Abstract
A series of N-substituted 2-phenylcyclopropylmethylamines were designed and synthesized, with the aim of finding 5-HT2C-selective agonists with preference for Gq signaling. A number of these compounds exhibit 5-HT2C selectivity with preference for Gq-mediated signaling compared with β-arrestin recruitment. Furthermore, the N-methyl compound (+)-15a, which displayed an EC50 of 23 nM in the calcium flux assay while showing no β-arrestin recruitment activity, is the most functionally-selective 5-HT2C agonist reported to date. The N-benzyl compound (+)-19, which showed an EC50 of 24 nM at the 5-HT2C receptor, is fully selective over the 5-HT2B receptor. In an amphetamine-induced hyperactivity model, compound (+)-19 showed significant antipsychotic drug-like activity. These novel compounds shed light on the role of functional selectivity at the 5-HT2C receptor with respect to antipsychotic activity.
Graphical Abstract
INTRODUCTION
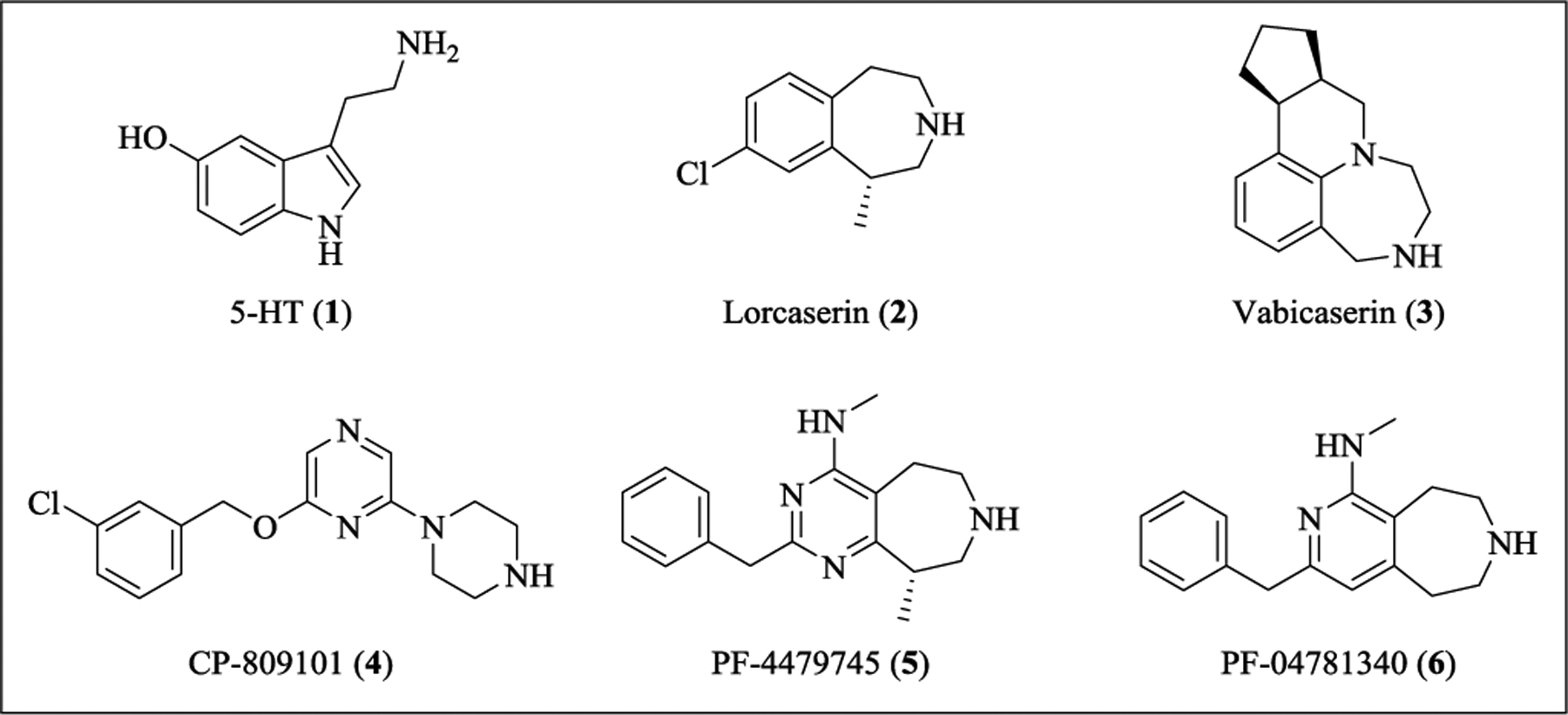
The serotonin 2C (5-HT2C) receptor, a serotonin (5-HT, 1, Figure 1), G protein-coupled receptor (GPCR), has been identified as a promising drug target for obesity and other central nervous system (CNS) disorders, such as schizophrenia and drug addiction.1–6 The 5-HT2C receptor shares approximately 50% homology similarity with the other two 5-HT2 subtypes, namely the 5-HT2A and 5-HT2B receptors, where agonists respectively mediate hallucinogenic activity7 and cardiac valvulopathy.8, 9
Figure 1.
Selected 5-HT2C agonists.
Furthermore, the classical understanding of GPCR signaling has undergone important changes in recent years with the recognition of the phenomenon of “functional selectivity”, namely the ability of a specific agonist to differentially mediate multiple receptor signaling events (i.e., Gq-linked calcium flux vs. β-arrestin recruitment in the case of 5-HT2C receptors).10 β-Arrestin was named after its initially discovered ability to arrest (turn off) GPCR signaling, and is an important downstream signaling and regulatory factor. β-Arrestin recruitment is responsible for desensitization, internalization, and eventual degradation of GPCRs.11 It has furthermore been demonstrated that the β-arrestin mediated signaling pathway functions can provide signaling independent of G-protein pathways.12 Thus, a GPCR agonist that has minimal capability to activate the β-arrestin signaling pathway could display long-term efficacy in regard to tolerance mediated by receptor desensitization and downregulation. Recently, biased GPCR ligands have been suggested to offer great therapeutic benefits as new generation drugs with enhanced efficacy and functional selectivity, resulting in reduced side effects.13, 14
Thus, to develop 5-HT2C agonists as potential antipsychotic medications, it is essential to explore ligands that are both G protein-biased and highly selective over 5-HT2B and 5-HT2A. Most importantly, the high selectivity for 5-HT2C over 5-HT2B has emerged as paramount due to the fact that chronic 5-HT2B agonism leads to irreversible cardiac valvulopathy, as illustrated by the withdrawal of fenfluramine and pergolide and the restriction of sales of cabergoline owing to their off-target activities at the 5-HT2B receptor.9 To date, a number of selective 5-HT2C ligands have been disclosed (Figure 1). In particular, lorcaserin (2) was approved by the FDA for the treatment of obesity in 2012. It shows excellent 5-HT2C agonist activity (EC50 = 9 nM, Emax = 99%), but only moderate (100-fold) selectivity over 5-HT2B (EC50 = 943 ± 90 nM, Emax = 100%), which is relevant to understanding the higher incidence of cardiac valve disorders compared to placebo in clinical trails.15 Vabicaserin (3) with partial agonism (Emax = 50%, EC50 = 12 or 102 nM depending on receptor expression level) at the 5-HT2B receptor has failed to achieve its primary endpoints in clinical trails, although a proof-of-concept has been achieved for the use of 5-HT2C agonists in treating schizophrenia.16, 17 The pyrimido[3,4-d]azepine, PF-4479745 (5), a hybrid of lorcaserin and CP-809101 (4),18 displays high potency (EC50 = 10 nM) and moderate efficacy (Emax = 67%) at 5-HT2C while possessing no measurable agonism at either the 5-HT2A or 5-HT2B receptor.19 The pyrido[3,4-d]azepine, PF-04781340 (6), is a potent 5-HT2C ligand (EC50 = 9 nM, Emax = 99%), with about 160-fold selectivity over 5-HT2B.20 Both compounds 5 and 6 were reported to have excellent ADME properties commensurate with an orally bioavailable and CNS penetrant profile. However, to the best of our knowledge, few biased 5-HT2c agonists have been reported to date.21
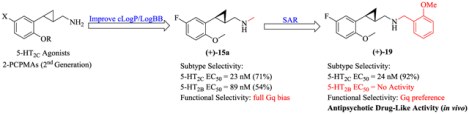
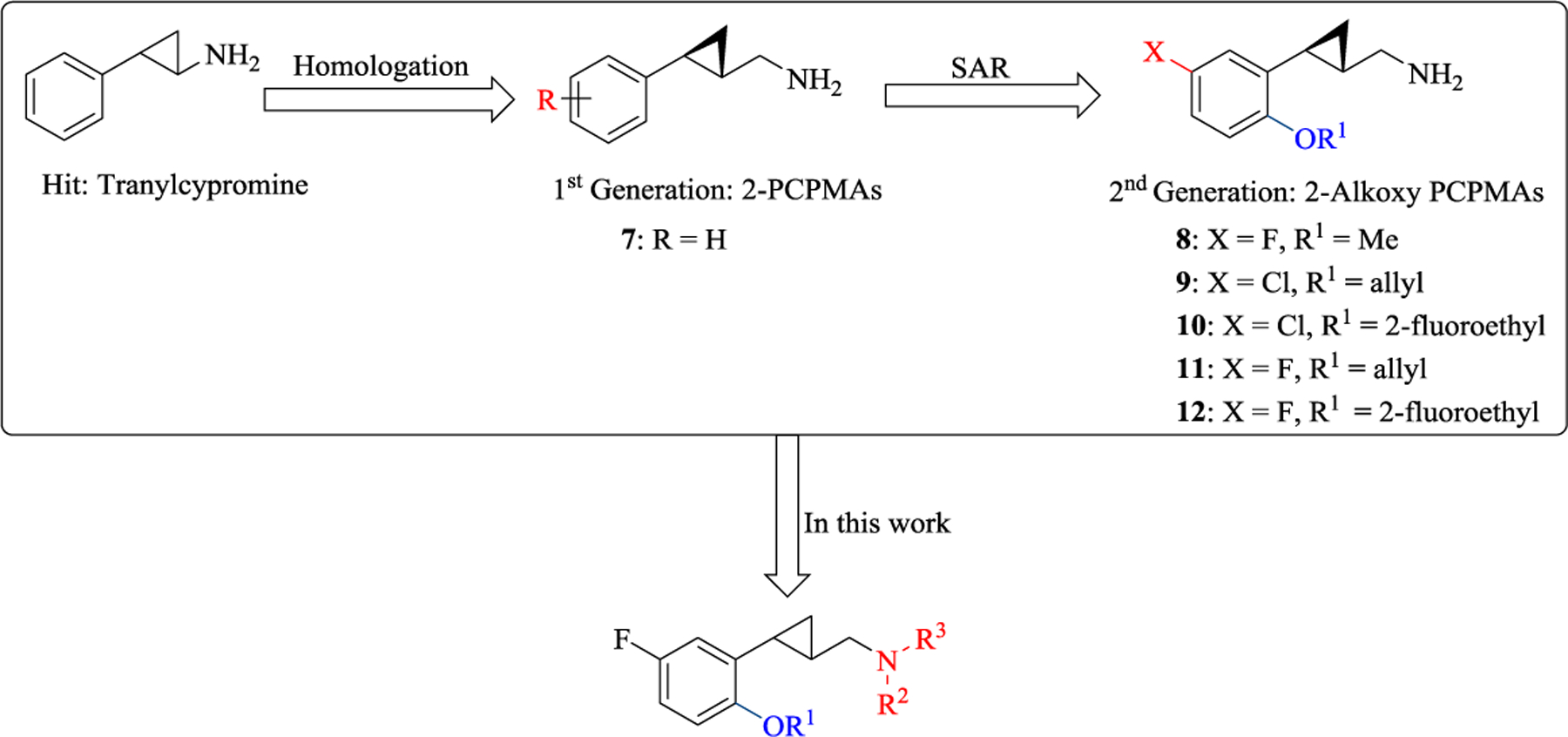
In our prior work, tranylcypromine was identified as a hit compound in an initial high-throughput screening (HTS) assay using a library containing 800 compounds. We developed our first-generation potent 5-HT2C agonists by homologation of the side chain of tranylcypromine to 2-phenylcyclopropylmethylamine (2-PCPMA).22 Subsequent structure-activity relationship (SAR) studies indicated that a 2-alkoxy substituent is a beneficial functional group for maintaining potency and selectivity (Figure 2). Further fine-tuning of the 2-alkoxy and the halogen substituents led to the identification of 11 and 12,23, 24 which showed excellent pharmacological profiles with regard to 5-HT2C potency and selectivity over 5-HT2A and 5-HT2B. In addition, compounds 11 and 12 showed only weak β-arrestin recruitment efficacy (Emax = 23% and 26%, respectively; unpublished data) similar to a series of structurally similar benzofuran-based compounds reported recently.21
Figure 2.
Selected 5-HT2C agonists based on the 2-phenylcyclopropylmethylamine scaffold, and new N-substituted derivatives
The achievement of optimal brain exposure, which is a critical and challenging task in CNS drug discovery, requires a more restrictive selection of physicochemical properties compared to orally active, peripheral drugs (Rule of 5).25–27 Due to the relatively low molecular weights (MW) of the 2-phenylcyclopropylmethylamines (for example, MW < 230 for 11 and 12), the addition of halogen atoms to the phenyl ring has previously been demonstrated to improve brain penetrance.23, 24 To further optimize drug-like properties, alkylation at the basic primary amino group of the 2-PCPMA scaffold was envisioned to provide opportunities for additional non-covalent interactions with the 5-HT2C receptor while also increasing the ligands’ lipophilicity. In earlier studies we found that N-methylation of 2-(2-methoxyphenyl)cyclopropylmethylamine (8, cLogP = 1.59, LogBB = –0.04) gave a fully Gq-biased 5-HT2c agonist with good potency (EC50 = 23 nM, Emax = 71%; cLogP = 2.07, LogBB = 0.26), while N-methylation of our first-generation 2-phenylcyclopropylmethylamine (7) led to a dramatic loss in activity.22 Moreover, it has been reported that N-benzylation of phenethylamines and 5-methoxytryptamines leads to improved agonism at 5-HT2 receptors.28, 29 Given that the additional N-substitution could improve the physicochemical properties in terms of further enhancing brain penetration, a new series of N-substituted 2-(2-alkoxyphenyl)cyclopropylmethylamines (Figure 2) was developed and evaluated for their potency and bias profiles in continuation of our previous SAR studies, along with behavioral tests and in vitro ADMET studies of a promising 5-HT2C receptor agonist which showed no activity at the 5-HT2B receptor.
RESULTS AND DISCUSSION
Chemistry
N-Monomethylation of compounds 8, 11, and 12 was carried out starting from Boc-protected 2-(2-alkoxyphenyl)cyclopropylmethylamines (–)-13a-c reported previously by our group as synthetic intermediates.23 As shown in Scheme 1, introduction of an N-methyl group with NaH and iodomethane followed by deprotection under acidic condition (2M HCl/Et2O) afforded the N-methylamines (+)-15a-c. Since the N-methylamine (+)-15a possessing a methoxy group at the 2-position of the phenyl ring maintained potency at 5-HT2C, the present work was focused on N-substituted analogs of compound 8.
Scheme 1. Synthesis of N-Methyl Analogs (+)-15a-ca.
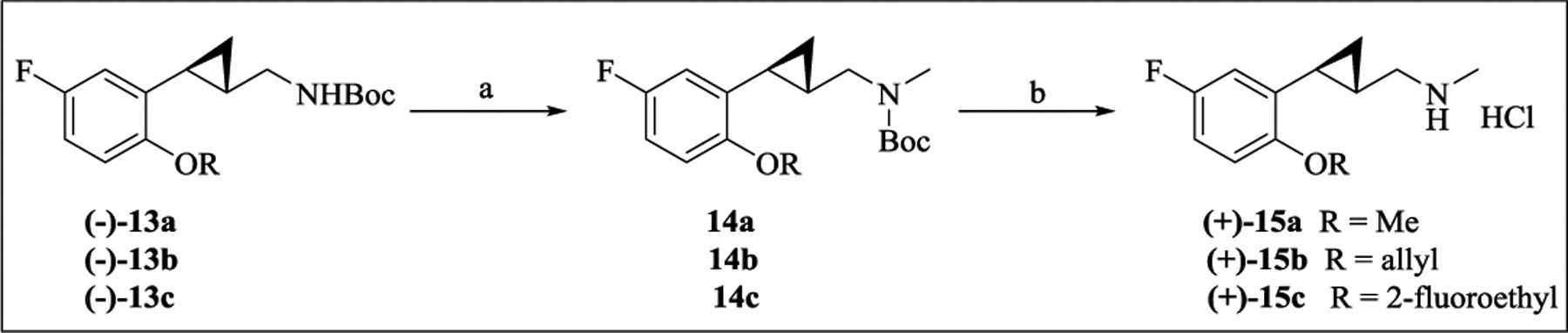
aReagents and conditions: (a) MeI, NaH, THF, rt; (b) 2M HCl/Et2O, rt.
The synthesis of these compounds was accomplished from the cyclopropane-bearing aldehyde D and primary amine G (Scheme 2)30 by reductive amination or N-substitution reactions (Scheme 3). For example, the N-alkyl derivatives 16–18 and N-arylmethyl/heteroarylmethyl derivatives 19–22, 26–32, and 34–35 were synthesized starting from the aldehyde D and amines by treatment with NaBH4 or NaBH(OAc)3. The tertiary amine 23 was prepared from the phenol 22 using a Mitsunobu reaction with 2-fluoroethanol. The N-arylmethyl/heteroarylmethyl derivatives 24, 33, and 36–39 were synthesized from the primary amine G and appropriate aldehydes following the same approach, while the derivative 24 was prepared from the intermediate tosylate 24b (Scheme 4) via a nucleophile substitution reaction. Hydrolysis of 24 under basic condition (NaOH/H2O2) smoothly afforded the amide 25. The intermediate cyclopropylamine 30b for the synthesis of compound 30 was obtained from 2-methoxybenzonitrile 30a using EtMgBr and Ti(OiPr)4, followed by treatment with a Lewis acid (BF3•Et2O) (Scheme 4).31 The intermediate thiophenylmethanamines 34e and 35e required for the synthesis of compounds 34 and 35 were prepared in four steps as illustrated in Scheme 5. The commercial thiophenes 34a and 35a were brominated with NBS followed by replacement of bromide with tritylamine to provide 34c and 35c in high yield. The bromides 34c and 35c were reacted with CH3ONa/CuBr to afford 34d and 35d,32 followed by the removal of the Trt group under acidic conditions to give 34e and 35e as acetic acid salts (Scheme 5).
Scheme 2. Synthesis of Intermediates D and Ga.
