Abstract
Amygdala abnormalities are widely documented in bipolar spectrum disorders (BSD). Amygdala volume typically is measured after BSD onset; thus, it is not known whether amygdala abnormalities predict BSD risk or relate to the disorder. Additionally, past literature often treated the amygdala as a homogeneous structure, and did not consider its distinct subnuclei and their differential connectivity to other brain regions. To address these issues, we used a behavioral high‐risk design and diffusion‐based subsegmentation to examine amygdala subnuclei among medication‐free individuals with, and at risk for, BSD. The behavioral high‐risk design (N = 114) included low‐risk (N = 37), high‐risk (N = 47), and BSD groups (N = 30). Diffusion‐based subsegmentation of the amygdala was conducted to determine whether amygdala volume differences related to particular subnuclei. Individuals with a BSD diagnosis showed greater whole, bilateral amygdala volume compared to Low‐Risk individuals. Examination of subnuclei revealed that the BSD group had larger volumes compared to the High‐Risk group in both the left medial and central subnuclei, and showed larger volume in the right lateral subnucleus compared to the Low‐Risk group. Within the BSD group, specific amygdala subnuclei volumes related to time since first episode onset and number of lifetime episodes. Taken together, whole amygdala volume analyses replicated past findings of enlargement in BSD, but did not detect abnormalities in the high‐risk group. Examination of subnuclei volumes detected differences in volume between the high‐risk and BSD groups that were missed in the whole amygdala volume. Results have implications for understanding amygdala abnormalities among individuals with, and at risk for, a BSD.
Keywords: amygdala, bipolar disorder, diffusion‐based subsegmentation, gray matter volume, high‐risk design, morphometry
Amygdala volume abnormalities may be a feature of BSD, as opposed to reflecting a pre‐existent marker of risk. Amygdala subnuclei volume increased our sensitivity to detect differences between BSD and High‐Risk groups that were not observable at the whole volume level; the BSD group had larger left medial and central subnuclei volumes than the High‐Risk group and a larger right lateral subnucleus than the Low‐Risk group. In the BSD group, amygdala subnuclei volumes related to clinical metrics of severity, including time since first BSD episode and the number of lifetime hypo/manic episodes
1. INTRODUCTION
Bipolar spectrum disorders (BSD) affect ~4.4% of the global population (Angst, Stassen, Clayton, & Angst, 2002; Merikangas et al., 2007; Nusslock & Alloy, 2017). Previous neuroimaging research on BSD typically has compared individuals with and without bipolar disorder (e.g., Bipolar I). This approach, however, has done little to discriminate markers of risk for BSD from features of BSD (i.e., disease sequalae). Unfortunately, fewer studies have examined individuals at elevated risk for BSD to identify markers of such risk. Additionally, individuals with BSD frequently are misdiagnosed, thus leading to improper or inadequate treatment (Hirschfeld, Lewis, & Vornik, 2003). This problem emphasizes a need for biomarkers that can distinguish BSD from other disorders. Therefore, examining individuals at risk for BSD has important implications for facilitating accurate and timely diagnosis.
Past studies have documented that amygdala abnormalities distinguish BSDs from schizophrenia and major depressive disorder (Beyer & Krishnan, 2002; Kempton, Geddes, Ettinger, Williams, & Grasby, 2008; McDonald et al., 2004; Phillips & Swartz, 2014; Post, Fleming, & Kapczinski, 2012; Soares & Mann, 1997). Furthermore, BSDs have shown distinct amygdala features with respect to volume (Altshuler et al., 2000; Fournier, Keener, Almeida, Kronhaus, & Phillips, 2013; Hibar et al., 2016), structural connectivity (Jalbrzikowski et al., 2017), and cellular structure (Gigante et al., 2011; Rubinow et al., 2016). Questions remain whether amygdala abnormalities precede the onset of BSD (Bechdolf et al., 2012), or arise as a result of the mood episodes (Bitter, Mills, Adler, Strakowski, & DelBello, 2011; Schneider, DelBello, McNamara, Strakowski, & Adler, 2012). Although a few studies address this question, the conclusions are inconsistent. Possible sources of inconsistency in the BSD amygdala literature include the impact of medication use (Savitz et al., 2010), the effects of normative brain development on BSD studies, and age‐of‐onset of the illness (Doty et al., 2008; Savitz et al., 2010; Usher, Leucht, Falkai, & Scherk, 2010). Another source of inconsistency may arise from a lack of consideration of the amygdala's distinct subnuclei and their differential connectivity to other regions (Hibar et al., 2016; Schneider et al., 2012; Usher et al., 2010). Abnormalities in subnuclei volume may be connected to specific amygdala subnetworks and provide a basis for integration into the larger amygdala connectivity literature. To address these issues, the present study had three objectives. First, we used a behavioral high‐risk design to compare amygdala volume in individuals at risk for BSD to individuals with a BSD diagnosis. Second, we used diffusion‐based subsegmentation to examine amygdala subnuclei in both BSD risk and BSD individuals. Finally, within the BSD group, we conducted descriptive analyses on the relationship between subnuclei volumes and clinical metrics of severity, including time since first onset of a BSD episode and the number of BSD episodes an individual has had during their lifetime.
1.1. Inconsistency in the BSD amygdala literature
The most notable inconsistency in the amygdala literature is that adults with a BSD typically display an enlarged amygdala volume (Arnone et al., 2009; Brambilla et al., 2003; Pearlson et al., 1997), whereas children with a BSD have smaller amygdala volume (Blumberg et al., 2005; Blumberg et al., 2006; Chang et al., 2005; DelBello, Zimmerman, Mills, Getz, & Strakowski, 2004; Frazier et al., 2005; Getz et al., 2002; Post et al., 2012; Rosso et al., 2007; Schneider et al., 2012). Although there is consistency within the adult and child literatures, questions remain about whether amygdala volume abnormalities are a risk factor or a consequence of BSD onset. Bechdolf et al. (2012) showed that children at high‐risk for BSD have a smaller amygdala volume than children at high‐risk for psychosis, suggesting that amygdala volume abnormalities are a marker that distinguishes risk for BSD from psychosis (Bechdolf et al., 2012). In contrast, Bitter et al. (2011) found normal amygdala volume at the time of first BSD episode and decreased volume at 1‐year follow‐up, suggesting volumetric differences are related to time since BSD onset, and thus, are not an early marker of risk (Bitter et al., 2011). Similarly, an adult onset BSD study found that an increased number of BSD episodes related to a larger amygdala volume, suggesting amygdala abnormalities mark a longer length or more severe course of illness (Schneider et al., 2012).
1.2. Amygdala subnuclei in BSD
Past studies have treated the amygdala as a homogenous structure (Schneider et al., 2012), despite the distinct structural connectivity of amygdala subnuclei. Considerable evidence shows that the basal subnucleus is more connected to frontal regions, the lateral subnucleus is connected to more temporal regions, and medial and central subnuclei are connected to distinct midbrain structures (Mori et al., 2017; Saygin, Osher, Augustinack, Fischl, & Gabrieli, 2011; Solano‐Castiella et al., 2010) (Table 1). Unfortunately, the literature on whole volume amygdala is not easily integrated into the connectivity literature because of its limited specificity with respect to network connections. The functional connectivity literature also relates the amygdala to BSD risk (Forde et al., 2015; Roberts et al., 2017), reward sensitivity underlying BSD (Manelis et al., 2016), and mood episodes (Brady et al., 2016; Brady et al., 2017; Spielberg et al., 2016). Volumetrically, amygdala subnuclei largely have remained unexamined in BSD because of the limited resolution of MRI images to differentiate amygdala subnuclei (Saygin et al., 2011). Furthermore, attempts to measure amygdala subnuclei with hand‐traced subsegmentation are labor intensive and error‐prone (Saygin et al., 2011). However, amygdala subnuclei can be examined in vivo using a multimodal approach that leverages the unique structural connectivity of amygdala subnuclei using diffusion‐based subsegmentation (Mori et al., 2017; Saygin et al., 2011; Solano‐Castiella et al., 2010). Diffusion based subsegmentation has been validated in vivo against hand tracings (Saygin et al., 2011; Solano‐Castiella et al., 2010), in vitro against postmortem histology (Mori et al., 2017), and in functional connectivity (Balderston, Schultz, Hopkins, & Helmstetter, 2015), suggesting that this multimodal approach is highly robust. There are two additional advantages of diffusion‐based subsegmentaion. First, it takes a personalized perspective and each participant's amygdala is subsegmented based on their own structural connectivity and anatomy (Mori et al., 2017). This personalized approach increases sensitivity and power to model individual differences in subnuclei volume. Second, it can facilitate our ability to contextualize abnormalities in amygdala subnuclei volume within the larger amygdala connectivity literature.
