Abstract
The third trimester of pregnancy is a period of rapid development of fiber bundles in the fetal white matter. Using a recently developed motion‐tracked slice‐to‐volume registration (MT‐SVR) method, we aimed to quantify tract‐specific developmental changes in apparent diffusion coefficient (ADC), fractional anisotropy (FA), and volume in third trimester healthy fetuses. To this end, we reconstructed diffusion tensor images from motion corrected fetal diffusion magnetic resonance imaging data. With an approved protocol, fetal MRI exams were performed on healthy pregnant women at 3 Tesla and included multiple (2–8) diffusion scans of the fetal head (1–2 b = 0 s/mm2 images and 12 diffusion‐sensitized images at b = 500 s/mm2). Diffusion data from 32 fetuses (13 females) with median gestational age (GA) of 33 weeks 4 days were processed with MT‐SVR and deterministic tractography seeded by regions of interest corresponding to 12 major fiber tracts. Multivariable regression analysis was used to evaluate the association of GA with volume, FA, and ADC for each tract. For all tracts, the volume and FA increased, and the ADC decreased with GA. Associations reached statistical significance for: FA and ADC of the forceps major; volume and ADC for the forceps minor; FA, ADC, and volume for the cingulum; ADC, FA, and volume for the uncinate fasciculi; ADC of the inferior fronto‐occipital fasciculi, ADC of the inferior longitudinal fasciculi; and FA and ADC for the corticospinal tracts. These quantitative results demonstrate the complex pattern and rates of tract‐specific, GA‐related microstructural changes of the developing white matter in human fetal brain.
Keywords: developing white matter, diffusion tensor imaging, diffusion weighted MRI, fetal brain, fetal MRI, tractography, tract‐specific analysis, white matter microstructure
Using a recently developed motion‐tracked slice‐to‐volume registration method, we aimed to quantify tract‐specific developmental changes in apparent diffusion coefficient, fractional anisotropy, and volume in third trimester healthy fetuses. To this end, we reconstructed diffusion tensor images from motion corrected fetal diffusion magnetic resonance imaging data. Our results show a complex pattern and rates of tract‐specific, gestational age‐related microstructural changes of the developing white matter in human fetal brain.
1. INTRODUCTION
The third trimester is a period of rapid fetal brain growth and refinement of the neural networks, as a prelude to postnatal life (Guihard‐Costa & Larroche, 1992; Kyriakopoulou et al., 2017). MRI volumetric analyses show that the rate of growth of the supratentorial brain ranges from 10 to 17% per week between the 22nd and 38th postconceptional weeks (Clouchoux, Guizard, Evans, du Plessis, & Limperopoulos, 2012; Kyriakopoulou et al., 2017; Scott et al., 2011). Fast growth renders the developing brain vulnerable to injury. Multiple studies have shown impaired global and regional cerebral development as a result of insults or decreased substrate delivery during these early stages of development (Bouyssi‐Kobar et al., 2016; Chang et al., 2011; Jaimes et al., 2018; Ortinau et al., 2018). Accordingly, validation of new tools that can identify abnormalities and localize them to known anatomic structures would greatly enhance our ability to prognosticate postnatal outcomes.
Understanding normal white matter development in the third trimester is of critical importance for neuroscience and neurology. The complex network of structural connections that arise in this period will shape functional connectivity and high order neurocognitive functions for the life span of an individual (Ferradal et al., 2019; Hagmann et al., 2006). It is also during this period that regional variability starts to arise, favoring maturation of networks that support functions early in life (e.g., vital reflexes in the dorsal brainstem, sensory input) (Guihard‐Costa & Larroche, 1992; Kostović & Jovanov‐Milosević, 2006; Kyriakopoulou et al., 2017; Scott et al., 2011). The oligodendrocyte precursor cells, which are abundant in the second half of pregnancy, are extremely vulnerable to injury which can lead to conditions such as periventricular leukomalacia (often manifesting clinically as cerebral palsy). Despite the latter, little is currently done to analyze tract specific white matter changes in the fetal brain in vivo; and that is mainly because of the technical challenges in imaging the fetus.
Diffusion tensor imaging (DTI) is an established tool to evaluate white matter anatomy, microstructure, and maturation. Postnatally, DTI has been used to characterize white matter changes from early infancy into late adulthood using scalar maps and tractography (Mukherjee et al., 2002; Watanabe, Sakai, Ozonoff, Kussman, & Jara, 2013; Zöllei, Jaimes, Saliba, Grant, & Yendiki, 2019). Due to the many technical challenges of performing DTI of the gravid abdomen (artifacts, low SNR, fetal motion, maternal respiration), diffusion imaging of the fetus remains difficult. Several groups, including Zanin et al. (2011); Kasprian et al. (2013); Song et al. (2015); Mitter, Prayer, Brugger, Weber, and Kasprian (2015); and Mitter, Jakab, et al. (2015), have demonstrated the feasibility of performing fetal tractography in vivo. However, these studies did not seek a technical solution for the inherent challenges of the acquisition, in particular fetal motion, which dramatically reduces the success rates, the quality of the fiber tracking, and the accuracy of quantitative diffusion metrics. Furthermore, many of the fetuses imaged in the previous studies had abnormalities, which precludes proper analysis of development‐related changes and utilization of these data as normative reference.