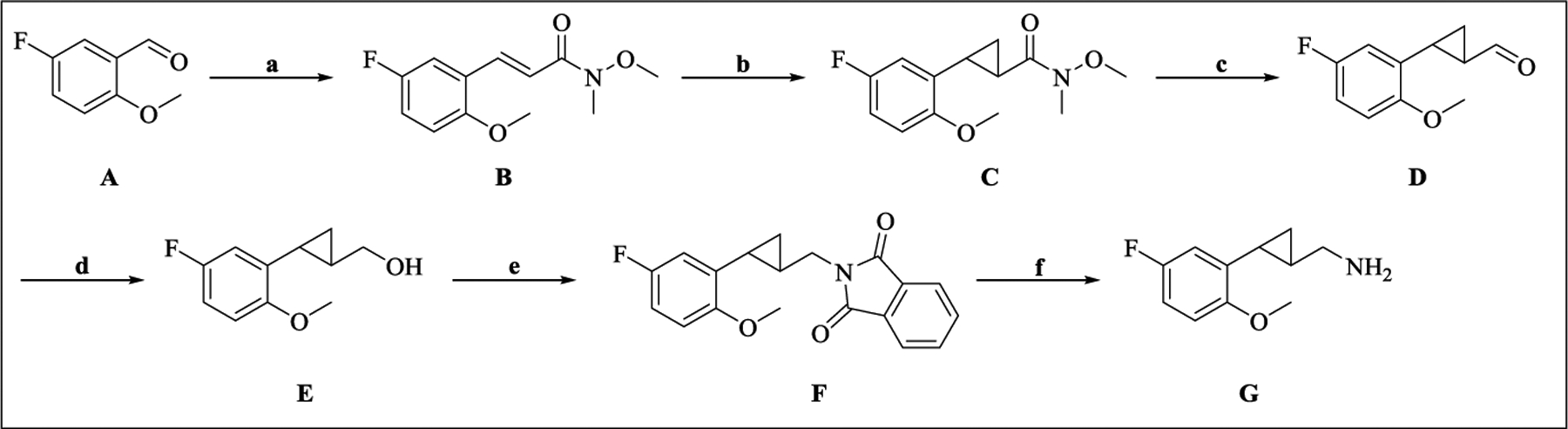
aReagents and conditions: (a) Ph3P=CHC(O)N(OMe)Me, CH2Cl2, rt; (b) Me3S+(O)I−, NaH, DMSO, rt; (c) DIBAL-H, THF, −78 oC; (d) NaBH4, MeOH, 0 oC to rt; (e) phthalimide, PPh3, DEAD, THF, rt; (f) N2H4•H2O, EtOH, reflux.
Scheme 3. Synthesis of Target Compounds 16–39a .
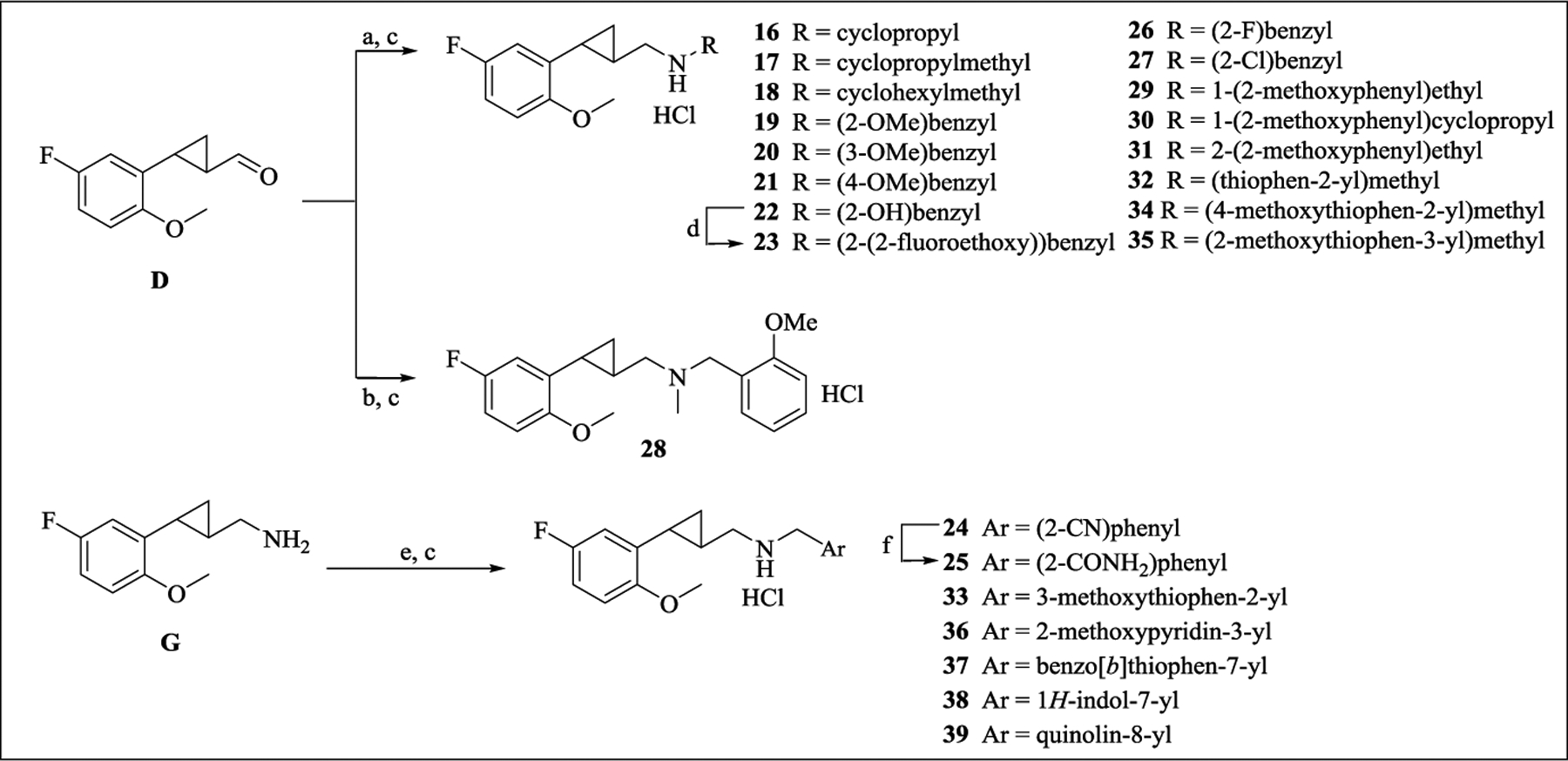
aReagents and conditions: (a) RNH2, NaBH4, MeOH; (b) 2-methoxy-N-methylbenzylamine, NaBH4, MeOH; (c) 2M HCl/Et2O, rt; (d) Ph3P, DEAD, 2-fluoroethanol, THF; (e) ArCH2OTs, K2CO3, CH3CN for 24; ArCHO, NaBH(OAc)3, DCE for 33, 37-39; ArCHO, NaBH4, MeOH for 36; (f) NaOH, H2O2, MeOH.
Scheme 4. Synthesis of Intermediates 24b and 30b for Analogs 24 and 30a.

aReagents and conditions: (a) i. NaBH4, MeOH; ii. TsCl, TEA, DCM; (b) i. EtMgBr, Ti(OiPr)4, Et2O, −78 oC; ii. BF3•Et2O, Et2O.
Scheme 5. Synthesis of Intermediates 34e-35e for Analogs 34–35a.
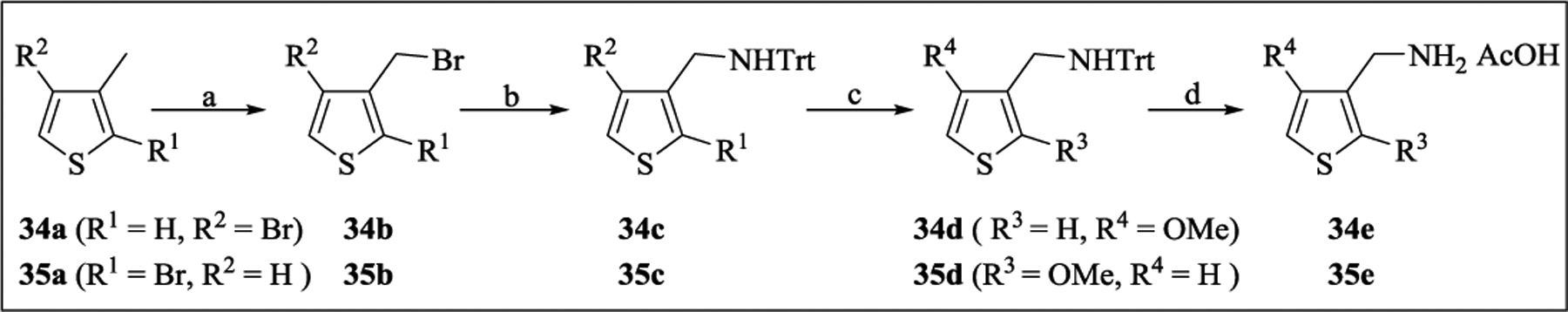
aReagents and conditions: (a) NBS, AIBN, CCl4, 60 oC; (b) TrtNH2, DCM, rt; (c) CH3ONa, CH3OH, CuBr, 100 oC; (d) AcOH, 50 oC.
Unless otherwise noted, the N-substituted derivatives were tested as racemic mixtures. The N-benzyl derivatives of (+)-11 and (+)-12, namely 40 and 41, respectively, were directly prepared from the enantiomers (+)-(S,S)-11 and (+)-(S,S)-12 via reductive alkylation with 2-methoxybenzaldehyde, while the pure (–)- and (+)-isomers of 19–21, 26–27, and 32 were obtained by preparative HPLC on a chiral stationary phase (Scheme 6). Compounds 40 and 41 were found to show the same sign of optical rotation as their parent compounds (+)-11 and (+)-12. Moreover, our finding that the (+)-enantiomers of all new N-arylmethyl/thiophenylmethyl compounds are more potent than their (–)-enantiomers is consistent with that previously observed for similar scaffolds.22–24 Thus, the absolute configuration of the (+)-enantiomers was assigned as 1S, 2S and that of the (–)-enantiomers as 1R, 2R.
Scheme 6. Preparation of Enantiomers 19–21, 26–27, 32, and 40–41a.
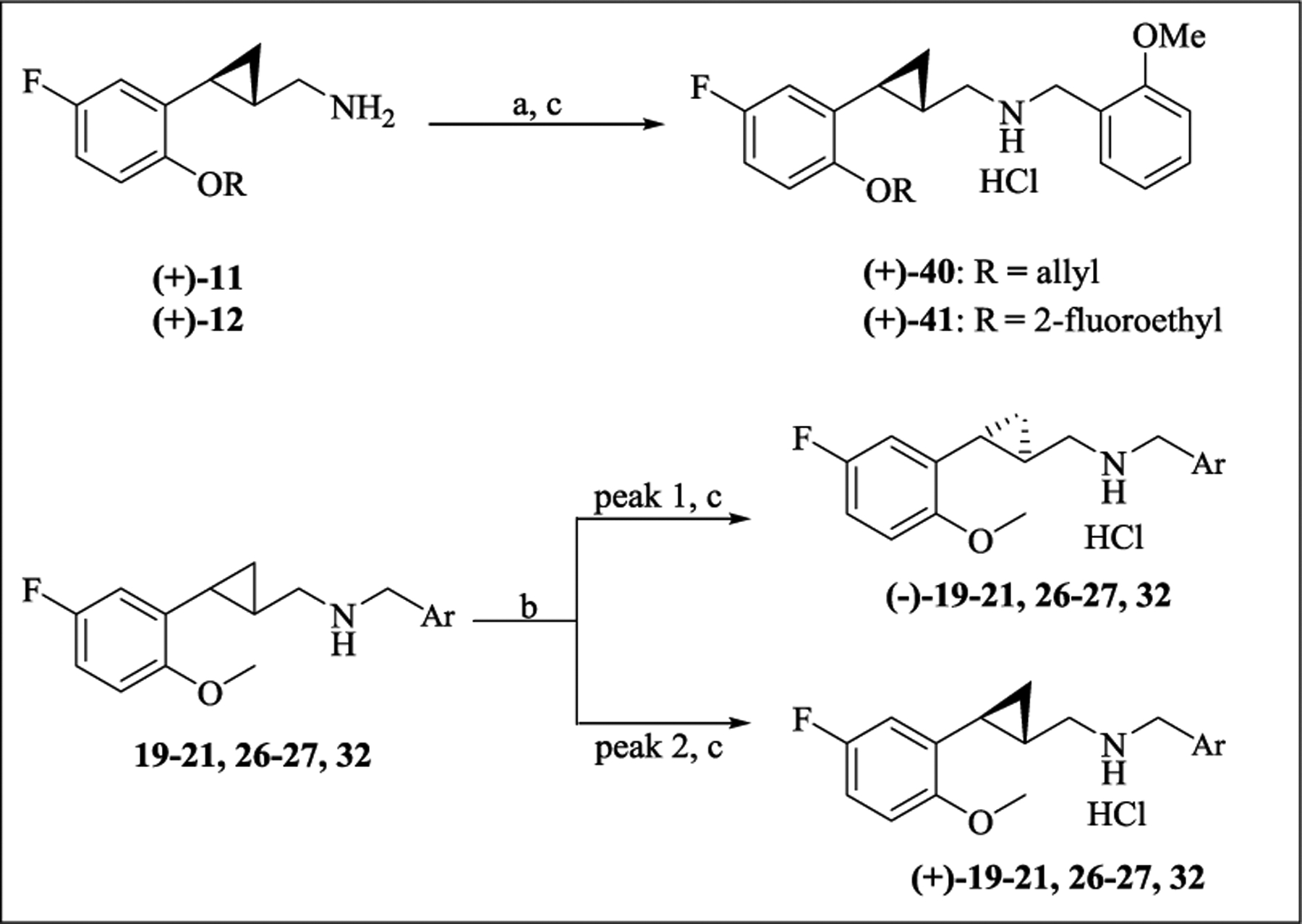
aReagents and conditions: (a) 2-methoxybenzaldehyde, NaBH4, MeOH; (b) chiral preparative HPLC separation; (c) 2M HCl/Et2O, rt.
In vitro Pharmacology
Activity at 5-HT2 Receptors
Preliminary studies demonstrated that 2-phenylcyclopropylmethylamines with the phenyl ring bearing 2-alkoxy and 5-fluoro substituents provided excellent 5-HT2C potency and selectivity over 5-HT2A and 5-HT2B receptors. Moreover, the initial N-methylation of 2-(2-methoxyphenyl)cyclopropylmethylamine (8) maintained potency at 5-HT2C (compound (+)-15a: EC50 = 23 nM, Emax = 71%, Table 1), which was not the case with the best ligands 11 and 12 (resulting in compounds (+)-15b and (+)-15c). Further binding studies showed that (+)-15a had high affinity for 5-HT2C (Ki = 81 nM, see Supporting Information Table S3). Thus, the parent compound 8 was taken as a lead to develop N-substituted derivatives. Physicochemical properties such as cLogP and LogBB were calculated to predict blood-brain barrier (BBB) permeability.
Table 1.