TABLE 1.
Amygdala subnuclei connectivity, behavior, and mood episode associations
| Amygdala subnuclei | Postmortem/functional connectivity | Anatomical connectivity |
|---|---|---|
| Basal | Enlarged in BSD compared to healthy individuals (Rosso et al., 2007), mania connectivity (Chang et al., 2005; DelBello et al., 2004) | Para/hippocampus, rostral ACC, mOFC, occipital, insula, subnucleus accumbens, and frontal areas (Forde et al., 2015; Anticevic et al., 2013; Torrisi et al., 2013; Chai et al., 2011) |
| Lateral | Enlarged in BSD compared to healthy individuals, (Fournier et al., 2013; Getz et al., 2002) schizophrenia (Mori et al., 2017), and major depression (Rubinow et al., 2016; Stefanacci & Amaral, 2000) | Temporal pole, fusiform, lOFC, superior temporal, inferior temporal (Brady et al., 2016; Spielberg et al., 2016; Anticevic et al., 2013) |
| Medial | Abnormal BSD compared to major Depression (Blumberg et al. 2005; Blumberg et al., 2006; Stefanacci et al., 2002) and schizophrenia; (Fournier et al., 2013; Endicott & Spitzer, 1978) associated with hypomanic risk traits (Damme et al., 2017) | Striatal and hippocampal regions (Anticevic et al., 2013; Brady et al., 2016; Brady, Margolis, Masters, Keshavan, & Öngür, 2017; Spielberg et al., 2016) |
| Central | ‐ | Hypothalamus, basal forebrain, and brainstem (Brady et al., 2016; Forde et al., 2015; Manelis et al., 2016; Roberts et al., 2017; Spielberg et al., 2016) |
Abbreviations: ACC, anterior cingulate cortex; BSD, bipolar spectrum disorder; mOFC, medial orbitofrontal cortex; lOFC, lateral orbitofrontal cortex.
Although no in vivo study has examined BSD amygdala subsegmentation, postmortem evidence suggests that cellular and volumetric amygdala abnormalities in BSD may arise from specific subnuclei (Berretta, Pantazopoulos, & Lange, 2007; Bezchlibnyk et al., 2007; Chance, Esiri, & Crow, 2002; Fournier et al., 2013; Li et al., 2015; Mamah, Alpert, Barch, Csernansky, & Wang, 2016; Rubinow et al., 2016). Some of these postmortem studies found that an enlargement in adult onset BSD amygdala volumes is specific to the basal and lateral subnuclei (Chance et al., 2002; Rubinow et al., 2016). Additional evidence points to abnormal cell structure and density selectively in the medial and lateral subnuclei (Berretta et al., 2007; Bezchlibnyk et al., 2007). Finally, postmortem amygdala abnormalities were unique to individuals with a BSD compared to individuals with schizophrenia (Chance et al., 2002; Mamah et al., 2016) or major depression (Fournier et al., 2013; Li et al., 2015). Subnuclei abnormalities may have specific effects on related functional connectivity as a result of the distinct structural connectivity of each subnucleus. For example, BSD individuals in a manic episode showed increased functional connectivity between the amygdala and superior frontal areas (Brady et al., 2016; Brady et al., 2017; Spielberg et al., 2016) and rostral anterior cingulate cortex (ACC), Table 1 (Brady et al., 2016; Brady et al., 2017). These functional connectivity abnormalities may relate to the basal subnucleus, which has distinct connectivity with superior frontal and rostral ACC (Amaral & Price, 1984; Carmichael & Price, 1995; Gloor, 1994; Stefanacci & Amaral, 2000). However, current functional connectivity findings rely on connectivity among whole volumes, and it is not easily integrated into parallel, postmortem literature that examines subnuclei. If subnuclei were examined in vivo, then these literatures may be integrated to form a better understanding of the role of the amygdala in BSD. Therefore, examining amygdala subnuclei may refine our understanding of the role of the amygdala in BSD onset and course and increase our sensitivity to profiles of connectivity associated with BSD risk.
1.3. The current study
The present study will elucidate the role of the amygdala in BSD in three critical ways. First, a behavioral high‐risk design will examine whether amygdala volume relates to risk for developing BSD or to the presence of BSD illness. In a novel analysis, we will examine a behavioral high‐risk group. If the high‐risk group has significantly larger amygdala volume than the low‐risk group, this would suggest that amygdala enlargement is a marker of risk that predates the onset of the illness, in line with risk models laid out in Wiggins et al. (2020). If, however, the high‐risk group does not differ from the low‐risk group and has significantly smaller volumes than the BSD group, then this suggests that amygdala volume reflects features of BSD alone. Second, diffusion‐based subsegmentation of the amygdala will examine the specificity of the volume findings. Based on amygdala functional connectivity abnormalities during manic episodes (Brady et al., 2016; Brady et al., 2017; Spielberg et al., 2016), we expect that the presence of a BSD will be related to enlarged basal subnuclei compared with groups who have never had a hypo/manic episode (e.g., low‐risk and high‐risk groups). We also expect to replicate postmortem data that found enlarged amygdala volume in both basal and lateral subnuclei among participants with a BSD compared with low‐risk individuals (Chance et al., 2002; Rubinow et al., 2016). Given that postmortem abnormalities in lateral and medial subnuclei distinguished BSD from clinically similar disorders (Chance et al., 2002; Li et al., 2015; Mamah et al., 2016; Mori et al., 2017), we expect that these subnuclei will distinguish the BSD group from the high‐risk group. Finally, we conduct a set of descriptive analyses within the BSD group examining the relationship between amygdala subnuclei volumes and time since first BSD episode (Bechdolf et al., 2012) and number of lifetime BSD episodes (Bitter et al., 2011). If amygdala volume relates to these clinical metrics of severity, this would provide further evidence that amygdala volumes relate to features of BSD onset, rather than reflecting preexistent risk factors (Bechdolf et al., 2012; Bitter et al., 2011).
2. METHODS AND MATERIALS
2.1. High‐risk design
Participants were recruited from a large, ongoing longitudinal project. In the longitudinal study, participants (ages 14–19) were recruited from the Philadelphia area, and completed three measures: the expanded Schedule for Affective Disorders and Schizophrenia‐Lifetime interview (Alloy et al., 2012; Endicott & Spitzer, 1978) (exp‐SADS‐L), Behavioral Inhibition System/Behavioral Activation System scales (Carver & White, 1994) (BIS/BAS), and the Sensitivity to Punishment/Sensitivity to Reward Questionnaire (Torrubia, Ávila, Moltó, & Caseras, 2001) (SPSRQ). The exp‐SADS‐L was used to identify any BSDs, exclude individuals with a primary psychotic disorder, determine age of BSD onset and the number of lifetime BSD episodes, and identify other past diagnoses and family history of BSD, Table 2.
TABLE 2.
Demographic and clinical features of the sample
| Demographics | Low‐risk | High‐risk | BSD | Total | Statistics | p‐value |
|---|---|---|---|---|---|---|
| Sample size (n) | 37 | 47 | 30 | 114 | ||
| Age—M(StD) | 21.11(2.04) | 20.64(2.03) | 21.33(2.36) | 20.71(2.00) | F(1,113) = 2.6 | p = .11 |
| Gender (% female) | 51% | 43% | 50% | 47% | χ2(113) = 0.59 | p = .75 |
| Familial bipolar diagnosis | 2.70% | 6.38% | 0.00% | 3.51% | ||
| Comorbid diagnoses | Low‐risk | High‐risk | BSD | Total | χ2 | p‐value |
| Depression | 45.95% | 57.45% | NA | 39.47% | 22.74 | >.0001 |
| Anxiety disorder | 21.62% | 29.79% | 36.67% | 28.95% | 1.85 | .39 |
| ADHD | 0.00% | 8.51% | 3.33% | 4.39% | 3.78 | .15 |
| Substance use disorder | 18.92% | 17.02% | 30.00% | 21.05% | 1.9 | .39 |
Note: Familial bipolar diagnosis includes any person with a first‐ or second‐degree relative; Depression category includes any depressive diagnosis (e.g., dysthymia, major depression, depression not otherwise).
Abbreviations: BSD, bipolar spectrum disorder; NA, not applicable as depression symptoms are contained within the BSD diagnosis.
