Abstract
Background:
Limited information exists regarding longitudinal trends in midlife women’s exposure to per- and polyfluoroalkyl substances (PFAS). Further, little is known about how patterns of exposure differ by race/ethnicity and reproductive characteristics including parity and menopause.
Objective:
We aimed to examine temporal variations in serum PFAS concentrations among midlife women from the Study of Women’s Health Across the Nation.
Methods:
Serum concentrations of 11 PFAS homologues were measured in 75 White, Black and Chinese women with blood samples collected in 1999–2000, 2002–2003, 2005–2006, and 2009–2011. Rates of changes in PFAS concentrations were calculated assuming a first-order elimination model. Associations between PFAS concentrations and race/ethnicity, menstruation and parity were evaluated with linear mixed models, adjusting for age, body mass index and study site.
Results:
Serum concentrations of linear-chain perfluorooctanoic acid (n-PFOA), linear- and branched-chain perfluorooctane sulfonic acid (n-PFOS and sm-PFOS) decreased significantly (−6.0%, 95% CI: −8.3%, −3.6% per year for n-PFOA; −14.8%, 95% CI: −17.3%, −12.3% per year for n-PFOS; −16.9%, 95% CI: −19.1%, −14.6% per year for sm-PFOS); whereas perfluorononanoic acid (PFNA) increased (16.0%, 95% CI: 10.6%, 21.6% per year). Detection rates of perfluorodecanoic acid (PFDeA) and perfluoroundecanoic acid (PFUA) doubled. Temporal trends varied significantly by race/ethnicity. Chinese women tended to have consistently higher PFNA concentrations at each follow-up visit, compared with White and Black women. Serum PFHxS concentrations significantly decreased in White and Black women, but not in Chinese. Menstruating women consistently had lower concentrations. Parity was associated with lower concentrations at baseline but the differences between nulliparous and parous women became smaller over time.
Conclusions:
Our results suggest longitudinal declines in serum concentrations of legacy PFAS and increases in serum concentrations of emerging compounds from 1999 to 2011 in midlife women. Temporal trends in PFAS concentrations are not uniform across race/ethnicity and parity groups.
Keywords: Per- and polyfluoroalkyl substances, Biomonitoring, Midlife women, Racial/ethnic disparities, Menstruation, Parity
1. INTRODUCTON
Per- and polyfluoroalkyl substances (PFAS) are anthropogenic compounds that have been widely used since the discovery of polytetrafuoroethylene (commonly known as Teflon) (Kotthoff et al., 2015; Prevedouros et al., 2006). Due to the strong electronegativity and small atomic size of fluorine, the perfluoroalkyl moiety imparts unique water- and oil-repellency, and thermal and chemical stability to these compounds, compared to their hydrocarbon counterparts. Many consumer products contain specific members of this family of chemicals, such as nonstick cookware, weatherproof clothing, surface protectants, carpets, greaseproof food packaging, aqueous film-forming foams, and etc. (Begley et al., 2005; Butenhoff et al., 2006; Kantiani et al., 2010; Kissa, 2011; Trudel et al., 2008).
PFAS are of particular concern as these compounds have been linked to hepatocellular damage (Darrow et al., 2016), chronic kidney disease (Shankar et al., 2011) and metabolic disorders (Liu et al., 2018; Sun et al., 2018), and have also been identified as potential reproductive toxicants (Jensen and Leffers, 2008). Perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) (so-called C8 compounds) have been the most extensively produced and studied chemicals. Epidemiological studies have shown associations between exposure to elevated concentrations of PFOA and PFOS with menstrual cycle irregularity (Lyngsø et al., 2014; Zhou et al., 2017), premature ovarian insufficiency and accelerated ovarian aging (Knox et al., 2011; Taylor et al., 2014; Zhang et al., 2018), steroidogenic defects (Barrett et al., 2015), and infertility (Bach et al., 2016). Given the toxicity, persistence and bioaccumulation of PFAS, government and regulatory bodies in some parts of the world have been working towards agreements and regulations to limit the production of PFOA and PFOS since 2000 (Significant New Use Rule Final Rule and Supplemental Proposed Rule, 2002; Stockholm Convention, 2016; US EPA, 2016, 2000). At the same time, several other PFAS homologues have increased steadily in the general population (Calafat et al., 2007). Therefore, the quantitation of multiple PFAS in human serum is important to adequately assess human exposure and associated health risks.
Previous human biomonitoring studies primarily focused on repeated cross-sectional data and have shown declines in PFOS, PFOA and their precursors (Calafat et al., 2007, 2006; Kato et al., 2011b; Liu et al., 2015; Olsen et al., 2012). To date, few published studies have reported longitudinal trends in the United States (Kato et al., 2014; Wu et al., 2015). These longitudinal studies (Kato et al., 2014; Wu et al., 2015) included only two recorded time points within a short time frame (~6–12 months) among pregnant women in Ohio and children and adults in California, respectively. Given that many PFAS are slowly eliminated, e.g., half-lives can exceed 2 years, it is important to have multiple measurements over a relatively long follow-up period to adequately describe within-person changes.
Understanding the health effects of common exposures is challenging as exposures may vary by participant characteristics such as race/ethnicity and geography. Serum concentrations also cannot easily be related to probable ongoing background exposures in midlife women, since factors such as menstruation and parity may not properly be accounted for in female elimination rates. To improve our understanding of exposure to PFAS in midlife women, we describe the longitudinal changes in PFAS concentrations during the menopausal transition and evaluate whether time trends differed by reproductive aging (i.e. menstruation status), parity, or race/ethnicity. The present study was based on four repeated measurements of serum PFAS concentrations collected 1999 through 2011 in a cohort of 75 multiethnic midlife women aged 45–56 years at baseline (1999–2000) from the United States.
2. METHODS
2.1. Study populations.
Participants were drawn from the Study of Women’s Health Across the Nation (SWAN), a multicenter, multi-ethnic, community-based cohort of midlife women. Detailed study designs were described elsewhere (Sowers et al., 2000). Briefly, 3,302 women were recruited at baseline during 1996–1997 from 7 study sites in the United States (Boston, MA; Chicago, IL; southeast Michigan, MI; Los Angeles, CA; Newark, NJ; Oakland, CA; Pittsburgh, PA). Each site recruited White women and women from a specified minority group (black in Boston, Chicago, southeast Michigan, and Pittsburgh; Chinese in Oakland; Japanese in Los Angeles; Hispanic in Newark). Baseline eligibility criteria for enrollment into the longitudinal cohort included the following: aged 42 to 52 years, having an intact uterus and at least one ovary, not currently using exogenous hormones affecting ovarian functions, having a menstrual period in the previous 3 months, and self-identified with a site’s designated racial/ethnic group.
The SWAN Multi-Pollutant Study (MPS) was initiated in 2016 to examine multiple environmental chemical exposures, including PFAS, polychlorinated biphenyls (PCBs), organochlorine pesticides, polybrominated diphenyl ethers (PBDEs), metals, phenols, phthalates, and organophosphate pesticide among midlife women. A schematic diagram of the SWAN MPS sampling procedure is shown in Supplemental Materials Figure A.1. Repository samples available from the third follow-up visit (V03, 1999–2000) were used for environmental exposure assessments. Of 2,694 women enrolled at V03, we excluded women from Chicago (n=368) and Newark (n=278) because urine samples were not available in these two sites. We additionally excluded 648 women with insufficient serum or urine samples at V03 or insufficient urine samples at V06 (for the assessment of non-persistent phenols and phthalates), yielding the sample size of 1400. Details of the study design are described elsewhere (Park et al., 2019).
We also designed a pilot project at the SWAN V03 (1999–2000), V06 (2002–2003), V09 (2005–2006) and V12 (2009–2011) to examine temporal trends in a panel of persistent organic pollutants, including PCBs, PBDEs, and PFAS. Of 1,400 participants with serum samples available at V03, we picked three study sites (Boston, MA in the East; southeast Michigan in the Midwest; Oakland, CA in the West), to capture temporal variations in different race/ethnic groups and geographical locations with limited resources. Because the menopausal transition and related body composition changes may impact chemical distributions of persistent organic pollutants, we then conducted random sampling to get a subsample of women (n=75) with 4 follow-up visits at V03, V06, V09 and V12 (n=300 observations in total) stratified on changes in waist circumferences and race/ethnicity. The sampling procedure of this pilot project is given in Supplemental Materials Figure A.2.
Characteristics among 1,400 participants at V03 were compared to those included in the temporal trend study. Women who were followed until V12 had a higher level of education (P<0.05, Supplemental Materials Table A.2). No significant differences were found for other socio-demographic factors (P>0.05, Supplemental Materials Table A.2) or for PFAS serum concentrations at the baseline (P>0.05, Supplemental Materials Table A.3). In addition, waist circumference changes did not impact the temporal trends of PFAS concentrations (data not shown).
2.2. PFAS measurements.
We obtained serum samples from 75 women at SWAN V03 (1999/2000), V06 (2002/2003), V09 (2005/2006), and V12 (2009/2011). Serum samples were sent to the Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention.
Perfluoroalkyl acids (PFAAs) are some of the most simple PFAS molecules, which are essentially non-degradable. The PFAAs contain two major groups, perfluoroalkyl carboxylic acids (PFCAs) and perfluoroalkane sulfonic acids (PFSAs). PFCAs included PFOA, perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDeA), perfluoroundecanoic acid (PFUA), and perfluorododecanoic acid (PFDoA). PFSAs included perfluorohexane sulfonic acid (PFHxS), and PFOS. PFOA and PFOS may be present as mixtures of linear- (n-) and branched- (sm-) chain isomers depending on the manufacturing process used. A linear isomer is composed of carbon atoms bonded to only one or two carbons, which form a straight backbone. A branched isomer consists of at least one carbon atom bonded to more than two carbon atoms. Note that 2-(N-ethyl-perfluorooctane sulfonamide) acetate (EtFOSAA) and 2-(N-methyl-perfluorooctane sulfonamide) acetate (MeFOSAA) are perfluoroalkane sulfonamide acetic acids (FASAAs) which belong to polyfluorinated compounds and act as intermediate environmental transformation products of PFOS. Chemical names and formulas of PFAS analyzed in this study are shown in Supplemental Materials Table A.1.