Functional Activity and Selectivity of N-substituted Derivatives 15a-c and 16–41 at 5-HT2 Receptors in the Calcium Flux Assaya
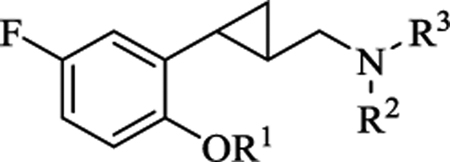 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Compd. | R1 | R2 | R3 | cLogP | LogBB | 5-HTic | 5-HTib | 5-HTia | |||
| pEC50 (EC50, nM) |
Emax (%) |
pEC50 (EC50, nM) |
Emax (%) |
pEC50 (EC50, nM) |
Emax (%) |
||||||
| serotonin | - | 9.78 ± 0.02 (0.17) |
100 −0.5 | 8.84 ± 0.03 (1.46) | 100 ±1.1 | 8.63 ± 0.02 (2.35) |
100 ± 0.7 | ||||
| lorcaserin | - | 8.58 ± 0.01 (2.64) |
100 ±0.7 | 6.36 ± 0.03 (433) |
80 ± 1.6 | 6.61 ± 0.01 (248) |
68 ± 0.5 | ||||
| (+)-15a | Me | H | Me | 2.07 | 0.26 | 7.65 ± 0.05 (23) | 71±1.5 | 7.05 ± 0.04 (89) |
54 – 1.1 | 6.57 ± 0.02 (271) |
79 ± 1.0 |
| (+)-15b | allyl | H | Me | 2.63 | 0.40 | 7.89 ± 0.25 (13) |
15 ± 1.4 | NA | NA | 6.55 ± 0.25 (284) |
19 ± 2.5 |
| (+)-15c | 2-fluoroethyl | H | Me | 2.16 | 0.31 | NA | NA | NA | NA | NA | NA |
| (±)-16 | Me | H | 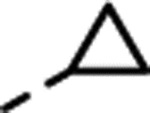 |
2.42 | 0.37 | 6.10 ± 1.40 (399) |
70 ± 0.7 | 6.19 ± 0.52 (641) |
14 – 0.2 | 5.96 ± 1.22 (1090) |
69 ± 0.4 |
| (±)-17 | 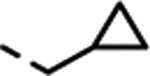 |
2.84 | 0.56 | NA | NA | NA | NA | 5.37 ± 4.33 (4260) |
43 ± 0.9 | ||
| (±)-18 | 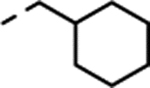 |
4.35 | 1.07 | 7.02 ± 0.25 (95) |
15 ± 1.8 | NA | NA | NA | NA | ||
| (−)-19 | 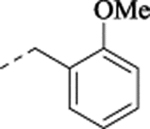 |
3.86 | 0.72 | 6.99 ± 0.05 (103) |
104 ±2.1 | 6.24 ± 0.06 (570) |
72 – 2.3 | 7.15 ± 0.04 (72) |
94 ± 1.4 | ||
| (+)-19 | 7.63 ± 0.04 (23.5) |
92 ± 1.3 | NA | NA | 6.86 ± 0.07 (139) |
63 ± 2 1 | |||||
| (−)-20 | 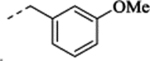 |
3.86 | 0.72 | 5.58 ± 0.11 (2600) |
85 ± 6.5 | NA | NA | 5.17 ± 0.08 (6840) |
97 ± 6.8 | ||
| (+)-20 | 6.64 ± 0.08 (231) |
83 ± 2.9 | NA | NA | 5.43 ± 0.06 (3700) |
70 ± I 2 | |||||
| (−)-21 | 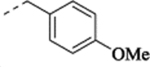 |
3.86 | 0.72 | NA | NA | NA | NA | NA | NA | ||
| (+)-21 | 5.92 ± 0.09 (1200) |
71 ± 3.4 | NA | NA | NA | NA | |||||
| (±)-22 | 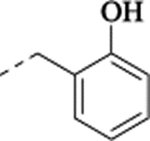 |
3.06 | 0.90 | 6.52 ± 0.06 (304) |
63 ± 2.2 | NA | NA | 7.11 ± 0.06 (77) |
76 ± 2.0 | ||
| (±)-23 | 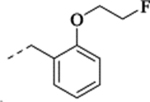 |
3.91 | 0.79 | 7.55 ± 0.17 (28) |
16 ± 1.1 | 7.25 ± 0.15 (56) |
11 ± 0.7 | 6.40 ± 0.03 (398) |
80 ± 1.2 | ||
| (±)-24 | 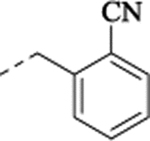 |
3.30 | 0.81 | 6.61 ± 0.11 (245) |
30 ± 2.7 | 6.62 ± 0.04 (238) |
82 ± 1.7 | 6.14 ± 0.06 (721) |
63 ± 2.3 | ||
| (±)-25 | 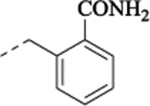 |
2.24 | 0.16 | 6.39 ± 0.25 (409) |
31 ± 0.5 | 6.48 ± 0.30 (335) |
82 ± 0.8 | 5.88 ± 0.02 (1310) |
67 ± 0.8 | ||
| (−)-26 | 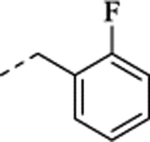 |
3.82 | 0.80 | 5.79 ± 0.07 (1640) |
90 ± 4.1 | NA | NA | 5.73 ± 0.06 (1880) |
78 ± 2.9 | ||
| (+)-26 | 6.30 ± 0.06 (502) |
78 ± 2.5 | NA | NA | 5.73 ± 0.07 (1880) |
63 ± 3.0 | |||||
| (−)-27 | 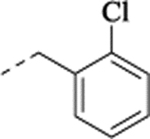 |
4.57 | 0.90 | 5.73 ± 0.1 (1860) |
72 ± 4.4 | NA | NA | 5.53 ± 0.06 (2990) |
84 ± 3.9 | ||
| (+)-27 | 6.28 ± 0.06 (529) |
94 ± 2.7 | NA | NA | 5.45 ± 0.07 (3530) |
64 ± 3.5 | |||||
| (±)-28 | Me | Me | 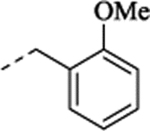 |
4.17 | 0.94 | 6.17 ± 0.10 (670) |
49 ± 2.6 | NA | NA | 5.60 ± 0.08 (2540) |
24 ± 1.4 |
| (±)-29 | Me | H | 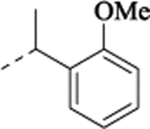 |
4.12 | 0.83 | 5.59 – 0.05 (2550) |
81 ± 2.6 | NA | NA | 6.62 – 0.06 (238) |
106 ± 2.9 |
| (±)-30 | 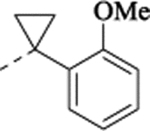 |
4.07 | 1.07 | 6.06 – 0.05 (874) |
77 ± 2.2 | NA | NA | 6.18 – 0.05 (655) |
102 ± 2.9 | ||
| (±)-31 | 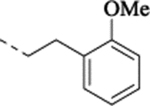 |
4.06 | 0.78 | 6.21 – 0.1 (615) |
51 ± 3.1 | 7.02 – 0.14 (96) | 17 ± 1.1 | 6.51 – 0.06 (307) |
59 ± 1.8 | ||
| (−)-32 | 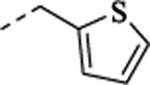 |
3.31 | 0.66 | 6.51 ± 0.03 (308) |
81 ± 1.5 | NA | NA | 6.17 ± 0.04 (674) |
59 ± 1.6 | ||
| (+)-32 | 6.92 ± 0.06 (120) |
60 ± 1.7 | NA | NA | 6.36 ± 0.08 (438) |
34 ± 1.5 | |||||
| (±)-33 | 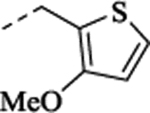 |
3.30 | 0.68 | 6.92 ± 0.06 (121) |
66 ± 1.8 | NA | NA | 6.83 ± 0.06 (148) |
102 ± 2.7 | ||
| (±)-34 | 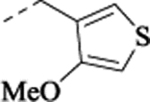 |
3.30 | 0.68 | 6.64 ± 0.03 (228) |
65 ± 1.0 | NA | NA | 6.84 ± 0.04 (145) |
89 ± 1.5 | ||
| (±)-35 | 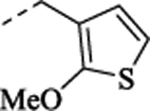 |
3.30 | 0.68 | 6.26 ± 0.04 (556) |
65 ± 1..3 | NA | NA | 6.33 ± 0.03 (463) |
100 1.5 | ||
| (±)-36 | 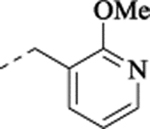 |
2.83 | 0.44 | 6.36 ± 0.07 (433) |
65 ± 2.5 | 6.56 ± 0.20 (274) |
32 ± 3.3 | 5.89 ± 0.06 (1290) | 66 ± 2.9 | ||
| (±)-37 | 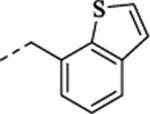 |
5.00 | 0.94 | 6.11 ± 0.06 (777) |
94 ± 3.0 | 6.09 ± 0.04 (807) |
79 – 1.7 | 5.65 ± 0.06 (2220) |
70 ± 2.8 | ||
| (±)-38 | 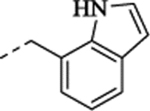 |
3.81 | 0.55 | NA | NA | NA | NA | NA | NA | ||
| (±)-39 |  |
3.75 | 0.31 | 6.28 ± 0.07 (530) |
84 ± 3.1 | 0.04 (87) |
73 ± 1.4 | 0.03 (460) |
|||
| (+)-40 | allyl | H |  |
4.48 | 0.91 | 8.05 ± 0.04 (9.2) |
97 ± 1.3 | 6.95 – 0.17 (113) |
28 ± 2.2 | 7.56 ± 0.05 (28) |
80 ± 1.7 |
| (+)-41 | 2-fluoroethyl | H |  |
3.91 | 0.79 | 8.65 ± 0.03 (2.4) |
93 ± 1.1 | 7.61 – 0.22 (24) |
22 ± 2.0 | 7.62 ± 0.03 (24) |
78 ± 1.1 |
All new compounds were tested as HCl salts except compound 36, which was tested as the free base. Pharmacological data were acquired with recombinant, stably expressed human 5-HT receptors in the HEK-293 cell line, using a fluorescence imaging plate reader (FLIPR) assay. pEC50 and Emax values are shown as the mean ± SEM (n = 3). EC50 values were calculated from averaged pEC50 values. “NA” indicates no activity up to 10 μM. “−” indicates structures of 5-HT and lorcaserin are not shown. cLogP and LogBB values were calculated for the free bases using the ACD Percepta program.
Substitution with larger alkyl groups, such as cyclopropyl (compound 16) and cyclopropylmethyl (compound 17), led to decreased potency at 5-HT2C (EC50 > 300 nM; Table 1). However, in view of the lack of activity for the cyclopropylmethyl derivative 17, it was intriguing that the even bulkier cyclohexylmethyl derivative 18 showed moderate potency (EC50 = 95 nM) and superior selectivity over 5-HT2B and 5-HT2A, but with extremely low efficacy (Emax = 15%). We therefore saw an opportunity for the introduction of an aromatic ring (Table 1), which could possibly engage in a π-π stacking interaction with the receptor.
Furthermore, Nichols et al. have demonstrated that N-(2-methoxybenzyl)-substituted 2, 5-dimethoxyphenethylamines exhibited enhanced agonism at 5-HT2 receptors.28, 29 We therefore first prepared the N-(2-methoxybenzyl) analog 19. The compound (+)-19 proved to have high potency at 5-HT2C (EC50 = 24 nM, Emax = 92%) and full selectivity against 5-HT2B. In addition, it was hypothesized that a hydrogen bond acceptor (HBA) at the ortho position of the benzyl moiety was beneficial to improve the potency of N-benzylated 2,5-dimethoxyphenethylamines.29 Movement of the o-methoxy group to the meta and para positions as in compounds (+)-20 and (+)-21, respectively, led to decreased potency (EC50 = 231 nM and 1200 nM). Furthermore, substituents with different electronic and steric properties acting as HBA groups at the 2-position of the benzyl group were investigated. The phenol 22 gave modest activity at 5-HT2C (EC50 = 304 nM, Emax = 63%) but poor selectivity (0.25-fold) against 5-HT2A, although it showed full selectivity over 5-HT2B. The slightly bulkier 2-fluoroethyl33 derivative 23 showed good potency at 5-HT2C (EC50 = 28 nM), but only 2-fold selectivity against 5-HT2B (EC50 = 56 nM). The cyano and carbamoyl derivatives 24 and 25 displayed attenuated activity (EC50 > 200 nM, Emax < 35%). Moreover, introduction of other electron-withdrawing substituents (EWG) such as F (compound (+)-26) and Cl (compound (+)-27) resulted in significant loss of potency at 5-HT2C (EC50 > 500 nM), which suggested that an EWG attached to the phenyl ring of the benzyl group was disadvantageous for 5-HT2C activity. Additional N-methylation of the benzylamine moiety as in analog 28 resulted in a 15-fold reduction of 5-HT2C potency (EC50 = 670 nM for the racemate) compared to the parent compound (+)-19. Additional substitution at the benzylic position of the 2-methoxybenzyl moiety with a methyl and ethylene group led to the sterically hindered analogs 29 and 30, respectively, with predicted increase in BBB penetration based on the calculated LogBB values. Their potency at 5-HT2C was, however, reduced. Moreover, homologation of the N-(2-methoxybenzyl) moiety as in compound 31 led to reduced potency at 5-HT2C (EC50 = 615 nM) and increased potency at 5-HT2B (EC50 = 96 nM).
To explore further structural diversity, N-heteroarylmethyl derivatives with altered electron densities in the aromatic ring that might result in different pharmacokinetic properties were investigated as depicted in Table 1. The isosteric thiophene derivative (+)-32 showed 120 nM potency at 5-HT2C and full selectivity against 5-HT2B. In line with the N-benzyl derivatives above, (+)-32 was more potent at 5-HT2C than its (–)-enantiomer (EC50 = 308 nM). Upon introduction of an additional methoxy group at the 3-position of the thiophene ring (compound 33), a 2-fold increased potency at 5-HT2C (EC50 = 121 nM for the racemate) and good selectivity against 5-HT2B were obtained. The regioisomers 34 and 35 displayed weaker potency at 5-HT2C. In contrast, the electron-deficient pyridine derivative 36 was less potent at 5-HT2C (EC50 = 433 nM) compared to (+)-32. Moreover, several benzene-fused heterocyclic derivatives (37–39) with various heteroatoms (N and S) at the 2-position of the benzene ring were also investigated. Intriguingly, in contrast with the modest potency at 5-HT2C of the benzo[b]thiophene derivative 37 (EC50 = 777 nM) and quinoline derivative 39 (EC50 = 530 nM), the indole derivative 38 was entirely inactive at 5-HT2C, which possibly resulted from the absence of a hydrogen bond acceptor at the ortho position of the aryl ring.
In an effort to discover potent and selective 5-HT2C agonists in a series of N-substituted 2-(2-methoxyphenyl)cyclopropylmethylamines, the N-(2-methoxybenzyl) derivative (+)-19 has been established as the best ligand in terms of selectivity for 5-HT2C. Introduction of the N-(2-methoxybenzyl) group onto the previously reported 5-HT2C-selective ligands 11 and 12 also gave very high 5-HT2C potency (40, EC50 = 9.2 nM, Emax = 97%; 41, EC50 = 2.4 nM, Emax = 93%), but poor selectivity against 5-HT2B (40, EC50 = 113 nM; 41, EC50 = 24 nM) and 5-HT2A (40, EC50 = 28 nM; 41, EC50 = 24 nM). Furthermore, to identify potential off-target activity, compound (+)-19 was profiled against a panel of serotonin receptors, dopamine receptors, monoamine transporters, and other selected CNS targets (see Supporting Information Table S2). Compound (+)-19 showed good selectivity with much higher binding affinity for 5-HT2C (Ki = 78 nM) than 5-HT2B (Ki = 411 nM) and 5-HT2A (Ki = 492 nM). None of the other screened targets were found to display any significant off-target affinity for compound (+)-19. Taken together, compound (+)-19 represents a good candidate for further studies in terms of both its 5-HT2C and selectivity.
5-HT2C Functional Selectivity
Recently, we have discovered benzofuran-based compounds as functionally selective 5-HT2C agonists with weak β-arrestin recruitment activities.21 However, no fully biased 5-HT2C agonist has been disclosed to date. The functional selectivity of the above 5-HT2C-selective N-substituted 2-phenylcyclopropylmethylamines was investigated and compared to lorcaserin and 5-HT. β-Arrestin recruitment activity was tested using reported methods21, 34 in parallel with a Gq-mediated calcium flux assay using the same drug dilutions. The relative activities (log Emax/EC50) were calculated to account for partial agonist differences. As shown in Table 2 and Figure 3, compounds (+)-15a and (+)-32 showed no β-arrestin recruitment activity, which indicates that these compounds exclusively signal via Gq-mediated calcium flux. The N-(2-methoxybenzyl) derivative (–)-19 also displayed preference for Gq-mediated calcium flux with weak potency (> 1 μM) for β-arrestin recruitment activity, whereas its (+)-enantiomer showed stronger potency for β-arrestin recruitment (EC50 = 70 nM) albeit with much reduced efficacy (Emax = 43%) compared to the reference ligand, lorcaserin (EC50 = 40 nM, Emax = 97%). The N-(2-methoxybenzyl) derivatives of 11 and 12, namely (+)-40 and (+)-41, respectively, had a preference for Gq signaling driven mainly by their weaker β-arrestin recruitment efficacy (Emax = 56 and 54%, respectively) compared to lorcaserin (Emax = 97%).
Table 2.
Functional Selectivity for 5-HT2C-Selective Agonistsa
| Compd. | Gq calcium flux | β-arrestin-2 | ||||
|---|---|---|---|---|---|---|
| pEC50 ( EC50, nM) |
Emax (%) |
Log(Emax / EC50) | pEC50 ( EC50, nM) |
Emax (%) |
Log(Emax / EC50) | |
| 5-HT | 9.74 ± 0.02 (0.18) |
100 ± 0.9 | 9.85 | 7.82 ± 0.03 (15) |
100 ± 1.0 | 7.82 |
| Lorcaserin | 8.68 ± 0.04 (2.1) |
101 ± 1.4 | 8.68 | 7.40 ± 0.05 (40) |
97 ± 2.1 | 7.38 |
| (+)−15a | 7.64 ± 0.05 (23) |
71 ± 1.4 | 7.49 | NA | NA | - |
| (+)−19 | 7.62 ± 0.04 (24) |
92 ± 1.3 | 7.58 | 7.16 ± 0.04 (70) |
43 ± 0.9 | 6.79 |
| (−)−19 | 6.99 ± 0.05 (103) |
104 ± 2.1 | 7.00 | > 1,000 | ND | - |
| (+)−32 | 6.92 ± 0.06 (120) |
60 ± 0.7 | 6.70 | NA | NA | - |
| (+)−40 | 8.04 ± 0.04 (9.2) | 97 ± 1.3 | 8.02 | 7.64 ± 0.05 (22.7) |
56 ± 1.0 | 7.39 |
| (+)−41 | 8.62 ± 0.03 (2.4) |
93 ± 1.1 | 8.59 | 7.96 ± 0.05 (11) |
54 ± 1.0 | 7.69 |
Data were acquired with the human 5-HT2C-INI receptor isoform measuring Gq calcium flux (FLIPR) and β-arrestin-2 recruitment (Tango). Emax values are shown as the mean ± SEM (n = 3), and assays were conducted in parallel with the same drug dilutions. “NA” indicates no activity up to 10 μM. “ND” indicates not determined because of no saturable Emax.
Figure 3.
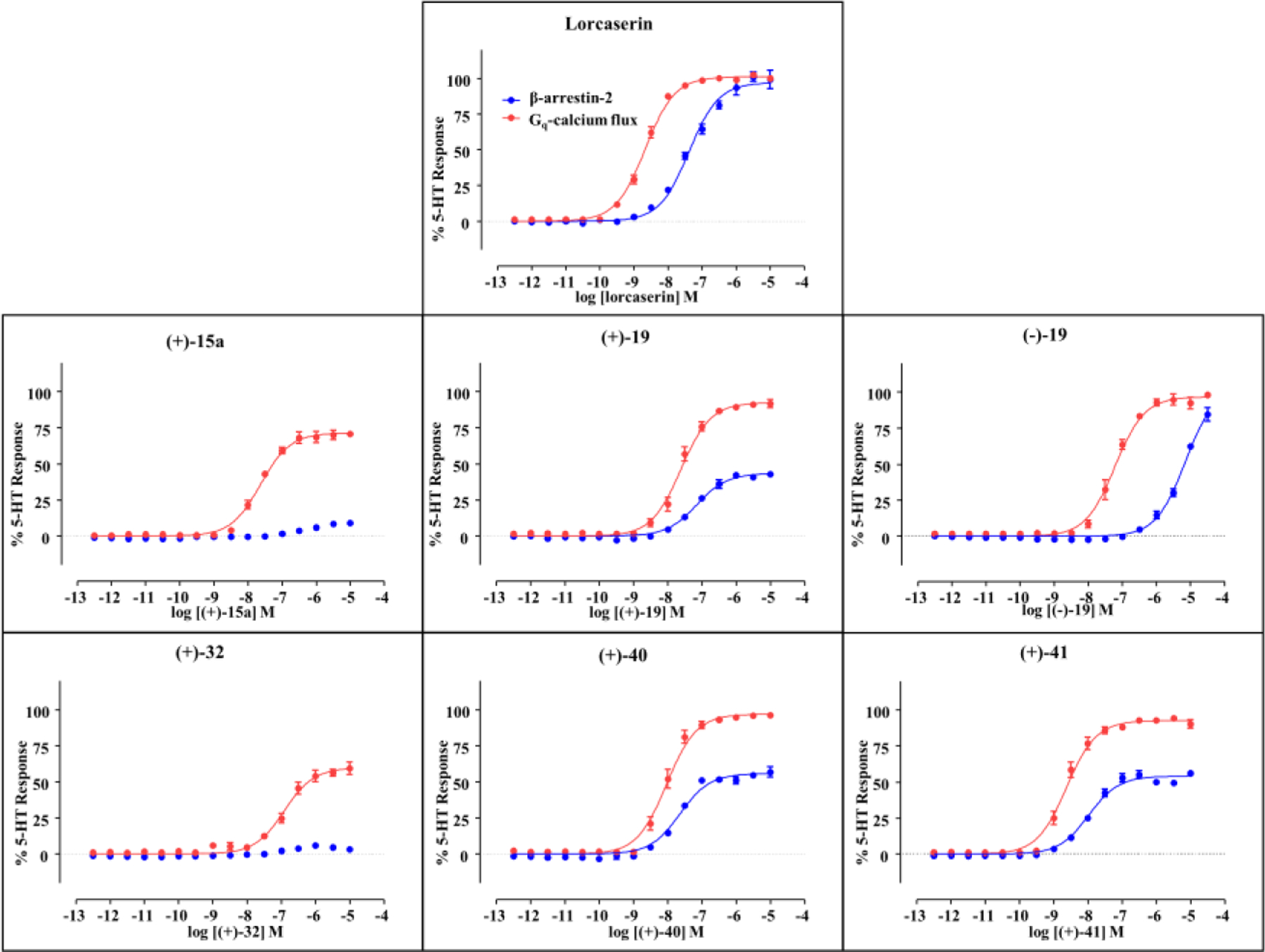
Profiling of 5-HT2C functional selectivity measuring Gq-calcium flux (FLIPR, red) and β-arrestin-2 recruitment (Tango, blue). Data were acquired with the human 5-HT2C-INI receptor isoform. Emax values are shown as the mean (n = 3), and assays were conducted in parallel with the same drug dilutions.
