Abstract
Vaccines against COVID-19 have the potential to protect people before they are exposed to the infective form of the virus. However, because of the involvement of pathogenic immune processes in many severe presentations of COVID-19, eliciting an immune response with a vaccine must strike a delicate balance to achieve viral clearance without also inducing immune-mediated harm. This Outlook synthesizes current laboratory findings to define which parts of the immune system help with recovery from and protection against the virus and which can lead to adverse outcomes. To inform our understanding, we analyze research about the immune mechanisms implicated in SARS-CoV, from the 2003 outbreak, and SARS-CoV-2, the virus causing COVID-19. The impact of how innate immunity, humoral immunity, and cell-mediated immunity play a role in a harmful versus helpful response is discussed, establishing principles to guide the development and evaluation of a safe but effective COVID-19 vaccine. The principles derived include (i) targeting the appropriate specificity and effector function of the humoral response, (ii) eliciting a T cell response, especially a cytotoxic T cell response, to achieve safe, yet effective, immune protection from COVID-19, and (iii) monitoring for the possibility of acute lung injury during SARS-CoV-2 infection post-vaccination in preclinical and clinical studies. These principles can not only guide efforts toward a safe and effective COVID-19 vaccine, but also the development of effective vaccines for viral pandemics to come.
Short abstract
A synthesis of studies about immune mechanisms during SARS-CoV and SARS-CoV-2 infection yields guiding principles for the evaluation and design of a safe and effective COVID-19 vaccine.
According to the World Health Organization, there are 17 COVID-19 vaccine candidates1−24 reported to be in clinical trials as of June 29, 2020.25 Much anticipation exists around whether these candidates are the answer to this pandemic, especially since a COVID-19 vaccine will require such a delicate balance. Since many of the severe presentations of COVID-19—involving acute respiratory distress syndrome, pulmonary edema, acute lung injury, and pulmonary fibrosis—are related to immune processes, it is important to invoke an immune response that is protective without also inducing immune-mediated lung damage. Based on previous work on SARS-CoV, the virus responsible for the 2003 outbreak, and on SARS-CoV-2, the virus causing COVID-19, this Outlook aims to establish important principles to consider in the evaluation of current vaccine candidates and in the development of new vaccine candidates that may enter the pipeline.
In the case of COVID-19, it is not clear that a broad robust immune response is always better and that antibody titers (specifically immunoglobulin isotype G (IgG)) are the best indicators for an effective response. Studies have shown that a broad immune response may actually be harmful and that other arms of the immune system—beyond an IgG response—are key determinants of an effective versus harmful immune response. In fact, based on current research, the importance of both humoral antibody specificity and T cell responses has emerged as key for clearing SARS-CoV-2 without harmful inflammation in the lung. Even though what defines an optimal immune response to SARS-CoV-2 is not yet certain, the principles that have already emerged can be used to guide our efforts forward. Ultimately, the data support (i) consideration of the specificity and isotype of the humoral response to ensure effective clearance while minimizing inflammatory activation, (ii) consideration of how to target the cytotoxic T cell response, which has been increasingly associated with favorable outcomes, and (iii) evaluation for the possibility of lung injury during SARS-CoV-2 infection after vaccination.
Early indicators that making a COVID-19 vaccine would be a delicate dance came from nonhuman primate studies that followed the first SARS outbreak in 2003. Studies showed that vaccination of Chinese macaques with modified vaccinia Ankara virus encoding intact spike glycoprotein from SARS-CoV led to acute lung injury upon viral challenge. Acute lung injury, which is inflammation-mediated damage to the lining of the lungs, was made evident by pathologic changes such as exudate, hyaline membrane formation and cellular infiltrate in lung tissue of vaccinated Chinese macaques.26 In a study with African green monkeys, prior infection with SARS-CoV—instead of being protective—was associated with more severe lung inflammation upon SARS-CoV challenge when compared to African green monkeys who had not been previously infected.27 These studies suggest that a vaccine against COVID-19 may pose a real risk of mediating acute lung injury in patients. Thus, an understanding of the immunopathology of COVID-19 is key (Figure 1).