The longitudinal study employed a behavioral high‐risk design in which participants are selected because they possess a behavioral characteristic associated with risk for developing a particular disorder (Alloy, Bender, et al., 2012). Participants were selected for this research based on how sensitive they are to rewarding stimuli in their environment. Self‐reported reward sensitivity has predictive validity for the onset and course of BSDs (Nusslock & Alloy, 2017). Specifically, elevated self‐reported reward sensitivity is associated with a greater likelihood of having a lifetime BSD (Alloy et al., 2006), a shorter time to recurrences of hypo/manic episodes (Alloy et al., 2008), an increase in manic symptoms among recovered individuals with bipolar I disorder (Meyer, Johnson, & Winters, 2001), and a greater likelihood of progressing to a more severe bipolar diagnosis among those with milder BSDs (Alloy et al., 2012). Furthermore, Alloy, Bender, et al. (2012) showed that individuals with elevated self‐reported reward sensitivity had a significantly greater likelihood of first lifetime onset of BSD than individuals with moderate reward sensitivity across an average of 12.8 months of follow‐up (12.9 vs. 4.2%). With further follow‐up (31.7 months on average), Alloy, Boland, Ng, Whitehouse, and Abramson (2015) found the rate of first onset of BSD was 14.5% in the high reward group versus 4.2% in the moderate reward group. Finally, Alloy, Bender, et al. (2012) reported that reward hypersensitivity prospectively predicts the likelihood of developing a first onset of BSD above and beyond a family history of bipolar disorder. This suggests that reward sensitivity is a risk factor above and beyond family history, and a very appropriate variable to select high‐risk participants for the present study. BIS/BAS scales and SPSRQ were used to define moderate reward sensitivity (low‐risk) and high reward sensitivity (high‐risk) groups. Participants between the 40th and 60th percentile on the BAS and sensitivity to reward (SR) scales were classified as having moderate reward sensitivity and considered low‐risk for BSD (Alloy, Bender, et al., 2012). Participants in the 85th to 100th percentile on both measures were classified as high reward and high‐risk for BSD (see Appendix S1).
Finally, for the current study subsample, we used the exp‐SADS‐L (Endicott & Spitzer, 1978) to further classify high reward, high‐risk participants based on whether they had a BSD at the time of fMRI scanning for the present study. This provided us with a unique opportunity to examine whether amygdala abnormalities are associated with risk for BSD or arise with the disorder. Group differences will be interpreted using the risk and resilience nosology described by Wiggins et al. (2020), whereby amygdala abnormalities will be described as risk markers if they appear in the high‐risk and BSD groups, but not in the low‐risk group. Similarly, amygdala abnormalities will be described as resilience markers if they appear in the high‐risk group, but not in the low‐risk or BSD groups (Wiggins et al., 2020). Finally, amygdala abnormalities will be described as markers of disorder sequelae if they appear in the BSD group, but not in the low‐risk or high‐risk groups.
2.2. Participants
Participants in this study were recruited from the larger longitudinal study discussed above (n = 539). A total of 131 young adults from the longitudinal study (52% female; age 18–27) completed the MRI portion of this study. Participants were excluded from the MRI study if they had contraindications for MRI scanning (e.g., head trauma, pregnancy), were left‐handed based on the Chapman Handedness Scale (Chapman & Chapman, 1987), or were taking a psychiatric medication at the time of fMRI scanning (see Appendix S1). We excluded six participants for use of psychiatric medication. All data were visually inspected for quality of both amygdala labeling and diffusion tensor imaging (DTI); 11 participants were excluded due to poor MRI data quality. Final analyses were based on a sample of 114 participants: 37 low‐risk (i.e., moderate reward sensitivity), 47 high‐risk (i.e., high reward sensitivity), and 30 individuals with high reward sensitivity and a BSD (9 Bipolar not otherwise specified [NOS]; 3 cyclothymia; 18 bipolar II; 4 bipolar I). We used the exp‐SADS‐L (Endicott & Spitzer, 1978) to assess for family history of a BSD in first‐ and second‐degree relatives for all participants. Among BSD participants, we used the exp‐SADS‐L to assess the amount of time since first BSD episode and the number of BSD episodes a participant had in their life prior to MRI scanning. Participants provided informed written consent and were compensated $100. All protocols were approved by the Temple University IRB.
2.3. MRI data acquisition
All MRI data were collected on a 3 T Verio MR scanner (Siemens, Erlangen, Germany) at Temple University. Structural images were collected using a T1‐weighted anatomical image (sagittal plane; repetition time [TR] 1,600 ms; echo time [TE] 2.46 ms; .5 mm3 isomorphic voxels, 176 interleaved slices; FOV 515; flip angle 57). Diffusion weighted images were collected using axial brain slices with the following parameters: 64 diffusion‐weighted (b = 1,000 s/mm2) and 1 nondiffusion weighted scan; field of view 190 × 190 mm; voxel size 2 × 2 × 2 mm; TR = 9,900 ms; TE = 90 ms.
2.4. Structural subsegmentation
Structural images were used to create anatomical labels in individuals' native space. FreeSurfer version 5.3.0 automatic segmentation software was used to extract surfaces (http://surfer.nmr.mgh.harvard.edu/) (Fischl, 2012). FreeSurfer defined the amygdala and 25 target masks per hemisphere. Amygdala labels were used to compare volumes across risk groups, and as a seed point for diffusion‐based subsegmentation. The additional 25 targets were used as structural connectivity targets to segment the amygdala into subnuclei (Appendix S1) (Saygin et al., 2011).
2.5. Diffusion‐based subsegmentation
Diffusion preprocessing was corrected for field heterogeneities detected in the initial unweighted image, eddy current correction, masking the brain, and extracting the skull using the FMRIB Software Library (FSL). DTI tractography and a connectivity‐based seed classification analysis were used to segment the amygdala based on the unique anatomical connectivity of the subnuclei (Saygin et al., 2011). Probabilistic diffusion tractography calculated 25,000 streamline samples in each amygdala seed voxel to create a distribution to each of the 25 cortical and subcortical target masks. This tractography process resulted in 25 classification matrices indexing voxel by voxel amygdala connectivity with a corresponding target mask. These values were converted to percentiles, which then were thresholded and binarized (Saygin et al., 2011). Boolean expressions, which described the unique anatomical connectivity patterns, allowed subsegmentation of the amygdala into four subnuclei: (a) lateral subnucleus, (b) basal subnucleus, (c) medial subnucleus, and (d) central subnucleus, based in their relative connectivity to ipsilateral targets (Saygin et al., 2011). These expressions and methods have been validated against hand tracings (Saygin et al., 2011) and are based on known anatomical connections. The advantages of diffusion‐based subsegmentation over other methods (e.g., Freesurfer's segmention algorithm) include: (a) It takes a personalized approach and each participant's amygdala is subsegmented based on their own structural connectivity and anatomy, thus increasing power to assess individual differences, which is a focus of the present paper, and (b) it facilitates our ability to contextualize amygdala subnuclei volume findings within the larger amygdala connectivity literature.
2.6. Data analysis
For group analyses of whole amygdala volume and amygdala subnuclei, we first examined if there was a group by hemisphere interaction. If no such effect was observed, we combined the hemispheres to examine bilateral amygdala volume in an effort to minimize family wise error rate. If there was an effect of hemisphere, we examined the left and right amygdala volumes separately. We employed Tukey's correction method to control for multiple comparisons in all group‐based analyses. We conducted a set of descriptive analyses within the BSD group examining the relationship between amygdala subnuclei volumes and metrics of clinical severity, including the amount of time since first BSD episode and the total number of BSD episodes a person has had in their lifetime. These analyses with clinical metrics are for descriptive purposes and thus we did not control for multiple comparisons. We provide the r‐values for these descriptive analyses as a metric of direction and effect size. With respect to covariates, we followed the suggestion of Miller and Chapman (2001) and corrected for relevant demographic variables that were related to group status or the outcome variable of interest (i.e., amygdala volume).
3. RESULTS
3.1. Sample characteristics and clinical symptoms
Analyses of variance (ANOVA) and Chi‐square tests examined demographic differences between groups. There were no significant between‐group differences in age, F(1,113) = 2.6, p = .11, handedness score (58), F (1,113) = .17, p = .68, or sex, χ2(1) = 0.59, p = .75. Furthermore, males and females did not significantly differ on amygdala volume, t(114) = 1.69, p = .094, and thus we did not correct for sex in analyses. Follow‐up analyses indicated that the addition of sex as a covariate did not change the magnitude or direction of our reported effect sizes. In particular, the inclusion of sex as a variable resulted in an average change in partial‐eta2 of .002, which is a small effect size. Thus, sex had a negligible effect on results. By contrast, age was related to amygdala volume, r(114) = .23, p = .02, and thus we control for age in all group‐based analyses. In a comparison of lifetime diagnoses (see Table 2), more individuals in the high‐risk group had a lifetime diagnosis of unipolar depression compared to individuals in the Low‐Risk group, χ2(1) = 29.36, p < .001, and thus, we control for lifetime depression in all group‐based analyses. As expected given our selection criteria, the groups were significantly different on BAS (56) and SPSRQ‐SR scores (57), such that the low‐risk group scored significantly lower than both the High‐Risk and BSD groups (p's < .001; see Appendix S1). The High‐Risk and BSD groups did not differ from one another on either the BAS (p = .14) or SPSRQ‐SR (p = .19). Four participants had a family history of BSD (three high‐risk participants and one low‐risk participant); removing these four participants from analyses did not impact the magnitude or direction of the effects.
