BACKGROUND
Gastric cancer is the third leading cause of cancer-related mortality and the fifth most common cancer globally.1 There is clear racial and ethnic and geographic variation in disease burden worldwide with populations and regions of high and low incidence.1,2 An estimated 26,240 new cases of gastric cancer and 10,800 related deaths occur annually in the United States (US), representing 1.5% and 1.8% of all new cancer diagnoses and deaths, respectively.3 Recent estimates confirm that the incidence and mortality rates of gastric cancer are increasing in the US among some groups, including minority populations.3–5
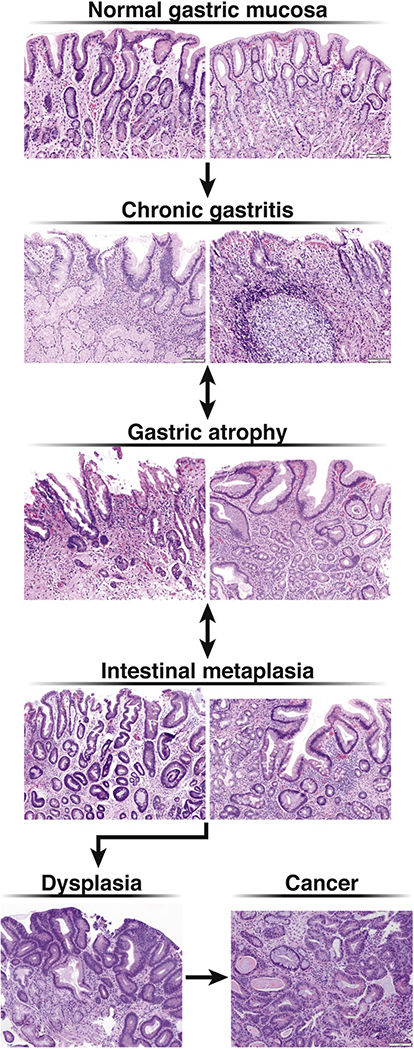
Gastric adenocarcinoma is classified by anatomic location as cardia versus noncardia and by the Lauren histologic classification as intestinal-type and diffuse-type.6 Intestinal-type noncardia gastric adenocarcinoma (NCGA) develops as a stepwise progression of discrete histopathologic stages from normal mucosa to chronic nonatrophic gastritis to chronic atrophic gastritis (AG) to gastric intestinal metaplasia (GIM) and dysplasia, prior to final malignant transformation (Figure 1). Infection with Helicobacter pylori (H. pylori) is accepted to be the primary driver for this progression, termed the Correa cascade, although other triggers such as autoimmunity, are possible.7–10 Risk factors for advancement along the Correa cascade are incompletely understood given that only a minority of individuals (~1–2%) will develop cancer.11 By contrast, no precursor lesions are definitively identified for diffuse-type gastric adenocarcinoma, although mixed intestinal- and diffuse-type histology is noted in up to 10% of patients.11 The recent NIH-funded Cancer Atlas Genome Project (TCGA) delineated molecular subtypes of gastric and confirmed both the intestinal and diffuse subtypes as distinct, as well as two lesser subtypes (EBV-associated, and Microsatellite Instability).12 AG and GIM are precancerous lesions and are associated with an increased risk of intestinal-type NCGA (hereafter referred to as “NCGA”).13 The combination of identifiable precancerous stages and stepwise neoplastic progression offers the potential opportunity for screening and surveillance with the goal of early detection of neoplasia and opportunity for endoscopic resection, to thereby reduce disease-related morbidity and mortality.
Figure 1:
Correa Cascade with histology
Current US-based guidelines do not recommend endoscopic screening for NCGA nor do they recommend universal surveillance of gastric precancerous lesions for the purpose of early NCGA detection.14GIM is usually encountered incidentally in patients undergoing esophagogastroduodenoscopy (EGD) and biopsy for nonspecific symptoms (e.g. dyspepsia). Limited awareness of risk factors for NCGA, uncertainty regarding the risk factors for neoplastic progression of GIM, and unadjudicated risk versus benefit of GIM surveillance, have resulted in wide clinical practice variability in the evaluation and management of patients with GIM.15,16 The American Society for Gastrointestinal Endoscopy (ASGE) and the European Society for Gastrointestinal Endoscopy (ESGE) have position statements on the management of gastric precancerous lesions, with the European guidelines based on a comprehensive review of the literature at the time (through November 2010) and expert consensus vote.14,17 By contrast, the ASGE position statement is limited in its recommendations on GIM management without a formal systematic review of the available literature.14 There is an unmet need for updated comprehensive guidelines for GIM management that are practical and relevant for the US population, particularly given the diversity of the population and potentially variable risk profiles for NCGA.18,19 Our aim is to provide a systematic and comprehensive synthesis of the literature to inform the AGA guideline panel in formulating evidence-based recommendations on the management of GIM in the absence of concurrent neoplasia (dysplasia or cancer), with a focus on the potential role of H. pylori eradication and endoscopic surveillance.
METHODS
Overview
The technical review team systematically synthesized the literature to inform pre-defined questions proposed by the AGA guideline panel using standard systematic review methodology. We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework to evaluate the certainty of evidence (also known as quality of evidence).20 The technical review team included a GRADE methodologist (RM), and six clinical domain experts (three gastroenterologists, one pathologist, and two gastroenterology fellows with outcomes research expertise).
The AGA guideline panel identified four clinically relevant questions and the technical review team used the PICO format (population (P), intervention (I), comparator (C), and outcomes (O)) to guide the evidence synthesis. Table 1 summarizes the PICO questions, patient important outcomes, and direct and indirect evidence needed to inform the systematic review and the AGA guideline statement. The primary objective of the first PICO question (“PICO 1”) was to synthesize the data assessing the need to empirically test for H. pylori infection (and treat if positive) in patients with GIM. The primary objectives of PICO 2 and PICO 3 were to synthesize the data informing the need for surveillance upper endoscopy once GIM is diagnosed in groups at otherwise low versus high risk for NCGA, respectively, in the US. The primary objective of PICO 4 was to synthesize the data informing the need for short-term follow up with EGD and histologic assessment in patients with GIM diagnosed incidentally on EGD.
Table 1:
PICO questions, outcomes, and evidence needed to inform PICO questions
| PICO Question | Patient-Important Outcomes | Evidence needed to inform PICO questions |
|---|---|---|
| 1. Among patients with GIM, does testing for H. pylori and treating if positive vs no testing affect outcomes? | Early cancer detection Reduced gastric cancer morbidity/mortality Endoscopy complications Costs Psychological harms |
1. Incidence and prevalence of GIM in the US population 2. Incidence of stomach cancer in the general population 3. Prevalence of concurrent stomach cancer in patients with GIM 4. Incidence of stomach cancer in patients with GIM after GIM diagnosis 5. Risk of progression to gastric cancer in patients with GIM Subgroups: Family history of gastric cancer, Race/Ethnicity, smoking status, histologic features, extent of GIM, biomarkers. 6. Potential adverse consequences of performing surveillance upper endoscopy for patients with GIM 7. Benefits of performing surveillance upper endoscopy for patients with GIM |
| 2. Among patients with GIM who are identified as low risk, does subsequent upper endoscopic surveillance vs no follow up affect outcomes? | ||
| 3. Among patients with GIM who are identified as high risk, does subsequent upper endoscopic surveillance vs no follow up affect outcomes? | ||
| 4. Among patients with GIM without dysplasia does short term upper endoscopic follow up (< 1 year) to determine the extent (using biopsies) of GIM vs no short term follow up affect outcomes? |
After finalizing the PICO questions, the technical review team and the guideline panel ranked outcomes by importance and prioritized outcomes critical for decision making (Table 1). Patient important outcomes of interest included both benefits and harms such as early NCGA detection, reduced morbidity/mortality from NCGA, complications associated with endoscopy, psychological outcomes (e.g. anxiety and stress related to endoscopic surveillance, coping with a precancerous condition), and resource implications (e.g. cost of surveillance).
A comprehensive list of direct and indirect evidence needed to inform the questions was developed (Table 1). The desired evidence included incidence and prevalence data for GIM, incidence of NCGA in individuals with GIM, and risk factors associated with progression to NCGA in patients with GIM compared to individuals without GIM. This “wish list of needed evidence” guided the systematic literature search. Given the presumed paucity of robust direct data on GIM in the US, evidence from all regions of the world was considered relevant in the evidence-gathering phase. Details related to the management and natural progression of dysplasia were considered outside the scope of this technical review, unless there was clear discernible clinical relevance to outcomes of GIM.
The Systematic Review Process
We reported the systematic review and the related meta-analyses results in concordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Meta-analysis Of Observational Studies in Epidemiology (MOOSE) statements.21,22 The technical review team scheduled weekly meetings to conduct the systematic review and develop the GRADE evidence profiles for each PICO question.20 The weekly meetings clarified and addressed issues that arose during the review process. Decisions were documented and input from the guideline panel was requested for key decisions.
Literature Search Strategy
In collaboration with a medical librarian, we defined a systematic search strategy and searched three electronic databases including Ovid MEDLINE ® Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE® from 1946; Embase Classic+Embase from 1947; and Wiley’s Cochrane Library. The initial search was conducted in July 2017 and updated in September 2018. The primary search terms included “stomach,” “precancerous conditions,” “neoplasms,” “gastric/stomach mucosa,” and “metaplasia.” The full literature search strategy is provided in Supplementary Table 1. Additionally, we checked the references of prior systematic reviews and guidance documents to identify additional studies that met our inclusion criteria.
Inclusion / Exclusion Criteria
We included randomized controlled trials (RCTs), prospective and retrospective cohort studies, case-control studies, and cross-sectional studies as long as they informed any PICO question. We excluded studies without data on GIM or if we were not able to separate the results by GIM status. We excluded studies that were not performed in humans or those that did not include primary data (e.g. narrative reviews, opinion pieces, letters). We excluded studies in pediatric only populations, studies conducted to evaluate the diagnostic accuracy of H. pylori tests, studies that compared different H. pylori treatment regimens, and studies that focused solely on gastric dysplasia or cancer without data on GIM. For studies that informed the incidence or prevalence of GIM, we included studies with at least 100 patients. After the data gathering phase, the threshold was modified to 250 patients due to the large number of studies identified in our search; prior to this modification, we performed a sensitivity analysis calculation that confirmed the unlikelihood that the prevalence estimates would be affected if smaller studies were included. For studies that informed the risk or rate of progression from GIM to NCGA, we included studies with at least 20 GIM patients.
We also obtained and searched the full texts of potentially relevant abstracts. If the abstract was published before 2015 and no accompanying publication was identified, we excluded the reference. For abstracts published in or after 2015, we contacted the authors to inquire about publication status and additional data; we excluded the reference if we received no response.
Study Screening and Selection
Two technical review committee members independently screened the search results for articles based on titles and abstracts. The full text-article was retrieved for any citation considered potentially relevant by any investigator. Each of the investigators then independently assessed the eligibility of each article by using a pilot-tested, standardized form with written instructions generated and maintained on the Research Electronic Data Capture (REDCap®) platform23 hosted at The University of Kansas. If at least one of the prespecified inclusion criteria was not met, the article was excluded. Any disagreement was resolved by consensus or arbitration.
Data Items and Definitions
Due to the vast amount of literature focused on gastric cancer and H. pylori, but not specifically GIM, it was necessary to agree upon definitions of certain concepts prior to data abstraction in order to limit confusion and disagreement. Box 1 summarizes the definition of different concepts that were used for this review.
Box 1. Definitions used throughout the process of the systematic reviews.
Global histologic progression or regression of GIM were deemed to be the more clinically relevant outcome, since scoring and staging systems are not used routinely in clinic practice and their implications are not well-defined. Similarly, we focused on incomplete versus complete as the most clinically relevant histologic classification for GIM.24–26 We acknowledge another classification system utilized predominately for research that divides GIM into three types: Type I GIM (non-secretory absorptive cells and sialomucin secreting goblet cells), Type 2 GIM (few absorptive cells, columnar cells secreting sialomucin, and goblet cells secreting mainly sialomucin but some sulphomucin), and Type 3 GIM (columnar cells secreting predominantly sulphomucin and goblet cells secreting sialomucin or sulphomucin).26 In accordance with the literature, Type I GIM was categorized as complete GIM, while Type II and Type III were categorized as incomplete GIM.26–28
Histologic Staging and Scoring Systems
Gastric histologic staging and scoring systems are primarily used in research settings, since issues such as pathologist time investment and interobserver variability in histopathologic grading (e.g. differentiating moderate versus severe) limits routine clinical use. Importantly, the systems require biopsies of both the antrum and corpus a priori. Two systems are prominent in the literature: OLGA/OLGIM and the Correa histopathology score.
The OLGA and OLGIM are histopathologic staging standards that incorporate both the severity (mild, moderate, severe) and extent (antrum/incisura, body) of AG and GIM, respectively, and range from Stage 0 to IV.29 Stage III/IV, which necessitate both antrum and corpus involvement (with the exception of Stage III, severe antral atrophy), are considered higher risk for neoplastic progression.25,30–32 These stages are derived from the semiquantitative scoring of AG or GIM on a Sydney System-compliant set of gastric biopsies, including biopsies from at least the gastric corpus and antrum. Epidemiology data as well as a recently published meta-analysis of prospective case-control studies support an increased risk of gastric cancer in OLGA/OLGIM Stage III/IV.32
The Correa histopathology score has been shown to correlate with the OLGA/OLGIM system.33 It is an ordinal system that incorporates data on GIM extent and complete/incomplete GIM status. The Correa histopathology score is useful for progression/regression analyses in cohort studies.
