Abstract
Introduction
Imeglimin, a glucose‐lowering agent targeting mitochondrial bioenergetics, decreases reactive oxygen species (ROS) overproduction and improves glucose homeostasis. We investigated whether this is associated with protective effects on metabolic syndrome‐related left ventricular (LV) and vascular dysfunctions.
Methods
We used Zucker fa/fa rats to assess the effects on LV function, LV tissue perfusion, LV oxidative stress and vascular function induced by imeglimin administered orally for 9 or 90 days at a dose of 150 mg/kg twice daily.
Results
Compared to untreated animals, 9‐ and 90‐day imeglimin treatment decreased LV end‐diastolic pressure and LV end‐diastolic pressure‐volume relation, increased LV tissue perfusion and decreased LV ROS production. Simultaneously, imeglimin restored acetylcholine‐mediated coronary relaxation and mesenteric flow‐mediated dilation. One hour after imeglimin administration, when glucose plasma levels were not yet modified, imeglimin reduced LV mitochondrial ROS production and improved LV function. Ninety‐day imeglimin treatment reduced related LV and kidney fibrosis and improved kidney function.
Conclusion
In a rat model, mimicking Human metabolic syndrome, imeglimin immediately countered metabolic syndrome‐related cardiac diastolic and vascular dysfunction by reducing oxidative stress/increased NO bioavailability and improving myocardial perfusion and after 90‐day treatment myocardial and kidney structure, effects that are, at least in part, independent from glucose control.
Keywords: cardiomyopathy, imeglimin, rat, type‐2 diabetes
This study shows that imeglimin, the 1st representative of the new class of oral antidiabetic agents, glimins, immediately countered metabolic syndrome‐related cardiac diastolic and vascular dysfunction by reducing oxidative stress/increased NO bioavailability and improving myocardial perfusion, and over the long term by countering myocardial and kidney structural modifications, effects that are at least in part, independent from glucose control.

1. INTRODUCTION
Metabolic syndrome refers to metabolic impairments such as insulin resistance, hyperglycaemia and dyslipidemia associated with obesity and hypertension and increases cardiovascular morbidity and mortality. 1 The latter is related to development of a progressive deterioration of left ventricular (LV) diastolic function and vascular endothelial function. 2 , 3 , 4 Of note is that several studies have demonstrated the implication of mitochondrial dysfunction in metabolic syndrome‐related cardiac diastolic 5 as well as vascular endothelial dysfunctions. 6
Imeglimin, a glucose‐lowering agent targeting mitochondrial bioenergetics, improves hyperinsulinemia, glucose tolerance and insulin sensitivity in experimental models of diabetes as well as in people with diabetes. 7 , 8 , 9 , 10 Moreover, imeglimin decreases reactive oxygen species (ROS) overproduction and delays mitochondrial permeability transition pore opening in endothelial cells, preventing cell death during oxidative stress due to hyperglycaemia. 11 However, there is a paucity of data to suggest whether these beneficial effects are associated with a prevention of metabolic syndrome‐related cardiovascular dysfunctions.
Thus, the goal of the present study was to assess the imeglimin's effects on systemic and cardiac hemodynamics, cardiovascular function and morphological alterations in a rat model mimicking human metabolic syndrome‐related cardiovascular dysfunctions, 12 , 13 , 14 1 hour, after imeglimin administration, as well as after a 9‐ and 90‐day treatment.
2. METHODS AND MATERIALS
2.1. Animals and treatment
This study was performed in 12‐week‐old male Zucker fa/+ (lean) and Zucker fa/fa rats (Charles River Laboratory, France). Metabolic‐related cardiovascular dysfunctions and cardiac structure were assessed after a 90‐day treatment period (study 1). However, because ‘long‐term’ structural effects can mask ‘short‐term’ effects on cardiac/vascular function, effects of imeglimin on metabolic‐related cardiovascular dysfunctions and cardiac structure were also assessed after a 9‐day treatment period. This experimental design allowed the evaluation of the acute effects of imeglimin independently of the potential beneficial effects induced by the improvement of cardiac remodelling after chronic treatment (study 2).
Study 1: Zucker fa/fa rats were randomized at the age of 12 weeks into two groups, either untreated (n = 15) or treated with imeglimin (n = 15; 150 mg/kg/d twice daily, gavage at 09:00 and 18:00) for a 90‐day period. Twelve untreated Zucker fa/+ animals were used as healthy controls. Study 2: Zucker fa/fa rats were randomized at the age of 12 weeks into two groups, either untreated (n = 15) or treated with imeglimin (n = 15) at the same dose for a 9‐day period. Nine untreated Zucker fa/+ animals were used as healthy controls.
Since glycemic control is involved in effects induced by 9‐ and 90‐day imeglimin treatment, 10 , 11 , 12 a third study evaluated the cardiovascular effects of imeglimin as soon as 1 hour after a single imeglimin administration (150 mg/kg) in 13‐week‐old Zucker fa/fa rats, allowing evaluation of imeglimin effects independently of glycemic control.
2.2. Investigated parameters
All parameters investigated after single or multiple imeglimin dose(s) were measured 1h after the last (or single) morning dose.
2.3. Left ventricular function
2.3.1. Echocardiography
Studies were performed in rats before and after 9, 30 and 90 days of treatment. For this purpose, rats were anesthetized with methohexital, the chest shaved and echocardiograms were performed with a Vivid 7 ultrasound echograph equipped with an M12L, as described previously. 14 In brief, a two‐dimensional short axis view of the LV was obtained at the level of the papillary muscle in order to record M‐mode tracings. Left ventricular diameters were measured by the American Society of Echocardiology leading‐edge method. 15 In addition, LV outflow velocity was measured by pulsed‐wave Doppler, and cardiac output was calculated as CO = aortic VTI (velocity time interval) x [π x (LV outflow diameter/2)2] x heart rate.
2.3.2. Left ventricular hemodynamics
Left ventricular hemodynamics were assessed 1 hour after a single imeglimin administration as well as after a 9‐ and a 90‐day imeglimin treatment using LV pressure‐volume curves, as previously described. 14 Animals were anesthetized with methohexital; a conductance‐micromanometer catheter (model SPR‐819, Millar Instruments) was connected to a pressure‐conductance unit and advanced retrograde via the carotid artery into the LV. Left ventricular pressure‐volume loops were recorded at baseline and during loading by gently occluding the abdominal aorta with a cotton swab. Data were stored and analysed by using Millar conductance data acquisition/analysis software and the following parameters were measured/calculated from the pressure‐volume curves: LV end‐systolic and end‐diastolic pressures, LV end‐systolic pressure‐volume and end‐diastolic pressure‐volume relations, and LV relaxation constant Tau.