We measured PFHxS, n-PFOS, sum of sm-PFOS, EtFOSAA, MeFOSAA, n-PFOA, sum of sb-PFOA, PFNA, PFDeA, PFUA, and PFDoA in 1 mL of serum, using an online solid phase extraction-high performance liquid chromatography-isotope dilution-tandem mass spectrometry (on-line SPE-HPLC-MS/MS) method (Kato et al., 2011a) that allows for selective analyses of serum. The limits of detection (LODs) were determined during method validation by running 5 repeated measurements of low-level standards spiked onto calf serum (Taylor, 1981) and then calculating the standard deviation of the instrument response. The limit of detection was then defined as three times the standard deviation. The LODs were 0.1 ng/mL for all PFAS analytes.
In parallel with sample analyses, the following quality control (QC) procedures were conducted: (a) standard reference materials (SRMs) and spike and surrogate recoveries were tested periodically; (b) linearity and drift checks were performed with each sample batch; (c) internal standards consisting of deuterated standards are used on each sample; (d) duplicates were analyzed in each batch; (e) method detection limits (MDLs) for each target compound were determined for each matrix; and (f) blanks (instrumental, field and laboratory) were run with each sample batch. Each sample run contained 9 calibration standards, two low-concentration QCs (QCL), two high-concentration QCs (QCH), three serum blanks, and two reagent blanks. All solvents and other materials contacting samples are proved to be clean, as confirmed using blanks. The coefficient of variation was 5.9–12.1% for the low QC pools; and 5.9–10.6% for the high QC pools.
2.3. Covariates.
Time-independent covariates included race/ethnicity, study site, and baseline age, measured body mass index, and parity. Parity, which represents the sum of the number of live births and stillbirths, was classified into nulliparous or parous. Time-varying variables included menstruation status. Menstruation was determined from self-administered questionnaires. At each visit women were asked: “Did you have any menstrual bleeding since your last study visit?” All these variables were fully observed in the study sample.
2.4. Comparison with NHANES data.
We used the NHANES 1999–2010 data to compare temporal trends in PFAS between our study and the contemporary US representative population. Only women with the same age range at each cycle matched to our longitudinal follow-up visits were included in the analyses. Thus, participants included in the comparison were 91 women aged 45–56 years from NHANES 1999–2000, 119 women aged 48–59 years from NHANES 2003–2004, 124 women aged 51–62 years from NHANES 2005–2006, and 232 women aged 55–68 years from NHANES 2009–2010 (a total of 566). Survey-weighted medians and interquartile ranges were computed at each cycle.
2.5. Statistical analysis.
The concentrations of 11 PFAS were described using geometric mean (GM), geometric standard deviation (GSD), median, interquartile range (IQR), 95th and 99th percentiles, and range. For measurements below the LODs, the values were substituted with . An intra-class correlation coefficient (ICC) was calculated to assess reliability of serum PFAS measurements using the following formula , where σB2 is the between-subject variation and σW2 is the within-subject variance. An ICC is very useful for analyzing continuous measures. A high ICC indicates that differences in PFAS serum concentration between subjects have greater variability than that within subjects over the study period (Enderlein, 2007).
PFAS homologues that were detected in at least 70% of the samples were included in the trend analysis. Under the assumption of a first-order elimination model, halving time (T1/2) for PFAS were calculated by , where β was the fixed effect coefficient of time; (eβ − 1) × 100% was expressed as the excretion constant rate. Repeated measure analysis of variance (ANOVA) tests were conducted to compare serum PFAS concentrations by participant characteristics.
In the adjusted analyses, linear mixed models (LMMs) were fitted with random intercepts to calculate effect estimates and standard errors (SEs) for assessment of time-varying PFAS serum concentrations by participant characteristics. PFAS concentrations were modeled as a function of follow-up visits with baseline visit as the reference, to capture non-linear trends. A natural logarithmic transformation was applied to serum PFAS concentrations to approximate a normal distribution. Both crude analyses and analyses with adjustment for age at baseline, race/ethnicity, study site (Santoro et al., 2011), BMI at baseline, parity, and menstrual bleeding were conducted. To explore temporal variations by covariates, interactions terms between time and these covariates were included in the regressions of SWAN follow-up visits. Likelihood ratio tests were used to compare models with and without interaction terms to determine whether time trajectories differed by these factors.
Given the limited sample size we used the most parsimonious adjusted model as shown below,
where PFASi,j is serum concentrations for the ith subject with the jth observation; X × Y is the interaction term between X and Y; V06 is SWAN visit 06 during 2002 – 2003, V09 is SWAN visit 09 during 2005 – 2006, and V12 is SWAN visit 12 during 2009 – 2011; Covariates include age at baseline, BMI at baseline, and study site.
Statistical analyses were conducted by R 3.4.4 (R Core Team (2018). R Foundation for Statistical Computing, Vienna, Austria) and SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina).
3. RESULTS
3.1. Participant characteristics.
Characteristics of 75 women at SWAN V03 (1999/2000), V06 (2002/2003), V09 (2005/2006) and V12 (2009/2011) are described in Table 1. The average age of participants was 49 years (range = 45 to 56 years) at V03, the baseline period for this analysis, in 1999–2000. The median (IQR) BMI was 26.1 (22.7–32.0) kg/m2 at baseline and did not change much during the follow-up visits. All 75 women lived in the same place during the follow-up visits. Among participants, 25.3% resided in southeast Michigan, 30.7% in Boston, and 44.0% in Oakland; 49.3% were White, 25.3% were Black and 25.3% were Chinese. 89.3% of participants experienced menstrual bleeding since their last visit at V03 (on average, approximately 12 months after V02) decreasing to 4.0% at V12. Sixteen percent were nulliparous at V03 and no one became pregnant or breastfed during the follow-up visits.
Table 1.
Characteristics of participants in the Study of Women’s Health Across the Nation (SWAN) 1999–2011.
| SWAN visit | V03 | V06 | V09 | V12 |
|---|---|---|---|---|
| Year of sample collection | 1999–2000 | 2002–2003 | 2005–2006 | 2009–2011 |
| No. of participants | 75 | 75 | 75 | 75 |
| Age at interview1, years | 49.4 (47.1–51.2) | 52.5 (50.1–54.2) | 55.4 (53.2–57.3) | 59.6 (57.8–62.1) |
| Body mass index1, kg/m2 | 26.1 (22.7–32.0) | |||
| Study site | ||||
| Southeast Michigan | 19 (25.3%) | |||
| Boston, MA | 23 (30.7%) | |||
| Oakland, CA | 33 (44.0%) | |||
| Race/ethnicity | ||||
| Black | 19 (25.3%) | |||
| White | 37 (49.3%) | |||
| Chinese | 19 (25.3%) | |||
| Menstrual bleeding since last visit | 67 (89.3%) | 46 (61.3%) | 23 (30.7%) | 3 (4.0%) |
| Parity | ||||
| Nulliparous | 12 (16.0%) | |||
| Parous | 63 (84.0%) | |||
Median (interquartile range).
3.2. Longitudinal trends of serum PFAS concentrations from 1999 to 2011.
Distributions of serum PFAS concentrations across follow-up visits are displayed for selected compounds with detection rates >70% in Figure 1, and presented for all the PFAS analytes in Supplemental Materials Table A.4. Over 70% of the serum samples had detectable concentrations above LODs at each time point for n-PFOA, n-PFOS, sm-PFOS, PFHxS, and PFNA. Substantial decreases were observed in median concentrations of n-PFOA (3.30 to 2.60 ng/mL; P=0.001), n-PFOS (17.00 to 7.50 ng/mL; P<.0001), and sm-PFOS (6.20 to 2.50 ng/mL; P<.0001) during 1999 and 2011. Over 98% of the samples had detectable EtFOSAA and MeFOSAA at V03 while the detectable percentages decreased to 1.33% and 50.67%, respectively, during the follow-up visits. PFHxS serum concentrations did not change significantly during 1999 and 2011 (1.50 to 1.20 ng/mL; P=0.05).
Figure 1.
