Summary
Background
When direct nerve coaptation is impossible after peripheral nerve injury, autografts, processed allografts, or conduits are used to bridge the nerve gap. The purpose of this study was to examine if human adipose-derived Mesenchymal Stromal/Stem Cells (MSCs) could be introduced to commercially available nerve graft substitutes and to determine cell distribution and the seeding efficiency of a dynamic seeding strategy.
Methods
MTS assays examined the viability of human MSCs after introduction to the Avance® Nerve Graft and the NeuraGen® Nerve Guide. MSCs were dynamically seeded on nerve substitutes for either 6, 12, or 24 h. Cell counts, live/dead stains, Hoechst stains, and Scanning Electron Microscopy (SEM) revealed the seeding efficiency and the distribution of MSCs after seeding.
Results
The viability of MSCs was not affected by nerve substitutes. Dynamic seeding led to uniformly distributed MSCs over the surface of both nerve substitutes and revealed MSCs on the inner surface of the NeuraGen® Nerve Guides. The maximal seeding efficiency of NeuraGen® Nerve Guides (94%), obtained after 12 h was significantly higher than that of Avance® Nerve Grafts (66%) (p=0.010).
Conclusion
Human MSCs can be dynamically seeded on Avance® Nerve Grafts and NeuraGen® Nerve Guides. The optimal seeding duration was 12 h. MSCs were distributed in a uniform fashion on exposed surfaces. This study demonstrates that human MSCs can be effectively and efficiently seeded onto commercially available nerve autograft substitutes in a timely fashion and sets the stage for the clinical application of MSC-seeded nerve graft substitutes clinically.
Keywords: MSCs, seeding, Avance® Nerve Graft, NeuraGen® Nerve Guide
INTRODUCTION
Peripheral nerve discontinuities that cannot be restored by direct end-to-end coaptation of nerve ends remain a clinical challenge. Despite many efforts to find an equivalent replacement, the autologous nerve graft, which results in donor site morbidity, remains the gold standard in peripheral nerve reconstruction. To minimize donor site morbidity, increase the number of reconstructive options and improve the outcomes of peripheral nerve repair, further improvement of the commercially available “off-the-shelf” nerve graft substitutes is essential.
Two commercially available nerve autograft substitutes are the NeuraGen® Nerve Guide (approved by the Food and Drug Administration in 2001) and the Avance® Nerve Graft (approved by the FDA in 2007). The NeuraGen® Nerve Guide is an artificial bioabsorbable hollow conduit made of purified bovine type I collagen. It has demonstrated effective nerve regeneration in a small diameter, short (<3 cm) sensory nerve defects, but evidence for its effective use in sensory nerve defects of longer size and/or larger diameter, or in motor nerve defects, remains scarce and inconsistent. (1–3)
The Avance® Nerve Graft is a decellularized human nerve allograft that has been processed to remove cellular debris. This process includes decellularization with chondroitinase and gamma irradiation, leading to a natural human product with a remaining ultrastructure that does not necessitate the use of immunosuppression. While clinical outcomes have been mostly with case reports and series (1, 4–6), there has been a lack of prospective clinical trials and valid comparisons to autografts. Their application in large motor-nerve defects remains controversial. (1, 4, 7, 8)
The hypothesis that the addition of cells that improve growth factors at the nerve regeneration site could result in enhanced nerve regeneration has been confirmed by studies reporting a synergistic effect of mesenchymal stromal/stem cells (MSCs) added to a nerve conduit, leading to improved functional outcomes in various types of nerve gaps. (9–14) Similarly, the enhancing effect of added MSCs or Schwann cells to processed nerve allografts has also been demonstrated on a gene expression and functional outcome level. (15–20)
A variety of nonvalidated delivery methods have been reported. Microinjection has been extensively described to deliver the MSCs. Acute microinjection is known to be traumatic to MSCs even though cells remain metabolically active. (21) Microinjection also damages the ultrastructure of the allograft and when placed in the center of a hollow conduit, leakage occurs, which may alter the effective dose of MSCs in an unpredictable manner. (22–27) Dynamic seeding is a novel, recently described delivery method that successfully adheres MSCs in a uniform matter on the surface of processed nerve grafts. (27, 28)
Application and validation of this dynamic seeding strategy when applied to commercially available products like the Avance® Nerve Graft and the NeuraGen® Nerve Guide could enhance the clinical applicability of the described method and would enable a valid comparison of the two products and their capability to enhance nerve regeneration when supported by MSCs.
To determine the clinical potential of dynamic seeding of MSCs, this study was designed to examine the interaction between MSCs and two commercially available nerve graft substitutes; the Avance® Nerve Graft and the NeuraGen® Nerve Guide. The purpose of this study was to determine (I) if the interaction of human adipose-derived MSCs with the NeuraGen® Nerve Guide and the Avance® Nerve Graft influences cell viability, (II) if human adipose-derived MSCs can be dynamically seeded and distributed uniformly onto these nerve substitutes, and (III) if dynamic seeding and optimized timing improves the efficiency of MSC seeding.
METHODS
General design
Two experiments were designed to ascertain the interaction of the nerve graft substitutes with human adipose-derived MSCs and to determine the optimal seeding times, survivability, and the distribution of MSCs.
Adipose-Derived Mesenchymal Stem Cell Collection and Preparation
Passage five human MSCs, isolated from abdominal lipoaspirates of a male donor were used in this experiment. Cells were provided by the Mayo Clinical Human Cellular Therapy Laboratory (Rochester, Minnesota, USA). These MSCs have been tested extensively for multilineage potential, cell surface markers (CD73, CD90, CD105, CD44, CD14, and CD45), and RNA-sequence transcriptome profiles previously. (23–25) For MSC culture, growth media consisting of a-MEM (Advanced MEM (1x); Gibco by Life TechnologiesTM, Cat #12492013), 5% platelet lysate (PLTMax®; Mill Creek Life Sciences), 1% penicillin/streptomycin (Penicillin-Streptomycin (10.000 U/mL); Gibco by Life TechnologiesTM Cat #15140148), 1% GlutaMAX (GlutaMAXTM Supplement 100X; Gibco by Life Technologies, Cat #35050061) and 0.2% heparin (Heparin Sodium Injection, USP, 1.000 USP units per mL; NOVAPLUS®) was used and media was changed every 72 h. (29–31)
Experimental Design and Measurement of Mesenchymal Stem Cell Viability
To test whether the chemical products used during processing of the Avance® Nerve Graft and the NeuraGen® Nerve Guide are harmful to MSCs, cell metabolic activity was measured using (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) (MTS) assays that were performed according to the manufacturer’s protocol (CellTiter 96® AQueous One Solution Cell Proliferation Assay, Promega®). Twenty-four 2 mm segments of the Avance® Nerve Graft and the NeuraGen® Nerve Guide were soaked in a-MEM for 2 h prior to the MTS assay. The soaked nerve substitute segments and 5,000 MSCs dissolved in 100 μL growth medium were placed into wells, which were coated with pHEMA (Poly 2-hydroxyethyl methacrylate; Sigma Cat # P3932) to prevent the migration of MSCs to the plastic well. Any influence of the pHEMA coating on cell viability was eliminated by measuring the viability of two extra groups (MSCs + Avance® Nerve Graft and MSCs + NeuraGen® Nerve Guide) at each time point. After 1, 2, 3, and 7 days of incubation at 37°C, the metabolic activity of three samples of each group were measured with the Infinite® 200 Pro TECAN Reader (Tecan Trading AG, Switzerland) at an absorbance wavelength of 490 nm. The metabolic activity of group I (pHEMA + MSCs + Avance® Nerve Graft) and group II (pHEMA + MSCs + NeuraGen® Nerve Guide) were expressed as a ratio of the metabolic activity of the control group (pHEMA + MSCs) and compared to each other.
Experimental Design and Measurement of Cell distribution, Migration, and Seeding Efficiency
In total, 20 Avance® Nerve Grafts and 20 NeuraGen® Nerve Guides of 10 mm in length were used in this experiment.