2.4. Myocardial perfusion
One hour after a single imeglimin administration as well as after a 9‐ and a 90‐day imeglimin treatment, myocardial perfusion was assessed in methohexital‐anesthetized animals using a Bruker Biospec 4.7 Tesla MRI, and an acquisition T1 sequence that does not need contrast agent application, as previously described. 16 , 17
2.5. Exercise tolerance test
Exercise capacity was determined in randomly selected animals of each group after the last imeglimin administration in the 90‐day protocol (study 1). The animals were exercised on a treadmill (Bioseb, Paris France) at a speed of 2.4 m/min at a 5° incline. The speed was increased by 2.4 m/min every 1 minutes to a maximum speed of 14.4 m/min. Fatigue was considered to occur when a rat started to lower its hindquarters and raise its snout, resulting in a significantly altered gait and an inability to remain on the treadmill. When this degree of fatigue was noted, the animal was taken off the treadmill, and running time was recorded.
2.6. Oral glucose tolerance test
At the end of the 9‐day treatment period, rats were fasted 16 hours before testing. A blood sample was collected from the tail vein, and blood glucose was measured with a glucometer (StatStrip Xpress, Nova Biomedical) before the OGTT. The OGTT was performed by oral gavage of D‐glucose 2 g/kg body weight, blood samples were collected from the tail vein, and glucose levels were measured for 2 hours.
2.7. Vascular endothelial function
Coronary endothelial function was assessed, using a DMT wire myograph (DMT, Denmark), as previously described. 14 Following the LV function assessment, the heart was removed and placed in cold‐oxygenated Krebs buffer. A 1.5‐ to 2‐mm‐long segment of coronary arteries was dissected and mounted in a vessel myograph. Segments with an internal diameter <170 µm were excluded to avoid mechanical endothelial injury and unspecific dysfunction. Concentration‐response curves to acetylcholine (10−8 to 3 x 10−5 mol/L) were performed in serotonin‐precontracted segments (10‐5 mol/L). Endothelium‐independent relaxation to increasing concentrations of sodium nitroprusside (10−9 to 3 x 10−5 mol/L) was also obtained in serotonin‐precontracted arteries. Peripheral endothelial function. At the end of 9 and 90 days of treatment, mesenteric artery dilatation was assessed, as previously described. 18 After assessment of the hemodynamic parameters, a third order mesenteric artery was isolated and transferred to a pressure myograph (DMT). Arteries were preconstricted by the phenylephrine, and flow‐mediated dilatation was assessed by increasing stepwise intraluminal perfusate flow rate.
2.8. Urinary biochemical assessment
After 90 days of treatment, randomly selected animals in each group were placed in metabolic cages for a 24‐hour urine collection. Urinary creatinine‐excretion and proteinuria were determined as indicators of renal function.
2.9. Kidney histology
Kidneys were harvested at the end of study 1. Masson's trichrome staining of paraffin‐embedded kidney sections was used for semi‐quantitative scoring of glomerulosclerosis, tubular injury (necrosis and atrophy), interstitial inflammation and interstitial fibrosis, as previously described. 19 A minimum of 20 glomeruli (range: 20 to 30) in each specimen were examined, and the severity of the lesion was graded from 0 to 4 and expressed as a percentage of number of the glomeruli examined.
2.10. Cardiac histology
After hemodynamic assessment, the heart was harvested and LV weight was measured. Sirius red staining of paraffin‐embedded LV sections was used for collagen determination using an image analysis system. Collagen density was calculated in the LV as the surface occupied by collagen divided by the surface of the image. 20
Left ventricular ROS levels were evaluated after 1h, 9 and 90 days of treatment in LV homogenates by electron paramagnetic resonance spectroscopy using the spin probe 1‐hydroxy‐3‐methoxycarbonyl‐2,2,5,5‐tetramethylpyrrolidine (Noxygen), as previously described. 21 Besides ‘overall’ LV ROS production, LV interfibrillar and LV subsarcolemmal mitochondria ROS production were determined at 1 hour after a single imeglimin administration, as previously described. 22
Plasma nitrite concentrations, a marker of nitric oxide (NO) production, were determined in plasma samples obtained after 1h, 9 and 90 days of treatment, using tri‐iodide based chemiluminescence, as previously described. 23 The NO signal was quantified using a nitric oxide analyser (NOA™ 280, Sievers Instruments).
2.11. Plasma biochemical
Plasma creatinine, glucose, insulin, total cholesterol and triglycerides levels were measured after 1h, 9 and 90 days of treatment, using the Catalyst Analyzer (IDEXX, France).
2.12. Statistical analysis
All results are given as mean ± SEM.
Left ventricular diastolic/systolic diameters and hemodynamic parameters were assessed as a primary endpoint, whereas all other parameters, that is the molecular mechanisms, were assessed as exploratory end‐points. Based upon historical data obtained with other drugs in Zucker fa/fa rats, 14 , 18 we made a simulation for each parameter obtained in untreated animals to demonstrate a statistical significance (P < .05) with a minimal power of 80%. The minimal expected effect size or difference between untreated and treated was fixed to 10% and 30%, with the coefficient of variation to 2% and 15% for echocardiographic and hemodynamic studies, respectively. For all significant differences concerning primary end‐points, a posteriori powers higher than 80% were also checked.
In order to evaluate the effect of metabolic syndrome, all parameters obtained in untreated Zucker fa/fa and lean rats were compared by Student's unpaired two‐tailed t test. In order to evaluate the effects of long‐ or short‐term imeglimin, all parameters obtained 1 hour, or 9‐ and 90‐day treated Zucker fa/fa rats were compared with age‐matched untreated Zucker fa/fa using Student's unpaired two‐tailed t test.
Before applying parametric tests as Student's unpaired two‐tailed t test, the Gaussian distribution of data was assessed by the Shapiro‐Wilk test for normality and the Kolmogorov‐Smirnov test, and graphically by Q‐Q plot and normal probability plot.
3. RESULTS
3.1. Biological parameters
Body weight was increased in 13‐ and 24‐week‐old untreated Zucker rats when compared to age‐matched lean rats. While plasma glucose concentration was increased in 13‐week‐old Zucker rats, plasma insulin, cholesterol and triglycerides concentrations were increased in 24‐week‐old untreated Zucker rats compared to lean rats. Imeglimin did not modify body weight, plasma cholesterol, triglycerides or insulin concentrations at any time, but reduced fasting plasma glucose after both 9 and 90 days of administration when compared to untreated Zucker rats (Table 1).
Table 1.