Marami et al. (2017) introduced a postprocessing algorithm that (a) performs temporal slice‐to‐volume registration (SVR) using a robust state space model to compensate for the fetal motion during acquisitions, by putting data from MRI slices back into their correct relative locations, (b) detects and rejects data corrupted by intraslice motion, and (c) generates high‐resolution motion‐corrected DTI from high‐quality, transformed slices. This approach has been recently used to create a spatio‐temporal DTI atlas of the fetal brain that characterizes global developmental trajectories in great detail Khan et al. (2019).
In this study, we hypothesize that fetal DTI processed with this novel motion correction approach will enable robust region of interest (ROI)‐based deterministic tractography that can demonstrate tract‐specific developmental trajectories in a cohort of typically developing fetuses.
2. METHODS
2.1. Subjects
This HIPPA compliant study was approved by the Institutional Review Board. A retrospective analysis was performed on MRI previously acquired for other research studies, where written informed consent was obtained from all subjects. Inclusion criteria were: maternal age 18–45 years, GA of at least 18 weeks gestation. Exclusion criteria were: (a) maternal contraindication to MRI, (b) known congenital infection in the fetus, (c) known or diagnosed brain or other organ abnormalities in the fetus, (d) chromosomal abnormalities, and (e) multiple‐gestation pregnancy. For this study, we only included MRI data acquired during the third trimester.
2.2. Image acquisition and processing
Subjects underwent imaging without sedation or breath holding. Subjects were scanned on 3 Tesla (T) MRI scanners (Skyra or Prisma, Siemens Medical Solutions, Erlangen, Germany) using a body array of 18 channels. Structural imaging protocol comprised multiple T2‐weighted (T2w) Half‐Fourier Single Shot Turbo Spin Echo scans of the fetal brain in orthogonal planes obtained with: TR = 1,400–2,000 ms, TE = 100–120 ms, 0.9–1.1 mm in‐plane resolution, 2 mm thickness with no interslice space, acquisition matrix size = 256 × 204, 256 × 256, or 320 × 320 with two‐ or four‐slice interleaved acquisition. Diffusion imaging protocol comprised two to eight scans, each along one of the orthogonal planes with respect to the fetal head. In each scan, 1 or 2 b = 0 s/mm2 images, and 12 diffusion‐sensitized images at b = 500 s/mm2 were acquired. Acquisition parameters were: TR = 3,000–4,000 ms, TE = 60 ms, in‐plane resolution = 2 mm, slice thickness = 2–4 mm.
Structural data were processed applying an automatic motion‐robust SVR that resulted in a 3D Isotropic intensity normalized T2w reconstruction of the fetal brain (Kainz et al., 2015). The diffusion data were then processed using a previously validated SVR algorithm with a motion trajectory estimation framework, referred to as motion‐tracked SVR (MT‐SVR) (Marami et al., 2017). This algorithm registered every diffusion‐sensitized image into a common space (to the reconstructed T2w image) and estimated a diffusion tensor model at every voxel using motion‐compensated data. The estimated diffusion tensor images were then processed using the Diffusion Toolkit (Version 0.6.4.1, Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Boston, MA) with a deterministic fiber assignment by a continuous tracking algorithm (35° angle threshold; no apparent diffusion coefficient [ADC] or FA threshold) to perform tractography by user‐defined ROI. The average time of each DTI acquisition was 1 min 40 s.
2.3. Image segmentation and analysis
To draw ROIs, we used TrackVis (Trackvis 0.6.1 Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital). To segment each tract, a trained research fellow (F. M.) placed the ROIs on anatomically relevant landmarks adjusted from the previous description by de Macedo Rodrigues et al. (2015), using a combination of T2‐w reconstructed images and scalar diffusion maps (trace and ADC). Both inclusion and exclusion ROIs were used, as appropriate. A pediatric neuroradiologist (C. J.) with 5 years of experience in fetal imaging reviewed and adjusted the ROIs. For each tract and subject, we exported tract volume, fractional anisotropy (FA), and ADC. We segmented 12 tracts: forceps major, forceps minor, right and left inferior fronto‐occipital fasciculus (IFOF), right and left inferior longitudinal fasciculus (ILF), right and left cingulum, right and left uncinate fasciculus (UF), and right and left corticospinal tracts (CST). We also estimated the average magnitude of motion for each subject using the motion parameters obtained from the temporal motion tracking method.
To estimate intraobserver agreement the neuroradiologist performed the same segmentation process in 10% of subjects (without assistance) at least 2 months after the original segmentations were refined, to avoid recall bias.
2.4. Statistical analysis
We used means of central tendency to evaluate the population and describe the results of the tractography analysis. Level of significance was set at an α value of .05 for all tests. A multivariable linear regression analysis was used to evaluate the association of GA with tract volume, FA and ADC, accounting for sex, the estimated average magnitude of motion, and the side of the tract (right or left for tracts with double representation). Interobserver agreement was estimated using a two‐way mixed‐effects model intraclass correlation coefficient (ICC).
3. RESULTS
3.1. Sample characteristics
Fifty subjects met the criteria for inclusion; 18 of these subjects were excluded on the basis of: image distortion or artifacts (n = 12), failure to identify all of the evaluated tracts (n = 3), failure of image reconstruction (n = 2), and incomplete clinical data (n = 1). Tractography was successful in the remaining subjects (n = 32), 19 of whom were male and 13 were female. The median GA was 32 weeks and 4 days old (interquartile range 5 weeks and 1 day); the youngest subject was 28 weeks old and the oldest was 36 weeks and 6 days old (Figure 1).
FIGURE 1.
