Abstract
Background
Over 70–85% of men with advanced prostate cancer (PCa) develop bone metastases characterized by severe bone pain and increased likelihood of bone fracture. These clinical features result in decreased quality of life and act as a predictor of higher mortality. Mechanistically, the skeletal pathologies such as osteolytic lesions and abnormal osteoblastic activity drive these symptoms. The role of immune cells in bone cancer pain remains under-studied, here we sought to recapitulate this symptomology in a murine model.
Methods
The prostate cancer bone metastasis-induced pain model (CIBP) was established by transplanting a mouse prostate cancer cell line into the femur of immunocompetent mice. Pain development, gait dynamics, and the changes in emotional activities like depression and anxiety were evaluated. Animal tissues including femurs, dorsal root ganglion, and spinal cord were collected at sacrifice and micro-CT, histology/immunohistochemistry, and quantitative immunofluorescent analysis were performed.
Results
Mice receiving prostate cancer cells showed a significantly lower threshold for paw withdrawal responses induced by mechanical stimulation compared to their control counterparts. Zero maze and DigiGait analyses indicated reduced and aberrant movement associated emotional activity compared with sham control at 8-weeks post-injection. Micro-CT analysis showed osteolytic and osteoblastic changes and a 50% reduction of the trabecular volumes within the prostate cancer group. Neurologically we demonstrate, increased CGRP and neuronal p75NTR immune-reactivities in both the projected terminals of the superficial dorsal horn and partial afferent neurons in dorsal root ganglion (DRG) at L2-L4 level in tumor-bearing mice. Furthermore, our data show elevated NGF and TrkA immunoreactivities in the same segment of the superficial dorsal horn that were, however, not colocalized with CGRP and p75NTR.
Conclusions
This study describes a novel immunocompetent model of CIBP and demonstrates the contribution of NGF and p75NTR to chronic pain in bone metastasis.
Keywords: Prostate Cancer, Neuroimmune, Bone Metastasis, Bone Pain, Neuropeptide
Introduction
Prostate cancer is the most common type of cancer among men 1, and over 80–90% of patients with advanced prostate cancer (PCa) develop bone metastases. Bone metastasis is characterized by an increased likelihood of bone fracture and chronic refractory pain 2 and remains mostly incurable. Due to a limited understanding of the pathophysiology driving cancer-induced bone pain (CIBP), current treatments result in inadequate pain relief and dose-limiting side-effects 3. Metastatic prostate CIBP has a profound impact on patient quality of life, and more studies and preclinical animal models are needed to develop new analgesics that target these effects 4.
Induction and maintenance of CIBP potentially arise via a combination of nociceptive, neuropathic, and inflammatory pathways 5–7. It was also reported that tumor-induced acidosis, nociceptive mediators released by interactions between tumor cells and bone stromal cells as well as tumor cell-induced nociceptive afferent fiber sprouting are associated with the development of CIBP 2. These factors act on the peripheral nociceptors and lead to the sensitization in both peripheral and central nerve afferent neurons. Neuropeptides such as CGRP and NGF play essential roles in the transmission, transduction, integration, and modulation of nociceptive signals via their receptor signaling pathways 8–12. Clinical studies have shown that chronic pain syndromes, such as migraine can be prevented by anti-CGRP and anti-NGF monoclonal antibodies 13–15. CGRP receptor antagonists and anti-NGF antibody were reported to peripherally attenuate inflammatory pain and tolerance to the analgesic effect of morphine 16–18. The role of the immune system in modulating pain pathways is not understood and under-researched with studies reporting sometimes conflicting data of its influence in neuropathic pain development 19–33.
This study addresses these concerns and describes, for the first time, a novel, newly developed syngeneic, immunocompetent mouse model of bone metastasis and CIBP. The B6 Hi-Myc transgenic mouse model of prostate cancer expresses the oncogene Myc in prostate epithelium and is backcrossed into the C57BL/6 mouse strain 34,35. It was developed as an allograft cell line by a serial subcutaneous passage of a tumor identified and excised from a metastatic lesion. The tumor cell line implants readily into C57BL/6 mice and is phenotypically characterized as an admixture of luminal and epithelial cells that express androgen receptor and are androgen-dependent35. Unlike other xenograft tumor models, here we developed a model of prostate cancer bone metastasis by direct intra-femoral inoculation of B6 Hi-Myc cells into immunocompetent C57BL/6 mice. This allows us to comprehensively explore the role of the immune repertoire on CIBP onset and progression and more importantly, the impact of bone-pain on neural mechanisms meditating nociception. We envisage that this model will potentially contribute to the discovery of new targets and analgesic strategies for CIBP. Notably, our primary data describe a prostate cancer cell-originated bone pain model and demonstrates pain responses to be associated with increased expressions of CGRP and p75NTR in primary afferent neurons and enhanced expression of NGF and TrkA in a population of neurons in the superficial dorsal horn.
Materials and methods
Ethics Statement
Animal studies were conducted under protocols 2013–3015 and 2013–2068 approved by the Institutional Animal Care and Use Committee (IACUC) at Northwestern University. IACUC at Northwestern is AAALAC accredited. Northwestern University has an Animal Welfare Assurance on file with the Office of Laboratory Animal Welfare (A3283–01). Northwestern University conducts its reviews in accordance with United States Public Health Service (USPHS) regulations and applicable federal and local laws. The composition of the IACUC meets the requirements of the USPHS policy and the Animal Welfare Act Regulations.
Animal Use
All experimental animals used in this study were purchased from Jackson Laboratory. Animals had free access to sterilized food and water ad libitum. Male eight weeks old C57BL6 (B6) mice were used for receiving saline and B6 Hi-Myc cells injection to femurs. All experimental procedures were performed in Class II biological safety cabinet until animals recovered from anesthesia. Experimental protocols were approved by the Northwestern University Feinberg School of Medicine Institutional Animal Care and Use Committee.
Establishment of Prostate Cancer Bone Pain Model
The C57BL/6 mouse-originated prostate cancer cell line B6 Hi-Myc ( a gift from Dr. Brian Simons) was used to establish the prostate cancer-induced bone pain model 34,35. The bone cancer pain model in this study was prepared as described previously 36–38 with some slight modifications. Briefly, C57BL/6 mice were anesthetized by isoflurane and the skin around the knee joint disinfected with 70% v/v ethanol. Prior to injection, a 17G needle was used for drilling a track on the right distal femoral epiphysis followed by direct injection of 5×103 B6 Hi-Myc cells in 6 μl PBS, or 6 μl PBS only, using a BD Veo™ insulin syringe with BD Ultra-Fine™ 6mm x 31G needle. The syringe needle was left in the medullary cavity for 2 minutes to prevent leakage. All animals recovered from inoculation surgery for at least two days prior to any experiment.
Mechanical Allodynia
Mechanical allodynia was measured by hind paw withdrawal from mechanical stimuli, as described previously39. Briefly, prior to the testing, mice were allowed to acclimate in a 6×12×12-cm chamber on a wire mesh grid for at least 15 min in a quiet, warm, and environmentally clean procedure room. Then, a calibrated set of von Frey filaments (0.02–6.00g) were applied from below to the plantar hind paw to determine the 50% force withdrawal threshold using an up-down iterative method39. The filament was applied to its tip at a right angle to the mid-plantar of the rodent’s hind foot through the mesh floor until the filament bends. After about 3 seconds, the response to the stimuli with foot withdrawal or flinching was recorded as a positive response, and the filament with the next lower force was applied next. If no response was induced, the next higher successive filament was applied. Calculations were performed, as previously described 39.