3.2. Whole amygdala volume
An analysis of covariance (ANCOVA) compared bilateral whole amygdala volume between the low‐risk, high‐risk, and BSD groups, controlling for extracted brain volume, age, and lifetime depression diagnoses. We examined bilateral whole amygdala volume given that there was no hemisphere by group interaction, F(113,2) = .098, p = .91. The overall model was significant, F(1,113) = 16.49, p < .001, and there was a significant main effect of group F(1,113) = 3.67, p = .03, Table 3. Pairwise comparisons revealed that the BSD group had significantly enlarged amygdala volume compared to the low‐risk group, t (66)= 1.87, p = .04. The high‐risk group was not significantly different from the low‐risk or BSD groups, p's > .36, Figure 1.
TABLE 3.
Significant group effects in whole amygdala volume and amygdala subnuclei volumes
| Statistic | p‐value | ||||
|---|---|---|---|---|---|
| Main effect of bilateral whole amygdala volume | F(113,2) = 3.67 | .03* | |||
| Post hoc comparisons on right lateral subnucleus volume | |||||
| Low‐risk | High‐risk | BSD | Contrast | Statistic | |
| M (SEM) uncorrected | 3,477.95(77.69) | 3,551.64(54) | 3,563.91(71.3) | BSD > low‐risk* | t (1,66)= 1.87* |
|
Z‐score (SEM)uncorrected |
−.16(.17) | −.10(.12) | .22(.21) | High‐risk > low‐risk | t(1,83) = −0.83 |
| BSD > high‐risk | t(1,76) = −0.86 | ||||
| Statistic | p‐value | ||||
| Main effect of right lateral subnucleus volume | F(113,1) = 5.897 | .01** | |||
| Post hoc comparisons on right lateral subnucleus volume | |||||
| Low‐risk | High‐risk | BSD | Contrast | Statistic | |
| M (SEM) uncorrected | 586.18(36.56) | 657.66(30.16) | 702.93(39.17) | BSD > low‐risk* | t (1,66) = 2.79 |
|
Z‐score (SEM) uncorrected |
−.29(.17) | .04(.14) | .16(.21) | High‐risk > low‐risk | t(1,83) = 1.63 |
| BSD > high‐risk | t(1,76) = .80 | ||||
| Statistic | p‐value | ||||
| Main effect of group on left medial subnucleus volume | F(113,1) = 3.42 | .03* | |||
| Post hoc comparisons on left medial subnucleus volume | |||||
| Low‐risk | High‐risk | BSD | Contrast | Statistic | |
| M (SEM) uncorrected | 295.37(19.30) | 268.59(18.09) | 347.29(27.16) | BSD > low‐risk | t (1,66) = 1.25 |
|
Z‐score (SEM) uncorrected |
−.02(.15) | −.24(.14) | .29(.23) | High‐risk > low‐risk | t(1,83) = 0.79 |
| BSD > high‐risk* | t(1,76) = 1.98* | ||||
| Statistic | p‐value | ||||
| Main effect of group on left central subnucleus volume | F(113,1) = 5.42 | .0005*** | |||
| Post hoc comparisons on left central subnucleus volume | |||||
| Low‐risk | High‐risk | BSD | Contrast | Statistic | |
| M (SEM) uncorrected | 123.72(20.12) | 77.4(9.72) | 174.15(33.81) | BSD > low‐risk | t (1,66) = −1.60 |
|
Z‐score (SEM) uncorrected |
0.02(.15) | −.33(.08) | 0.32(.26) | High‐risk > low‐risk | t(1,83) = −1.68 |
| BSD > high‐risk* | t(1,76) = 3.09* | ||||
*<.05 **.01 ***.005. Abbreviation: BSD, bipolar spectrum disorders.
FIGURE 1.
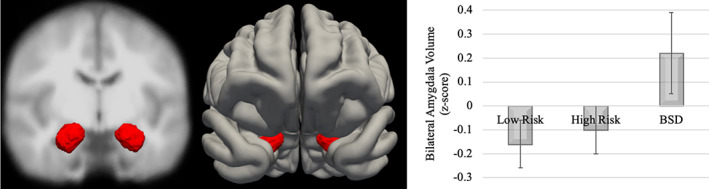
Bilateral whole amygdala volume by group (Note: Error bars display SE of Mean; BSD, bipolar spectrum disorder)
3.3. Amygdala subnuclei volume
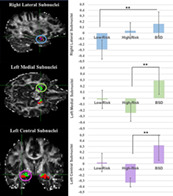
Separate ANCOVAs correcting for extracted brain volume, age, and lifetime depression diagnoses were run for each of the subnuclei for each hemisphere (given a significant hemisphere by group interaction, F(113,2) = 2.99, p = .03), Table 3. These analyses revealed a significant main effect of group in the right lateral subnucleus, F(113,2) = 5.897, p = .01, Figure 2. Pairwise comparisons indicated significantly larger right lateral subnuclei in the BSD group (M = 702.93, SD = 39.17) compared to the Low‐Risk group, t (66)= 2.79, p = .03, Table 3. No other right hemisphere subnuclei (i.e., basal, medial, or central) differed across groups, all p's > .14. There also was a significant main effect of group in both the left medial subnucleus, F(113,2) = 3.42, p = .03, and left central subnucleus, F(113,2) = 5.42, p = .0005, Figure 2. Pairwise comparisons in the left medial subnucleus revealed a significantly larger left medial subnuclei in the BSD group than the high‐risk group, t(76) = 1.98 p = .02. Similarly, pairwise comparisons in the left central subnucleus revealed significantly larger left central subnuclei in the BSD than the High‐Risk group, t(76) = 3.09, p = .01, Table 3. Neither the left basal nor lateral subnuclei were significantly different across the groups, all p's > .21.
FIGURE 2.
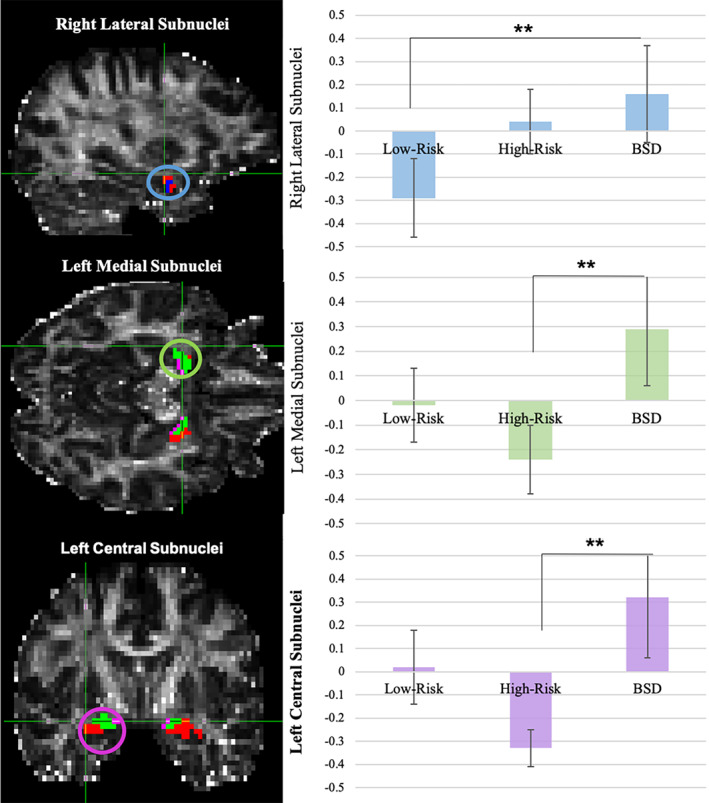
Amygdala subnuclei volume by group: (Note: Left Panel: The medial subnucleus is depicted in green, the basal subnucleus is depicted in red, the lateral subnucleus is depicted in blue, and the central subnucleus is depicted in purple; Right Panel: Significant group effects are depicted here as uncorrected amygdala z‐scores; Error Bars are SE of the Mean; significant effects are noted with asterisks; BSD, bipolar spectrum disorders)
3.4. Relationships between amygdala subnuclei volume and clinical severity metrics among BSD participants
We next conducted a series of descriptive simultaneous multiple regression analyses within the BSD group examining the relationship between amygdala subnuclei volumes and time since first BSD episode and the number of lifetime BSD episodes, controlling for extracted brain volume and current age. These analyses examined relationships between subnuclei volumes with the length in years of illness for both first depression episode and first hypo/mania episode. Similarly, the number of episodes was examined separately for both depression episodes and hypo/mania episodes. Time since first depression episode was significantly related to right central subnucleus volume, r = −.41, t (25)= − 2.21, p = .03, such that a smaller volume corresponded to longer length of illness. Time since first hypo/mania episode was related to the volume of the left central subnucleus, r = .45, t (23)=2.17, p = .04, with a greater volume being associated with a longer length of illness. A greater number of lifetime hypo/mania episodes was associated with larger right central subnuclei, r = .56, t (26)=2.91, p = .008, and smaller right basal subnuclei, r = −.42, t (26)=2.56, p = .01, Figure 3. The number of lifetime depressive episodes was not related to any of the amygdala subnuclei volumes.