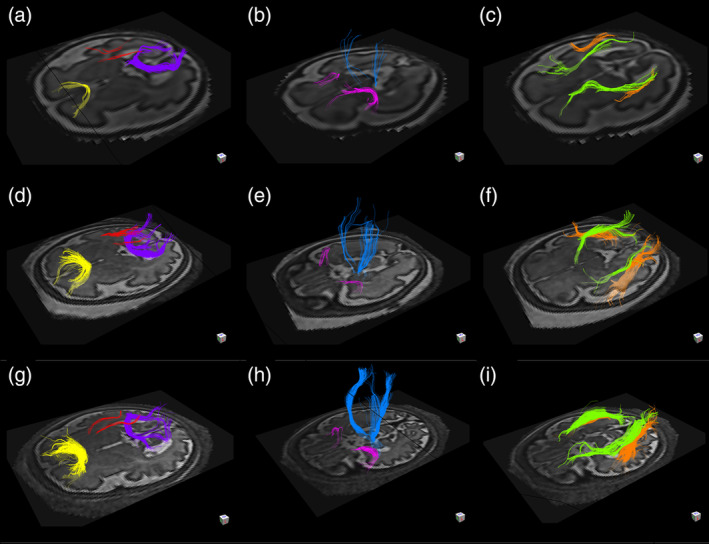
Results of in vivo tractography analysis of fetuses at 29 weeks and 3 days (a–c), 32 weeks and 5 days (d–f), and 36 weeks and 1 day (g–i) gestational age. The forceps minor (yellow), the forceps major (purple), and the superior cingulum (red) are depicted in figure parts (a,d,g). The corticospinal tracts (blue) and the uncinate fasciculi (pink) are depicted in figure parts (b,e,h). The inferior longitudinal fasciculi (orange) and the inferior fronto‐occipital fasciculi (green) are depicted in figure parts (c,f,i)
3.2. Commissural tracts
Increasing GA was associated with increasing FA in the forceps major (p = .048), decreasing ADC in the forceps major (p = .016), and increasing volume in the forceps minor (p = .035). Volume in the forceps major and FA in the forceps minor showed a trend toward increase with GA and ADC in the forceps minor showed a trend toward decrease with GA, although these did not reach statistical significance (p > .082) (Figure 2a,b) (Table 1). We also found a significant relationship between absolute motion and higher ADC in the forceps minor (p = .025). There was no association between sex and the parameters evaluated in either tract (all p > .445).
FIGURE 2.
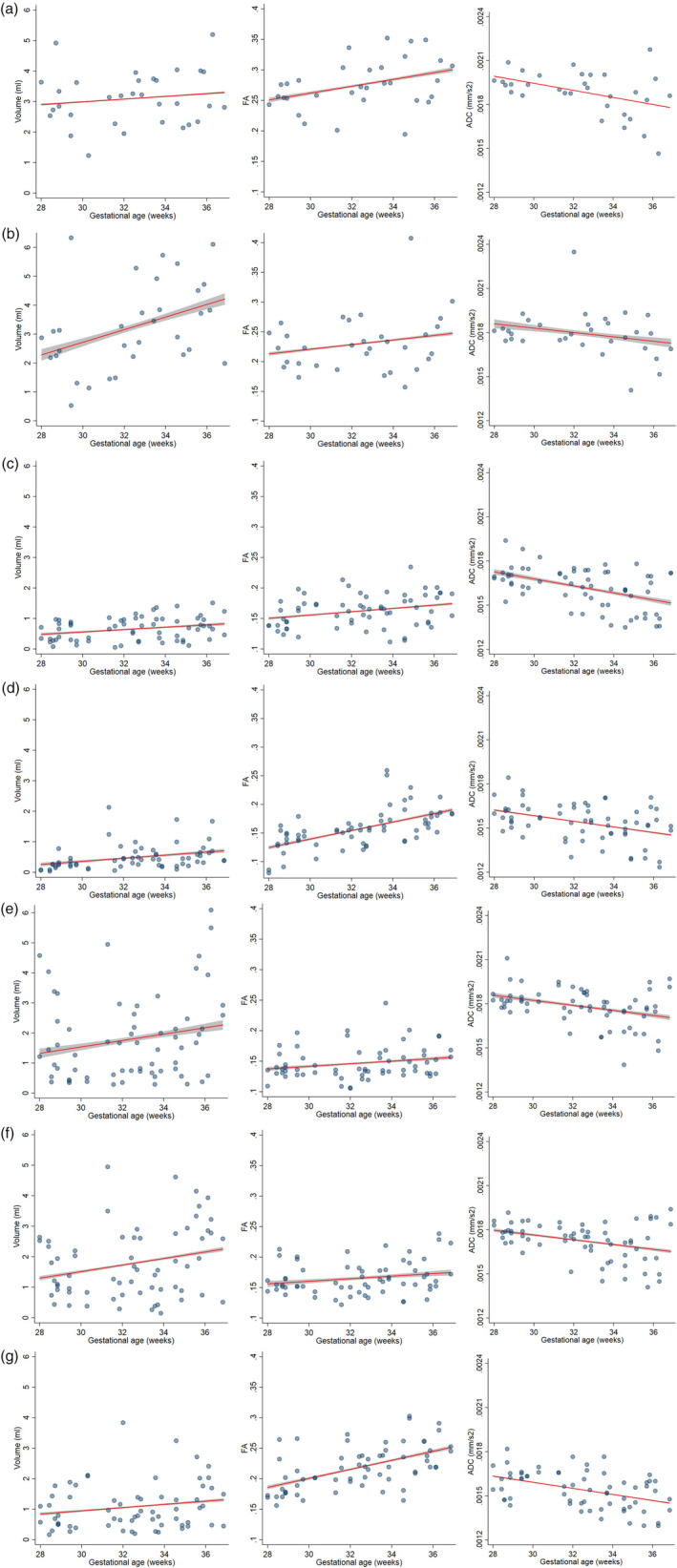
Changes in volume, FA, and ADC with GA of forceps major (2a) and minor (2b) of the corpus callosum, UF (2c), cingulum (2d), ILF(2e), IFOF (2f) and CSTs (2 g). Fitted linear regression models controlling for sex, motion, and laterality (when applicable) for tract volume, FA, and ADC. 95% confidence intervals of fitted models are shown in gray. ADC, apparent diffusion coefficient; CST, corticospinal tract; FA, fractional anisotropy; IFOF, inferior fronto‐occipital fasciculus, ILF, inferior longitudinal fasciculus; UF, uncinate fasciculus
TABLE 1.