Categorization of Population Subgroups
We defined certain population subgroups to assess risk of developing GIM and of GIM progression. The subgroups were selected a priori based on discussion with the guidelines panel and established risk factors for NCGA. These categorical subgroups included: race and ethnicity, first-degree family history of gastric neoplasia, smoking history, concomitant autoimmune gastritis, concomitant pernicious anemia, histologic features (e.g. incomplete versus complete GIM), topographic extent of GIM (extensive versus limited), and specific biomarkers. Given the limited and highly heterogeneous biomarker data, the technical review team in consultation with the guidelines panel unanimously agreed to formally evaluate only biomarkers with potential clinical relevance; this ultimately included established H. pylori virulence factors (CagA, VacA) and pepsinogen. These determinants were used to inform risk stratification into “high risk” and “low risk” groups for PICO 3 and 4; having at least one risk factor was considered “high risk”.
Data Collection Process
We abstracted data using a separately constructed, pre-piloted and standardized form generated and maintained on the REDCap platform. The form was tailored according to study design and tailored to abstract data relevant to the PICO questions. We abstracted the following data: baseline demographics and characteristics of the patients included in the studies, the management they received, clinically relevant outcomes and outcome measures.
We assessed methodologic quality. We also abstracted data for the pre-defined subgroups above. Two investigators independently abstracted all relevant data from each included study. The results of data abstraction were then compared and any discrepancies were resolved by discussion and consensus or arbitration. When the same results were presented in more than one publication, we collated and summarized the data from all relevant publications and available time points. If results were incomplete or unclear, we contacted study authors for additional information. In cases of non-response, we included the study in the systematic review but not in the statistical pooling in the meta-analyses. Data abstraction for dysplasia or gastric cancer were beyond the scope of this technical review.
Summary Measures
For comparative binary outcomes, such as the risk of developing NCGA in patients with GIM who were treated versus not treated for H. pylori infection, we used relative risks (RR) to compare the different interventions. When the outcomes were reported as person-time, we used the incidence rate ratio (IRR). For estimating the incidence of progression from GIM to NCGA, we used the incidence rate (number of events per person-time unit) and, if not reported, the cumulative incidence (number of events over a specified period). The prevalence of GIM was reported as the proportion of patients with GIM in the population of interest (population at risk) for each study.
Statistical Analysis and Synthesis of Results
For comparative studies, we expected these to originate from heterogeneous populations and therefore used the DerSimonian-Laird random-effects model to pool the relative risks and incidence rate ratios.34 For prevalence and incidence data, we used the Freeman-Tukey transformation and then pooled the results using the inverse variance fixed-effects model.35,36 We elected to use the fixed-effects model despite our anticipation of heterogeneity between the studies, as we presumed that larger studies would likely be more representative and inclusive versus smaller studies. To assess the robustness of our model, we performed sensitivity analyses using the generalized linear mixed models and random-effects models.37 We used the I2 statistic to quantify statistical heterogeneity.38 When subgroups were presented as binary outcomes in individual studies, we pooled them as RR or IRR as appropriate. When the subgroups were presented as proportions of the population of interest, we pooled them as proportions and compared them using interaction tests.38 If sufficient studies were available for an outcome with no significant statistical heterogeneity, we assessed for publication bias using asymmetry tests and visual inspection of funnel plots.38 The statistical analyses were conducted using the package meta in R version 3.4.4.38
Risk of Bias and Quality of Evidence
We used the Cochrane Collaboration’s tool for assessing the risk of bias in RCTs.39 For comparative non-randomized observational studies, including cohort studies, case-control studies, and cross-sectional studies, we used a modified Newcastle-Ottawa Scale for quality assessment.40 For studies of prevalence or incidence, we used the pertinent Joanna Briggs Institute tool.33 We used RevMan statistical software to produce risk of bias summaries and tables.40
We used the GRADE framework to evaluate the certainty (quality) of the body of evidence for each outcome. The quality assessment is a reflection of certainty and confidence in the evidence and can be categorized as: very low, low, moderate, and high certainty. Evidence from RCTs starts as high certainty and then can be downgraded according to the risk of bias, inconsistency, imprecision, indirectness, or publication bias.41 Evidence originating from observational studies starts as low certainty, and can be downgraded according to the same domains as RCTs. The certainty of evidence from observational studies, with no concerns about study validity, can also be upgraded if there is dose-response relationship, large magnitude of effect or if all plausible confounders and bias would reduce a demonstrable effect. The quality of evidence for studies of prevalence or incidence starts as high, regardless of study design, and follows the same gradation rubric as detailed above.
RESULTS
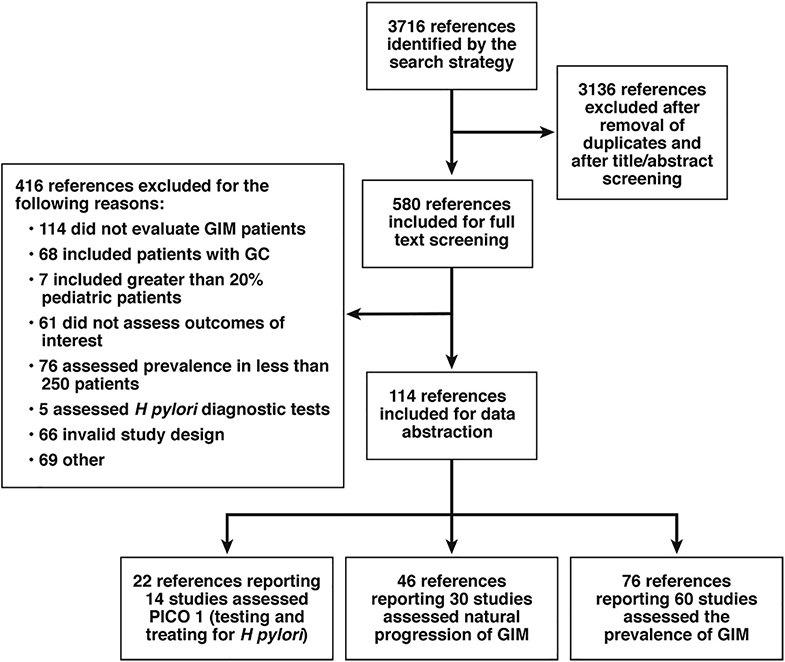
A total of 3716 articles were identified in the literature search, from which 3136 articles were excluded after removal of duplicates, conference abstracts without full text publication and title/abstract screening. The full texts of 580 articles were reviewed for eligibility. Of these, 329 studies were excluded for not meeting full inclusion criteria. Thus, we abstracted data from 121 articles. Figure 2, summarizes the PRISMA flow diagram of the studies screened and included in this review. The baseline characteristics and risk of bias of the individual studies, the results of the meta-analyses, and the quality of evidence are summarized below according to the relevant PICO question(s). Detailed risk of bias summaries for all studies are available in Supplementary Figure 1, Supplementary Figure 2, Supplementary Figure 3, Supplementary Figure 4, Supplementary Figure 5, Supplementary Figure 6.
Figure 2:
PRISMA flow diagram
PICO 1: In patients with GIM does testing and treating for H. pylori vs no testing and treating affect patient-important outcomes?
The benefit of H. pylori testing and treatment has been established and addressed previously for NCGA prevention and for the management of dyspepsia.42 The specific focus of PICO 1 was to identify direct evidence to assess the effects of testing for and treating H. pylori infection, when diagnosed, in patients with GIM when compared to a no empiric testing and treatment strategy. Among H. pylori-related studies meeting inclusion criteria, only those studies which specifically included patients with GIM were included to answer this question. Studies which assessed the effect of H. pylori treatment on the different outcomes (e.g. NCGA incidence) but did not specify the inclusion of GIM patients, were excluded to limit heterogeneity and potential for bias. We acknowledge that the analysis focuses on the efficacy of the primary intervention of an H. pylori treatment regimen versus placebo, but not necessarily H. pylori infection status over the entire time course nor at the time of the endpoint of interest. To this end, confirmation of eradication following H. pylori treatment was variably documented. Table 2 summarizes the included studies that informed PICO1.
Table 2:
Study characteristics, objectives, patients characteristics, intervention, comparison and outcomes (PICO 1)
| Study / reference | Design and Objectives | Intervention and Comparison Group | Total subjects (N), full study | Countries / (Ethnicity) | Mean Age, yrs (SD and range) | % Males | Follow up protocol and GIM-specific Outcomes |
|---|---|---|---|---|---|---|---|
| Colombian Chemoprevention Trial56–58 |
RCT To determine if treatment of H. pylori infection and/or supplementation with ascorbic acid and β-carotene prevents gastric precancerous lesion progression |
Intervention: H. Pylori treatment with or without-long term supplements of ascorbic acid and β-carotene. Comparison: Placebo |
852 | Colombia (Hispanic) | 51.1 (SD 8.5) | 46.1% | Endoscopy at 3, 6, 12, and 16 yrs with biopsies to determine the prevalence of precancerous lesions and progression to gastric cancer HP eradication confirmed: Yes |
| Shandong Intervention Trial43–45,48 |
RCT To test the effects of one-time H. pylori treatment and long-term vitamin or garlic supplements in reducing the prevalence and progression of gastric precancerous lesions. |
Intervention: H. Pylori treatment, 7 years of oral supplementation with garlic, or with a mixture of vitamin C, vitamin E, and selenium. Comparison: Placebo |
4326 | China | 47.2 Range 35–64 |
51.4% | Endoscopy with biopsies to determine the prevalence of gastric precancerous lesions and incidence of cancer at 3 and 7 yrs. HP eradication confirmed: Yes |
| Yantai County Trial49–52 |
RCT To determine if H. pylori screening and treatment improves histologic severity and decreases risk of gastric cancer progression |
Intervention: H. pylori screening and treatment if positive Comparison: Placebo |
587 | China | 52 (SD 8.1) Range (16–75) |
48% | Endoscopy with biopsies 1, 5, 10 yrs to determine who developed or died of gastric cancer HP eradication confirmed: Yes |
| Ley, 200446 | RCT To determine if H. pylorieradication is associated with regression of gastric pre-neoplastic conditions over 1 year |
Intervention:
H. pylori treatment Comparison: Placebo |
316 | Mexico (Hispanic) | Intervention: 51.0 (SD 9.2) Control: 52.0 (SD 9.8) |
36.7% | Endoscopy with biopsies at 6wks and 12 mo to assess regression vs progression of gastric precancerous lesions HP eradication confirmed: Yes |
| Wong, 200453 |
RCT To determine whether H. pylori eradication is associated with reduced gastric cancer incidence |
Intervention: H. pylori screening and treatment if positive Comparison: Placebo |
1630 | China | 42.2 (SD 9.0) | 54% | Endoscopic follow up at 7.5 yrs, to determine incidence of gastric cancer HP eradication confirmed: Yes |
| Wong 201254 |
RCT To assess whether celecoxib alone or combined with H. pylori treatment is associated with progression or regression of gastric precancerous lesions |
Intervention: H. pylori screening and treatment if positive, with or without celecoxib. Comparison: Placebo |
1024 | China | 53 (SD 6.5) | 46% | Endoscopic follow up at 5 yrs to assess change in progression/regression of gastric precancerous lesions and gastric cancer incidence HP eradication confirmed: Yes |
| Massarrat, 201255 |
RCT To evaluate histologic change and topography of precancerous lesions after H. pylori eradication |
Intervention: H. pylori testing and treatment if positive Comparison: Placebo |
521 | Iran | 47.8 Range (38–70) |
49% | Endoscopic follow up at 2.5 or 4.5 yrs to assess regression or progression of GIM HP eradication confirmed: Yes |
| Kuipers, 200447 |
RCT To determine if H. pylori eradication in patients with GERD on long-term omeprazole therapy influences gastritis progression |
Intervention: H. pylori positive patients treated omeprazole based triple therapy followed by omeprazole only Comparison: H. pylori positive patients treated with omeprazole only |
234 | Netherlands, France, UK, Denmark,Sweden, Austraila, Germany | Intervention: 61.8 (SD 12.3) Control: 62.4 (SD12.0) |
54.5% | Endoscopic follow up with biopsies at 1 and 2 yrs to determine severity and extent of gastric pathology HP eradication confirmed: Yes |
| Lee, 201359 |
Cohort To evaluate the effect of H, pylori eradication on the prevalence of gastric precancerous lesions, and secular trends in the incidence of gastric precancer and cancer. |
Intervention: H. pylori screening and treatment if positive Comparison: 1995–2003 (pre-HP eradication campaign) vs. 2004–2008 (post HP eradication campaign) |
Total 1762, 841 with endoscopic follow up | Taiwan | 49.2 (SD 12.8) | 46% | Endoscopic follow up with biopsies to determine incidence of GIM pre versus post treatment HP eradication confirmed: Yes |
| Ciok, 199790 |
Cohort To evaluate the effect of H. pylori eradication on antral GIM |
Intervention: H. pylori treatment if positive Comparison: Eradicated vs. Not eradicated |
35 | Poland | 44 Range (29–63) |
54.3% | Endoscopic follow up with biopsies at 2 yrs to determine presence and severity of antral GIM HP eradication confirmed: Yes |
| Salih, 200591 |
Cohort 1) To evaluate the effect of H. pylori and H. pylori genotypes on gastric histology 2) To evaluate the effect of H. pylori eradication on histology |
Intervention: H. pylori positive, treated Comparison: H. pylori positive, not treated |
28 | Turkey | 46 Range (24–66) |
57.1% | Endoscopic follow up with biopsies at 1 and 12 mo to determine change in average histologic score HP eradication confirmed: Yes |
| Lu, 200592 |
Cohort To evaluate the effect of H. pylori eradication on atrophic gastritis and GIM |
Intervention: H. pylori positive, treated Comparison: Untreated cohort (all HP +) |
179 | China | Range (30–74) | 45.8% | Endoscopic follow up with biopsies at 3 years_ to determine change in histology score HP eradication confirmed: Yes |
| Mansour-Ghanaei, 201360 |
Case series To determine histology change in GIM over 1 year |
Intervention: H. Pylori treatment Comparison: H Pylori eradicated vs. not eradicated after treatment |
71 | Iran | 48 (SD 12) Range (21–79) |
56% | Endoscopic follow up with biopsies at 1 year to determine progression to dysplasia in GIM HP eradication confirmed: Yes |
| Yamada, 200393 |
Cohort To evaluate the effect of H. pylori eradication on atrophic gastritis |
Intervention: H. Pylori treatment Comparison: eradicated vs. infected |
116 | Japan | Intervention: 52.6 (SD 1.2) Control: 57.1 (SD 2.1) |
61.2 | Endoscopic follow up with biopsies at 10–49 mo to determine if gastric atrophy improves HP eradication confirmed: Not reported |
Of the 121 studies included for this technical review, 22 studies informed PICO 1 (Figure 2). This included 7 RCTs and 3 cohort studies.43–55 Three large studies with multiple related publications and timepoints were included, and due to their complexity, warrant further description below:
-
Shandong Intervention Trial
This RCT was conducted in China (Linqu County) across 14 villages.43,44,48 Individuals were invited to participate in a gastric cancer screening program with EGD and gastric biopsies (antrum and corpus). Participants (N=4326) age 35–64 years were randomized in a 23 factorial design to one of three interventions: treatment for H. pylori infection vs. vitamin C/vitamin E/selenium dietary supplementation vs. garlic dietary supplementation.