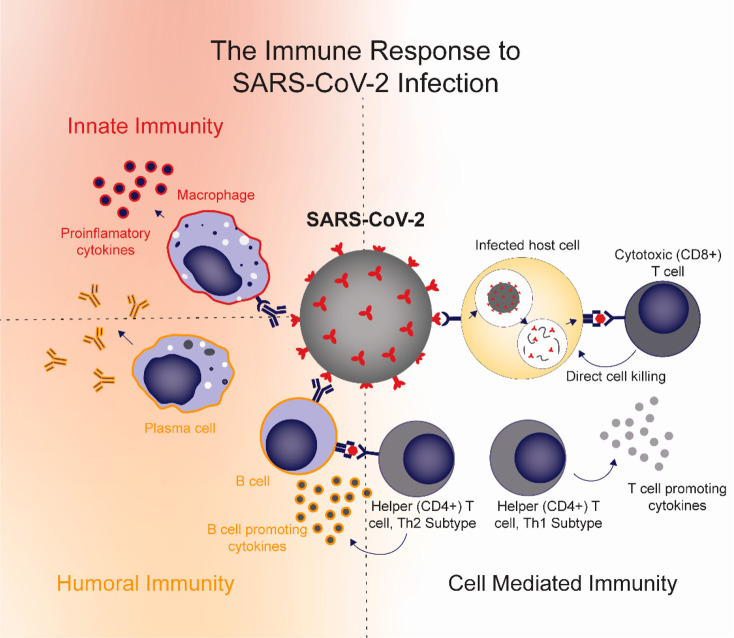
Figure 1.
Immune response to SARS-CoV-2 infection. Activation of inflammatory macrophages and subsequent production of proinflammatory cytokines is associated with adverse outcomes, including lung injury, during SARS-CoV-2 infection. Antibodies from humoral immunity can link adaptive immunity and innate immunity through Fc receptor interactions, leading to inflammatory activation of macrophages. Humoral immunity can also help promote viral clearance when targeted to appropriate parts of the virus. T cells have been shown to play an important role in viral clearance and recovery. CD8+ cytotoxic T cells, in particular, have been associated with favorable outcomes. Helper T cells can be of the Th1 subtype, which promote T cell activity, or the Th2 subtype, which link cell-mediated and humoral immunity by promoting B cell activity.
First, it is known that inflammation is a mediator of acute lung injury. Indeed, proinflammatory cytokines such as IL-1, IL-6, and IL-8 were associated with immunopathology in the lungs of SARS-CoV infected mice.28 More specifically, inflammatory monocytes/macrophages, which are high producers of those proinflammatory cytokines, have been repeatedly associated with more severe lung disease with SARS-CoV infection.26,28 In fact, treatment with a neutralizing antibody that depletes inflammatory monocytes/macrophages improved survival of SARS-CoV infected mice.26 These studies imply that an important feature of a safe and effective vaccine is the ability to elicit an immune response that achieves viral clearance without activating inflammatory monocytes and the production of proinflammatory cytokines.
Second, cases have been observed where humoral (antibody-mediated) immunity is also implicated in lung injury. An early clue that humoral immunity has potential for harm came from a study where Chinese macaques were given IgG antibodies specific to the spike glycoprotein of SARS-CoV (2003). The tissue from these treated Chinese macaques showed pathologic findings consistent with acute lung injury that paralleled the pathology findings after vaccination against intact spike glycoprotein.26 Interestingly, this parallel is observed in patients as well. In a prospective study of 75 patients, the severity of respiratory disease coincided with IgG seroconversion—despite decreases in viral load—in 80% of patients with SARS-CoV infection.29 Consistent with these clues, blockade of the receptor that binds to the constant (Fc) region of immunoglobulin reduced the proinflammatory signals associated with lung injury in Chinese macaques treated with antispike glycoprotein IgG.26 This trend was also observed with antibody-induced Fc blockade of SARS-CoV (+) patient sera when stimulated with SARS-CoV pseudovirus infected monocytes.26
Studies seem to suggest that this humoral-related pathology is due to Fc receptor interactions linking adaptive immunity to inflammation. Antibody-Fc receptor interactions have been shown to induce an inflammatory cascade, invoking proinflammatory cytokines and inflammatory macrophages implicated in lung injury. Antibodies have also been shown to facilitate antibody-dependent enhancement, where virus-specific antibodies increase uptake of the virus into macrophages, leading to macrophage activation and secretion of chemokine and cytokines.30−36 Whereas many vaccine candidates are evaluated on IgG titer alone, the production of IgG has the capacity to mediate lung injury during coronavirus infection.