Volume, FA, and ADC rate of change for GA, accounting for sex and motion
| GA regression coefficients [p value] | |||
|---|---|---|---|
| Volume (ml/week) | FA (units/week) | ADC (mm2/s/week) | |
| Commissural tracts | |||
| Forceps major | 0.0450898 [.460] | 0.0053362 [.048] a | −0.0000243 [.016] a |
| Forceps minor | 0.2051243 [.035] a | 0.0032402 [.220] | −0.0000158 [.102] |
| Association tracts | |||
| UF | 0.0362653 [.033] a | 0.0025293 [.043] a | −0.0000235 [<.001] a |
| Cingulum | 0.0455081 [.012] a | 0.0073717 [<.001] a | −0.0000192 [.001] a |
| ILF | 0.1017471 [.129] | 0.0020341 [.102] | −0.0000169 [.003] a |
| IFOF | 0.1016163 [.065] | 0.0020171 [.098] | −0.0000156 [.008] a |
| Projection tracts | |||
| CST | 0.0504272 [.169] | 0.0071398 [<.001] a | −0.0000208 [<.001] a |
Note: Multiple linear regression of GA and volume, FA and ADC, accounting for sex, magnitude of motion and laterality (for association and projection tracts). p‐Values are displayed.
Abbreviations: ADC, apparent diffusion coefficient; CST, corticospinal tract; FA, fractional anisotropy; GA, gestational age; IFOF, inferior fronto‐occipital fasciculus, ILF, inferior longitudinal fasciculus; UF, uncinate fasciculus.
Statistical significance at alpha of .05.
3.3. Association tracts
For the UF and cingulum, there was a significant increase in volume (p = .033 and p = .012, respectively) and FA (p = .043 and p < .001, respectively) with GA, and a decrease in ADC (p < .001 and p = .001, respectively) with GA (Figure 2c,d) (Table 1). However, in the IFOF and the ILF, only the inverse relationship between ADC and GA reached statistical significance (p = .008 and p = .003, respectively) (Figure 2e,f) (Table 1); volume and FA in the IFOF and ILF trended toward and increase with GA, without reaching significance. Higher estimated absolute motion was significantly associated with higher ADC in the ILF (p = .047), and lower FA in the IFOF (p = .012). Of all tracts, only the ADC of UF was associated with laterality (0.0000725 mm2/s more in right‐sided tracts, p = .017). There was no association between sex and the parameters evaluated for any of the tracts (all p > .081).
3.4. Projection tracts
For the CST, there was a significant increase in FA (p < .001) with GA and a decrease in ADC (p = <.001) with GA. There was a trend toward increase in volume with GA which did not reach statistical significance (p = .169) (Figure 2g) (Table 1). There was no association with sex, estimated absolute motion, and laterality with any of the parameters (all p > .258).
Data for mean volume, FA, and ADC by tract are available in supplementary Table S1.
3.5. Interobserver agreement
A sample of four subjects (48 tracts) corresponding to 13% of subjects had a two‐way mixed ICC between readers of 0.788 (95% CI .621–.881) (F = 4.71, p < .001) for volume, 0.923 (95% CI .863–.957) (F = 12.99, p < .0001) for FA, and 0.982 (95% CI .967–.990) (F = 54.63, p < .001) for ADC. All of the evaluated tracts were successfully identified in both attempts at segmentation. Data for specific tract ICC are available in supplementary Table S2.
4. DISCUSSION
Historically, the technical challenges that exist in imaging the gravid abdomen have limited the study of emerging cerebral connectivity with DTI in the fetus. In this study, we utilized diffusion data processed with a recently introduced MT‐SVR algorithm to perform deterministic tractography in TD fetuses in the third trimester. The MT‐SVR algorithm estimates and corrects the relative position of every acquired slice by modeling the dynamics of fetal motion estimated directly from slices, detects and removes data that are adversely affected by intraslice motion, and reconstructs diffusion tensor images with high spatial resolution. The MT‐SVR reconstructed DTI data resulted in successful tractography in a high percentage of subjects (64%) in this study. Our tractography analysis revealed a complex process of gestational age (GA)‐related and tract‐specific changes in volume, FA, and ADC that lay the foundation for the developmental landscape of the brain at birth and for the developmental trajectories that follow in the first years of life.