Eighteen samples per group were dynamically seeded according to the dynamic seeding strategy described by Rbia and colleagues. (27) Prior to seeding, all nerve substitute segments were soaked in a-MEM for 2 h as an equilibration step to restore the salt balance. Conical tubes containing nerve samples and one million MSCs per nerve sample in growth medium were rotated in a bioreactor placed in an incubator (37°C) for 6, 12, and 24 h (n=6 per group per seeding duration).
The viability of adherent MSCs was evaluated by live/dead Cell Viability Assays (Invitrogen, Life Technologies Corporation, NY, USA) after each of the different seeding durations. Hoechst staining was performed according to standard protocols (Hoechst stain solution; Sigma-Aldrich Corp., MO, USA) on the surface of the nerve substitutes to show the distribution and migration of cells after dynamic seeding. Both Live/Dead and Hoechst stains were performed on three samples per group per seeding duration and were visualized directly after seeding with a confocal microscope (Zeiss LSM 780 confocal microscope).
To study cell distribution, Scanning Electron Microscopy (SEM) (n=3 per group per seeding duration) was performed. Samples were fixed in 2% Trump’s fixative solution (37% formaldehyde and 25% glutaraldehyde) directly after seeding. After 24 h, samples were washed with phosphate buffer, rinsed in water, processed through graded series of ethanol (final 100% ethanol), critical point dried with carbon and mounted on an aluminum stub. After sputter-coating for 60 s using gold-palladium, sample images were taken with a Hitachi S-4700 cold field emission SEM (Hitachi High Technologies America, Inc., IL, USA) at 5 kV accelerating voltage. After obtaining images of the graft surface, samples were cut longitudinally and imaged to reveal cell distribution on the inside of nerve substitutes.
To reinforce the SEM findings, two extra 10 mm samples per group were seeded with 1 million MSCs according to the estimated optimal seeding duration, fixed in 10% formalin and processed and embedded in paraffin. Three sections of 5 μM of the proximal and mid-nerve substitutes were taken and Hoechst stained to evaluate for cells that migrated inside the nerve substitute (i.e., within the nerve allograft or nerve conduit material itself). The cells were visualized with a confocal microscope (Zeiss LSM 780 confocal microscope; Zeiss, Germany).
To quantify seeding efficiency, cell counts of the cell supernatant after the rotation duration was completed, provided the number of MSCs that remained free floating in the media. This indirectly led to the number and percentages of MSCs that were attached to the nerve sample, in this manuscript expressed as seeding efficiency. (32)
In addition to cell counts in the supernatant, seeding efficiency of MSCs after the various seeding durations was further evaluated by Hoechst fluorescence staining of the seeded nerve substitutes using the Infinite® 200 Pro TECAN Reader (Tecan Trading AG, Switzerland) at an absorbance wavelength of 340/458 nm.
Statistical analysis
Cell counts were performed in triplicate per sample and are expressed as the mean percentage ± standard deviation. The different measurements were analyzed using a two-way ANOVA. When ANOVA indicated a significant interaction, both within and between group comparisons were analyzed with the Kruskal-Wallis test, followed by pairwise comparisons using Wilcoxon rank-sum tests with Bonferroni correction. Significance was set at α≤0.05.
RESULTS
Cell Viability upon interaction of MSCs with Nerve Graft Substitute:
Analysis revealed no significant effect on cell viability of the pHEMA coating that was used in this experiment. The viability of MSCs when in the presence of Avance® Nerve Graft or the NeuraGen® Nerve Guide, expressed as a ratio of the viability of MSCs without either of the nerve substitutes in figure 1, was not affected by the presence of both nerve substitutes indicating that there was no detrimental interaction of the manufacturing process to MSCs. There were no significant differences in cell viability between and within the two groups over time as well (p=0.450).
Figure 1.
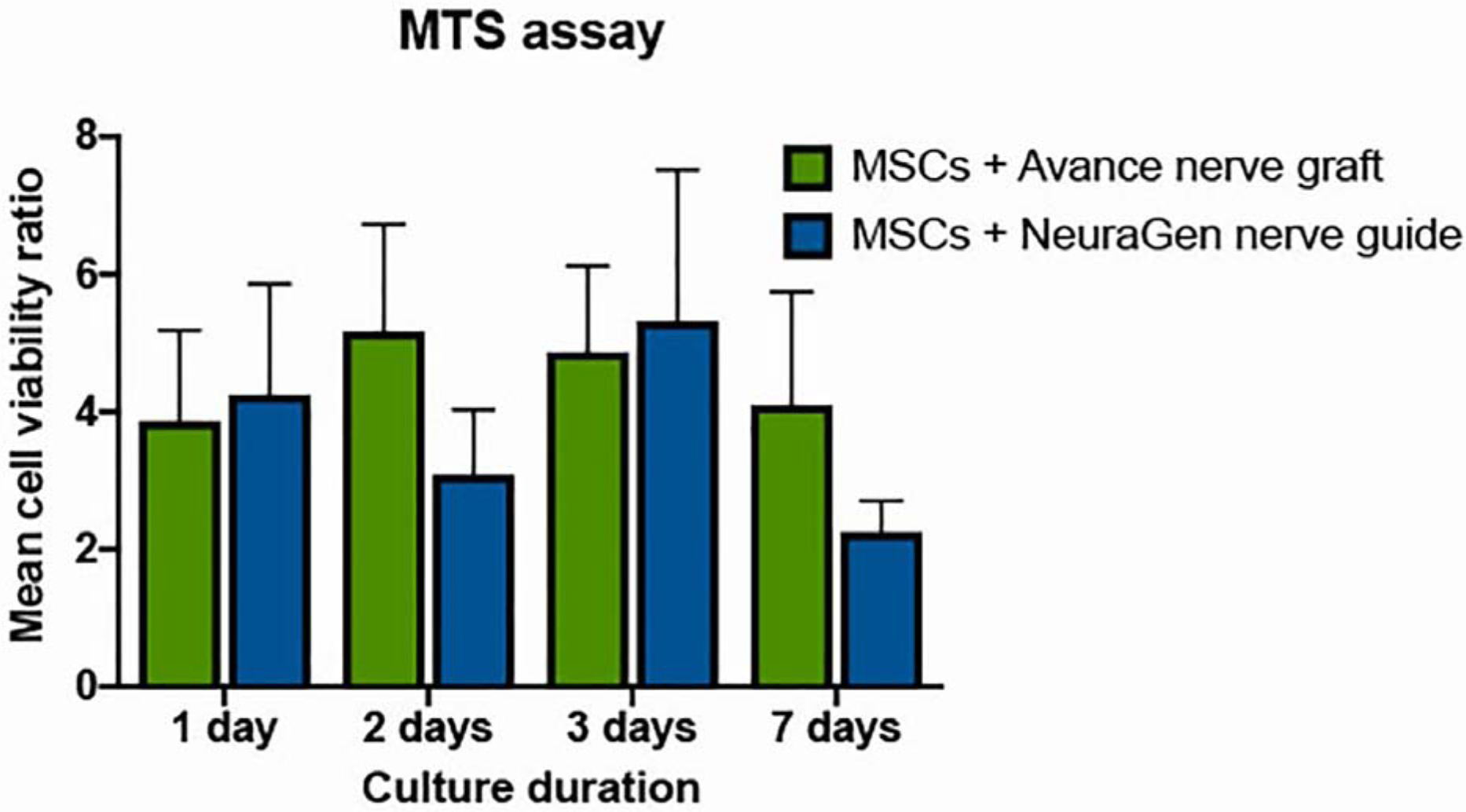
Cell viability over time of MSCs when combined with the Avance® Nerve Graft and the NeuraGen® Nerve Guide (n=3 per group per time point). Viability of MSCs in the presence of the Avance® Nerve Graft and the NeuraGen® Nerve Guide is expressed as a ratio of the viability of MSCs without any of the nerve substitutes. pHEMA coating was used in all groups presented in this figure. There were no significant differences between and within groups in 7 days of follow-up. Error bars: SEM. SEM = standard error of the mean
Cell distribution, migration, and seeding efficiency of MSCs on Nerve Graft Substitute
Live/dead staining and nuclear staining using Hoechst dye revealed a uniform distribution of viable MSCs over the entire surface of both nerve substitutes after all seeding durations. Figures 2 and 3 demonstrate example images after 12 h of seeding of live/dead staining and Hoechst staining, respectively. Hoechst fluorescent measurements demonstrated increased fluorescence as seeding duration time increases (Figure 4) (p=0.001), but there were no significant differences in Hoechst fluorescence between groups.