FIGURE 3.
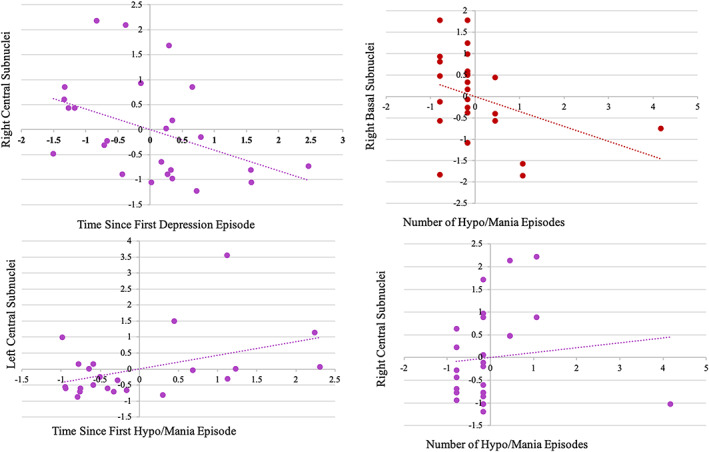
Relationship between amygdala subnuclei and time since first lifetime BSD Episode and Number of BSD Episodes among participants in BSD group (Note: BSD, bipolar spectrum disorders)
4. DISCUSSION
The present study elucidates the role of the amygdala in BSD in three critical ways. First, our high‐risk design suggests that amygdala volume abnormalities are a feature of BSD, as opposed to reflecting a pre‐existent marker of risk. Second, analyzing amygdala subnuclei volume increased our sensitivity to detect differences between BSD and high‐risk groups that were not observable at the whole volume level. Specifically, the BSD group had larger left medial and central subnuclei volumes than the High‐Risk group (Alloy, Bender, et al., 2012) and a larger right lateral subnucleus than the low‐risk group. Finally, within the BSD group, amygdala subnuclei volumes related to clinical metrics of severity, including time since first BSD episode and the number of lifetime hypo/manic episodes. Collectively, these findings suggest that an enlarged amygdala is a marker of BSD sequelae (i.e., a potential disease related scar of the disorder) (Wiggins et al., 2020) that is associated with the duration of the illness and number of lifetime episodes, as opposed to reflecting a pre‐existent marker of risk for BSD.
Bilateral amygdala volume was significantly larger in the BSD group than the low‐risk group, replicating past findings (Arnone et al., 2009; Brambilla et al., 2003; Pearlson et al., 1997). Individuals at High‐Risk for BSD (i.e., elevated reward sensitivity) were intermediate between the low‐risk and BSD participants, and did not significantly differ from either group. One possibility is that the high‐risk group includes a mixed sample of healthy individuals with elevated reward sensitivity who are likely to never go on to develop a BSD, and individuals with elevated reward sensitivity who are, in fact, at risk for a BSD. The combination of these two types of participants in the High‐Risk group may lead to an average of the Low‐Risk and BSD volume profiles, and suppress our ability to detect effects for High‐Risk participants. If the high‐risk group contained both of these types of individuals (i.e., those who likely will and will not go on to develop a BSD), then one might expect a bimodal distribution of amygdala volumes among high‐risk participants. We did not, however, observe such a bimodal distribution of amygdala volumes in the high‐risk group, thus challenging this hypothesis (see Appendix S1). Alternatively, an enlarged amygdala volume in BSD indeed may emerge with the onset of BSD, and may increase in size over the course of the illness. In this situation, one might expect that the volume of the amygdala among high‐risk participants is intermediate between the Low‐Risk and BSD groups, as observed in the present study. The relationships among BSD participants in the present study between amygdala subnuclei volumes and time since first BSD episode and number of lifetime BSD episodes supports this latter scenario. Collectively, however, these findings highlight the need for longitudinal studies comparing individuals at behavioral high‐risk who later do and do not develop a BSD.
The present study used diffusion tensor classification to segment the amygdala volume into four subnuclei: basal, lateral, medial, and central (Saygin et al., 2011). Consistent with prior functional connectivity research, the right amygdala significantly differed between the BSD and low‐risk groups (Li et al., 2015; Spielberg et al., 2016). This finding was specific to the right lateral subnucleus, consistent with postmortem studies of volume and cell structure abnormalities in BSD (Berretta et al., 2007; Chance et al., 2002; Rubinow et al., 2016). This finding also is consistent with functional connectivity between the right amygdala and lateral prefrontal cortex that has been linked to increased symptom severity in BSD (Anticevic et al., 2013; Chai et al., 2011; Torrisi et al., 2013). In contrast, the left central and medial subnuclei may be markers of resilience to BSD as these volumes distinguished high‐risk individuals from individuals with a BSD, Figure 2. These findings are consistent with functional connectivity in BSD involving increased connectivity in midbrain subnetworks related to central and medial subnuclei during active man (Saygin et al., 2011). Similarly, they also are consistent with structural connectivity work that relates increased amygdala‐striatal connectivity (originating in the medial subnuclei) to elevated hypomania personality traits (Damme, Young, & Nusslock, 2017). Although the basal subnuclei did not significantly differ between groups, as expected based on functional connectivity findings (Brady et al., 2016; Brady et al., 2017; Spielberg et al., 2016), abnormalities in lateral subnucleus volume may impact the functional connectivity in basal subnucleus networks through high internuclei connectivity between the basal and lateral subnuclei (Mori et al., 2017). Collectively, these analyses indicate that amygdala enlargement in BSD may be specific to certain subnuclei.
Descriptive analyses examined the relationships between amygdala subnuclei volumes and clinical severity metrics among BSD participants. We report that central amygdala volume related negatively to time since first depression episode (on the right side), positively to time since first hypo/mania episode (on the left side), and positively to number of hypo/mania episodes (on the right side). As noted, these findings support the idea that abnormalities in amygdala volume emerge with the onset of the illness, and relate to features of BSD course and severity, as opposed to reflecting a preexistent marker of risk for BSD. Furthermore, whereas past amygdala volume studies on clinical course focused on hypo/manic episodes alone (Bechdolf et al., 2012; Bitter et al., 2011), it may be important to examine all mood episodes (Brady et al., 2016; Brady et al., 2017; Spielberg et al., 2016). The present study suggests that time since depression and time since hypo/mania have opposite associations with central subnuclei volumes. Future studies should examine whether these distinct relations of amygdala subnuclei to episode type may provide further insight into why individuals with a BSD show distinct amygdala volumes from individuals with a major depressive diagnosis (Fournier et al., 2013; Li et al., 2015). However, given the descriptive nature of these analyses, they should be interpreted cautiously and viewed as preliminary until they can be further replicated in future studies.
Taken together, these findings suggest that examining amygdala subnuclei may increase sensitivity to differences between markers of disease sequelae and markers of risk that are otherwise obscured at the whole volume level. Importantly, leveraging structural connectivity of the amygdala to segment the whole amygdala into subnuclei provides a unique opportunity to contextualize volumetric findings in a larger neural connectome and functional connectivity literature. The current study is consistent with functional connectivity literature (Anticevic et al., 2013; Chai et al., 2011; Li et al., 2015; Spielberg et al., 2016; Torrisi et al., 2013) and provides further evidence in support of amygdala subnetwork specificity in the pathophysiology of BSD. Furthermore, the convergence of the current findings across cellular (postmortem) (Berretta et al., 2007; Bezchlibnyk et al., 2007; Chance et al., 2002; Rubinow et al., 2016), functional connectivity (Anticevic et al., 2013; Chai et al., 2011; Torrisi et al., 2013), structural connectivity (Damme et al., 2017), and whole volume literatures (Bechdolf et al., 2012; Bitter et al., 2011) builds a complex, multi‐modal understanding of amygdala dysfunction underlying BSD sequelae and resilience.
