Abstract
Introduction
Patients with NAFLD have a two‐fold increased risk of diabetes, and conversely, NAFLD affects up to 80% of patients with type 2 diabetes. Due to the co‐occurrence of both diseases and the lack of approved pharmacotherapy for NAFLD, the anti‐steatogenic potential of diabetes‐related drugs is being explored. In this study, we aim to monitor liver fat noninvasively during treatment with SGLT‐2 inhibitors or GLP‐1 analogues in a real‐world setting.
Methods
Overall, 39 patients (49% women, age 57.7 ± 10.9 years) with type 2 diabetes and hepatic steatosis (defined by controlled attenuation parameter [CAP] values ≥ 215 dB/m) were observed for 6 months and routinely monitored with respect to hepatic fat contents and liver stiffness (VCTE); body composition (BIA); and blood biochemistry, including liver function tests (LFTs), serum lipids and glucose metabolism markers.
Results
Median liver fat contents were significantly (P = .026) reduced by 9% in patients taking either SGLT‐2 (n = 22) or GLP‐1 (n = 17) for 6 months (absolute median CAP decrease: −32 dB/m [−58 to 32 dB/m]). In parallel, serum ALT and γ‐GT activities decreased significantly (P = .002 and P = .049, respectively). These improvements were accompanied by significant (P < .0001) changes to body weight and BMI (−2.5 ± 3.3 kg and −0.9 ± 1.2 kg/m2, respectively) and glucose homeostasis, with significant reductions in HbA1c and fasting plasma glucose (FDG) (both P < .0001). Of note, significant reductions of intrahepatic lipid contents occured in patients receiving SGLT‐2 inhibitors only.
Conclusions
In this real‐world observational evaluation of fatty liver monitored noninvasively in patients with type 2 diabetes treated with either SGLT2 or GLP‐1, improvements in measures of hepatic steatosis, glucose and weight parameters were observed after 6 months, with significant reductions of intrahepatic lipid contents seen specifically in the SGLT2 subgroup.
Keywords: CAP, elastography, hepatic steatosis
In this observational study, we aim to monitor liver fat noninvasively during treatment with SGLT‐2 inhibitors or GLP‐1 analogues in a real‐world setting. The findings demonstrated that SGLT‐2 inhibitors are associated with improved body weight and glucose‐related parameters but also hepatic steatosis, which frequently occurs in patients with type 2 diabetes.

1. INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) comprises simple steatosis and, when accompanied by hepatic inflammation, nonalcoholic steatohepatitis (NASH). Environmental factors such as obesity and diabetes play a prominent role in NAFLD risk. 1 The global prevalence of NAFLD is currently estimated at almost 25% 2 and is diagnosed in up to 80% of patients with type 2 diabetes. 3 Patients with concurrent diabetes are at risk of more aggressive NAFLD. 3
Given the current lack of approved pharmacotherapy for treating NAFLD, drugs for associated comorbidities, such as diabetes, are being investigated for possible liver‐related benefits. The PIVENS trial observed the insulin‐sensitizing drug pioglitazone to improve liver histopathology and liver enzymes 4 ; however, it was associated with side effects, including weight gain. Sodium‐glucose co‐transporter‐2 (SGLT‐2) inhibitors are also approved for use in patients with diabetes. They prevent glucose from being reabsorbed in the kidney and increase the amount of glucose excreted in urine. 5 Both experimental 6 , 7 , 8 , 9 and first clinical studies conducted primarily in Asian populations have reported liver‐related benefits with SGLT‐2 inhibitors in the setting of diabetes and NAFLD. 9 , 10 , 11 , 12 , 13 , 14 Glucagon‐like peptide‐1 (GLP‐1) analogues induce weight loss and insulin sensitivity and, like SGLT2 inhibitors, improve liver histopathology and serum surrogate markers in mouse NASH models. 15 , 16 , 17 , 18 Reductions in hepatic fat contents and in LFTs have been demonstrated in some 19 , 20 but not all 21 studies in patients with diabetes and NAFLD.
The aim of this present study was to monitor liver fat noninvasively during treatment with SGLT‐2 inhibitors or GLP‐1 analogues in a real‐world setting based on data collected in a tertiary outpatient clinic, as well as to explore possible liver‐specific differences in therapeutic outcomes.
2. PATIENTS AND METHODS
2.1. Patients
This prospective real‐world data study included 39 patients with type 2 diabetes and hepatic steatosis from the Department of Medicine II (Gastroenterology and Endocrinology), at Saarland University Medical Center. Women and men over 18 years of age were included if the following criteria were fulfilled: ability to provide informed consent; type 2 diabetes with or without current anti‐diabetic medication; HbA1c ≥ 6.5% (48 mmol/mol); presence of hepatic steatosis by controlled attenuation parameter (CAP value ≥215 dB/m using transient elastography) 22 ; and were about to begin therapy with SGLT‐2 inhibitors or GLP‐1 analogues. The choice of specific drugs was made by physicians board licensed for internal medicine and/or endocrinology, who supervised the treatment of the individual patients. The treatment regimens included the following: for GLP‐analogues: 5 μg s.c. exenatide twice/d for 1 month, then if necessary 10 μg s.c twice/d, in each case 1 hour before meal; 0.6 mg s.c. liraglutide once/d for 1 week, then increase to 1.2 mg and if necessary after 1 week to 1.8 mg; or 0.75‐1.5 mg s.c. dulaglutide once weekly. Alternatively, patients were included if SGLT‐2 inhibitors were initiated as follows: 5‐10 mg dapagliflozin once/d or 10 mg empagliflozin once/d and if well tolerated with a dosage increase to 25 mg.
Exclusion criteria included the following: liver cirrhosis (liver stiffness measurement during elastography ≥13 kPa); viral hepatitis or drug‐induced hepatopathy; alcohol consumption ≥21 drinks/wk (30 g alcohol/d) in men and ≥14 drinks/wk (20 g alcohol/d) in women, as assessed using the Alcohol Use Disorders Identification Test questionnaire 23 ; noncompliance with medical therapy; glomerular filtration rate (GFR) <30 or <60 mL/min for those prescribed GLP‐1 analogues and SGLT‐2 inhibitors, respectively; or pregnancy. The study conformed to the Declaration of Helsinki. The study was approved by the Ärztekammer des Saarlandes ethics committee (ref. 271/11), and patients provided written informed consent prior to participation.
2.2. Study procedures
A baseline assessment was conducted in patients meeting the study criteria, and upon initiation of GLP‐1 analogues or SGLT‐2 inhibitors, patients were followed up after 4 weeks and 3 and 6 months. Changes to existing medications during the study were documented as was compliance with the aforementioned prescribed medications using the pill‐count (or equivalent) method. As summarized in Figure 1, the following data were obtained during each visit:
FIGURE 1.