Figure 2.
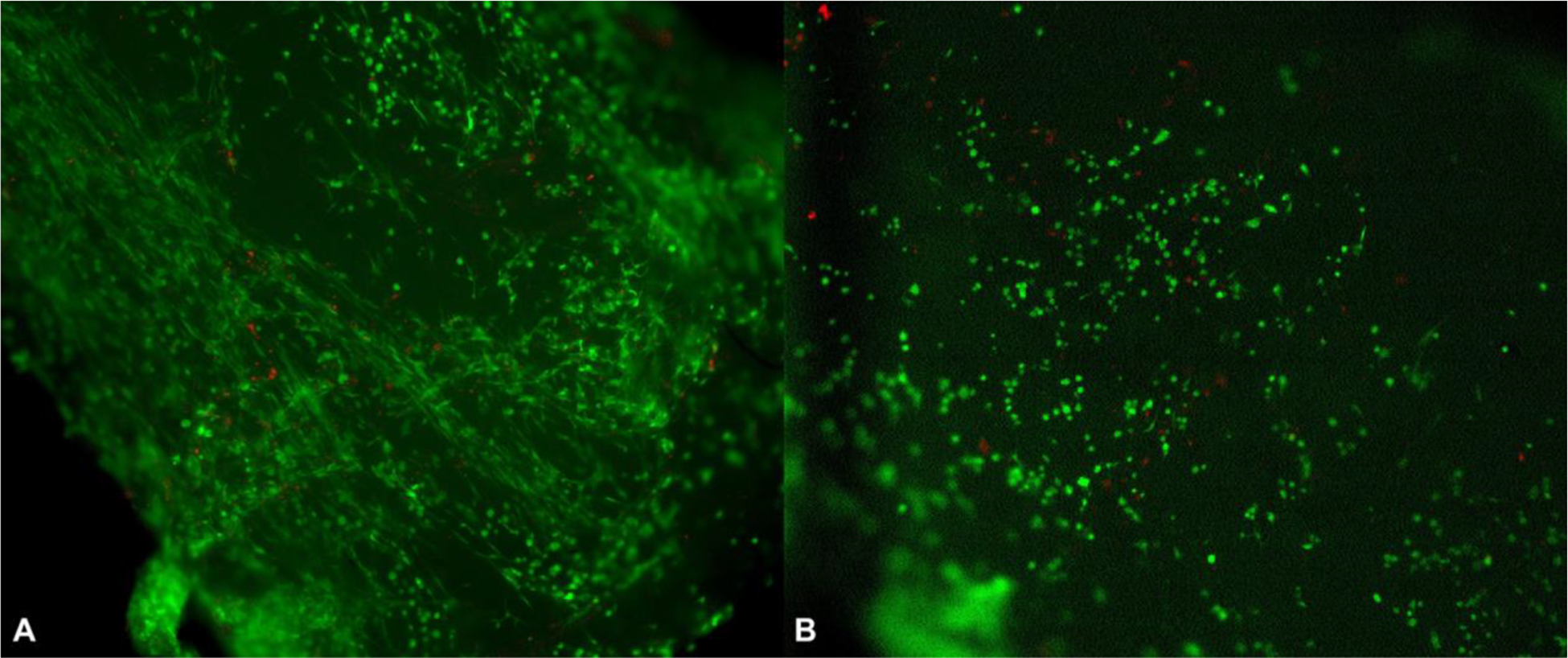
Live/Dead stains of a seeded Avance® Nerve Graft (A) and a seeded NeuraGen® Nerve Guide (B) after 12 h of seeding with MSCs, show mainly living cells (green) mixed with only a few dead cells (red) on the surface of both nerve substitutes.
Figure 3.
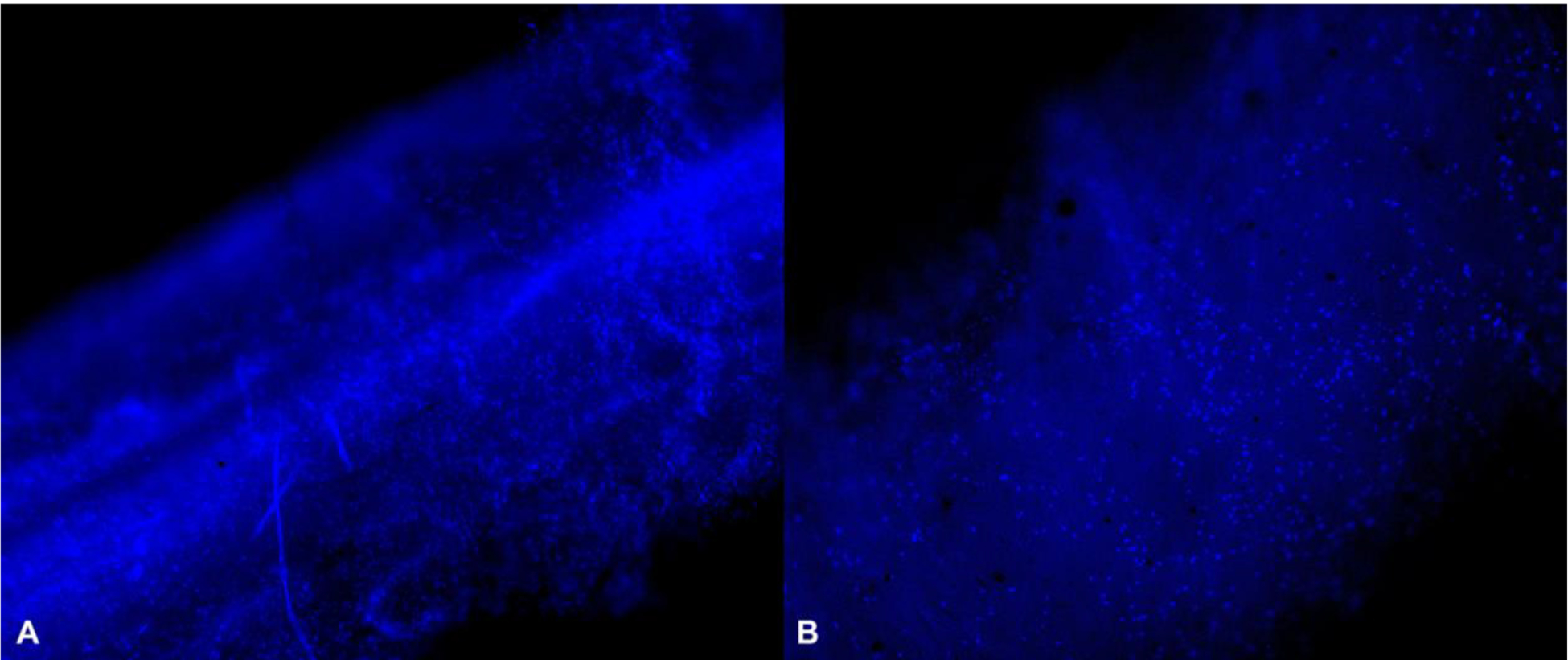
Hoechst-stained Avance® Nerve Graft (A) and NeuraGen® Nerve Guide (B) after 12 h of dynamic seeding with MSCs show a uniform distribution of cell nuclei among both nerve substitutes (10X). Cell nuclei are displayed in bright blue.
Figure 4.
