed.2019.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nachvak SM, Moradi S, Anjom-Shoae J, et al. Soy, soy isoflavones, and protein intake in relation to mortality from all causes, cancers, and cardiovascular diseases: a systematic review and dose-response meta-analysis of prospective cohort studies. J Acad Nutr Diet 2019;119:1483-500.e17. 10.1016/j.jand.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons; 2019. https://training.cochrane.org/handbook
- 22. World Health Organization International statistical classification of diseases and related health problems, 10th revision (ICD–10). World Health Organization, 2008. [Google Scholar]
- 23. Bero L, Chartres N, Diong J, et al. The risk of bias in observational studies of exposures (ROBINS-E) tool: concerns arising from application to observational studies of exposures. Syst Rev 2018;7:242. 10.1186/s13643-018-0915-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parohan M, Anjom-Shoae J, Nasiri M, Khodadost M, Khatibi SR, Sadeghi O. Dietary total antioxidant capacity and mortality from all causes, cardiovascular disease and cancer: a systematic review and dose-response meta-analysis of prospective cohort studies. Eur J Nutr 2019;58:2175-89. 10.1007/s00394-019-01922-9. [DOI] [PubMed] [Google Scholar]
- 25. Sadeghi O, Sadeghian M, Rahmani S, Maleki V, Larijani B, Esmaillzadeh A. Whole-grain consumption does not affect obesity measures: an updated systematic review and meta-analysis of randomized clinical trials. Adv Nutr 2020;11:280-92. 10.1093/advances/nmz076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sadeghi O, Saneei P, Nasiri M, Larijani B, Esmaillzadeh A. Abdominal obesity and risk of hip fracture: a systematic review and meta-analysis of prospective studies. Adv Nutr 2017;8:728-38. 10.3945/an.117.015545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zahedi H, Djalalinia S, Sadeghi O, et al. Dietary inflammatory potential score and risk of breast cancer: systematic review and meta-analysis. Clin Breast Cancer 2018;18:e561-70. 10.1016/j.clbc.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 28. Parohan M, Sadeghi A, Khatibi SR, et al. Dietary total antioxidant capacity and risk of cancer: a systematic review and meta-analysis on observational studies. Crit Rev Oncol Hematol 2019;138:70-86. 10.1016/j.critrevonc.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 29. Anjom-Shoae J, Sadeghi O, Larijani B, Esmaillzadeh A. Dietary intake and serum levels of trans fatty acids and risk of breast cancer: a systematic review and dose-response meta-analysis of prospective studies. Clin Nutr 2020;39:755-64. 10.1016/j.clnu.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 30. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose–response data. Stata J 2006;6:40-57 10.1177/1536867X0600600103. [DOI] [Google Scholar]
- 31. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992;135:1301-9. 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 32. Jackson D, White IR, Thompson SG. Extending DerSimonian and Laird’s methodology to perform multivariate random effects meta-analyses. Stat Med 2010;29:1282-97. 10.1002/sim.3602. [DOI] [PubMed] [Google Scholar]
- 33. Stobäus N, Müller MJ, Küpferling S, Schulzke JD, Norman K. Low recent protein intake predicts cancer-related fatigue and increased mortality in patients with advanced tumor disease undergoing chemotherapy. Nutr Cancer 2015;67:818-24. 10.1080/01635581.2015.1040520. [DOI] [PubMed] [Google Scholar]
- 34. van den Brandt PA. Red meat, processed meat, and other dietary protein sources and risk of overall and cause-specific mortality in The Netherlands Cohort Study. Eur J Epidemiol 2019;34:351-69. 10.1007/s10654-019-00483-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Farvid MS, Malekshah AF, Pourshams A, et al. Dietary protein sources and all-cause and cause-specific mortality: the Golestan Cohort Study in Iran. Am J Prev Med 2017;52:237-48. 10.1016/j.amepre.2016.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ding M, Li J, Qi L, et al. Associations of dairy intake with risk of mortality in women and men: three prospective cohort studies. BMJ 2019;367:l6204. 10.1136/bmj.l6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Takata Y, Shu XO, Gao YT, et al. Red meat and poultry intakes and risk of total and cause-specific mortality: results from cohort studies of Chinese adults in Shanghai. PLoS One 2013;8:e56963. 10.1371/journal.pone.0056963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tanaka H, Date C, Hayashi M, et al. Trends in death and consultation rates of ischemic heart disease in Japan and the risk factors in a rural community. Jpn Circ J 1987;51:306-13. 10.1253/jcj.51.306. [DOI] [PubMed] [Google Scholar]