Evaluation in Animal Models of Antipsychotic Drug-like Activity.
In vitro pharmacology profiles identified the N-(2-methoxybenzyl) compound (+)-19 as the most potent 5-HT2C agonist (EC50 = 24 nM, Emax = 92%) with full selectivity over 5-HT2B in the present series of compounds. As the absence of 5-HT2B agonism is necessary to avoid potential cardiovascular side effects, compound (+)-19 was selected for further in vivo studies in the amphetamine (AMPH)-induced and phencyclidine (PCP)-induced hyperactivity models (details of the behavioral studies are provided in the Experimental Section), which are both well-recognized models to evaluate the possible antipsychotic activities of compounds.
Amphetamine (AMPH)-induced Hyperactivity Model.
In this model, adult male C57BL/6J mice were administered saline (vehicle), lorcaserin (as the positive control, 3 mg/kg), or the test compound (+)-19 (10 and 20 mg/kg), and locomotor activity was monitored for 15 min (baseline). As shown in Figure 4A, lorcaserin decreased baseline locomotion while the compound (+)-19 (10 and 20 mg/kg) had no effect. Next the mice were given saline or amphetamine (3 mg/kg), and activity was measured for an additional 90 min. The results showed that lorcaserin reduced amphetamine-induced hyperactivity consistent with other 5-HT2C agonists,35, 36 whereas responses to 10 and 20 mg/kg of (+)-19 were differentiated by dose (Figure 4A and 4B). Although the low dose (10 mg/kg) of (+)-19 had no significant effect, the higher dose (20 mg/kg) decreased the hyperlocomotion such that its effects were similar to those of lorcaserin. Thus, compound (+)-19 (20 mg/kg) showed an antipsychotic action by blocking amphetamine-induced hyperactivity with no effect on spontaneous motor activity.
Figure 4.
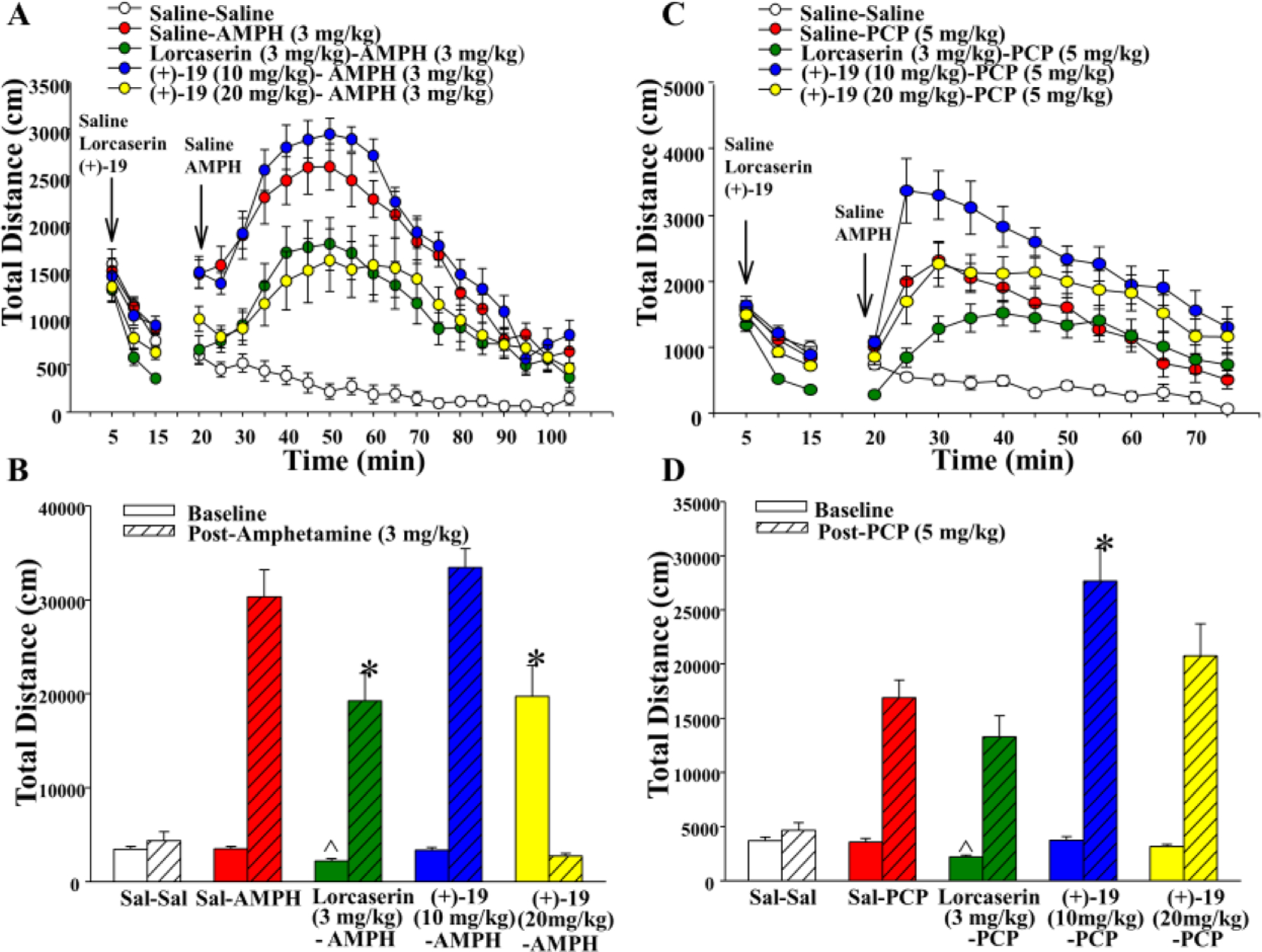
Evaluation of compound (+)-19 in animal models of antipsychotic drug-like activity. (A) Locomotor activity reflecting baseline responses (0–15 min) and AMPH-induced hyperactivity with reductions by lorcaserin and (+)-19 (20–105 min). (B) Cumulative baseline locomotor activities (0–15 min), AMPH-induced hyperactivities, and reductions by lorcaserin and (+)-19 (20–105 min); ^ p < 0.05, compared to Sal-Sal group, baseline; * p < 0.05 compared to Sal-AMPH, Post-AMPH. (C) Locomotor activity reflecting baseline responses (0–15 min) and PCP-induced hyperactivity with effects by lorcaserin and (+)-19 (20–75 min). (B) Cumulative baseline locomotor activities (0–15 min), PCP-induced hyperactivities, and effects by lorcaserin and (+)-19 (20–75 min); ^ p < 0.05, compared to Sal-Sal group, baseline; * p < 0.05 compared to Sal-PCP, Post-PCP. N = 8–10 mice/group.
Phencyclidine (PCP)-induced hyperactivity model.
In contrast to amphetamine-induced hyperactivity, lorcaserin showed a tendency to suppress PCP-induced hyperactivity at the beginning of the test session, but there was no overall reduction in activity that was statistically significant. While the low dose (10 mg/kg) of (+)-19 increased locomotion, the higher dose (20 mg/kg) had no effect on PCP-induced hyperlocomotion (Figure 4C and 4D). In general, it has been demonstrated that 5-HT2C agonists decrease PCP-induced hyperactivity.37, 38 The locomotion enhancement observed with the lower dose of (+)-19 could result from other off-target effects on PCP. For example, from our in vitro ADMET results (Table 3), (+)-19 exhibits strong inhibition (85.6%, Table 3) of human cytochrome P450 (CYP) 3A4 (in the mouse mainly its highly homologous 3A11)39 which is the same enzyme that has been reported to metabolize PCP.40 Alternatively, the 5-HT2A agonism of (+)-19 (EC50 = 139 nM, Emax = 63%, Table 1) might also explain its effect on PCP-induced locomotor activity. The 5-HT2A/2C agonist DOI (5-HT2A, EC50 = 57 nM, Emax = 46%; 5-HT2C, EC50 = 178 nM, Emax = 90%)41 has been shown to potentiate the locomotor effects of PCP.42 These results indicate that (+)-19 at doses of either 10 or 20 mg/kg does not possess an antipsychotic-like profile in the PCP-induced locomotion model.
Table 3.
In Vitro ADMET Data for Compound (+)-19
| Assay | (+)-19 | |
|---|---|---|
| human | 82.0 | |
| mouse | 92.0 | |
| CYP inhibition at 10 μM (%)a | 1A2 | 20.5 |
| 2C9 | 9.3 | |
| 2D6 | 68.9 | |
| 3A4 | 85.6 | |
| T1/2 (min)b | human | 43.0 |
| mouse | 7.8 | |
| hERG IC50 (μM)c | 1.4 | |
The CYP inhibition test was performed using human liver microsomes. Phenacetin, tolbutamide, dextromethorphan, and midazolam were used as test substrates for the 1A2, 2C9, 2D6, and 3A4 isoforms, respectively.
The concentration of hepatocytes was 0.5 × 106 cells/mL, and (+)-19 was tested at 1 μM.
hERG inhibition was tested on CHO cells using the automated patch-clamp method.
In summary, (+)-19 has superiority over lorcaserin based on its behavioral profile in decreasing amphetamine-induced hyperactivity without having an effect on spontaneous activity. Further studies are required to determine the precise mechanism of the enhancement of PCP using the lower dose (+)-19.
ADMET Studies (In vitro).
Due to its efficacy in the amphetamine-induced hyperactivity model, compound (+)-19 was evaluated for selected in vitro ADMET properties (Table 3) to qualify it as a possible candidate for further development. Compared with the structurally similar parent 2-(5-chlorophenyl)cyclopropylmethylamines (+)-9 and (+)-10 previously reported by us, the N-benzylated derivative (+)-19 displayed higher human plasma protein binding (82%, (+)-19; 75%, (+)-9; 57%, (+)-10)23 as a result of its enhanced lipophilicity, as the cLogP is a highly correlated indicator that determines protein binding properties. In the recombinant CYP inhibition assay, (+)-19 showed low inhibition of CYP 1A2 and CYP 2C9, and relatively higher inhibition of CYP 2D6 and CYP 3A4 at 10 μM (> 50%). Compound (+)-19 was found to have a half-life of 43 min in the human hepatocyte stability assay, which may be a consequence of the presence of the electron-rich N-benzyl group.43 Moreover, moderate hERG inhibition (IC50 = 1.4 μM) was detected.
Conclusions
New N-substituted 2-phenylcyclopropylmethylamines have been characterized as reasonably selective 5-HT2C receptor agonists with novel patterns of functional selectivity. The N-methyl compound (+)-15a, which displayed an EC50 of 23 nM at 5-HT2C with no β-arrestin recruitment activity, is the first potent and at the same time fully Gq-biased 5-HT2C agonist reported to date, while the N-benzyl compound (+)-19 with an EC50 of 24 nM at 5-HT2C is fully selective over 5-HT2B. The potency and lack of detectable arrestin recruitment of (+)-15a make it a valuable chemical probe to better understand biased 5-HT2C signaling. Moreover, although (+)-19 had a relatively short half-life in the hepatocyte stability assay, preliminary in vivo studies in an amphetamine-induced hyperactivity model indicate that (+)-19 shows potential antipsychotic effects. Further compound optimization to develop better drug-like analogs is in progress.
EXPERIMENTAL SECTION
General.
All chemicals and solvents were purchased from Sigma-Aldrich or Fisher Scientific, and were used as obtained without further purification. Microwave reactions were run in a Biotage Initiator microwave reactor. Synthetic intermediates were purified on 230−400 mesh silica gel using a Teledyne CombiFlash Rf flash chromatograph. 1H and 13C NMR spectra were recorded on Bruker DPX-400 or AVANCE-400 spectrometers at 400 MHz and 100 MHz, respectively. NMR chemical shifts are reported in δ (ppm) using residual solvent peaks as standards (CDCl3–7.26 (H), 77.16 (C); CD3OD–3.31 (H), 49.00 (C)). Mass spectra were measured using an LCMS-IT-TOF (Shimadzu) mass spectrometer in ESI mode. Preparative HPLC purification of synthetic intermediates was performed on a Shimadzu LC-8A instrument with an ACE 5AQ column (150 × 21.2 mm, particle size 5 μm; eluent: 8 – 100% MeOH (0.05% TFA)/ H2O (0.05% TFA) gradient, 30 min; flow rate: 17 mL/min; UV detection at 254 and 280 nm). Chiral separation of racemic intermediates was conducted by preparative HPLC on RegisPack (25 cm × 21.1 mm, particle size 10 μm) or ChromegaChiral CCJ (25 cm × 20 mm, particle size 10 μm) chiral columns with isopropanol (0.05% diethylamine, DEA)/ n-hexane (0.05% DEA) as the eluent. The purity of all final compounds (greater than 95% in all cases) was determined by analytical HPLC on an ACE 3AQ C18 column (150 × 4.6 mm, particle size 3 μm; eluent: MeOH (0.05% TFA)/ H2O (0.05% TFA) gradient, 25 min; flow rate: 1.0 mL/min). Specific rotations were recorded on a Rudolph Research Autopol IV automatic polarimeter.
The synthetic procedures, chiral separation methods, and characterization data of all intermediates can be found in the Supporting Information. All intermediates subjected to chiral preparative HPLC separation were prepared with an optical purity of > 90% ee (determined by analytical HPLC using a RegisPack (25 cm × 4.6 mm, 10 μm) or ChromegaChiral CCJ (25 cm × 4.6 mm, 10 μm) chiral column and isopropanol (0.05% DEA)/ n-hexane (0.05% DEA) as the eluent).
General Method A: Preparation of HCl Salts 15a-c.
The N-Boc-amines 14a-c were dissolved in 2M HCl (g) in diethyl ether (5 equiv.) and stirred at room temperature for 24–48 h. The white solids formed were collected by filtration, washed with diethyl ether, and dried under vacuum to give the HCl salts as white solids.
General Method B: Preparation of HCl Salts 16–22, 26–32, and 34–35 from the Intermediate Aldehyde D.
The aldehyde D was prepared according to the reported procedure.24 Aldehyde D (1.0 equiv.) and amines (1.2 equiv.) were reacted under reductive amination condition (NaBH4 (1.5 equiv.)/MeOH). The reaction mixtures were quenched with water and extracted with DCM. The organic phases were washed with brine, dried over sodium sulfate, concentrated, and purified by preparative HPLC to give the trifluoroacetate salts. The salts were neutralized with aq. NaHCO3, and the resulting solutions were extracted with DCM. The organic layers were dried over sodium sulfate and concentrated to provide the desired compounds as free bases. For 19–21, 26–27, and 32, the obtained racemic free bases were separated by chiral preparative HPLC to afford their enantiomers (see Supporting Information). The racemic or enantiopure free bases were dissolved in 2M HCl (g) in diethyl ether (3 equiv.) and stirred at room temperature for 1–2 h. The white solids formed were collected by filtration, washed with diethyl ether, and dried under vacuum to give the HCl salts as white solids in high yields (80–95%).
General Method C: Preparation of HCl Salts 24–25, 33, and 36–39 from the Intermediate Cyclopropylmethylamine G.
The cyclopropylmethylamine G was prepared according to the reported procedure.24 The listed compounds were prepared from the cyclopropylmethylamine G (1.0 equiv.) and ArCHO (1.0 equiv.) or ArCH2OTs (1.0 equiv.) via reductive amination (NaBH4 (1.5 equiv.)/MeOH or NaBH(OAc)3 (2.0 equiv.)/DCE) or nucleophile substitution reactions (base/CH3CN), respectively. The crude products were purified by preparative LC and reacted with 2M HCl/Et2O to afford the HCl salts as white solids according to the similar procedure described in General Method B.
(+)-1-[(1S,2S)-2-(5-Fluoro-2-methoxyphenyl)cyclopropyl]-N-methylmethanamine Hydrochloride (15a).
Obtained from the intermediate 14a (56 mg, 0.18 mmol) employing General Method A (40 mg, 90% yield). 1H NMR (CDCl3) δ 9.61 (s, 2H), 6.88 – 6.82 (m, 1H), 6.76 (dd, J = 9.0, 4.6 Hz, 1H), 6.66 (dd, J = 9.3, 3.0 Hz, 1H), 3.85 (s, 3H), 3.10 (dd, J = 13.1, 7.1 Hz, 1H), 3.02 (dd, J = 13.0, 7.6 Hz, 1H), 2.75 (s, 3H), 2.25 – 2.07 (m, 1H), 1.52 – 1.37 (m, 1H), 1.21 – 1.09 (m, 2H); 13C NMR (CDCl3) δ 157.2 (d, J = 238.2 Hz), 154.5 (s), 130.6 (d, J = 7.4 Hz), 113.3 (d, J = 23.9 Hz), 113.1 (d, J = 22.5 Hz), 111.2 (d, J = 8.5 Hz), 56.2 (s), 52.9 (s), 32.1 (s), 17.8 (s), 16.9 (s), 13.0 (s); HRMS (ESI) calculated for C12H17FNO ([M+H]+), 210.1289; found, 210.1269. [α]D20 +18.0 (c 0.1, CHCl3).
(+)-1-[(1S,2S)-2-[2-(Allyloxy)-5-fluorophenyl]cyclopropyl]-N-methylmethanamine Hydrochloride (15b).
Obtained from the intermediate 14b (188 mg, 0.56 mmol) employing General Method A (140 mg, 93% yield). 1H NMR (CDCl3) δ 9.62 (s, 2H), 6.82 (td, J = 8.4, 3.0 Hz, 1H), 6.75 (dd, J = 8.9, 4.6 Hz, 1H), 6.65 (dd, J = 9.3, 3.0 Hz, 1H), 6.14 – 6.02 (m, 1H), 5.41 (dd, J = 17.2, 1.4 Hz, 1H), 5.30 (dd, J = 10.5, 1.4 Hz, 1H), 4.54 (d, J = 5.3 Hz, 2H), 3.21 – 3.06 (m, 1H), 3.05 – 2.92 (m, 1H), 2.73 (s, 3H), 2.25 – 2.17 (m, 1H), 1.53 – 1.41 (m, 1H), 1.15 (t, J = 7.1 Hz, 2H); 13C NMR (CDCl3) δ 157.3 (d, J = 238.7 Hz), 153.4 (s), 133.4 (s), 131.0 (d, J = 7.3 Hz), 118.0 (s), 113.2 (d, J = 23.8 Hz), 113.1 (d, J = 22.6 Hz), 112.7 (d, J = 8.4 Hz), 69.9 (s), 52.8 (s), 32.2 (s), 17.9 (s), 17.0 (s), 13.0 (s); HRMS (ESI) calculated for C14H19FNO ([M+H]+), 236.1445; found, 236.1408. [α]D20 +20.3 (c 0.1, CHCl3).