-
Yantai County Trial
This RCT was conducted in 11 rural villages in Yantai County, China.49–52 Individuals age 16–75 years were invited to participate in a gastric cancer screening program with EGD and gastric biopsies (antrum and corpus). Participants (N=587) were also randomized to H. pylori treatment vs. placebo.
-
Colombia Chemoprevention Trial
This RCT included individuals (N=852) from Colombia with gastric precancerous lesions who were randomized to receive H. pylori treatment with vs. without antioxidant supplementation.56–58 At the end of six years of intervention, those who did not receive H. pylori treatment were offered treatment. After the intervention, 795 adults were followed prospectively. Endoscopy with gastric biopsies (antrum and corpus) was performed at study entry and at 3, 6, 12 and 16 years of follow up. Although the publications did not specify the number of patients with GIM in each arm, the authors provided us with the necessary data for risk estimates when we contacted them.
A summary of the pooled estimates of effect for H. pylori testing and treatment is shown in Table 3 and Figures 3–6 according to patient outcomes. These are further summarized below:
Table 3:
Evidence profile and pooled effect estimates to inform PICO 1 (H Pylori)
| Certainty Assessment | No of Patients | Effect | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other | Test/Treat for H Pylori | No Test/Treat | Relative (95% CI) | Absolute (95% CI) | Certainty | Importance |
| Relative risk of death (All-cause mortality) among patients with H. pylori infection after H. pylori treatment versus placebo (all patients, with or without GIM) | ||||||||||||
| 3 | RCTs | Not serious | Not serious | Serious2 | Serious3 | None | 180/2202 (8.2%) | 169/2199 (7.7%) | RR=1.07 (0.88–1.31) | 5 more per 1000 (from 9 fewer to 24 more) | ⊕⊕○○ Low |
Critical |
| Relative risk of gastric cancer-related mortality among patients with H. pylori infection after H pylori treatment versus placebo (all patients, with or without GIM) | ||||||||||||
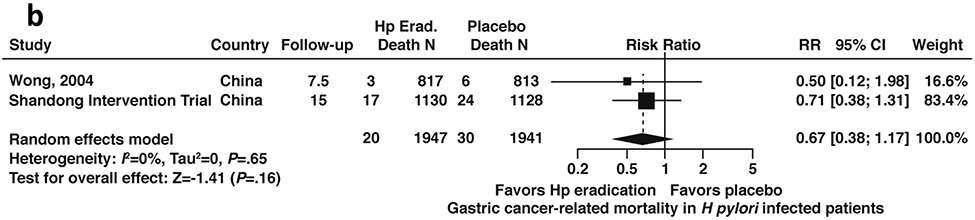
| 2 | RCTs | Not serious | Not serious | Serious2 | Serious3 | None | 20/1947 (1.0%) | 30/1941 (1.5%) | RR=0.67 (0.38–1.17) | 5 fewer per 1000 (from 3 more to 10 fewer) | ++ Low |
Critical |
| Relative risk of incident gastric cancer among patients with H. pylori infection after H pylori treatment versus placebo (all patients, with or without GIM) | ||||||||||||
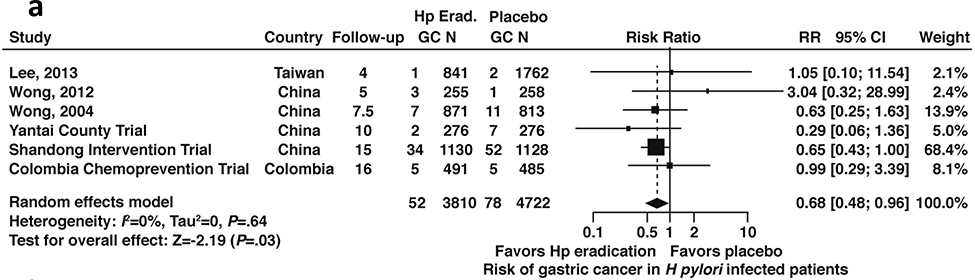
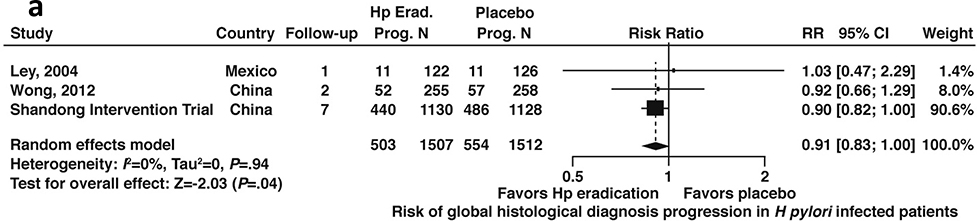
| 6 | RCTs and cohort1 | Not serious | Not serious | Serious2 | Not serious | None | 52/3810 (1.4%) | 78/4722 (1.7%) | RR=0.68 (0.48–0.96) | 6 fewer per 1000 (1 fewer to 9 fewer) | +++ Moderate |
Critical |
| Relative risk of incident gastric cancer among patients with H. pylori infection and confirmed GIM after H pylori treatment versus placebo | ||||||||||||
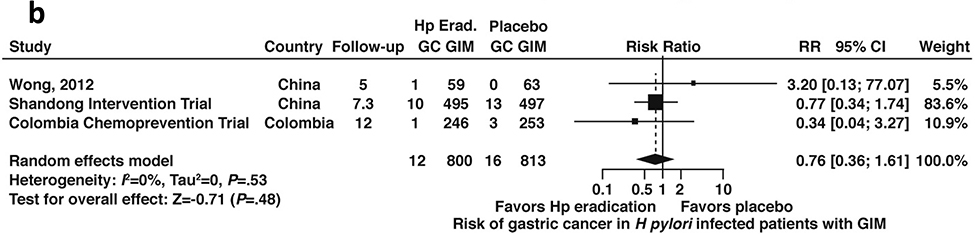
| 3 | RCTs | Not serious | Not serious | Not serious | Very serious3 | None | 12/800 (1.5%) | 16/813 (2.0%) | RR=0.76 (0.36–1.61) | 3 fewer per 1000 (from 14 fewer to 20 more) | ++ Low |
Critical |
CI: Confidence interval; RR: Risk Ratio
4 RCTs and 2 observational cohort. Results are driven by RCTs findings
Data considered in all patients, not just patients with GIM
Few events, confidence intervals are wide encompassing both considerable benefit and harm.
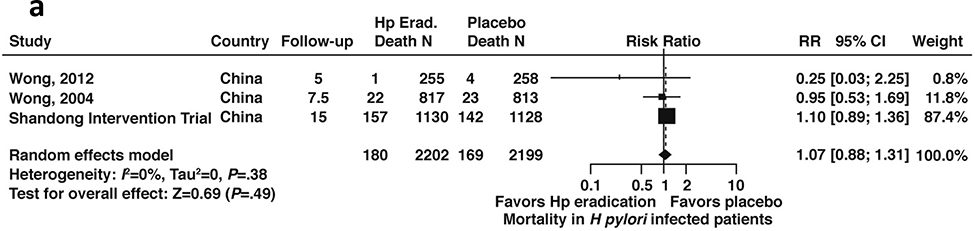
Figure 3a:
Relative risk of all-cause mortality among patients with H. pylori infection after H. pylori treatment versus placebo (all patients, with or without GIM)
Figure 6a:
Relative risk of regression to improved global histology from baseline among patients with H. pylori infection after H. pylori treatment versus placebo (all patients, with or without GIM)
Mortality (All cause and gastric cancer-related)
Among patients with H. pylori infection, including patients with or without GIM, based on data from three RCTs (N= 2199), the relative risk of all-cause mortality was 1.07; 95% CI: 0.88–1.31 for those patients who received H. pylori treatment compared to placebo) (Figure 3a, low certainty in evidence).43,44,48,53,54 Conversely, based on data derived from 2 RCTs (N=1941), among patients with H. pylori infection including patients with or without GIM, the relative risk of gastric cancer-related mortality was 0.67; 95% CI: 0.38–1.17 compared to placebo (Figure 3b, low certainty in evidence).43,44,48,53
Figure 3b:
Relative risk of gastric cancer-related mortality among patients with H. pylori infection after H. pylori treatment versus placebo (all patients, with or without GIM)
Risk of incident gastric cancer
Data were available from 6 total studies43,44,49–54,56,59 that each included patients with or without GIM (N=8,532); 3 studies43,44,48,54,56 reported results limited only to patients with GIM (N=1,613). Among patients with H. pylori infection with or without GIM, H. pylori treatment was associated with a lower risk of incident gastric cancer compared to placebo (RR 0.68, 95% CI: 0.48–0.96) (Figure 4a, moderate certainty in evidence).
Figure 4a:
Relative risk of incident gastric cancer among patients with H. pylori infection after H. pylori treatment versus placebo (all patients, with or without GIM)
Among patients with H. pylori infection and confirmed GIM, compared to placebo, H. pylori treatment trended toward a lower risk of incident gastric cancer but this was not statistically significant (RR 0.76; 95% CI: 0.36–1.61) (Figure 4b, low certainty in evidence).
Figure 4b:
Relative risk of incident gastric cancer among patients with H. pylori infection and confirmed GIM after H. pylori treatment versus placebo
Risk of progression to worse global histology (from baseline)
Data were available from 4 studies that reported discrete data on progression to worsened global histology (based on the Correa histopathology cascade) from baseline enrollment; 3 of these studies included patients with or without GIM (N=3,019)43,44,46,48,54 and 2 studies included data limited only to patients with GIM (N=1,044).43,44,48,60 Among patients with H. pylori infection with or without GIM, H. pylori treatment trended toward lower risk of histological progression, but this was not statistically significant (RR 0.91; 95%CI: 0.83–1.00) (Figure 5a, moderate certainty in evidence).
Figure 5a:
Relative risk of progression to worse global histology from baseline among patients with H. pylori infection after H. pylori treatment versus placebo (all patients, with or without GIM)
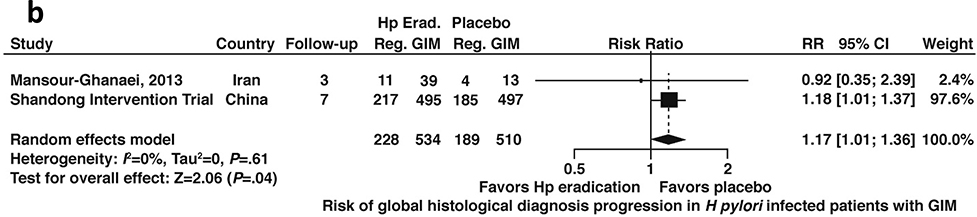
Conversely, among patients with H. pylori infection and confirmed GIM, H. pylori treatment was associated with a 17% higher risk of progression to worse histology compared to placebo (RR 1.17; 95%CI: 1.01–1.36) (Figure 5b, moderate certainty in evidence). Notably, these estimates were driven by data from the same trial, the Shandong Intervention Trial.