- 39. Cirillo M, Cavallo P, Bilancio G, Lombardi C, Terradura Vagnarelli O, Laurenzi M. Low protein intake in the population: low risk of kidney function decline but high risk of mortality. J Ren Nutr 2018;28:235-44. 10.1053/j.jrn.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 40. Chen Y, McClintock TR, Segers S, et al. Prospective investigation of major dietary patterns and risk of cardiovascular mortality in Bangladesh. Int J Cardiol 2013;167:1495-501. 10.1016/j.ijcard.2012.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nilsson LM, Winkvist A, Eliasson M, et al. Low-carbohydrate, high-protein score and mortality in a northern Swedish population-based cohort. Eur J Clin Nutr 2012;66:694-700. 10.1038/ejcn.2012.9. [DOI] [PubMed] [Google Scholar]
- 42. Lagiou P, Sandin S, Weiderpass E, et al. Low carbohydrate-high protein diet and mortality in a cohort of Swedish women. J Intern Med 2007;261:366-74. 10.1111/j.1365-2796.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- 43. Trichopoulou A, Psaltopoulou T, Orfanos P, Hsieh CC, Trichopoulos D. Low-carbohydrate-high-protein diet and long-term survival in a general population cohort. Eur J Clin Nutr 2007;61:575-81. 10.1038/sj.ejcn.1602557. [DOI] [PubMed] [Google Scholar]
- 44. Sanders CL, Wengreen HJ, Schwartz S, et al. Cache County Investigators Nutritional status is associated with severe dementia and mortality: the Cache County Dementia Progression Study. Alzheimer Dis Assoc Disord 2018;32:298-304. 10.1097/WAD.0000000000000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Palli D, Russo A, Saieva C, Salvini S, Amorosi A, Decarli A. Dietary and familial determinants of 10-year survival among patients with gastric carcinoma. Cancer 2000;89:1205-13. . [DOI] [PubMed] [Google Scholar]
- 46. Argos M, Melkonian S, Parvez F, et al. A population-based prospective study of energy-providing nutrients in relation to all-cause cancer mortality and cancers of digestive organs mortality. Int J Cancer 2013;133:2422-8. 10.1002/ijc.28250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bates CJ, Mansoor MA, Pentieva KD, Hamer M, Mishra GD. Biochemical risk indices, including plasma homocysteine, that prospectively predict mortality in older British people: the National Diet and Nutrition Survey of People Aged 65 Years and Over. Br J Nutr 2010;104:893-9. 10.1017/S0007114510001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Borugian MJ, Sheps SB, Kim-Sing C, et al. Insulin, macronutrient intake, and physical activity: are potential indicators of insulin resistance associated with mortality from breast cancer? Cancer Epidemiol Biomarkers Prev 2004;13:1163-72. [PubMed] [Google Scholar]
- 49. Campmans-Kuijpers MJ, Sluijs I, Nöthlings U, et al. Isocaloric substitution of carbohydrates with protein: the association with weight change and mortality among patients with type 2 diabetes. Cardiovasc Diabetol 2015;14:39. 10.1186/s12933-015-0202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Courand P-Y, Lesiuk C, Milon H, et al. Association between protein intake and mortality in hypertensive patients without chronic kidney disease in the OLD-HTA cohort. Hypertension 2016;67:1142-9. 10.1161/HYPERTENSIONAHA.116.07409. [DOI] [PubMed] [Google Scholar]
- 51. Dehghan M, Mente A, Zhang X, et al. Prospective Urban Rural Epidemiology (PURE) study investigators Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet 2017;390:2050-62. 10.1016/S0140-6736(17)32252-3. [DOI] [PubMed] [Google Scholar]
- 52. Dwyer JT, Madans JH, Turnbull B, et al. Diet, indicators of kidney disease, and later mortality among older persons in the NHANES I epidemiologic follow-up study. Am J Public Health 1994;84:1299-303. 10.2105/AJPH.84.8.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Esrey KL, Joseph L, Grover SA. Relationship between dietary intake and coronary heart disease mortality: lipid research clinics prevalence follow-up study. J Clin Epidemiol 1996;49:211-6. 10.1016/0895-4356(95)00066-6. [DOI] [PubMed] [Google Scholar]