Biology and systemic hemodynamics after 9 (D9) and 90 d (D90) imeglimin treatment
| Group | Time | Lean | Zucker fa/fa | |
|---|---|---|---|---|
| Untreated | Imeglimin | |||
| Body Weight (g) | D9 | 361 ± 12 | 426 ± 7* | 421 ± 16 |
| D90 | 415 ± 25 | 568 ± 11* | 545 ± 13 | |
| Plasma glucose (mmol/L) | D9 | 4.98 ± 0.17 | 8.19 ± 0.47* | 6.64 ± 0.39† |
| D90 | 7.17 ± 0.61 | 6.73 ± 0.25 | 5.50 ± 0.31† | |
| Plasma insulin (Unit/mL) | D9 | ‐ | 14.1 ± 3.5 | 13.3 ± 2.4 |
| D90 | 1.27 ± 0.53 | 4.88 ± 0.59* | 4.76 ± 0.57 | |
| Plasma cholesterol (mmol/L) | D9 | ‐ | 4.11 ± 0.24 | 4.19 ± 0.40 |
| D90 | 2.85 ± 0.12 | 5.66 ± 0.23* | 5.51 ± 0.33 | |
| Plasma triglycerides (mMol/L) | D9 | ‐ | 2.62 ± 0.22 | 1.66 ± 0.41 |
| D90 | 0.96 ± 0.12 | 5.02 ± 0.31* | 5.24 ± 0.64 | |
| Systolic Blood Pressure (mm Hg) | D9 | 130 ± 6 | 143 ± 5 | 146 ± 4 |
| D90 | 108 ± 6 | 131 ± 6* | 125 ± 5 | |
| Diastolic Blood Pressure (mm Hg) | D9 | 102 ± 4 | 101 ± 5 | 106 ± 2 |
| D90 | 82 ± 7 | 96 ± 6 | 90 ± 4 | |
| Heart rate (beats/min) | D9 | 354 ± 12 | 370 ± 18 | 381 ± 17 |
| D90 | 343 ± 9 | 345 ± 13 | 340 ± 14 | |
P < .05 vs Lean zucker fa/+.
P < .05 vs untreated Zucker fa/fa.
3.2. Systemic hemodynamics
In 13 and 24‐week‐old untreated Zucker rats, systolic blood pressure was increased compared to age‐matched lean rats, reaching statistical significance after 90 days, while diastolic blood pressure and heart rate were not different when compared with age‐matched lean rats. Neither 9‐ nor 90‐day imeglimin treatment modified systolic and diastolic blood pressure or heart rate (Table 1).
3.3. Left ventricular remodelling
Left ventricular diastolic and systolic diameters as well as LV fractional shortening were similar in 12‐week‐old untreated Zucker rats compared to age‐matched lean rats. Left ventricular diastolic and systolic diameters increased during ageing, resulting in a significant decrease in LV fractional shortening compared to age‐matched lean rats after 90 days. Imeglimin slightly reduced LV diastolic and systolic diameters after 9, 30 and 90 days of treatment, reaching statistical significance after 30 days for LV diastolic diameter and after 90 days for systolic diameter. Imeglimin also increased LV fractional shortening, reaching statistical significance after 90 days (Figure 1).
Figure 1.
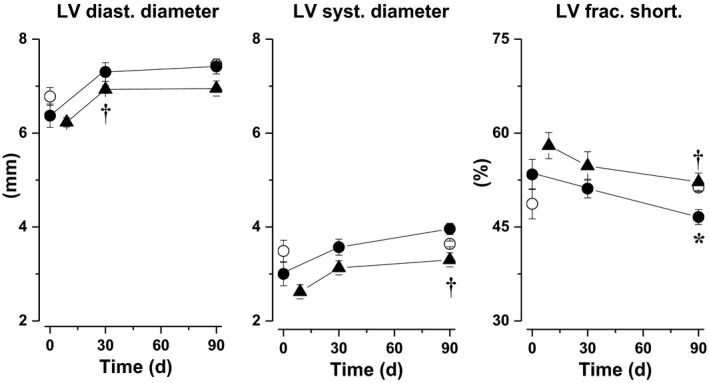
Left ventricular (LV) diastolic diameter and systolic diameter as well as LV fractional shortening determined before and after 30 and 90 d in untreated Zucker fa/+ (open circles) and untreated Zucker fa/fa/ rats (filled circles) and after 9, 30 and 90 d in imeglimin‐treated Zucker fa/fa (filled triangles). *: P<.05 vs untreated Zucker fa/+; †: P<.05 vs untreated Zucker fa/fa
3.4. Left ventricular hemodynamics
Left ventricular end‐systolic pressure was slightly increased in 13‐ and 24‐week‐old Zucker rats when compared to age‐matched lean rats, and this increase was statistically significant only in 24‐week‐old rats. Left ventricular end‐systolic pressure‐volume relationship was significantly increased in 13‐week‐old Zucker rats, while LV end‐diastolic pressure, relaxation constant Tau, and LV end‐diastolic pressure‐volume relationship were all significantly increased in both 13‐ and 24‐week‐old Zucker rats compared to age‐matched lean animals (Figure 2).
Figure 2.
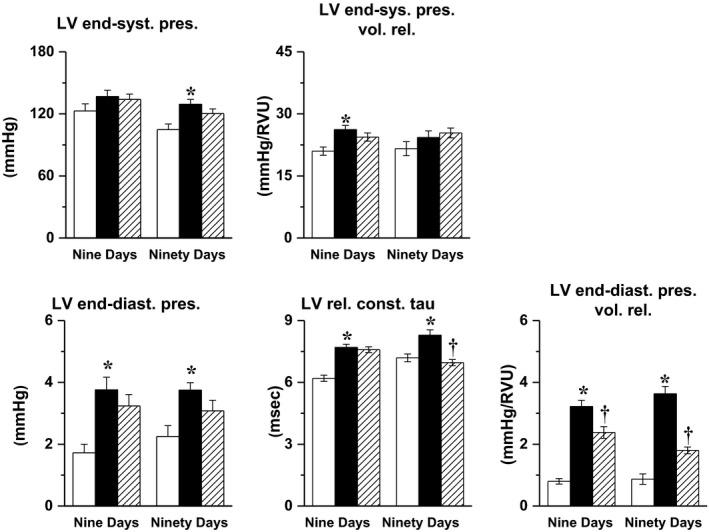
Left ventricular (LV) end‐systolic pressure, LV end‐systolic pressure‐volume relation, LV end‐diastolic pressure, LV relaxation constant Tau and LV end‐diastolic pressure‐volume relation determined after 9 or 90 d in untreated Zucker fa/+ rats (white bars), as well as in untreated (black bars) and imeglimin‐treated Zucker fa/fa (hatched bars). *: P<.05 vs untreated Zucker fa/+; †: P<.05 vs untreated Zucker fa/fa
Administration of imeglimin for 9 and 90 days modified neither LV end‐systolic pressure nor LV end‐systolic pressure‐volume relation. The 9‐day imeglimin administration decreased LV end‐diastolic pressure and LV end‐diastolic pressure‐volume relationship, reaching statistical significance for the latter, without modifying LV Tau. The 90‐day imeglimin treatment reduced LV end‐diastolic pressure, LV end‐diastolic pressure‐volume relationship and Tau, reaching statistical significance for LV end‐diastolic pressure‐volume relationship and Tau (Figure 2).