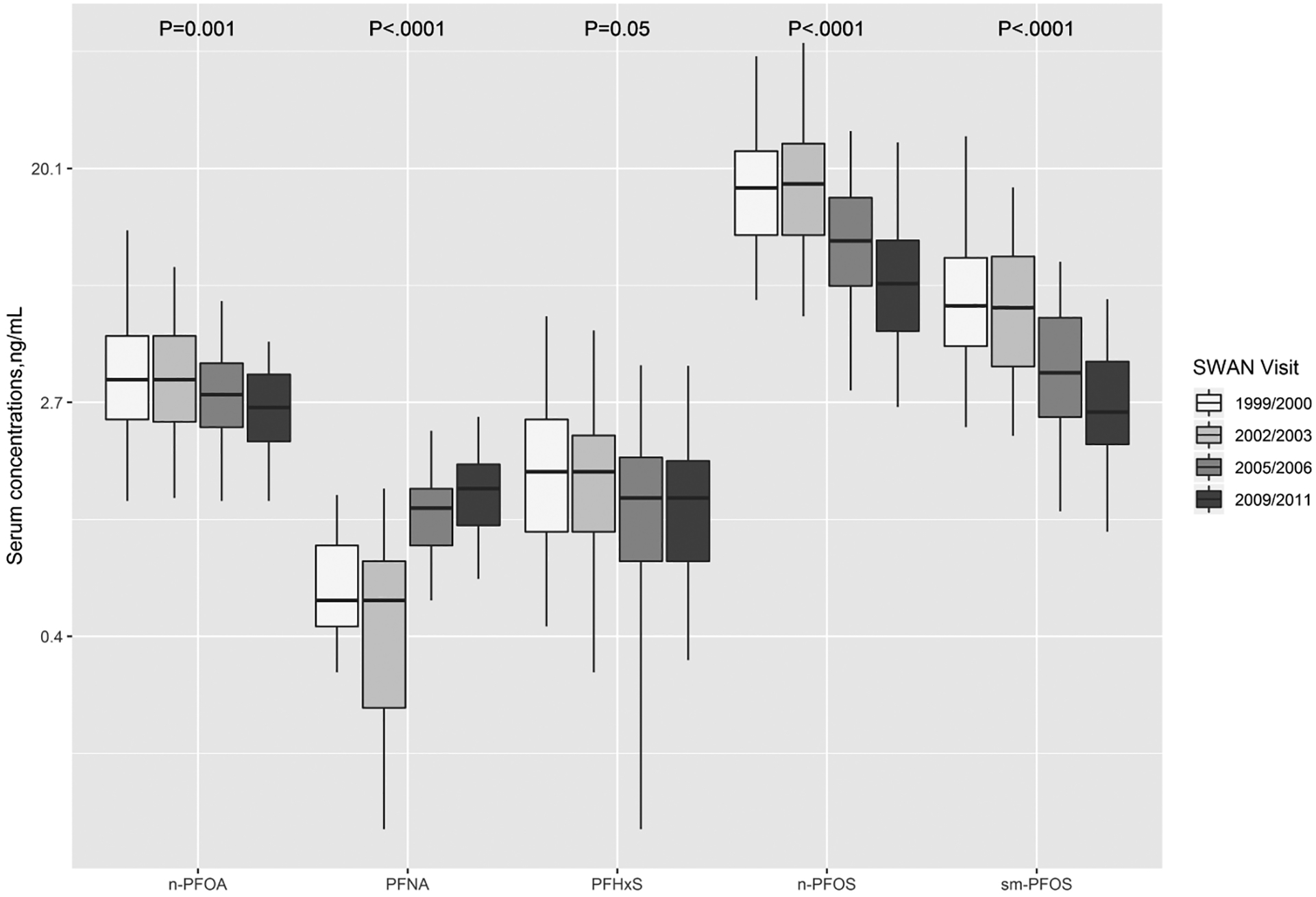
Concentrations of selected PFAS with detection rates >70% analyzed in repeated serum samples of women (n=75) across the United States for four SWAN visits. Boxes represent the 25th-75th percentiles, horizontal lines represent the median, and whiskers indicate 5th and 95th percentiles, respectively. Note that a log scale is used for Y axis. The limits of detection were 0.1 ng/mL for all PFAS analytes. Abbreviations: n-PFOA, linear-chain perfluorooctanoic acid; PFNA, perfluorononanoic acid; PFHxS, perfluorohexane sulfonic acid; n-PFOS, linear-chain perfluorooctane sulfonic acid; sm-PFOS, sum of branched-chain perfluorooctane sulfonic acid.
In contrast to other PFAS homologues, PFNA showed increase steeply (0.50 to 1.30 ng/mL), comparing V03 (1999/2000) to V12 (2009/2011) (P<.0001). PFDeA and PFUA concentrations increased during 1999–2011 with a detection rate of 89.3% and 66.7% at V12, respectively, from 42.7% and 36% at V03. Fewer than 20% of the samples had detectable levels of sb-PFOA and PFDoA. Intraclass correlation coefficients ranged 0.16 to 0.48, indicating low similarity and highly variable values from the same subject over time.
On average, serum concentrations of n-PFOA, n-PFOS, sm-PFOS and PFHxS were estimated to be decreased by 6.0% (95% CI: −8.3%, −3.6%), 14.8% (95% CI: −17.3%, −12.3%), 16.9% (95% CI: −19.1%, −14.6%) and 6.2% (95% CI: −9.1%, −3.2%) per year, respectively, as shown in Table 2. The halving time was estimated to be 11.2 (95% CI: 8.0–19.0) years for n-PFOA, and 4.3 (95% CI: 3.6–5.3), 3.7 (95% CI: 3.3–4.4) and 10.8 (95% CI: 7.2–21.5) years and for n-PFOS, sm-PFOS and PFHxS, respectively. On the contrary, PFNA increased by 16.0% (95% CI: 10.6%, 21.6%) during follow-up visits with a doubling time of 4.7 (95% CI: 3.5–6.9) years. Adjustment for age at baseline, race and study site and menstruation did not account for temporal variations of PFAS serum concentrations, except for PFNA, of which the time effects became insignificant (P=0.12).
Table 2.
Halving or doubling time for serum PFAS1 concentrations among 75 women (300 observations in total) in SWAN 1999–2011.
| n-PFOA | n-PFOS | sm-PFOS | PFHxS | PFNA | |
|---|---|---|---|---|---|
| Unadjusted | |||||
| Percent change per year | −6.0% | −14.8% | −16.9% | −6.2% | 16.0% |
| 95% CI | −8.3%, −3.6% | −17.3%, −12.3% | −19.1%, −14.6% | −9.1%, −3.2% | 10.6%, 21.6% |
| p-value | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 |
| Halving or doubling time, year | 11.2 | 4.3 | 3.7 | 10.8 | −4.73 |
| 95% CI | 8.0, 19.0 | 3.6, 5.3 | 3.3, 4.4 | 7.2, 21.5 | −6.9, −3.53 |
| Adjusted2 | |||||
| Percent change per year | −6.9% | −12.0% | −14.7% | −6.3% | 4.7% |
| 95% CI | −10.0%, −3.7% | −15.4%, −8.4% | −17.7%, −11.6% | −10.2%, −2.1% | −1.2%, 10.8% |
| p-value | <.0001 | <.0001 | <.0001 | 0.004 | 0.12 |
| Halving or doubling time, year | 9.6 | 5.4 | 4.4 | 10.7 | −15.33 |
| 95% CI | 6.6, 18.3 | 4.1, 7.9 | 3.6, 5.6 | 6.4, 32.9 | −58.4, 6.73 |
PFAS with serum concentrations above limit of detection more than 60% were included in the analyses.
Adjusting for age at baseline, body mass index at baseline, race/ethnicity, study site, menstrual bleeding, and parity.
Negative numbers indicate doubling time.
3.3. Comparison with NHANES data.
Comparisons of the present study with the contemporary NHANES data of general U.S. women with the same age range are displayed in Supplemental Materials Figure A.3. Both showed serum concentrations of PFOA, PFOS, PFHxS, and PFNA with detection rates>70%. SWAN participants had lower median concentrations over time, but the spread of IQRs in SWAN and the NHANES datasets generally overlapped. Both had decreasing trends of PFOA, PFOS and PFHxS, and increasing trends of PFNA. Detection rates of PFDeA and PFUA increased in both SWAN and NHANES data but decreased for EtFOSAA (data not shown). Neither showed detectable concentrations of PFDoA.
3.4. Determinants of temporal changes in PFAS concentrations.
Unadjusted median (IQR) log-transformed serum concentrations of n-PFOA, n-PFOS, sm-PFOS, PFHxS and PFNA by race/ethnicity, menstruation status and parity over time are displayed in Figures 2–4. In the unadjusted analyses, temporal trends differed significantly by race/ethnicity for n-PFOA (P=0.007), n-PFOS (P=0.02), and PFHxS (P=0.04) but not for sm-PFOS (P=0.13) and PFNA (P=0.19). Menstruating women had lower PFAS concentrations and the differences remain almost the same over time during the follow-up visits (P=0.31 for n-PFOA, P=0.29 for n-PFOS, P=0.80 for sm-PFOS, P=0.36 for PFHxS, and P=0.07 for PFNA). Nulliparous women had higher serum PFAS concentrations at baseline but the time trajectories of n-PFOA and n-PFOS had changed significantly during the follow-up visits (P=0.03 for n-PFOA, P=0.03 for n-PFOS). In contrast, the trajectories of other PFAS homologues did not differ by parity (P=0.19 for sm-PFOS, P=0.99 for PFHxS, and P=0.28 for PFNA).
Figure 2.