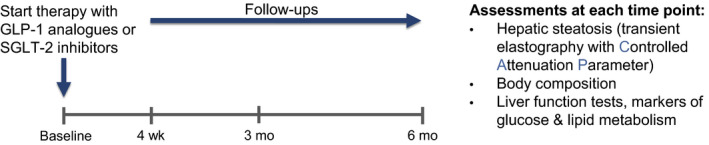
Description of the study design including the assessments carried out during this real‐world observational study
2.2.1. Serum biochemistry and clinical assessments
Blood was collected for biochemical analyses: liver function tests (LFTs) including serum aminotransferase activities (alanine aminotransferase [ALT], aspartate aminotransferase [AST]), alkaline phosphatase (AP), γ‐glutamyl transferase (γ‐GT), albumin, bilirubin, urea, creatinine, GFR‐creatinine clearance and international normalized ratio (INR). In addition, lipid status was assessed by total serum cholesterol (TC), low‐density lipoprotein (LDL) cholesterol, high‐density lipoprotein (HDL) cholesterol and serum triglyceride (TG) concentrations. Glucose metabolism‐related measurement was carried out and included the following: C‐peptide, HbA1c, FPG, fasting insulin, homeostatic model assessment measuring insulin resistance (HOMA‐IR) index (calculated using fasting glucose [mg/dL] × fasting insulin [µU/mL/405]). 24 Serum virology tests excluded the presence of hepatitis A, B, C or D virus infections.
2.2.2. Transient elastography
Vibration‐controlled transient elastography (VCTE) (FibroScan®, Echosens, Paris) with controlled attenuation parameter (CAP) quantified hepatic fat contents, and concurrent liver stiffness measurements (LSM) were documented. This technique has been recommended by the joint clinical practice European guidelines. 25 VTCE with CAP was repeated 10 times to obtain the final value, and values ≥215 dB/m determined the presence of hepatic steatosis. 26 As recommended previously, liver stiffness measurement (LSM) results were included in the analysis if the success rate was ≥ 60% based on at least 10 valid measurements and the interquartile range (IQR)/median LSM ≤ 30%, 27 unless the LSM < 7.1 kPa, in which case the IQR/LSM ratio was not considered. 28
2.2.3. Anthropometry including body composition
The stadiometer seca217 (seca) was used to measure height and a measuring tape for waist circumference. The eight‐electrode segmental multifrequency Medical Body Composition Analyzer mBCA515 (Seca GmbH) quantified body composition through bioelectrical impedance analysis (BIA). This BIA instrument can measure fat mass (FM), fat‐free mass (FFM), total body water (TBW), phase angle (PA) and visceral fat (VF) using frequencies of 5 kHz and 50 kHz and empirical linear regression models. Impedance is determined through body resistance (R) and reactance (Xc) to a flow of alternating electrical current. MetS was diagnosed when three of the following five conditions were present: abdominal adiposity, elevated serum triglycerides, decreased HDL cholesterol, hypertension and elevated fasting glucose. 29
2.3. Study outcomes and statistics
Changes in hepatic steatosis using CAP after 4 weeks and 3 and 6 months of medical management with GLP‐1 analogues or SGLT‐2 inhibitors formed the primary outcome. Secondary outcomes included changes to body composition, LFTs, lipid status and glucose metabolism markers as well as to LSM. Subgroup analysis explored the specific effects of the two types of medications (GLP‐1 analogues and SGLT‐2 inhibitors). The Kolmogorov‐Smirnov and Shapiro‐Wilk tests were used to test for normality of data distributions, and depending on the outcome, either mean ± standard deviation or median (interquartile range) was used to report results and to guide the appropriate statistical tests. Paired Student's t test and Wilcoxon signed rank test assessed differences between two dependent samples, and t test or Mann‐Whitney U test evaluated independent samples. Repeated measures ANOVA and the Friedman test assessed for changes over time for related samples. Data for the subgroup analyses are represented by median (interquartile range), given a significant rejection of normal distribution assumption. Post hoc linear regression was conducted with absolute changes to CAP at 6 months as the dependent variable and the following covariates as independent variables: (CAP at baseline, BMI at baseline, glucose at baseline, and changes in weight at 6 months). The above analyses were conducted with SPSS 20.0 (IBM) and GraphPad Prism 7.0 (GraphPad Software). A two‐sided P value ≤ 0.05 determined statistical significance.
3. RESULTS
3.1. Patient characteristics
In total, 39 patients (49% women, mean age 57.7 ± 10.9 years) with type 2 diabetes and evidence of hepatic steatosis were recruited into this prospective study. Table 1 summarizes the patient characteristics. Patients presented with marked hepatic fat accumulation, as evidenced by a median CAP of 338 dB/m (311‐363 dB/m), which indicates severe histological steatosis grade (S3). 22
TABLE 1.
Baseline and follow‐up data for the entire cohort
| Baseline (n = 39) | 4 wk (n = 39) | 3 mo (n = 39) | 6 mo (n = 36) | P | Change | Relative change | |
|---|---|---|---|---|---|---|---|
| Sex (M/F) | 20/19 | ||||||
| Age (y) | 57.7 ± 10.9 | ||||||
| Transient elastography | |||||||
| CAP (dB/m) | 338 (311‐363) | 313 (296‐341) | 316 (284‐341) | 337 (280‐360) | .026 | −32 (−58 to 32) | −8.9 (−16.9 to 10.0) |
| LSM (kPa) | 6.9 (5.3‐8.4) | 6.4 (5.4‐7.6) | 6.8 (5.7‐7.9) | 6.1 (5.4‐8.1) | .857 | −0.5 (−2.6 to 0.9) | −7.7 (−31.5 to 17.6) |
| Anthropometry/body composition | |||||||
| Weight (kg) | 100.8 ± 17.7 | 99.0 ± 18.2 | 98.7 ± 19.0 | 97.5 ± 18.3 | .000 | −2.5 ± 3.3 | −2.8 ± 3.4 |