3.5. Left ventricular remodelling and myocardial perfusion
While LV weight was similar between Zucker and lean rats at the age of 13 and 24 weeks, LV interstitial collagen density was significantly increased in the 24‐week‐old Zucker rats. Imeglimin did not modify LV weight at any time, but significantly reduced LV collagen density after 90 days treatment. Additionally, myocardial perfusion was decreased in both 13‐ and 24‐week‐old untreated Zucker rats. Both 9‐ and 90‐day imeglimin treatments significantly increased myocardial tissue perfusion (Table 2).
Table 2.
LV remodelling, perfusion and ROS after 9 and 90 d of imeglimin treatment
| Group | Time | Lean | Zucker fa/fa | |
|---|---|---|---|---|
| Untreated | Imeglimin | |||
| LV weight (g) | D9 | 0.832 ± 0.030 | 0.813 ± 0.013 | 0.813 ± 0.03 |
| D90 | 1.017 ± 0.048 | 1.048 ± 0.034 | 1.126 ± 0.042 | |
| LV collagen density (%) | D9 | 2.26 ± 0.21 | 3.48 ± 0.48 | 3.33 ± 0.22 |
| D90 | 2.06 ± 0.11 | 3.07 ± 0.08* | 2.52 ± 0.20† | |
| LV collagen representative pictures at D90 |
|

|

|
|
| LV myocardial perfusion (mL/min/g) | D9 | 6.31 ± 0.31 | 4.88 ± 0.26* | 5.71 ± 0.26† |
| D90 | 6.16 ± 0.26 | 4.28 ± 0.35* | 5.68 ± 0.12† | |
| LV ROS production (AU/µg/h) | D9 | 32.25 ± 0.90 | 39.51 ± 1.77* | 27.61 ± 1.29† |
| D90 | 26.69 ± 3.06 | 40.96 ± 3.29* | 34.02 ± 1.49 | |
| Plasma nitrite | D9 | 407 ± 20 | 270 ± 27* | 438 ± 51† |
| D90 | 450 ± 43 | 292 ± 26* | 424 ± 15† | |
P < .05 vs Lean Zucker fa/+.
P < .05 vs untreated Zucker fa/fa.
3.6. Left ventricular oxidative stress
Left ventricular ROS production was increased, while plasma nitrite levels were decreased in 13‐ and 24‐week‐old untreated Zucker rats when compared to age‐matched lean rats. Both 9‐ and 90‐day imeglimin treatments decreased LV ROS production levels, reaching statistical significance after 9 days and increased plasma nitrite levels (Table 2).
3.7. Coronary artery endothelium‐dependent relaxation
Imeglimin administration for 9 and 90 days prevented the impairment in acetylcholine‐induced relaxation of septal coronary artery observed in 24‐week‐old untreated Zucker rats (Figure 3, middle and right panel). Moreover, 1 hour after a single imeglimin administration, endothelium‐dependent relaxation was slightly improved compared to age‐matched untreated Zucker rats (Figure 3, left panel), reaching statistical significance at the concentrations of 10−7‐3 x 10−6 M acetylcholine.
Figure 3.
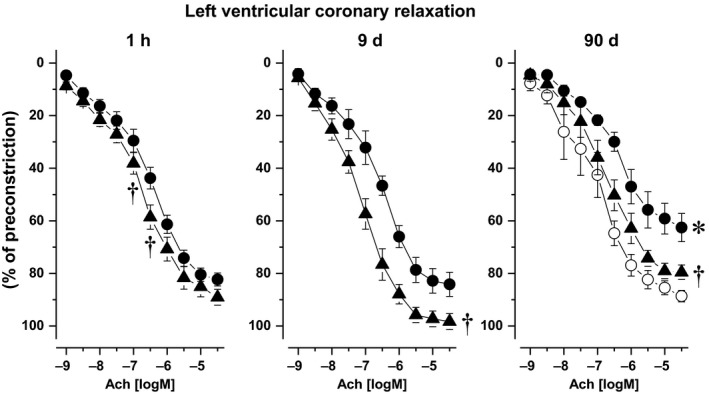
Septal coronary relaxation induced by acetylcholine in imeglimin‐treated Zucker fa/fa rats (filled triangles) either 1 h after single administration (left), and after 9 (center) as well as 90‐d treatment (right), and in age‐matched untreated Zucker fa/fa rats (filled circles). Twenty‐four wk‐old untreated Zucker fa/+ (open circles) were used as healthy control *: P<.05 vs Zucker fa/+ rats; †: P<.05 vs untreated Zucker fa/fa
Coronary relaxation induced by sodium nitroprusside was identical in all groups (data not shown).
3.8. Mesenteric endothelium‐dependent dilatation
Imeglimin administered for 9 or 90 days prevented the impairment of flow‐mediated dilation of mesenteric arteries when compared to time‐matched untreated Zucker rats. (Figure 4).
Figure 4.
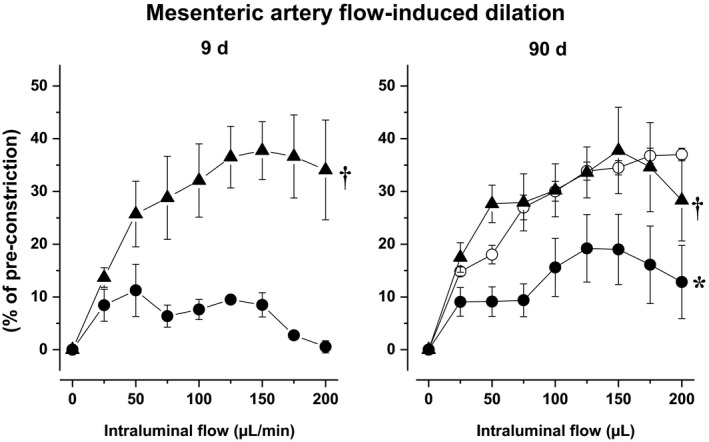
Mesenteric artery flow‐mediated dilation in untreated (filled circles) and imeglimintreated Zucker fa/fa (filled triangles), after 9 (left panel) and 90 d (right panel). Twenty‐four week‐old untreated Zucker fa/+ (open circles) were used as healthy control. *: P<.05 vs Zucker fa/+ rats; †: P<.05 vs untreated Zucker fa/fa
3.9. Oral glucose tolerance test
Oral glucose tolerance was impaired in 13‐week‐old untreated Zucker rats when compared to age‐matched lean rats. Imeglimin normalized OGTT after 9 days of treatment (Figure 5).
Figure 5.