DigiGait Analysis
DigiGait imaging system (Mouse Specifics, Inc., Boston) was used for the evaluation of bone cancer-associated nociception. Gait dynamics were recorded and analyzed, as previously described40. Briefly, to acclimatize animals to the testing environment, the mouse was introduced to treadmill belt before the motorized began moving. Mice walked on the treadmill belt and recordings were taken at three gradually increasing speeds, 10cm/s, 17cm/s, and 24cm/s. A 3-minute interval between recordings was set up to ensure mice were not over-exerted. The Gait dynamics were collected on video taken from a high-speed digital video camera mounted underneath the rig. For dynamic Gait analysis, the video was reviewed and the noise was manually corrected from dynamic gait signals. A minimum of four seconds (150 frames/s) of the movie was required.
Elevated Zero Maze
The basic maze is set up as an elevated (33 cm), grey annular (46 cm inner diameter) runway (5.5 cm width) consisting of 2 pairs of opposing quadrants. One pair is the “closed” quadrants with 14 cm inner and outer walls made of gray polyvinyl-chloride. The other pair of quadrants without walls is set as the “open” quadrants. A digital video camera is mounted above the maze on the ceiling of the test chamber. After the focus and field for video camera and the orientation and position of maze are set up, the mice were placed in the middle of one closed quadrant and followed using a 5-min video recording on LimeLight software41.
Open Field Test
The open field arena (56 × 56 × 30cm) is used to assess ambulation levels as well as anxiety. A mouse is placed in the center of the arena, and its ambulation activity is collected on a digital video camera mounted on the top of the field by the LimeLight software for 5 minutes. For data analysis, the arena is divided as 25 grids assigned to two zones. Zone 1 consists of 16 grids along the wall and zone 2 was assigned the remaining nine grids in the center. At the beginning of the experiment, mice were placed in the center, and the 5-min video collected for offline analysis.
Micro-Computed Tomography (μCT)
Prior to histologic processing, femurs of C57BL6 wild-type mice injected intra-femorally with B6 Hi-Myc cells were scanned by micro-computed tomography (μCT) with a μCT-35 cone-beam scanner (Scanco Medical) with a 55 kVp source and a 145 μAmp current. Mouse legs were scanned at a resolution of 12μm. To assess tumor cells-induced bone mass changes, the morphometry was reconstructed and bone volume density (BV/TV) was analyzed.42
Immunohistochemistry and immunofluorescence
Mice were anesthetized with 2.5% isoflurane (Isothesia; Butler) and transcardially perfused with cold PBS, followed by ice-cold 4% paraformaldehyde. DRGs, spinal cords and femurs were removed and post-fixed for 1, 12, and 48 hours, respectively, in 4% paraformaldehyde. Then DRG and spinal cord were transferred to 30% sucrose solution overnight, and the decalcification of the femur was carried out in 14% EDTA for five days with a daily change of solution. Tissues were placed in ornithine carbamyl transferase embedding medium (Tissue Tek) and frozen by immersion in a dry ice-chilled isopentane bath and stored at −80°C for further use. L3–5 DRG and corresponding spinal cord segments were sectioned on a cryostat (Leica), mounted on precleaned superfrost-plus slides, and stored at −80°C or 4°C in PBS respectively. The femur samples were cut and mounted on slides and stored at −80°C. Tissue slices were first fixed in 4% paraformaldehyde in PBS for 10 min, and followed by antigen retrieval treatment with 10mM sodium citrate buffer, pH 9.0, for 10 min at 80°C. Sections were then incubated in blocking buffer (0.3% Triton X-100 mixed with 5% donkey serum in PBS) for 30 min at room temperature, followed by overnight incubation with primary antibody in blocking buffer (4°C). Sections were then washed in PBS for 3 × 10 min and incubated in Alexa Fluor 405-, 488-, 555- or 647-conjugated secondary antibodies for 2 hours. After washing, slices were mounted on superfrost/plus slides with Prolong™ Diamond Antifade mounting medium (Invitrogen). IB4 was selected as a marker of non-peptidergic afferent neurons that are different from CGRP-positive peptidergic neurons. NGF is known to play a critical role in neuropathic pain and has been implicated in CIBP. TrkA and p75NTR were examined as major receptor molecules known to interact with NGF to mediate nociceptive signaling. The antibodies and labeling molecules used in this study were as follows: mouse monoclonal(AE1/AE3) anti-pan-cytokeratin (1:500, Novus), rat monoclonal(AN1–15) anti-androgen receptor (1:500, Invitrogen), rabbit anti-NGF (1:200), Rabbit polyclonal anti-p75NTR (1:1,000, Abcam), rabbit anti-TrkA (1:1,000), mouse monoclonal(4901) anti-CGRP (1:1,000, Abcam). For labeling non-peptidergic unmyelinated primary afferent neurons, axons and terminals projected to spinal cord, 1:500 fluorescein-labeled GSL I-B4 (Vector Laboratories).
Confocal Microscopy and Image Analysis
Images were obtained on a Nikon A1 Confocal Laser Microscope System (Plan Apo 20× NA 0.75 and Plan Apo 60× NA1.4 Oil, objectives). For quantitative immunofluorescent analysis, the comparable tissues/regions were selected. Images were taken on the same confocal setting as the template for imaging on all groups. After pixel and average lines were selected, laser intensity and gain on each channel were optimized. For z-series imaging, the same settings were applied to total slice thickness, step value, and the thickness to the surfaces of the section. For image analysis, several custom macros were independently run with NIH ImageJ software to automatically carry out background correction and thresholding for different slices. For z-stacks images analysis, after thresholding on image, the targeted regions of interest were determined in combination with manual correction.
Statistical Analysis
Statistical analysis was performed using the student’s t test for the comparison between two groups. Multiple comparisons (more than two groups) were carried out using a one-way analysis of variance (ANOVA) followed by post hoc Newman. All data are presented as mean ± SD, and the differences are considered statistically significant with a p-value<0.05. Data analyses and statistical calculations were performed using Microsoft Excel™ and GraphPad Prism 5.43
Results
Intra-femoral injection of prostate cancer cells induces bone pathology
We established a preclinical model of prostate cancer induced bone pain by intra-femoral injection of murine derived B6 Hi-Myc prostate cancer cells into the right distal femoral epiphysis of male C57BL/6 mice. At 60–90 days after injection, animals were sacrificed and the femurs were collected for histological and morphological analyses. We utilized pancytokeratin expression on the B6 Hi-Myc prostate epithelial cells 35 to confirm the presence of viable cells within the medullary cavity of the femurs of B6 Hi-Myc injected mice but not in saline controls (Fig. 1a). We observed the clonal growth of cytokeratin-positive prostate cells close to the cortical bone as well as cytokeratin positive cells distributed singly in the medullary cavity. To further confirm the presence of the B6 Hi-Myc prostate cancer cells we performed immunohistochemistry for androgen receptor (AR) expression (Fig. 1b). AR immunoreactivity was found to be associated with single cells as well as clusters in the femoral medulla. Interestingly, these cells or cell clusters were commonly found adjoining the trabecular bone, suggesting a direct or indirect role of the injected prostate cancer cells in the observed bone pathology. To determine the morphological changes that occurred upon tumor engraftment, we performed μCT on mouse femurs at day 90 following injection. Representative 3D reconstructions of femurs in different orientations indicated new bone formation in B6 Hi-Myc injected femurs (Fig. 1d). Transverse images of femoral bone showed typical osteoblastic and osteolytic changes and qualitative loss of trabecular bone volume (Fig 1e). Quantitative analysis of this data demonstrated a pronounced and significant reduction in the trabecular bone volume (BV) to total volume (TV) ratio indicative of bone-cancer induced pathology (Figure 1e&f). These data demonstrate that the B6 Hi-Myc intra-femoral model is capable of engrafting and inducing bone pathology reminiscent of metastatic prostate cancer.