Granted not all humoral responses are bad. After all, passive immunity by administration of convalescent plasma from recovered COVID-19 patients has been shown as a promising strategy to treat COVID-19.37,38 In fact, data suggest that antibody targeting is key. Mice vaccinated with recombinant vaccinia virus that raised antibodies against the nucleocapsid protein of SARS-CoV exhibited exacerbated pneumonia and subsequent lung injury upon challenge,39 whereas vaccination of mice with receptor binding domain-based vaccines have been shown to induce neutralizing antibody responses that protect mice both from infection and from lung injury upon SARS-CoV challenge.40,41 Studies in patients have also pointed to antibody targeting as a possible determinant of recovery. For patients who recovered from COVID-19, a recent study showed that elderly patients were more likely to have higher neutralizing antibody titers than younger patients and that neutralizing antibody titers correlate negatively with blood lymphocyte counts and correlate positively with C-reactive protein, a marker of inflammation.42 This observation is consistent with the first study this Outlook describes where administration of a vaccine formulated with intact spike glycoprotein (which elicits spike glycoprotein-specific antibodies) and administration of spike glycoprotein-specific IgG both led to acute lung injury in Chinese macaques.26 Perhaps early, robust antibodies with broad specificities are at least partially responsible for mediating lung injury.
Efforts to isolate neutralizing antibodies from patients who have recovered from COVID-19 have shed light on possible antibody features that define an optimal immune response.43−46 Serum collected from 29 convalescent patients diagnosed with COVID-19 showed that 100% of patients had IgG against the S1 subunit of the spike glycoprotein (as well as against the N protein), but not against the S2 subunit of the spike glycoprotein.47 Studies that have applied large screening platforms for rapid antibody discovery have shown that the most neutralizing antibodies map to the receptor binding domain (RBD)43 (which is contained in the S1 subunit of spike glycoprotein), and the most potent neutralizers are targeted to the ACE2 (the receptor recognized by SARS-CoV-2 for viral entry) binding site, in particular.44 In fact, only those antibodies that bound the RBD with an equilibrium dissociation constant (KD) smaller or close to that of ACE2-RBD binding had significant neutralizing effects in a SARS-CoV-2 pseudovirus system.45 These potent RBD neutralizing antibodies were shown to protect animals from signs of clinical disease and reduce viral loads in lung tissue when administered at high doses before and during viral challenge.44,45 It should be noted that one of these studies noted a trend—although not statistically significant—of worsened clinical disease when lower doses of antibodies were administered, pointing to the importance of balancing immune-mediated disease and viral clearance with humoral immunity.44 Another report found that the convalescent plasma from 149 COVID-19 patients, who did not require hospitalization, contained highly potent antibodies converging at three distinct epitopes on the RBD. Interestingly, these antibodies were not at particularly high titers (96% had titers <1:1000) but exhibited potent neutralizing activity at low concentrations (half-maximal inhibitory concentrations were as low as a few ng/mL).48 This suggests that effective immunity may be defined by sufficient production of potent, but specific, antibodies instead of broad, high-titer antibody production. While specificity of the antibody response may be one of the defining features of an effective versus harmful humoral response, specificity cannot always be distinguished with an IgG titer alone.
The importance of antibody isotype cannot be distinguished with an IgG titer either. IgA is known to be an important immune mediator in the respiratory tract. Indeed, a study of four COVID-19 patients showed that patients with more severe progression had a delayed IgA response, hinting at a possible role for antibody isotype in mediating effective viral clearance.49 Also, intranasal vaccination against SARS-CoV in mice elicited higher IgA titers, which were associated with decreased viral loads and decreased lung damage.41 Likely, antibody isotype—in addition to specificity—is a defining feature of an effective versus harmful response. While the exact role for IgA in COVID-19 remains to be defined, it may prove important in both the design and evaluation of vaccine candidates.