The results of our tractography analysis are consistent with the expected anatomy of known white matter pathways based on adult and neonatal tractography (Kaur, Powell, He, Pierson, & Parikh, 2014; Zöllei et al., 2019) and with other studies from in vivo fetal tractography by Mitter, Jakab, et al. (2015), Song et al. (2018), and Kasprian et al. (2013), as well as ex vivo fetal tractography (Song et al., 2015). The use of the MT‐SVR reconstructions enabled a high success rate of reconstructions of individual tracts; from the initial cohort of 50 patients, we identified all tracts in 32 subjects. We purposedly excluded subjects for whom a tract was missing or those where artifacts degraded the reconstruction. In doing so, we maximized the potential of using this unique cohort of typically developing fetuses to study normal emerging cerebral connectivity. Our success rate compares favorably to that reported by Mitter et al. that ranged between 23 and 100% depending on the tract of interest and on the GA (Mitter, Jakab, et al., 2015); in addition, many of these studies only acquired the DTI sequence in fetuses that remained still during the MRI whereas we acquired DTI in all subjects independent of fetal motion. Although we did observe some differences in the fiber tracking between individuals of similar GAs, these were minor and similar to those observed in newborn tractography where constraints of spatial resolution and motion occur frequently (Zöllei et al., 2019).
We found an age‐related increase in FA and decrease in ADC in all tracts, although the trend did not reach statistical significance for every tract. This observation is consistent with the findings from in vivo tractography (Mitter, Jakab, et al., 2015), ex vivo tractography (Song et al., 2015), and atlas‐based analyses (Khan et al., 2019). Importantly, the ADC and FA values and the rate of change varied between tracts, resulting from a complex amalgam of tract‐specific microstructural changes. A differential rate of white matter development postnatally has long been recognized and attributed to early maturation of brain networks that are needed to support vital functions (Geng et al., 2012). Our results build upon these observations by demonstrating that differential developmental trajectories exist during the third trimester and that analogous to postnatal development, these localize to anatomically and functionally distinct white matter pathways. For example, the CST showed high FA, low ADC, and rapid age‐related changes relative to other tracts. These trends correlate with known myelination milestones late in pregnancy and with the developmental stage of the human brain at term, where the posterior limb of the internal capsule and the perirolandic white matter (which correspond to the CST) are some of the few myelinated supratentorial white matter tracts at term (Barkovich, Kjos, Jackson, & Norman, 1988; Brody, Kinney, Kloman, & Gilles, 1987; Kinney, Brody, Kloman, & Gilles, 1988). On the other hand, tracts that demonstrate low ADC, FA, and slow rate of change such as the UF, ILF, and IFOF, correspond to nonmyelinated white matter tracts at term on diffusion and structural images (Kaur et al., 2014).
An interesting observation from our tractography analysis is that changes in volume and microstructural parameters are not always coupled. For example, the forceps minor, despite showing rapid growth, demonstrated only modest changes in ADC and FA; lack of rapidly progressing myelination in this tract is, however, not unexpected as this process is known to occur between the 6th and 12th month of postnatal life (Brody et al., 1987). The ILF and IFOF show a similar pattern of rapid volumetric growth and slow microstructural maturation; the changes in ADC and FA are concordant with the expected postnatal myelination of these deep white matter association fibers. The CST and the forceps major demonstrate a different developmental trajectory characterized by rapid change in ADC/FA with only modest volumetric growth. Our observations in the CST are consistent with the results on tractography in the first 2 years of postnatal life reported by Bragga et al. who identified a similar trend of dynamic microstructural change and slow volumetric growth (Braga et al., 2015). Rapid microstructural changes in the forceps major also correlate well with the known earlier onset of myelination in the splenium of the corpus callosum relative to the genu of the corpus callosum (Barkovich et al., 1988).
The tract‐specific trajectories that we observed also appear to lay the foundation for other developmental changes that occur during infancy. For instance, the rapid rate of ADC and FA changes in the CST and forceps major respectively conform to the known anterior‐to‐posterior and central‐to‐peripheral progression of myelination in postnatal life, respectively (Barkovich et al., 1988; Kinney et al., 1988). The UF showed a distinct pattern of development characterized by slow volumetric growth and slow microstructural changes which is consistent with the expected course of maturation of this tract. The UF is known to be one of the last white matter bundles to reach peak maturation, attaining its highest FA and lowest ADC well into the third decade of life (Lebel et al., 2012; Olson, Von Der Heide, Alm, & Vyas, 2015). The slow volumetric and microstructural changes of the UF in utero could be indicative of a relatively quiescent behavior during what is otherwise a dynamic developmental stage.
Although all the analyzed tracts increased in volume with age, the trend only reached statistical significance for three tracts. A small sample size may have limited our power to detect significant associations. We also believe that tractography may underestimate the true volume of the white matter tracts by one of several mechanisms. First, the deterministic FACT method used in this study terminates the fiber tracking if it encounters a voxel outside of the pre‐set tracking conditions (difference in directionality of the main eigenvector, diffusivity). Second, despite using MT‐SVR, it is conceivable that even small residual artifacts could affect propagation of the tractography and underestimate the volume of the tract and consequently its change with GA (Anastasopoulos et al., 2014). Third, it is also possible that our experiments underestimated the volume of the tracts due to the presence of areas of crossing fibers which cannot be resolved using the diffusion tensor model and the limited directions of diffusion acquired.
We did not observe a widespread association between motion parameters and tract metrics; average motion was only significantly associated with higher ADC in the forceps minor and ILF, and lower FA in the IFOF. It is possible that some motion effects remained despite the use of the MT‐SVR. This observation highlights the need for utilization of robust motion‐mitigation strategies for analysis of fetal DTI, as motion is ubiquitous in fetal MRI and it can introduce significant bias to these analyses (Yendiki, Koldewyn, Kakunoori, Kanwisher, & Fischl, 2014).