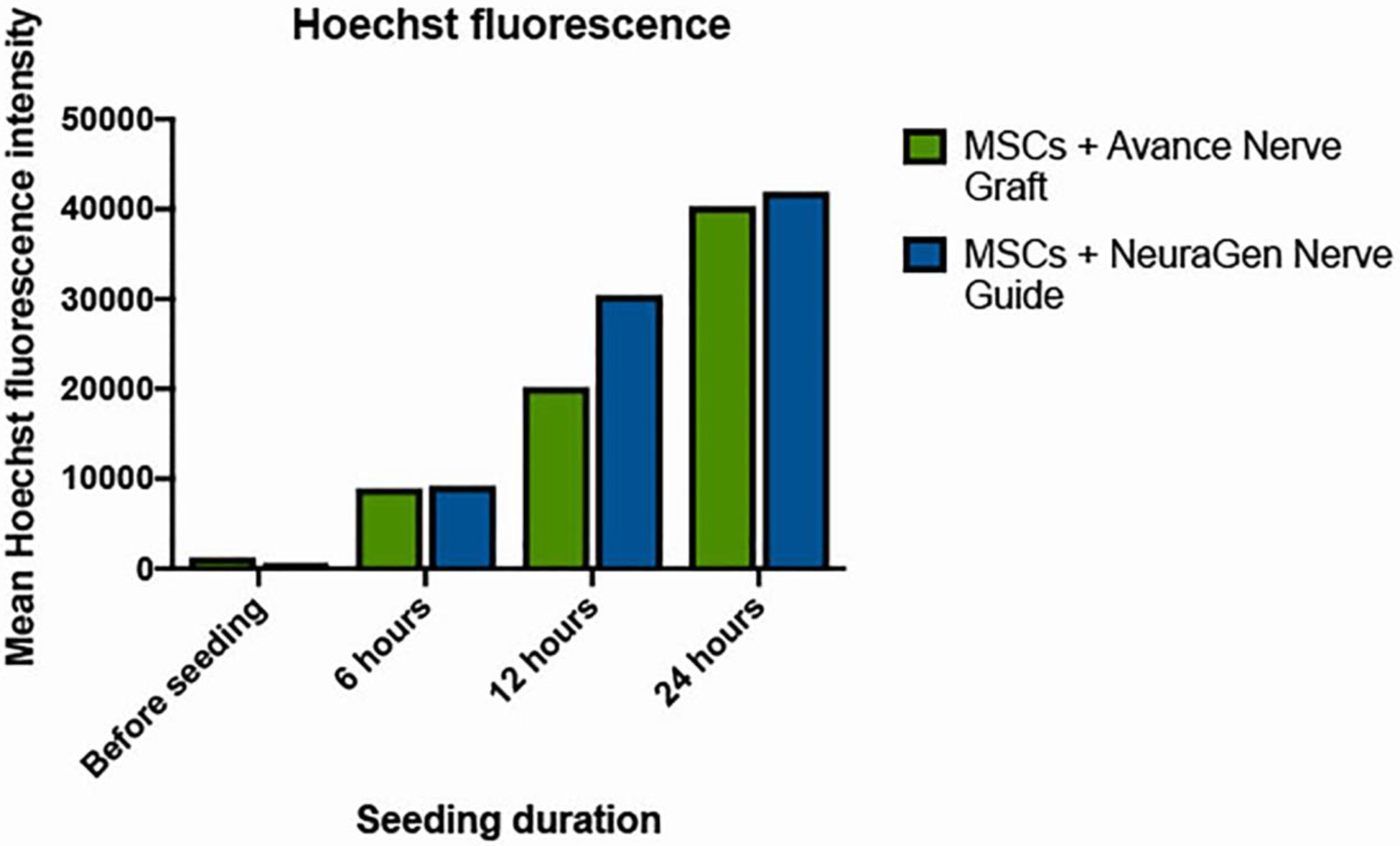
Hoechst fluorescence intensity of the Avance® Nerve Graft and the NeuraGen® Nerve Guide when seeding with MSCs according to increasing seeding durations (n=3 per group per time point). ANOVA analysis did not demonstrate a significant interaction between seeding duration and Hoechst fluorescence (p=0.001) when merging groups, but within groups analysis did not demonstrate any significant increases between time points (p>0.221 for the Avance® Nerve Grafts and p>0.083 for the NeuraGen® Nerve Guides).
Despite the different compositions of the surfaces of the Avance® Nerve Graft and the NeuraGen® Nerve Guide, SEM images revealed a similar distribution of cells among their surface (Figure 5). Manual quantification could not be carried out reliably due to cell aggregation, but the assessment of samples showed a marked increase in the cell coverage of both nerve substitutes between 6 and 12 h of seeding. Between 12 and 24 h of dynamic seeding, there were no appreciable differences in cell coverage. The morphology of cells did not change over time. SEM images of longitudinally cut segments of the Avance® Nerve Graft did not show any MSCs in the inner ultrastructure of the graft after 6, 12, and 24 h of seeding (Figure 6, left side). MSCs were present throughout the inner surface of the NeuraGen® Nerve Guides after 6, 12, and 24 h, although the coverage of MSCs was clearly less than on the outside of the Nerve Guide (Figure 6, right side). Additionally, the MSC did not migrate into the substrate of the NeuraGen® Nerve Guides.
Figure 5.
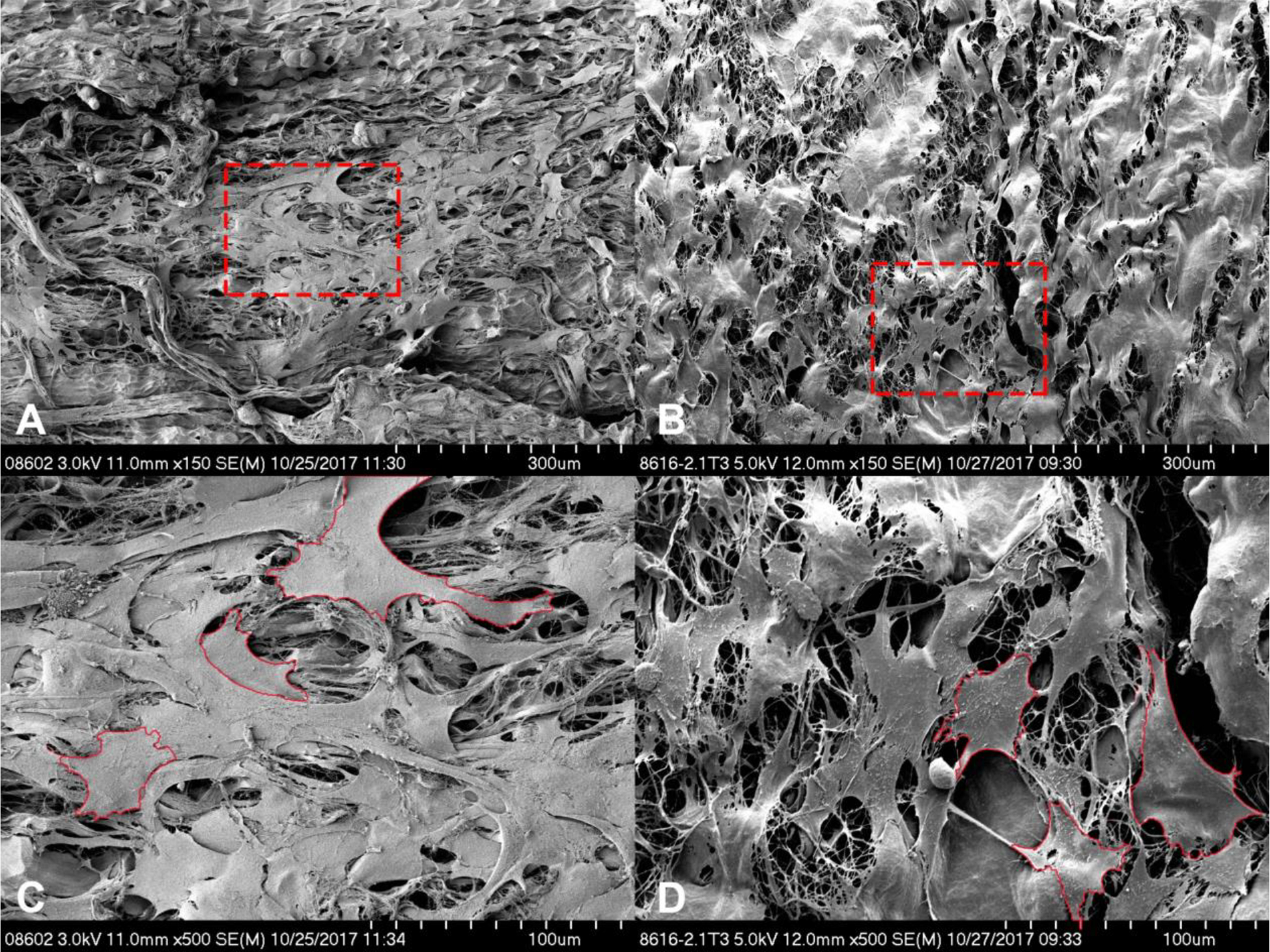
Scanning electron microscopy images showing the cell coverage of the Avance® Nerve Graft (A and C) and the NeuraGen® Nerve Guide (B and D) after being dynamically seeded with human MSCs for 12 h. Images A and B display overview images with 150X magnification. Images C and D display the areas that are encircled in red in images A and B, 500X magnification. Shown is a uniform distribution of partly aggregating MSCs on the porous surface of both nerve substitutes. Examples of cell contours are displayed in red in C and D.