Third, studies have identified a T cell response as simultaneously effective for viral clearance and protective of lung injury. Across studies, decreased CD4+ and CD8+ T cell counts are observed in COVID-19 patients with severe disease when compared to patients with moderate disease or healthy controls.50−56 Low CD3+CD8+ T cell counts have even been suggested as predictors of mortality.57 Of note, studies have observed restored T cell counts during convalescence and in COVID-19 patients who reach an undetectable viral load.58,59 Further evidence that an optimal vaccine design would involve induction of a T cell response, and especially a CD8+ cytotoxic T cell response, has come from SARS-CoV vaccination studies in mice. Dendritic cells loaded with immunodominant epitopes are a vaccine platform shown to exclusively activate CD8+ T cells without inciting an innate immune response. When loaded with SARS-CoV epitopes, this vaccine has been shown to protect BALB/c and B6 mice from lethal mouse-adapted SARS-CoV challenge and reduce lung pathology.60,61 Even more, transfer of SARS-CoV specific T cells from vaccinated mice to infected, immunocompromised mice effectively cleared the virus and reversed clinical disease, ultimately showing that virus-specific T cells are necessary and sufficient for viral clearance and protection from clinical disease.62 Eliciting a CD8+ T cell response has the added advantage of tempering the innate immune response and suppressing a cytokine surge.63 In SARS-CoV recovered patients, memory T cells have been shown to persist for up to 6 years, even after SARS-specific IgG titers have declined.62,64,65 These findings suggest that eliciting a strong CD8+ T cell response may be a promising approach for developing a vaccine that achieves effective viral clearance and immunologic memory, while circumventing the inflammatory processes associated with lung injury. Evaluating T cell responses in addition to IgG titer could improve our understanding of current vaccine candidates and inform future efforts.
Recent results have demonstrated some of the concepts discussed so far. A DNA vaccine, INO-4800, encoding SARS-CoV-2 spike glycoprotein with an N-terminal IgE leader sequence was tested in guinea pigs and mice. This vaccine was shown to elicit a T cell response as well as production of antispike glycoprotein antibodies capable of blocking ACE2 binding in in vitro assays. While no challenge study was reported as of June 29, 2020, sera of vaccinated mice demonstrated neutralization in a SARS-CoV-2 pseudovirus system.66 An mRNA vaccine, mRNA-1273, encoding a prefusion stabilized SARS-CoV-2 spike trimer, has been shown to elicit both a humoral and T cell response in mice. The elicited antispike glycoprotein antibodies had potent neutralizing activity even with a single 10 μg vaccine dose, and the T cell response was characterized by a robust CD8+ T cell response and predominant Th1 (T cell supporting) CD4+ T cell response. This vaccine reduced viral replication in the lungs and nasal turbinates of mice after viral challenge. While mild inflammation was present in the lung tissue of mice given a standard vaccine dose, findings were less severe than in controls, and it was noted that subprotective doses were not associated with more severe lung pathology when compared to controls.67 In another study, an adenovirus vaccine vector (ChAdOx1) encoding full length SARS-CoV-2 spike glycoprotein has been shown to elicit neutralizing antibodies and a robust T cell response with Th1 predominance that protected mice and rhesus macaques from pneumonia upon viral challenge. Importantly, no immune-enhanced disease was observed with viral challenge after vaccination as made evident by the absence of lung pathology in vaccinated animals while control animals’ lung tissue exhibited features of lung injury.68 This same platform has shown efficacy and safety in early clinical trials in the prevention of Ebola, another virus for which there’s evidence of antibody-dependent enhancement.34,69
It is exciting to see how quickly the scientific community has responded to COVID-19, producing at least 17 vaccine candidates already in human trials—with many more in the pipeline—in a matter of months of the SARS-CoV-2 viral genome being published.25 While it is still uncertain what the target immune response should be, the current data support (i) targeting a humoral response with appropriate specificity and effector function and (ii) the importance of eliciting a T cell response. In addition, the data support (iii) careful monitoring of the possibility of humoral-related, inflammation-mediated lung injury after vaccination and upon SARS-CoV-2 infection. Moving forward, issues to be explored further include the nature of vaccine delivery vehicles, the mode of administration, the nature of the antigen, and importantly the spatial presentation of the antigen to cells and its chemical stability in vivo, to determine which vaccine features lead to which types of immune responses. Coupling the principles established here with global mining of expertise across multiple fields (in addition to vaccine experts) to explore these issues can facilitate the development of an effective COVID-19 vaccine that precisely balances the humoral versus cell-mediated response for long-term efficacy. Even more, this will ultimately establish principles and strategies for rapid vaccine development for viral pandemics to come.
Acknowledgments
We would like the acknowledge the Defense Threat Reduction Agency Grant HDTRA1-15-10052/P00001, the National Institutes of Health Grant 1U19AI142780-01, the David and Lucile Packard Foundation, the Camille Dreyfus Teacher-Scholar Program, and the Center for Regenerative Nanomedicine at the Simpson Querrey Institute. We also thank Ashty Karim for figure development, and we are grateful to some colleagues who wished to remain anonymous and the reviewers for their input during review.