| BMI (kg/m2) | 34.3 ± 4.9 | 33.7 ± 5.0 | 33.5 ± 5.3 | 33.3 ± 5.3 | .000 | −0.9 ± 1.2 | −2.7 ± 3.6 |
| Fat‐free mass (kg) | 60.2 ± 11.9 | 59.3 ± 11.9 | 59.4 ± 12.0 | 58.3 ± 12.0 | .013 | −1.1 ± 1.9 | −1.9 ± 3.2 |
| Fat mass (kg) | 40.6 ± 11.7 | 39.7 ± 11.8 | 39.3 ± 12.0 | 38.7 ± 12.1 | .027 | −1.4 ± 3.1 | −4.0 ± 7.7 |
| Total body water (L) | 45.0 ± 8.0 | 44.3 ± 8.4 | 44.0 ± 9.0 | 43.5 ± 8.4 | .005 | −0.9 ± 1.5 | −2.1 ± 3.3 |
| Visceral fat (L) | 4.6 (3.2‐6.9) | 4.7 (3.5‐6.3) | 4.3 (3.2‐6.1) | 4.0 (3.0‐6.0) | .056 | −0.6 ± 0.8 | −9.5 ± 17.1 |
| WC (cm) | 116 ± 13 | 114 ± 12 | 113 ± 14 | 113 ± 13 | .073 | −0.3 ± 0.5 | −2.8 ± 4.5 |
| Phase angle | 5.1 (4.6‐5.6) | 5.3 (4.8‐5.5) | 5.2 (4.6‐5.7) | 5.2 (4.9‐5.7) | .105 | −0.3 ± 0.3 | −0.5 ± 5.2 |
| Biochemistry | |||||||
| Liver function tests | |||||||
| AST (U/L) | 24 (18‐32) | 25 (20‐34) | 22 (18‐28) | 24 (20‐28) | .059 | −2 (−6 to 2) | −7.4 (−22.2 to 8.3) |
| ALT (U/L) | 28 (22‐43) | 29 (22‐44) | 25 (20‐32) | 29 (20‐41) | .002 | −2 (−14 to 2) | −12.5 (−30.2 to 7.4) |
| γ‐GT (U/L) | 36 (24‐54) | 31 (24‐49) | 31 (23‐47) | 33 (24‐49) | .049 | −4 (−15 to 1) | −11.1 (−28.9 to 4.4) |
| AP (U/L) | 79 (63‐87) | 74 (58‐89) | 73 (62‐95) | 73 (60‐95) | .623 | −1 (−7 to 3) | −1.2 (−8.8 to 4.8) |
| Glucose‐related parameters | |||||||
| HbA1c (%) | 8.0 (7.2‐9.0) | N/A | 7.3 (7.0‐7.7) | 7.4 (6.7‐7.8) | .000 | −0.6 (−1.4 to −0.4) | −7.1 (−17.5 to −4.9) |
| FPG (mg/dL) | 184 (140‐212) | 142 (135‐174) | 143 (130‐160) | 135 (126‐151) | .000 | −35.5 (−69.0 to −7.0) | −21.6 (−33.3 to −6.3) |
| Insulin (mIU/ml) | 12.3 (10.7‐22.6) | 9.7 (7.2‐19.2) | 12.5 (8.5‐22.1) | 12.2 (7.1‐17.9) | .138 | −2.1 (−6.3 to 2.3) | −14.7 (−49.2 to 20.9) |
| C‐Peptide (ng/mL) | 2.7 (1.8‐3.3) | 2.3 (1.5‐2.9) | 2.7 (2.2‐3.7) | 2.3 (1.4‐3.0) | .050 | −0.1 (−0.7 to 0.5) | −6.5 (−33.3 to 16.2) |
| HOMA‐IR score (FPGXIns/405) | 5.9 (3.8‐10.6) | 4.1 (2.4‐6.7) | 4.6 (2.8‐8.1) | 4.1 (2.2‐7.2) | .029 | −1.8 (−4.9 to −0.2) | −36.8 (−60.2 to 5.0) |
| Lipid status | |||||||
| TG (mg/dL) | 153 (118‐259) | 159 (124‐217) | 142 (106‐192) | 155 (106‐195) | .464 | −26 (−58 to 8) | −14.5 (−30.6 to 8.0) |
| TC (mg/dL) | 181 ± 42 | 176 ± 40 | 179 ± 41 | 180.3 ± 46.2 | .857 | −1.2 ± 27.1 | −0.3 ± 14.4 |
| LDL‐C (mg/dL) | 106 ± 28 | 104 ± 30 | 111 ± 33 | 108.3 ± 36.9 | .699 | 3.2 ± 25.4 | 3.5 ± 25.2 |
| HDL‐C (mg/dL) | 43 (37‐49) | 42 (36‐48) | 44 (40‐51) | 45 (42‐56) | .004 | 3 (−1 to 7) | 5.7 (−2.5 to 15.8) |
Significant P‐values are highlighted in bold.
Abbreviations: ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; BMI, body mass index; CAP, controlled attenuation parameter; FPG, fasting plasma glucose; HbA1c, haemoglobin A1c; HDL, high‐density lipoprotein; HOMA‐IR, homeostatic model assessment measuring insulin resistance; LDL, low‐density lipoprotein; LSM, liver stiffness measurement; TC, total cholesterol; TG, triglyceride; WC, waist circumference; γ‐GT, gamma‐glutamyl transpeptidase.
Liver stiffness measurements ranged from absent to minimal liver fibrosis for most patients (7.2 kPa, 5.3‐8.4 kPa). Serum aminotransferase activities as surrogate markers of liver injury were elevated in 12 patients only (31%; n = 3 for AST; n = 9 for ALT) and γ‐GT levels in 12 (31%) patients. Additionally, three patients (8%) presented with raised AP activities.
All patients apart from one were either overweight (n = 7) or obese (n = 31), and 35 patients had visceral adiposity as reflected by elevated WC measurements, based on the standard European cut‐offs of 94 cm for men and 80 cm for women. 30 In terms of biochemical metabolic parameters, the HOMA‐IR score was elevated in 36 patients. Lipid status was assessed using TC, which was elevated in 12 patients (31%), and a total of 6 (15%) and 14 (36%) patients displayed increased LDL‐C and reduced HDL‐C concentrations, respectively. Finally, raised serum TG concentrations were present in 22 patients (56%).
All patients except for one were diagnosed with MetS, as defined by Alberti et al. 29 The concomitant medications were as follows: fourteen patients were taking insulin only, 30 metformin, ten of whom were also receiving insulin and three were also receiving DPP4 inhibitors; one patient was taking DPP4 inhibitors only. With regard to lipid‐lowering medications, one patient took a fibrate, and statins and ezetimibe were prescribed for 16 and two patients, respectively. The regimens for these mediations remained unchanged throughout the duration of the study.
3.2. Changes of hepatic phenotypes
Figure 2 illustrates a significant (P = .026) reduction of CAP in the entire cohort during the 6‐month observation period, including individual CAP reductions for each point in time. This overall CAP reduction corresponds to a median relative reduction of 8.9%. Table 1 shows the values for each time point (4 weeks, 3 and 6 months) as well as the overall absolute and relative changes for all liver‐related parameters. Furthermore, when comparing CAP at specific time points, both the values at 4 weeks (T1) and at 3 months (T2) differed significantly as compared to baseline (P = .043 and P = .029, respectively). The greatest reduction in CAP occurred after 4 weeks, with a median CAP change of −26 dB/m (−62 to 24 dB/m). No changes to LSM were observed. For serum surrogate markers, significant reductions were observed for ALT (P = .002) and γ‐GT (P = .049).
FIGURE 2.