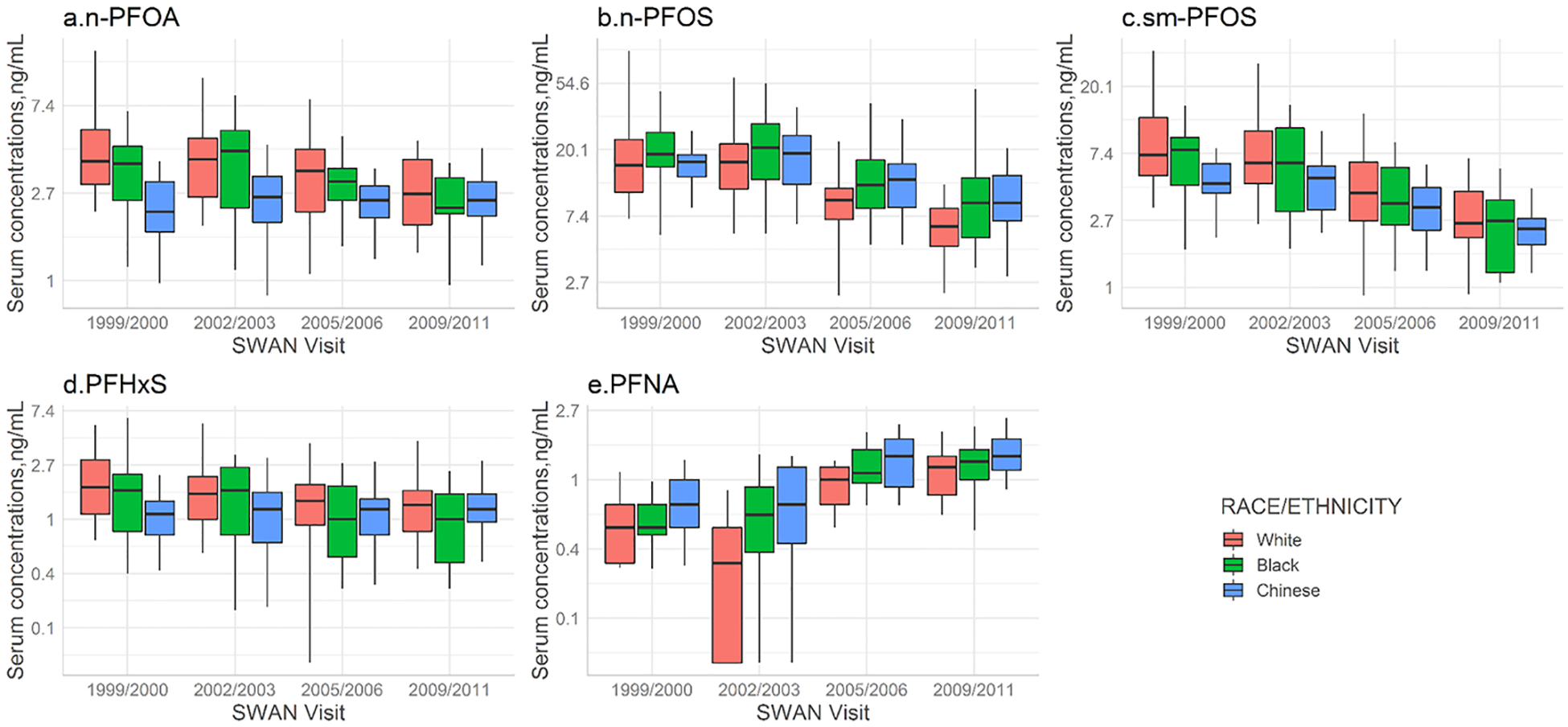
Serum concentrations of selected PFAS with detection rates >70% by race/ethnicity in women (n=75) across the United States for four SWAN visits. Boxes represent the 25th-75th percentiles, horizontal lines represent the median, and whiskers indicate 5th and 95th percentiles, respectively. Repeated measure analysis of variance tests was conducted to compare temporal variations of PFAS concentrations by racial/ethnic groups: P=0.007 for n-PFOA; P=0.02 for n-PFOS; P=0.13 for sm-PFOS; P=0.04 for PFHxS; and P=0.19 for PFNA. Note that a log scale is used for Y axis. The limits of detection were 0.1 ng/mL for all PFAS analytes. Abbreviations: n-PFOA, linear-chain perfluorooctanoic acid; PFNA, perfluorononanoic acid; PFHxS, perfluorohexane sulfonic acid; n-PFOS, linear-chain perfluoroctane sulfonic acid; sm-PFOS, sum of branched-chain perfluorooctane sulfonic acid.
Figure 4.

Serum concentrations of selected PFAS with detection rates >70% by parity status (nulliparous or parous) in women (n=75) across the United States for four SWAN visits. Boxes represent the 25th-75th percentiles, horizontal lines represent the median, and whiskers indicate 5th and 95th percentiles, respectively. Repeated measure analysis of variance tests was conducted to compare temporal variations of PFAS concentrations by parity group: P=0.03 for n-PFOA; P=0.03 for n-PFOS; P=0.19 for sm-PFOS; P=0.99 for PFHxS; P=0.28 for PFNA. Note that a log scale is used for Y axis. The limits of detection were 0.1 ng/mL for all PFAS analytes. Abbreviations: n-PFOA, linear-chain perfluorooctanoic acid; PFNA, perfluorononanoic acid; PFHxS, perfluorohexane sulfonic acid; n-PFOS, linear-chain perfluorooctane sulfonic acid; sm-PFOS, sum of branched-chain perfluorooctane sulfonic acid.
Figure 5 depicts the adjusted trends of n-PFOA, n-PFOS, sm-PFOS, PFHxS and PFNA across the four visits stratified by race/ethnicity, menstrual bleeding and parity, controlling for age at baseline, study site, and BMI at baseline (see effect estimates and standard errors from mixed regression models in Supplemental Materials Table A.5).
Figure 5.
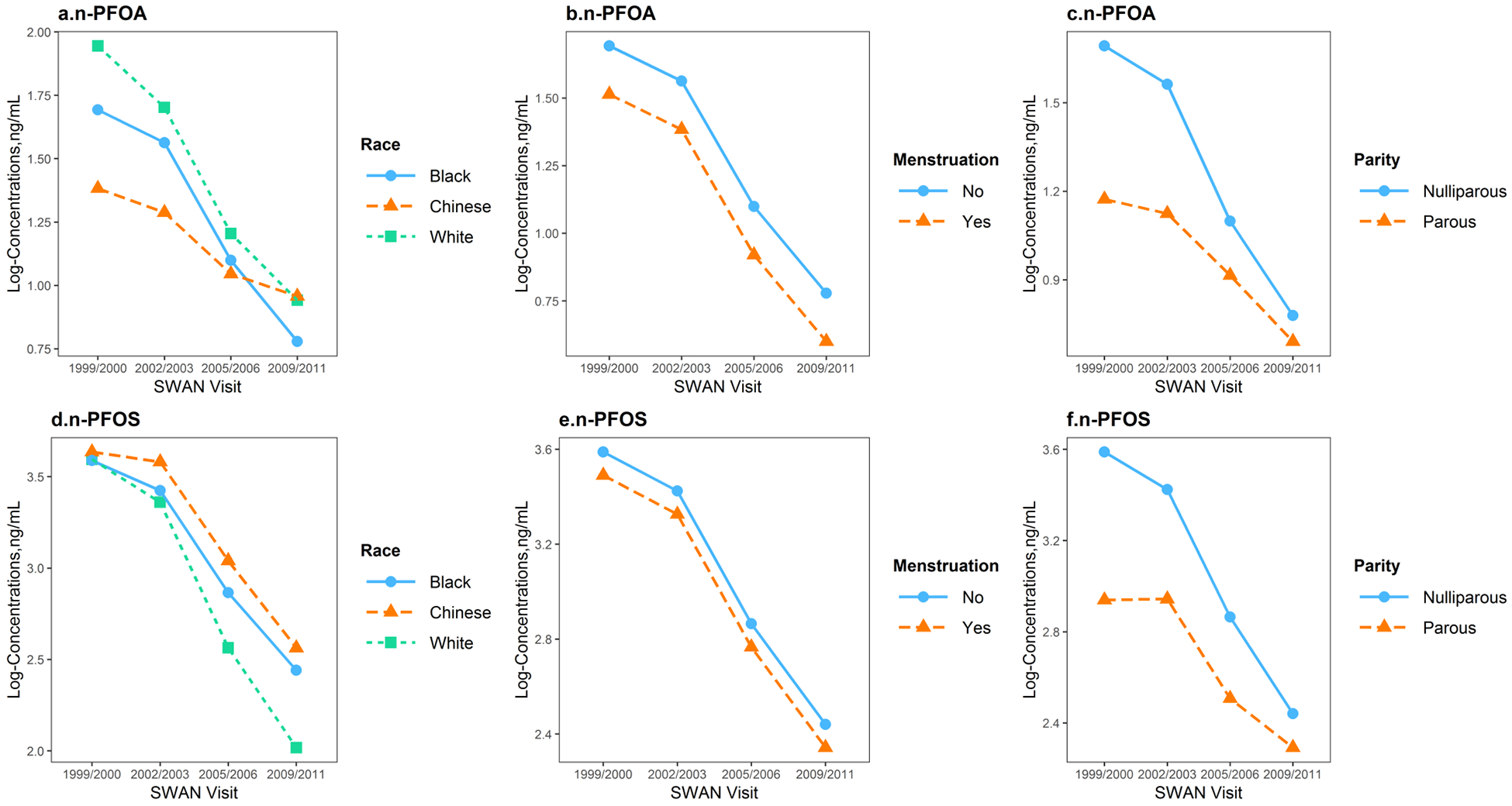
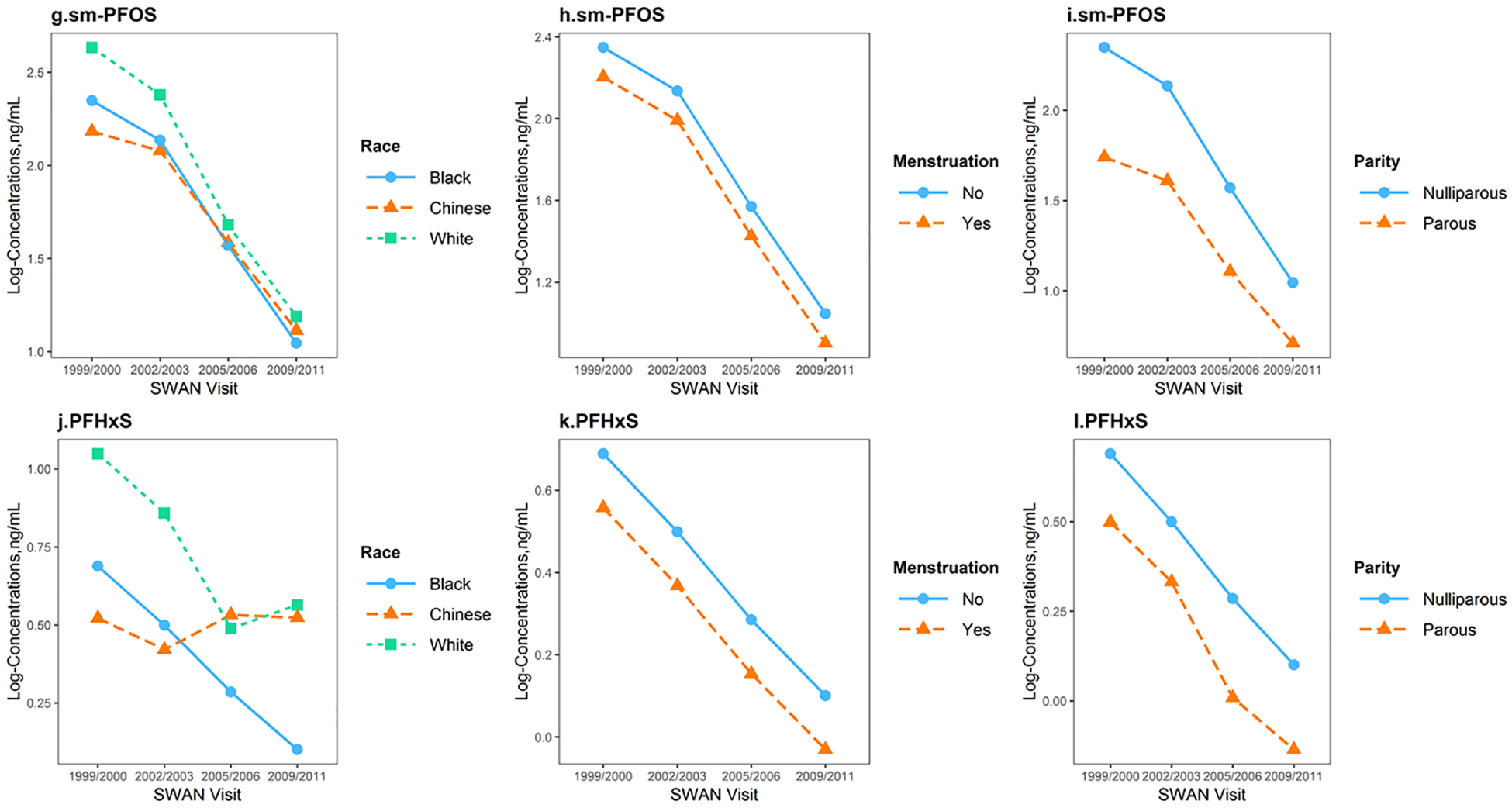
Predicted temporal trends of log-transformed n-PFOA, n-PFOS, sm-PFOS, PFHxS and PFNA serum concentrations at SWAN V03 (1999/2000), V06 (2002/2003), V09 (2005/2006) and V12 (2009/2011), stratified by race/ethnicity, menstruation status and parity, adjusting for age at baseline, study site and body mass index at baseline, based on fixed effects estimated from mixed regression models. Abbreviations: n-PFOA, linear-chain perfluorooctanoic acid; PFNA, perfluorononanoic acid; PFHxS, perfluorohexane sulfonic acid; n-PFOS, linear-chain perfluorooctane sulfonic acid; sm-PFOS, sum of branched-chain perfluorooctane sulfonic acid.
Race/ethnicity was an independent predictor of PFAS concentrations and their trends over time. For n-PFOA, women had significantly lower concentrations from baseline to V12, but trends differed significantly by race/ethnicity (P for interaction=0.001). White women had the highest exposures to n-PFOA at baseline; however, time mitigated the racial/ethnic differences. Chinese women showed a much slower decline in from V03 to V12. For n-PFOS, all three racial/ethnic groups had significantly lower concentrations at V12 compared to V03, but White women had a more rapid decline from baseline to V12, compared with that of Chinese and Black (P for interaction=0.0007). Temporal trends of sm-PFOS and PFHxS also differed significantly by race/ethnicity (P for interaction=0.03 and 0.008, respectively), with racial/ethnic differences decreased over time. However, PFNA serum concentrations did not change significantly over time across racial/ethnic groups (P for interaction=0.13) but Chinese women showed consistently higher concentrations compared to other racial/ethnic groups (P=0.03 at baseline for race/ethnicity).
Compared to women without menstrual bleeding since the last visit, menstruating women had 16.4% (95% CI: −26.7%, −4.7%), 18.3% (95% CI: −31.9%, −1.9%), 13.4% (95% CI: −22.8%, −2.9%) lower serum concentrations of n-PFOA, PFNA, and sm-PFOS, respectively, during follow-up visits. n-PFOS or PFHxS were not associated with menstruation status (P=0.14 and 0.15, respectively). Interaction terms between time and menstrual bleeding were not significant.