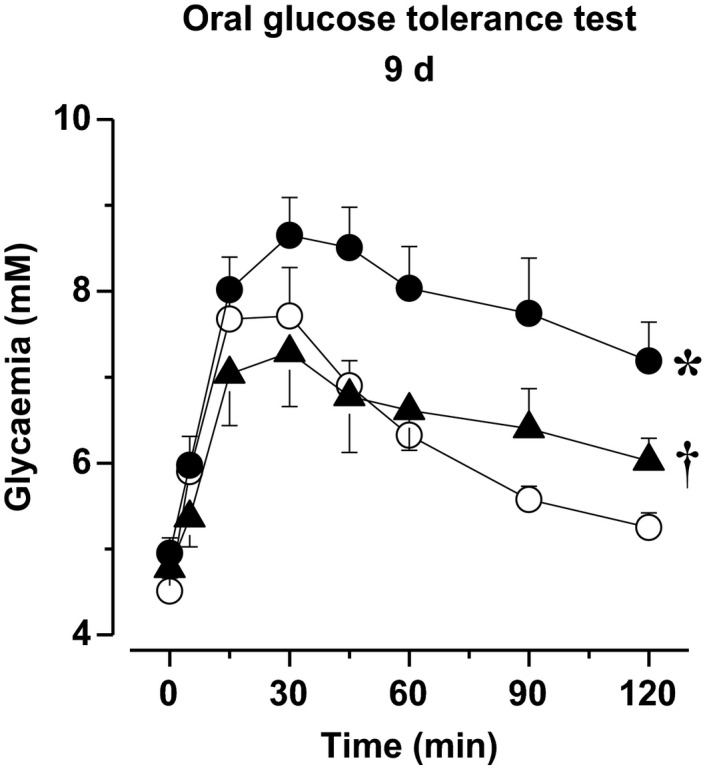
Oral glucose tolerance test in untreated Zucker fa/+ (open circles) and Zucker fa/fa (filled circles) as well as in imeglimin‐treated Zucker fa/fa (filled triangles) 1 h after the last imeglimin administration of the 9‐d treatment period. *: P<.05 vs Zucker fa/+ rats; †: P<.05 vs untreated Zucker fa/fa
3.10. Kidney function
Albuminuria was significantly increased in 24‐week‐old untreated Zucker rats compared to age‐matched lean rats, while urinary volume and creatinuria were not modified. While imeglimin treatment for 90 days reduced albuminuria, there was no reduction in creatinuria or urinary volume (Table 3).
Table 3.
Kidney structure and function after 90 d of imeglimin treatment
| Group | Lean | Zucker fa/fa | |
|---|---|---|---|
| Untreated | Imeglimin | ||
| Urinary volume (ml/24 h) | 7.60 ± 0.61 | 8.93 ± 0.64 | 9.37 ± 0.60 |
| Creatiniuria (mg/24 h) | 834 ± 71 | 692 ± 77 | 659 ± 78 |
| Albuminuria (mg/24 h) | 108 ± 8 | 385 ± 58* | 251 ± 27† |
| Glomerular injury (AU) | 0.19 ± 0.09 | 2.10 ± 0.56* | 1.43 ± 0.30 |
| Glomerular injury representative pictures at D90 |
|
|
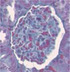
|
| Tubular injury (AU) | 0.19 ± 0.09 | 2.30 ± 0.30* | 2.43 ± 0.23 |
| Interstitial fibrosis (AU) | 0.38 ± 0.13 | 2.00 ± 0.22* | 1.50 ± 0.11† |
| Interstitial fibrosis representative pictures at D90 |
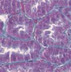
|
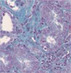
|
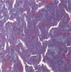
|
| Interstitial inflammation (AU) | 0.06 ± 0.06 | 1.60 ± 0.29* | 1.14 ± 0.18 |
P < .05 vs Lean Zucker fa/+;
P < .05 vs untreated Zucker fa/fa
3.11. Kidney histology
Glomeruli and tubular injury scores, interstitial fibrosis, and interstitial inflammation were increased in 24‐week‐old untreated Zucker rats compared to age‐matched lean rats. Treatment with imeglimin for 90 days significantly reduced interstitial fibrosis; while the glomerular injury score and interstitial inflammation were also reduced, the reduction did not reach statistical significance (Table 3).
3.12. Exercise tolerance
Maximal running distance was 438 ± 2 m in 24‐week‐old lean animals (n = 4) and was reduced to 123 ± 13 m in 24‐week‐old untreated Zucker animals (n = 7). 90 days of treatment with imeglimin resulted in an increase of maximal running distance to 161 ± 19 m (n = 5), without reaching statistical significance.
3.13. Acute effects of imeglimin
As soon as 1 hour after imeglimin administration, plasma fasting glucose was not modified. However, there was an improvement in myocardial tissue perfusion, as well as LV diastolic function (as illustrated by the decreases in Tau and LV end‐diastolic pressure‐volume relation and the small reduction in LV end‐diastolic pressure) while LV end‐systolic pressure‐volume relation was not changed (Table 4). Moreover, this acute administration slightly improved coronary endothelium‐dependent relaxation, compared to age‐matched untreated Zucker rats (Figure 3, left panel), reaching statistical significance at concentrations of 10−8‐3 x 10−7‐M acetylcholine. Furthermore, after 1 hour of imeglimin administration, there was a small reduction in LV ROS production, while ROS production was significantly decreased in an isolated subsarcolemma mitochondria population (Table 4). Finally, the acute treatment increased plasma nitrites.
Table 4.
Acute effects of imeglimin 1 h after administration
| Group | Zucker fa/fa | |
|---|---|---|
| Untreated | Imeglimin | |
| Fasting plasma glucose (mmol/L) | 5.98 ± 0.46 | 5.15 ± 0.62 |
| Systolic blood pressure (mm Hg) | 145 ± 4 | 133 ± 4† |
| Diastolic blood pressure (mm Hg) | 105 ± 3 | 97 ± 3 |
| Heart rate (beats/min) | 371 ± 11 | 374 ± 9 |
| LV end‐systolic pressure (mm Hg) | 140 ± 4 | 123 ± 6† |
| LV end‐systolic pressure‐volume relation (mm Hg/RVU) | 25.2 ± 1.1 | 27.7 ± 1.9 |
| LV end‐diastolic pressure (mm Hg) | 3.57 ± 0.35 | 2.80 ± 0.43 |
| LV relaxation constant Tau (msec) | 7.89 ± 0.18 | 7.03 ± 0.24† |
| LV end‐diastolic pressure‐volume relation (mmHg/RVU) | 3.38 ± 0.18 | 1.90 ± 0.07† |
| LV myocardial tissue perfusion (ml/min/g) | 4.51 ± 0.26 | 6.24 ± 0.52† |
| LV ROS production (AU/µg/h) | 20.64 ± 0.77 | 18.21 ± 0.83 (P = .06) |
| Plasma Nitrite levels (nmol/L) | 297 ± 26 | 648 ± 92† |
| LV mitochondrial ROS (AU/µg/h) | ||
| Subsarcolemma | 6407 ± 779 | 4319 ± 485† |
| Interfibrillar | 2953 ± 413 | 2116 ± 532 |
P < .05 vs untreated Zucker fa/fa.