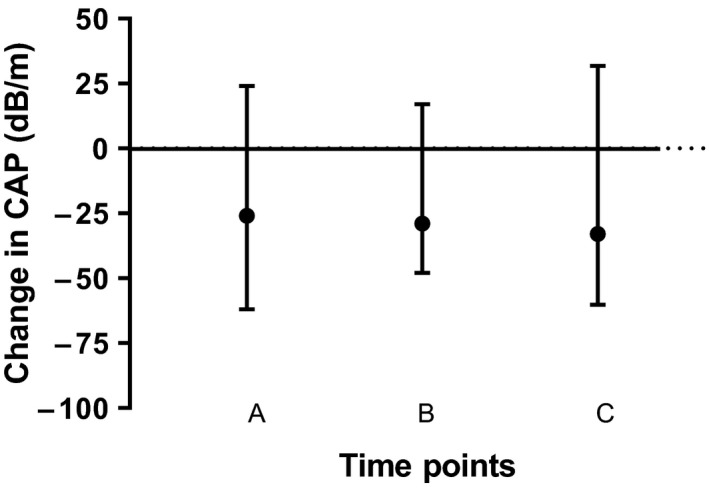
Change in CAP values during the observation period for the entire cohort (n = 39). There was a significant (P = .026) reduction in hepatic steatosis (as reflected by CAP) during the 6 mo that patients received either SGLT‐2 inhibitors or GLP‐1 analogues. The following comparisons are depicted in the Figure: A = change at 4 weeks compared to baseline; B = change at 3 mo compared to baseline; C = change at 6 mo compared to baseline
3.3. Changes to body composition and other metabolic markers
Table 1 summarizes the baseline and follow‐up values, in addition to the change score for the parameters related to body composition and metabolic markers. Specifically, a significant reduction of weight (2.5 ± 3.3 kg) and BMI (−0.9 ± 1.2 kg/m2) occured during follow‐up (both P < .0001). BIA analysis revealed the weight reductions to result from significant decreases in FFM (P = .013), FM (P = .027) and TBW (P = .005), with the following mean relative reductions observed: −1.9 ± 3.2 kg, −4.0 ± 7.7 kg, and −2.1 ± 3.3 L, respectively. WC decreased marginally in the follow‐up period (−0.3 ± 0.5 cm, P > .05). Marked improvements of glucose‐related parameters occurred during the 6‐month period, with significant reductions in HbA1c and fasting plasma glucose (FPG) (both P < .0001), and C‐peptide (P = .05). For instance, FPG improved by 35.5 mg/dL (−69.0 to −7.0 mg/dL), which corresponded to a median relative reduction of 22%. Lipid status improvements only occurred for HDL cholesterol concentration, which significantly (P = .004) increased by 3 mg/dL (−1 to 7 mg/dL).
3.4. Association of SGLT‐2 inhibitors and GLP‐1 analogues with liver‐related effects
Table 2 presents the values at baseline and after 6 months for all parameters based on the type of medication (SGLT‐2 inhibitors or GLP‐1 analogues). No differences between patient characteristics were demonstrated at baseline between the two groups. Stratified analysis revealed significant (P = .014) reductions in CAP to occur in patients receiving SGLT‐2 inhibitors but not for those receiving GLP‐1 analogues (P = .562). Here, patients taking SGLT‐2 inhibitors had a median absolute CAP reduction of 38 dB/m (−58 to 9 dB/m), which corresponded to a 11.3% reduction (−17.1% to 2.7%). Further analysis revealed significant differences to occur at T1 and T2 as compared to baseline CAP (P = .009 and P = .035, respectively). Figure 3 shows the baseline and follow‐up CAP values for each of the two groups, in addition to the corresponding individual patient data.
TABLE 2.
Data for subgroup analysis assessing patients receiving SGLT‐2 inhibitors and GLP‐1 analogues
| SGLT‐2 Group | P | GLP‐1 Group | P | |||
|---|---|---|---|---|---|---|
| Baseline (n = 22) | 6 mo (n = 21) | Baseline (n = 17) | 6 mo (n = 15) | |||
| Sex (M/F) | 12/10 | 8/9 | ||||
| Age (y) | 56 (50‐62) | 66 (56‐68) | ||||
| Transient elastography | ||||||
| CAP (dB/m) | 338 (311‐371) | 336 (274‐353) | .014 | 341 (316‐360) | 337 (295‐368) | .562 |
| LSM (kPa) | 6.4 (5.3‐7.4) | 6.1 (5.6‐8.1) | .438 | 7.4 (6.7‐1.5) | 6.1 (5.0‐7.8) | .568 |
| Anthropometry/body composition | ||||||
| Weight (kg) | 95.6 (89.3‐108.2) | 94.0 (85.9‐107.8) | .006 | 101.7 (91.0‐116.5) | 96.8 (87.1‐114.0) | .000 |
| BMI (kg/m2) | 33.2 (29.6‐37.0) | 32.1 (29.3‐36.7) | .069 | 34.8 (33.0‐38.7) | 33.1 (31.2‐39.0) | .000 |
| Fat‐free mass (kg) | 63.3 (48.8‐65.9) | 6.8 (49.5‐65.8) | .052 | 61.5 (48.8‐72.2) | 59.2 (48.4‐67.4) | .296 |
| Fat mass (kg) | 38.7 (28.8‐48.2) | 37.8 (26.7‐44.9) | .108 | 41.0 (34.0‐51.2) | 36.5 (3.1‐5.0) | .107 |
| Total body water (L) | 46.4 (36.7‐49.1) | 44.3 (37.4‐48.5) | .016 | 46.3 (38.3‐53.7) | 44.3 (36.5‐49.8) | .300 |
| Visceral fat (L) | 4.1 (3.2‐6.9) | 4.0 (3.3‐5.4) | .305 | 5.2 (3.8‐7.4) | 4.5 (3.9‐5.5) | .211 |
| WC (cm) | 111 (106‐121) | 112 (104‐117) | .427 | 120 (112‐129) | 118 (114‐120) | .160 |
| Phase angle | 5.3 (5.0‐5.6) | 5.2 (4.9‐5.5) | .093 | 4.8 (4.5‐5.3) | 5.0 (4.8‐5.7) | .414 |
| Biochemistry | ||||||
| Liver function tests | ||||||
| AST (U/L) | 25 (18‐35) | 25 (17‐32) | .045 | 21 (19‐28) | 21 (20‐25) | .508 |
| ALT (U/L) | 32 (25‐52) | 29 (23‐43) | .052 | 26 (21‐30) | 25 (18‐35) | .029 |
| γ‐GT (U/L) | 48 (28‐71) | 34 (24‐49) | .385 | 33 (24‐51) | 32 (25‐39) | .082 |
| AP (U/L) | 80 (66‐87) | 70 (58.5‐87.5) | .264 | 75 (61‐106) | 74 (65‐121) | .714 |
| Glucose‐related parameters | ||||||
| HbA1c (%) | 8.4 (7.2‐9.2) | 7.6 (7.1‐8.0) | .000 | 7.6 (7.2‐8.8) | 7.2 (6.7‐7.6) | .006 |
| FPG (mg/dL) | 160 (139‐221) | 143 (129‐165) | .001 | 186 (146‐198) | 129 (117‐137) | .006 |
| Insulin (mIU/mL) | 11.6 (10.7‐16.1) | 1.9 (4.6‐15.4) | .188 | 17.1 (1.9‐25.7) | 14.8 (9.7‐31.0) | .720 |
| C‐Peptide (ng/mL) | 2.6 (1.9‐3.1) | 2.3 (1.5‐2.8) | .075 | 2.8 (1.8‐3.3) | 2.2 (1.4‐3.0) | .470 |
| HOMA‐IR score (FPGXIns/405) | 4.9 (3.8‐8.9) | 4.0 (1.5‐4.8) | .026 | 8.0 (5.0‐12.3) | 4.7 (2.7‐1.4) | .277 |
| Lipid status | ||||||
| TG (mg/dL) | 165 (123‐259) | 163 (121‐197) | .104 | 153 (92‐255) | 121 (93‐179) | .580 |
| TC (mg/dL) | 182 (166‐205) | 165 (123‐259) | .463 | 171 (141‐192) | 163 (127‐177) | .101 |
| LDL‐C (mg/dL) | 105 (86‐122) | 112 (87‐147) | .105 | 102 (87‐122) | 98 (63‐114) | .055 |
| HDL‐C (mg/dL) | 46 (38‐51) | 47 (42‐61) | .040 | 42 (37‐47) | 43 (42‐48) | .093 |
Significant P‐values are highlighted in bold.