(+)-1-[(1S,2S)-2-[5-Fluoro-2-(2-fluoroethoxy)phenyl]cyclopropyl]-N-methylmethanamine Hydrochloride (15c).
Obtained from the intermediate 14c (70 mg, 0.20 mmol) employing General Method A (52 mg, 95% yield). 1H NMR (CDCl3) δ 9.53 (s, 2H), 6.86 (td, J = 8.5, 2.7 Hz, 1H), 6.77 (dd, J = 8.9, 4.5 Hz, 1H), 6.68 (dd, J = 9.2, 2.8 Hz, 1H), 5.02 – 4.68 (m, 2H), 4.36 – 4.12 (m, 2H), 3.19 – 2.99 (m, 2H), 2.74 (s, 3H), 2.29 – 2.17 (m, 1H), 1.52 – 1.36 (m, 1H), 1.26 – 1.07 (m, 2H); 13C NMR (CDCl3) δ 157.7 (d, J = 239.6 Hz), 153.3 (s), 131.4 (d, J = 7.4 Hz), 113.7 (d, J = 23.8 Hz), 113.3 (d, J = 22.9 Hz), 112.8 (d, J = 8.5 Hz), 82.4 (d, J = 169.9 Hz), 68.5 (d, J = 19.4 Hz), 53.0 (s), 32.5 (s), 17.8 (s), 17.3 (s), 12.8 (s). HRMS (ESI) calculated for C13H18F2NO ([M+H]+), 242.1351; found, 242.1328. [α]D20 +8.2 (c 0.1, CHCl3).
N-[[2-(5-Fluoro-2-methoxyphenyl)cyclopropyl]methyl]cyclopropanamine Hydrochloride (16).
Prepared from cyclopropanamine employing Method B. 1H NMR (CDCl3) δ 9.72 (s, 2H), 6.87 – 6.80 (m, 1H), 6.75 (dd, J = 8.9, 4.6 Hz, 1H), 6.63 (dd, J = 9.3, 3.0 Hz, 1H), 3.83 (s, 3H), 3.20 (dd, J = 13.0, 6.9 Hz, 1H), 3.03 (dd, J = 13.0, 7.8 Hz, 1H), 2.78 – 2.66 (m, 1H), 2.28 – 2.15 (m, 1H), 1.59 – 1.46 (m, 1H), 1.36 – 1.07 (m, 4H), 0.91 – 0.75 (m, 2H); 13C NMR (CDCl3) δ 157.2 (d, J = 238.2 Hz), 154.4 (s), 130.9 (d, J = 7.3 Hz), 113.0 (d, J = 24.8 Hz), 112.9 (d, J = 21.2 Hz), 111.1 (d, J = 8.4 Hz), 56.1 (s), 52.2 (s), 29.5 (s), 17.8 (s), 17.0 (s), 13.5 (s), 4.1 (s), 3.8 (s); HRMS (ESI) calculated for C14H19FNO ([M+H]+), 236.1445; found, 236.1405.
1-Cyclopropyl-N-[[2-(5-fluoro-2-methoxyphenyl)cyclopropyl]methyl]methanamine Hydrochloride (17).
Prepared from cyclopropylmethanamine employing Method B. 1H NMR (CDCl3) δ 9.65 (s, 2H), 6.84 (td, J = 8.5, 2.9 Hz, 1H), 6.75 (dd, J = 8.9, 4.5 Hz, 1H), 6.62 (dd, J = 9.3, 2.9 Hz, 1H), 3.84 (s, 3H), 3.26 – 3.01 (m, 2H), 3.00 – 2.88 (m, 2H), 2.23 – 2.09 (m, 1H), 1.56 – 1.40 (m, 1H), 1.39 – 1.22 (m, 1H), 1.19 – 1.07 (m, 2H), 0.76 – 0.64 (m, 2H), 0.55 – 0.40 (m, 2H); 13C NMR (CDCl3) δ 157.3 (d, J = 238.3 Hz), 154.4 (s), 130.8 (d, J = 7.4 Hz), 113.1 (d, J = 24.0 Hz), 113.0 (d, J = 24.4 Hz), 111.2 (d, J = 8.4 Hz), 56.2 (s), 51.3 (s), 50.7 (s), 17.8 (s), 17.2 (s), 13.2 (s), 7.2 (s), 4.9 (s), 4.7 (s); HRMS (ESI) calculated for C15H21FNO ([M+H]+), 250.1602; found, 250.1545.
1-Cyclohexyl-N-[[2-(5-fluoro-2-methoxyphenyl)cyclopropyl]methyl]methanamine Hydrochloride (18).
Prepared from cyclohexylmethylamine employing Method B. 1H NMR (CDCl3) δ 9.52 (s, 1H), 9.43 (s, 1H), 6.88 – 6.80 (m, 1H), 6.76 (dd, J = 9.0, 4.6 Hz, 1H), 6.59 (dd, J = 9.3, 3.0 Hz, 1H), 3.83 (s, 3H), 3.17 – 3.03 (m, 2H), 2.98 – 2.81 (m, 2H), 2.22 – 2.11 (m, 1H), 2.04 – 1.85 (m, 3H), 1.81 – 1.62 (m, 3H), 1.50 – 1.38 (m, 1H), 1.36 – 1.09 (m, 5H), 1.08 – 0.94 (m, 2H); 13C NMR (CDCl3) δ 157.3 (d, J = 238.3 Hz), 154.3 (s), 130.8 (d, J = 7.3 Hz), 113.0 (d, J = 22.8 Hz), 112.8 (d, J = 23.8 Hz), 111.1 (d, J = 8.4 Hz), 56.2 (s), 52.5 (s), 51. 6 (s), 34.8 (s), 31.1 (s), 31.0 (s), 26.0 (s), 25.5 (s), 25.4 (s), 17.8 (s), 17.3 (s), 13.0 (s); HRMS (ESI) calculated for C18H27FNO ([M+H]+), 292.2071; found, 292.2073.
(+)-1-[(1S,2S)-2-(5-Fluoro-2-methoxyphenyl)cyclopropyl]-N-(2-methoxybenzyl)methanamine Hydrochloride ((+)-19).
Prepared from 2-methoxybenzylamine employing Method B including chiral separation. 1H NMR (CDCl3) δ 10.02 (s, 1H), 9.15 (s, 1H), 7.48 (d, J = 6.7 Hz, 1H), 7.33 (t, J = 7.8 Hz, 1H), 6.95 (t, J = 7.4 Hz, 1H), 6.88 (d, J = 8.3 Hz, 1H), 6.81 (td, J = 8.6, 2.9 Hz, 1H), 6.72 (dd, J = 8.9, 4.5 Hz, 1H), 6.59 (dd, J = 9.2, 2.9 Hz, 1H), 4.19 (m, 2H), 3.84 (s, 3H), 3.78 (s, 3H), 3.08 – 2.72 (m, 2H), 1.99 – 1.83 (m, 1H), 1.47 – 1.37 (m, 1H), 1.09 – 0.88 (m, 1H); 13C NMR (CDCl3) δ 157.9 (s), 157.2 (d, J = 238.3 Hz), 154.4 (d, J = 2.0 Hz), 132.2 (s), 131.2 (s), 130.7 (d, J = 7.4 Hz), 121.2 (s), 119.0 (s), 113.5 (d, J = 23.8 Hz), 113.1 (d, J = 22.7 Hz), 111.1 (d, J = 8.4 Hz), 110.7 (s), 56.2 (s), 55.7 (s), 50.3 (s), 45.8 (s), 17.9 (s), 17.3 (s), 13.2 (s); HRMS (ESI) calculated for C19H23FNO2 ([M+H]+), 316.1707; found, 316.1703; [α]D20 +34.0 (c 0.6, CHCl3).
(–)-1-[(1R,2R)-2-(5-Fluoro-2-methoxyphenyl)cyclopropyl]-N-(2-methoxybenzyl)methanamine Hydrochloride ((–)-19).
Prepared from 2-methoxybenzylamine employing General Method B including chiral separation. 1H NMR (CDCl3) δ 10.02 (s, 1H), 9.15 (s, 1H), 7.48 (d, J = 6.7 Hz, 1H), 7.33 (t, J = 7.8 Hz, 1H), 6.95 (t, J = 7.4 Hz, 1H), 6.88 (d, J = 8.3 Hz, 1H), 6.81 (td, J = 8.6, 2.9 Hz, 1H), 6.72 (dd, J = 8.9, 4.5 Hz, 1H), 6.59 (dd, J = 9.2, 2.9 Hz, 1H), 4.19 (m, 2H), 3.84 (s, 3H), 3.78 (s, 3H), 3.07 – 2.72 (m, 2H), 1.99 – 1.83 (m, 1H), 1.47 – 1.37 (m, 1H), 1.09 – 0.87 (m, 1H); 13C NMR (CDCl3) δ 157.9 (s), 157.2 (d, J = 238.3 Hz), 154.4 (d, J = 2.0 Hz), 132.2 (s), 131.2 (s), 130.7 (d, J = 7.4 Hz), 121.2 (s), 119.0 (s), 113.5 (d, J = 23.8 Hz), 113.1 (d, J = 22.7 Hz), 111.1 (d, J = 8.4 Hz), 110.7 (s), 56.2 (s), 55.7 (s), 50.3 (s), 45.8 (s), 17.9 (s), 17.3 (s), 13.2 (s); HRMS (ESI) calculated for C19H23FNO2 ([M+H]+), 316.1707; found, 316.1710; [α]D20 –39.0 (c 0.2, CHCl3).
(+)-1-[(1S,2S)-2-(5-Fluoro-2-methoxyphenyl)cyclopropyl]-N-(3-methoxybenzyl)methanamine Hydrochloride ((+)-20).
Prepared from 3-methoxybenzylamine employing General Method B including chiral separation. 1H NMR (CDCl3) δ 10.00 (s, 2H), 7.42 – 7.23 (m, 2H), 7.19 – 7.06 (m, 1H), 6.89 (d, J = 8.1 Hz, 1H), 6.82 (td, J = 8.6, 2.3 Hz, 1H), 6.73 (dd, J = 8.8, 4.4 Hz, 1H), 6.63 (dd, J = 9.1, 2.4 Hz, 1H), 4.12 (s, 2H), 3.83 (s, 3H), 3.79 (s, 3H), 3.08 – 2.75 (m, 2H), 2.20 – 2.03 (m, 1H), 1.57 – 1.36 (m, 1H), 1.15 – 0.96 (m, 2H); 13C NMR (CDCl3) δ 160.3 (s), 157.3 (d, J = 238.5 Hz), 154.4 (s), 132.0 (s), 130.7 (d, J = 7.2 Hz), 130.3 (s), 122.3 (s), 115.8 (s), 115.1 (s), 113.2 (d, J = 23.4 Hz), 113.0 (d, J = 22.2 Hz), 111.2 (d, J = 8.3 Hz), 56.2 (s), 55.7 (s), 49.9 (s, 2C), 18.0 (s), 17.0 (s), 13.4 (s); HRMS (ESI) calculated for C19H23FNO2 ([M+H]+), 316.1707; found, 316.1706; [α]D20 +13.6 (c 0.3, MeOH).
(–)-1-[(1R,2R)-2-(5-Fluoro-2-methoxyphenyl)cyclopropyl]-N-(3-methoxybenzyl)methanamine Hydrochloride ((–)-20).
Prepared from 3-methoxybenzylamine employing General Method B including chiral separation. 1H NMR (CDCl3) δ 10.01 (s, 2H), 7.42 – 7.23 (m, 2H), 7.19 – 7.06 (m, 1H), 6.89 (d, J = 8.1 Hz, 1H), 6.82 (td, J = 8.6, 2.3 Hz, 1H), 6.73 (dd, J = 8.8, 4.4 Hz, 1H), 6.63 (dd, J = 9.1, 2.4 Hz, 1H), 4.12 (s, 2H), 3.83 (s, 3H), 3.79 (s, 3H), 3.08 – 2.75 (m, 2H), 2.20 – 2.03 (m, 1H), 1.57 – 1.36 (m, 1H), 1.15 – 0.96 (m, 2H).; 13C NMR (CDCl3) δ 160.3 (s), 157.3 (d, J = 238.5 Hz), 154.4 (s), 132.0 (s), 130.7 (d, J = 7.2 Hz), 130.3 (s), 122.3 (s), 115.8 (s), 115.1 (s), 113.2 (d, J = 23.4 Hz), 113.0 (d, J = 22.2 Hz), 111.2 (d, J = 8.3 Hz), 56.2 (s), 55.7 (s), 49.9 (s, 2C), 18.0 (s), 17.1 (s), 13.4 (s); HRMS (ESI) calculated for C19H23FNO2 ([M+H]+), 316.1707; found, 316.1703; [α]D20 –15.0 (c 0.3, MeOH).
(+)-1-[(1S,2S)-2-(5-Fluoro-2-methoxyphenyl)cyclopropyl]-N-(4-methoxybenzyl)methanamine Hydrochloride ((+)-21).
Prepared from 4-methoxybenzylamine employing General Method B including chiral separation. 1H NMR (CDCl3) δ 9.90 (s, 2H), 7.54 (d, J = 8.5 Hz, 2H), 6.90 (d, J = 8.5 Hz, 2H), 6.81 (td, J = 8.4, 3.0 Hz, 1H), 6.72 (dd, J = 8.9, 4.5 Hz, 1H), 6.62 (dd, J = 9.3, 3.0 Hz, 1H), 4.09 – 4.01 (m, 2H), 3.78 (s, 3H), 3.74 (s, 3H), 3.00 – 2.74 (m, 2H), 2.10 – 2.06 (m, 1H), 1.49 – 1.46 (m, 1H), 1.06 – 1.00 (m, 2H); 13C NMR (CDCl3) δ 160.4 (s), 157.2 (d, J = 238.1 Hz), 154.3 (s), 131.9 (s, 2C), 130.8 (d, J = 7.2 Hz), 122.5 (s), 114.5 (s, 2C), 113.2 (d, J = 24.1 Hz), 112.9 (d, J = 23.0 Hz), 111.1 (d, J = 8.4 Hz), 56.1 (s), 55.3 (s), 49.6 (s), 49.3 (s), 17.9 (s), 17.0 (s), 13.4 (s); HRMS (ESI) calculated for C19H23FNO2 ([M+H]+), 316.1707; found, 316.1700; [α]D20 +20.6 (c 1.0, MeOH).
(–)-1-[(1R,2R)-2-(5-Fluoro-2-methoxyphenyl)cyclopropyl]-N-(4-methoxybenzyl)methanamine Hydrochloride ((–)-21).
Prepared from 4-methoxybenzylamine employing General Method B including chiral separation. 1H NMR (CDCl3) δ 9.90 (s, 2H), 7.54 (d, J = 8.5 Hz, 2H), 6.90 (d, J = 8.5 Hz, 2H), 6.81 (td, J = 8.4, 3.0 Hz, 1H), 6.72 (dd, J = 8.9, 4.5 Hz, 1H), 6.62 (dd, J = 9.3, 3.0 Hz, 1H), 4.09 – 4.01 (m, 2H), 3.78 (s, 3H), 3.74 (s, 3H), 3.00 – 2.74 (m, 2H), 2.10 – 2.05 (m, 1H), 1.49 – 1.46 (m, 1H), 1.06 – 1.02 (m, 2H); 13C NMR (CDCl3) δ 160.4 (s), 157.2 (d, J = 238.1 Hz), 154.3 (s), 131.9 (s, 2C), 130.8 (d, J = 7.2 Hz), 122.5 (s), 114.5 (s, 2C), 113.2 (d, J = 24.1 Hz), 112.9 (d, J = 23.0 Hz), 111.1 (d, J = 8.4 Hz), 56.1 (s), 55.3 (s), 49.6 (s), 49.3 (s), 17.9 (s), 17.0 (s), 13.4 (s); HRMS (ESI) calculated for C19H23FNO2 ([M+H]+), 316.1707; found, 316.1702; [α]D20 –22.3 (c 1.0, MeOH).
2-[[[[2-(5-Fluoro-2-methoxyphenyl)cyclopropyl]methyl]amino]methyl]phenol Hydrochloride (22).
Prepared from 2-hydroxybenzylamine employing General Method B. 1H NMR (CDCl3) δ 9.52 (s, 1H), 9.07 (s, 1H), 8.75 (s, 1H), 7.27 – 7.10 (m, 3H), 6.88 – 6.75 (m, 2H), 6.69 (dd, J = 8.8, 4.3 Hz, 1H), 6.52 (dd, J = 9.1, 2.4 Hz, 1H), 4.32 – 4.01 (m, 2H), 3.76 (s, 3H), 3.14 – 2.79 (m, 2H), 2.09 – 1.90 (m, 1H), 1.42 – 1.28 (m, 1H), 1.10 – 0.76 (m, 2H); 13C NMR (CDCl3) δ 157.1 (d, J = 238.2 Hz), 155.8 (s), 154.4 (s), 131.5 (s), 131.4 (s), 130.3 (d, J = 7.3 Hz), 120.5 (s), 117.1 (s, 2C), 113.4 (d, J = 23.7 Hz), 113.1 (d, J = 22.3 Hz), 111.1 (d, J = 8.2 Hz), 56.2 (s), 50.7 (s), 47.5 (s), 17.8 (s), 17.4 (s), 12.7 (s); HRMS (ESI) calculated for C18H21FNO2 ([M+H]+), 302.1551; found, 302.1554.
N-[2-(2-Fluoroethoxy)benzyl]-1-[2-(5-fluoro-2-methoxyphenyl)cyclopropyl] methanamine Hydrochloride (23).
To a solution of the free base 22 (30 mg, 0.1 mmol), 2-fluoroethanol (10 mg, 0.15 mmol), and triphenylphosphine (52 mg, 0.2 mmol) in anhydrous THF (5 mL) at 0 °C was slowly added diethyl azodicarboxylate (35 mg, 0.2 mmol), and the solution was then heated in a microwave reactor at 60 °C for 45 min. The mixture was concentrated, and the residue was purified by flash chromatography to give the free base, which was further treated with 2M HCl in ether to afford the title compound as a white solid (25 mg, 63% yield). 1H NMR (CDCl3) δ 9.86 (s, 1H), 9.23 (s, 1H), 7.58 (dd, J = 7.5, 1.4 Hz, 1H), 7.40 – 7.31 (m, 1H), 7.02 (t, J = 7.3 Hz, 1H), 6.91 (d, J = 8.2 Hz, 1H), 6.87 – 6.81 (m, 1H), 6.74 (dd, J = 8.9, 4.5 Hz, 1H), 6.61 (dd, J = 9.2, 3.0 Hz, 1H), 4.93 – 4.82 (m, 1H), 4.82 – 4.68 (m, 1H), 4.42 – 4.26 (m, 2H), 4.26 – 4.11 (m, 2H), 3.77 (s, 3H), 3.04 – 2.83 (m, 2H), 2.07 – 1.95 (m, 1H), 1.49 – 1.36 (m, 1H), 1.04 (t, J = 7.1 Hz, 2H); 13C NMR (CDCl3) δ 157.3 (d, J = 238.2 Hz), 156.8 (s), 154.4 (s), 132.6 (s), 131.3 (s), 130.8 (d, J = 7.4 Hz), 121.9 (s), 119.6 (s), 113.4 (d, J = 23.9 Hz), 113.0 (d, J = 22.7 Hz), 111.6 (s), 111.1 (d, J = 8.4 Hz), 82.0 (d, J = 170.5 Hz), 67.6 (d, J = 19.6 Hz), 56.1 (s), 50.8 (s), 45.7 (s), 17.7 (s), 17.3 (s), 13.2 (s); HRMS (ESI) calculated for C20H24F2NO2 ([M+H]+), 348.1770; found, 348.1754.