The authors declare no competing financial interest.
References
- A Phase 2a, Randomized, Observer-Blind, Placebo Controlled, Dose-Confirmation Study to Evaluate the Safety, Reactogenicity, and Immunogenicity of mRNA-1273 SARS-COV-2 Vaccine in Adults Aged 18 Years and Older; Identifier, NCT04405076; ClinicalTrials.gov. U.S. National Library of Medicine, 2020. [Google Scholar]
- A 2-Part, Phase 1/2, Randomized, Observer-Blinded Study To Evaluate The Safety And Immunogenicity Of A SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine (SARS-CoV-2 rS) With Or Without MATRIX-M Adjuvant In Healthy Subjects; Identifier, NCT04368988; ClinicalTrials.gov. U.S. National Library of Medicine, 2020. [Google Scholar]
- A Multi-site Phase I/II, 2-Part, Dose-Escalation Trial Investigating the Safety and Immunogenicity of four Prophylactic SARS-CoV-2 RNA Vaccines Against COVID-19 Using Different Dosing Regimens; Identifier, 2020-001038-36; EU Clinical Trials Register, 2020. [Google Scholar]
- A randomized, double-blinded, placebo-controlled phase II clinical trial for Recombinant Novel Coronavirus (2019-nCOV) Vaccine (Adenovirus Vector) in healthy adults aged above 18 years; Identifier, ChiCTR2000031781; Chinese Clinical Trial Register, 2020. [Google Scholar]
- Evaluation of the safety and immunogenicity of inactivated Novel Coronavirus Pneumonia (COVID-19) vaccine (Vero cells) in healthy population aged 6 years and above: a randomized, double-blind, placebo parallel-controlled phase I/II clinical trial; Identifier, ChiCTR2000031809; Chinese Clinical Trial Register, 2020. [Google Scholar]
- A single-center, open and dose-escalation phase I clinical trial for recombinant novel coronavirus vaccine (adenoviral vector) in healthy adults aged between 18 and 60 years; Identifier, ChiCTR2000030906; Chinese Clinical Trial Register, 2020. [Google Scholar]
- Randomized, double-blind, placebo parallel controlled phase I/II clinical trial to evaluate the safety and immunogenicity of the new inactivated coronavirus vaccine (2019-CoV) (Vero cells) in healthy people aged 3 years and older; Identifier, ChiCTR2000032459; Chinese Clinical Trial Register, 2020. [Google Scholar]
- A Phase 1, Partially Blind, Placebo-controlled, Dose-escalation, First-in-human, Clinical Trial to Evaluate the Safety, Reactogenicity and Immunogenicity After 1 and 2 Doses of the Investigational SARS-CoV-2 mRNA Vaccine CVnCoV Administered Intramuscularly; Identifier, NCT04449276; ClinicalTrials.gov. U.S. National Library of Medicine, 2020. [Google Scholar]
- An Open Study of the Safety, Tolerability and Immunogenicity of the Drug ‘Gam-COVID-Vac Lyo’ Lyophilizate for the Preparation of a Solution for Intramuscular Injection With the Participation of Healthy Volunteers; Identifier, NCT04437875; ClinicalTrials.gov. U.S. National Library of Medicine, 2020. [Google Scholar]
- An Open Study of the Safety, Tolerability and Immunogenicity of the Drug ‘Gam-COVID-Vac’ a Solution for Intramuscular Injection With the Participation of Healthy Volunteers; Identifier, NCT04436471; ClinicalTrials.gov. U.S. National Library of Medicine, 2020. [Google Scholar]
- A Phase I clinical trial to evaluate the safety, tolerance and preliminary immunogenicity of different doses of a SARS-CoV-2 mRNA vaccine in population aged 18–59 years and 60 years and above; Identifier, ChiCTR2000034112; Chinese Clinical Trial Register, 2020. [Google Scholar]
- Clinical trial to assess the safety of a coronavirus vaccine in healthy men and women; Identifier, ISRCTN17072692; ISRCTN International Trial Registry, 2020. [Google Scholar]