4. DISCUSSION
This study highlights that imeglimin, a new glucose‐lowering agent targeting mitochondrial bioenergetics, improves metabolic syndrome‐related cardiac and vascular dysfunctions. Importantly, these improvements of both cardiac and vascular functions are observed after 9‐ and 90‐day treatment, as well as at 1 hour after imeglimin administration, when glycaemia was not yet modified but ROS production was already reduced.
We choose the Zucker rats since this rat model of metabolic syndrome‐related cardiovascular dysfunctions presents classical characteristics observed in human metabolic syndrome. 2 Indeed, as already reported by others, 24 , 25 we observed in 13‐ and 24‐week‐old Zucker rats obesity, hypertension, hyperlipidemia, hypercholesterolaemia and impaired tolerance to glucose. Moreover, as previously reported by us and others, this was associated with LV diastolic dysfunction, 14 , 26 , 27 as well as vascular dysfunctions, that is impairments of both coronary endothelium‐dependent vasorelaxation 12 and mesenteric endothelium‐dependent vasodilatation, 18 both characteristic complications observed in diabetic patients. 28 , 29
Our first major finding is that imeglimin improves both LV diastolic and vascular dysfunctions. The improvement of diastolic dysfunction was independent of modifications in systemic hemodynamics since no major modification of blood pressure was observed, but clearly involved a decrease in oxidative stress. Moreover, this decrease in oxidative stress is independent of glycemic control as evidenced by the fact that in 13‐week‐old Zucker rats 1 hour after imeglimin acute administration, plasma glucose level was not changed, whereas LV ROS production was already decreased. Interestingly, the decrease in LV oxidative stress probably results from a decrease in mitochondrial ROS production, as already shown in the liver mitochondria following imeglimin treatment, 9 as the decrease in mitochondrial ROS production was more marked than in ‘whole’ LV tissue. Moreover, we cannot exclude contribution of others ROS sources, such as NADPH oxidase, in reducing ROS production after 9 or 90 days of imeglimin treatment, since reduction in ROS will reduce ROS‐induced ROS production. 30
This reduction in oxidative stress is also probably involved in the improvement of vascular function by imeglimin. Indeed, limiting NO neutralization by reducing endothelial ROS overproduction increases NO bioavailability, as illustrated in our study by the improvement in NO‐mediated acetylcholine‐induced coronary relaxation and flow‐mediated mesenteric dilatation, as well as the increase in nitrite plasma level, altogether demonstrating a restored NO bioavailability. 31
Moreover, the reduction in ROS/increase in NO bioavailability and the resulting improved coronary function probably contributes to the increase in myocardial perfusion. This likely limits the metabolic syndrome induced hypoperfusion observed in this study and thus re‐establishes LV tissue O2 supply. Such effect is beneficial as it reduces hypoxia‐induced ROS production and thus probably inflammation, 32 , 33 , 34 and as a consequence, breaks the vicious circle of ROS/inflammation/ROS production.
Simultaneously with the improvement of coronary function, imeglimin also improves LV diastolic function. This likely results, at least in part, from the reduction of ROS and increase in NO bioavailability, as NO directly improves LV diastolic dysfunction by promoting Ca 2 + handling through protein kinase GMPc dependent activation. 35 , 36
The results obtained after acute (1 hour) imeglimin treatment demonstrate beneficial effects of imeglimin on both LV diastolic and coronary dysfunctions that are similar to those of chronic treatment. Whereas the acute and chronic effects share several mechanisms, for example the reduction of ROS/increase in NO bioavailability, other additional mechanisms are involved in the ‘long‐term’ effects of imeglimin. Indeed, in addition to direct beneficial effects of the reduction in oxidative stress on LV diastolic function, indirect effects due to chronic decreases in LV ROS production contribute to the cardiovascular protective effect of imeglimin. For example, reduced oxidative stress will limit LV collagen accumulation over the long term by reducing myocardial ROS‐induced collagen synthesis. 37
In addition to direct limitation of oxidative stress, imeglimin might also prevent hyperglycaemia‐related toxicity/ROS production 38 through a restoration in glucose tolerance and a reduction in plasma fasting glucose, thus participating in the sustained decrease in oxidative stress and increase in NO bioavailability.
Interestingly, at the vascular level, the imeglimin‐related increased in NO bioavailability improved coronary function and also restored mesenteric artery flow‐mediated dilation. Thus, imeglimin opposes the aggravation of vascular endothelium‐dependent dysfunction observed during ageing in untreated Zucker rats, 39 a vascular effect already observed in humans with metabolic syndrome. 40
In parallel to the improvement in peripheral artery endothelial function, we observed a decrease in albuminuria after 90 days of imeglimin administration. Moreover, interstitial fibrosis was decreased in kidney tubules, probably also resulting from a persistent reduction in oxidative/inflammatory status as observed in Zucker fa/fa rats. 37 Taken together, these preclinical results demonstrate that in addition to mitigating cardiac and vascular effects, a 90‐day imeglimin treatment initiated ‘early’ also limits end‐stage kidney damage, but whether this is solely related to imeglimin's glucose‐lowering effect remains to be elucidated, and whether this renal protection occurs in humans remains to be confirmed in prospective clinical trials.
Intolerance to physical exercise is a frequent phenomenon observed in patients with diastolic dysfunction as well as in experimental models of metabolic syndrome 41 and this excercise intolerance was also observed in 24‐week‐old untreated Zucker rats. Despite the effect on cardiac function as well as on peripheral artery dilatation and thus the possible improvement of the metabolic syndrome‐related impairment of skeletal muscle perfusion, 42 , 43 imeglimin did not clearly improve exercise tolerance. Indeed, the 90‐day imeglimin treatment increases running distance by 20% in obese Zucker fa/fa rats but without reaching statistical significance. This may be related to the fact that imeglimin did not modify body weight and that obesity probably remains the most important factor in effort intolerance in such a model.
4.1. Study limitations
In this study, imeglimin was given as a single medication at the dose of 150 mg/kg bid as previously administered in others rodent models of diabetes, 9 , 10 a dosing regimen known to control glycaemia. However, it may be that another dosing regimen, for example continuous administration, will be more effective. Furthermore, this preclinical study evaluated the effects of imeglimin as a mono‐therapy, but patients with metabolic syndrome often receive several medications that each target a specific component of metabolic syndrome, such as antihypertensive drugs, statins and other glucose‐lowering agents. Further investigation to the effects of imeglimin on cardiac, vascular and renal functions when administered with other treatments of metabolic syndrome needs to address these questions.
Moreover, in our experimental conditions, as already mentioned above, improvement of LV function and LV tissue perfusion has been observed 1 hour after imeglimin administration without a marked modification in glycaemia, suggesting that imeglimin's cardiac effects might be, at least in part, independent of glycemic control, but further investigation is needed to confirm this hypothesis.