Even though widespread asymmetries in diffusion parameters have been documented early in postnatal life (Dean et al., 2017), these asymmetries were not apparent in our data. The only asymmetry that reached statistical significance was lower ADC in the left UF, which is consistent with known and expected asymmetries observed in infancy and childhood (Hasan et al., 2009). Prior work in the field has suggested the presence of scattered tract‐based asymmetries, but profound differences in technique and populations between the studies hampers direct comparison. Specifically the study by Song et al. (2015) utilized a combination of high angular resolution diffusion imaging on ex vivo samples and postnatal DTI of infants from 30 postconceptional weeks to 3 years of age. The emerging asymmetries detected in that study span a much broader range of developmental stages than the population analyzed in our study. In the study by Mitter, Prayer, et al. (2015), the only significant asymmetry observed was higher ADC in the left ILF, which does not correlate with our findings or the findings on the subjects of corresponding gestational and postconceptional age from the study by Song et al. (2015). One can hypothesize that the asymmetries are emerging in the later part of gestation based on their presence on postnatal DTI and the presence of some early hemispheric asymmetries on fetal structural imaging; however, additional work with a larger sample and innovative techniques is needed to characterize the precise onset of these developmental changes (Dean et al., 2017; Vasung et al., 2019).
There are several limitations to our study. Our findings are based on a relatively small sample size which limits the power of statistical analyses and increases the likelihood of Type II error. Furthermore, fetal DTI data are limited by the quality of acquisition and the reconstruction. Although a robust MT‐SVR algorithm was used, some subjects were excluded from the analyses because of motion‐corrupted data. We elected to use a diffusion tensor model and a deterministic FACT algorithm for this exploratory study, but future analysis that relies on improved diffusion acquisitions and tractography approaches should be undertaken. Finally, the specific neurobiological processes driving these microstructural changes (e.g., myelination, premyelination, axonal pruning) cannot be determined given the absence of pathology correlates or other sequences.
5. CONCLUSION
Tractography analysis based on fetal DTI processed with an MT‐SVR algorithm demonstrates a complex landscape of growth and microstructural changes in the third trimester. Tract‐specific changes correspond to known cellular processes that occur during late gestation and enable the study of normal and abnormal development in vivo with high anatomic specificity.
AKNOWLEDGMENTS
Research reported in this study was supported in part by the National Institute of Biomedical Imaging and Bioengineering and the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (NIH) under Award Numbers R01EB018988, R01NS106030, and R01EB013248; by a Technological Innovations in Neuroscience Award from the McKnight Foundation; Schlaeger Fellowship for Neuroscience Research; and by the Fetal Health Foundation (FHF). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, FHF, or the McKnight Foundation.
Supporting information
Supplementary Table S1 Average Volume, FA and ADC values.
Supplementary Table S2 Interobserver agreement
Jaimes C, Machado‐Rivas F, Afacan O, et al. In vivo characterization of emerging white matter microstructure in the fetal brain in the third trimester. Hum Brain Mapp. 2020;41:3177–3185. 10.1002/hbm.25006
Funding information American Academy of Neurology, Grant/Award Number: Clinical Research Training Fellowship; Fetal Health Foundation; McKnight Foundation; National Alliance for Research on Schizophrenia and Depression, Grant/Award Number: Young Investigator; National Heart, Lung, and Blood Institute, Grant/Award Number: Scholar Award from the Pediatric Heart Network; National Institute of Neurological Disorders and Stroke, Grant/Award Number: K23NS101120; National Institutes of Health, Grant/Award Numbers: R01EB013248, R01EB018988, R01NS106030; Schlaeger Fellowship for Neuroscience Research
DATA AVAILABILITY STATEMENT
Fetal Brain Atlas data that support the findings of this study are openly available in http://crl.med.harvard.edu/research/fetal_brain_atlas/. Individual subject data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Anastasopoulos, C. , Reisert, M. , Kiselev, V. G. , Nguyen‐Thanh, T. , Schulze‐Bonhage, A. , Zentner, J. , & Mader, I. (2014). Local and global fiber tractography in patients with epilepsy. AJNR. American Journal of Neuroradiology, 35(2), 291–296. 10.3174/ajnr.A3752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkovich, A. J. , Kjos, B. O. , Jackson, D. E. , & Norman, D. (1988). Normal maturation of the neonatal and infant brain: MR imaging at 1.5 T. Radiology, 166(1 Pt 1), 173–180. 10.1148/radiology.166.1.3336675 [DOI] [PubMed] [Google Scholar]