Figure 1: Intra-femoral injection of prostate cancer cells induces bone pathology.
C57BL/6 Mice received direct injection of 5×103 B6 Hi-Myc cells in 6 μl PBS, or 6 μl PBS alone into the right distal femoral epiphysis. (a) Low power images on the left (top and bottom panels) show the expansion of cytokeratin-positive cells close to cortical bone in the B6 Hi-Myc injected femur but not in the saline group. Note, the black circle indicates cytokeratin-positive clustering cells, and the blue dashed line is the border of the medulla and cortical bone. Right top and bottom panels are high power images of the same areas. (b) High power images show androgen receptor positive cells present singly or as clusters (arrows) in the B6 Hi-Myc injected femurs but not the saline group. (c) H&E stained sections of the femur showing prostate cancer associated pathology with increased cell infiltration (see inset) and loss of trabecular bone in the B6 Hi-Myc injected femurs. (d) Reconstructed 3-dimensional μCT images of femurs in different orientations demonstrate bone cancer pathology in B6 Hi-Myc injected femurs. Red dotted lines with red arrowhead indicate new bone formation. L, Lateral; M, Medial. (e) Transverse images of femoral bone showing osteoblastic and osteolytic changes, white arrowheads and arrows, respectively (bottom left panel) Reconstructed 3D μCT images demonstrating the loss of trabecular bone volume (bottom right panel). (f) Quantitative analysis of ratio of trabecular bone volume. Values are expressed as mean ± SD, **p<0.01, n=3/per group.
Metastatic bone lesions induce pain associated behavioral responses
To assess whether the morphological changes and tumor engraftment described above were associated with pain responses we examined mechanical allodynia by von Frey foot withdrawal assay prior to and at multiple time-points post-injection. Analysis of foot withdrawal thresholds on the ipsilateral side of animals injected with cancer cells versus sham revealed significantly decreased threshold responses at day ten post-injection that continued until the experimental endpoint at day 90 (Figure 2). These data are indicative of the induction and maintenance of allodynia that is associated with tumor engraftment. These responses are notable for both their chronicity and time of induction, suggesting that pain develops in these animals before/during morphological distortion. We next examined whether the pain response in mice-bearing B6 Hi-Myc cells had corresponding anxiety and depression-like behavioral changes. 7-weeks post-injection, the animals were evaluated using both the elevated zero maze and an open field test. The data displayed in Table 1, demonstrate emotional adaptive changes associated with bone cancer-induced pain responses, specifically the distance traveled in open area (OA) of the zero maze. The direct effect of bone-cancer engraftment and altered bone density on gait was then examined by DigiGait analyses (Table 2). Here we show that both paw angle and midline distance were significantly different comparing between sham and tumor-bearing mice. These data indicate that both behavioral responses to pain and gait dynamics were altered in injected animals.
Figure 2: Prostate cancer bone lesions induce pain-associated behavioral responses.
Hind-paw withdrawal threshold-associated mechanical allodynia response was induced by von Frey filament forced stimulation. Testing was performed every ten days for 90 days. Responses are depicted as a withdrawal response to filaments of increasing rigidities. Mice receiving B6 Hi-Myc cells (closed inverted triangle) are compared with age-matched saline-injected C57BL/6 mice (open triangle). Values are expressed as mean ± SD, **p<0.01, ***p<0.001, n=10–12/per group.
Table 1.
Effects of B6 Hi-Myc cells injected into right femur on behavioral tests#
| Saline | B6 Hi-Myc cells | |
|---|---|---|
| Open Field | ||
| Time in OZ, -sec | 40.26 ± 16.39 | 39.38 ± 18.69 |
| Time % in OZ | 13.14 ± 5.44 | 13.01 ± 6.27 |
| Distance, -cm | 3122.68 ± 460.50 | 2867.72 ± 712.97 |
| Distance % in OZ | 21.65 ± 7.20 | 19.64 ± 7.84 |
| Crossings | 20.30 ± 7.35 | 20.42 ± 9.11 |
| Zero Maze | ||
| Time in OA, -sec | 46.27 ± 27.21 | 31.64 ± 20.12 |
| Time % in OA | 15.45 ± 9.08 | 10.56 ± 6.72 |
| Distance, -cm | 982.62 ± 254.30 | 760.65 ± 184.72 * |
| Distance % in OA | 28.86 ± 9.13 | 19.78 ± 10.69 * |
| Crossings | 26.80 ± 13.20 | 17.80 ± 7.90 |
Values are expressed as means ± SD. n=10–12 mice per group.
p<0.05
Data were collected on 53 days post-injection
Table 2.
Effects of B6 Hi-Myc cells injected into right femur on DigiGait dynamics#
| Saline | B6CaP cells | |
|---|---|---|
| % Swing Stride | 29.025 ± 8.722 | 24.029 ± 4.866 |
| % Stance Stride | 70.975 ± 8.722 | 75.971 ± 4.866 |
| Stance/Swing | 2.675 ± 0.830 | 3.329 ± 0.930 |
| Paw Area at Peak Stance in sq. cm | 0.218 ± 0.071 | 0.269 ± 0.064 |
| Paw Angle | 16.925 ± 9.099 | 23.918 ± 4.604 * |
| Absolute Paw Angle | 17.908 ± 6.740 | 23.918 ± 4.604 * |
| Midline Distance (Left Hind) | 2.301 ± 0.531 | 1.855 ± 0.465 * |
| MAX dA/dT | 14.025 ± 5.648 | 17.842 ± 5.034 |
| Hind Limb Shared Stance Time | 0.196 ± 0.053 | 0.232 ± 0.049 |
Values are expressed as means ± SD. n=10–12 mice per group.