In addition, serum concentrations of n-PFOA, n-PFOS and sm-PFOS also varied significantly across parity. Concentrations decreased by −40.5% (95% CI: −58.5%, −14.6%) for n-PFOA, −47.7% (95% CI: −65.6%, −20.7%) for n-PFOS, and −45.5% (95% CI: −64.0%, −17.4%) for sm-PFOS, comparing parous to nulliparous women at baseline; whereas the differences became significantly smaller for n-PFOA and n-PFOS (P for interaction=0.02 for n-PFOA, and 0.008 for n-PFOS) over time, but did not change for sm-PFOS (P for interaction=0.21). No significant differences by parity status were observed for PFHxS (P=0.50) and PFNA (P=0.31) at baseline, or during follow-up visits (P for interaction= 0.95 for PFHxS, and 0.47 for PFNA). Further adjustment for education, employment status, and difficulty paying for basics did not eliminate the observed differences by race/ethnicity, menstruation, or parity (data not shown).
4. DISCUSSION
This study updates the existing knowledge on human exposure to PFAS. It provides valuable data on midlife women’s exposure to PFAS as they transition through menopause and temporal trends of PFAS serum concentrations, which have rarely been reported before. This study also provides new evidences on the contribution of race/ethnicity, menstruation, and parity to the temporal variations of PFAS concentrations.
4.1. Longitudinal trends of serum PFAS concentrations from 1999 to 2011.
Overall, serum concentrations of legacy compounds, including n-PFOA, n-PFOS, sm-PFOS, EtFOSAA and MeFOSAA peaked at baseline. In contrast, increasing trends were observed for PFNA, PFUA, and PFDeA from 1999 to 2011.
Along with a recent study summarizing PFAS data in NHANES (Calafat et al., 2007), our findings indicated effectiveness of deliberate efforts to reduce the production of PFOA, PFOS and its precursors in the United States. Unlike the majority of legacy PFAS, the current study and previous studies have suggested increases in serum concentrations of other long-chain PFAS, including PFNA (Calafat et al., 2006, 2007; Kato et al., 2011; Spliethoff et al., 2008), PFUA (Calafat et al., 2006, 2007; Kato et al., 2011; Spliethoff et al., 2008), and PFDeA (Calafat et al., 2006, 2007; Kato et al., 2011; Spliethoff et al., 2008).These increasing trends indicate an ongoing exposure. For example, PFNA was found to be present in several commonly used consumer products, e.g. paper-based food contact materials and textiles (Kotthoff et al., 2015). In addition, PFNA and PFUA are believed to be manufactured through a transformation of fluorotelomer olefins (Buck et al., 2011), which could be formed by telomere-derived PFAS precursors. It is also possible that the observed increase in PFNA concentrations was related to internal metabolisms (e.g. cessation of menstruation) in midlife women. However, the routes of exposure and control mechanisms for these compounds remain obscure, as the main exposure pathway for PFCAs varies according to homologues and exposure scenario (Gebbink et al., 2015).
No significant changes were observed for serum PFHxS concentrations among the midlife women. However, PFHxS was the second most abundant PFSAs next to PFOS during 2003 and 2011. Previous research indicates that higher prevalence of PFHxS could be associated with increased use of stovetop Teflon cookware (Hu et al., 2018), preheated packaged/microwavable foods (Hu et al., 2018; Wu et al., 2015), as well as indoor dust (Wu et al., 2015) and lower vacuuming frequency (Siebenaler et al., 2017).
4.2. Differential changes in PFAS concentrations by race/ethnicity from 1999–2011.
Race/ethnicity has previously been correlated with PFAS exposures (Boronow et al., 2019; Calafat et al., 2007; Park et al., 2019). Although serum concentrations of n-PFOA, n-PFOS, and sm-PFOS have declined over all, to our knowledge no longitudinal study to date had assessed whether temporal changes in serum PFAS concentrations differ by race/ethnicity. The present study addresses this gap. Our findings suggest that temporal trends in PFAS exposure are not uniform across racial/ethnic groups, and subpopulations with higher initial PFAS exposures often experienced the greater change over the study period. For example, we observed a more rapid decline in n-PFOA and n-PFOS concentrations among White women, who had higher baseline concentrations compared with other racial/ethnic groups, possibly reflecting differences in consumer product use. A scenario-based risk assessment study (Trudel et al., 2008) reported that in female adults, the most dominant source of PFOA exposure was likely from consumer products including impregnation spray, treated carpets in homes, and coated food contact materials, while a large proportion of PFOS exposure was through intake of contaminated food. Substantial declines in serum concentrations of n-PFOA and n-PFOS among White women might result from changes in product preferences and food consumption. Conversely, White women had a slight increase in PFHxS serum concentrations in 2009–2011. Another recent study of middle-aged women also found that White women with higher serum concentrations of PFHxS compared with Black, likely attributable to exposure from dental floss with Oral-B Glide (Boronow et al., 2019).