The current study had a number of strengths. We minimized the confounding effects of medication and gray matter changes related to normative aging by only including individuals who were free from psychiatric medication at the time of the scan and who were in the young adult age range. Our findings do not appear to be affected by familial risk for BSD, given that only four participants in the present study had a family history of BSD. Furthermore, removing these four participants had no impact on the direction or magnitude of the results. Although this study has multiple strengths, there are also some limitations. First, although the structural classification of amygdala subnuclei has been validated against high‐resolution hand tracing, there may be limitations of this approach compared to high‐resolution structural scans and in vitro, histological tracing of subnuclei. If this approach does not faithfully reflect subcortical volumes, it may still highlight important information about differences in the structural connectivity of the amygdala subnuclei and facilitate our ability to contextualize abnormalities in amygdala subnuclei volume within the larger amygdala connectivity literature. Furthermore, the personalized perspective that diffusion‐based subsegmentation takes, where each person's amygdala is subsegmented based on their own anatomy, can increase sensitivity to model individual differences. Second, although the current study is similar in size to previous research on this topic (Brady et al., 2016; Brady et al., 2017; Spielberg et al., 2016), larger sample sizes may be more effective in examining moderators of the relationship between amygdala volume and BSD risk, including sex and BSD symptom profiles (Wiggins et al., 2020). Third, the BSD group included individuals across the bipolar spectrum, including bipolar I, bipolar II, cyclothymia, and bipolar NOS, and future studies should examine variance between BSD subtypes. Finally, despite the strengths of our behavioral‐high risk design, the cross‐sectional nature of our study precludes any strong conclusions about causality. Future research using a multiwave longitudinal design is needed to fully understand whether abnormalities in amygdala volume emerge with the onset of BSD, or reflect a preexistent marker of risk for the illness.
5. DISCLOSURES
Authors have nothing to disclose.
Supporting information
Appendix S1: Supporting information
ACKNOWLEDGMENTS
This project was supported by National Institute of Mental Health grants (R01 MH077908 and R01 MH102310) to L. B. A. The National Institute of Mental Health (T32NS047987) supported the work of K. S. D for this project. Support for R. N. comes from the National Institute of Mental Health (R01 MH100117 and R01 MH077908), the Ryan Licht Sang Bipolar Foundation, and the Chauncey and Marion D. McCormick Family. We would like to thank Michael Weston, Wan Kwok, Laura Padilla, Virginia Hoch, and Ajay Nadig for their assistance on quality assurance on segmentation label accuracy.
Damme KSF, Alloy LB, Young CB, et al. Amygdala subnuclei volume in bipolar spectrum disorders: Insights from diffusion‐based subsegmentation and a high‐risk design. Hum Brain Mapp. 2020;41:3358–3369. 10.1002/hbm.25021
Funding information National Institute of Mental Health, Grant/Award Numbers: MH077908, MH100117‐01, MH102310, R01 MH077908, R01 MH100117, R01 MH102310, T32NS047987
DATA AVAILABILITY STATEMENT
Data preparation scripts are available at https://github.com/katedamme/Amygdala‐Subnuclei‐Volume‐Abnormalities‐across‐the‐Bipolar‐Spectrum‐Insight‐from‐Diffusion‐based‐S. Data may be made available upon request to the PIs (Robin Nusslock and Lauren Alloy).
REFERENCES
- Alloy, L. B. , Abramson, L. Y. , Walshaw, P. D. , Cogswell, A. , Grandin, L. D. , Hughes, M. E. , … Hogan, M. E. (2008). Behavioral approach system and behavioral inhibition system sensitivities and bipolar spectrum disorders: Prospective prediction of bipolar mood episodes. Bipolar Disorders, 10(2), 310–322. 10.1111/j.1399-5618.2007.00547.x [DOI] [PubMed] [Google Scholar]
- Alloy, L. B. , Abramson, L. Y. , Whitehouse, W. G. , Hogan, M. E. , Panzarella, C. , & Rose, D. T. (2006). Prospective incidence of first onsets and recurrences of depression in individuals at high and low cognitive risk for depression. Journal of Abnormal Psychology, 115(1), 145–156. 10.1037/0021-843X.115.1.145 [DOI] [PubMed] [Google Scholar]
- Alloy, L. B. , Bender, R. E. , Whitehouse, W. G. , Wagner, C. A. , Liu, R. T. , Grant, D. A. , … Abramson, L. Y. (2012). High behavioral approach system (BAS) sensitivity, reward responsiveness, and goal‐striving predict first onset of bipolar Spectrum disorders: A prospective behavioral high‐risk design. Journal of Abnormal Psychology, 121(2), 339–351. 10.1037/a0025877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy, L. B. , Boland, E. M. , Ng, T. H. , Whitehouse, W. G. , & Abramson, L. Y. (2015). Low social rhythm regularity predicts first onset of bipolar spectrum disorders among at‐risk individuals with reward hypersensitivity. Journal of Abnormal Psychology, 124(4), 944–952. 10.1037/abn0000107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy, L. B. , Urošević, S. , Abramson, L. Y. , Jager‐Hyman, S. , Nusslock, R. , Whitehouse, W. G. , & Hogan, M. (2012). Progression along the bipolar Spectrum: A longitudinal study of predictors of conversion from bipolar Spectrum conditions to bipolar I and II disorders. Journal of Abnormal Psychology, 121(1), 16–27. 10.1037/a0023973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler, L. L. , Bartzokis, G. , Grieder, T. , Curran, J. , Jimenez, T. , Leight, K. , … Mintz, J. (2000). An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biological Psychiatry, 48(2), 147–162. 10.1016/S0006-3223(00)00836-2 [DOI] [PubMed] [Google Scholar]
- Amaral, D. G. , & Price, J. L. (1984). Amygdalo‐cortical projections in the monkey (Macaca fascicularis). The Journal of Comparative Neurology, 230(4), 465–496. 10.1002/cne.902300402 [DOI] [PubMed] [Google Scholar]
- Angst, F. , Stassen, H. H. , Clayton, P. J. , & Angst, J. (2002). Mortality of patients with mood disorders: Follow‐up over 34–38 years. Journal of Affective Disorders, 68(2), 167–181. 10.1016/S0165-0327(01)00377-9 [DOI] [PubMed] [Google Scholar]
- Anticevic, A. , Brumbaugh, M. S. , Winkler, A. M. , Lombardo, L. E. , Barrett, J. , Corlett, P. R. , … Glahn, D. C. (2013). Global prefrontal and Fronto‐amygdala dysconnectivity in bipolar I disorder with psychosis history. Biological Psychiatry, 73(6), 565–573. 10.1016/j.biopsych.2012.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone, D. , Cavanagh, J. , Gerber, D. , Lawrie, S. M. , Ebmeier, K. P. , & McIntosh, A. M. (2009). Magnetic resonance imaging studies in bipolar disorder and schizophrenia: Meta‐analysis. The British Journal of Psychiatry, 195(03), 194–201. 10.1192/bjp.bp.108.059717 [DOI] [PubMed] [Google Scholar]
- Balderston, N. L. , Schultz, D. H. , Hopkins, L. , & Helmstetter, F. J. (2015). Functionally distinct amygdala subregions identified using DTI and high‐resolution fMRI. Social Cognitive and Affective Neuroscience, 10(12), 1615–1622. 10.1093/scan/nsv055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechdolf, A. , Wood, S. J. , Nelson, B. , Velakoulis, D. , Yücel, M. , Takahashi, T. , … McGorry, P. D. (2012). Amygdala and insula volumes prior to illness onset in bipolar disorder: A magnetic resonance imaging study. Psychiatry Research: Neuroimaging, 201(1), 34–39. 10.1016/j.pscychresns.2011.06.010 [DOI] [PubMed] [Google Scholar]
- Berretta, S. , Pantazopoulos, H. , & Lange, N. (2007). Neuron numbers and volume of the amygdala in subjects diagnosed with bipolar disorder or schizophrenia. Biological Psychiatry, 62(8), 884–893. 10.1016/j.biopsych.2007.04.023 [DOI] [PubMed] [Google Scholar]
- Beyer, J. L. , & Krishnan, K. R. R. (2002). Volumetric brain imaging findings in mood disorders. Bipolar Disorders, 4(2), 89–104. 10.1034/j.1399-5618.2002.01157.x [DOI] [PubMed] [Google Scholar]