- 54. Halbesma N, Bakker SJ, Jansen DF, et al. PREVEND Study Group High protein intake associates with cardiovascular events but not with loss of renal function. J Am Soc Nephrol 2009;20:1797-804. 10.1681/ASN.2008060649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Holmes MD, Stampfer MJ, Colditz GA, Rosner B, Hunter DJ, Willett WC. Dietary factors and the survival of women with breast carcinoma. Cancer 1999;86:826-35. . [DOI] [PubMed] [Google Scholar]
- 56. Holmes MD, Wang J, Hankinson SE, Tamimi RM, Chen WY. Protein intake and breast cancer survival in the Nurses’ Health Study. J Clin Oncol 2017;35:325-33. 10.1200/JCO.2016.68.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kurihara A, Okamura T, Sugiyama D, et al. NIPPON DATA90 Research Group Vegetable protein intake was inversely associated with cardiovascular mortality in a 15-year follow-up study of a general Japanese population. J Atheroscler Thromb 2019;26:198-206. 10.5551/jat.44172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nagata C, Wada K, Tamura T, et al. Dietary intakes of glutamic acid and glycine are associated with stroke mortality in Japanese adults. J Nutr 2015;145:720-8. 10.3945/jn.114.201293. [DOI] [PubMed] [Google Scholar]
- 59. Payette H, Coulombe C, Boutier V, Gray-Donald K. Weight loss and mortality among free-living frail elders: a prospective study. J Gerontol A Biol Sci Med Sci 1999;54:M440-5. 10.1093/gerona/54.9.M440. [DOI] [PubMed] [Google Scholar]
- 60. Rohan TE, Hiller JE, McMichael AJ. Dietary factors and survival from breast cancer. Nutr Cancer 1993;20:167-77. 10.1080/01635589309514283. [DOI] [PubMed] [Google Scholar]
- 61. Smit E, Garcia-Palmieri MR, Figueroa NR, et al. Protein and legume intake and prostate cancer mortality in Puerto Rican men. Nutr Cancer 2007;58:146-52. 10.1080/01635580701328206. [DOI] [PubMed] [Google Scholar]
- 62. Tharrey M, Mariotti F, Mashchak A, Barbillon P, Delattre M, Fraser GE. Patterns of plant and animal protein intake are strongly associated with cardiovascular mortality: the Adventist Health Study-2 cohort. Int J Epidemiol 2018;47:1603-12. 10.1093/ije/dyy030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zaslavsky O, Zelber-Sagi S, Hebert JR, et al. Biomarker-calibrated nutrient intake and healthy diet index associations with mortality risks among older and frail women from the Women’s Health Initiative. Am J Clin Nutr 2017;105:1399-407. 10.3945/ajcn.116.151530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Preis SR, Stampfer MJ, Spiegelman D, Willett WC, Rimm EB. Dietary protein and risk of ischemic heart disease in middle-aged men. Am J Clin Nutr 2010;92:1265-72. 10.3945/ajcn.2010.29626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mendonça N, Kingston A, Granic A, Hill TR, Mathers JC, Jagger C. Contribution of protein intake and its interaction with physical activity to transitions between disability states and to death in very old adults: the Newcastle 85+ Study. Eur J Nutr 2019. 10.1007/s00394-019-02041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Miller V, Mente A, Dehghan M, et al. Prospective Urban Rural Epidemiology (PURE) study investigators Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): a prospective cohort study. Lancet 2017;390:2037-49. 10.1016/S0140-6736(17)32253-5. [DOI] [PubMed] [Google Scholar]
- 67. Aune D, Keum N, Giovannucci E, et al. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective studies. BMC Med 2016;14:207. 10.1186/s12916-016-0730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. van Nielen M, Feskens EJ, Mensink M, et al. InterAct Consortium Dietary protein intake and incidence of type 2 diabetes in Europe: the EPIC-InterAct case-cohort study. Diabetes Care 2014;37:1854-62. 10.2337/dc13-2627. [DOI] [PubMed] [Google Scholar]