Figure 6.
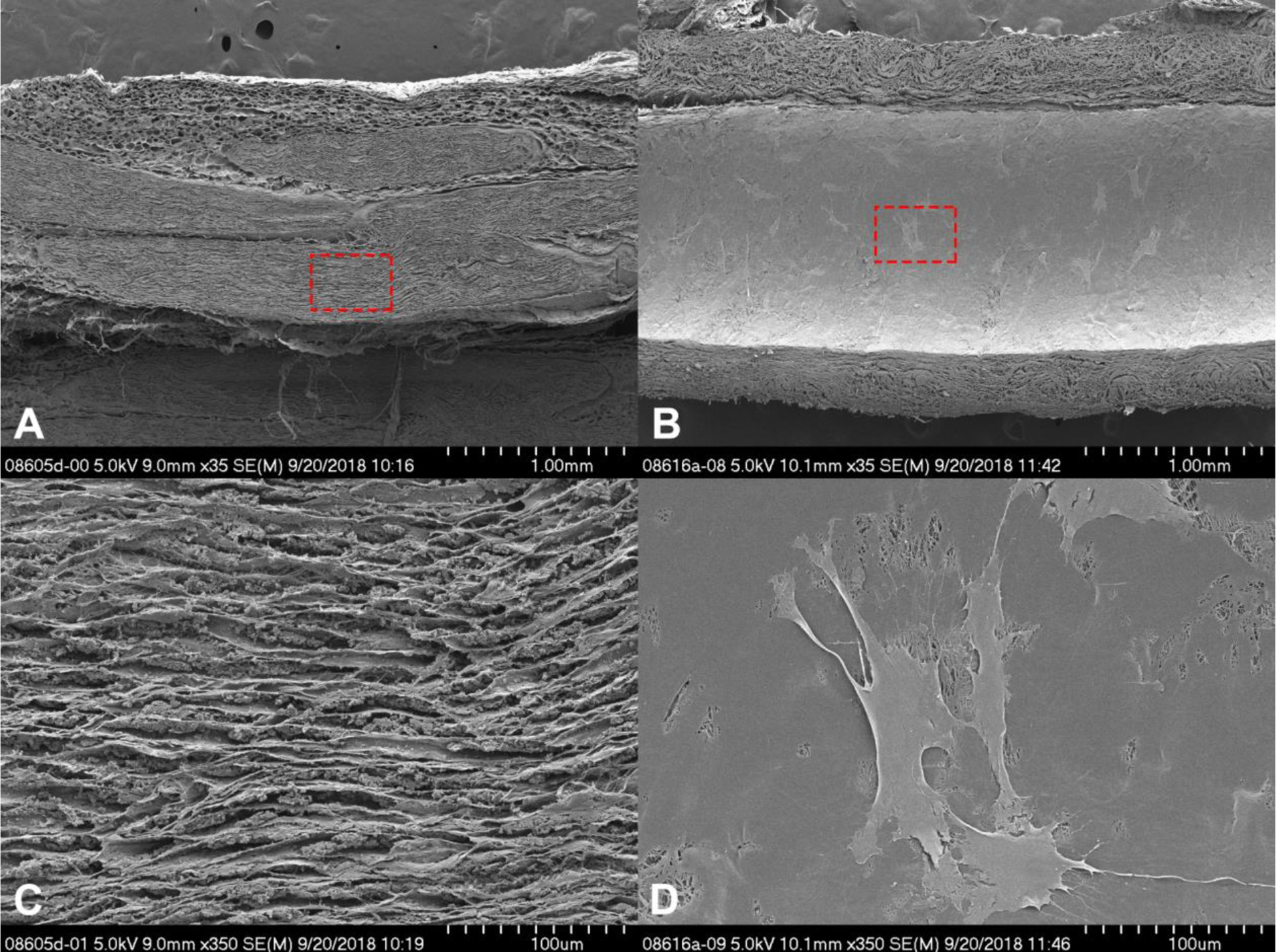
Cross-sectional scanning electron microscopy images of the Avance® Nerve Graft (A and C) and the NeuraGen® Nerve Guide (B and D) after 12 h of dynamic seeding with human MSCs. Both nerve substitutes were cut longitudinally. The cross-section of the Avance® Nerve Graft shows aligned fascicles without the presence of any cells. The cross-section of the NeuraGen® Nerve Guide demonstrates the smooth inner surface of the hollow conduit, with MSCs spread out among the entire length of the nerve guide.
These findings were confirmed by Hoechst staining of cross-sectional images of both groups that revealed no staining of nuclei in cells inside of the Avance® Nerve Grafts but detectable nuclear staining of cells within the NeuraGen® Nerve Guides (Figure 7).
Figure 7.
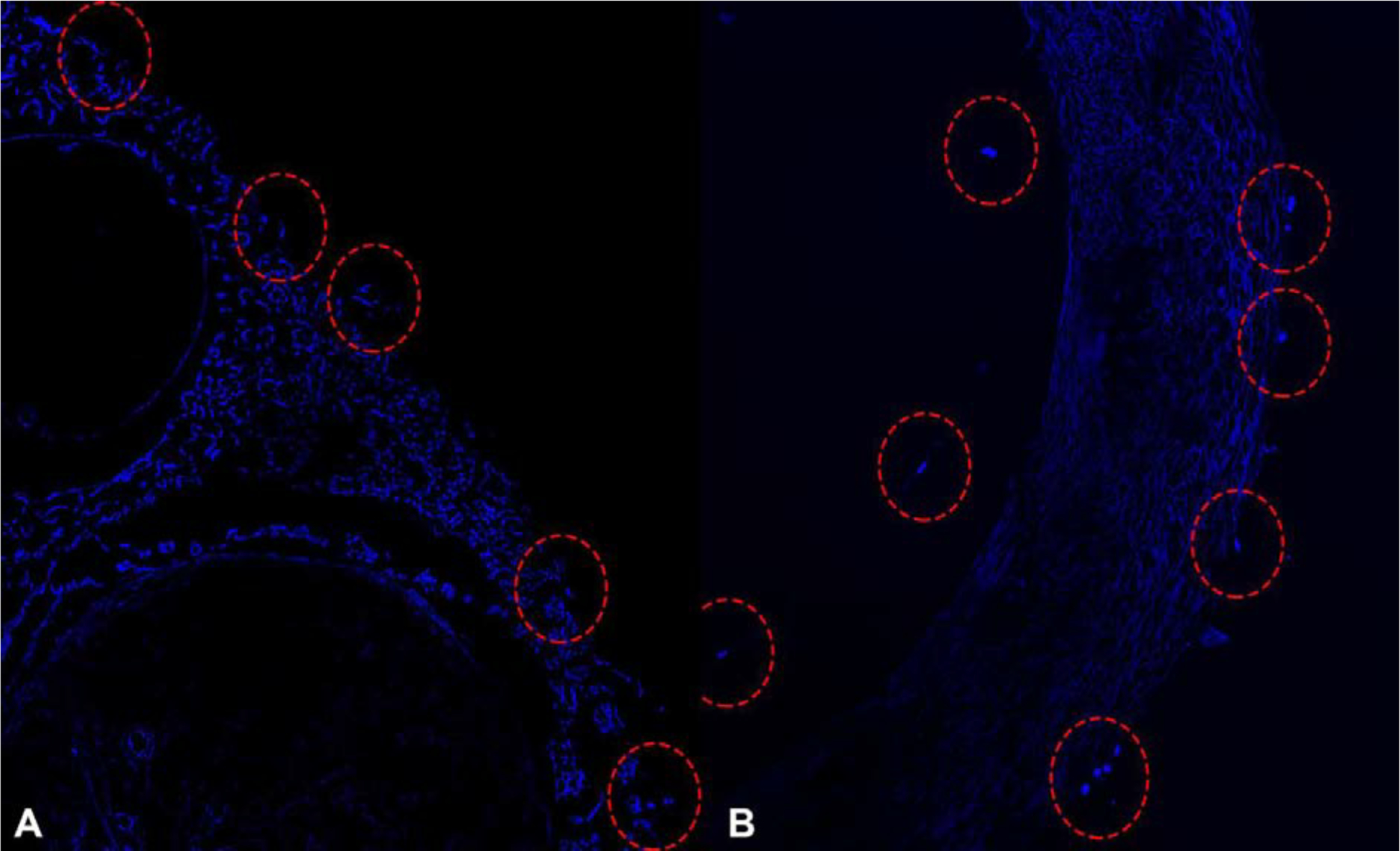
Hoechst-stained cross-sectional segments of the Avance® Nerve Graft (A) and the NeuraGen® Nerve Guide (B) after 12 h of dynamic seeding with human MSCs. Cell nuclei, labeled in bright blue, are displayed among the outer surface of the Avance® Nerve Graft (left) and on both the inner and outer surface of the NeuraGen® Nerve Guide (right).