p<0.05
Data were collected on 50 days post-injection
Prostate cancer bone metastasis is associated with increased CGRP-positive neurons in the DRG
CGRP-positive sensory neurons are a major type of peptidergic neuron widely reported to play important roles in noxious sensitization and inflammatory, neuropathic pain. IB4-positive neurons are non-peptidergic afferent neurons. To determine whether CGRP-positive cells are involved in the pain responses induced by bone metastasis, we performed CGRP immunostaining on DRG sections from B6 Hi-Myc and sham injected mice. CGRP-immunoreactivity was significantly increased (~20%) in ipsilateral DRGs at the L3–5 level in tumor bearing mice compared to their control counterparts (Figure 3(a–b)). To further characterize this difference, we performed IB4, and CGRP double immuno-labeling to differentially count the number of neurons that were IB4 and CGRP positive (Figure 3(b–d)). In contrast to the CGRP data, there was no significant difference in the IB4-labeled population (p=0.2798) between these animal groups (Figure 3c). We did, however, determine that the numbers of double-positive neurons (IB4 & CGRP) were significantly increased in tumor-bearing mice, Figure 3d. To verify if there is the same pattern of expression in the spinal cord as in the peripheral afferent pathway, we performed the same double-labeling on spinal cord sections. The projections from peripheral CGRP-positive cells in the DRG to the superficial dorsal horn had an increase of approximately 20% in the laminae I & II regions (Figure 3(e & f)). Still, we observed no change in IB4-labeled cells. Taken together, these data suggest that increased CGRP-positivity in the DRG and spinal cord might be a specific response to B6 Hi-Myc injection and engraftment.
Figure 3: Prostate cancer bone lesions are associated with increased CGRP-positive neurons in the DRG.
(a) Representative images of saline (top) and B6 Hi-Myc-cell (bottom) injected ipsilateral DRG sections with CGRP-positive neurons shown in red, IB4-labeled neuronal population in green and DAPI nuclear staining in blue. Co-localization is shown as yellow puncta. Quantitation by differential counting of IB4-, CGRP-, and dual-labeled cells is shown in graphs (b-d). Values are expressed as mean ± SD, *p<0.05, **p<0.01. (e) Representative images showing primary afferent neurons with CGRP (red), IB4 (green), and DAPI nuclear stain in blue on superficial dorsal horn sections of the ipsilateral spinal cord. (f) Quantitative analysis of immunoreactivity of CGRP and IB4 labeling. Values were expressed as mean ± SD, **p<0.01.
CGRP-positive neurons show increased p75NTR expression in the DRG and superficial dorsal horn of mice with bone pain
p75NTR is the low-affinity receptor of NGF and has been shown to play a key role in nociceptive input in multiple inflammatory and neuropathic pain models and clinical conditions. To assess whether p75NTR contributes to bone metastasis-associated nociceptive input, transmission, and signal integration, we examined expression, distribution and cell type-specific colocalization of p75NTR by immunofluorescent staining and confocal microscopy. Immunoreactivity was measured based on expression in CGRP-positive, IB4-positive and IB4 and CGRP double negative cells. We demonstrated that enhanced p75NTR immunoreactivity was in CGRP-positive cells without exception (Figure 4a & b). To verify whether the same could be shown in the superficial dorsal horn, we also performed the same analyses on spinal cord sections. Enhanced p75NTR immunoreactivity was found in the same regions that the CGRP-positive cells projected into, specifically laminae I & IIouter (Figure 4c). Further colocalization analyses using high magnification of confocal images (x 240) revealed that p75NTR only colocalized with CGRP-labeled terminals, but not with IB4 labeled processes in both laminae I & II (rt= 0.766) (Figure 4d–f).
Figure 4: CGRP-positive neurons show increased p75NTR expression in the DRG and superficial dorsal horn of mice with bone cancer-associated pain.
(a) Representative images of cell type-specific expression and differential immunoreactivity of p75NTR (red) in ipsilateral DRG of saline (top left panel) and B6 Hi-Myc-cell injected (bottom left panel) animal at L2-L4 level. p75NTR colocalization with CGRP-positive cells is shown in white and IB4-labeled in green. Images of higher magnification (right panel) from the insets of the middle panel in control (top) and B6 Hi-Myc (bottom) show the distribution and differential immunoreactivities of p75NTR. Asterisk (*) in the right panel indicates IB4-labeled neurons and symbol (#) IB4/CGRP double negative neurons. Dapi (blue), scale bar=50μm. (b) Quantitative analysis of p75NTR immunoreactivity intensity based on individual cells of IB4/CGRP double negative, IB4-positive, and CGRP-positive. Values were expressed as mean ± SD, ****p<0.0001. (c) Representative images of p75NTR (red) immunoreactivity in the ipsilateral dorsal horn in saline control and B6 Hi-Myc cell-bearing mice 2 months post-injection. Cyan dotted lines are marked as the border between laminae I/IIouter (presented as “LI/II-outer”) and laminae IIinner (presented as “LII-inner”) in the superficial dorsal horn of the spinal cord. The images corresponding to p75NTR immunoreactivity are shown in bottom panels. Dapi (blue), scale bar=100μm. (d) The higher power images show the differential p75NTR (red) immunoreactivity intensity and the colocalization information between p75NTR and IB4-labeled (green) and CGRP-immunoreactive (white) punctum respectively in saline control (left panel) and B6 Hi-Myc cell-bearing mice (right panel). Cyan dotted lines are presented as the border between LI/II-outer and LII-inner. Dapi is shown in blue, scale bar=5μm. (e) The higher magnification from the inset in (d) shows the colocalization between p75NTR and CGRP (cyan arrowhead) and IB4 labeling (yellow arrow), respectively. Note that several condensate punctum colocalizing with IB4-labeling in different sizes of purple circles are reviewed as CGRP-positive. Scale bar=1μm. (f) Colocalization analysis between p75NTR and CGRP or IB4-labeling signal. Pearson’s correlation coefficient, p75NTR to CGRP on the left panel: Rt=0.766.
Prostate cancer bone pain induced neuronal NGF and TrkA expression in neurons of the superficial dorsal horn
NGF is crucial for neurite growth, differentiation, and survival during the development of the nervous system. To mediate these effects, NGF acts as the main ligand of p75NTR and another high-affinity receptor involved in nociception, TrkA. To evaluate whether these factors were also increased in the superficial dorsal horn, spinal cord sections from the L3–5 levels were immunolabeled with antibodies against CGRP and NGF or IB4 and TrkA. We observed increased expression of both NGF and TrkA in B6 Hi-Myc-bearing mice compared to control animals. Strikingly, unlike p75NTR, the increased NGF and TrkA signals did not colocalize with either CGRP- or IB4-labeled primary afferent terminals but were observed in local neurons of the dorsal spinal cord (Figure 5a & b). It is also noteworthy that almost all NGF-immuno-reactive puncta greater than 1–2μm are seen in the cytoplasm around the soma of neurons in the superficial dorsal horn and are not in the projected CGRP-labeled fibers.
Figure 5: Prostate cancer bone lesion-associated pain induced neuronal NGF and TrkA expression in neurons of the superficial dorsal horn.
(a) Representative images show NGF (red) and CGRP (green) immunoreactivities in the ipsilateral superficial dorsal horn in saline control (left panel) and B6 Hi-Myc-bearing (right panel) animals 2 months post-injection. In low power images (top panel), the dash lines are the border between LI/II-outer and LII-inner. Scale bar=50μm. Higher magnification (bottom panel) shows that the majority of enhanced NGF immune-reactive punctum with different sizes distribute in the whole dorsal horn. (b) Representative images of the distribution and intensity of TrkA (red) immunoreactivity in the ipsilateral superficial dorsal horn in saline control (left panel) and B6 Hi-Myc-bearing animals (right panel). IB4 (green) is used for labeling non-peptide primary afferent neurons and their fibers. Scale bar=5μm.