Figure 5b:
Relative risk of progression to worse global histology from baseline among patients with H. pylori infection and confirmed GIM after H. pylori treatment versus placebo
Risk of regression to improved global histology (from baseline)
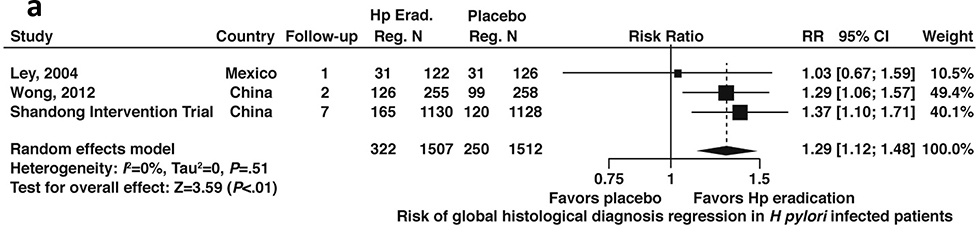
Data were available from 3 studies that reported discrete data on regression to improved global histologic diagnosis from baseline enrollment; 2 of these studies included all patients with or without GIM (N= 3,019) and 1 study included data limited only to patients with GIM (N=992).43,44,46,48,54 Among patients with H. pylori infection with or without GIM, H. pylori treatment, H. pylori treatment was associated with a higher likelihood of regression compared to placebo (RR 1.29; 95%CI: 1.12–1.48) (Figure 6a, moderate certainty in evidence).
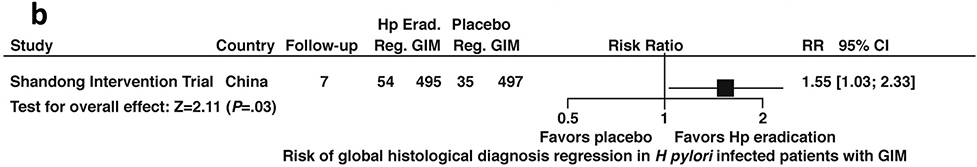
Similarly, among patients with H. pylori infection and confirmed GIM, H. pylori treatment was associated with a higher likelihood of regression compared to placebo (RR 1.55; 95%CI: 1.03–2.33) (Figure 6b, low certainty in evidence). Notably these estimates were informed by a single trial, the Shandong Intervention Trial.
Figure 6b:
Relative risk of regression to improved global histology from baseline among patients with H. pylori infection and confirmed GIM after H. pylori treatment versus placebo
Summary
The evidence profiles and effect estimates for PICO 1 were driven mainly by the three large RCTs, all of which were conducted in countries of high H. pylori prevalence and high NCGA incidence. Due to the few number of events (gastric cancer development in patients with GIM) the confidence intervals are wide. However, there were protective effects for the key outcomes of gastric cancer-related mortality (irrespective of histology) and incident gastric cancer (irrespective of histology as well as among patients confirmed to have GIM) in patients who received treatment for H. pylori infection versus placebo. It is important to acknowledge that the H. Pylori test sensitivity varies for each modality and depends both on the test itself but other factors including the use of proton-pump inhibitors and the density of H pylori colonization, for example. One recent small study found that of 19 subjects who had gastric biopsies containing H pylori DNA, only 12 tested positive for H pylori by conventional testing.61
Further analyses of surrogate outcomes such as progression to worse global histology or regression of GIM after H. pylori treatment showed inconsistent results. Specifically, among patients with GIM in a single trial, eradication of H. pylori was associated with both increased risk for progression to worse histology (RR 1.17; 95%CI: 1.01–1.36) as well as increased risk of regression to improved histology (RR 1.55; 95%CI: 1.03–2.33). These paradoxical findings leave uncertainty with regard to the potential mechanisms of the observed reduced risk for incident gastric cancer among patients with GIM, and contribute to the moderate quality of evidence to support our recommendations for H. pylori testing and eradication. One hypothesis could be that the higher risk estimate for global histologic progression among patients with GIM who receive H. pylori treatment versus placebo, which is distinct compared to the protective risk estimate among all patients irrespective of histology, supports the generally accepted theory that some patients with GIM are “past the point of no return” in the Correa cascade (Figure 1); for reasons that are poorly understood, these patients remain at risk for neoplastic progression irrespective of H. pylori infection status. For similarly poorly understood reasons, regression to improved histology does appear possible in a subset of patients with GIM.
PICO 2: In patients with GIM who are identified as low risk for NCGA, does subsequent surveillance upper endoscopy with biopsies vs no follow up affect outcomes?
PICO 3: In patients with GIM who are identified as high risk for NCGA, does subsequent surveillance upper endoscopy with biopsies vs no follow up affect patient-important outcomes?
GIM is an established precancerous precursor lesion for intestinal-type gastric adenocarcinoma; unfortunately, it is currently not possible to definitively predict who will progress to gastric neoplasia. Surveillance of people with GIM might allow for the earlier diagnosis of neoplasia when endoscopic or surgical resection is curative. Whether GIM surveillance is associated with this and other patient important outcomes, particularly in a low incidence region, such as the US, is not established. Furthermore, whether outcomes of GIM surveillance are distinct for patients deemed higher risk versus lower risk for NCGA is also not established but would be important for informing surveillance recommendations and intervals. Because categorization as “high-risk” or “low-risk” is somewhat arbitrary, a complementary focus of PICO 2 and PICO 3 was to provide evidence-based guidance for risk-stratification based on predefined risk determinants (e.g. family history, racial / ethnicity background, smoking history, pernicious anemia or autoimmune gastritis, GIM topographic extent, GIM histologic subtype, predictive biomarkers), and the magnitude of association with progression of GIM to neoplasia.
The ideal studies to inform these two questions would be RCTs or prospective cohort studies with comparator arms. Unfortunately, our comprehensive systematic literature search yielded no studies that compared patient important outcomes of endoscopic GIM surveillance versus no surveillance among patients considered low-risk (PICO 2) or high-risk (PICO 3) for gastric cancer. Therefore, the technical review team and guidelines panel reached consensus regarding which indirect evidence would be informative including:
The overall prevalence of GIM in order to determine the population burden of GIM.
The overall risk of neoplastic progression (or global regression) of GIM without concomitant dysplasia.
The overall risk of neoplastic progression (or global regression) of GIM without concomitant dysplasia stratified by presence or absence of predefined risk determinants.
Risk of progression of GIM to any dysplasia, high grade dysplasia, or gastric cancer (overall)
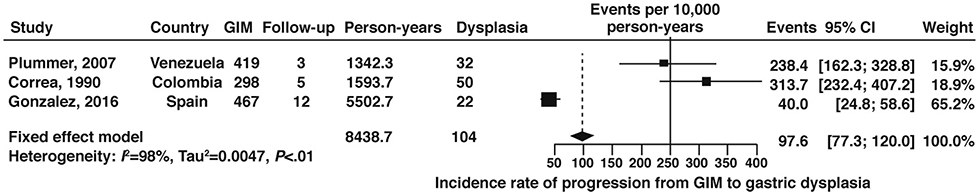
A total of 3 cohort studies,62–64 which included 1184 patients with GIM with 8439 patient-years of follow up time, reported on the global progression of GIM to any dysplasia. The fixed-effects pooled incidence rate was 97.9 (95% CI: 77.3–120.0) dysplasia cases per 10,000 person-years follow-up time (Figure 7, low certainty of evidence). No studies were available from the US.
Figure 7:
Incidence rate per 10,000 person-years of any dysplasia in patients with GIM
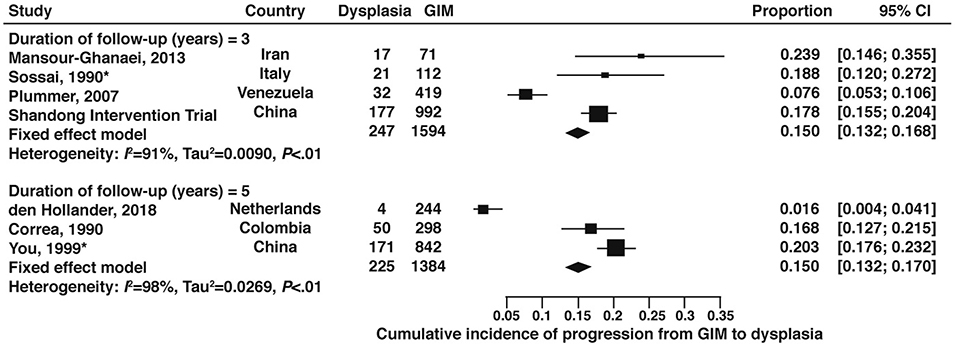
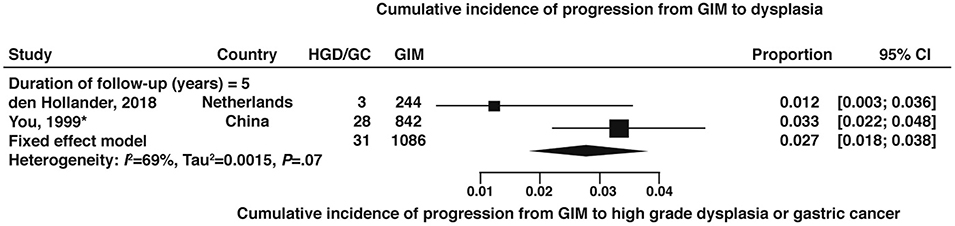
The cumulative incidence of dysplasia at 3-years and 5-years were both 15% based on studies from outside the US.43,44,48,60,62,65–67 (Figure 8, very low certainty of evidence) In the Shandong Intervention Trial, among patients with GIM, the cumulative incidence of dysplasia at 7-years was 38%. One study from the US which enrolled 79 patients with GIM at the outset, reported no cases of dysplasia in 36 patients followed out to 8 years (cumulative incidence 0; 95% CI: 0.0–9.7%).28 The cumulative incidence of high-grade dysplasia or gastric cancer among GIM patients at 5-years was 2.7% (95% CI:1.8–3.8) based on 2 non-US studies.66,67 (Figure 9, very low certainty of evidence)
Figure 8:
Cumulative incidence of any dysplasia at 3-, 5-year, and 8-years in patients with GIM
Figure 9:
Cumulative incidence of HGD or gastric cancer at 5-years in patients with GIM
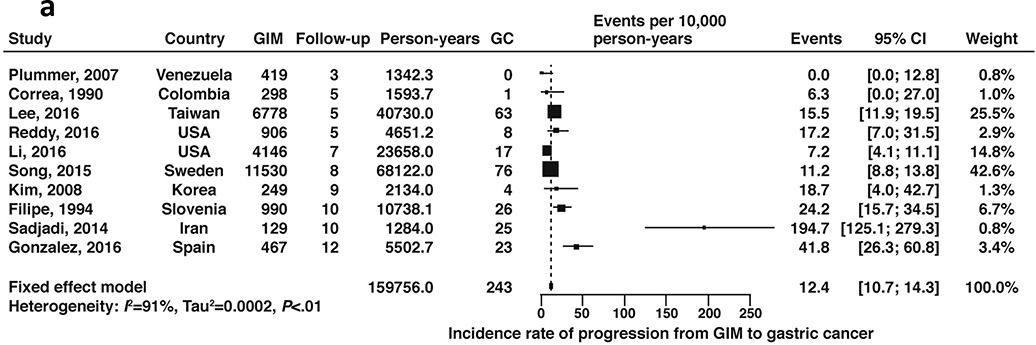
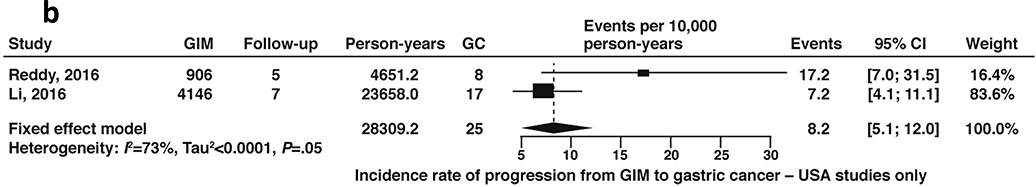
A total of 10 cohort studies, which included 25,912 patients with GIM with 159,756 patient-years of follow-up time, reported on the natural progression of GIM (without concomitant diagnosis of dysplasia) to gastric cancer. The fixed-effects pooled incidence rate was 12.4 (95% CI 10.7–14.3) gastric cancer cases per 10,000 patient-years’ time (Figure 10a, low certainty in evidence). Two of these studies included US populations only (Reddy 2016, Li 2016); the rate of gastric cancer among US-based patients with GIM was 8.2 cases (95% CI: 5.1–12.0) per 10,000 patient-years’ time (Figure 10b, low certainty of evidence). We performed a sensitivity analysis excluding gastric cancer cases diagnosed within 1 year of GIM diagnosis as these could be considered to be prevalent cases missed at the prior endoscopy. Of the two US-based studies, only one reported a case of gastric cancer at 7 months after the endoscopy diagnosing GIM, with all other cases being diagnosed after 1 year.68 Exclusion of this patient from the events and total number of patients with GIM resulted in no significant change to the pooled estimates.