Abbreviations: ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; BMI, body mass index; CAP, controlled attenuation parameter; FPG, fasting plasma glucose; HbA1c, haemoglobin A1c; HDL, high‐density lipoprotein; HOMA‐IR, homeostatic model assessment measuring insulin resistance; LDL, low‐density lipoprotein; LSM, liver stiffness measurement; TC, total cholesterol; TG, triglyceride; WC, waist circumference; γ‐GT, gamma‐glutamyl transpeptidase.
FIGURE 3.
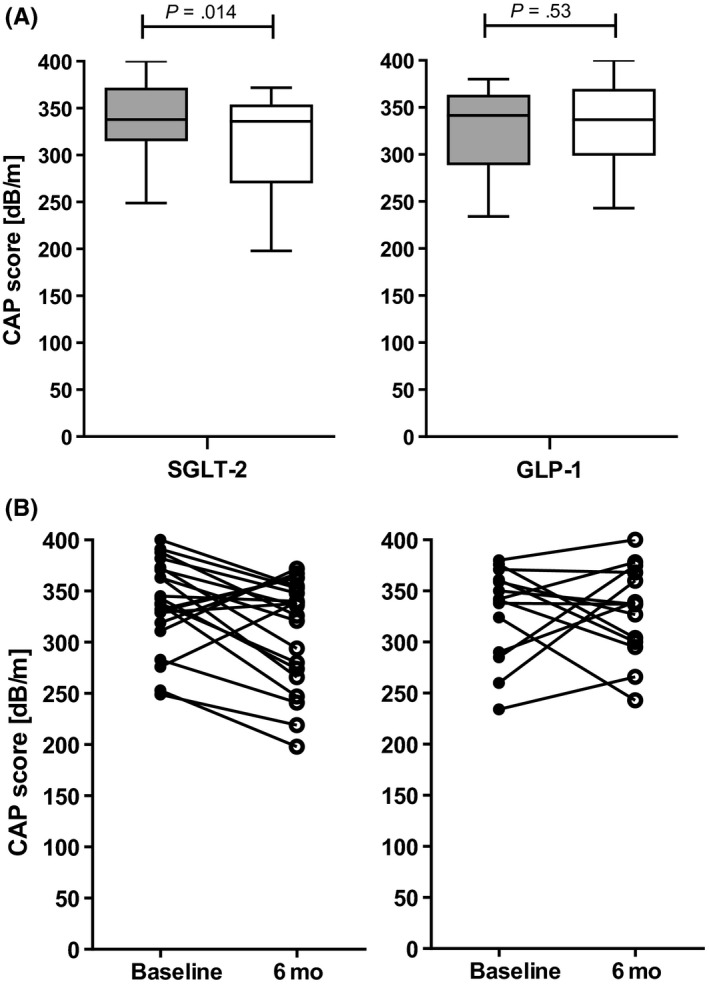
Changes to hepatic steatosis using TE with CAP based on treatment with SGLT‐2 inhibitors or GLP‐1 analogues. There was a significant reduction in CAP in patients receiving SGLT‐2 inhibitors but not GLP‐1 analogues (A, B). Grey boxes represent CAP measurements at baseline, and white boxes represent CAP measurements at the 6‐mo follow‐up (A). Individual patient data for baseline and 6‐mo measurements as quantified using CAP (B)
LSM values did not change in either groups during the observation period. A small but significant median absolute reduction of 2 U/L (−10 to 4 U/L) in serum AST activities was demonstrated for patients taking SGLT‐2 inhibitors (P = .045). Conversely, ALT levels decreased significantly by 2 U/L (−9 to 0 U/L) in those receiving GLP‐1 analogues (P = .029).
3.5. Association of SGLT‐2 inhibitors and GLP‐1 analogues with changes of body composition and metabolic markers
Body weight (but not BMI) decreased significantly (P = .006) in the SGLT‐2 group (mean reduction 2.3 ± 3.8 kg). Both body weight and BMI decreased significantly (P < .0001) by 2.8 ± 2.5 kg and 1.0 ± 1.0 kg/m2, respectively, in the GLP‐1 group; however, body composition (FM, FFM, TBW and WC) did not change. In the SGLT‐2 group, TBW significantly (P = .016) decreased.
Glucose‐related parameters improved in both groups: FPG and HbA1c were significantly (P < .0001) reduced in the SGLT‐2 group. These values decreased by 31 mg/dL (−62 to −7 mg/dL) and 0.5% (−1.9% to −0.4%), respectively. Greater reductions of FPG and HbA1c occurred in the GLP‐1 group (both P = .006), with absolute mean decreases of 49.8 ± 35.1 mg/dL and 0.8 ± 0.6%, respectively. HDL‐C concentrations increased significantly (3.9 ± 6.4 mg/dL; P = .040) in patients receiving SGLT‐2 inhibitors only.
3.6. Hepatic response associated with SGLT‐2 inhibitors and GLP‐1 analogues
Two‐thirds of the patients demonstrated a hepatic response (defined as lower CAP value at 6 months as compared to baseline). This equated to 71% in the SGLT‐2 group and 60% in the GLP‐1 group, respectively. A comparison of the baseline characteristics revealed patients with a hepatic response to differ from those without with respect to BMI, which was lower in the responders (P = .049). Moreover, baseline CAP values were markedly higher: 360 (338‐372) dB/m vs 315 (281‐331) dB/m (P = .002). This illustrates that the response occured in patients with more severe hepatic steatosis (see Table 3). These patients presented with lower baseline LSM (P = .008) as well as lower AST and ALT activities (P = .029 and P = .026, respectively).
TABLE 3.