Unlike other racial/ethnic groups, Chinese women had increasing exposures to PFHxS since 2002, and consistently higher serum concentrations of PFNA during the follow-up visits. However, little is known about potential sources of exposure in Chinese populations. Socioeconomic characteristics, lifestyle factors or genetics may account for the observed disparities. Compared to White women, both Black and Chinese had lower education attainment, less physical activity; Black women had more difficulty paying for basics; Chinese women had more fish intake (Supplemental Materials Table A.6). Although biomonitoring studies are useful for documenting population exposures to environmental chemicals, they are limited in their ability to identify the contribution of specific sources to personal exposure. Nonetheless, our understanding of sources of PFAS exposure remains incomplete, however, these findings prompt follow-up in future studies.
4.3. PFAS concentrations by parity.
Parity was a significant determinant of PFAS serum concentrations, especially with parous women having lower concentrations of n-PFOA, n-PFOS and sm-PFOS than nulliparous women. This is the first study examining longitudinal trends of PFAS serum concentrations by parity in midlife women. Previous studies found that nulliparous women had higher PFAS maternal concentrations (Berg et al., 2014; Brantsæter et al., 2013; Fei et al., 2007; Jensen et al., 2015; Lewin et al., 2017; Ode et al., 2013). PFAS can be transferred to infants or to the placenta through the umbilical cord (Beesoon et al., 2011; Hanssen et al., 2010; Kato et al., 2014). Blood loss during delivery might also decrease maternal body burden of PFAS (Lorber et al., 2015). After birth, PFAS may gradually re-accumulate in women, and as a result the differences at baseline narrowed over time (Fei et al., 2009). On the other hand, given the longer half-life of long-chain PFAS (e.g. PFNA) they might not be easily excreted during delivery.
4.4. PFAS concentrations by menstruation status.
This study strengthens the evidence that PFAS concentrations are influenced by menstruation status. Menstruating women tended to have lower serum concentrations compared to those without. The results are consistent with a previous pharmacokinetics modeling, in which Wong et al. found that approximately 30% of the PFOS elimination half-life difference between females and males (Wong et al., 2014). Decreased serum concentrations have been shown in premenopausal versus postmenopausal women (Dhingra et al., 2017; Taylor et al., 2014) and, analogously, in men undergoing venesections for medical treatment (Lorber et al., 2015). Given that approximately 90% to 99% of these compounds in the blood are bound to serum albumin (Han et al., 2003; Ylinen and Auriola, 1990), blood loss through menstruation may be an important elimination pathway.
Kudo et al. examined the role of sex hormones and transport proteins on the renal clearance and concluded that: in ovariectomized female rats, 1) estradiol could facilitate transporting PFOA across the membranes of kidney tubules into the glomerular filtrate; 2) treatments with testosterone reduced the clearance of PFOA (Kudo et al., 2002). This conforms to humans. Zhang et al. study reported that the rate of renal elimination was 0.024 mL/day/kg among women >50 years while 0.043 mL/day/kg among women ≤50 years (Zhang et al., 2013). We cannot rule out the possibility of other unknown elimination routes that might elucidate the change of PFAS serum concentrations among midlife women during menopausal transition.
4.5. Strengths and limitations.
Our study is limited by its small sample size. We do not have sufficient power to examine the changes over time stratified by participant characteristics. Instead, we relied on the tests of statistical interactions between time and covariates. We also oversampled Chinese women to better capture racial/ethnic differences. In addition, we did not have information on important sources of exposure including food contact materials, microwavable or packaged food consumption, or use of carpet treatment procedures because no such information was available in this cohort. We were also unable to fully assess the impact of genetics and lifestyle factors which may account for racial/ethnic disparities. Future studies with a larger sample size would provide a clearer picture of the complex relationships between race/ethnicity in PFAS exposure. Lastly, our study participants were 75 midlife women from three geographic locations, so results might not be generalizable to people living in other areas. The study sample is not representative of the base population of the SWAN Multipollutant Study. Women who have completed all 4 visits and provided serum samples were eligible. Also, by design, only women from Oakland, South Michigan, and Boston were included in selection.
A major strength of this study was the opportunity to include persons from different geographical locations in the United States, and Chinese, whose exposures have not been characterized. The repeated measurements also allow for examination of longitudinal intra-individual changes in PFAS serum concentrations. As the ICCs for repeated measurements were relatively low, indicating that one single serum measurement may not be enough to provide a reliable biomarker of PFAS exposures.
5. CONCLUSIONS
In summary, our results depict longitudinal declines in legacy PFAS (i.e. PFOA and PFOS), as well as their branched isomers and precursors MeFOSAA and EtFOSAA among midlife women living in the US during 1999–2011. The findings are consistent with reduced environmental exposures since 2000–2002. We also identified differential patterns of exposure by race/ethnicity, which can provide useful information for developing hypotheses about possible sources of exposure that, especially for PFHxS and PFNA, are poorly understood. Additional analyses should be performed nationwide to examine whether similar racial/ethnic disparities exist across different regions of the country and which compounds (e.g. behaviors, consumer products) are the primary determinants of this risk disparity.
Supplementary Material
Figure 3.
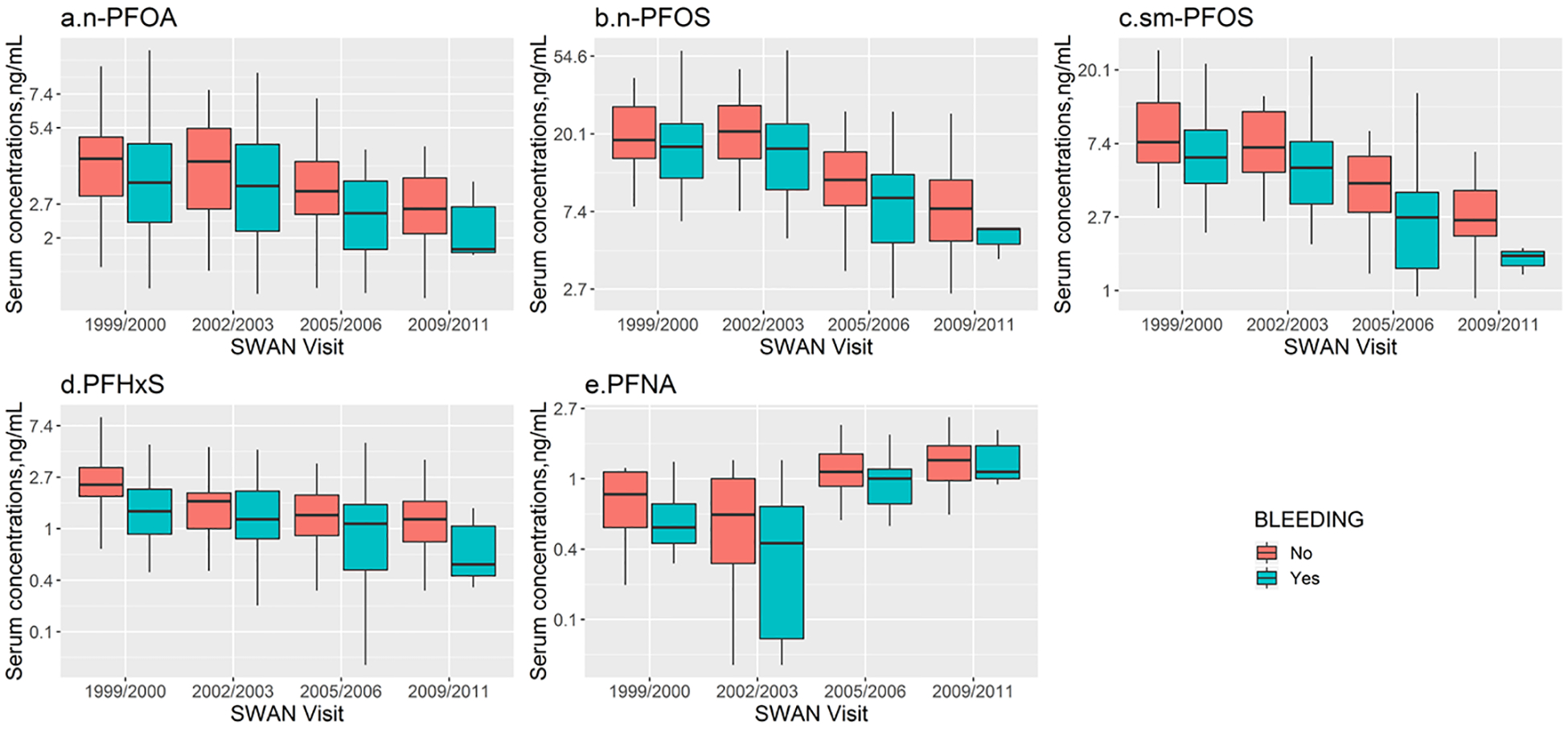
Serum concentrations of selected PFAS with detection rates >70% by menstruation status (i.e. whether had menstrual bleeding since last visit) in women (n=75) across the United States for four SWAN visits. Boxes represent the 25th-75th percentiles, horizontal lines represent the median, and whiskers indicate 5th and 95th percentiles, respectively. Repeated measure analysis of variance tests was conducted to compare temporal variations of PFAS concentrations by menstruation status: P=0.31 for n-PFOA; P=0.29 for n-PFOS; P=0.80 for sm-PFOS; P=0.36 for PFHxS; P=0.07 for PFNA. Note that a log scale is used for Y axis. The limits of detection were 0.1 ng/mL for all PFAS analytes. Abbreviations: n-PFOA, linear-chain perfluorooctanoic acid; PFNA, perfluorononanoic acid; PFHxS, perfluorohexane sulfonic acid; n-PFOS, linear-chain perfluorooctane sulfonic acid; sm-PFOS, sum of branched-chain perfluorooctane sulfonic acid.
ACKNOWLEDGEMENTS
This study was supported by grants from the National Institute of Environmental Health Sciences (NIEHS) R01-ES026578, R01-ES026964 and P30-ES017885, and by the Center for Disease Control and Prevention (CDC)/National Institute for Occupational Safety and Health (NIOSH) grant T42-OH008455.