- A Phase 1/2, Placebo-Controlled, Randomized, Observer-Blind, Dose-Finding Study To Describe The Safety, Tolerability, Immunogenicity, And Potential Efficacy Of Sars-Cov-2 Rna Vaccine Candidates Against Covid-19 In Healthy Adults; Identifier, NCT04368728; ClinicalTrials.gov. U.S. National Library of Medicine, 2020. [Google Scholar]
- A phase III randomized controlled trial to determine safety, efficacy, and immunogenicity of the non-replicating ChAdOx1 nCoV-19 vaccine; Identifier, ISRCTN89951424; ISCRTN International Trial Registry, 2020. [Google Scholar]
- A Multi-center, Double-blind, Randomized, Placebo Parallel Controlled, Safety and Tolerability Phase I Clinical Trial of Recombinant Novel Coronavirus Vaccine (CHO Cells) in Healthy People Between 18 and 59; Identifier, NCT04445194; ClinicalTrials.gov. U.S. National Library of Medicine, 2020. [Google Scholar]
- A Phase 1/2a, Multi-center, Randomized, Double-blind, Placebo-controlled Study to Investigate the Safety, Tolerability, and Immunogenicity of GX-19, a COVID-19 Preventive DNA Vaccine in Healthy Subjects; Identifier, NCT04445389; ClinicalTrials.gov. U.S. National Library of Medicine, 2020. [Google Scholar]
- A Phase 1, Randomized, Double-blind, Placebo-controlled, First-in-human Study to Evaluate the Safety and Immunogenicity of SCB 2019, a Recombinant SARS-CoV-2 Trimeric S Protein Subunit Vaccine for COVID-19 in Healthy Volunteers; Identifier, NCT04405908; ClinicalTrials.gov. U.S. National Library of Medicine, 2020. [Google Scholar]
- Phase 1 Open-label Study to Evaluate the Safety, Tolerability and Immunogenicity of INO-4800, a Prophylactic Vaccine Against SARS-CoV-2, Administered Intradermally Followed by Electroporation in Healthy Volunteers; Identifier, NCT04336410; ClinicalTrials.gov. U.S. National Library of Medicine, 2020. [Google Scholar]
- A Randomized, Double-Blinded, Placebo-Controlled, Phase I/II Clinical Trial, to Evaluate the Safety and Immunogenicity of the SARS-CoV-2 Inactivated Vaccine in Healthy Adults Aged 18 ∼ 59 Years; Identifier NCT04352608; ClinicalTrials.gov. U.S. National Library of Medicine, 2020. [Google Scholar]
- A Randomized, Double-Blinded, Placebo-Controlled, Phase I/II Clinical Trial, to Evaluate the Safety and Immunogenicity of the SARS-CoV-2 Inactivated Vaccine (Vero Cell) in Healthy Population Aged ≥ 60 Years Estimated; Identifier, NCT04383574; ClinicalTrials.gov. U.S. National Library of Medicine, 2020. [Google Scholar]
- A Randomized, Double-blind, Placebo-controlled, Phase Ia/IIa Trial of an Inactivated SARS-CoV-2 Vaccine in Healthy People Aged 18 to 59 Years; Identifier NCT04412538; ClinicalTrials.gov. U.S. National Library of Medicine, 2020. [Google Scholar]
- Phase I, Open-Label, Dose-Ranging Study of the Safety and Immunogenicity of 2019-nCoV Vaccine (mRNA-1273) in Healthy Adults; Identifier, NCT04283461; ClinicalTrials.gov. U.S. National Library of Medicine, 2020. [Google Scholar]
- A phase 2/3 study to determine the efficacy, safety and immunogenicity of the candidate Coronavirus Disease (COVID-19) vaccine ChAdOx1 nCoV-19; Identifier, 2020-001228-32; EU Clinical Trials Register, 2020. [Google Scholar]
- A phase I/II study to determine efficacy, safety and immunogenicity of the candidate Coronavirus Disease (COVID-19) vaccine ChAdOx1 nCoV-19 in UK healthy adult volunteers; Identifier, 2020-001072-15 A; EU Clinical Trials Register, 2020. [Google Scholar]
- World Health Organization . Draft landscape of COVID-19 candidate vaccines - 29 June 2020; WHO, 2020.