- Bezchlibnyk, Y. B. , Sun, X. , Wang, J.‐F. , MacQueen, G. M. , McEwen, B. S. , & Young, L. T. (2007). Neuron somal size is decreased in the lateral amygdalar nucleus of subjects with bipolar disorder. Journal of Psychiatry & Neuroscience, 32(3), 203–210. [PMC free article] [PubMed] [Google Scholar]
- Bitter, S. M. , Mills, N. P. , Adler, C. M. , Strakowski, S. M. , & DelBello, M. P. (2011). Progression of amygdala volumetric abnormalities in adolescents following their first manic episode. Journal of the American Academy of Child and Adolescent Psychiatry, 50(10), 1017–1026. 10.1016/j.jaac.2011.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg, H. P. , Donegan, N. H. , Sanislow, C. A. , Collins, S. , Lacadie, C. , Skudlarski, P. , … Krystal, J. H. (2005). Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology, 183(3), 308–313. 10.1007/s00213-005-0156-7 [DOI] [PubMed] [Google Scholar]
- Blumberg, H. P. , Krystal, J. H. , Bansal, R. , Martin, A. , Dziura, J. , Durkin, K. , … Peterson, B. S. (2006). Age, rapid‐cycling, and pharmacotherapy effects on ventral prefrontal cortex in bipolar disorder: A cross‐sectional study. Biological Psychiatry, 59(7), 611–618. 10.1016/j.biopsych.2005.08.031 [DOI] [PubMed] [Google Scholar]
- Brady, R. O. , Margolis, A. , Masters, G. A. , Keshavan, M. , & Öngür, D. (2017). Bipolar mood state reflected in cortico‐amygdala resting state connectivity: A cohort and longitudinal study. Journal of Affective Disorders, 217, 205–209. 10.1016/j.jad.2017.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady, R. O. , Masters, G. A. , Mathew, I. T. , Margolis, A. , Cohen, B. M. , Öngür, D. , & Keshavan, M. (2016). State dependent cortico‐amygdala circuit dysfunction in bipolar disorder. Journal of Affective Disorders, 201, 79–87. 10.1016/j.jad.2016.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla, P. , Harenski, K. , Nicoletti, M. , Sassi, R. B. , Mallinger, A. G. , Frank, E. , … Soares, J. C. (2003). MRI investigation of temporal lobe structures in bipolar patients. Journal of Psychiatric Research, 37(4), 287–295. 10.1016/S0022-3956(03)00024-4 [DOI] [PubMed] [Google Scholar]
- Carmichael, S. T. , & Price, J. L. (1995). Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. The Journal of Comparative Neurology, 363(4), 615–641. 10.1002/cne.903630408 [DOI] [PubMed] [Google Scholar]
- Carver, C. S. , & White, T. L. (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology, 67(2), 319–333. 10.1037/0022-3514.67.2.319 [DOI] [Google Scholar]
- Chai, X. J. , Whitfield‐Gabrieli, S. , Shinn, A. K. , Gabrieli, J. D. E. , Nieto Castañón, A. , McCarthy, J. M. , … Öngür, D. (2011). Abnormal medial prefrontal cortex resting‐state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology, 36(10), 2009–2017. 10.1038/npp.2011.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance, S. A. , Esiri, M. M. , & Crow, T. J. (2002). Amygdala volume in schizophrenia: Post‐mortem study and review of magnetic resonance imaging findings. The British Journal of Psychiatry., 180(4), 331–338. 10.1192/bjp.180.4.331 [DOI] [PubMed] [Google Scholar]
- Chang, K. , Karchemskiy, A. , Barnea‐Goraly, N. , Garrett, A. , Simeonova, D. I. , & Reiss, A. (2005). Reduced Amygdalar gray matter volume in familial pediatric bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 44(6), 565–573. 10.1097/01.chi.0000159948.75136.0d [DOI] [PubMed] [Google Scholar]
- Chapman, L. J. , & Chapman, J. P. (1987). The measurement of handedness. Brain and Cognition, 6(2), 175–183. [DOI] [PubMed] [Google Scholar]
- Damme, K. S. , Young, C. B. , & Nusslock, R. (2017). Elevated nucleus accumbens structural connectivity associated with proneness to hypomania: A reward hypersensitivity perspective. Social Cognitive and Affective Neuroscience, 12(6), 928–936. 10.1093/scan/nsx017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelBello, M. P. , Zimmerman, M. E. , Mills, N. P. , Getz, G. E. , & Strakowski, S. M. (2004). Magnetic resonance imaging analysis of amygdala and other subcortical brain regions in adolescents with bipolar disorder. Bipolar Disorders, 6(1), 43–52. 10.1046/j.1399-5618.2003.00087.x [DOI] [PubMed] [Google Scholar]
- Doty, T. J. , Payne, M. E. , Steffens, D. C. , Beyer, J. L. , Krishnan, K. R. R. , & LaBar, K. S. (2008). Age‐dependent reduction of amygdala volume in bipolar disorder. Psychiatry Research, 163(1), 84–94. 10.1016/j.pscychresns.2007.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott, J. , & Spitzer, R. L. (1978). A diagnostic interview: The schedule for affective disorders and schizophrenia. Archives of General Psychiatry, 35(7), 837–844. [DOI] [PubMed] [Google Scholar]
- Fischl, B. (2012). FreeSurfer. NeuroImage, 62(2), 774–781. 10.1016/j.neuroimage.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde, N. J. , O'Donoghue, S. , Scanlon, C. , Emsell, L. , Chaddock, C. , Leemans, A. , … McDonald, C. (2015). Structural brain network analysis in families multiply affected with bipolar I disorder. Psychiatry Research: Neuroimaging, 234(1), 44–51. 10.1016/j.pscychresns.2015.08.004 [DOI] [PubMed] [Google Scholar]
- Fournier, J. C. , Keener, M. T. , Almeida, J. , Kronhaus, D. M. , & Phillips, M. L. (2013). Amygdala and whole brain activity to emotional faces distinguishes major depressive disorder and bipolar disorder. Bipolar Disorders, 15(7), 741–752. 10.1111/bdi.12106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier, J. A. , Chiu, S. , Breeze, J. L. , Makris, N. , Lange, N. , Kennedy, D. N. , … Biederman, J. (2005). Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. American Journal of Psychiatry, 162(7), 1256–1265. 10.1176/appi.ajp.162.7.1256 [DOI] [PubMed] [Google Scholar]
- Getz, G. E. , DelBello, M. P. , Fleck, D. E. , Zimmerman, M. E. , Schwiers, M. L. , & Strakowski, S. M. (2002). Neuroanatomic characterization of schizoaffective disorder using MRI: A pilot study. Schizophrenia Research, 55(1), 55–59. 10.1016/S0920-9964(01)00210-9 [DOI] [PubMed] [Google Scholar]
- Gigante, A. D. , Young, L. T. , Yatham, L. N. , Andreazza, A. C. , Nery, F. G. , Grinberg, L. T. , … Lafer, B. (2011). Morphometric post‐mortem studies in bipolar disorder: Possible association with oxidative stress and apoptosis. The International Journal of Neuropsychopharmacology, 14(8), 1075–1089. 10.1017/S146114571000146X [DOI] [PubMed] [Google Scholar]
- Gloor, P. (1994). Mesial temporal lobe structures In Shorvon S. D., Fish D. R., Andermann F., Bydder G. M., & Stefan H. (Eds.), Magnetic resonance scanning and epilepsy. NATO ASI series (pp. 33–36). Boston, MA: Springer US; 10.1007/978-1-4615-2546-2_5 [DOI] [Google Scholar]
- Hibar, D. P. , Westlye, L. T. , van Erp, T. G. M. , Rasmussen, J. , Leonardo, C. D. , Faskowitz, J. , … Andreassen, O. A. (2016). Subcortical volumetric abnormalities in bipolar disorder. Molecular Psychiatry, 21(12), 1710–1716. 10.1038/mp.2015.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfeld, R. M. A. , Lewis, L. , & Vornik, L. A. (2003). Perceptions and impact of bipolar disorder: How far have we really come? Results of the national depressive and manic‐depressive association 2000 survey of individuals with bipolar disorder. The Journal of Clinical Psychiatry, 64(2), 161–174. [PubMed] [Google Scholar]
- Jalbrzikowski, M. , Larsen, B. , Hallquist, M. N. , Foran, W. , Calabro, F. , & Luna, B. (2017). Development of white matter microstructure and intrinsic functional connectivity between the amygdala and ventromedial prefrontal cortex: Associations with anxiety and depression. Biological Psychiatry, 82(7), 511–521. 10.1016/j.biopsych.2017.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempton, M. J. , Geddes, J. R. , Ettinger, U. , Williams, S. C. R. , & Grasby, P. M. (2008). Meta‐analysis, database, and meta‐regression of 98 structural imaging studies in bipolar disorder. Archives of General Psychiatry, 65(9), 1017–1032. 10.1001/archpsyc.65.9.1017 [DOI] [PubMed] [Google Scholar]