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH. The SWAN Repository (U01AG017719). This publication was supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 RR024131.
Clinical Centers: University of Michigan, Ann Arbor - Siobán Harlow, PI 2011 - present, MaryFran Sowers, PI 1994-2011; Massachusetts General Hospital, Boston, MA - Joel Finkelstein, PI 1999 - present; Robert Neer, PI 1994 - 1999; Rush University, Rush University Medical Center, Chicago, IL - Howard Kravitz, PI 2009 - present; Lynda Powell, PI 1994 - 2009; University of California, Davis/Kaiser - Ellen Gold, PI; University of California, Los Angeles - Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY - Carol Derby, PI 2011 - present, Rachel Wildman, PI 2010 - 2011; Nanette Santoro, PI 2004 - 2010; University of Medicine and Dentistry - New Jersey Medical School, Newark - Gerson Weiss, PI 1994 - 2004; and the University of Pittsburgh, Pittsburgh, PA - Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD - Chhanda Dutta 2016- present; Winifred Rossi 2012-2016; Sherry Sherman 1994 - 2012; Marcia Ory 1994 - 2001; National Institute of Nursing Research, Bethesda, MD - Program Officers.
Central Laboratory: University of Michigan, Ann Arbor - Daniel McConnell (Central Ligand Assay Satellite Services).
SWAN Repository: University of Michigan, Ann Arbor - Siobán Harlow 2013 - Present; Dan McConnell 2011 - 2013; MaryFran Sowers 2000 - 2011.
Coordinating Center: University of Pittsburgh, Pittsburgh, PA - Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 - 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 - 2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
We also thank Drs. Antonia Calafat and Xiaoyun Ye at Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention, for their support in PFAS assessment.
Footnotes
Disclosure: The authors declare no competing financial interest.
REFERENCES
- Bach CC, Vested A, Jørgensen KT, Bonde JPE, Henriksen TB, Toft G, 2016. Perfluoroalkyl and polyfluoroalkyl substances and measures of human fertility: a systematic review. Crit. Rev. Toxicol 46, 735–755. 10.1080/10408444.2016.1182117 [DOI] [PubMed] [Google Scholar]
- Barrett ES, Chen C, Thurston SW, Haug LS, Sabaredzovic A, Fjeldheim FN, Frydenberg H, Lipson SF, Ellison PT, Thune I, 2015. Perfluoroalkyl substances and ovarian hormone concentrations in naturally cycling women. Fertil. Steril 103, 1261–1270.e3. 10.1016/J.FERTNSTERT.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesoon S, Webster GM, Shoeib M, Harner T, Benskin JP, Martin JW, 2011. Isomer Profiles of Perfluorochemicals in Matched Maternal, Cord, and House Dust Samples: Manufacturing Sources and Transplacental Transfer. Environ. Health Perspect 119, 1659–1664. 10.1289/ehp.1003265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley TH, White K, Honigfort P, Twaroski ML, Neches R, Walker RA, 2005. Perfluorochemicals: Potential sources of and migration from food packaging. Food Addit. Contam 22, 1023–1031. 10.1080/02652030500183474 [DOI] [PubMed] [Google Scholar]
- Berg V, Nøst TH, Huber S, Rylander C, Hansen S, Veyhe AS, Fuskevåg OM, Odland JØ, Sandanger TM, 2014. Maternal serum concentrations of per- and polyfluoroalkyl substances and their predictors in years with reduced production and use. Environ. Int 69, 58–66. 10.1016/J.ENVINT.2014.04.010 [DOI] [PubMed] [Google Scholar]
- Boronow KE, Brody JG, Schaider LA, Peaslee GF, Havas L, Cohn BA, 2019. Serum concentrations of PFASs and exposure-related behaviors in African American and non-Hispanic white women. J. Expo. Sci. Environ. Epidemiol 1 10.1038/s41370-018-0109-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantsæter AL, Whitworth KW, Ydersbond TA, Haug LS, Haugen M, Knutsen HK, Thomsen C, Meltzer HM, Becher G, Sabaredzovic A, Hoppin JA, Eggesbø M, Longnecker MP, 2013. Determinants of plasma concentrations of perfluoroalkyl substances in pregnant Norwegian women. Environ. Int 54, 74–84. 10.1016/j.envint.2012.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SPJ, 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr. Environ. Assess. Manag 7, 513–41. 10.1002/ieam.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenhoff JL, Olsen GW, Pfahles-Hutchens A, 2006. The applicability of biomonitoring data for perfluorooctanesulfonate to the environmental public health continuum. Environ. Health Perspect 114, 1776–82. 10.1289/ehp.9060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Caudill SP, Reidy JA, Needham LL, 2006. Perfluorochemicals in pooled serum samples from United States residents in 2001 and 2002. Environ. Sci. Technol 40, 2128–2134. 10.1021/es0517973 [DOI] [PubMed] [Google Scholar]
- Calafat AM, Wong L-Y, Kuklenyik Z, Reidy JA, Needham LL, 2007. Polyfluoroalkyl Chemicals in the U.S. Population: Data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and Comparisons with NHANES 1999–2000. Environ. Health Perspect 115, 1596–1602. 10.1289/ehp.10598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow LA, Groth AC, Winquist A, Shin HM, Bartell SM, Steenland K, 2016. Modeled perfluorooctanoic acid (PFOA) exposure and liver function in a Mid-Ohio Valley community. Environ. Health Perspect 124, 1227–1233. 10.1289/ehp.1510391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra R, Winquist A, Darrow LA, Klein M, Steenland K, 2017. A Study of Reverse Causation: Examining the Associations of Perfluorooctanoic Acid Serum Levels with Two Outcomes. Environ. Health Perspect 125, 416–421. 10.1289/EHP273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enderlein G, 2007. Fleiss, J. L.: The Design and Analysis of Clinical Experiments. Wiley, New York - Chichester - Brislane - Toronto - Singapore 1986, 432 S., £38.35. Biometrical J. 30, 304–304. 10.1002/bimj.4710300308 [DOI] [Google Scholar]
- Fei C, McLaughlin JK, Lipworth L, Olsen J, 2009. Maternal levels of perfluorinated chemicals and subfecundity. Hum. Reprod 24, 1200–1205. 10.1093/humrep/den490 [DOI] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Tarone RE, Olsen J, 2007. Perfluorinated Chemicals and Fetal Growth: A Study within the Danish National Birth Cohort. Environ. Health Perspect 115, 1677–1682. 10.1289/ehp.10506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebbink WA, Berger U, Cousins IT, 2015. Estimating human exposure to PFOS isomers and PFCA homologues: The relative importance of direct and indirect (precursor) exposure. Environ. Int 74, 160–169. 10.1016/J.ENVINT.2014.10.013 [DOI] [PubMed] [Google Scholar]
- Han X, Snow TA, Kemper RA, Jepson GW, 2003. Binding of perfluorooctanoic acid to rat and human plasma proteins. Chem. Res. Toxicol 16, 775–781. 10.1021/tx034005w [DOI] [PubMed] [Google Scholar]
- Hanssen L, Röllin H, Odland JØ, Moe MK, Sandanger TM, 2010. Perfluorinated compounds in maternal serum and cord blood from selected areas of South Africa: results of a pilot study. J. Environ. Monit 12, 1355 10.1039/b924420d [DOI] [PubMed] [Google Scholar]
- Hu XC, Dassuncao C, Zhang X, Grandjean P, Weihe P, Webster GM, Nielsen F, Sunderland EM, 2018. Can profiles of poly- and Perfluoroalkyl substances (PFASs) in human serum provide information on major exposure sources? Environ. Health 17, 11 10.1186/s12940-018-0355-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AA, Leffers H, 2008. Emerging endocrine disrupters: Perfluoroalkylated substances. Int. J. Androl 31, 161–169. 10.1111/j.1365-2605.2008.00870.x [DOI] [PubMed] [Google Scholar]
- Jensen TK, Andersen LB, Kyhl HB, Nielsen F, Christesen HT, Grandjean P, 2015. Association between perfluorinated compound exposure and miscarriage in Danish pregnant women. PLoS One 10, e0123496 10.1371/journal.pone.0123496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantiani L, Llorca M, Sanchís J, Farré M, Barceló D, 2010. Emerging food contaminants: a review. Anal. Bioanal. Chem 398, 2413–2427. 10.1007/s00216-010-3944-9 [DOI] [PubMed] [Google Scholar]
- Kato K, Basden BJ, Needham LL, Calafat AM, 2011a. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J. Chromatogr. A 1218, 2133–2137. 10.1016/J.CHROMA.2010.10.051 [DOI] [PubMed] [Google Scholar]
- Kato K, Wong L-Y, Chen A, Dunbar C, Webster GM, Lanphear BP, Calafat AM, 2014. Changes in Serum Concentrations of Maternal Poly- and Perfluoroalkyl Substances over the Course of Pregnancy and Predictors of Exposure in a Multiethnic Cohort of Cincinnati, Ohio Pregnant Women during 2003–2006. Environ. Sci. Technol 48, 9600–9608. 10.1021/es501811k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Wong L-Y, Jia LT, Kuklenyik Z, Calafat AM, 2011b. Trends in Exposure to Polyfluoroalkyl Chemicals in the U.S. Population: 1999–2008 †. Environ. Sci. Technol 45, 8037–8045. 10.1021/es1043613 [DOI] [PubMed] [Google Scholar]
- Kissa E, 2011. Fluorinated Surfactants and Repellents, Textile Research Journal. Marcel Dekker. 10.1177/004051750107100823 [DOI] [Google Scholar]
- Knox, Jackson T, Javins B, Frisbee SJ, Shankar A, Ducatman AM, 2011. Implications of early menopause in women exposed to perfluorocarbons. J. Clin. Endocrinol. Metab 96, 1747–1753. 10.1210/jc.2010-2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotthoff M, Müller J, Jürling H, Schlummer M, Fiedler D, 2015. Perfluoroalkyl and polyfluoroalkyl substances in consumer products. Environ. Sci. Pollut. Res. Int 22, 14546–59. 10.1007/s11356-015-4202-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo N, Katakura M, Sato Y, Kawashima Y, 2002. Sex hormone-regulated renal transport of perfluorooctanoic acid. Chem. Biol. Interact 139, 301–16. [DOI] [PubMed] [Google Scholar]
- Lewin A, Arbuckle TE, Fisher M, Liang CL, Marro L, Davis K, Abdelouahab N, Fraser WD, 2017. Univariate predictors of maternal concentrations of environmental chemicals: The MIREC study. Int. J. Hyg. Environ. Health 220, 77–85. 10.1016/j.ijheh2017.01.001. [DOI] [PubMed] [Google Scholar]
- Liu G, Dhana K, Furtado JD, Rood J, Zong G, Liang L, Qi L, Bray GA, DeJonge L, Coull B, Grandjean P, Sun Q, 2018. Perfluoroalkyl substances and changes in body weight and resting metabolic rate in response to weight-loss diets: A prospective study. PLoS Med. 15 10.1371/journal.pmed.1002502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Pereira AS, Beesoon S, Vestergren R, Berger U, Olsen GW, Glynn A, Martin JW, 2015. Temporal trends of perfluorooctanesulfonate isomer and enantiomer patterns in archived Swedish and American serum samples. Environ. Int 75, 215–222. 10.1016/J.ENVINT.2014.11.014 [DOI] [PubMed] [Google Scholar]
- Lorber M, Eaglesham GE, Hobson P, Toms L-ML, Mueller JF, Thompson JS, 2015. The effect of ongoing blood loss on human serum concentrations of perfluorinated acids. Chemosphere 118, 170–177. 10.1016/j.chemosphere.2014.07.093 [DOI] [PubMed] [Google Scholar]
- Lyngsø J, Ramlau-Hansen CH, Høyer BB, Støvring H, Bonde JP, Jönsson BAG, Lindh CH, Pedersen HS, Ludwicki JK, Zviezdai V, Toft G, 2014. Menstrual cycle characteristics in fertile women from Greenland, Poland and Ukraine exposed to perfluorinated chemicals: a cross-sectional study. Hum. Reprod 29, 359–367. 10.1093/humrep/det390 [DOI] [PubMed] [Google Scholar]
- Ode A, Rylander L, Lindh CH, Källén K, Jönsson BAG, Gustafsson P, Olofsson P, Ivarsson SA, Rignell-Hydbom A, 2013. Determinants of maternal and fetal exposure and temporal trends of perfluorinated compounds. Environ. Sci. Pollut. Res 20, 7970–7978. 10.1007/s11356-013-1573-5 [DOI] [PubMed] [Google Scholar]
- Olsen GW, Lange CC, Ellefson ME, Mair DC, Church TR, Goldberg CL, Herron RM, Medhdizadehkashi Z, Nobiletti JB, Rios JA, Reagen WK, Zobel LR, 2012. Temporal Trends of Perfluoroalkyl Concentrations in American Red Cross Adult Blood Donors, 2000–2010. Environ. Sci. Technol 46, 6330–6338. 10.1021/es300604p [DOI] [PubMed] [Google Scholar]
- Park SK, Peng Q, Ding N, Mukherjee B, Halow S, 2019. Distributions and determinants of per- and polyfluoroakyl substances (PFASs) in midlife women : Evidence of racial / ethnic residential segregation of PFAS exposure. [DOI] [PMC free article] [PubMed]
- Prevedouros K, Cousins IT, Buck RC, Korzeniowski SH, 2006. Sources, fate and transport of perfluorocarboxylates. Environ. Sci. Technol 10.1021/es0512475 [DOI] [PubMed] [Google Scholar]
- Santoro N, Taylor ES, Sutton-Tyrrell K, 2011. The SWAN Song: Study of Women’s Health Across the Nation’s Recurring Themes. Obstet. Gynecol. Clin. North Am 38, 417–423. 10.1016/j.ogc.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar A, Xiao J, Ducatman A, 2011. Perfluoroalkyl chemicals and chronic kidney disease in US adults. Am. J. Epidemiol 174, 893–900. 10.1093/aje/kwr171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenaler R, Cameron R, Butt CM, Hoffman K, Higgins CP, Stapleton HM, 2017. Serum perfluoroalkyl acids (PFAAs) and associations with behavioral attributes. Chemosphere 184, 687–693. 10.1016/j.chemosphere.2017.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Significant New Use Rule Final Rule and Supplemental Proposed Rule, 2002. 40 CFR Part 721 Perfluoroalkyl Sulfonates; Significant New Use Rule; Final Rule and Supplemental Proposed Rule. [Google Scholar]
- Sowers MF, Crawford S, Sternfeld B, Morganstein D, Gold E, Greendale G, Evans D, Neer R, Matthews K, Sherman S, Lo A, Weiss G, Kelsey J, 2000. SWAN: A Multicenter, Multiethnic, Community-Based Cohort Study of Women and the Menopausal Transition. Women’s Heal. Res. Fac. Publ [Google Scholar]
- Spliethoff HM, Tao L, Shaver SM, Aldous KM, Pass KA, Kannan K, Eadon GA, 2008. Use of Newborn Screening Program Blood Spots for Exposure Assessment: Declining Levels of Perfluorinated Compounds in New York State Infants. Environ. Sci. Technol 42, 5361–5367. 10.1021/es8006244 [DOI] [PubMed] [Google Scholar]
- Stockholm Convention, 2016. Information on PFOA, its salts and PFOA-related compounds.
- Sun Q, Zong G, Valvi D, Nielsen F, Coull B, Grandjean P, 2018. Plasma Concentrations of Perfluoroalkyl Substances and Risk of Type 2 Diabetes: A Prospective Investigation among U.S. Women. Environ. Health Perspect 126, 037001 10.1289/EHP2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JK, 1981. Quality assurance of chemical measurements. Anal. Chem 53, 1588A–1596A. 10.1021/ac00237a001 [DOI] [Google Scholar]
- Taylor KW, Hoffman K, Thayer KA, Daniels JL, 2014a. Polyfluoroalkyl chemicals and menopause among women 20–65 years of age (NHANES). Environ. Health Perspect 122, 145–50. 10.1289/ehp.1306707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KW, Hoffman K, Thayer KA, Daniels JL, 2014b. Polyfluoroalkyl chemicals and menopause among women 20–65 years of age (NHANES). Environ. Health Perspect 122, 145–50. 10.1289/ehp.1306707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel D, Horowitz L, Wormuth M, Scheringer M, Cousins IT, Hungerbühler K, 2008. Estimating Consumer Exposure to PFOS and PFOA. Risk Anal. 28, 251–269. 10.1111/j.1539-6924.2008.01017.x [DOI] [PubMed] [Google Scholar]
- US EPA, 2016. Fact Sheet: 2010/2015 PFOA Stewardship Program.
- US EPA, 2000. EPA and 3M announce phase out of PFOS [WWW Document]. US Environ. Prot. Agency; URL https://archive.epa.gov/epapages/newsroom_archive/newsreleases/33aa946e6cb11f35852568e1005246b4.html (accessed 9.23.18). [Google Scholar]
- Wang X, Mukherjee B, Batterman S, Harlow SD, Park SK, 2019. Urinary metals and metal mixtures in midlife women: The Study of Women’s Health Across the Nation (SWAN). Int. J. Hyg. Environ. Health 222, 778–789. 10.1016/j.ijheh.2019.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong F, MacLeod M, Mueller JF, Cousins IT, 2014. Enhanced Elimination of Perfluorooctane Sulfonic Acid by Menstruating Women: Evidence from Population-Based Pharmacokinetic Modeling. Environ. Sci. Technol 48, 8807–8814. 10.1021/es500796y [DOI] [PubMed] [Google Scholar]
- Wu X (May), Bennett DH, Calafat AM, Kato K, Strynar M, Andersen E, Moran RE, Tancredi DJ, Tulve NS, Hertz-Picciotto I, 2015. Serum concentrations of perfluorinated compounds (PFC) among selected populations of children and Adults in California. Environ. Res 136, 264–273. 10.1016/j.envres.2014.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylinen M, Auriola S, 1990. Tissue Distribution and Elimination of Perfluorodecanoic Acid in the Rat after Single Intraperitoneal Administration. Pharmacol. Toxicol 66, 45–48. 10.1111/j.1600-0773.1990.tb00700.x [DOI] [PubMed] [Google Scholar]
- Zhang S, Tan R, Pan R, Xiong J, Tian Y, Wu J, Chen L, 2018. Association of Perfluoroalkyl and Polyfluoroalkyl Substances With Premature Ovarian Insufficiency in Chinese Women. J. Clin. Endocrinol. Metab 103, 2543–2551. 10.1210/jc.2017-02783 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Beesoon S, Zhu L, Martin JW, 2013. Biomonitoring of Perfluoroalkyl Acids in Human Urine and Estimates of Biological Half-Life. Environ. Sci. Technol 47, 10619–10627. 10.1021/es401905e [DOI] [PubMed] [Google Scholar]
- Zhou W, Zhang L, Tong C, Fang F, Zhao S, Tian Y, Tao Y, Zhang J, Shanghai Birth Cohort Study, 2017. Plasma Perfluoroalkyl and Polyfluoroalkyl Substances Concentration and Menstrual Cycle Characteristics in Preconception Women. Environ. Health Perspect 125, 067012 10.1289/EHP1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