5. Conclusion
In an animal model of metabolic syndrome‐related cardiomyopathy imeglimin, a glucose‐lowering agent targeting mitochondrial bioenergetics mitigates various clinically relevant parameters of end‐organ damage also observed in patients with metabolic syndrome, that is LV diastolic, vascular endothelial and renal dysfunctions, at least in part through a reduction in oxidative stress. While 2 Japanese ongoing clinical trials have proven imeglimin's efficacy and safety in Japanese patients with type‐2 diabetes, either alone or in combination with classical antidiabetic treatment, 42 , 43 new clinical studies are needed to test the possible beneficial effects of this compound on heart failure with preserved ejection fraction observed in type‐2 diabetes, as suggested recently. 44
CONFLICT OF INTERESTS
Marianne Lachaux, Matthieu Soulié, Mouad Hamzaoui, Anaëlle Bailly, Lionel Nicol, Isabelle Rémy‐Jouet, Sylvanie Renet, Cathy Vendeville, Christelle Monteil, Vincent Richard and Paul Mulder (being the guarantor) have nothing to declare. Pascale Gluais‐Dagorn and Sophie Hallakou‐Bozec are employee Poxel SA.
AUTHOR CONTRIBUTIONS
Marianne Lachaux, Matthieu Soulié, Mouad Hamzaoui, Anaëlle Bailly, Lionel Nicol, Isabelle Rémy‐Jouet, Sylvanie Renet, Cathy Vendeville made all substantial contributions to the acquisition, analysis or interpretation of data for the work and contributed to the drafting the work. Pascale Gluais‐Dagorn, Sophie Hallakou‐Bozec, Christelle Monteil, Vincent Richard, Paul Mulder made all substantial contributions to the conception, the design, the acquisition, the analysis or/and the interpretation of data for the work and all contributed to the drafting the manuscript.
ETHICS APPROVAL
The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85‐23, revised 1996) and was approved by the local ethical comity CENOMEXA n°54 (authorization number 05 231.01).
ACKNOWLEDGEMENTS
This work was funded by a research grant from Poxel SA, France, and co‐supported by the European Union and the Région Normandie.(Europe gets involved in Normandy with European Regional Development Fund; PACT‐CBS), the European Fibro‐Targets Project (grant agreement No. FP7#602904), the FP7‐funded COST ADMIRE network (BM1301) and the Fédération Française de Cardiology. Marianne Lachaux was a recipient of a PhD grant from Rouen Normandy University and the FHU REMOD‐VHF, while Matthieu Soulié and Mouad Hamzaoui were recipients of a PhD grant from Rouen Normandy University.
Lachaux M, Soulié M, Hamzaoui M, et al. Short‐and long‐term administration of imeglimin counters cardiorenal dysfunction in a rat model of metabolic syndrome. Endocrinol Diab Metab. 2020;3:e00128 10.1002/edm2.128
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle‐aged men. JAMA. 2002;288:2709‐2716. [DOI] [PubMed] [Google Scholar]
- 2. Wilson PW, D'Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066‐3072. [DOI] [PubMed] [Google Scholar]
- 3. von Bibra H, St John Sutton M. Diastolic dysfunction in diabetes and the metabolic syndrome: Promising potential for diagnosis and prognosis. Diabetologia. 2010;53:1033‐1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Katakam PV, Tulbert CD, Snipes JA, Erdos B, Miller AW, Busija DW. Impaired insulin‐induced vasodilation in small coronary arteries of zucker obese rats is mediated by reactive oxygen species. Am J Physiol Heart Circ Physiol. 2005;288:H854‐860. [DOI] [PubMed] [Google Scholar]
- 5. van Bilsen M, Smeets PJ, Gilde AJ, van der Vusse GJ. Metabolic remodelling of the failing heart: the cardiac burn‐out syndrome? Cardiovasc Res. 2004;61:218‐226. [DOI] [PubMed] [Google Scholar]
- 6. Katakam PV, Snipes JA, Tulbert CD, Mayanagi K, Miller AW, Busija DW. Impaired endothelin‐induced vasoconstriction in coronary arteries of zucker obese rats is associated with uncoupling of [ca2+]i signaling. Am J Physiol Regul Integr Comp Physiol. 2006;290:R145‐153. [DOI] [PubMed] [Google Scholar]
- 7. Fouqueray P, Pirags V, Diamant M, et al. The efficacy and safety of imeglimin as add‐on therapy in patients with type 2 diabetes inadequately controlled with sitagliptin monotherapy. Diabetes Care. 2014;37:1924‐1930. [DOI] [PubMed] [Google Scholar]
- 8. Fouqueray P, Pirags V, Inzucchi SE, et al. The efficacy and safety of imeglimin as add‐on therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy. Diabetes Care. 2013;36:565‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vial G, Chauvin MA, Bendridi N, et al. Imeglimin normalizes glucose tolerance and insulin sensitivity and improves mitochondrial function in liver of a high‐fat, high‐sucrose diet mice model. Diabetes. 2015;64:2254‐2264. [DOI] [PubMed] [Google Scholar]
- 10. Perry RJ, Cardone RL, Petersen MC, et al. Imeglimin lowers glucose primarily by amplifying glucose‐stimulated insulin secretion in high‐fat‐fed rodents. Am J Physiol Endocrinol Metab. 2016;311:E461‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Detaille D, Vial G, Borel AL, et al. Imeglimin prevents human endothelial cell death by inhibiting mitochondrial permeability transition without inhibiting mitochondrial respiration. Cell death discovery. 2016;2:15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bender SB, DeMarco VG, Padilla J, et al. Mineralocorticoid receptor antagonism treats obesity‐associated cardiac diastolic dysfunction. Hypertension. 2015;65:1082‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Agouni A, Lagrue‐Lak‐Hal AH, Mostefai HA, et al. Red wine polyphenols prevent metabolic and cardiovascular alterations associated with obesity in zucker fatty rats (fa/fa). PLoS One. 2009;4:e5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fang Y, Nicol L, Harouki N, et al. Improvement of left ventricular diastolic function induced by beta‐blockade a comparison between nebivolol and metoprolol. J Mol Cell Cardiol. 2011;51:168‐176. [DOI] [PubMed] [Google Scholar]
- 15. Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in m‐mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072‐1083. [DOI] [PubMed] [Google Scholar]
- 16. Waller C, Hiller KH, Voll S, Haase A, Ertl G, Bauer WR. Myocardial perfusion imaging using a non‐contrast agent mr imaging technique. Int J Cardiovasc Imaging. 2001;17:123‐132. [DOI] [PubMed] [Google Scholar]