Discussion
Current models of prostate cancer-induced bone metastases and pain primarily utilize immunodeficient animals 4. With the potential of new immune modulation therapies, there is a critical need for modeling bone pain in an immune competent background. The data we present here details for the first time a novel immunocompetent model of prostate-cancer metastasis of the bone and the neurological contribution to chronic pain. Our study demonstrates that injection of B6 Hi-Myc cells into the femur allows for engraftment and resultant bone pathology. In contrast to most immunodeficient models, we do not observe a large space occupying tumor mass that obliterates the medullary cavity or causes dramatic morphological changes. We observed the presence of distributed cancer cells in the femur medulla and foci of clonally expanded cells that adjoin the cortical bone. We hypothesize that the immunocompetent environment may be responsible for limiting the outgrowth of the tumor 44. Despite the distributed nature of the tumor, μCT and histology of bone tissue in the B6 Hi-Myc instilled mice shows osteoblastic and osteolytic changes, including new bone formation and extensive loss of trabecular bone. These are features commonly associated with the development of metastatic bone cancer 45–48 These first analyses serve to demonstrate that intra-femoral injection of B6 Hi-Myc in C57BL/6 mice leads to successful cancer cell engraftment and associated bone pathology.
Bone metastasis has a profound impact on patient quality of life, which is characterized by excruciating chronic pain, mobility issues, and altered behavioral/emotional responses 2. In order to demonstrate that experimental CIBP simulates this phenotype, we first evaluated mechanical allodynia by von Frey filament-induced withdrawal responses. Two days following injection, both saline and tumor-cell injected animals show similar responses, indicative of the induction of acute pain following surgery. This pain is quickly resolved in the saline cohort of animals, whereas tumor-bearing mice show significantly decreased withdrawal thresholds from 10 days post-surgery until the experimental endpoint at 90 days. Assessing behavioral changes in animals that mirror the depression/anxiety associated with chronic pain in humans is difficult, but taken together with the mechanical allodynia data forms a robust picture of the detrimental effects of chronic pain. Here, at 8-weeks post-injection we demonstrate that tumor-bearing mice have altered locomotor movement capacity and altered gait dynamics that are limited to the ipsilateral side of the animal. Our behavioral findings of emotional alterations by decreased movement in both Open Field and Elevated Zero-maze tests demonstrate that tumor-bearing mice showed depression/anxiety-like responses. We considered whether these changes in gait and behavior may be due to large tumor masses affecting physiological function. However, our review of tumor pathology (Fig. 1) does not support the existence of gross tissue changes in the model sufficient to impact movement or behavior. This can be evidenced in free movement of tumor-injected animals in the DigiGait analysis (Supplementary Fig. 1). We therefore conclude that the presence of altered withdrawal thresholds and significantly reduced gait dynamics indicate the response to chronic pain and closely recapitulates the symptomology observed in human patients. The presence of depression/anxiety like behavior could be attributed to the chronic pain as well as the potential lack of mobility associated with the presence of pain in this model.
To further understand and characterize the neural mechanisms associated with CIBP, we first evaluated the status of nociceptive neurons in ipsilateral DRG at the L3–5 level. We interrogated our model by examining two nociceptive neuron-related markers, IB4 and CGRP49–51, to identify CIBP-associated subsets of primary sensory neurons. The number of CGRP+ peptidergic neurons was increased but we observed no change in the IB4-labeled nonpeptidergic population. CGRP is a 37-amino acid neuropeptide detected in multiple regions innervated by the peripheral and central nervous systems and its widespread expression is indicative of diverse regulatory activity 52. Notably, CGRP has been shown to play important roles in different pain conditions and pain models such as migraine, osteoarthritis, neuropathic pain, inflammatory pain and cancer bone pain where it has been shown to promote peripheral and central afferent sensitization and nociceptive input 8,53–59. Extending this finding, we demonstrate an increase in CGRP+ neurons projecting central processes/terminals into the superficial dorsal horn in CIBP.
Previous publications have shown that CGRP expression in a subset of primary afferent neurons is modulated by the signaling of nerve growth factor via the low-affinity receptor p75NTR 60–63. Under the condition of noxious stimuli-induced nociceptive sensitization, CGRP expression in the primary nociceptors is regulated by enhanced p75NTR. Takahashi et al., administered anti-p75NTR neutralizing antibody to a punctured IVD injury model and determined that blocking p75NTR suppresses CGRP in primary sensory neurons 64. It is worth noting that our data indicated that p75NTR expression increased in both L3–5 DRG and the spinal dorsal horn, moreover the increased p75NTR appears to be mainly in CGRP-positive neurons and their projection in the dorsal horn. In the DRG, mice-bearing tumor cells showed a 25% higher p75NTR immunoreactivity than control, but IB4-labeled and IB4 & CGRP double negative neurons do not. Colocalization analysis demonstrated that p75NTR only colocalizes with CGRP-positive projections in laminae I/II of the superficial dorsal horn (Rt=0.776). Our data implies that p75NTR plays a key role in mediating pain responses in the immunocompetent CIBP model in this study.
NGF is widely known to control the growth and survival of peripheral sensory and sympathetic neurons 65. A naturally occurring point mutation in hereditary sensory and autonomic neuropathy (HSAN V) patients, NGFR100W, retains the ability to bind and signal through TrkA receptors and supports the neurotropic effect of primary sensory neurons but fails to bind or activate p75NTR. This results in attenuated TrkA-mediated acute sensitization when compared to wtNGF 66. In vivo, bradykinin, one of the most powerful endogenous pain-producing substances, is released following tissue injury. p75NTR markedly increased NGF-induced expression of endogenous bradykinin binding sites on sensory neurons 67. Saporin, a toxin used to destroy p75NTR, has been shown to suppress the expression of CGRP and attenuate nociceptive input in a puncture injury-induced discogenic pain model64. These reports demonstrated the application of p75NTR inhibition in multiple injury or pain-related models and showed that blocking p75NTR reduced CGRP expression, mechanical hyperalgesia, and peripheral and central nociceptor sensitization 61,68–71. Alternately, elevated levels of NGF 72 or direct application of NGF or NGF expression constructs in pain models can induce hyperalgesia, peripheral and central nociceptor sensitization, CGRP expression, and afferent process extension73–78. In our immunocompetent prostate cancer model, the fact that neuronal p75NTR and CGRP highly colocalize in common nociceptors suggests a role in nociceptive input, synaptic transmission, and sensitization in CIBP.