- Liu L. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI insight 2019, 4, e123158. 10.1172/jci.insight.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay C.; et al. Primary Severe Acute Respiratory Syndrome Coronavirus Infection Limits Replication but Not Lung Inflammation upon Homologous Rechallenge. J. Virol. 2012, 86, 4234–4244. 10.1128/JVI.06791-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R.; et al. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe 2016, 19, 181–193. 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J. S. M.; et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: A prospective study. Lancet 2003, 361, 1767–1772. 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B.; O’Rourke E. J. Antibody-enhanced dengue virus infection in primate leukocytes [21]. Nature 1977, 265, 739–741. 10.1038/265739a0. [DOI] [PubMed] [Google Scholar]
- Haslwanter D.; Blaas D.; Heinz F. X.; Stiasny K. A novel mechanism of antibody-mediated enhancement of flavivirus infection. PLoS Pathog. 2017, 13, e1006643. 10.1371/journal.ppat.1006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai H.; et al. Infection enhancement of influenza A NWS virus in primary murine macrophages by anti-hemagglutinin monoclonal antibody. J. Med. Virol. 1992, 36, 217–221. 10.1002/jmv.1890360312. [DOI] [PubMed] [Google Scholar]
- Takada A.; Kawaoka Y. Antibody-dependent enhancement of viral infection: Molecular mechanisms and in vivo implications. Rev. Med. Virol. 2003, 13, 387–398. 10.1002/rmv.405. [DOI] [PubMed] [Google Scholar]
- Takada A.; Feldmann H.; Ksiazek T. G.; Kawaoka Y. Antibody-Dependent Enhancement of Ebola Virus Infection. J. Virol. 2003, 77, 7539–7544. 10.1128/JVI.77.13.7539-7544.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S.; Dandekar A. A. Immunopathogenesis of coronavirus infections: Implications for SARS. Nat. Rev. Immunol. 2005, 5, 917–927. 10.1038/nri1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y.; Cheng Y.; Wu Y. Understanding SARS-CoV-2-Mediated Inflammatory Responses: From Mechanisms to Potential Therapeutic Tools. Virol. Sin. 2020, 35, 266. 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C.; et al. Treatment of 5 Critically Ill Patients with COVID-19 with Convalescent Plasma. JAMA - J. Am. Med. Assoc. 2020, 323, 1582–1589. 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan K.et al. The feasibility of convalescent plasma therapy in severe COVID-19 patients: a pilot study. medRxiv, 03-16-2020, 20036145. 10.1101/2020.03.16.20036145. [DOI]
- Yasui F.; et al. Prior Immunization with Severe Acute Respiratory Syndrome (SARS)-Associated Coronavirus (SARS-CoV) Nucleocapsid Protein Causes Severe Pneumonia in Mice Infected with SARS-CoV. J. Immunol. 2008, 181, 6337–6348. 10.4049/jimmunol.181.9.6337. [DOI] [PubMed] [Google Scholar]
- Du L.; et al. Receptor-binding domain of SARS-CoV spike protein induces long-term protective immunity in an animal model. Vaccine 2007, 25, 2832–2838. 10.1016/j.vaccine.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L.; et al. Intranasal Vaccination of Recombinant Adeno-Associated Virus Encoding Receptor-Binding Domain of Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) Spike Protein Induces Strong Mucosal Immune Responses and Provides Long-Term Protection against SARS-. J. Immunol. 2008, 180, 948–956. 10.4049/jimmunol.180.2.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J.et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. Lancet Infect. Dis. 2020, in press. [Google Scholar]
- Zost S. J.et al. Rapid isolation and profiling of a diverse panel of human monoclonal antibodies targeting the SARS-CoV-2 spike protein. Bioarxiv, 2020, 091462. https://www.biorxiv.org/content/10.1101/2020.05.12.091462v1. [DOI] [PMC free article] [PubMed]
- Rogers T. F.; et al. Rapid isolation of potent SARS-CoV-2 neutralizing antibodies and protection in a small animal model. Science (Washington, DC, U. S.) 2020, eabc7520. 10.1126/science.abc7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y.; et al. Potent Neutralizing Antibodies against SARS-CoV-2 Identified by High-Throughput Single-Cell Sequencing of Convalescent Patients’ B Cells. Cell 2020, 1–12. 10.1016/j.cell.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D.Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 2020, in press. 10.1038/s41586-020-2349-y [DOI] [PubMed] [Google Scholar]