Figure 10a:
Incidence rate of gastric cancer per 10,000 person-years in patients with GIM (all geographies)
Figure 10b:
Incidence rate of gastric cancer per 10,000 person-years in patients with GIM (US geography only)
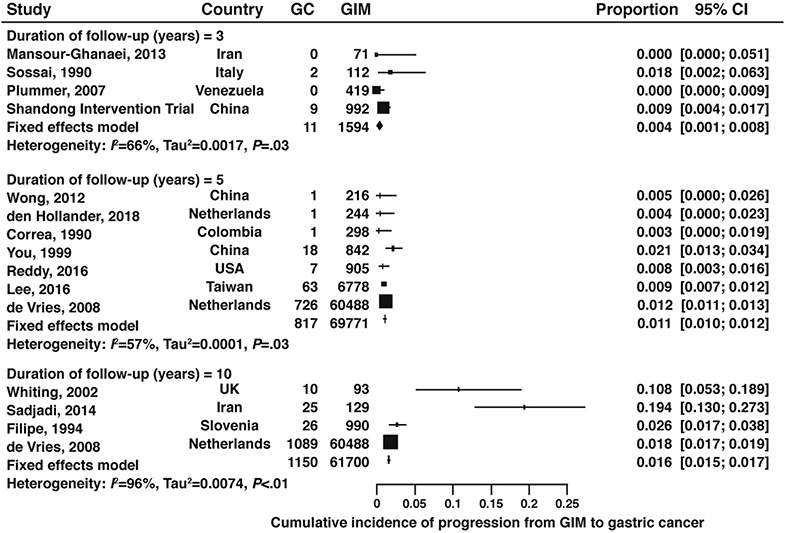
Among patients with GIM, the cumulative incidence of gastric cancer at 3-years, 5-years, and 10-years was 0.4%, 1.1%, and 1.6%, respectively (Figure 11, 3-years: very low certainty of evidence; 5-years: low certainty of evidence; 10-years: very low certainty of evidence). Only one of these studies was from the US (0.9% 5-year cumulative incidence).68
Figure 11:
Cumulative incidence of gastric cancer at 3-, 5-, and 10-years in patients with GIM
Risk of progression of GIM to dysplasia or gastric cancer (subgroup analyses)
We identified comparative studies which informed the risk of neoplastic progression among patients with GIM in the presence versus absence of our pre-specified risk determinants (i.e. race and ethnicity, first-degree family history of gastric neoplasia, smoking history, concomitant autoimmune gastritis, concomitant pernicious anemia, histologic features (e.g. incomplete versus complete GIM), topographic extent of GIM (extensive versus limited), and specific biomarkers) as available based on the systematic literature search.
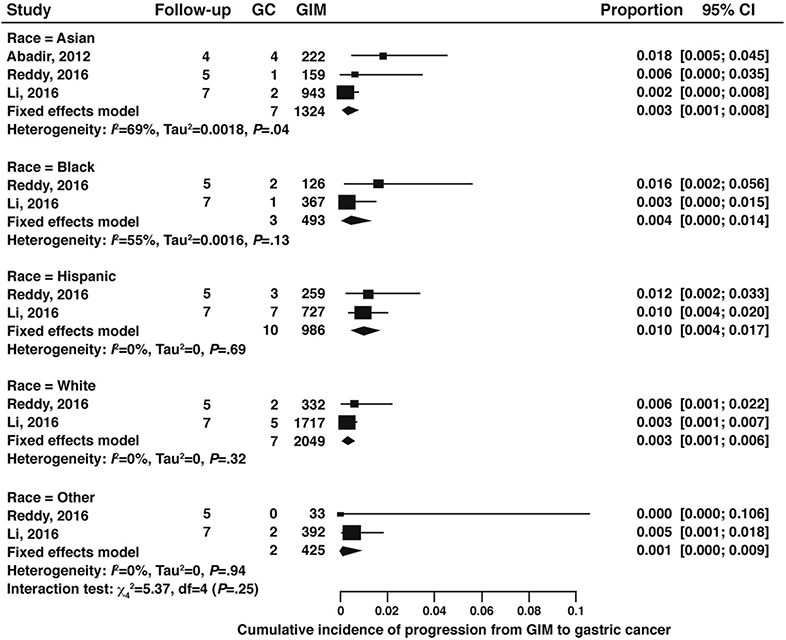
For progression of GIM according to race and ethnicity, we considered studies that were from North America and that reported on GIM progression to gastric cancer stratified by racial and ethnic background (non-Hispanic white, black, Hispanic, Asian, and other). One study from Canada reported only on Asian populations and included mostly immigrant populations69, while the two studies from the US included Asian, non-Hispanic white, black, Hispanic, and “other” racial or ethnic subgroups. Meta-analysis of these studies revealed no significant difference in GIM progression according to race or ethnicity on subgroup interaction test. (Figure 12, very low certainty of evidence).
Figure 12:
Cumulative incidence of gastric cancer in GIM patients according to race and ethnicity (4–7 years followup)
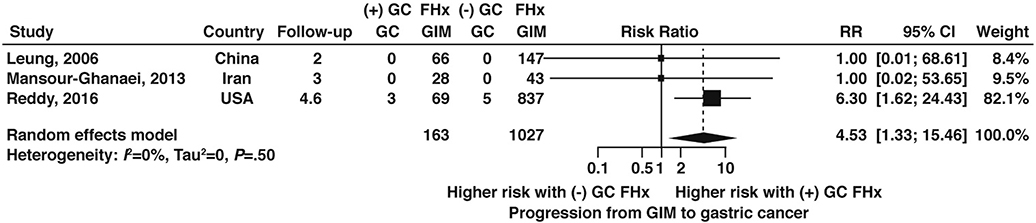
Four studies60,68–70 reported data on GIM progression to gastric cancer according to family history, only one of which was from the US. Among patients with GIM, having a history of a first-degree relative with gastric cancer was associated with over 4.5-fold higher odds (OR 4.53, 95% CI: 1.33–15.46) of gastric cancer compared to patients with GIM and a negative family history (Figure 13, very low certainty of evidence).
Figure 13:
Relative risk of progression to gastric cancer in GIM patients with versus without a family history of gastric cancer
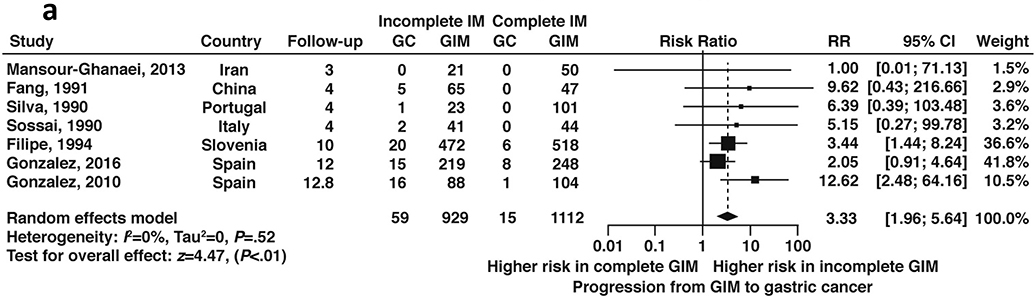
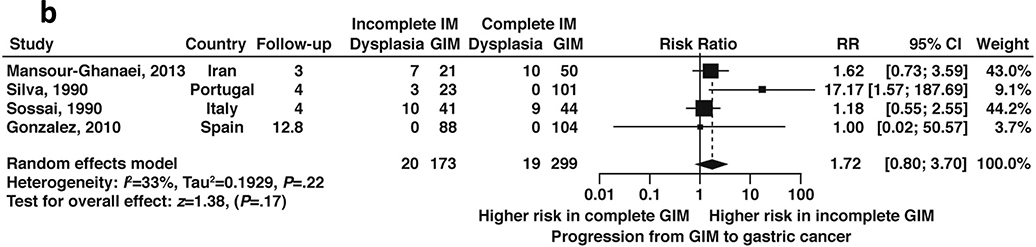
Seven studies, all non-US based, reported data on GIM progression to dysplasia or cancer according to histologic subtype. Among patients with GIM, incomplete GIM was associated with a 3.3-fold (RR 3.33, 95%CI: 1.96–5.64) higher risk of incident gastric cancer compared to complete GIM during follow-up ranging from 3–12.8 years (Figure 14a, low certainty of evidence). Four studies60,65,71,72 reported on the risk of dysplasia in a total of 472 patients with incomplete versus complete GIM; overall, there was a 1.7-fold higher risk of progression to dysplasia in patients with incomplete versus complete GIM (RR 1.7, 95% CI: 0.8–3.7) (Figure 14b, low certainty in evidence). As noted, histologic subtyping for GIM is not routinely done in the US and is reflected in the dearth of US-based studies on the topic.
Figure 14a:
Relative risk of progression to gastric cancer according to incomplete versus complete GIM on baseline histology
Figure 14b:
Relative risk of progression to any dysplasia according to incomplete versus complete GIM on baseline histology
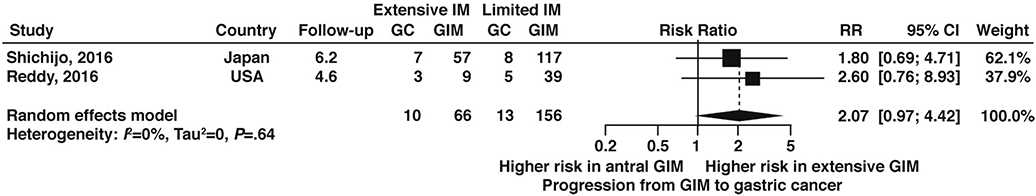
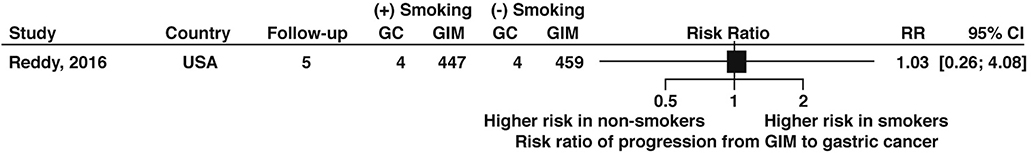
Two studies68,73 informed the relative risk of neoplastic progression in patients with extensive versus limited topographic extent of GIM according to our prespecified definitions, with extensive GIM associated with a nonstatistically significant 2-fold increased risk of progression compared to limited GIM (RR 2.07, 95% CI: 0.97–4.42) (Figure 15, very low certainty of evidence). Only one study, which was from the US, informed the risk of GIM progression to gastric cancer among patients with versus without a history of tobacco use and found no difference in risk of progression, albeit based on limited data (RR 1.03; 95% CI: 0.26—4.08) and with no other details for smoking status provided (Figure 16, very low certainty in evidence).68
Figure 15:
Relative risk of progression to gastric cancer according to topographic extent of GIM, extensive versus limited1
Figure 16:
Relative risk of progression of GIM to gastric cancer according to tobacco smoker versus non-smoker status
Other than for histologic subtype of GIM, no data on progression of GIM to dysplasia according to the pre-defined subgroups were available.
No data were available for risk of progression according to history of pernicious anemia or autoimmune gastritis. Biomarker data were abstracted but significant heterogeneity precluded meaningful meta-analysis; currently no biomarkers are approved nor readily available for the purpose of gastric preneoplasia diagnosis, prognosis, and management. One study by Plummer et al. did report on the severity of gastric mucosal lesions according to H. pylori CagA+ versus H. pylori CagA- status, as well as compared to uninfected patients. Among 268 H. pylori infected patients, CagA seropositivity showed a ‘dose-response’ as the grade of the lesion increased; that is, there was a 2.7-fold (OR 2.71, 95% CI: 1.46–5.04), 3.2-fold (OR 3.15, 95% CI: 1.71–5.82), 7.4- (OR 7.35, 95% CI: 3.45–15.6) to 14-fold (OR 14.0, 95% CI: 6.22–31.4) and 16.7-fold (OR16.7, 95%CI: 7.75–35.9) higher odds of atrophic gastritis, complete intestinal GIM (type I), incomplete GIM (type II/III), and dysplasia, respectively, among patients infected with H. pylori CagA+ versus H. pylori CagA-. Notably, a similar magnitude of ‘dose-response’ was seen in H. pylori CagA+ infected patients compared to uninfected controls; however, among H. pylori CagA- infected patients, there was a higher likelihood of chronic gastritis but not more advanced lesions compared to uninfected controls.62
Global histologic regression of GIM to normal mucosa or (non)atrophic gastritis
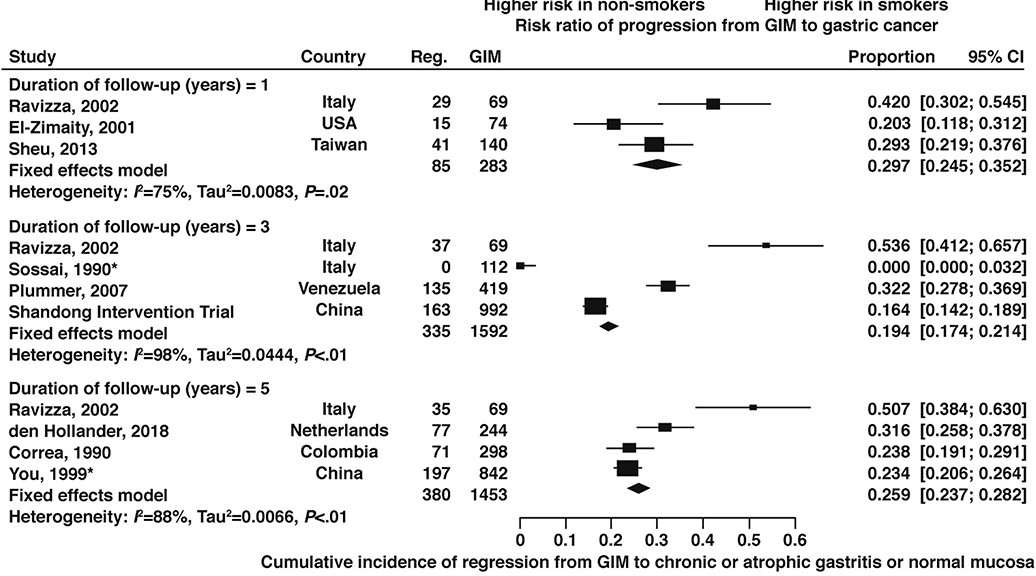
A total of 15 cohort studies reported on the global regression of GIM to normal mucosa or non-atrophic gastritis. The fixed-effect cumulative risk of regression in patients with GIM at 1-year, 3-years, 5-years, and 10-years was 29.7% (95% CI: 24.5–35.2%), 19.4% (95% CI: 17.4–21.4%), 25.9% (95% CI: 23.7–28.2%), and 19.4% (95% CI: 7.9–34.2%), respectively. Only one study was from the US, which reported similar estimates overall (Figure 17, very low certainty of evidence). There were no data on risk of regression according to the predefined risk determinants.