Comparison of baseline characteristics for hepatic responders a vs nonresponders
| Hepatic | ||
|---|---|---|
| Responders | Nonresponders | |
| Sex (M/F) | 11/13 | 8/4 |
| Age (y) | 56 (54‐67) | 60 (50‐65) |
| Transient elastography | ||
| CAP (dB/m) | 360 (338‐372) | 315 (281‐331)§ |
| LSM (kPa) | 5.9 (4.9‐7.4) | 8.3 (6.7‐11.6)§ |
| Anthropometry/body composition | ||
| Weight (kg) | 95 (84‐110) | 106 (98‐120) |
| BMI (kg/m2) | 33.2 (29.4‐37.0) | 34.8 (33.0‐40.5)* |
| Fat‐free mass (kg) | 58.3 (47.9‐65.5) | 69.6 (51.8‐75.5) |
| Fat mass (kg) | 37.6 (29.8‐46.2) | 48.2 (34.9‐54.5) |
| Total body water (L) | 44 (36‐48) | 51 (40‐55) |
| Visceral fat (L) | 4.1 (3,2‐6.8) | 6.2 (3,5‐9.2) |
| WC (cm) | 114 (105‐123) | 122 (112‐134) |
| Phase angle | 5.1 (4.6‐6.7) | 6.2 (4.6‐5.6) |
| Biochemistry | ||
| Liver function parameters | ||
| AST (U/L) | 21 (18‐27) | 28 (26‐40)* |
| ALT (U/L) | 27 (22‐32) | 42 (27‐72)* |
| γ‐GT (U/L) | 37 (24‐53) | 43 (29‐71) |
| AP (U/L) | 76 (62‐101) | 80 (69‐84) |
| Glucose‐related parameters | ||
| HbA1c (%) | 8.0 (7.3‐8.9) | 8.2 (7.2‐20.0) |
| FPG (mg/dL) | 169 (140‐200) | 188 (141‐230) |
| Insulin (mIU/mL) | 12.0 (10.0‐23.6) | 15.3 (11.9‐17.5) |
| C‐Peptide (ng/mL) | 2.3 (1.5‐3.1) | 2.5 (1.9‐3.8) |
| HOMA‐IR score (FPGXIns/405) | 5.8 (3.8‐10.6) | 7.8 (3.8‐10.9) |
| Lipid status | ||
| TC (mg/dL) | 179 (144‐209) | 171 (156‐202) |
| LDL‐C (mg/dL) | 104 (77‐122) | 94 (88‐121) |
| HDL‐C (mg/dL) | 44 (38‐49) | 45 (37‐52) |
| TG (mg/dL) | 152 (124‐238) | 165 (96‐258) |
P value between the two groups determined with the Mann‐Whitney U test: *P ≤ .05, $ P ≤ .01.
Abbreviations: ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; BMI, body mass index; CAP, controlled attenuation parameter; FPG, fasting plasma glucose; HbA1c, haemoglobin A1c; HDL, high‐density lipoprotein; HOMA‐IR, homeostatic model assessment measuring insulin resistance; LDL, low‐density lipoprotein; LSM, liver stiffness measurement; TC, total cholesterol; TG, triglyceride; WC, waist circumference; γ‐GT, gamma‐glutamyl transpeptidase.
Hepatic responders were defined as those having a lower CAP value at the final follow‐up as compared to baseline (CAP values were available for N = 36 patients at 6 mo).
As summarized in Table 4, a post hoc univariate linear regression analysis found baseline CAP and BMI to be significant determinants of absolute CAP change (P = .003 and P = .012, respectively), but no influence for baseline glucose concentrations or change in body weight at 6 months (both P > .05). Multivariate regression analysis subsequently confirmed both CAP and BMI at baseline to be independent predictors of CAP changes during the intervention with anti‐diabetics (P = .001 and P = .002, respectively).
TABLE 4.
Univariate and multivariate analysis of determinants of absolute changes to CAP
| β coefficient | P | |
|---|---|---|
| A ‐ Univariate analysis | ||
| CAP at baseline | −0.480 | .003 |
| BMI at baseline | 0.421 | .012 |
| Glucose at baseline | 0.255 | >.05 |
| Change in weight at 6 mo | 0.111 | >.05 |
| B ‐ Multivariate analysis | ||
| CAP at baseline | −0.483 | .001 |
| BMI at baseline | 0.377 | .002 |
Linear regression was conducted with absolute changes to CAP at 6 mo as the dependent variable and the following covariates as independent variables: (CAP at baseline, BMI at baseline, glucose at baseline, changes in weight at 6 mo). The β‐coefficient is the resulting effect estimate and P‐values refer to respective Null hypotheses that β = 0. Significant P‐values are highlighted in bold.
Abbreviations: BMI, body mass index; CAP, controlled attenuation parameter.
4. DISCUSSION
In this study based on real‐world data, anti‐diabetic therapies were associated with reductions in liver fat contents of almost 10% in patients after 6 months, with significant reductions demonstrated for patients taking SGLT2‐analogues. Overall, an absolute median CAP decrease of −32 dB/m (−58 to 32 dB/m) was observed for the entire cohort. Serum surrogate markers paralleled these improvements with decreases reported for liver enzymes, as well as for glucose‐related markers (HbA1c and FPG). The analysis of hepatic response predictors showed patients with higher baseline CAP and lower baseline BMI to benefit most from treatment, consistent with findings from others. 31 Of note, changes in weight after 6 months did not appear to have an influence on changes to CAP, as assessed using multivariate regression analysis.
When conducting stratified analysis, the beneficial effects on liver fat appeared to primarily occur in patients receiving SGLT‐2 inhibitors. These findings support other studies in patients receiving SGLT‐2 inhibitors. 9 , 10 , 12 , 14 , 32 , 33 , 34 , 35 An open‐label trial in Japan randomized 33 patients with NAFLD and type 2 diabetes to 5 mg dapagliflozin per day for 24 weeks, and 24 similar patients to a control group. 14 CAP values significantly reduced from 315 ± 61 dB/m at baseline to 290 ± 73 dB/m at 24 weeks. Additionally, the study reported similar reductions to HbA1c to that observed in our cohort, and improvements to body composition and LFTs were also noted.
Transient elastography with CAP values was also reported in six Japanese patients with NASH that received 50 mg oral ipragliflozin/d for 24 weeks. 36 CAP values significantly decreased from 286 (222‐338) dB/m to 258 (163‐320) dB/m. Another study reported a histopathological benefit in nine Japanese cases with diabetes mellitus and NAFLD who received canagliflozin 100 mg once/daily for 24 weeks. 10 In addition to improvements to body composition and glucose metabolism markers, SGLT‐2 inhibitors are suggested to possess anti‐inflammatory properties and to reduce oxidative stress, thus benefiting NAFLD patients. 37 , 38 Another randomized trial has reported a larger reduction when taking SGLT‐2 inhibitors vs placebo, in the secretion of the transmembrane hepatic protein, soluble dipeptidyl peptidase‐4, which triggers both inflammation and insulin resistance. 39
In our study, patients receiving GLP‐1 analogues displayed nonsignificant decreases of CAP after 6 months. This is consistent with other studies reporting no significant benefits to hepatic steatosis for GLP‐1 analogues. 20 , 21 For example, the 12‐week randomized placebo‐controlled Dutch trial administering 1.8 mg liraglutide/d in 17 overweight patients with diabetes did not observe liver fat reduction, as assessed by proton magnetic resonance spectroscopy (1H‐MRS), 21 and liver function tests also remained unchanged.