- Jiang H.et al. Global profiling of SARS-CoV-2 specific IgG/ IgM responses of convalescents using a proteome microarray. medRxiv, 03-20-2020, 20039495. 10.1101/2020.03.20.20039495. [DOI]
- Robbiani D. F. Convergent Antibody Responses to SARS-CoV-2 Infection in Convalescent Individuals. Nature 2020, s41586. 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlke C.et al. Distinct early IgA profile may determine severity of COVID-19 symptoms: an immunological case series. medRxiv, 2020, 20059733. 10.1101/2020.04.14.20059733. [DOI]
- Zheng Y.et al. Study of the lymphocyte change between COVID-19 and non-COVID-19 pneumonia cases suggesting other factors besides uncontrolled inflammation contributed to multi-organ injury. medRxiv, 2020, 20024885. 10.1101/2020.02.19.20024885. [DOI]
- Shi W.Deep Learning-Based Quantitative Computed Tomography Model in Predicting the Severity of COVID-19: A Retrospective Study in 196 Patients. SSRN Electron. J. 2020, in press. 10.2139/ssrn.3546089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z.; Wenzheng Y.; Kesheng H.; Qingbo W.; Shuang Y.; Wei L.; Li L.; T F.. Clinical features and short-term outcomes of 114 elderly patients with COVID-19 in Wuhan, China: a single-center, retrospective, observational study. Lancet Infect. Dis. 2020, in press. [Google Scholar]
- Song C. Y.; Xu J.; He J. Q.; Lu Y. Q.. COVID-19 early warning score: a multi-parameter screening tool to identify highly suspected patients. medRxiv, 2020, 20031906. 10.1101/2020.03.05.20031906. [DOI]
- Diao B.; et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020, 55, 102763. 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du R. H.; et al. Predictors of Mortality for Patients with COVID-19 Pneumonia Caused by SARS-CoV-2: A Prospective Cohort Study. Eur. Respir. J. 2020, 55, 2000524. 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M.; et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020, 17, 533. 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.et al. Restoration of leukomonocyte counts is associated with viral clearance in COVID-19 hospitalized patients. medRxiv, 03-03-2020, 20030437. 10.1101/2020.03.03.20030437. [DOI]
- Zhao J.; Zhao J.; Perlman S. T Cell Responses Are Required for Protection from Clinical Disease and for Virus Clearance in Severe Acute Respiratory Syndrome Coronavirus-Infected Mice. J. Virol. 2010, 84, 9318–9325. 10.1128/JVI.01049-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R.; Fett C.; Zhao J.; Meyerholz D. K.; Perlman S. Virus-Specific Memory CD8 T Cells Provide Substantial Protection from Lethal Severe Acute Respiratory Syndrome Coronavirus Infection. J. Virol. 2014, 88, 11034–11044. 10.1128/JVI.01505-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L. T.; et al. Long-lived effector/central memory T-cell responses to severe acute respiratory syndrome coronavirus (SARS-CoV) S antigen in recovered SARS patients. Clin. Immunol. 2006, 120, 171–178. 10.1016/j.clim.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Kim K.; et al. Adaptive immune cells temper initial innate responses. Nat. Med. 2007, 13, 1248–1252. 10.1038/nm1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F.; et al. Lack of Peripheral Memory B Cell Responses in Recovered Patients with Severe Acute Respiratory Syndrome: A Six-Year Follow-Up Study. J. Immunol. 2011, 186, 7264–7268. 10.4049/jimmunol.0903490. [DOI] [PubMed] [Google Scholar]
- Peng H.; et al. Long-lived memory T lymphocyte responses against SARS coronavirus nucleocapsid protein in SARS-recovered patients. Virology 2006, 351, 466–475. 10.1016/j.virol.2006.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. R. F.; et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat. Commun. 2020, 11, 2601. 10.1038/s41467-020-16505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett K. S.et al. SARS-CoV-2 mRNA Vaccine Development Enabled by Prototype Pathogen Preparedness. bioRxiv, 2020, 145920. https://www.biorxiv.org/content/10.1101/2020.06.11.145920v1. [DOI] [PMC free article] [PubMed]
- Van Doremalen N.ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. bioRxiv, 2020, 093195. 10.1101/2020.05.13.093195 [DOI]
- Venkatraman N.; et al. Safety and immunogenicity of a heterologous prime-boost ebola virus vaccine regimen in healthy adults in the United Kingdom and Senegal. J. Infect. Dis. 2019, 219, 1187–1197. 10.1093/infdis/jiy639. [DOI] [PMC free article] [PubMed] [Google Scholar]