- 17. Nahrendorf M, Wiesmann F, Hiller KH, et al. In vivo assessment of cardiac remodeling after myocardial infarction in rats by cine‐magnetic resonance imaging. J Cardiovasc Magn Reson. 2000;2:171‐180. [DOI] [PubMed] [Google Scholar]
- 18. Merabet N, Fang Y, Nicol L, et al. Selective heart rate reduction improves metabolic syndrome‐related left ventricular diastolic dysfunction. J Cardiovasc Pharmacol. 2015;66:399‐408. [DOI] [PubMed] [Google Scholar]
- 19. Jung GO, Moon JI, Kim JM, et al. Can preemptive kidney transplantation guarantee longer graft survival in living‐donor kidney transplantation? Single‐center study. Transplant Proc. 2010;42:766‐774. [DOI] [PubMed] [Google Scholar]
- 20. Mulder P, Barbier S, Chagraoui A, et al. Long‐term heart rate reduction induced by the selective i(f) current inhibitor ivabradine improves left ventricular function and intrinsic myocardial structure in congestive heart failure. Circulation. 2004;109:1674‐1679. [DOI] [PubMed] [Google Scholar]
- 21. Fang Y, Debunne M, Vercauteren M, et al. Heart rate reduction induced by the if current inhibitor ivabradine improves diastolic function and attenuates cardiac tissue hypoxia. J Cardiovasc Pharmacol. 2012;59:260‐267. [DOI] [PubMed] [Google Scholar]
- 22. Crochemore C, Mekki M, Corbiere C, et al. Subsarcolemmal and interfibrillar mitochondria display distinct superoxide production profiles. Free Radical Res. 2015;49:331‐337. [DOI] [PubMed] [Google Scholar]
- 23. Mulder P, Mellin V, Favre J, et al. Aldosterone synthase inhibition improves cardiovascular function and structure in rats with heart failure: a comparison with spironolactone. Eur Heart J. 2008;29:2171‐2179. [DOI] [PubMed] [Google Scholar]
- 24. Debin R, Lauzier B, Sicard P, et al. Are zucker obese rats a useful model for cardiovascular complications in metabolic syndrome? Physical, biochemical and oxidative stress considerations. Fundam Clin Pharmacol. 2009;23:59‐67. [DOI] [PubMed] [Google Scholar]
- 25. Luo H, Wang X, Wang J, et al. Chronic nf‐kappab blockade improves renal angiotensin ii type 1 receptor functions and reduces blood pressure in zucker diabetic rats. Cardiovasc Diabetol. 2015;14:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ayalon N, Gopal DM, Mooney DM, et al. Preclinical left ventricular diastolic dysfunction in metabolic syndrome. Am J Cardiol. 2014;114:838‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lachaux M, Barrera‐Chimal J, Nicol L, et al. Short‐ and long‐term administration of the non‐steroidal mineralocorticoid receptor antagonist finerenone opposes metabolic syndrome‐related cardio‐renal dysfunction. Diabetes Obes Metab. 2018;20:2399‐2407. [DOI] [PubMed] [Google Scholar]
- 28. Matsutani D, Sakamoto M, Kayama Y, Takeda N, Horiuchi R, Utsunomiya K. Effect of canagliflozin on left ventricular diastolic function in patients with type 2 diabetes. Cardiovasc Diabetol. 2018;17:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Duflot T, Moreau‐Grange L, Roche C, et al. Altered bioavailability of epoxyeicosatrienoic acids is associated with conduit artery endothelial dysfunction in type 2 diabetic patients. Cardiovasc Diabetol. 2019;18:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS‐induced ROS release: an update and review. Biochim Biophys Acta. 2006;1757:509‐517. [DOI] [PubMed] [Google Scholar]
- 31. Lauer T, Preik M, Rassaf T, et al. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci U S A. 2001;98:12814‐12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Serpillon S, Floyd BC, Gupte RS, et al. Superoxide production by NAD(P)H oxidase and mitochondria is increased in genetically obese and hyperglycemic rat heart and aorta before the development of cardiac dysfunction. The role of glucose‐6‐phosphate dehydrogenase‐derived NADPH. Am J Physiol Heart Circ Physiol. 2009;297:H153‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rosen P, Osmers A. Oxidative stress in young zucker rats with impaired glucose tolerance is diminished by acarbose. Horm Metab Res. 2006;38:575‐586. [DOI] [PubMed] [Google Scholar]
- 34. Zhou X, Ma L, Habibi J, et al. Nebivolol improves diastolic dysfunction and myocardial remodeling through reductions in oxidative stress in the zucker obese rat. Hypertension. 2010;55:880‐888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Heerebeek L, Franssen CP, Hamdani N, Verheugt FW, Somsen GA, Paulus WJ. Molecular and cellular basis for diastolic dysfunction. Curr Heart Fail Rep. 2012;9:293‐302. [DOI] [PubMed] [Google Scholar]
- 36. van Heerebeek L, Hamdani N, Handoko ML, et al. Diastolic stiffness of the failing diabetic heart: Importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43‐51. [DOI] [PubMed] [Google Scholar]
- 37. Zhao W, Zhao T, Chen Y, Ahokas RA, Sun Y. Oxidative stress mediates cardiac fibrosis by enhancing transforming growth factor‐beta1 in hypertensive rats. Mol Cell Biochem. 2008;317:43‐50. [DOI] [PubMed] [Google Scholar]
- 38. Busik JV, Mohr S, Grant MB. Hyperglycemia‐induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes. 2008;57:1952‐1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oltman CL, Richou LL, Davidson EP, Coppey LJ, Lund DD, Yorek MA. Progression of coronary and mesenteric vascular dysfunction in zucker obese and zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol. 2006;291:H1780‐1787. [DOI] [PubMed] [Google Scholar]
- 40. Fornoni A, Raij L. Metabolic syndrome and endothelial dysfunction. Curr Hypertens Rep. 2005;7:88‐95. [DOI] [PubMed] [Google Scholar]
- 41. Wong CY, O'Moore‐Sullivan T, Fang ZY, Haluska B, Leano R, Marwick TH. Myocardial and vascular dysfunction and exercise capacity in the metabolic syndrome. Am J Cardiol. 2005;96:1686‐1691. [DOI] [PubMed] [Google Scholar]
- 42. DeFronzo RA. 2019. https://www.easd.org/virtualmeeting/home.html#!resources/the‐role‐of‐mitochondrial‐dysfunction‐in‐the‐pathophysiology‐of‐diabetes. Accessed September 09, 2019.
- 43. Dubourg J. 2019. https://www.easd.org/virtualmeeting/home.html#!resources/clinical‐evidence‐to‐support‐the‐safety‐and‐efficacy‐of‐imeglimin‐in‐various‐populations‐of‐patients‐with‐type‐2‐diabetes. Accessed September 09, 2019.
- 44. Sakamoto M, Matsutani D, Kayama Y. Possibility of a new therapeutic strategy for left ventricular dysfunction in type 2 diabetes. J Clin Med Res. 2018;10:799‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