Figure 17:
Cumulative risk of regression to improved global histology at 1-year, 3-year, and 5-year follow up in patients with GIM
PICO 4: In patients with GIM without dysplasia, does short-term follow up (< 1 year) to determine the extent of gastric preneoplasia (using biopsies) vs no short-term follow up affect patient-important outcomes?
The histologic extent of gastric preneoplastic lesions (AG, GIM) is generally accepted to be an important risk factor for neoplastic progression, although robust supporting data and effect estimates are limited. Accurate histopathologic staging necessitates biopsies from both the antrum and corpus. Non-targeted biopsies of the antrum and corpus increases the sensitivity of the endoscopic evaluation for the detection of GIM and allows more accurate determination of histologic extent of disease as well as histologic subtype (e.g. complete versus incomplete). The prior and recently updated European (ESGE) guidelines for GIM surveillance emphasize the extent of mucosal involvement to delineate a higher risk GIM population, and advocate endoscopic surveillance for extensive GIM (i.e. antrum and corpus involvement) at a 3-year surveillance interval; surveillance is not recommended if corpus biopsies are negative and GIM is limited to the antrum.74,75 The ESGE acknowledges the mucosal subtlety and patchiness of GIM and state that at least four non-targeted biopsies from the antrum and corpus labelled in separate biopsy jars are needed for adequate assessment. While some higher incidence regions, such as East Asia, customarily diagnose GIM using image-enhancing techniques such as magnification chromoendoscopy with or without formal pathologic assessment, these technologies and expertise are in routine use in the US. Similarly, while serologic markers of gastric atrophy, such as pepsinogen I and pepsinogen I:II ratio, are used variably in some European and Asian countries,76,77 there are currently no validated noninvasive biomarkers for GIM, GIM topographic extent, or GIM progression risk in clinical use in the US. Thus, the accurate diagnosis and staging of GIM in the US hinges on EGD with biopsies of the corpus and antrum. This is potentially problematic in the US as an overall low incidence country, and has led to wide clinical practice variation, since the diagnosis of GIM is usually made incidentally on limited biopsies taken for other clinical indications.
Thus, the motivation for PICO 4, which is most relevant for populations where NCGA incidence is overall low and the topographic extent might not have been assessed a priori, was to determine whether a repeat short-term follow up (<12 months) endoscopy with biopsies of antrum and corpus in patients diagnosed incidentally with GIM and no dysplasia is associated with improved patient-important outcomes compared to patients who do not undergo short-term follow up examination.
Unfortunately, no studies provided direct evidence to inform PICO 4. That is, no studies compared patient important outcomes in patients with GIM and no dysplasia who underwent a repeat short-interval endoscopy (<12 months) to define the topographic extent of GIM with those who did not. Therefore, the technical review team and guidelines panel reached consensus regarding the indirect evidence that would be needed to inform PICO 4, which includes:
The overall prevalence of GIM in order to quantify the magnitude of the potentially at-risk population.
The risk of progression of GIM to incident dysplasia or NCGA in patients with limited versus extensive GIM.
Identification of high-risk subgroups who might benefit from short-interval endoscopy to define the topographic extent of GIM.
The risk of having high-grade dysplasia or NCGA diagnosed within the first year of follow up after GIM diagnosis, as these are very likely to be prevalent cases not identified on the initial endoscopy diagnosing GIM (i.e. “missed neoplasia”).
Prevalence of GIM
We identified six studies that reported data on GIM prevalence in the US and included at least 250 people.78 Among 897,371 people, the pooled prevalence of GIM was 4.8% (95% CI: 4.8% - 4.9%). Individual studies ranged from 4.9% up to 19.1%. Further details regarding the prevalence of GIM and the associated predisposing risk factors are detailed in the second part of the technical review.78
Risk of progression of GIM to incident gastric neoplasia among patients with limited versus extensive GIM
As noted above, we identified two retrospective cohort analyses—one from Japan73 and one from the US68 — that specifically reported the risk of progression to gastric cancer among patients who underwent antral and corpus biopsies and were found to have limited GIM (total N=156) versus extensive GIM (total N=66), according to the definitions decided a priori for this technical review. We have provided the context of these studies and highlighted key considerations below. Extensive GIM was associated with higher risk of progression to gastric cancer compared to limited GIM (RR 2.07; 95% CI: 0.97–4.42), although this was statistically nonsignificant (Figure 15, very low certainty in evidence). Discrete data on the progression of GIM to dysplasia according to topographic extent of GIM were not available for analysis.
The Shichijo et al. study reported the incidence of gastric cancer among 573 patients from Japan following H. pylori eradication according to presence and topography of GIM over a follow up period of 6.2+/−4.8 years.79 No patients had dysplasia or cancer at the outset, but over 30% had gastric ulcers (24% gastric ulcers only, 6.3% gastric and duodenal ulcers). Annual EGD was recommended for all patients although the adherence and time between EGDs was not explicitly stated in the study. For this cohort, the cumulative incidence of gastric cancer at 1 year was 0.3% in patients without GIM, 0% in patients with antral GIM only, and 5.6% in patients with GIM of the corpus (1.5%, 3.7%, and 9.8% at 5-years; and 1.5%, 11%, and 16% at 10-years, respectively). After adjusting for age and sex, there was a 3.6- (95%CI: 1.2–11.0) and 3.7-fold (95% CI: 1.1–12.0) increased risk of gastric cancer in patients with antral GIM only and corpus GIM, respectively, compared to patients without GIM.
Using a retrospective cohort design and concomitant nested case-control analysis, Reddy et al. reported the outcomes of 923 patients with GIM from the US Kaiser Health System.68 The definitions of extensive versus limited GIM that we defined a priori for this technical review differed from the definitions in their study; the authors defined extensive GIM as moderate/marked GIM in at least 2 biopsy specimens (which could have been from the same gastric anatomic location) or GIM in at least 2 anatomic gastric locations (which could have included cardia GIM, notably), while limited GIM was not explicitly defined. Notably, it was not clear if all included patients in the cohort analysis had antral and corpus biopsies obtained. Among the 25 patients diagnosed with gastric cancer, only 32% (N=8) were incident gastric cancers following the GIM diagnosis, whereas the remaining 68% were diagnosed at the time of GIM diagnosis. Of these 8 incident cases, 6 occurred at least 2 years after the index endoscopy diagnosing GIM, while two cases occurred at 7 months and 13 months of follow up. Four of these patients had extensive GIM according to our definition. Using a nested case-control design (8 gastric cancer cases matched with 40 age- and sex-matched controls without gastric cancer selected from the cohort of patients with GIM), the authors reported that extensive GIM, according to their definition, was associated with over 9-fold higher likelihood for gastric cancer (OR 9.4, 95% CI: 1.8–50.4); however, they also report that the specific anatomic location of GIM was not statistically significantly associated with risk of gastric cancer. Importantly, the location of the gastric cancers (cardia versus non-cardia) was not specified.
Additional studies do report on the risk of neoplastic progression in extensive GIM, but the studies are heterogeneous with respect to reference group, definitions of extensive GIM, and protocol (e.g. not all patients had biopsies taken from both antrum and corpus for adequate determination of GIM extent), among other factors that limit the quality and interpretability of available data for PICO 4 specifically. A descriptive analysis of these studies is provided here, as they are still informative. A recent prospective cohort study from the Netherlands and Norway, both low risk geographic regions, found that among 279 patients undergoing endoscopic surveillance (87% with varying severity of GIM), 4 (1.4%) progressed to high-grade dysplasia or invasive neoplasia, with these 4 cases occurring at 11, 43, 58, and 80 months following the baseline endoscopy; GIM (N=3) and HGD (N=1) were the most severe lesions in these patients at baseline.67 The one case that was diagnosed at 11 months was notably in a 53-year-old woman with a first-degree relative with gastric cancer who had a low-risk baseline OLGIM (I) score and no visible endoscopic lesions; histopathology from gastrectomy confirmed diffuse-type adenocarcinoma and not intestinal-type adenocarcinoma. Although it was not possible to isolate neoplastic progression outcomes according to extensive versus limited GIM specifically, a nonstatistically significantly higher percentage of patients with high OLGIM scores (Stage III-IV) had progression to HGD or gastric cancer compared to the group with low OLGIM scores (Stage 0-II) (2/56 [3.6%] vs. 1/155 [0.6%, P=0.11) on follow-up.
Benefit of short-interval endoscopy in high-risk subgroups
We did not identify any studies that specifically compared the rate of neoplastic progression in patients with limited versus extensive GIM stratified by additional potential risk factors for progression. The study by den Hollander et al. reported that adjunctive use of the pepsinogen biomarker (PG I/II ratio) with OLGIM score appropriately identified a low- and high-risk group in this cohort (as defined by histopathologic OLGIM stage and PG I/II ratio) overall.67 Other studies reported neoplastic progression for subgroups variably defined as high- and low-risk based on risk factors other than extensive versus limited GIM; these studies were used for indirect evidence informing PICO 2 and 3.
Risk of gastric neoplasia diagnosis within the first year following a diagnosis of GIM
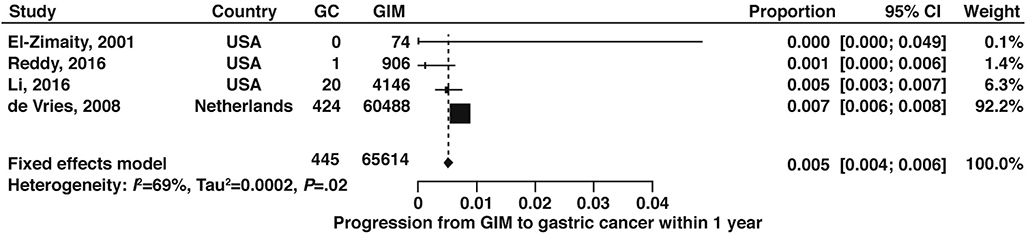
In addition to determining the topographic extent of GIM, a repeat short interval endoscopy allows for a “second look” in the context of knowing a patient is at higher risk and potentially the opportunity to diagnose a previously missed lesion at a stage when curative resection is still possible. Early gastric neoplastic lesions are often subtle and might be overlooked, particularly in the absence of a careful systematic examination and clinical protocols such as magnification chromoendoscopy. In higher-risk populations, such as patients with preneoplasia, there is higher pretest probability for synchronous gastric neoplasia, although the exact risk is not known and depends on the population and presence of endoscopic findings.80,81 With adherence to appropriate quality metrics, including full mucosal visualization, adequate air insufflation, photo-documentation, and duration of focused luminal examination82–84 the risk of missed lesions is lower. No studies which reported the endoscopic miss rate for gastric neoplasia specifically in the setting of GIM were identified in our search. As indirect evidence, we analyzed the risk of gastric neoplasia within the first year of a GIM diagnosis. It should be noted that there is potential for unmeasured bias since it is not uncommon for cancer incidence studies to exclude patients who are diagnosed with cancer at enrollment or within a certain time interval thereafter; these potentially prevalent or short interval incident cases would thus not be captured. Based on 4 cohort studies,28,68,85,86 the cumulative incidence of gastric cancer at one year in patients with GIM was low (5 per 1000 persons; 95% CI: 0.004–0.006) (Figure 18, low certainty of evidence), with the estimate driven primarily by a large study from the Netherlands, a low incidence nation, which included 60,488 patients with GIM.86
Figure 18:
Cumulative incidence of gastric cancer at 1 year in patients with GIM
Summary and Conclusions
The comprehensive literature search, review, and data abstraction process showed that there was direct evidence to inform only one of four a priori PICO questions—i.e., PICO 1, which focused on the benefit of empiric H. pylori testing and treatment in patients with GIM. We relied on indirect evidence to inform PICO 2–4. Extensive work was done to determine if indirect evidence, including data on pertinent patient subgroups, could further inform the evidence profiles for PICO 2–4 to inform the AGA guidelines. Overall, the results highlight the limited published literature about patients with GIM without concurrent neoplasia, particularly with respect to the utility of an endoscopic surveillance program.
The diagnosis of gastric cancer in the curable stage prior to submucosal invasion is uncommon in the US since early-stage gastric cancer is typically asymptomatic or associated with nonspecific symptoms such as dyspepsia. The goal of surveillance of preneoplastic lesions at defined intervals is to diagnose early-stage cancer and facilitate endoscopic or surgical resection with curative intent. That the overall 5-year survival rates for gastric cancer have improved to nearly 70% in East Asian countries with gastric cancer screening and cancer surveillance programs validates this approach and is in marked contrast to the 31% 5-year survival rate for gastric cancer in the US.87 Because the US is a low incidence nation, population-based gastric cancer screening and surveillance has been neither recommended nor commonly performed in clinical practice. Focused screening strategies in high-risk populations, with or without biomarkers, also has not been recommended due to the lack of evidence.