Seeding efficiency
With the Avance® Nerve Graft, a seeding efficiency of 18.23% (± 28.12) was obtained after 6 h, reaching a maximum of 66.46% (± 16.01) after 12 h that was sustained at 59.90% (± 28.81) after 24 h of dynamic seeding. With the NeuraGen® Nerve Guide, the seeding efficiency increased from 52.08% (± 14.81) after 6 h to 94.17% (± 4.03) after 12 h but decreased to 52.50% (± 19.27) after 24 h.
Two-way ANOVA showed a significant interaction between seeding duration, the different groups, and the seeding efficiency (p=0.004). Kruskal-Wallis analysis of within-group differences for the Avance® Nerve Grafts was significant (p=0.007). Pairwise comparisons showed that the increase in seeding efficiency between the first (6 h) and second (12 h) time points was statistically significant (p=0.006), but that the decrease between the second (12 h) and third (24 h) time points was not (p=0.589). The Kruskal-Wallis analysis of within-group differences of the NeuraGen® Nerve Guide showed a significant interaction (p=0.024). Pairwise comparisons revealed a significant increase in seeding efficiency between time points 1 and 2 (p=0.029) and a significant decrease between time points 2 and 3 (P=0.029). Seeding efficiencies obtained with the NeuraGen® Nerve Guide were significantly higher than the seeding efficiencies obtained with the Avance® Nerve Graft after 12 h of seeding (p=0.010). No significant differences were found at time point 1 (p=0.055) and time point 3 (p=0.522). The seeding efficiency over time for both groups is depicted in Figure 8.
Figure 8.
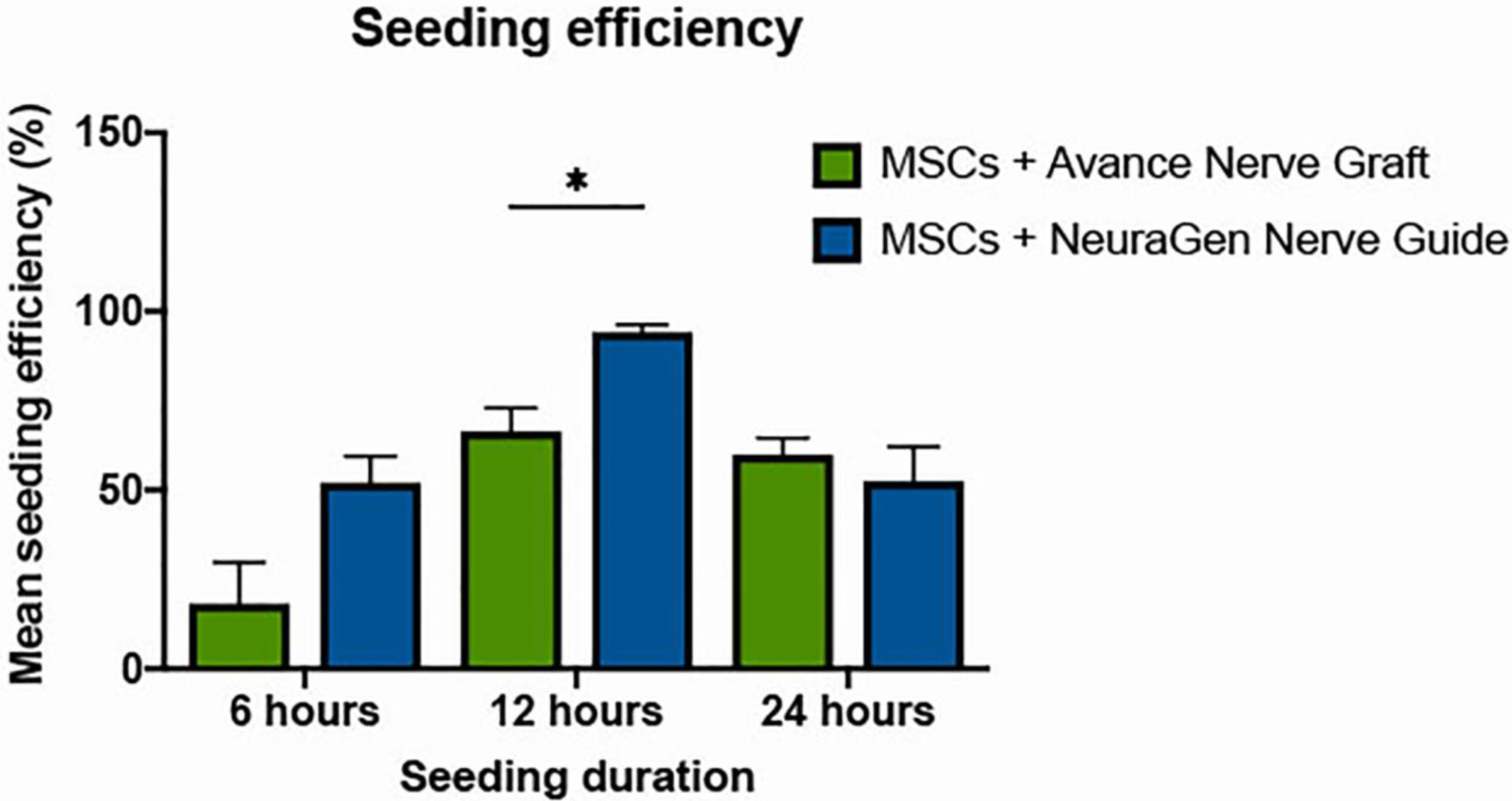
Seeding efficiencies of the Avance® Nerve Graft and the NeuraGen® Nerve Guide after the completion of 6, 12, and 24 h of dynamic seeding with human MSCs (n=6 per group per time point). Both groups obtained an optimal seeding efficiency after 12 h of dynamic seeding; the Avance® Nerve Graft reached a seeding efficiency of 66.46% (± 16.01), the NeuraGen® Nerve Guide reacheda seeding efficiency of 94.17% (± 4.03). Error bars: SEM. * = significant difference, with α≤0.05. SEM = standard error of the mean.