- Li, M. , Huang, C. , Deng, W. , Ma, X. , Han, Y. , Wang, Q. , … Li, T. (2015). Contrasting and convergent patterns of amygdala connectivity in mania and depression: A resting‐state study. Journal of Affective Disorders, 173, 53–58. 10.1016/j.jad.2014.10.044 [DOI] [PubMed] [Google Scholar]
- Mamah, D. , Alpert, K. I. , Barch, D. M. , Csernansky, J. G. , & Wang, L. (2016). Subcortical neuromorphometry in schizophrenia spectrum and bipolar disorders. NeuroImage: Clinical, 11, 276–286. 10.1016/j.nicl.2016.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manelis, A. , Almeida, J. R. C. , Stiffler, R. , Lockovich, J. C. , Aslam, H. A. , & Phillips, M. L. (2016). Anticipation‐related brain connectivity in bipolar and unipolar depression: A graph theory approach. Brain, 139(9), 2554–2566. 10.1093/brain/aww157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, C. , Zanelli, J. , Rabe‐Hesketh, S. , Ellison‐Wright, I. , Sham, P. , Kalidindi, S. , … Kennedy, N. (2004). Meta‐analysis of magnetic resonance imaging brain morphometry studies in bipolar disorder. Biological Psychiatry, 56(6), 411–417. 10.1016/j.biopsych.2004.06.021 [DOI] [PubMed] [Google Scholar]
- Merikangas, K. R. , Akiskal, H. S. , Angst, J. , Greenberg, P. E. , Hirschfeld, R. M. A. , Petukhova, M. , & Kessler, R. C. (2007). Lifetime and 12‐month prevalence of bipolar Spectrum disorder in the national comorbidity survey replication. Archives of General Psychiatry, 64(5), 543–552. 10.1001/archpsyc.64.5.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, B. , Johnson, S. L. , & Winters, R. (2001). Responsiveness to threat and incentive in bipolar disorder: Relations of the BIS/BAS scales with symptoms. Journal of Psychopathology and Behavioral Assessment, 23(3), 133–143. 10.1023/A:1010929402770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, G. A. , & Chapman, J. P. (2001). Misunderstanding analysis of covariance. Journal of Abnormal Psychology, 110(1), 40–48. [DOI] [PubMed] [Google Scholar]
- Mori, S. , Kageyama, Y. , Hou, Z. , Aggarwal, M. , Patel, J. , Brown, T. , … Troncoso, J. C. (2017). Elucidation of white matter tracts of the human amygdala by detailed comparison between high‐resolution postmortem magnetic resonance imaging and histology. Frontiers in Neuroanatomy, 11 10.3389/fnana.2017.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock, R. , & Alloy, L. B. (2017). Reward processing and mood‐related symptoms: An RDoC and translational neuroscience perspective. Journal of Affective Disorders, 216, 3–16. 10.1016/j.jad.2017.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlson, G. D. , Barta, P. E. , Powers, R. E. , Menon, R. R. , Richards, S. S. , Aylward, E. H. , … Tien, A. Y. (1997). Medial and superior temporal gyral volumes and cerebral asymmetry in schizophrenia versus bipolar disorder. Biological Psychiatry, 41(1), 1–14. 10.1016/S0006-3223(96)00373-3 [DOI] [PubMed] [Google Scholar]
- Phillips, M. L. , & Swartz, H. A. (2014). A critical appraisal of neuroimaging studies of bipolar disorder: Toward a new conceptualization of underlying neural circuitry and roadmap for future research. The American Journal of Psychiatry, 171(8), 829–843. 10.1176/appi.ajp.2014.13081008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post, R. M. , Fleming, J. , & Kapczinski, F. (2012). Neurobiological correlates of illness progression in the recurrent affective disorders. Journal of Psychiatric Research, 46(5), 561–573. 10.1016/j.jpsychires.2012.02.004 [DOI] [PubMed] [Google Scholar]
- Roberts, G. , Lord, A. , Frankland, A. , Wright, A. , Lau, P. , Levy, F. , … Breakspear, M. (2017). Functional dysconnection of the inferior frontal gyrus in young people with bipolar disorder or at genetic high risk. Biological Psychiatry, 81(8), 718–727. 10.1016/j.biopsych.2016.08.018 [DOI] [PubMed] [Google Scholar]
- Rosso, I. M. , Killgore, W. D. S. , Cintron, C. M. , Gruber, S. A. , Tohen, M. , & Yurgelun‐Todd, D. A. (2007). Reduced amygdala volumes in first‐episode bipolar disorder and correlation with cerebral White matter. Biological Psychiatry, 61(6), 743–749. 10.1016/j.biopsych.2006.07.035 [DOI] [PubMed] [Google Scholar]
- Rubinow, M. J. , Mahajan, G. , May, W. , Overholser, J. C. , Jurjus, G. J. , Dieter, L. , … Stockmeier, C. A. (2016). Basolateral amygdala volume and cell numbers in major depressive disorder: A postmortem stereological study. Brain Structure & Function, 221(1), 171–184. 10.1007/s00429-014-0900-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz, J. , Nugent, A. C. , Bogers, W. , Liu, A. , Sills, R. , Luckenbaugh, D. A. , … Drevets, W. C. (2010). Amygdala volume in depressed patients with bipolar disorder assessed using high resolution 3T MRI: The impact of medication. NeuroImage, 49(4), 2966–2976. 10.1016/j.neuroimage.2009.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saygin, Z. M. , Osher, D. E. , Augustinack, J. , Fischl, B. , & Gabrieli, J. D. E. (2011). Connectivity‐based segmentation of human amygdala nuclei using probabilistic tractography. NeuroImage, 56(3), 1353–1361. 10.1016/j.neuroimage.2011.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, M. R. , DelBello, M. P. , McNamara, R. K. , Strakowski, S. M. , & Adler, C. M. (2012). Neuroprogression in bipolar disorder. Bipolar Disorders, 14(4), 356–374. 10.1111/j.1399-5618.2012.01024.x [DOI] [PubMed] [Google Scholar]
- Soares, J. C. , & Mann, J. J. (1997). The anatomy of mood disorders—Review of structural neuroimaging studies. Biological Psychiatry, 41(1), 86–106. 10.1016/S0006-3223(96)00006-6 [DOI] [PubMed] [Google Scholar]
- Solano‐Castiella, E. , Anwander, A. , Lohmann, G. , Weiss, M. , Docherty, C. , Geyer, S. , … Turner, R. (2010). Diffusion tensor imaging segments the human amygdala in vivo. NeuroImage, 49(4), 2958–2965. 10.1016/j.neuroimage.2009.11.027 [DOI] [PubMed] [Google Scholar]
- Spielberg, J. M. , Beall, E. B. , Hulvershorn, L. A. , Altinay, M. , Karne, H. , & Anand, A. (2016). Resting state brain network disturbances related to hypomania and depression in medication‐free bipolar disorder. Neuropsychopharmacology, 41(13), 3016–3024. 10.1038/npp.2016.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanacci, L. , & Amaral, D. G. (2000). Topographic organization of cortical inputs to the lateral nucleus of the macaque monkey amygdala: A retrograde tracing study. The Journal of Comparative Neurology, 421(1), 52–79. [DOI] [PubMed] [Google Scholar]
- Torrisi, S. , Moody, T. D. , Vizueta, N. , Thomason, M. E. , Monti, M. M. , Townsend, J. D. , … Altshuler, L. L. (2013). Differences in resting corticolimbic functional connectivity in bipolar I euthymia. Bipolar Disorders, 15(2), 156–166. 10.1111/bdi.12047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrubia, R. , Ávila, C. , Moltó, J. , & Caseras, X. (2001). The sensitivity to punishment and sensitivity to reward questionnaire (SPSRQ) as a measure of Gray's anxiety and impulsivity dimensions. Personality and Individual Differences, 31(6), 837–862. 10.1016/S0191-8869(00)00183-5 [DOI] [Google Scholar]
- Usher, J. , Leucht, S. , Falkai, P. , & Scherk, H. (2010). Correlation between amygdala volume and age in bipolar disorder — A systematic review and meta‐analysis of structural MRI studies. Psychiatry Research: Neuroimaging, 182(1), 1–8. 10.1016/j.pscychresns.2009.09.004 [DOI] [PubMed] [Google Scholar]
- Wiggins, J. , Briggs‐Gowan, M. , Brotman, M. A. , Leibenluft, E. , & Wakschlag, L. S. (in press). Don't miss the boat: Towards a developmental nosology for disruptive mood dysregulation disorder (DMDD) in early childhood. The American Journal of Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information
Data Availability Statement
Data preparation scripts are available at https://github.com/katedamme/Amygdala‐Subnuclei‐Volume‐Abnormalities‐across‐the‐Bipolar‐Spectrum‐Insight‐from‐Diffusion‐based‐S. Data may be made available upon request to the PIs (Robin Nusslock and Lauren Alloy).