The impact of established risk factors for NCGA on the natural course of GIM are not well-established; these include age, gender, race/ethnicity, host genetics and family history, dietary factors (e.g., salt intake, processed meats), smoking, and H. pylori infection with considerations for genetic variants, timing of infection, and cumulative duration of infection. Up to 10% of the risk of gastric cancer is attributed to germline mutations and familial clustering, while less than 1–3% of all gastric cancer cases are inherited as part of specific familial syndromes, including hereditary diffuse gastric cancer, Lynch syndrome, and Peutz-Jeghers syndrome.67,88 Germline mutations are increasingly recognized as a factor in intestinal-type gastric cancer. The importance of the interface between these germline mutations and the natural course of GIM are unknown. Many uncertainties similarly exist as to the relative contribution of non-genetic determinants on the natural history of GIM. Because NCGA results from cumulative exposure of disease determinants and their interaction over time, it is difficult (if even possible) to isolate the effect of a single determinant on GIM regression or progression. Risk factors will also vary by populations and geographic regions. For example, because it is consistently demonstrated that H. pylori eradication reduces the risk of incident and metachronous intestinal-type NCGA overall,89 H. pylori eradication in the presence of GIM has been recommended if there are no competing contraindications.74 However, the actual effect of H. pylori eradication or persistence on the natural course of GIM alone is unknown and highlighted by the evidence profile for PICO 1. That neoplastic progression occurs even after the resolution of H. pylori infection implicates H. pylori-independent factors. A better understanding of the biology of progression has implications for improved risk stratification, patient counseling, and disease prevention.
One key strength of this technical review is its comprehensiveness, including organized efforts to identify and analyze indirect evidence and the consistent application of GRADE methodology. In order to ensure high fidelity of the data, every step of the data selection process was performed in duplicate with adjudication when disagreements arose. In addition, we were able to obtain previously unpublished data related to GIM from the Colombia Chemoprevention study56 and cohort for this technical review. An obvious limitation of this review is related to the dearth of published evidence that is specific to GIM in the absence of neoplasia, manifest by our inability to provide direct evidence to inform three of the PICO questions proposed by the AGA. The indirect evidence we abstracted to inform these questions was heterogenous and of variable quality.
In conclusion, we achieved the primary objective of this technical review and qualitatively and quantitatively summarized the available evidence to inform the AGA guidelines panel on GIM management for cancer surveillance, with a prespecified focus on the intestinal-type gastric adenocarcinoma pathway. Our extensive systematic literature review and data synthesis identified direct evidence to inform one of four PICO questions, and indirect evidence to inform the remaining three. In general, empiric H. pylori testing and treatment in patients with confirmed infection (with or without GIM) favored a protective effect against incident gastric cancer, and was also associated with improved gastric cancer-related mortality when compared to patients who received placebo. Based on low quality evidence, family history of gastric cancer, extensive GIM and incomplete GIM on histology are associated with increased risk of progression to gastric cancer in patients with GIM. This technical review has highlighted the paucity of direct evidence to inform endoscopic surveillance of GIM, but also provides enormous opportunity for future work and efforts. Future work on GIM should use standardized protocols and definitions for defining outcomes.
Supplementary Material
Table 4:
Evidence profile and pooled effect estimates to inform PICO questions 2–4
| Outcome | Estimate (95% CI) | Certainty of Evidence | Risk of Bias | Inconsistency | Imprecision | Indirectness | |
|---|---|---|---|---|---|---|---|
| Incidence rate of any dysplasia in patients with GIM | 97.6/10,000 p-y (77.3 – 120.0) | ⊕⊕○○ Low |
Not serious | Serious | Not serious | Serious | |
| Cumulative incidence of any dysplasia in patients with GIM | at 3 years | 15.0% (13.2 – 16.8) | ⊕○○○ Very low |
Serious | Serious | Not serious | Serious |
| at 5 years | 15.0% (13.2 – 17.0) | ⊕⊕○○ Low |
Not serious | Serious | Not serious | Serious | |
| Cumulative incidence of HGD or gastric cancer in patients with GIM at 5 years | 2.7% (1.8 – 3.8) | ⊕○○○ Very low |
Not serious | Not serious | Very serious | Serious | |
| Incidence rate of gastric cancer in patients with GIM | all geographies | 12.4/10,000 p-y (10.7 – 14.3) | ⊕⊕○○ Low |
Serious1 | Serious1 | Not serious | Serious |
| US geography only | 8.2/10,000 p-y (5.1 – 12.0) | ⊕⊕○○ Low |
Serious | Not serious | Serious | Not serious | |
| Cumulative incidence of gastric cancer in patients with GIM | within 1 year | 0.5% (0.4 – 0.6) | ⊕⊕○○ Low |
Serious | Not serious | Not serious | Serious |
| at 3 years | 0.4% (0.1 – 0.8) | ⊕○○○ Very low |
Not serious | Not serious | Very serious | Serious | |
| at 5 years | 1.1% (1.0 – 1.2) | ⊕⊕○○ Low |
Serious | Not serious | Not serious | Serious | |
| at 10 years | 1.6% (1.5 – 1.7) | ⊕○○○ Very low |
Serious | Serious | Not serious | Serious | |
| Cumulative incidence of gastric cancer in patients with GIM based on race/ethnicity | Asian | 0.3% (0.1 – 0.8) | ⊕○○○ Very low |
Serious | Not serious | Very serious | Not serious |
| Black | 0.4% (0.0 – 1.4) | ⊕○○○ Very low |
Serious | Not serious | Very serious | Not serious | |
| Hispanic | 1.0% (0.4 – 1.7) | ⊕○○○ Very low |
Serious | Not serious | Very serious | Not serious | |
| White | 0.3% (0.1 – 0.6) | ⊕○○○ Very low |
Serious | Not serious | Very serious | Not serious | |
| Other | 0.1% (0.0 – 0.9) | ⊕○○○ Very low |
Serious | Not serious | Very serious | Not serious | |
| Relative risk of incident gastric cancer in patients with GIM and first degree family history of gastric cancer vs. no first degree family history | 4.5 (1.3 – 15.5) | ⊕○○○ Very low |
Serious | Not serious | Very serious | Not serious | |
| Relative risk of incident gastric cancer in patients with incomplete vs. complete GIM on histology | 3.3 (2.0 – 5.6) | ⊕⊕○○ Low |
Not serious | Not serious | Serious | Serious | |
| Relative risk of incident gastric dysplasia in patients with incomplete vs. complete GIM on histology | 1.7 (0.8 – 3.7) | ⊕⊕○○ Low |
Not serious | Not serious | Serious2 | Serious | |
| Relative risk of incident gastric cancer in patients with extensive vs. limited GIM | 2.1 (1.0 – 4.4) | ⊕○○○ Very low |
Serious | Not serious | Very serious | Not serious | |
| Relative risk of incident gastric cancer in patients with GIM who smoke(d) tobacco vs. none smokers | 1.0 (0.3 – 4.1) | ⊕○○○ Very low |
Serious | Not serious | Very serious | Not serious | |
| Cumulative risk of GIM regression to improved global histology | at 1 year | 29.7% (24.5 – 35.2) | ⊕○○○ Very low |
Serious | Not serious | Serious | Serious |
| at 3 years | 19.4% (17.4 – 21.4) | ⊕○○○ Very low |
Serious | Serious | Not serious | Serious | |
| at 5 years | 25.9% (23.7 – 28.2) | ⊕○○○ Very low |
Serious | Serious | Not serious | Serious | |
we rated down one level only for both risk of bias (few studies lacked protocolized biopsies) and inconsistency (most of the evidence was consistent except two small studies) together.
despite the narrow confidence interval, we elected to rate down for imprecision due to the small number of events.
Abbreviations: GIM, gastric intestinal metaplasia; HGD, high grade dysplasia.
Acknowledgements
1. We would like to acknowledge Drs. Luis Eduardo Bravo and Robertino Mera for their assistance in obtaining unpublished data from the Columbia Chemoprevention Cohort for use in this technical review.
Acronyms
- NCGA
Intestinal-type noncardia gastric adenocarcinoma
- AG
atrophic gastritis
- H. Pylori
Helicobacter pylori
- TCGA
Cancer Atlas Genome Project
- GIM
gastric intestinal metaplasa
- EGD
esophagogastroduodenoscopy
- ASGE
American Society for Gastrointestinal Endoscopy
- ESGE
European Society for Gastrointestinal Endoscopy
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- PICO
population (P), intervention (I), comparator (C), and outcomes (O)
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- MOOSE
Meta-analysis Of Observational Studies in Epidemiology
- RCT
randomized controlled trial
- REDCap
Research Electronic Data Capture
- OLGA
Operative Link on Gastritis Assessment
- OLGIM
Operative Link on Gastric Intestinal Metaplasia Assessment
- RR
Relative Risk
- IRR
incidence rate ratio
- ROB
risk of bias
- HGD
high drade dysplasia
- US
United States
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;September 12. ep. [DOI] [PubMed] [Google Scholar]
- 2.Anderson WF, Rabkin CS, Turner N, et al. The Changing Face of Noncardia Gastric Cancer Incidence Among US Non-Hispanic Whites. J. Natl. Cancer Inst 2018;110:608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA. Cancer J. Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 4.Merchant SJ, Kim J, Choi AH, et al. A rising trend in the incidence of advanced gastric cancer in young Hispanic men. Gastric Cancer 2017;20:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson WF, Rabkin CS, Turner N, et al. The Changing Face of Noncardia Gastric Cancer Incidence Among US Non-Hispanic Whites. JNCI J. Natl. Cancer Inst 2018;110:608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Correa P, Schneider BG. Etiology of Gastric Cancer: What Is New? Cancer Epidemiol Biomarkers Prev 2005;14:1865–1868. [DOI] [PubMed] [Google Scholar]
- 7.Minalyan A, Benhammou JN, Artashesyan A, et al. Autoimmune atrophic gastritis: current perspectives. Clin. Exp. Gastroenterol 2017;10:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jencks DS, Adam JD, Borum ML, et al. Overview of Current Concepts in Gastric Intestinal Metaplasia and Gastric Cancer. Gastroenterol. Hepatol. (N. Y) 2018;14:92–101. [PMC free article] [PubMed] [Google Scholar]
- 9.Correa P Helicobacter pylori and gastric cancer: state of the art. Cancer Epidemiol. Biomarkers Prev 1996;5:477–81. [PubMed] [Google Scholar]
- 10.Polk DB, Peek RM. Helicobacter pylori: gastric cancer and beyond. Nat. Rev. Cancer 2010;10:403–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wroblewski LE, Peek RM, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin. Microbiol. Rev 2010;23:713–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bass AJ, Thorsson V, Shmulevich I, et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Correa P Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–40. [PubMed] [Google Scholar]
- 14.Evans JA, Chandrasekhara V, Chathadi K V., et al. The role of endoscopy in the management of premalignant and malignant conditions of the stomach. Gastrointest Endosc 2015;82:1–8. [DOI] [PubMed] [Google Scholar]
- 15.Vance RB, Kubiliun N, Dunbar KB. How Do We Manage Gastric Intestinal Metaplasia? A Survey of Clinical Practice Trends for Gastrointestinal Endoscopists in the United States. Dig Dis Sci 2016;61:1870–8. [DOI] [PubMed] [Google Scholar]
- 16.Shah SC, Itzkowitz SH, Jandorf L. Knowledge Gaps among Physicians Caring for Multiethnic Populations at Increased Gastric Cancer Risk. Gut Liver 2018;12:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dinis-Ribeiro M, Areia M, Vries A de, et al. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy 2012;44:74–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang JH. Understanding Gastric Cancer Risk Factors: We Need to Close the Gap. Gut Liver 2018;12:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim GH, Liang PS, Bang SJ, et al. Screening and surveillance for gastric cancer in the United States: Is it needed? Gastrointest Endosc 2016;84:18–28. [DOI] [PubMed] [Google Scholar]
- 20.Guyatt GH, Oxman AD, Schünemann HJ, et al. GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology. J. Clin. Epidemiol 2011;64:380–382. [DOI] [PubMed] [Google Scholar]
- 21.Stroup D, Berlin J, Morton S, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535–b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cassaro M, Rugge M, Gutierrez O, et al. Topographic patterns of intestinal metaplasia and gastric cancer. Am. J. Gastroenterol 2000;95:1431–1438. [DOI] [PubMed] [Google Scholar]
- 25.Capelle LG, de Vries AC, Haringsma J, et al. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest. Endosc 2010;71:1150–1158. [DOI] [PubMed] [Google Scholar]
- 26.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996;20:1161–81. [DOI] [PubMed] [Google Scholar]