2-[[[[2-(5-Fluoro-2-methoxyphenyl)cyclopropyl]methyl]amino]methyl]benzonitrile Hydrochloride (24).
Prepared from the tosylate 24b employing General Method C (K2CO3 (3.0 equiv.)/CH3CN, 60 oC). 1H NMR (CDCl3) δ 14.79 (s, 1H), 9.36 (t, J = 7.8 Hz, 1H), 7.84 (m, 1H), 7.71 (t, J = 7.6 Hz, 1H), 7.63 (d, J = 7.7 Hz, 1H), 7.28 (m, 1H), 6.80 (td, J = 8.8, 2.9 Hz, 1H), 6.70 (dd, J = 8.9, 4.6 Hz, 1H), 6.58 (dd, J = 9.4, 2.9 Hz, 1H), 5.85 (s, 2H), 3.96 – 3.84 (m, 1H), 3.75 – 3.63 (m, 1H), 3.67 (s, 3H), 2.29 – 2.18 (m, 1H), 1.64 – 1.50 (m, 1H), 1.23 – 1.05 (m, 2H); 13C NMR (CDCl3) δ 158.4 (s, J = 237.6 Hz), 154.5 (s), 136.5 (s), 131.4 (s, J = 7.7 Hz), 130.8 (s), 128.8 (s), 126.5 (s), 124.0 (s), 121.6 (s), 113.1 (d, J = 23.7 Hz), 112.9 (s), 112.8 (s, J = 22.9 Hz), 111.2 (d, J = 8.4 Hz), 56.2 (s), 48.3 (s), 48.2 (s), 20.4 (s), 17.4 (s), 13.3 (s); HRMS (ESI) calculated for C19H20FN2O ([M+H]+), 311.1554; found, 312.1563.
2-[[[[2-(5-Fluoro-2-methoxyphenyl)cyclopropyl]methyl]amino]methyl]benzamide Hydrochloride (25).
To a solution of the free base 24 (35 mg, 0.1 mmol) and 5N sodium hydroxide (30 μL) in methanol (5 mL) was added 30% hydrogen peroxide (0.2 mL). The mixture was heated to reflux for 1 h. The reaction mixture was cooled, treated with water, and extracted with EtOAc. The organic layer was washed with brine, dried over Na2SO4, and concentrated. The residue was purified by flash chromatography to give the free base, which was further treated with 2M HCl in ether to afford the title compound as a white solid (30 mg, 73% yield). 1H NMR (CDCl3) δ 14.54 (s, 1H), 9.32 (s, 1H), 7.90 – 7.84 (m, 1H), 7.77 – 7.68 (m, 1H), 7.68 – 7.58 (m, 1H), 7.35 – 7.30 (m, 1H), 6.81 (td, J = 8.7, 2.0 Hz, 1H), 6.71 (dd, J = 8.7, 4.4 Hz, 1H), 6.59 (dd, J = 9.1, 2.1 Hz, 1H), 5.85 (s, 2H), 5.20 (s, 2H), 4.02 – 3.83 (m, 1H), 3.80 – 3.60 (m, 1H), 3.68 (s, 3H), 2.31 – 2.18 (m, 1H), 1.67 – 1.51 (m, 1H), 1.24 – 1.03 (m, 2H); 13C NMR (CDCl3) δ 172.5 (s), 157.2 (d, J = 238.0 Hz), 154.5 (s), 143.5 (s), 139.4 (s), 136.5 (s), 131.5 (d, J = 7.3 Hz), 130.9 (s), 128.8 (s), 121.7 (s), 113.1 (d, J = 23.8 Hz), 112.7 (d, J = 22.7 Hz), 111.2 (d, J = 8.4 Hz), 56.3 (s), 48.5 (s), 48.3 (s), 20.4 (s), 17.4 (s), 13.4 (s); HRMS (ESI) calculated for C19H22FN2O2 ([M+H]+), 329.1660; found, 329.1660.
(+)-N-(2-Fluorobenzyl)-1-[(1S,2S)-(+)-2-(5-fluoro-2-methoxyphenyl)cyclopropyl] methanamine Hydrochloride ((+)-26).
Prepared from 2-fluorobenzylamine employing General Method B including chiral separation. 1H NMR (CDCl3) δ 10.18 (s, 1H), 9.98 (s, 1H), 7.89 (t, J = 7.2 Hz, 1H), 7.37 (m, 1H), 7.21 (t, J = 7.3 Hz, 1H), 7.11 (t, J = 9.0 Hz, 1H), 6.81 (td, J = 8.5, 3.0 Hz, 1H), 6.72 (dd, J = 8.9, 4.5 Hz, 1H), 6.63 (dd, J = 9.3, 3.0 Hz, 1H), 4.25 (s, 2H), 3.79 (s, 3H), 3.12 – 2.80 (m, 2H), 2.16 – 2.04 (m, 1H), 1.56 – 1.40 (m, 1H), 1.13 – 0.98 (m, 2H); 13C NMR (CDCl3) δ 161.3 (d, J = 248.6 Hz), 157.2 (d, J = 238.1 Hz), 154.4 (s), 132.7 (s), 131.7 (d, J = 8.2 Hz), 130.7 (d, J = 7.3 Hz), 125.2 (d, J = 3.0 Hz), 118.0 (d, J = 14.0 Hz), 115.9 (d, J = 21.5 Hz), 113.3 (d, J = 28.5 Hz), 113.0 (d, J = 27.1 Hz), 111.1 (d, J = 8.3 Hz), 56.2 (s), 50.2 (s), 42.6 (s), 17.9 (s), 16.9 (s), 13.5 (s); HRMS (ESI) calculated for C18H20F2NO ([M+H]+), 304.1507; found, 304.1508; [α]D20 +12.6 (c 0.6, MeOH).
(–)-N-(2-Fluorobenzyl)-1-[(1R,2R)-2-(5-fluoro-2-methoxyphenyl)cyclopropyl] methanamine Hydrochloride ((–)-26).
Prepared from 2-fluorobenzylamine employing General Method B including chiral separation. 1H NMR (CDCl3) δ 10.18 (s, 1H), 9.98 (s, 1H), 7.89 (t, J = 7.2 Hz, 1H), 7.37 (m, 1H), 7.21 (t, J = 7.3 Hz, 1H), 7.11 (t, J = 9.0 Hz, 1H), 6.81 (td, J = 8.5, 3.0 Hz, 1H), 6.72 (dd, J = 8.9, 4.5 Hz, 1H), 6.63 (dd, J = 9.3, 3.0 Hz, 1H), 4.25 (s, 2H), 3.79 (s, 3H), 3.11 – 2.81 (m, 2H), 2.16 – 2.04 (m, 1H), 1.56 – 1.41 (m, 1H), 1.13 – 0.98 (m, 2H); 13C NMR (CDCl3) δ 161.3 (d, J = 248.6 Hz), 157.2 (d, J = 238.1 Hz), 154.4 (s), 132.7 (s), 131.7 (d, J = 8.2 Hz), 130.7 (d, J = 7.3 Hz), 125.2 (d, J = 3.0 Hz), 118.0 (d, J = 14.0 Hz), 115.9 (d, J = 21.5 Hz), 113.3 (d, J = 28.5 Hz), 113.0 (d, J = 27.1 Hz), 111.1 (d, J = 8.3 Hz), 56.2 (s), 50.2 (s), 42.6 (s), 17.9 (s), 16.9 (s), 13.5 (s); HRMS (ESI) calculated for C18H20F2NO ([M+H]+), 304.1507; found, 304.1508; [α]D20 –11.7 (c 0.6, MeOH).
(+)-N-(2-Chlorobenzyl)-1-[(1S,2S)-2-(5-fluoro-2-methoxyphenyl)cyclopropyl]methanamine Hydrochloride ((+)-27).
Prepared from 2-chlorobenzylamine employing General Method B including chiral separation. 1H NMR (CDCl3) δ 10.25 (s, 1H), 9.84 (s, 1H), 7.97 (dd, J = 7.0, 1.8 Hz, 1H), 7.42 (dd, J = 7.0, 2.2 Hz, 1H), 7.37 – 7.29 (m, 2H), 6.85 – 6.77 (m, 1H), 6.71 (dd, J = 8.9, 4.5 Hz, 1H), 6.60 (dd, J = 9.2, 3.0 Hz, 1H), 4.35 (s, 2H), 3.76 (s, 3H), 3.10 – 2.90 (m, 2H), 2.14 – 2.02 (m, 1H), 1.55 – 1.39 (m, 1H), 1.08 (t, J = 7.1 Hz, 2H); 13C NMR (CDCl3) δ 157.1 (d, J = 238.2 Hz), 154.2 (s), 134.7 (s), 132.4 (s), 130.9 (s), 130.5 (d, J = 7.2 Hz), 129.9 (s), 128.8 (s), 127.8 (s), 113.2 (d, J = 24.4 Hz), 112.9 (d, J = 23.4 Hz), 111.0 (d, J = 8.3 Hz), 56.0 (s), 50.4 (s), 46.5 (s), 17.8 (s), 17.0 (s), 13.3 (s); HRMS (ESI) calculated for C18H20ClFNO ([M+H]+), 320.1212; found, 320.1166; [α]D20 +4.2 (c 1.4, MeOH).
(–)-N-(2-Chlorobenzyl)-1-[(1R,2R)-2-(5-fluoro-2-methoxyphenyl)cyclopropyl]methanamine Hydrochloride ((–)-27).
Prepared from 2-chlorobenzylamine employing General Method B including chiral separation. 1H NMR (CDCl3) δ 10.25 (s, 1H), 9.84 (s, 1H), 7.97 (dd, J = 7.0, 1.8 Hz, 1H), 7.42 (dd, J = 7.0, 2.2 Hz, 1H), 7.37 – 7.29 (m, 2H), 6.85 – 6.77 (m, 1H), 6.71 (dd, J = 8.9, 4.5 Hz, 1H), 6.61 (dd, J = 9.2, 3.0 Hz, 1H), 4.35 (s, 2H), 3.76 (s, 3H), 3.11 – 2.90 (m, 2H), 2.14 – 2.02 (m, 1H), 1.55 – 1.39 (m, 1H), 1.07 (t, J = 7.1 Hz, 2H); 13C NMR (CDCl3) δ 157.1 (d, J = 238.2 Hz), 154.2 (s), 134.7 (s), 132.4 (s), 130.9 (s), 130.5 (d, J = 7.2 Hz), 129.9 (s), 128.8 (s), 127.8 (s), 113.2 (d, J = 24.4 Hz), 112.9 (d, J = 23.4 Hz), 111.0 (d, J = 8.3 Hz), 56.0 (s), 50.4 (s), 46.5 (s), 17.8 (s), 17.0 (s), 13.3 (s); HRMS (ESI) calculated for C18H20ClFNO ([M+H]+), 320.1212; found, 320.1206; [α]D20 –3.6 (c 1.4, MeOH).
1-[2-(5-Fluoro-2-methoxyphenyl)cyclopropyl]-N-(2-methoxybenzyl)-N-methylmethanamine Hydrochloride (28).
Prepared from 2-methoxy-N-methylbenzylamine employing General Method B. 1H NMR (CDCl3, racemic mixture of two pairs of enantiomeric salts) δ 11.62 (s, 1H), 11.43 (s, 1H), 7.48 – 7.34 (m, 4H), 7.02 (t, J = 7.5 Hz, 2H), 6.96 (d, J = 8.3 Hz, 2H), 6.91 – 6.82 (m, 2H), 6.77 (dd, J = 8.9, 4.5 Hz, 2H), 6.65 – 6.54 (m, 2H), 4.53, 4.44 (ABq, J = 12.9 Hz, 2H), 4.30, 4.22 (ABq, J = 12.9 Hz, 2H), 3.84 (s, 6H), 3.81 (s, 6H), 3.39 (dd, J = 13.3, 6.5 Hz, 1H), 3.29 – 3.15 (m, 2H), 3.00 (dd, J = 13.3, 7.7 Hz, 1H), 2.82 (s, 6H), 2.29 – 2.15 (m, 2H), 1.43 – 1.32 (m, 2H), 1.25 – 1.13 (m, 2H), 1.07 – 0.97 (m, 2H); 13C NMR (CDCl3) δ 158.29 (s), 158.24 (s), 157.28 (d, J = 238.3 Hz, 2C), 154.35 (s), 158.31 (s), 133.04 (s), 132.92 (s), 132.07 (s, 2C), 130.28 (d, J = 7.3 Hz, 2C), 121.36 (s, 2C), 117.26 (s, 2C), 113.22 (d, J = 22.7 Hz, 2C), 112.83 (d, J = 24.0 Hz), 112.80 (d, J = 23.9 Hz), 111.46 (s, 2C), 111.19 (d, J = 8.5 Hz, 2C), 59.43 (s), 59.23 (s), 56.00 (s, 2C), 55.52 (s, 2C), 53.81 (s), 53.43 (s), 39.34 (s), 38.82 (s), 17.98 (s), 17.74 (s), 15.95 (s), 15.90 (s), 12.90 (s), 12.77 (s); HRMS (ESI) calculated for C20H25FNO2 ([M+H]+), 330.1864; found, 330.1817.
N-[[2-(5-Fluoro-2-methoxyphenyl)cyclopropyl]methyl]-1-(2-methoxyphenyl)ethan-1-amine Hydrochloride (29).
Prepared from 1-(2-methoxyphenyl)ethylamine employing General Method B. 1H NMR (CD3OD, racemic mixture of two pairs of enantiomers) δ 7.45 (t, J = 7.9 Hz, 2H), 7.40 – 7.32 (m, 2H), 7.14 (dd, J = 8.1, 3.6 Hz, 2H), 7.08 (t, J = 7.6 Hz, 2H), 6.99 – 6.84 (m, 4H), 6.75 – 6.64 (m, 2H), 4.75 (m, 2H), 3.90 (s, 3H), 3.88 (s, 3H), 3.83 (s, 3H), 3.82 (s, 3H), 3.20 (dd, J = 13.0, 5.7 Hz, 1H), 3.01 (dd, J = 13.2, 7.4 Hz, 1H), 2.92 (dd, J = 13.2, 7.4 Hz, 1H), 2.82 (dd, J = 13.0, 7.9 Hz, 1H), 2.15 – 2.06 (m, 2H), 1.71 (d, J = 7.0 Hz, 3H), 1.68 (d, J = 7.0 Hz, 3H), 1.34 – 0.91 (m, 6H); 13C NMR (CD3OD) δ 158.61 (d, J = 237.0 Hz), 158.57 (d, J = 235.3 Hz), 158.43 (s), 158.32 (s), 155.76 (s, 2C), 132.22 (d, J = 9.7 Hz), 132.15 (s, 2C), 132.06 (d, J = 7.8 Hz), 129.76 (s, 2C), 124.97 (s), 124.90 (s), 122.56 (s), 122.52 (s), 114.05 (d, J = 19.8 Hz), 113.97 (d, J = 24.1 Hz), 113.91 (d, J = 22.7 Hz), 113.83 (d, J = 23.8 Hz), 112.71 (s, 2C), 112.44 (d, J = 8.3 Hz), 112.40 (d, J = 8.3 Hz), 56.51 (s), 56.46 (s), 56.10 (s, 2C), 55.35 (s), 55.26 (s), 51.01 (s), 50.92 (s), 18.74 (s), 18.32 (s), 18.28 (s, 2C), 18.14 (s), 18.01 (s), 13.75 (s), 13.05 (s); HRMS (ESI) calculated for C20H25FNO2 ([M+H]+), 330.1864; found, 330.1802.
N-[[2-(5-Fluoro-2-methoxyphenyl)cyclopropyl]methyl]-1-(2-methoxyphenyl)cyclopropan-1-amine Hydrochloride (30).
Prepared from 30b employing General Method B. 1H NMR (CD3OD) δ 7.49 – 7.45 (m, 1H), 7.42 (dd, J = 7.6, 1.6 Hz, 1H), 7.13 (d, J = 8.4 Hz, 1H), 7.04 (td, J = 7.6, 0.9 Hz, 1H), 6.92 – 6.89 (m, 2H), 6.64 (dd, J = 8.0, 2.8 Hz, 1H), 3.97 (s, 3H), 3.86 (s, 3H), 3.02 (dd, J = 13.2, 7.3 Hz, 1H), 2.89 (dd, J = 13.1, 7.6 Hz, 1H), 2.02 – 1.97 (m, 1H), 1.46 – 1.39 (m, 1H), 1.33 – 0.89 (m, 4H), 1.09 – 1.02 (m, 1H), 0.95 – 0.88 (m, 1H); 13C NMR (CD3OD) δ 160.2, 159.8 (d, J = 235.5 Hz), 155.7 (d, J = 2.0 Hz), 132.8, 132.2 (d, J = 7.4 Hz), 132.0, 122.7, 122.2, 114.1 (d, J = 22.8 Hz), 113.9 (d, J = 24.2 Hz), 112.5 (d, J = 8.4 Hz), 112.4, 56.5, 56.3, 51.6, 41.6, 18.5, 18.4, 14.2, 12.4, 11.8; HRMS (ESI) calculated for C21H25FNO2: [M+H]+, m/z 342.1864; found: 342.1833.
N-[[2-(5-Fluoro-2-methoxyphenyl)cyclopropyl]methyl]-2-(2-methoxyphenyl)ethan-1-amine Hydrochloride (31).
Prepared from 2-(2-methoxyphenyl)ethylamine employing General Method B. 1H NMR (CDCl3) δ 9.79 (s, 1H), 9.67 (s, 1H), 7.27 – 7.16 (m, 2H), 6.91 – 6.85 (m, 1H), 6.85 – 6.77 (m, 2H), 6.70 (dd, J = 9.0, 4.5 Hz, 1H), 6.60 (dd, J = 9.3, 3.0 Hz, 1H), 3.75 (s, 3H), 3.71 (s, 3H), 3.42 – 3.21 (m, 4H), 3.21 – 3.02 (m, 2H), 2.19 – 2.09 (m, 1H), 1.53 – 1.40 (m, 1H), 1.18 – 1.07 (m, 2H); 13C NMR (CDCl3) δ 157.5 (s), 157.2 (d, J = 238.2 Hz), 154.4 (d, J = 2.0 Hz), 130.8 (s), 130.7 (d, J = 7.2 Hz), 128.7 (s), 125.0 (s), 120.9 (s), 113.0 (d, J = 24.0 Hz), 112.9 (d, J = 24.5 Hz), 111.0 (d, J = 8.4 Hz), 110.4 (s), 56.0 (s), 55.3 (s), 51.0 (s), 46.1 (s), 27.8 (s), 17.8 (s), 17.2 (s), 13.0 (s); HRMS (ESI) calculated for C20H25FNO2 ([M+H]+), 330.1864; found, 330.1822.
(+)-1-[(1S,2S)-2-(5-Fluoro-2-methoxyphenyl)cyclopropyl]-N-(thiophen-2-ylmethyl)methanamine Hydrochloride ((+)-32).
Prepared from 2-thiophenylmethylamine employing General Method B including chiral separation. 1H NMR (CDCl3) δ 10.10 (s, 2H), 7.48 (d, J = 2.9 Hz, 1H), 7.34 (dd, J = 5.1, 1.1 Hz, 1H), 7.04 (dd, J = 5.1, 3.5 Hz, 1H), 6.86 – 6.79 (m, 1H), 6.74 (dd, J = 9.0, 4.6 Hz, 1H), 6.63 (dd, J = 9.3, 3.0 Hz, 1H), 4.40 (s, 2H), 3.81 (s, 3H), 3.02 (dd, J = 13.1, 7.2 Hz, 1H), 2.93 (dd, J = 13.1, 7.5 Hz, 1H), 2.19 – 2.09 (m, 1H), 1.55 – 1.42 (m, 1H), 1.13 – 1.02 (m, 2H); 13C NMR (CDCl3) δ 157.2 (d, J = 238.4 Hz), 154.4 (d, J = 2.0 Hz), 131.4 (s), 131.3 (s), 130.7 (d, J = 7.4 Hz), 128.0 (s), 127.9 (s), 113.3 (d, J = 22.3 Hz), 113.0 (d, J = 21.2 Hz), 111.2 (d, J = 8.3 Hz), 56.2 (s), 49.4 (s), 43.5 (s), 18.0 (s), 17.0 (s), 13.2 (s); HRMS (ESI) calculated for C16H19FNOS ([M+H]+), 292.1166; found, 292.1160; [α]D20 +30.6 (c 1.0, MeOH).
(–)-1-[(1R,2R)-2-(5-Fluoro-2-methoxyphenyl)cyclopropyl]-N-(thiophen-2-ylmethyl)methanamine Hydrochloride ((–)-32).
Prepared from 2-thiophenylmethylamine employing General Method B including chiral separation. 1H NMR (CDCl3) δ 10.10 (s, 2H), 7.48 (d, J = 2.9 Hz, 1H), 7.34 (dd, J = 5.1, 1.1 Hz, 1H), 7.04 (dd, J = 5.1, 3.5 Hz, 1H), 6.86 – 6.79 (m, 1H), 6.74 (dd, J = 9.0, 4.6 Hz, 1H), 6.63 (dd, J = 9.3, 3.0 Hz, 1H), 4.40 (s, 2H), 3.81 (s, 3H), 3.02 (dd, J = 13.1, 7.2 Hz, 1H), 2.93 (dd, J = 13.1, 7.5 Hz, 1H), 2.19 – 2.09 (m, 1H), 1.55 – 1.42 (m, 1H), 1.13 – 1.03 (m, 2H); 13C NMR (CDCl3) δ 157.2 (d, J = 238.4 Hz), 154.4 (d, J = 2.0 Hz), 131.4 (s), 131.3 (s), 130.7 (d, J = 7.4 Hz), 128.0 (s), 127.9 (s), 113.3 (d, J = 22.3 Hz), 113.0 (d, J = 21.2 Hz), 111.2 (d, J = 8.3 Hz), 56.2 (s), 49.5 (s), 43.5 (s), 18.0 (s), 17.0 (s), 13.2 (s); HRMS (ESI) calculated for C16H19FNOS ([M+H]+), 292.1166; found, 292.1160; [α]D20 –28.5 (c 1.0, MeOH).
1-[2-(5-Fluoro-2-methoxyphenyl)cyclopropyl]-N-[(3-methoxythiophen-2-yl)methyl]methanamine Hydrochloride (33).
Prepared from 3-methoxythiophene-2-carboxaldehyde employing General Method C (NaBH(OAc)3 (2.0 equiv.)/DCE). 1H NMR (CD3OD) δ 7.53 (d, J = 5.6 Hz, 1H), 7.08 (d, J = 5.6 Hz, 1H), 6.95 – 6.90 (m, 2H), 6.75 (dd, J = 9.6, 2.4 Hz, 1H), 4.40, 4.36 (ABq, J = 14.2 Hz, 2H), 3.92 (s, 3H), 3.84 (s, 3H), 3.19 (dd, J = 13.1, 6.9 Hz, 1H), 3.05 (dd, J = 13.1, 7.9 Hz, 1H), 2.23 – 2.18 (m, 1H), 1.34 – 1.28 (m, 1H), 1.19 – 1.14 (m, 1H), 1.07 – 1.02 (m, 1H); 13C NMR (CD3OD) δ 159.5 (s), 158.6 (d, J = 236.8 Hz), 155.9 (d, J = 2.0 Hz), 132.2 (d, J = 7.5 Hz), 127.8 (s), 117.1 (s), 114.1 (d, J = 24.2 Hz), 114.0 (d, J = 22.8 Hz), 112.4 (d, J = 8.5 Hz), 108.3 (s), 59.4 (s), 56.5 (s), 51.8 (s), 42.2 (s), 18.4 (s), 18.3 (s), 13.2 (s); HRMS (ESI) calculated for C17H21FNO2S ([M+H]+), m/z 322.1272; found: 322.1225.
1-[2-(5-Fluoro-2-methoxyphenyl)cyclopropyl]-N-[(4-methoxythiophen-3-yl)methyl]methanamine Hydrochloride (34).
Prepared from 34e employing General Method B. 1H NMR (CD3OD) δ 7.60 (d, J = 3.2 Hz, 1H), 6.93 – 6.90 (m, 2H), 6.75 (dd, J = 9.6, 2.4 Hz, 1H), 6.61 (d, J = 3.2 Hz, 1H), 4.24, 4.19 (ABq, J = 13.6 Hz, 2H), 3.89 (s, 3H), 3.83 (s, 3H), 3.20 (dd, J = 13.1, 7.0 Hz, 1H), 3.08 (dd, J = 13.0, 7.9 Hz, 1H), 2.23 – 2.16 (m, 1H), 1.38 – 1.30 (m, 1H), 1.19 – 1.14 (m, 1H), 1.07 – 1.03 (m, 1H); 13C NMR (CDCl3) δ 157.2 (d, J = 237.6 Hz), 156.2 (s), 154.4 (s), 130.7 (d, J = 7.4 Hz), 127.6 (s), 121.9 (s), 113.5 (d, J = 23.9 Hz), 113.1 (d, J = 22.7 Hz), 111.1 (d, J = 8.4 Hz), 97.7 (s), 57.8 (s), 56.2 (s), 50.0 (s), 41.7 (s), 17.9 (s), 17.1 (s), 13.2 (s); HRMS (ESI) calculated for C17H21FNO2S ([M+H]+), m/z 322.1272; found: 322.1241.
1-[2-(5-Fluoro-2-methoxyphenyl)cyclopropyl]-N-[(2-methoxythiophen-3-yl)methyl]methanamine Hydrochloride (35).
Prepared from 35e employing General Method B. 1H NMR (CD3OD) δ 6.95 – 6.90 (m, 3H), 6.83 (d, J = 6.0 Hz, 1H), 6.74 (dd, J = 9.6, 2.4 Hz, 1H), 4.19, 4.14 (ABq, J = 13.5 Hz, 2H), 4.00 (s, 3H), 3.83 (s, 3H), 3.16 (dd, J = 13.0, 7.0 Hz, 1H), 3.05 (dd, J = 13.1, 7.8 Hz, 1H), 2.23 – 2.18 (m, 1H), 1.31 – 1.28 (m, 1H), 1.19 – 1.14 (m, 1H), 1.07 – 1.03 (m, 1H); 13C NMR (CD3OD) δ 166.7 (s), 158.6 (d, J = 235.3 Hz), 155.8 (d, J = 2.0 Hz), 132.2 (d, J = 7.5 Hz), 127.3 (s), 114.0 (d, J = 23.0 Hz), 113.9 (d, J = 24.1 Hz), 113.1 (s), 112.4 (d, J = 8.5 Hz), 111.2 (s), 62.4 (s), 56.4 (s), 52.1 (s), 42.7 (s), 18.3 (s), 18.2 (s), 13.3 (s); HRMS (ESI) calculated for C17H21FNO2S ([M+H]+), m/z 322.1272; found: 322.1193.
1-[2-(5-Fluoro-2-methoxyphenyl)cyclopropyl]-N-[(2-methoxypyridin-3-yl]methyl)methanamine (36).
The free base 36 was obtained from 2-methoxynicotinaldehyde according to the similar procedure described in General Method C (NaBH4 (1.5 equiv.)/MeOH) as a colorless oil. 1H NMR (CD3OD) δ 8.10 (dd, J = 5.0, 1.8 Hz, 1H), 7.59 (dd, J = 7.2, 1.8 Hz, 1H), 6.88 (dd, J = 7.1, 5.1 Hz, 1H), 6.86 – 6.78 (m, 1H), 6.75 (dd, J = 8.9, 4.6 Hz, 1H), 6.58 (dd, J = 9.5, 3.0 Hz, 1H), 3.98 (s, 3H), 3.89, 3.84 (ABq, J = 14.0 Hz, 2H), 3.81 (s, 3H), 3.08 (br s, 1H), 2.86 (dd, J = 12.1, 6.0 Hz, 1H), 2.55 (dd, J = 12.1, 7.8 Hz, 1H), 1.97 – 1.88 (m, 1H), 1.31 – 1.20 (m, 1H), 0.99 – 0.91 (m, 1H), 0.88 – 0.80 (m, 1H); 13C NMR (CD3OD) δ 162.1 (s), 157.4 (d, J = 237.6 Hz), 154.4 (s), 145.7 (s), 138.0 (s), 132.7 (d, J = 6.6 Hz), 127.2 (s), 116.9 (s), 112.7 (d, J = 23.7 Hz), 112.3 (d, J = 22.7 Hz), 111.0 (d, J = 8.6 Hz), 56.1 (s), 53.6 (s), 53.5 (s), 48.3 (s), 22.0 (s), 16.8 (s), 12.8 (s); HRMS (ESI) calculated for C18H22FN2O2 ([M+H]+), 317.1660; found, 317.1626.
1-(Benzo[b]thiophen-7-yl)-N-[(2-(5-fluoro-2-methoxyphenyl)cyclopropyl)methyl]methanamine Hydrochloride (37).
Prepared from benzo[b]thiophene-7-carboxaldehyde employing General Method C (NaBH(OAc)3 (2.0 equiv.)/DCE). 1H NMR (CDCl3) δ 10.34 (s, 1H), 10.05 (s, 1H), 7.94 (d, J = 7.3 Hz, 1H), 7.84 (d, J = 7.9 Hz, 1H), 7.51 – 7.44 (m, 2H), 7.41 (d, J = 5.4 Hz, 1H), 6.83 – 6.76 (m, 1H), 6.67 (dd, J = 9.0, 4.5 Hz, 1H), 6.62 (dd, J = 9.2, 3.0 Hz, 1H), 4.46 (s, 2H), 3.64 (s, 3H), 3.12 – 2.94 (m, 2H), 2.08 – 1.97 (m, 1H), 1.53 – 1.42 (m, 1H), 1.15 – 1.01 (m, 2H); 13C NMR (CDCl3) δ 157.2 (d, J = 238.4 Hz), 154.3 (s), 140.5 (s), 140.4 (s), 130.5 (d, J = 7.3 Hz), 126.3 (s), 126.2 (s), 125.6 (s), 125.1 (s), 125.0 (s), 124.9 (s), 113.5 (d, J = 23.9 Hz), 113.1 (d, J = 22.6 Hz), 111.1 (d, J = 8.3 Hz), 56.0 (s), 50.6 (s), 48.5 (s), 18.0 (s), 17.3 (s), 13.2 (s); HRMS (ESI) calculated for C20H21FNOS ([M+H]+), 342.1322; found, 342.1288.
1-[2-(5-Fluoro-2-methoxyphenyl)cyclopropyl)-N-[(1H-indol-7-yl)methyl]methanamine Hydrochloride (38).
Prepared from indole-7-carboxaldehyde employing General Method C (NaBH(OAc)3 (2.0 equiv.)/DCE). 1H NMR (CDCl3) δ 11.25 (s, 1H), 9.65 (s, 1H), 9.22 (s, 1H), 7.73 (dd, J = 7.4, 1.3 Hz, 1H), 7.40 (t, J = 6.9 Hz, 1H), 7.13 – 7.03 (m, 2H), 6.80 – 6.72 (m, 1H), 6.64 – 6.56 (m, 3H), 4.61 – 4.38 (m, 2H), 3.49 (s, 3H), 3.19 – 3.06 (m, 1H), 2.87 – 2.75 (m, 1H), 1.98 – 1.90 (m, 1H), 1.44 – 1.33 (m, 1H), 1.16 – 1.06 (m, 1H), 1.03 – 0.92 (m, 1H); 13C NMR (CDCl3) δ 157.2 (d, J = 238.7 Hz), 154.2 (s), 134.5 (s), 123.0 (d, J = 7.3 Hz), 129.5 (s), 126.3 (s), 124.8 (s), 123.1 (s), 119.4 (s), 113.6 (d, J = 24.1 Hz), 113.3 (d, J = 23.0 Hz), 112.8 (s), 111.1 (d, J = 8.3 Hz), 102.7 (s), 55.8 (s), 50.8 (s), 48.9 (s), 18.1 (s), 17.8 (s), 12.7 (s); HRMS (ESI) calculated for C20H22FN2O ([M+H]+), 325.1711; found, 325.1664.
1-[2-(5-Fluoro-2-methoxyphenyl)cyclopropyl]-N-(quinolin-8-ylmethyl)methanamine Hydrochloride (39).
Prepared from 8-quinolinecarboxaldehyde employing General Method C (NaBH(OAc)3 (2.0 equiv.)/DCE). 1H NMR (CD3OD) δ 9.01 (dd, J = 4.8, 1.5 Hz, 1H), 8.69 (d, J = 7.8 Hz, 1H), 8.27 (d, J = 7.0 Hz, 1H), 8.12 (d, J = 8.1 Hz, 1H), 7.85 – 7.73 (m, 2H), 6.84 – 6.75 (m, 1H), 6.72 (dd, J = 8.9, 4.5 Hz, 1H), 6.59 (dd, J = 9.2, 3.0 Hz, 1H), 4.96, 4.87 (ABq, J = 13.7 Hz, 2H), 3.71 (s, 3H), 3.38 (dd, J = 12.5, 7.1 Hz, 1H), 3.18 – 3.09 (m, 1H), 2.11 – 1.99 (m, 1H), 1.47 – 1.33 (m, 1H), 1.13 – 0.96 (m, 2H); 13C NMR (CD3OD) δ 158.3 (s), 157.3 (d, J = 238.5 Hz), 154.5 (s), 147.6 (s), 142.9 (s), 136.7 (s), 130.9 (s), 130.6 (d, J = 7.5 Hz), 129.3 (s), 128.9 (s), 125.5 (s), 122.3 (s), 113.6 (d, J = 24.0 Hz), 113.2 (d, J = 22.5 Hz), 111.2 (d, J = 8.4 Hz), 55.9 (s), 52.0 (s), 47.8 (s), 17.6 (s), 17.5 (s), 12.7 (s); HRMS (ESI) calculated for C21H22FN2O ([M+H]+), 337.1711; found, 337.1689.
(+)-1-[(1S,2S)-2-[2-(Allyloxy)-5-fluorophenyl]cyclopropyl]-N-(2-methoxybenzyl)methanamine Hydrochloride ((+)-40).
Prepared from (+)-10 employing General Method C (NaBH4 (1.5 equiv.)/MeOH). 1H NMR (CD3OD) δ 7.52 – 7.44 (m, 1H), 7.41 (d, J = 7.4 Hz, 1H), 7.11 (d, J = 8.3 Hz, 1H), 7.04 (t, J = 7.5 Hz, 1H), 6.98 – 6.83 (m, 2H), 6.74 (dd, J = 9.5, 2.8 Hz, 1H), 6.11 – 6.07 (m, 1H), 5.38 (dd, J = 17.3, 1.5 Hz, 1H), 5.24 (dd, J = 10.5, 1.2 Hz, 1H), 4.63 – 4.49 (m, 2H), 4.36, 4.26 (ABq, J = 12.9 Hz, 2H), 3.36 (s, 3H), 3.16 (d, J = 7.3 Hz, 2H), 2.39 – 2.21 (m, 1H), 1.50 – 1.34 (m, 1H), 1.25 – 0.99 (m, 2H); 13C NMR (CD3OD) δ 159.3 (s), 158.7 (d, J = 237.2 Hz), 154.7 (d, J = 2.1 Hz), 134.7 (s), 132.8 (s), 132.7 (s), 132.6 (d, J = 7.4 Hz), 122.0 (s), 120.5 (s), 118.0 (s), 114.1 (d, J = 8.4 Hz), 113.9 (d, J = 23.0 Hz), 113.7 (d, J = 24.2 Hz), 112.0 (s), 70.8 (s), 56.1 (s), 52.4 (s), 49.8 (s), 47.6 (s), 18.5 (s), 13.4 (s); HRMS (ESI) calculated for C21H25FNO2 ([M+H]+), 342.1864; found, 342.1829; [α]D20 +10.0 (c 0.1, MeOH).
(+)-1-[(1S,2S)-2-[5-Fluoro-2-(2-fluoroethoxy)phenyl]cyclopropyl]-N-(2-methoxybenzyl)methanamine Hydrochloride ((+)-41).
Prepared from (+)-11 employing General Method C (NaBH4 (1.5 equiv.)/MeOH). 1H NMR (CD3OD) δ 7.46 (t, J = 7.9 Hz, 1H), 7.40 (d, J = 7.4 Hz, 1H), 7.11 (d, J = 8.3 Hz, 1H), 7.03 (t, J = 7.5 Hz, 1H), 6.96 (dd, J = 8.9, 4.7 Hz, 1H), 6.91 (td, J = 8.6, 2.9 Hz, 1H), 6.76 (dd, J = 9.5, 2.9 Hz, 1H), 4.85 – 4.61 (m, 2H), 4.36 – 4.21 (m, 4H), 3.89 (s, 3H), 3.20 (dd, J = 13.1, 7.2 Hz, 1H), 3.12 (dd, J = 13.1, 7.4 Hz, 1H), 2.34 – 2.22 (m, 1H), 1.45 – 1.30 (m, 1H), 1.28 – 1.17 (m, 1H), 1.14 – 1.03 (m, 1H); 13C NMR (CD3OD) δ 159.36 (s), 158.94 (d, J = 237.6 Hz), 154.76 (d, J = 2.1 Hz), 132.98 (d, J = 7.6 Hz), 132.71 (s), 132.70 (s), 122.03 (s), 120.44 (s), 114.26 (d, J = 8.5 Hz), 114.03 (d, J = 23.0 Hz), 113.98 (d, J = 24.1 Hz), 112.08 (s), 83.34 (d, J = 168.4 Hz), 69.78 (d, J = 19.3 Hz), 56.12 (s), 52.28 (s), 47.58 (s), 18.56 (s), 18.42 (d, J = 1.2 Hz), 13.35 (s); HRMS (ESI) calculated for C20H24F2NO2 ([M+H]+), 348.1770; found, 348.1764; [α]D20 +9.4 (c 0.5, MeOH).