- 69. Pounis GD, Tyrovolas S, Antonopoulou M, et al. Long-term animal-protein consumption is associated with an increased prevalence of diabetes among the elderly: the Mediterranean Islands (MEDIS) study. Diabetes Metab 2010;36:484-90. 10.1016/j.diabet.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 70. van Baak MA, Larsen TM, Jebb SA, et al. Dietary intake of protein from different sources and weight regain, changes in body composition and cardiometabolic risk factors after weight loss: the DIOGenes study. Nutrients 2017;9:1326. 10.3390/nu9121326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Halton TL, Willett WC, Liu S, et al. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N Engl J Med 2006;355:1991-2002. 10.1056/NEJMoa055317. [DOI] [PubMed] [Google Scholar]
- 72. Han MA, Zeraatkar D, Guyatt GH, et al. Reduction of red and processed meat intake and cancer mortality and incidence: a systematic review and meta-analysis of cohort studies. Ann Intern Med 2019;171:711-20. 10.7326/M19-0699. [DOI] [PubMed] [Google Scholar]
- 73. Zhao LG, Sun JW, Yang Y, Ma X, Wang YY, Xiang YB. Fish consumption and all-cause mortality: a meta-analysis of cohort studies. Eur J Clin Nutr 2016;70:155-61. 10.1038/ejcn.2015.72. [DOI] [PubMed] [Google Scholar]
- 74. Allen NE, Appleby PN, Davey GK, Kaaks R, Rinaldi S, Key TJ. The associations of diet with serum insulin-like growth factor I and its main binding proteins in 292 women meat-eaters, vegetarians, and vegans. Cancer Epidemiol Biomarkers Prev 2002;11:1441-8. [PubMed] [Google Scholar]
- 75. Holmes MD, Pollak MN, Willett WC, Hankinson SE. Dietary correlates of plasma insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations. Cancer Epidemiol Biomarkers Prev 2002;11:852-61. [PubMed] [Google Scholar]
- 76. Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet 2004;363:1346-53. 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 77. Cappola AR, Xue Q-L, Ferrucci L, Guralnik JM, Volpato S, Fried LP. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J Clin Endocrinol Metab 2003;88:2019-25. 10.1210/jc.2002-021694. [DOI] [PubMed] [Google Scholar]
- 78. Elliott P, Stamler J, Dyer AR, et al. Association between protein intake and blood pressure: the INTERMAP Study. Arch Intern Med 2006;166:79-87. 10.1001/archinte.166.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Carroll K, Hamilton R. Effects of dietary protein and carbohydrate on plasma cholesterol levels in relation to atherosclerosis. J Food Sci 1975;40:18-23 10.1111/j.1365-2621.1975.tb03726.x. [DOI] [Google Scholar]
- 80. Chalvon-Demersay T, Azzout-Marniche D, Arfsten J, et al. A systematic review of the effects of plant compared with animal protein sources on features of metabolic syndrome. J Nutr 2017;147:281-92. 10.3945/jn.116.239574. [DOI] [PubMed] [Google Scholar]
- 81. National Research Council Diet and health: implications for reducing chronic disease risk. National Academies Press, 1989. [PubMed] [Google Scholar]
- 82. Windey K, De Preter V, Verbeke K. Relevance of protein fermentation to gut health. Mol Nutr Food Res 2012;56:184-96. 10.1002/mnfr.201100542. [DOI] [PubMed] [Google Scholar]
- 83. Nourmohammadi E, Mahoonak AS. Health implications of bioactive peptides: a review. Int J Vitam Nutr Res 2018;88:319-43. 10.1024/0300-9831/a000418. [DOI] [PubMed] [Google Scholar]
- 84. Korhonen H, Pihlanto A. Bioactive peptides: production and functionality. Int Dairy J 2006;16:945-60 10.1016/j.idairyj.2005.10.012. [DOI] [Google Scholar]
- 85. Chatterjee C, Gleddie S, Xiao C-W. Soybean bioactive peptides and their functional properties. Nutrients 2018;10:1211. 10.3390/nu10091211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wang W, De Mejia EG. A new frontier in soy bioactive peptides that may prevent age‐related chronic diseases. Compr Rev Food Sci Food Saf 2005;4:63-78 10.1111/j.1541-4337.2005.tb00075.x. [DOI] [PubMed] [Google Scholar]
- 87. Potter SM. Overview of proposed mechanisms for the hypocholesterolemic effect of soy. J Nutr 1995;125(Suppl):606S-11S. 10.1093/jn/125.3_Suppl.606S. [DOI] [PubMed] [Google Scholar]
- 88. Razquin C, Ruiz-Canela M, Clish CB, et al. Lysine pathway metabolites and the risk of type 2 diabetes and cardiovascular disease in the PREDIMED study: results from two case-cohort studies. Cardiovasc Diabetol 2019;18:151. 10.1186/s12933-019-0958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. McCarty MF. Vegan proteins may reduce risk of cancer, obesity, and cardiovascular disease by promoting increased glucagon activity. Med Hypotheses 1999;53:459-85. 10.1054/mehy.1999.0784. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary material