SUMMARY SENTENCE
Discussion on the role of Kindlin-3 in the capacity of nonclassical monocytes to prevent malignant colonization of vascularized tissues by ensuring effective endothelial adhesion.
Monocytes are bone marrow-derived, circulating cells representing approximately 10% of peripheral leukocytes in humans (1). Classical CD14+CD16− monocytes constitute up to 90% of the monocyte pool. Traditionally, monocytes have been viewed as precursors of terminally differentiated macrophages and dendritic cells in tissues. In the context of cancer, for instance, it is now generally accepted that most tumor-associated macrophages (TAMs) originate from bone marrow-derived monocytic Myeloid-Derived Suppressor Cells (M-MDSCs) (2), although proliferating tissue-resident macrophages clearly contribute to the pool of TAMs (3). M-MDSC are similar to monocytes and appear to originate from the same progenitor, but they subsequently acquire immunosuppressive activity in tumor-bearing hosts, through mechanisms that remain incompletely understood (4).
The remaining monocytes include CD14+CD16+ monocytes and “nonclassical” monocytes, which in humans are identified as CD14lowCD16+ cells (CX3CR1highLy6C− monocytes in mice). Nonclassical monocytes are primarily found in peripheral blood and vascularized tissues. They have distinct functional activities that go beyond the role of monocytes as mere myeloid precursors, which depend on their capacity to patrol vascular compartments and interact with the endothelium. Those include their ability to clear pathogens (5); a role in vascular homeostasis and endothelial repair (6); and their anti-tumor activity by preventing metastatic colonization of the lung by removing tumor cells (7).
In this issue, Marcovecchio and colleagues unveil the crucial role of Kindlin-3, an adaptor protein that binds to the cytoplasmic tails of β-integrins, in adhesion of nonclassical monocytes to the vascular endothelium. In the absence of Kindlin-3, nonclassical monocytes “slip” out of the vascular wall and cannot effectively adhere to the endothelial layers. Accordingly, they cannot proficiently patrol to prevent colonization of tumor-free tissue by invading tumor cells; one of their major functions.
To investigate the role of Kindlin-3 in nonclassical monocytes, the authors generated an interferon-inducible Fermt3 (encodes Kindle-3) knockout model, to which they added a CX3CR1-GFP reporter. Ablation of Fermt3 prevented GFPhigh (nonclassical) monocytes from effectively patrolling the vasculature upon TNFα administration, as demonstrated by few contact points on the endothelium and defects in crawling through lung blood vessels. Furthermore, intravenous administration of melanoma cells evidenced a Fermt3-dependent defect in the accumulation of nonclassical monocytes in the proximity of tumor cells in invaded lung tissue. Accordingly, when different transgenic mice lacking nonclassical monocytes were reconstituted with patrolling-defective (Fermt3−/−GFPhigh) monocytes, lung metastases grew faster and in higher numbers. A theoretical caveat of these experiments is that tumor cells were administered in tumor-free mice, thus bypassing the mobilization of tumor-promoting myeloid progenitors universally associated with advanced solid tumors. Therefore, the pre-metastatic niches that provide a favorable microenvironment for sprouted tumor cells during the metastatic process (8) does not play a role in this experimental system. Similarly, how pathological myelopoiesis in advanced tumor-bearing hosts alters the mobilization and phenotype of nonclassical monocytes remains to be investigated. Nevertheless, it has been suggested that metastatic spreading could occur early during tumor development in at least some malignancies, and therefore the results of Marcovecchio and colleagues on the protective role of nonclassical monocytes are still relevant, in the absence of emergency/pathological myelopoiesis.
Importantly, there was not a basal reduction in the numbers of nonclassical monocytes upon interferon-inducible ablation of Fermt3, further supporting a true functional defect in their capacity to patrol and interact with other cell types. Interestingly, Geissmann and colleagues previously reported that nonclassical monocytes require LFA-1 to effectively patrol (9). The study of Marcovecchio et al identifies the additional requirement of Kindlin-3 for firm adhesion to vascular endothelium, thus preventing ineffective “slipping”, even in the presence of LFA-1.
Two important mechanistic aspects of how patrolling monocytes prevent metastatic colonization emerging from this work involve the crosstalk between patrolling monocytes, NK cells and other myeloid subsets: On the one hand, lack of Kindlin-3 in nonclassical monocytes results in decreased accumulation of NK cells upon tumor invasion. This is not surprising, given a recent report of the same group, showing that nonclassical monocytes drive recruitment and in situ activation of NK cells, which serve as effectors in immune protection against metastatic invasion (10). The chemokine and cytokine secretion patterns of Kindlin-3-defective versus competent patrolling monocytes (including possible basal defects) were not exhaustively dissected in this study. However, it seems plausible that Kindlin-3:β-integrin interactions between patrolling monocytes and endothelial surfaces drive the production of chemokines that attract NK cells, cytokines that activate them, or both.
The other interesting and potentially more novel finding is that nonclassical, patrolling monocytes are important for the recruitment and/or proliferation of CD11c+ cells during the metastatic process. CD11c expression is found in myeloid macrophages and dendritic cells, but also NK cells. Although the heterogeneity of decreased CD11c+ populations is not specifically addressed, the authors are correct in that it is more likely that the reduction in the accumulation of CD11c+ cells occurs at the expense of the myeloid compartment, which is simply more abundant. Interestingly, the frequency of migratory dendritic cells in the lung during metastasis did not change in a significant manner, although there was a trend. A theoretical concern of these experiments is that differences in the accumulation of both CD11c+ cells and migratory dendritic cells are expressed in percentages. However, bone marrow reconstitution with Fermt3-deficient hematopoietic cells causes inflammation in lung endothelium. This involves endothelial cell production of cytokines known to drive the mobilization of myeloid progenitors, including IL-1β and IL-6. It is therefore possible that the ratio of CD11c+ cells is decreased simply because more tumor-promoting, classical monocytes/M-MDSCs accumulate in higher numbers and create pre-metastatic niches in response to inflammation. This could provide an alternative explanation that would be more relevant to understand the balance between protective role of patrolling monocytes vs. the tumor-promoting role classical monocytes in patients with established tumors, which are universally associated with pathological myelopoiesis. In this context, it would be interesting to investigate, for instance, whether early colonization of the lung by malignant cells is associated with down-regulation of either LFA-1 or Kindlin-3 in nonclassical monocytes in a clinical setting. It would be also important to clarify whether the deffect in the capacity of nonclassical to effectively patrol the vascular endothelium is in turn associated with accumulation of M-MDSCs and other tumor-promoting cells, and the formation of pre-metastatic niches. Finally, future studies should also clarify how decreased NK activation, subsequent to impaired anchoring of patrolling monocytes, affects the phenotype of myeloid cells, as result, for instance, of reduced interferon gamma production. Addressing these questions opened by this study will provide a more comprehensive picture of the metastatic process, and could even lead to early diagnostic or preventive interventions in patients.
Overall, the work of Marcovecchio and colleagues provides a novel important clue about the importance of Kindlin-3 in the capacity of nonclassical monocytes to effectively patrol the vascular endothelium to prevent malignant colonization, in addition to LFA-1. While the activity of myeloid cells is usually associated with the promotion of malignant progression, the distinctive function of nonclassical monocytes makes them important antagonists of hematogenous metastatic spreading. Understanding how defects in the capacity of these monocytes to recruit and/or induce the proliferation of NK and myeloid cells at places of metastatic tumor invasion, as a function of their ability to effectively interact with endothelial cells, should offer new answers for the many questions open about how metastases occur in human cancer.
Expression of Kindlin-3 in Nonclassical Monocytes limits Cancer Cell Metastasis.
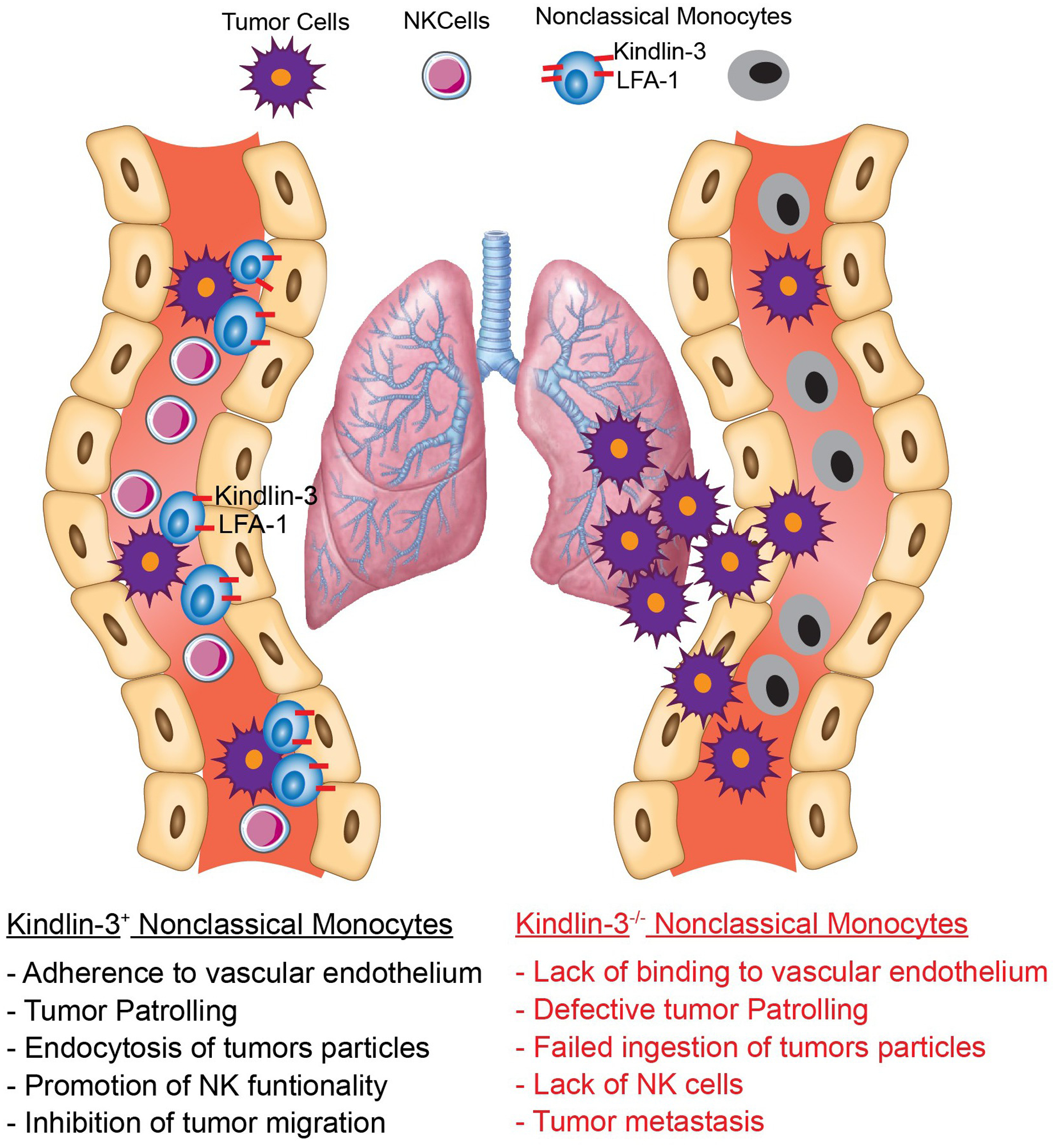
Left: Similar to the known effects of LFA-1 on myeloid cells, adherence of Kindlin-3-expressing Nonclassical Monocytes to the vascular endothelium activates tumor patrolling and ingestion of tumor particles, which promotes recruitment and function of NK cells and blunts extravasation of cancer cells to the metastatic area. Right: Conditional elimination of Kindlin-3 in Nonclassical Monocytes impairs their interaction with the vascular endothelium, which results in impaired tumor patrolling, reduced NK cell infiltration and promotion of tumor metastasis.
REFERENCES
- 1.Guilliams M, Mildner A, and Yona S. Developmental and Functional Heterogeneity of Monocytes. Immunity. 2018;49:595–613. [DOI] [PubMed] [Google Scholar]
- 2.Kumar V, Patel S, Tcyganov E, and Gabrilovich DI. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016;37:208–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laviron M, and Boissonnas A. Ontogeny of Tumor-Associated Macrophages. Front Immunol. 2019;10:1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tcyganov E, Mastio J, Chen E, and Gabrilovich DI. Plasticity of myeloid-derived suppressor cells in cancer. Curr Opin Immunol. 2018;51:76–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, et al. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153:362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanna RN, Cekic C, Sag D, Tacke R, Thomas GD, Nowyhed H, et al. Patrolling monocytes control tumor metastasis to the lung. Science. 2015;350:985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, et al. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. 2017;17:302–317. [DOI] [PubMed] [Google Scholar]
- 9.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. [DOI] [PubMed] [Google Scholar]
- 10.Narasimhan PB, Eggert T, Zhu YP, Marcovecchio P, Meyer MA, Wu R, et al. Patrolling Monocytes Control NK Cell Expression of Activating and Stimulatory Receptors to Curtail Lung Metastases. J Immunol. 2020;204:192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]