Despite the lack of significant reductions to CAP, we however did observe other benefits, such as improvements of body composition and serum ALT. These results reinforce findings of previously published studies. For example, significant reductions in transaminase activities, HbA1c and BMI were observed with the GLP‐1 receptor agonist, dulaglutide (0.75 μg/wk) given for 12 weeks and evaluated retrospectively in 15 Japanese patients with biopsy‐proven NAFLD and type 2 diabetes. 40 Five patients also received transient elastography together with body composition analysis before and after treatment, and reductions in body fat and LSM but not CAP were reported.
Unlike our findings, positive liver‐related benefits however have been reported for GLP‐1 analogues. 19 , 41 For instance, the phase II multicentre LEAN trial conducted in the UK demonstrated histopathological resolution of NASH in nine of 23 normal‐weight patients receiving 1.8 mg liraglutide/d for 48 weeks vs two of 22 patients on placebo. 19 Moreover, body weight improved and there was less worsening of fibrosis. Of note, the presence of diabetes did not influence these findings. In addition, the single‐centre Lira‐NAFLD study conducted in France, which availed of 1H‐MRS in 68 patients with NAFLD and uncontrolled type 2 diabetes (defined by HbA1c > 7%/53 mmol/mol), reported a significant reduction of 31% liver fat contents in patients receiving 1.2 mg liraglutide for 6 months. 20 Additionally, decreases in serum ALT, γ‐GT and TG activities occurred and were paralleled by an increase in HDL cholesterol concentrations. Similar reductions of body weight and BMI were reported as in our study, and HbA1c also improved.
The limitation with our single‐centre study is the lack of a control group, small sample size and the fact that dietary habits, which are potential confounders, were not documented systematically during the study. Of note, however, no serious adverse events were observed in those receiving SGLT‐2 inhibitors or GLP‐1 analogues, which is consistent with the lack of severe adverse events reported by others. 42
5. CONCLUSION
In this real‐world observational evaluation of fatty liver monitored noninvasively in patients with type 2 diabetes treated with either SGLT2 or GLP‐1, we observed improvements in measures of hepatic steatosis, glucose and weight parameters after 6 months, with significant reductions of intrahepatic lipid contents seen specifically in the SGLT2 subgroup. Such therapies are being explored further in randomized controlled trials, especially because NAFLD patients with co‐morbid diabetes are particularly at risk of progressive liver disease and complications. Thus, they represent a priority group for whom appropriate therapy of liver disease is urgently required.
CONFLICT OF INTEREST
The authors of the present study have nothing to declare regarding funding or conflict of interest.
AUTHOR CONTRIBUTIONS
C. S. Stokes and F. Lammert supervised the study. V. Mittag‐Roussou recruited patients, collected and analysed the data. S. Wagenpfeil provided statistical input. CS Stokes drafted the manuscript, which was then critically revised by all authors. The final draft submitted has been approved by all authors.
ACKNOWLEDGEMENTS
We are very grateful to the study nurses Ute Kemnade and Birgit Scheidweiler for their assistance with the preparation and storage of biological samples.
Mittag‐Roussou V, Wagenpfeil S, Lammert F, Stokes CS. Noninvasive monitoring of liver fat during treatment with GLP‐1 analogues and SGLT‐2 inhibitors in a real‐world setting. Endocrinol Diab Metab. 2020;3:e00131 10.1002/edm2.131
DATA AVAILABILITY STATEMENT
The data sets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Arab JP, Arrese M, Trauner M. Recent insights into the pathogenesis of nonalcoholic fatty liver disease. Annu Rev Pathol. 2018;13:321‐350. [DOI] [PubMed] [Google Scholar]
- 2. Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11‐20. [DOI] [PubMed] [Google Scholar]
- 3. Targher G, Lonardo A, Byrne CD. Nonalcoholic fatty liver disease and chronic vascular complications of diabetes mellitus. Nat Rev Endocrinol. 2018;14:99‐114. [DOI] [PubMed] [Google Scholar]
- 4. Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675‐1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kalra S. Sodium glucose co‐transporter‐2 (SGLT2) inhibitors: a review of their basic and clinical pharmacology. Diabetes Ther. 2014;5:355‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Honda Y, Imajo K, Kato T, et al. The selective SGLT2 inhibitor ipragliflozin has a therapeutic effect on nonalcoholic steatohepatitis in mice. PLoS ONE. 2016;11:e0146337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qiang S, Nakatsu Y, Seno Y, et al. Treatment with the SGLT2 inhibitor luseogliflozin improves nonalcoholic steatohepatitis in a rodent model with diabetes mellitus. Diabetol Metab Syndr. 2015;7:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jojima T, Tomotsune T, Iijima T, Akimoto K, Suzuki K, Aso Y. Empagliflozin (an SGLT2 inhibitor), alone or in combination with linagliptin (a DPP‐4 inhibitor), prevents steatohepatitis in a novel mouse model of non‐alcoholic steatohepatitis and diabetes. Diabetol Metab Syndr. 2016;8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Komiya C, Tsuchiya K, Shiba K, et al. Ipragliflozin improves hepatic steatosis in obese mice and liver dysfunction in type 2 diabetic patients irrespective of body weight reduction. PLoS ONE. 2016;11:e0151511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Akuta N, Kawamura Y, Watanabe C, et al. Impact of sodium glucose cotransporter 2 inhibitor on histological features and glucose metabolism of non‐alcoholic fatty liver disease complicated by diabetes mellitus. Hepatol Res. 2019;49:531‐539. [DOI] [PubMed] [Google Scholar]
- 11. Seko Y, Sumida Y, Tanaka S, et al. Effect of sodium glucose cotransporter 2 inhibitor on liver function tests in Japanese patients with non‐alcoholic fatty liver disease and type 2 diabetes mellitus. Hepatol Res. 2017;47:1072‐1078. [DOI] [PubMed] [Google Scholar]
- 12. Tobita H, Sato S, Miyake T, Ishihara S, Kinoshita Y. Effects of dapagliflozin on body composition and liver tests in patients with nonalcoholic steatohepatitis associated with type 2 diabetes mellitus: a prospective, open‐label, uncontrolled study. Curr Ther Res Clin Exp. 2017;87:13‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ohki T, Isogawa A, Toda N, Tagawa K. Effectiveness of ipragliflozin, a sodium‐glucose co‐transporter 2 inhibitor, as a second‐line treatment for non‐alcoholic fatty liver disease patients with type 2 diabetes mellitus who do not respond to incretin‐based therapies including glucagon‐like peptide‐1 analogs and dipeptidyl peptidase‐4 inhibitors. Clin Drug Investig. 2016;36:313‐319. [DOI] [PubMed] [Google Scholar]