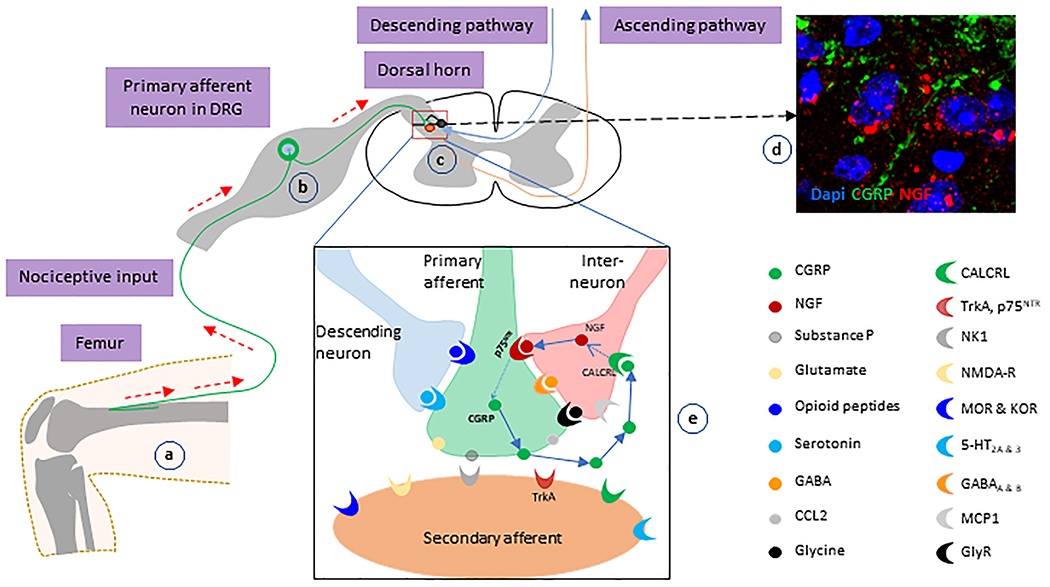
We also measured the immunoreactivities of NGF and TrkA in the ipsilateral superficial dorsal horn and found both NGF and TrkA to be increased. Unexpectedly, we observed no colocalization of p75NTR with either NGF or TrkA in the spinal dorsal horn. NGF and TrkA were expressed in the local neurons or other cells, rather than primary projected processes from the DRG. In a stressed-induced mouse depression model, CGRP has been shown to promote NGF mRNA expression 79. Our data suggest that there is a strong activation of the nociception-associated regulatory circuit in our immunocompetent CIBP model. We hypothesize that, in the metastatic bone microenvironment, elevated expression of p75NTR is induced in primary afferents which then initiates a “supra-vicious cycle” (Figure 6). As the first step, a number of nociceptive or noxious factors such as CCL2, IL-6, TNF, NGF, etc., are released from the metastatic bone environment, which mediate peripheral afferent sensitization. As the sensitization progresses, p75NTR expression is up-regulated and either alone or in combination with other signaling pathways promotes CGRP expression in primary afferent neurons. Next, CGRP is released from the primary terminals projecting to the dorsal spinal cord, where it induces NGF-expression in the local neurons in the superficial dorsal horn to form a “supra-vicious cycle.” We hypothesize that unchecked activation of this positive feedback loop results in CIBP that becomes persistent and refractory.
Figure 6: Schematic model of the involvement of NGF-p75NTR-CGRP-associated “supra-vicious cycle” in CIBP.
(a) Noxious factors released from the metastatic cancer-bone environment initiates the sensitization of peripheral afferents and the transduction of nociception. (b and c) CGRP-positive primary afferents conduct the nociceptive inputs to the second-ordered neurons in dorsal spinal cord. (d) In the dorsal spinal cord, CGRP-positive primary afferents project to the region of the local neurons-expressing NGF. (e) CGRP released from the projected terminals binds to its cognate receptors on local inter-neurons, and up-regulate NGF expression. Then, NGF stimulates CGRP expression in projected primary afferents via p75NTR. Finally, nociceptive transmission in the central circuits is elevated.
Conclusions
In this study, we established an immunocompetent prostate cancer bone pain model that induced bone morphological changes and mirrored symptoms typically reported by patients with pain. Elevated p75NTR and CGRP in primary afferent processes and increased expressions of NGF and TrkA in superficial dorsal spinal cord indicate that cross-talk and reciprocal regulation between these pain-related molecules may play essential roles in the pathophysiology of CIBP. Further study of neuro-immune regulation of CIBP may benefit the development of novel analgesic strategies for CIBP.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Brian Simons for the gift of the B6 Hi-Myc cell line. Funding for the study was from internal gifts and the U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases R01DK083609 and R01DK108127 (PT).
Footnotes
Additional information/Disclosure: There are no competing interests associated with this paper. All authors declare that they have no disclosures or competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Mantyh PW. Bone cancer pain: from mechanism to therapy. Curr Opin Support Palliat Care. 2014;8(2):83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman RE. Management of bone metastases. Oncologist. 2000;5(6):463–470. [DOI] [PubMed] [Google Scholar]
- 4.Berish RB, Ali AN, Telmer PG, Ronald JA, Leong HS. Translational models of prostate cancer bone metastasis. Nature Reviews Urology. 2018;15(7):403–421. [DOI] [PubMed] [Google Scholar]
- 5.Colvin L, Fallon M. Challenges in cancer pain management--bone pain. Eur J Cancer. 2008;44(8):1083–1090. [DOI] [PubMed] [Google Scholar]
- 6.Laird B, Colvin L, Fallon M. Management of cancer pain: basic principles and neuropathic cancer pain. Eur J Cancer. 2008;44(8):1078–1082. [DOI] [PubMed] [Google Scholar]
- 7.Clohisy DR, Mantyh PW. Bone cancer pain. Cancer. 2003;97(3 Suppl):866–873. [DOI] [PubMed] [Google Scholar]
- 8.Iyengar S, Ossipov MH, Johnson KW. The role of CGRP in peripheral and central pain mechanisms including migraine. Pain. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benemei S, Nicoletti P, Capone JG, Geppetti P. CGRP receptors in the control of pain and inflammation. Curr Opin Pharmacol. 2009;9(1):9–14. [DOI] [PubMed] [Google Scholar]
- 10.Pezet S [Neurotrophins and pain]. Biol Aujourdhui. 2014;208(1):21–29. [DOI] [PubMed] [Google Scholar]
- 11.Heppenstall PA, Lewin GR. Neurotrophins, nociceptors and pain. Curr Opin Anaesthesiol. 2000;13(5):573–576. [DOI] [PubMed] [Google Scholar]
- 12.Lewin GR, Ritter AM, Mendell LM. Nerve Growth-Factor Induced Hyperalgesia in the Neonatal and Adult-Rat. J Neurosci. 1993;13(5):2136–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melo-Carrillo A, Noseda R, Nir RR, et al. Selective Inhibition of Trigeminovascular Neurons by Fremanezumab: A Humanized Monoclonal Anti-CGRP Antibody. J Neurosci. 2017;37(30):7149–7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mason BN, Kaiser EA, Kuburas A, et al. Induction of Migraine-Like Photophobic Behavior in Mice by Both Peripheral and Central CGRP Mechanisms. J Neurosci. 2017;37(1):204–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Arco M, Giniatullin R, Simonetti M, et al. Neutralization of nerve growth factor induces plasticity of ATP-sensitive P2X3 receptors of nociceptive trigeminal ganglion neurons. J Neurosci. 2007;27(31):8190–8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirsch S, Corradini L, Just S, Arndt K, Doods H. The CGRP receptor antagonist BIBN4096BS peripherally alleviates inflammatory pain in rats. Pain. 2013;154(5):700–707. [DOI] [PubMed] [Google Scholar]
- 17.Menard DP, van Rossum D, Kar S, et al. A calcitonin gene-related peptide receptor antagonist prevents the development of tolerance to spinal morphine analgesia. J Neurosci. 1996;16(7):2342–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheppudira BP, Trevino AV, Petz LN, Christy RJ, Clifford JL. Anti-nerve growth factor antibody attenuates chronic morphine treatment-induced tolerance in the rat. BMC Anesthesiol. 2016;16(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boué J, Blanpied C, Djata-Cabral M, Pelletier L, Vergnolle N, Dietrich G. Immune conditions associated with CD4+ T effector-induced opioid release and analgesia. Pain. 2012;153(2):485–493. [DOI] [PubMed] [Google Scholar]