DISCUSSION
We examined the clinical potential of dynamic seeding of MSCs onto commercially available nerve graft substitutes by testing whether (I) the NeuraGen® Nerve Guide and the Avance® Nerve Graft affect the viability of human MSCs, (II) human MSCs can be dynamically seeded and distributed uniformly onto these nerve substitutes, and (III) dynamic seeding and optimized timing improve the efficiency of MSC seeding.
MTS assays demonstrated that both the Avance® Nerve Graft and the NeuraGen® Nerve Guide did not affect the metabolic activity or survivability of human adipose-derived MSCs. The human MSCs were able to adhere in a uniform manner of the surfaces of both nerve substitutes, with an optimal dynamic seeding duration of 12 h. The significantly better seeding efficiency of NeuraGen® Nerve Guides after 12 h of seeding is most likely due to their hollow conduit configuration, enabling MSCs to adhere to the outer and inner surfaces of the conduit. The MSCs did not migrate inside the Avance® Nerve Graft or into the substrate of the NeuraGen® Nerve Guides. It is hypothesized that the decrease in seeding efficiency after 12 h of dynamic seeding is related to cell damage due to the rotational forces of the seeding process, leading to a decreased ability of cells to adhere to the surfaces of the nerve substitutes.
The concept that MSCs inside a nerve substitute support the regeneration of axons is relevant for the applicability of the dynamic seeding strategy. MSCs used in peripheral nerve repair potentially differentiate into Schwann cells in vivo, and may have a structural function by replacing injured tissue. However, studies supporting that MSCs need to be delivered inside nerve substitutes are limited and most papers show that only a fraction of the added MSCs are differentiated into actual Schwann cells and survive in the long term.(33, 34) Other studies reported trophic effects of MSCs, producing proteins and molecules that stimulate tissue regeneration, form extracellular matrix components, enhance angiogenesis, inhibit scar formation, and attenuate inflammation without the MSCs actually being physically integrated into the regenerating tissue. (35–39) The robust expression of secreted proteins, including growth factors and morphogens by MSCs, when introduced to injured tissues supports the concept that MSCs may be more effective as tissue repair catalysts rather than architectural participants. (40, 41)
The trophic concept forms the basis for the proposed delivery method of MSCs. Considering the shown mismatch in size between the axon fascicules and the MSCs (Figure 6), the delivery of MSCs inside a graft may block the ingrowth of regenerating axons. As MSCs do not need to be delivered inside the nerve graft to produce growth factors and cytokines that support nerve regeneration, it is preferred that MSCs are added in a simple, efficient and systematic manner that is nontraumatic and avoids damage to the nerve substitute. In contrast to microinjection and soaking methods, the dynamic seeding strategy of Rbia and colleagues meets these requirements. (22, 26–28) This strategy has demonstrated to enhance the neuroregenerative gene expression in the MSCs (19, 20) and revealed an in vivo survival of the MSCs up to 29 days. (42) The absence of MSCs on the inner surface of the Avance® Nerve Graft, therefore does not implicate that this combination by definition results in less nerve regeneration enhancement of the combination of MSCs and the NeuraGen® Nerve Guide. In vivo studies using the described techniques should indicate whether MSC-seeding location (inner or outer surface) has implications for their effect on nerve regeneration.
Seeding efficiency was indirectly determined by cell counts in the supernatant after the seeding duration time passed. Although multiple cell counts were performed with small standard errors and subjective assessment of the obtained live/dead and Hoechst images imply high seeding efficiencies, seeding efficiency could theoretically be overestimated by this indirect strategy, as MSCs might have attached to the conical tube or have clumped together. (32) Group sizes were limited due to the costs associated with the use of various products. Another limitation is that we did not test our chimeric cell/graft models yet in animal models.
Despite the limitations, this study is a preliminary but essential step toward considering the use of dynamic seeding in a clinical setting. This study clearly demonstrates that MSCs can be seeded on the Avance® Nerve Graft and the NeuraGen® Nerve Guide. MSCs more efficiently adhered to the inner and outer surface of the NeuraGen® Nerve Guide than to the Avance® Nerve Graft, MSCs are evenly distributed on the surface and do not migrate into the nerve or substrate.
CONCLUSION
The NeuraGen® Nerve Guide and the Avance® Nerve Graft do not negatively influence the viability of human MSCs. After 12 h of dynamic seeding, 66% (Avance) to 94% (NeuraGen) of the administered dose of MSCs adhered to the nerve substitutes, with a statistically significant higher efficiency for the NeuraGen Nerve Guide. The vast majority of adhered MSCs survived and were distributed in a uniform manner among the surface of both nerve substitutes and did not migrate into the nerve or collagen material. Future human or animal studies will permit the determination of effects of MSCs seeded on nerve graft substitutes on motor regeneration or sensory reinnervation in large nerve deficits.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at:
ASSH 2018 Annual meeting, September 13–15, Boston, Massachusetts, USA; poster presentation.
APSN 2019 Annual meeting, February 2, Palm Desert, California, USA; podium presentation.
FESSH 2019, June 17–21, Berlin, Germany; podium presentation.
Conflicts of interest
The authors have nothing to disclose.
This study was funded by the NIH R01, “Bridging the gap: angiogenesis and stem cell seeding of processed nerve allograft.” 1 RO1 NS102360–01A1
The Avance® Nerve Grafts used in this study were provided by AxoGen Inc., Alachua, Florida, USA.
The NeuraGen® Nerve Guides used in this study were provided by Integra LifeSciences Holdings Corporation, Plainsboro, New Jersey, USA.
REFERENCES
- 1.Lin MY, Manzano G, Gupta R. Nerve allografts and conduits in peripheral nerve repair. Hand Clin 2013;29:331–48. [DOI] [PubMed] [Google Scholar]
- 2.Moore AM, Kasukurthi R, Magill CK, Farhadi HF, Borschel GH, Mackinnon SE. Limitations of conduits in peripheral nerve repairs. Hand 2009;4:180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isaacs J, Browne T. Overcoming short gaps in peripheral nerve repair: conduits and human acellular nerve allograft. Hand 2014;9:131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks DN, Weber RV, Chao JD, Rinker BD, Zoldos J, Robichaux MR, et al. Processed nerve allografts for peripheral nerve reconstruction: A multicenter study of utilization and outcomes in sensory, mixed, and motor nerve reconstructions. Microsurg 2012;32:1–14. [DOI] [PubMed] [Google Scholar]
- 5.Cho MS, Rinker BD, Weber RV, Chao JD, Ingari JV, Brooks D, et al. Functional outcome following nerve repair in the upper extremity using processed nerve allograft. J Hand Surg Am 2012;37:2340–9. [DOI] [PubMed] [Google Scholar]
- 6.Karabekmez FE, Duymaz A, Moran SL. Early clinical outcomes with the use of decellularized nerve allograft for repair of sensory defects within the hand. Hand 2009;4:245–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rbia N, Shin AY. The Role of Nerve Graft Substitutes in Motor and Mixed Motor/Sensory Peripheral Nerve Injuries. J Hand Surg Am 2017;42:367–77. [DOI] [PubMed] [Google Scholar]
- 8.Rinker B, Zoldos J, Weber RV, Ko J, Thayer W, Greenberg J, et al. Use of Processed Nerve Allografts to Repair Nerve Injuries Greater Than 25 mm in the Hand. Annals Plast Surg 2017;78(6S Suppl 5):S292–s5. [DOI] [PubMed] [Google Scholar]