- 14. Shimizu M, Suzuki K, Kato K, et al. Evaluation of the effects of dapagliflozin, a sodium‐glucose co‐transporter‐2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non‐alcoholic fatty liver disease. Diabetes Obes Metab. 2019;21:285‐292. [DOI] [PubMed] [Google Scholar]
- 15. Ding X, Saxena NK, Lin S, Gupta NA, Anania FA. Exendin‐4, a glucagon‐like protein‐1 (GLP‐1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. 2006;43:173‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mells JE, Fu PP, Sharma S, et al. Glp‐1 analog, liraglutide, ameliorates hepatic steatosis and cardiac hypertrophy in C57BL/6J mice fed a Western diet. Am J Physiol Gastrointest Liver Physiol. 2012;302:G225‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trevaskis JL, Griffin PS, Wittmer C, et al. Glucagon‐like peptide‐1 receptor agonism improves metabolic, biochemical, and histopathological indices of nonalcoholic steatohepatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G762‐G772. [DOI] [PubMed] [Google Scholar]
- 18. Moreira GV, Azevedo FF, Ribeiro LM, et al. Liraglutide modulates gut microbiota and reduces NAFLD in obese mice. J Nutr Biochem. 2018;62:143‐154. [DOI] [PubMed] [Google Scholar]
- 19. Armstrong MJ, Gaunt P, Aithal GP, et al. Hubscher SG & Newsome PN. Liraglutide safety and efficacy in patients with non‐alcoholic steatohepatitis (LEAN): a multicentre, double‐blind, randomised, placebo‐controlled phase 2 study. Lancet. 2016;387:679‐690. [DOI] [PubMed] [Google Scholar]
- 20. Petit JM, Cercueil JP, Loffroy R, et al. Effect of liraglutide therapy on liver fat content in patients with inadequately controlled type 2 diabetes: The Lira‐NAFLD Study. J Clin Endocrinol Metab. 2017;102:407‐415. [DOI] [PubMed] [Google Scholar]
- 21. Smits MM, Tonneijck L, Muskiet MHA, et al. Twelve week liraglutide or sitagliptin does not affect hepatic fat in type 2 diabetes: a randomised placebo‐controlled trial. Diabetologia. 2016;59:2588‐2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Karlas T, Petroff D, Sasso M, et al. Individual patient data meta‐analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022‐1030. [DOI] [PubMed] [Google Scholar]
- 23. AUDIT Questionnaire : Babor TF, Higgins‐Biddle JC, Saunders JB. The Alcohol Use Disorders Identification Test In: Guidelines for use in Primary Health Care. 2nd ed Geneva: World Health Organisation; 2001. [Google Scholar]
- 24. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412‐419. [DOI] [PubMed] [Google Scholar]
- 25. European Association for the Study of the L , European Association for the Study of D , European Association for the Study of O . EASL‐EASD‐EASO Clinical Practice Guidelines for the management of non‐alcoholic fatty liver disease. Diabetologia. 2016;59:1121‐1140. [DOI] [PubMed] [Google Scholar]
- 26. de Ledinghen V, Vergniol J, Foucher J, Merrouche W, le Bail B. Non‐invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int. 2012;32:911‐918. [DOI] [PubMed] [Google Scholar]
- 27. Liver EAfSot & Higado ALpeEd . EASL‐ALEH Clinical Practice Guidelines: non‐invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237‐264. [DOI] [PubMed] [Google Scholar]
- 28. Boursier J, Zarski JP, de Ledinghen V, et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology. 2013;57:1182‐1191. [DOI] [PubMed] [Google Scholar]
- 29. Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640‐1645. [DOI] [PubMed] [Google Scholar]
- 30. Alberti KG, Zimmet P, Shaw J, Group IDFETFC . The metabolic syndrome–a new worldwide definition. Lancet. 2005;366:1059‐1062. [DOI] [PubMed] [Google Scholar]
- 31. Cuthbertson DJ, Irwin A, Gardner CJ, et al. Improved glycaemia correlates with liver fat reduction in obese, type 2 diabetes, patients given glucagon‐like peptide‐1 (GLP‐1) receptor agonists. PLoS ONE. 2012;7:e50117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takeda A, Irahara A, Nakano A, et al. The improvement of the hepatic histological findings in a patient with non‐alcoholic steatohepatitis with type 2 diabetes after the administration of the sodium‐glucose cotransporter 2 inhibitor ipragliflozin. Intern Med. 2017;56:2739‐2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shibuya T, Fushimi N, Kawai M, et al. Luseogliflozin improves liver fat deposition compared to metformin in type 2 diabetes patients with non‐alcoholic fatty liver disease: a prospective randomized controlled pilot study. Diabetes Obes Metab. 2018;20:438‐442. [DOI] [PubMed] [Google Scholar]
- 34. Cusi K, Sattar N, Garcia‐Perez LE, et al. Dulaglutide decreases plasma aminotransferases in people with Type 2 diabetes in a pattern consistent with liver fat reduction: a post hoc analysis of the AWARD programme. Diabet Med. 2018;35:1434‐1439. [DOI] [PubMed] [Google Scholar]
- 35. Itani T, Ishihara T. Efficacy of canagliflozin against nonalcoholic fatty liver disease: a prospective cohort study. Obes Sci Pract. 2018;4:477‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miyake T, Yoshida S, Furukawa S, et al. Ipragliflozin ameliorates liver damage in non‐alcoholic fatty liver disease. Open Medicine. 2018;13:402‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bonnet F, Scheen AJ. Effects of SGLT2 inhibitors on systemic and tissue low‐grade inflammation: The potential contribution to diabetes complications and cardiovascular disease. Diabetes Metab. 2018;44:457‐464. [DOI] [PubMed] [Google Scholar]
- 38. Yaribeygi H, Atkin SL, Butler AE, Sahebkar A. Sodium‐glucose cotransporter inhibitors and oxidative stress: an update. J Cell Physiol. 2019;234:3231‐3237. [DOI] [PubMed] [Google Scholar]
- 39. Aso Y, Kato K, Sakurai S, et al. Impact of dapagliflozin, an SGLT2 inhibitor, on serum levels of soluble dipeptidyl peptidase‐4 in patients with type 2 diabetes and non‐alcoholic fatty liver disease. Int J Clin Pract. 2019;73(5):e13335. [DOI] [PubMed] [Google Scholar]
- 40. Seko Y, Sumida Y, Tanaka S, et al. Effect of 12‐week dulaglutide therapy in Japanese patients with biopsy‐proven non‐alcoholic fatty liver disease and type 2 diabetes mellitus. Hepatol Res. 2017;47:1206‐1211. [DOI] [PubMed] [Google Scholar]
- 41. Eguchi Y, Kitajima Y, Hyogo H, et al. Pilot study of liraglutide effects in non‐alcoholic steatohepatitis and non‐alcoholic fatty liver disease with glucose intolerance in Japanese patients (LEAN‐J). Hepatol Res. 2015;45:269‐278. [DOI] [PubMed] [Google Scholar]
- 42. Ludvik B, Frias JP, Tinahones FJ, et al. Dulaglutide as add‐on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD‐10): a 24‐week, randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2018;6:370‐381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