- Bouyssi‐Kobar, M. , du Plessis, A. J. , McCarter, R. , Brossard‐Racine, M. , Murnick, J. , Tinkleman, L. , … Limperopoulos, C. (2016). Third trimester brain growth in preterm infants compared with in utero healthy fetuses. Pediatrics, 138(5), e20161640 10.1542/peds.2016-1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga, R. M. , Roze, E. , Ball, G. , Merchant, N. , Tusor, N. , Arichi, T. , … Counsell, S. J. (2015). Development of the corticospinal and callosal tracts from extremely premature birth up to 2 years of age. PLoS One, 10(5), e0125681 10.1371/journal.pone.0125681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody, B. A. , Kinney, H. C. , Kloman, A. S. , & Gilles, F. H. (1987). Sequence of central nervous system myelination in human infancy. I. An autopsy study of myelination. Journal of Neuropathology and Experimental Neurology, 46(3), 283–301. 10.1097/00005072-198705000-00005 [DOI] [PubMed] [Google Scholar]
- Chang, K. T. E. , Keating, S. , Costa, S. , Machin, G. , Kingdom, J. , & Shannon, P. (2011). Third‐trimester stillbirths: Correlative neuropathology and placental pathology. Pediatric and Developmental Pathology, 14(5), 345–352. 10.2350/10-07-0882-OA.1 [DOI] [PubMed] [Google Scholar]
- Clouchoux, C. , Guizard, N. , Evans, A. C. , du Plessis, A. J. , & Limperopoulos, C. (2012). Normative fetal brain growth by quantitative in vivo magnetic resonance imaging. American Journal of Obstetrics and Gynecology, 206(2), 173.e1–173.e8. 10.1016/j.ajog.2011.10.002 [DOI] [PubMed] [Google Scholar]
- de Macedo Rodrigues, K. , Ben‐Avi, E. , Sliva, D. D. , Choe, M. , Drottar, M. , Wang, R. , … Zöllei, L. (2015). A FreeSurfer‐compliant consistent manual segmentation of infant brains spanning the 0†2 year age range. Frontiers in Human Neuroscience, 9 10.3389/fnhum.2015.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean, D. C. , Planalp, E. M. , Wooten, W. , Adluru, N. , Kecskemeti, S. R. , Frye, C. , … Alexander, A. L. (2017). Mapping white matter microstructure in the one month human brain. Scientific Reports, 7(1), 9759 10.1038/s41598-017-09915-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferradal, S. L. , Gagoski, B. , Jaimes, C. , Yi, F. , Carruthers, C. , Vu, C. , … Zöllei, L. (2019). System‐specific patterns of thalamocortical connectivity in early brain development as revealed by structural and functional MRI. Cerebral Cortex (New York, N.Y.: 1991), 29(3), 1218–1229. 10.1093/cercor/bhy028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng, X. , Gouttard, S. , Sharma, A. , Gu, H. , Styner, M. , Lin, W. , … Gilmore, J. H. (2012). Quantitative tract‐based white matter development from birth to age 2 years. NeuroImage, 61(3), 542–557. 10.1016/j.neuroimage.2012.03.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guihard‐Costa, A.‐M. , & Larroche, J. C. (1992). Growth velocity of some fetal parameters. Neonatology, 62(5), 309–316. 10.1159/000243887 [DOI] [PubMed] [Google Scholar]
- Hagmann, P. , Jonasson, L. , Maeder, P. , Thiran, J.‐P. , Wedeen, V. J. , & Meuli, R. (2006). Understanding diffusion MR imaging techniques: From scalar diffusion‐weighted imaging to diffusion tensor imaging and beyond. RadioGraphics, 26(Suppl 1), S205–S223. 10.1148/rg.26si065510 [DOI] [PubMed] [Google Scholar]
- Hasan, K. M. , Iftikhar, A. , Kamali, A. , Kramer, L. A. , Ashtari, M. , Cirino, P. T. , … Ewing‐Cobbs, L. (2009). Development and aging of the healthy human brain uncinate fasciculus across the lifespan using diffusion tensor tractography. Brain Research, 1276, 67–76. 10.1016/j.brainres.2009.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaimes, C. , Cheng, H. H. , Soul, J. , Ferradal, S. , Rathi, Y. , Gagoski, B. , … Zöllei, L. (2018). Probabilistic tractography‐based thalamic parcellation in healthy newborns and newborns with congenital heart disease: Thalamic parcellation in newborns. Journal of Magnetic Resonance Imaging, 47(6), 1626–1637. 10.1002/jmri.25875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kainz, B. , Steinberger, M. , Wein, W. , Kuklisova‐Murgasova, M. , Malamateniou, C. , Keraudren, K. , … Rueckert, D. (2015). Fast volume reconstruction from motion corrupted stacks of 2D slices. IEEE Transactions on Medical Imaging, 34(9), 1901–1913. 10.1109/TMI.2015.2415453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprian, G. , Brugger, P. C. , Schöpf, V. , Mitter, C. , Weber, M. , Hainfellner, J. A. , & Prayer, D. (2013). Assessing prenatal white matter connectivity in commissural agenesis. Brain, 136(1), 168–179. 10.1093/brain/aws332 [DOI] [PubMed] [Google Scholar]
- Kaur, S. , Powell, S. , He, L. , Pierson, C. R. , & Parikh, N. A. (2014). Reliability and repeatability of quantitative tractography methods for mapping structural white matter connectivity in preterm and term infants at term‐equivalent age. PLoS One, 9(1), e85807 10.1371/journal.pone.0085807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, S. , Vasung, L. , Marami, B. , Rollins, C. K. , Afacan, O. , Ortinau, C. M. , … Gholipour, A. (2019). Fetal brain growth portrayed by a spatiotemporal diffusion tensor MRI atlas computed from in utero images. NeuroImage, 185, 593–608. 10.1016/j.neuroimage.2018.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney, H. C. , Brody, B. A. , Kloman, A. S. , & Gilles, F. H. (1988). Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. Journal of Neuropathology and Experimental Neurology, 47(3), 217–234. 10.1097/00005072-198805000-00003 [DOI] [PubMed] [Google Scholar]