- 27.Correa P, Piazuelo MB, Wilson KT. Pathology of Gastric Intestinal Metaplasia: Clinical Implications. Am. J. Gastroenterol 2010;105:493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Zimaity HM, Ramchatesingh J, Saeed MA, et al. Gastric intestinal metaplasia: subtypes and natural history. J. Clin. Pathol 2001;54:679–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rugge M, Genta RM, OLGA Group. Staging Gastritis: An International Proposal. Gastroenterol 2005;129:1807–1808. [DOI] [PubMed] [Google Scholar]
- 30.Rugge M, De Boni M, Pennelli G, et al. Gastritis OLGA-staging and gastric cancer risk: A twelve-year clinico-pathological follow-up study. Aliment. Pharmacol. Ther 2010;31:1104–1111. [DOI] [PubMed] [Google Scholar]
- 31.Rugge M, Kim JG, Mahachai V, et al. OLGA gastritis staging in young adults and country-specific gastric cancer risk. Int. J. Surg. Pathol 2008;16:150–154. [DOI] [PubMed] [Google Scholar]
- 32.Yue H, Shan L, Bin L. The significance of OLGA and OLGIM staging systems in the risk assessment of gastric cancer: a systematic review and meta-analysis. Gastric Cancer 2018;21:579–587. [DOI] [PubMed] [Google Scholar]
- 33.Mera RM, Bravo LE, Camargo MC, et al. Dynamics of Helicobacter pylori infection as a determinant of progression of gastric precancerous lesions: 16-year follow-up of an eradication trial. Gut 2018;67:1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control. Clin. Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 35.Barendregt JJ, Doi SA, Lee YY, et al. Meta-analysis of prevalence. J. Epidemiol. Community Health 2013;67:974–8. [DOI] [PubMed] [Google Scholar]
- 36.Freeman MF, Tukey JW. Transformations Related to the Angular and the Square Root. Ann. Math. Stat 1950;21:607–611. [Google Scholar]
- 37.Stijnen T, Hamza TH, Ozdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat. Med 2010;29:3046–67. [DOI] [PubMed] [Google Scholar]
- 38.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928–d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wells G, Shea B, O’Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa, Canada; 2011. [Google Scholar]
- 41.Guyatt GH, Oxman AD, Schünemann HJ, et al. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J. Clin. Epidemiol 2011;64:380–2. [DOI] [PubMed] [Google Scholar]
- 42.Wang F, Meng W, Wang B, et al. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345:196–202. [DOI] [PubMed] [Google Scholar]
- 43.Gail MH, You WC, Chang YS, et al. Factorial trial of three interventions to reduce the progression of precancerous gastric lesions in Shandong, China: Design issues and initial data. Control. Clin. Trials 1998;19:352–369. [DOI] [PubMed] [Google Scholar]
- 44.You WC, Brown LM, Zhang L, et al. Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J. Natl. Cancer Inst 2006;98:974–983. [DOI] [PubMed] [Google Scholar]
- 45.You WC, Chang YS, Heinrich J, et al. An intervention trial to inhibit the progression of precancerous gastric lesions: compliance, serum micronutrients and S-allyl cysteine levels, and toxicity. Eur. J. Cancer Prev 2001;10:257–263. [DOI] [PubMed] [Google Scholar]
- 46.Ley C, Mohar A, Guarner J, et al. Helicobacter pylori Eradication and Gastric Preneoplastic Conditions: A Randomized, Double-Blind, Placebo-Controlled Trial. Cancer Epidemiol. Biomarkers Prev 2004;13:4–10. [DOI] [PubMed] [Google Scholar]
- 47.Kuipers EJ, Nelis GF, Klinkenberg-Knol EC, et al. Cure of Helicobacter pylori infection in patients with reflux oesophagitis treated with long term omeprazole reverses gastritis without exacerbation of reflux disease: Results of a randomised controlled trial. Gut 2004;53:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma JL, Zhang L, Brown LM, et al. Fifteen-year effects of helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J. Natl. Cancer Inst 2012;104:488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sung JJ, Lin SR, Ching JY, et al. Atrophy and intestinal metaplasia one year after cure of H. pylori infection: a prospective, randomized study. Gastroenterol 2000;119:7–14. [DOI] [PubMed] [Google Scholar]
- 50.Zhou L, Sung JJY, Lin S, et al. A five-year follow-up study on the pathological changes of gastric mucosa after H. pylori eradication. Chin. Med. J. (Engl) 2003;116:11–14. [PubMed] [Google Scholar]
- 51.Leung WK. Factors predicting progression of gastric intestinal metaplasia: results of a randomised trial on Helicobacter pylori eradication. Gut 2004;53:1244–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou LY, Lin SR, Ding SG, et al. Relationship of Helicobacter pylori eradication with gastric cancer and gastric mucosal histological changes: A 10-year follow-up study. Chin. Med. J. (Engl) 2014;127:1454–1458. [PubMed] [Google Scholar]
- 53.Wong BC-Y, Lam SK, Wong WM, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA 2004;291:187–94. [DOI] [PubMed] [Google Scholar]
- 54.Wong BCY, Zhang L, Ma J, et al. Effects of selective COX-2 inhibitor and Helicobacter pylori eradication on precancerous gastric lesions. Gut 2012;61:812–818. [DOI] [PubMed] [Google Scholar]
- 55.Massarrat S, Haj-Sheykholeslami A, Mohamadkhani A, et al. Precancerous conditions after H. pylori eradication: a randomized double blind study in first degree relatives of gastric cancer patients. Arch. Iran. Med 2012;15:664–669. [PubMed] [Google Scholar]
- 56.Correa P Chemoprevention of Gastric Dysplasia: Randomized Trial of Antioxidant Supplements and Anti-Helicobacter pylori Therapy. J. Natl. Cancer Inst 2000;92:1881–1888. [DOI] [PubMed] [Google Scholar]
- 57.Mera R, Fontham ETH, Bravo LE, et al. Long term follow up of patients treated for Helicobacter pylori infection. Gut 2005;54:1536–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mera RM, Bravo LE, Camargo MC, et al. Dynamics of Helicobacter pylori infection as a determinant of progression of gastric precancerous lesions: 16-year follow-up of an eradication trial. Gut 2017:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee Y-C, Chen TH-H, Chiu H-M, et al. The benefit of mass eradication of Helicobacter pylori infection: a community-based study of gastric cancer prevention. Gut 2013;62:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mansour-Ghanaei F, Joukar F, Soati F, et al. Outcome of Intestinal Metaplasia in Gastric Biopsy of Patients with Dyspepsia in Guilan Province, North Iran. Asian Pacific J. Cancer Prev 2013;14:3549–3554. [DOI] [PubMed] [Google Scholar]
- 61.Bik EM, Eckburg PB, Gill SR, et al. Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci USA 2006;103:732–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Plummer M, van Doorn LJ, Franceschi S, et al. Helicobacter pylori cytotoxin-associated genotype and gastric precancerous lesions. J. Natl. Cancer Inst 2007;99:1328–1334. [DOI] [PubMed] [Google Scholar]
- 63.Correa P, Haenszel W, Cuello C, et al. Gastric precancerous process in a high risk population: cohort follow-up. Cancer Res. 1990;50:4737–4740. [PubMed] [Google Scholar]
- 64.Gonzalez CA, Sanz-Anquela JM, Companioni O, et al. Incomplete type of intestinal metaplasia has the highest risk to progress to gastric cancer: Results of the Spanish follow-up multicenter study. J. Gastroenterol. Hepatol 2016;31:953–958. [DOI] [PubMed] [Google Scholar]
- 65.Sossai P, Barbazza R. Intestinal metaplasia and dysplasia in gastric ulcer and its tissue repair. Am. J. Gastroenterol 1990;85:829–832. [PubMed] [Google Scholar]
- 66.You WC, Li JY, Blot WJ, et al. Evolution of precancerous lesions in a rural Chinese population at high risk of gastric cancer. Int J Cancer 1999;83:615–619. [DOI] [PubMed] [Google Scholar]
- 67.den Hollander WJ, Holster IL, den Hoed CM, et al. Surveillance of premalignant gastric lesions: a multicentre prospective cohort study from low incidence regions. Gut 2018:gutjnl-2017–314498. [DOI] [PubMed] [Google Scholar]
- 68.Reddy KM, Chang JI, Shi JM, et al. Risk of Gastric Cancer Among Patients With Intestinal Metaplasia of the Stomach in a US Integrated Health Care System. Clin. Gastroenterol. Hepatol 2016;14:1420–1425. [DOI] [PubMed] [Google Scholar]
- 69.Abadir A, Streutker C, Brezden-Masley C, et al. Intestinal metaplasia and the risk of gastric cancer in an immigrant Asian population. Clin. Med. Insights Gastroenterol 2012;5:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leung WK, Ng EKW, Chan FKL, et al. Effects of long-term rofecoxib on gastric intestinal metaplasia: results of a randomized controlled trial. Clin. Cancer Res. 2006;12:4766–4772. [DOI] [PubMed] [Google Scholar]
- 71.Gonzalez CA, Pardo ML, Ruiz Liso JM, et al. Gastric cancer occurrence in preneoplastic lesions: A long-term follow-up in a high-risk area in Spain. Int. J. Cancer 2010;127:2654–2660. [DOI] [PubMed] [Google Scholar]
- 72.Silva S, Filipe M, Pinho A. Variants of intestinal metaplasia in the evolution of chronic atrophic gastritis and gastric ulcer. A follow up study. Gut 1990;31:1097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shichijo S, Hirata Y, Yoshida S, et al. Histological intestinal metaplasia and endoscopic atrophy are predictors of gastric cancer development after helicobacter pylori eradication. Gastroenterol 2015;1):S563–S564. [DOI] [PubMed] [Google Scholar]
- 74.Dinis-Ribeiro M, Areia M, de Vries AC, et al. ManaGement of precancerous conditions and lesions in the stomach (MAPS): Guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of PatholoGy (ESP), and the Sociedade PortuGuesa. Endoscopy 2012;44:74–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pimentel-Nunes P, Libânio D, Marcos-Pinto R, et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 2019;51:365–388. [DOI] [PubMed] [Google Scholar]
- 76.Wang X, Lu B, Meng L, et al. The correlation between histological gastritis staging- “OLGA/OLGIM” and serum pepsinogen test in assessment of gastric atrophy/intestinal metaplasia in China. Scand. J. Gastroenterol 2017;52:822–827. [DOI] [PubMed] [Google Scholar]
- 77.Chang C-H, Wu M-S, Chang Y-T, et al. Risk factors for intestinal metaplasia in adult residents of Matzu: a cross-sectional study. J. Formos. Med. Assoc. 2002;101:835–40. [PubMed] [Google Scholar]
- 78.Altayar O, Davitkov P, Shah S, et al. American Gastroenterological Association Technical Review on Gastric Intestinal Metaplasia Cancer Surveillance - Part 2. Gastroenterol 2019. [Google Scholar]
- 79.Shichijo S, Hirata Y, Niikura R, et al. Histologic intestinal metaplasia and endoscopic atrophy are predictors of gastric cancer development after Helicobacter pylori eradication. Gastrointest Endosc 2016;84:618–624. [DOI] [PubMed] [Google Scholar]
- 80.Hosokawa O, Watanabe K, Hatorri M, et al. Detection of gastric cancer by repeat endoscopy within a short time after negative examination. Endoscopy 2001;33:301–5. [DOI] [PubMed] [Google Scholar]
- 81.Chadwick G, Groene O, Riley S, et al. Gastric Cancers Missed During Endoscopy in England. Clin. Gastroenterol. Hepatol 2015;13:1264–1270.e1. [DOI] [PubMed] [Google Scholar]
- 82.Teh JL, Tan JR, Lau LJF, et al. Longer examination time improves detection of gastric cancer during diagnostic upper gastrointestinal endoscopy. Clin. Gastroenterol. Hepatol 2015;13:480–487.e2. [DOI] [PubMed] [Google Scholar]
- 83.Khor C, Chia CK, Lim LG, et al. Systematic endoscopic surveillance is feasible for the detection of early gastric neoplasia. J. Gastroenterol. Hepatol 2013;28:503. [Google Scholar]
- 84.Telford JJ, Enns RA. Editorial: Endoscopic Missed Rates of Upper Gastrointestinal Cancers: Parallels With Colonoscopy. Am. J. Gastroenterol 2010;105:1298–1300. [DOI] [PubMed] [Google Scholar]
- 85.Li D, Bautista MC, Jiang S-F, et al. Risks and Predictors of Gastric Adenocarcinoma in Patients with Gastric Intestinal Metaplasia and Dysplasia: A Population-Based Study. Am. J. Gastroenterol 2016;111:1104–1113. [DOI] [PubMed] [Google Scholar]
- 86.de Vries AC, van Grieken NCT, Looman CWN, et al. Gastric Cancer Risk in Patients With Premalignant Gastric Lesions: A Nationwide Cohort Study in the Netherlands. Gastroenterol 2008;134:945–952. [DOI] [PubMed] [Google Scholar]
- 87.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kupfer SS. Gaining Ground in the Genetics of Gastric Cancer. Gastroenterol 2017;152:926–928. [DOI] [PubMed] [Google Scholar]
- 89.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med 2001;345:784–9. [DOI] [PubMed] [Google Scholar]
- 90.Ciok J, Dzieniszewski J, Lucer C. Helicobacter pylori eradication and antral intestinal metaplasia--two years follow-up study. J. Physiol. Pharmacol 1997;48 Suppl 4:115–122. [PubMed] [Google Scholar]
- 91.Salih BA, Abasiyanik MF, Saribasak H, et al. A follow-up study on the effect of Helicobacter pylori eradication on the severity of gastric histology. Dig. Dis. Sci 2005;50:1517–1522. [DOI] [PubMed] [Google Scholar]
- 92.Lu B, Chen MT, Fan YH, et al. Effects of Helicobacter pylori eradication on atropic gastritis and intestinal metaplasia: A 3-year follow-up study. World J. Gastroenterol 2005;11:6518–6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yamada T, Miwa H, Fujino T, et al. Improvement of gastric atrophy after Helicobacter pylori eradication therapy. J. Clin. Gastroenterol 2003;36:405–410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.