- 20.Machelska H Control of neuropathic pain by immune cells and opioids. CNS Neurol Disord Drug Targets. 2011;10(5):559–570. [DOI] [PubMed] [Google Scholar]
- 21.Labuz D, Schreiter A, Schmidt Y, Brack A, Machelska H. T lymphocytes containing beta-endorphin ameliorate mechanical hypersensitivity following nerve injury. Brain Behav Immun. 2010;24(7):1045–1053. [DOI] [PubMed] [Google Scholar]
- 22.Labuz D, Schmidt Y, Schreiter A, Rittner HL, Mousa SA, Machelska H. Immune cell-derived opioids protect against neuropathic pain in mice. J Clin Invest. 2009;119(2):278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baddack-Werncke U, Busch-Dienstfertig M, Gonzalez-Rodriguez S, et al. Cytotoxic T cells modulate inflammation and endogenous opioid analgesia in chronic arthritis. J Neuroinflammation. 2017;14(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lane NE, Schnitzer TJ, Birbara CA, et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med. 2010;363(16):1521–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vega JA, Garcia-Suarez O, Hannestad J, Perez-Perez M, Germana A. Neurotrophins and the immune system. J Anat. 2003;203(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caroleo MC, Costa N, Bracci-Laudiero L, Aloe L. Human monocyte/macrophages activate by exposure to LPS overexpress NGF and NGF receptors. J Neuroimmunol. 2001;113(2):193–201. [DOI] [PubMed] [Google Scholar]
- 27.Leon A, Buriani A, Dal Toso R, et al. Mast cells synthesize, store, and release nerve growth factor. Proc Natl Acad Sci U S A. 1994;91(9):3739–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark AK, Old EA, Malcangio M. Neuropathic pain and cytokines: current perspectives. J Pain Res. 2013;6:803–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller RE, Miller RJ, Malfait AM. Osteoarthritis joint pain: the cytokine connection. Cytokine. 2014;70(2):185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol. 2009(194):417–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med. 2010;16(11):1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Austin PJ, Moalem-Taylor G. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol. 2010;229(1–2):26–50. [DOI] [PubMed] [Google Scholar]
- 33.Thacker MA, Clark AK, Marchand F, McMahon SB. Pathophysiology of peripheral neuropathic pain: immune cells and molecules. Anesth Analg. 2007;105(3):838–847. [DOI] [PubMed] [Google Scholar]
- 34.Barakat DJ, Suresh R, Barberi T, Pienta KJ, Simons BW, Friedman AD. Absence of myeloid Klf4 reduces prostate cancer growth with pro-atherosclerotic activation of tumor myeloid cells and infiltration of CD8 T cells. PLoS One. 2018;13(1):e0191188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simons BW, Kothari V, Benzon B, et al. A mouse model of prostate cancer bone metastasis in a syngeneic immunocompetent host. Oncotarget. 2019;10(64):6845–6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Honore P, Luger NM, Sabino MAC, et al. Osteoprotegerin blocks bone cancer-induced skeletal destruction, skeletal pain and pain-related neurochemical reorganization of the spinal cord. Nature Medicine. 2000;6(5):521–528. [DOI] [PubMed] [Google Scholar]
- 37.Godebu E, Muldong M, Strasner A, et al. PCSD1, a new patient-derived model of bone metastatic prostate cancer, is castrate-resistant in the bone-niche. Journal of translational medicine. 2014;12:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ono H, Nakamura A, Kanemasa T, Sakaguchi G, Shinohara S. Effect of estrogen on morphine- and oxycodone-induced antinociception in a female femur bone cancer pain model. Eur J Pharmacol. 2016;773:1–12. [DOI] [PubMed] [Google Scholar]
- 39.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. [DOI] [PubMed] [Google Scholar]
- 40.Hampton TG, Stasko MR, Kale A, Amende I, Costa AC. Gait dynamics in trisomic mice: quantitative neurological traits of Down syndrome. Physiol Behav. 2004;82(2–3):381–389. [DOI] [PubMed] [Google Scholar]
- 41.Heisler LK, Chu HM, Brennan TJ, et al. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci U S A. 1998;95(25):15049–15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao L, Zhang S, Gu J, et al. Deletion of Runx2 in Articular Chondrocytes Decelerates the Progression of DMM-Induced Osteoarthritis in Adult Mice. Sci Rep. 2017;7(1):2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sabsovich I, Wei T, Guo TZ, et al. Effect of anti-NGF antibodies in a rat tibia fracture model of complex regional pain syndrome type I. Pain. 2008;138(1):47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nature Reviews Cancer. 2011;11(6):411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sevcik MA, Luger NM, Mach DB, et al. Bone cancer pain: the effects of the bisphosphonate alendronate on pain, skeletal remodeling, tumor growth and tumor necrosis. Pain. 2004;111(1–2):169–180. [DOI] [PubMed] [Google Scholar]
- 46.Chirgwin JM, Mohammad KS, Guise TA. Tumor-bone cellular interactions in skeletal metastases. J Musculoskelet Neuronal Interact. 2004;4(3):308–318. [PubMed] [Google Scholar]
- 47.Schirrmeister H Detection of bone metastases in breast cancer by positron emission tomography. Radiol Clin North Am. 2007;45(4):669–676, vi. [DOI] [PubMed] [Google Scholar]
- 48.Honore P, Mantyh PW. Bone cancer pain: from mechanism to model to therapy. Pain Med. 2000;1(4):303–309. [DOI] [PubMed] [Google Scholar]
- 49.Han L, Ma C, Liu Q, et al. A subpopulation of nociceptors specifically linked to itch. Nature neuroscience. 2013;16(2):174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nature Reviews Neuroscience. 2001;2(2):83–91. [DOI] [PubMed] [Google Scholar]
- 51.McCoy ES, Taylor-Blake B, Street SE, Pribisko AL, Zheng J, Zylka MJ. Peptidergic CGRPalpha primary sensory neurons encode heat and itch and tonically suppress sensitivity to cold. Neuron. 2013;78(1):138–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenfeld MG, Mermod J-J, Amara SG, et al. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature. 1983;304:129. [DOI] [PubMed] [Google Scholar]