- 9.Whitlock EL, Tuffaha SH, Luciano JP, Yan Y, Hunter DA, Magill CK, et al. Processed allografts and type I collagen conduits for repair of peripheral nerve gaps. Muscle & Nerve 2009;39:787–99. [DOI] [PubMed] [Google Scholar]
- 10.Pfister LA, Papaloizos M, Merkle HP, Gander B. Nerve conduits and growth factor delivery in peripheral nerve repair. J Peripher Nerv Syst 2007;12:65–82. [DOI] [PubMed] [Google Scholar]
- 11.Cui Y, Yao Y, Zhao Y, Xiao Z, Cao Z, Han S, et al. Functional collagen conduits combined with human mesenchymal stem cells promote regeneration after sciatic nerve transection in dogs. J Tissue Eng Regen Med 2018;12:1285–96. [DOI] [PubMed] [Google Scholar]
- 12.Hsueh YY, Chang YJ, Huang TC, Fan SC, Wang DH, Chen JJ, et al. Functional recoveries of sciatic nerve regeneration by combining chitosan-coated conduit and neurosphere cells induced from adipose-derived stem cells. Biomat 2014;35:2234–44. [DOI] [PubMed] [Google Scholar]
- 13.Ao Q, Fung CK, Tsui AY, Cai S, Zuo HC, Chan YS, et al. The regeneration of transected sciatic nerves of adult rats using chitosan nerve conduits seeded with bone marrow stromal cell-derived Schwann cells. Biomat 2011;32:787–96. [DOI] [PubMed] [Google Scholar]
- 14.di Summa PG, Kingham PJ, Campisi CC, Raffoul W, Kalbermatten DF. Collagen (NeuraGen(R)) nerve conduits and stem cells for peripheral nerve gap repair. Neurosci Ltrs 2014;572:26–31. [DOI] [PubMed] [Google Scholar]
- 15.Brenner MJ, Lowe JB 3rd, Fox IK, Mackinnon SE, Hunter DA, Darcy MD, et al. Effects of Schwann cells and donor antigen on long-nerve allograft regeneration. Microsurg 2005;25:61–70. [DOI] [PubMed] [Google Scholar]
- 16.Ogden MA, Feng FY, Myckatyn TM, Jensen JN, Grand AG, Wood PW, et al. Safe injection of cultured schwann cells into peripheral nerve allografts. Microsurg 2000;20:314–23. [DOI] [PubMed] [Google Scholar]
- 17.Hess JR, Brenner MJ, Fox IK, Nichols CM, Myckatyn TM, Hunter DA, et al. Use of cold-preserved allografts seeded with autologous Schwann cells in the treatment of a long-gap peripheral nerve injury. Plast Reconstr Surg 2007;119:246–59. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Zhao Z, Ren Z, Zhao B, Zhang L, Chen J, et al. Recellularized nerve allografts with differentiated mesenchymal stem cells promote peripheral nerve regeneration. Neurosci Ltrs 2012;514:96–101. [DOI] [PubMed] [Google Scholar]
- 19.Rbia N, Bulstra LF, Lewallen EA, Hovius SER, van Wijnen AJ, Shin AY. Seeding decellularized nerve allografts with adipose-derived mesenchymal stromal cells: An in vitro analysis of the gene expression and growth factors produced. JPRAS 2019;72:1316–25. [DOI] [PubMed] [Google Scholar]
- 20.Mathot F, Rbia N, Thaler R, Bishop AT, van Wijnen AJ, Shin AY. Gene expression and growth factor analysis in early nerve regeneration following segmental nerve defect reconstruction with a mesenchymal stromal cell-enhanced decellularized nerve allograft. PRS Global Open (Accepted for publication 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onishi K, Jones DL, Riester SM, Lewallen EA, Lewallen DG, Sellon JL, et al. Human Adipose-Derived Mesenchymal Stromal/Stem Cells Remain Viable and Metabolically Active Following Needle Passage. PMR 2016;8:844–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jesuraj NJ, Santosa KB, Newton P, Liu Z, Hunter DA, Mackinnon SE, et al. A systematic evaluation of Schwann cell injection into acellular cold-preserved nerve grafts. J Neurosci Meth 2011;197:209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garvican ER, Cree S, Bull L, Smith RK, Dudhia J. Viability of equine mesenchymal stem cells during transport and implantation. Stem Cell Res Therap 2014;5:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agashi K, Chau DY, Shakesheff KM. The effect of delivery via narrow-bore needles on mesenchymal cells. Regen Med 2009;4:49–64. [DOI] [PubMed] [Google Scholar]
- 25.Mamidi MK, Singh G, Husin JM, Nathan KG, Sasidharan G, Zakaria Z, et al. Impact of passing mesenchymal stem cells through smaller bore size needles for subsequent use in patients for clinical or cosmetic indications. J Translat Med 2012;10:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson MJ, Patel G, Isaacs J, McMurtry J, Richards N, Daner W. Introduction of neurosupportive cells into processed acellular nerve allografts results in greater number and more even distribution when injected compared to soaking techniques. Neurolog Res 2017;39:189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rbia N, Bulstra LF, Bishop AT, van Wijnen AJ, Shin AY. A simple dynamic strategy to deliver stem cells to decellularized nerve allografts. Plast Reconstr Surg 2018;142:402–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathot F, Rbia N, Bishop AT, Hovius SER, Van Wijnen AJ, Shin AY. Adhesion, distribution, and migration of differentiated and undifferentiated mesenchymal stem cells (MSCs) seeded on nerve allografts. J Plast Reconstr Aesthet Surg 2019;May 22 Pii S1748–6815(19):30231–1. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crespo-Diaz R, Behfar A, Butler GW, Padley DJ, Sarr MG, Bartunek J, et al. Platelet lysate consisting of a natural repair proteome supports human mesenchymal stem cell proliferation and chromosomal stability. Cell Transplant 2011;20:797–811. [DOI] [PubMed] [Google Scholar]
- 30.Mader EK, Butler G, Dowdy SC, Mariani A, Knutson KL, Federspiel MJ, et al. Optimizing patient derived mesenchymal stem cells as virus carriers for a phase I clinical trial in ovarian cancer. J Transl Med 2013;11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahmoudifar N, Doran PM. Osteogenic differentiation and osteochondral tissue engineering using human adipose-derived stem cells. Biotechnol Prog 2013;29:176–85. [DOI] [PubMed] [Google Scholar]
- 32.Villalona GA, Udelsman B, Duncan DR, McGillicuddy E, Sawh-Martinez RF, Hibino N, et al. Cell-seeding techniques in vascular tissue engineering. Tissue Eng Part B Rev. 2010;16:341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orbay H, Uysal AC, Hyakusoku H, Mizuno H. Differentiated and undifferentiated adipose-derived stem cells improve function in rats with peripheral nerve gaps. J Plast Reconstr Aesthet Surg 2012;65:657–64. [DOI] [PubMed] [Google Scholar]
- 34.Tomita K, Madura T, Sakai Y, Yano K, Terenghi G, Hosokawa K. Glial differentiation of human adipose-derived stem cells: implications for cell-based transplantation therapy. Neurosci 2013;236:55–65. [DOI] [PubMed] [Google Scholar]
- 35.Caplan AI, Hariri R. Body Management: Mesenchymal Stem Cells Control the Internal Regenerator. Stem Cells Translat Med 2015;4:695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caplan AI. Adult Mesenchymal Stem Cells: When, Where, and How. Stem Cells Int’l 2015;2015:628767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castro-Manrreza ME, Montesinos JJ. Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications. J Immunol Res 2015;2015:394917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death and Diff 2014;21:216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kingham PJ, Kolar MK, Novikova LN, Novikov LN, Wiberg M. Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells and Develop 2014;23:741–54. [DOI] [PubMed] [Google Scholar]
- 40.Mathot F, Shin AY, van Wijnen AJ. Targeted stimulation of MSCs in peripheral nerve repair. Gene. 2019;710:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kingham PJ, Kolar MK, Novikova LN, Novikov LN, Wiberg M. Stimulating the neutrotrophic and angiogenic properties of human adispose-derived stem cells enchances nerve repair. Stem Cells and Develop. 2014;23:741–54. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Zhang Z, Qin Y, Wu H, Lv Q, Chen X, et al. A new method for Schwann-like cell differentiation of adipose derived stem cells. Neurosci Ltrs 2013;551:79–83. [DOI] [PubMed] [Google Scholar]
- 43.Rbia N, Bulstra LF, Thaler R, Hovius SER, van Wijnen AJ, Shin AY. In Vivo Survival of Mesenchymal Stromal Cell-Enhanced Decellularized Nerve Grafts for Segmental Peripheral Nerve Reconstruction. J Hand Surg 2019;44:514.e1–.e11. [DOI] [PubMed] [Google Scholar]