Calcium Flux Assay.
Calcium flux assays were performed on a FLIPRTETRA fluorescence imaging plate reader (Molecular Dynamics) with Flp-In-293 cells stably expressing the human 5-HT2A, 5-HT2B, or 5-HT2C-INI receptors as previously described.21 Cells were preincubated in 384-well poly-L-lysine plates at a density of 10,000 cells/well. Next day, cells were loaded with Fluo-4 Direct dye (Invitrogen, (20 μL/well) for 1 h at 37 °C in drug buffer (pH 7.4, 1× HBSS, 2.5 mM probenecid, and 20 mM HEPES). Dilutions of each tested drug were prepared at 3× final concentration in drug buffer (pH 7.4, 1× HBSS, 20 mM HEPES, 0.1% BSA, 0.01% ascorbic acid). The tested drugs in 10 μL assay buffer were added, and calcium flux was measured every second for 5 min. For assessment of functional selectivity, drug solutions for FLIPR assay were exactly the same as used for the Tango assay (below). Fluorescence in each well was normalized to the average of the first 10 reads (i.e., baseline fluorescence), then the maximum-fold increase was determined and fold over baseline was plotted as a function of drug concentration. Data were normalized to % 5-HT stimulation and analyzed using log(agonist) vs. response in Graphpad Prism 5.0.
Tango Arrestin Recruitment Assay.
The HEK cell line expressing TEV-fused β-Arrestin-2 (HTLA cells, kindly provided by Dr. Richard Axel) and a tetracycline transactivator (tTA)-driven luciferase were utilized for Tango assay testing β-arrestin-2 recruitment.24 HTLA cells were transfected with the 5-HT2C INI receptor fused to tTA containing a TEV cleavage site. Cells were incubated as for the FLIPR assay in 40 μL except into white 384-well plates, and stimulated with the same drugs used for FLIPR (3×, 20 μL per well in HBSS, 20 mM HEPES, 0.1% BSA, 0.01% ascorbic acid, pH 7.4). After incubation for 20 hours at 37° C and 5% CO2, medium containing drugs was decanted, and 20 μL of Bright-Glo reagent (Promega) was added per well. The plate was incubated for 20 min at room temperature for complete cell lysis before being counted using a Wallac MicroBeta Trilux luminescence counter (Perkin Elmer). Results (relative luminescence units) were plotted as a function of drug concentration, normalized to % 5-HT, and subjected to non-linear least-squares regression analysis using the sigmoidal dose-response function in GraphPad Prism 5.0.
Animal Behavioral Studies.
Materials and Methods.
Male C57BL/6J mice (approximately 9–10 week of age at the start of testing) were obtained from Jackson Laboratories (Bar Harbor, ME). Mice were group-housed in Tecniplast ventilated cages and were maintained on a 12/12 hour light/dark cycle (lights on 7 am). The room temperature was maintained at 20–23 °C with relative humidity at approximately 50%. Food and water were available ad libitum for the duration of the study, except during testing, and all testing was conducted during the light phase of the light/dark cycle. The behavioral tests were conducted according to established protocols approved by the Harvard Center for Comparative Medicine (HCCM) IACUC committee in AALAC-accredited facilities, and in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health 2011).
Drugs.
d-Amphetamine (Tocris Bioscience, Bristol, UK), PCP (Sigma-Aldrich, St. Louis, MO), lorcaserin (HCl salt, Carbosynth, San Diego CA), and compound (+)-19 were dissolved in physiological saline and administered by intraperitoneal injection (IP) at a concentration of 10 mL/kg.
Procedures.
Locomotor activity was measured in Plexiglas square chambers (27.3 × 27.3 × 20.3 cm; Med Associates Inc., St Albans, VT) surrounded by infrared photobeam sources and detectors. Mice were tested under ambient light, and data were collected by Med Associates software (Activity Monitor, version 5.9). Mice were injected with saline vehicle, lorcaserin (3 mg/kg), or (+)-19 (10 or 20 mg/kg), and locomotor activity was monitored for 15 min (baseline total distance). Mice were then administered saline or d-amphetamine (AMPH) (3 mg/kg), and activity was measured for an additional 90 min. In a second experiment mice were administered phencyclidine (PCP) (5 mg/kg), and activity was measured for an additional 60 min.
Statistics.
Locomotor activity was measured as total distance traveled (cm), assessed via infrared beam breaks. Locomotion prior to AMPH or PCP administration (baseline, 0–15 min) was analyzed by one-way analysis of variance (ANOVA) with drug treatment (doses of test compounds) as the independent variable. The effect of test compounds on AMPH- and PCP-induced hyperactivity was analyzed by one-way ANOVA with drug treatment after AMPH (Post Amphetamine, 15–105 min) or PCP (Post PCP, 15–75 min) as the independent variable. All significant effects were followed up with the Student Newman-Keuls post hoc test. An effect was considered significant if p<0.05 (Statview for Windows, Version 5.0).
Supplementary Material
Acknowledgments
Financial support from the National Institute of Mental Health (NIMH) (Grant R01MH99993) and Psychoactive Drug Screening Program (PDSP, Contract HHSN-271-2013-00017-C) is gratefully acknowledged. We thank Dr. Werner Tueckmantel for proofreading the manuscript and providing valuable suggestions.
Abbreviations
- 5-HT
serotonin
- GPCR
G protein-coupled receptor
- CNS
central nervous system
- FDA
U.S. Food and Drug Administration
- ADMET
absorption, distribution, metabolism, excretion, and toxicity
- HTS
high throughput screening
- 2-PCMPA
2-phenylcyclopropylmethylamine
- SAR
structure-activity relationship
- MW
molecular weight
- HPLC
high-performance liquid chromatography
- BBB
blood-brain barrier
- FLIPR
fluorescence imaging plate reader
- HEK-293
human embryonic kidney-293 cell
- CYP
cytochrome P450
- AMPH
amphetamine
- PCP
phencyclidine
- PPB
plasma protein binding
- hERG
human ether-a-go-go-related gene
Footnotes
The authors declare no competing financial interest.
Supporting information
Synthetic procedures, chiral separation methods, and characterization data of all intermediates; Molecular formula strings and related data.
REFERENCES
- 1.Meltzer HY; Roth BL Lorcaserin and pimavanserin: emerging selectivity of serotonin receptor subtype-targeted drugs. J. Clin. Invest 2013, 123, 4986–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith BM; Thomsen WJ; Grottick AJ The potential use of selective 5-HT2C agonists in treating obesity. Expert Opin. Invest. Drugs 2006, 15, 257–266. [DOI] [PubMed] [Google Scholar]
- 3.Sargent BJ; Henderson AJ Targeting 5-HT receptors for the treatment of obesity. Curr. Opin. Pharmacol 2011, 11, 52–58. [DOI] [PubMed] [Google Scholar]
- 4.Rosenzweig-Lipson S; Comery TA; Marquis KL; Gross J; Dunlop J 5-HT2C agonists as therapeutics for the treatment of schizophrenia. Handb. Exp. Pharmacol 2012, 213, 147–165. [DOI] [PubMed] [Google Scholar]
- 5.Berger M; Gray JA; Roth BL The expanded biology of serotonin. Annu. Rev. Med 2009, 60, 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCorvy JD; Roth BL Structure and function of serotonin G protein-coupled receptors. Pharmacol. Ther 2015, 150, 129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wacker D; Wang S; McCorvy JD; Betz RM; Venkatakrishnan AJ; Levit A; Lansu K; Schools ZL; Che T; Nichols DE; Shoichet BK; Dror RO; Roth BL Crystal structure of an LSD-bound human serotonin receptor. Cell 2017, 168, 377–389 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothman RB; Baumann MH; Savage JE; Rauser L; McBride A; Hufeisen SJ; Roth BL Evidence for possible involvement of 5-HT2B receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation 2000, 102, 2836–2841. [DOI] [PubMed] [Google Scholar]
- 9.Roth BL Drugs and valvular heart disease. N. Engl. J. Med 2007, 356, 6–9. [DOI] [PubMed] [Google Scholar]
- 10.Urban JD; Clarke WP; von Zastrow M; Nichols DE; Kobilka B; Weinstein H; Javitch JA; Roth BL; Christopoulos A; Sexton PM; Miller KJ; Spedding M; Mailman RB Functional selectivity and classical concepts of quantitative pharmacology. J. Pharmacol. Exp. Ther 2007, 320, 1–13. [DOI] [PubMed] [Google Scholar]
- 11.DeWire SM; Ahn S; Lefkowitz RJ; Shenoy SK Beta-arrestins and cell signaling. Annu. Rev. Physiol 2007, 69, 483–510. [DOI] [PubMed] [Google Scholar]
- 12.Luttrell LM; Lefkowitz RJ The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J. Cell Sci 2002, 115, 455–465. [DOI] [PubMed] [Google Scholar]
- 13.Violin JD; Lefkowitz RJ Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol. Sci 2007, 28, 416–422. [DOI] [PubMed] [Google Scholar]
- 14.Manglik A; Lin H; Aryal DK; McCorvy JD; Dengler D; Corder G; Levit A; Kling RC; Bernat V; Hübner H; Huang XP; Sassano MF; Giguère PM; Löber S; Da D; Scherrer G; Kobilka BK; Gmeiner P; Roth BL; Shoichet BK Structure-based discovery of opioid analgesics with reduced side effects. Nature 2016, 537, 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.[No authors listed]. Lorcaserin. In obesity: unacceptable risks. Prescrire Int. 2014, 23, 117–120. [PubMed] [Google Scholar]
- 16.Cheng J; Kozikowski AP We need 2C but not 2B: developing serotonin 2C (5-HT2C) receptor agonists for the treatment of CNS disorders. ChemMedChem 2015, 10, 1963–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunlop J; Watts SW; Barrett JE; Coupet J; Harrison B; Mazandarani H; Nawoschik S; Pangalos MN; Ramamoorthy S; Schechter L; Smith D; Stack G; Zhang J; Zhang G; Rosenzweig-Lipson S Characterization of vabicaserin (SCA-136), a selective 5-hydroxytryptamine 2C receptor agonist. J. Pharmacol. Exp. Ther 2011, 337, 673–680. [DOI] [PubMed] [Google Scholar]
- 18.Green MP; McMurray G; Storer RI Selective 5-HT2C receptor agonists: design and synthesis of pyridazine-fused azepines. Bioorg. Med. Chem. Lett 2016, 26, 4117–4121. [DOI] [PubMed] [Google Scholar]
- 19.Storer RI; Brennan PE; Brown AD; Bungay PJ; Conlon KM; Corbett MS; DePianta RP; Fish PV; Heifetz A; Ho DK; Jessiman AS; McMurray G; de Oliveira CA; Roberts LR; Root JA; Shanmugasundaram V; Shapiro MJ; Skerten M; Westbrook D; Wheeler S; Whitlock GA; Wright J Multiparameter optimization in CNS drug discovery: design of pyrimido[4,5-d]azepines as potent 5-hydroxytryptamine 2C (5-HT2C) receptor agonists with exquisite functional selectivity over 5-HT2A and 5-HT2B receptors. J. Med. Chem 2014, 57, 5258–5269. [DOI] [PubMed] [Google Scholar]
- 20.Rouquet G; Moore DE; Spain M; Allwood DM; Battilocchio C; Blakemore DC; Fish PV; Jenkinson S; Jessiman AS; Ley SV; McMurray G; Storer RI Design, synthesis, and evaluation of tetrasubstituted pyridines as potent 5-HT2C receptor agonists. ACS Med. Chem. Lett 2015, 6, 329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho SJ; Jensen NH; Kurome T; Kadari S; Manzano ML; Malberg JE; Caldarone B; Roth BL; Kozikowski AP Selective 5-hydroxytryptamine 2C receptor agonists derived from the lead compound tranylcypromine: identification of drugs with antidepressant-like action. J. Med. Chem 2009, 52, 1885–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng J; Giguere PM; Onajole OK; Lv W; Gaisin A; Gunosewoyo H; Schmerberg CM; Pogorelov VM; Rodriguiz RM; Vistoli G; Wetsel WC; Roth BL; Kozikowski AP Optimization of 2-phenylcyclopropylmethylamines as selective serotonin 2C receptor agonists and their evaluation as potential antipsychotic agents. J. Med. Chem 2015, 58, 1992–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng J; Giguere PM; Schmerberg CM; Pogorelov VM; Rodriguiz RM; Huang XP; Zhu H; McCorvy JD; Wetsel WC; Roth BL; Kozikowski AP Further advances in optimizing (2-phenylcyclopropyl) methylamines as novel serotonin 2C agonists: effects on hyperlocomotion, prepulse inhibition, and cognition models. J. Med. Chem 2016, 59, 578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng J; McCorvy JD; Giguere PM; Zhu H; Kenakin T; Roth BL; Kozikowski AP Design and discovery of functionally selective serotonin 2C (5-HT2C) receptor agonists. J. Med. Chem 2016, 59, 9866–9880. [DOI] [PubMed] [Google Scholar]
- 25.Ghose AK; Herbertz T; Hudkins RL; Dorsey BD; Mallamo JP Knowledge-based, central nervous system (CNS) lead selection and lead optimization for CNS drug discovery. ACS Chem. Neurosci 2012, 3, 50–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rankovic Z CNS drug design: balancing physicochemical properties for optimal brain exposure. J. Med. Chem 2015, 58, 2584–2608. [DOI] [PubMed] [Google Scholar]
- 27.Heffron TP Small molecule kinase inhibitors for the treatment of brain cancer. J. Med. Chem 2016, 59, 10030–10066. [DOI] [PubMed] [Google Scholar]
- 28.Rickli A; Luethi D; Reinisch J; Buchy D; Hoener MC; Liechti ME Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2,5-dimethoxy-substituted phenethylamines (2C drugs). Neuropharmacology 2015, 99, 546–553. [DOI] [PubMed] [Google Scholar]
- 29.Nichols DE; Sassano MF; Halberstadt AL; Klein LM; Brandt SD; Elliott SP; Fiedler WJ N-Benzyl-5-methoxytryptamines as potent serotonin 5-HT2 receptor family agonists and comparison with a series of phenethylamine analogues. ACS Chem. Neurosci 2015, 6, 1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen G; Cho SJ; Huang XP; Jensen NH; Svennebring A; Sassano MF; Roth BL; Kozikowski AP Rational drug design leading to the identification of a potent 5-HT2C agonist lacking 5-HT2B activity. ACS Med. Chem. Lett 2011, 2, 929–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertus P; Szymoniak J A direct synthesis of 1-aryl- and 1-alkenylcyclopropylamines from aryl and alkenyl nitriles. J. Org. Chem 2003, 68, 7133–7136. [DOI] [PubMed] [Google Scholar]
- 32.Cheng D; Li Y; Wang J; Sun Y; Jin L; Li C; Lu Y Fluorescence and colorimetric detection of ATP based on a strategy of self-promoting aggregation of a water-soluble polythiophene derivative. Chem. Commun. (Camb.) 2015, 51, 8544–8546. [DOI] [PubMed] [Google Scholar]
- 33.Prabhakaran J; Underwood MD; Kumar JS; Simpson NR; Kassir SA; Bakalian MJ; Mann JJ; Arango V Synthesis and in vitro evaluation of [18F]FECIMBI-36: a potential agonist PET ligand for 5-HT2A/2C receptors. Bioorg. Med. Chem. Lett 2015, 25, 3933–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kroeze WK; Sassano MF; Huang XP; Lansu K; McCorvy JD; Giguere PM; Sciaky N; Roth BL PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nat. Struct. Mol. Biol 2015, 22, 362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pogorelov VM; Rodriguiz RM; Cheng J; Huang M; Schmerberg CM; Meltzer HY; Roth BL; Kozikowski AP; Wetsel WC 5-HT2C agonists modulate schizophrenia-like behaviors in mice. Neuropsychopharmacology 2017. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Canal CE; Morgan D; Felsing D; Kondabolu K; Rowland NE; Robertson KL; Sakhuja R; Booth RG A novel aminotetralin-type serotonin (5-HT)2C receptor-specific agonist and 5-HT2A competitive antagonist/5-HT2B inverse agonist with preclinical efficacy for psychoses. J. Pharmacol. Exp. Ther 2014, 349, 310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siuciak JA; Chapin DS; McCarthy SA; Guanowsky V; Brown J; Chiang P; Marala R; Patterson T; Seymour PA; Swick A; Iredale PA CP-809,101, a selective 5-HT2C agonist, shows activity in animal models of antipsychotic activity. Neuropharmacology 2007, 52, 279–290. [DOI] [PubMed] [Google Scholar]
- 38.Marquis KL; Sabb AL; Logue SF; Brennan JA; Piesla MJ; Comery TA; Grauer SM; Ashby CR Jr.; Nguyen HQ; Dawson LA; Barrett JE; Stack G; Meltzer HY; Harrison BL; Rosenzweig-Lipson S WAY-163909 [(7bR,10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]diazepino[6,7,1hi ]indole]: A novel 5-hydroxytryptamine 2C receptor-selective agonist with preclinical antipsychotic-like activity. J. Pharmacol. Exp. Ther 2007, 320, 486–496. [DOI] [PubMed] [Google Scholar]
- 39.Martignoni M Species and Strain Differences in Drug Metabolism in Liver and Intestine. University Library Groningen: 2006; pp 26–32. [Google Scholar]
- 40.Laurenzana EM; Owens SM Metabolism of phencyclidine by human liver microsomes. Drug Metab. Dispos 1997, 25, 557–563. [PubMed] [Google Scholar]
- 41.Acuna-Castillo C; Villalobos C; Moya PR; Saez P; Cassels BK; Huidobro-Toro JP Differences in potency and efficacy of a series of phenylisopropylamine/phenylethylamine pairs at 5-HT2A and 5-HT2C receptors. Br. J. Pharmacol 2002, 136, 510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krebs-Thomson K; Lehmann-Masten V; Naiem S; Paulus MP; Geyer MA Modulation of phencyclidine-induced changes in locomotor activity and patterns in rats by serotonin. Eur. J. Pharmacol 1998, 343, 135–143. [DOI] [PubMed] [Google Scholar]
- 43.Nielsen LM; Holm NB; Leth-Petersen S; Kristensen JL; Olsen L; Linnet K Characterization of the hepatic cytochrome P450 enzymes involved in the metabolism of 25I-NBOMe and 25I-NBOH. Drug Test. Anal 2017, 9, 671–679. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