- 53.de Prado BM, Hammond DL, Russo AF. Genetic Enhancement of Calcitonin Gene-Related Peptide-Induced Central Sensitization to Mechanical Stimuli in Mice. J Pain. 2009;10(9):992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cornelison LE, Hawkins JL, Durham PL. Elevated Levels of Calcitonin Gene-Related Peptide in Upper Spinal Cord Promotes Sensitization of Primary Trigeminal Nociceptive Neurons. Neuroscience. 2016;339:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu P, Van Slambrouck C, Berti-Mattera L, Hall AK. Activin induces tactile allodynia and increases calcitonin gene-related peptide after peripheral inflammation. J Neurosci. 2005;25(40):9227–9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu T, Calvo L, Anta B, et al. In vivo regulation of NGF-mediated functions by Nedd4–2 ubiquitination of TrkA. J Neurosci. 2014;34(17):6098–6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Staton PC, Wilson AW, Bountra C, Chessell LP, Day NC. Changes in dorsal root ganglion CGRP expression in a chronic inflammatory model of the rat knee joint: Differential modulation by rofecoxib and paracetamol. Eur J Pain. 2007;11(3):283–289. [DOI] [PubMed] [Google Scholar]
- 58.Ma WY, Ramer MS, Bisby MA. Increased calcitonin gene-related peptide immunoreactivity in gracile nucleus after partial sciatic nerve injury: Age-dependent and originating from spared sensory neurons. Exp Neurol. 1999;159(2):459–473. [DOI] [PubMed] [Google Scholar]
- 59.Benschop RJ, Collins EC, Darling RJ, et al. Development of a novel antibody to calcitonin gene-related peptide for the treatment of osteoarthritis-related pain. Osteoarthritis Cartilage. 2014;22(4):578–585. [DOI] [PubMed] [Google Scholar]
- 60.Sugiura A, Ohtori S, Yamashita M, et al. Effect of applying p75NTR saporin to a punctured intervertebral disc on calcitonin gene-related peptide expression in rat dorsal root ganglion neurons. J Orthop Sci. 2010;15(3):407–413. [DOI] [PubMed] [Google Scholar]
- 61.Orita S, Ohtori S, Nagata M, et al. Inhibiting Nerve Growth Factor or Its Receptors Downregulates Calcitonin Gene-Related Peptide Expression in Rat Lumbar Dorsal Root Ganglia Innervating Injured Intervertebral Discs. J Orthop Res. 2010;28(12):1614–1620. [DOI] [PubMed] [Google Scholar]
- 62.Iwakura N, Ohtori S, Orita S, Yamashita M, Takahashi K, Kuniyoshi K. Role of Low-Affinity Nerve Growth Factor Receptor Inhibitory Antibody in Reducing Pain Behavior and Calcitonin Gene-Related Peptide Expression in a Rat Model of Wrist Joint Inflammatory Pain. J Hand Surg-Am. 2010;35a(2):267–273. [DOI] [PubMed] [Google Scholar]
- 63.Hannila SS, Kawaja MD. Nerve growth factor-mediated collateral sprouting of central sensory axons into deafferentated regions of the dorsal horn is enhanced in the absence of the p75 neurotrophin receptor. J Comp Neurol. 2005;486(4):331–343. [DOI] [PubMed] [Google Scholar]
- 64.Sugiura A, Ohtori S, Yamashita M, et al. Effect of applying p75NTR saporin to a punctured intervertebral disc on calcitonin gene-related peptide expression in rat dorsal root ganglion neurons. J Orthop Sci. 2010;15(3):407–413. [DOI] [PubMed] [Google Scholar]
- 65.Levi-Montalcini R, Hamburger V. Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J Exp Zool. 1951;116(2):321–361. [DOI] [PubMed] [Google Scholar]
- 66.Sung K, Ferrari LF, Yang W, et al. Swedish Nerve Growth Factor Mutation (NGF(R100W)) Defines a Role for TrkA and p75(NTR) in Nociception. J Neurosci. 2018.
- 67.Petersen M, Segond von Banchet G, Heppelmann B, Koltzenburg M. Nerve growth factor regulates the expression of bradykinin binding sites on adult sensory neurons via the neurotrophin receptor p75. Neuroscience. 1998;83(1):161–168. [DOI] [PubMed] [Google Scholar]
- 68.Iwakura N, Ohtori S, Orita S, Yamashita M, Takahashi K, Kuniyoshi K. Role of low-affinity nerve growth factor receptor inhibitory antibody in reducing pain behavior and calcitonin gene-related Peptide expression in a rat model of wrist joint inflammatory pain. J Hand Surg Am. 2010;35(2):267–273. [DOI] [PubMed] [Google Scholar]
- 69.Zhang YH, Nicol GD. NGF-mediated sensitization of the excitability of rat sensory neurons is prevented by a blocking antibody to the p75 neurotrophin receptor. Neurosci Lett. 2004;366(2):187–192. [DOI] [PubMed] [Google Scholar]
- 70.Watanabe T, Ito T, Inoue G, et al. The p75 receptor is associated with inflammatory thermal hypersensitivity. J Neurosci Res. 2008;86(16):3566–3574. [DOI] [PubMed] [Google Scholar]
- 71.Fukui Y, Ohtori S, Yamashita M, et al. Low affinity NGF receptor (p75 neurotrophin receptor) inhibitory antibody reduces pain behavior and CGRP expression in DRG in the mouse sciatic nerve crush model. J Orthop Res. 2010;28(3):279–283. [DOI] [PubMed] [Google Scholar]
- 72.Lanlua P, Decorti F, Gangula PRR, Chung K, Taglialatela G, Yallampalli C. Female steroid hormones modulate receptors for nerve growth factor in rat dorsal root ganglia. Biol Reprod. 2001;64(1):331–338. [DOI] [PubMed] [Google Scholar]
- 73.Tang XQ, Cai J, Nelson KD, Peng XJ, Smith GM. Functional repair after dorsal root rhizotomy using nerve conduits and neurotrophic molecules. Eur J Neurosci. 2004;20(5):1211–1218. [DOI] [PubMed] [Google Scholar]
- 74.Cameron AA, Smith GM, Randall DC, Brown DR, Rabchevsky AG. Genetic manipulation of intraspinal plasticity after spinal cord injury alters the severity of autonomic dysreflexia. J Neurosci. 2006;26(11):2923–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chaudhry N, de Silva U, Smith GM. Cell adhesion molecule L1 modulates nerve-growth-factor-induced CGRP-IR fiber sprouting. Exp Neurol. 2006;202(1):238–249. [DOI] [PubMed] [Google Scholar]
- 76.Tang XQ, Heron P, Mashburn C, Smith GM. Targeting sensory axon regeneration in adult spinal cord. J Neurosci. 2007;27(22):6068–6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kelamangalath L, Tang X, Bezik K, Sterling N, Son YJ, Smith GM. Neurotrophin selectivity in organizing topographic regeneration of nociceptive afferents. Exp Neurol. 2015;271:262–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin CL, Heron P, Hamann SR, Smith GM. Functional distinction between NGF-mediated plasticity and regeneration of nociceptive axons within the spinal cord. Neuroscience. 2014;272:76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hashikawa-Hobara N, Ogawa T, Sakamoto Y, et al. Calcitonin gene-related peptide pre-administration acts as a novel antidepressant in stressed mice. Sci Rep. 2015;5:12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.