- Kostović, I. , & Jovanov‐Milosević, N. (2006). The development of cerebral connections during the first 20‐45 weeks' gestation. Seminars in Fetal & Neonatal Medicine, 11(6), 415–422. 10.1016/j.siny.2006.07.001 [DOI] [PubMed] [Google Scholar]
- Kyriakopoulou, V. , Vatansever, D. , Davidson, A. , Patkee, P. , Elkommos, S. , Chew, A. , … Rutherford, M. A. (2017). Normative biometry of the fetal brain using magnetic resonance imaging. Brain Structure and Function, 222(5), 2295–2307. 10.1007/s00429-016-1342-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel, C. , Gee, M. , Camicioli, R. , Wieler, M. , Martin, W. , & Beaulieu, C. (2012). Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage, 60(1), 340–352. 10.1016/j.neuroimage.2011.11.094 [DOI] [PubMed] [Google Scholar]
- Marami, B. , Mohseni Salehi, S. S. , Afacan, O. , Scherrer, B. , Rollins, C. K. , Yang, E. , … Gholipour, A. (2017). Temporal slice registration and robust diffusion‐tensor reconstruction for improved fetal brain structural connectivity analysis. NeuroImage, 156, 475–488. 10.1016/j.neuroimage.2017.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitter, C. , Jakab, A. , Brugger, P. C. , Ricken, G. , Gruber, G. M. , Bettelheim, D. , … Kasprian, G. (2015). Validation of in utero tractography of human fetal commissural and internal capsule fibers with histological structure tensor analysis. Frontiers in Neuroanatomy, 9 10.3389/fnana.2015.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitter, C. , Prayer, D. , Brugger, P. C. , Weber, M. , & Kasprian, G. (2015). In vivo Tractography of fetal association fibers. PLoS One, 10(3), e0119536 10.1371/journal.pone.0119536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee, P. , Miller, J. H. , Shimony, J. S. , Philip, J. V. , Nehra, D. , Snyder, A. Z. , … McKinstry, R. C. (2002). Diffusion‐tensor MR imaging of gray and white matter development during normal human brain maturation. AJNR. American Journal of Neuroradiology, 23(9), 1445–1456. [PMC free article] [PubMed] [Google Scholar]
- Olson, I. R. , Von Der Heide, R. J. , Alm, K. H. , & Vyas, G. (2015). Development of the uncinate fasciculus: Implications for theory and developmental disorders. Developmental Cognitive Neuroscience, 14, 50–61. 10.1016/j.dcn.2015.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortinau, C. M. , Mangin‐Heimos, K. , Moen, J. , Alexopoulos, D. , Inder, T. E. , Gholipour, A. , … Smyser, C. D. (2018). Prenatal to postnatal trajectory of brain growth in complex congenital heart disease. NeuroImage: Clinical, 20, 913–922. 10.1016/j.nicl.2018.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, J. A. , Habas, P. A. , Kim, K. , Rajagopalan, V. , Hamzelou, K. S. , Corbett‐Detig, J. M. , … Studholme, C. (2011). Growth trajectories of the human fetal brain tissues estimated from 3D reconstructed in utero MRI. International Journal of Developmental Neuroscience, 29(5), 529–536. 10.1016/j.ijdevneu.2011.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, J. W. , Gruber, G. M. , Patsch, J. M. , Seidl, R. , Prayer, D. , & Kasprian, G. (2018). How accurate are prenatal tractography results? A postnatal in vivo follow‐up study using diffusion tensor imaging. Pediatric Radiology, 48(4), 486–498. 10.1007/s00247-017-3982-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, J. W. , Mitchell, P. D. , Kolasinski, J. , Ellen Grant, P. , Galaburda, A. M. , & Takahashi, E. (2015). Asymmetry of white matter pathways in developing human brains. Cerebral Cortex, 25(9), 2883–2893. 10.1093/cercor/bhu084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasung, L. , Rollins, C. K. , Yun, H. J. , Velasco‐Annis, C. , Zhang, J. , Wagstyl, K. , … Gholipour, A. (2020). Quantitative in vivo MRI assessment of structural asymmetries and sexual dimorphism of transient fetal compartments in the human brain. Cerebral Cortex, 30(3), 1752–1767. 10.1093/cercor/bhz200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, M. , Sakai, O. , Ozonoff, A. , Kussman, S. , & Jara, H. (2013). Age‐related apparent diffusion coefficient changes in the normal brain. Radiology, 266(2), 575–582. 10.1148/radiol.12112420 [DOI] [PubMed] [Google Scholar]
- Yendiki, A. , Koldewyn, K. , Kakunoori, S. , Kanwisher, N. , & Fischl, B. (2014). Spurious group differences due to head motion in a diffusion MRI study. NeuroImage, 88, 79–90. 10.1016/j.neuroimage.2013.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanin, E. , Ranjeva, J.‐P. , Confort‐Gouny, S. , Guye, M. , Denis, D. , Cozzone, P. J. , & Girard, N. (2011). White matter maturation of normal human fetal brain. An in vivo diffusion tensor tractography study: in vivo DTI tractography in human fetuses. Brain and Behavior, 1(2), 95–108. 10.1002/brb3.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zöllei, L. , Jaimes, C. , Saliba, E. , Grant, P. E. , & Yendiki, A. (2019). TRActs constrained by UnderLying INfant anatomy (TRACULInA): An automated probabilistic tractography tool with anatomical priors for use in the newborn brain. NeuroImage, 199, 1–17. 10.1016/j.neuroimage.2019.05.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1 Average Volume, FA and ADC values.
Supplementary Table S2 Interobserver agreement
Data Availability Statement
Fetal Brain Atlas data that support the findings of this study are openly available in http://crl.med.harvard.edu/research/fetal_brain_atlas/. Individual subject data are not publicly available due to privacy or ethical restrictions.


