Ceramides are a class of bioactive sphingolipids increasingly recognized in metabolic disease, heart failure (HF), atherosclerosis, and cardiovascular death (1). Despite accumulating evidence linking ceramides to HF risk, little is known about circulating ceramides and clinical outcomes in HF.
We performed targeted sphingolipidomics to test whether circulating ceramides are associated with death or heart failure admission (DHFA) among participants with heart failure with preserved ejection fraction (HFpEF) in the TOPCAT trial. TOPCAT data and samples were obtained from the National Heart, Lung, and Blood Institute Biolincc repository. Serum concentrations of 76 sphingolipids were determined for all participants with available samples (n = 433) using liquid chromatography-tandem mass spectrometry at the Virginia Commonwealth University Lipidomics Core Facility (Richmond, VA). Analyses were conducted in a blinded manner.
Compared to participants without available samples, the prevalence of myocardial infarction (25.1% vs 31.9%), coronary artery bypass surgery (12.1% vs 18.2%), atrial fibrillation (34.3% vs 42%), and a history of smoking (40.2% vs 47.9%) was higher in the subset with available samples (all P<0.05). Subjects in this subset also had higher body mass index (30.8 kg/m2 vs 32 kg/m2) BNP levels (mean 397 pg/ml vs 508 pg/ml) and were more frequently from the Americas (49.4% vs 44.1%; all P<0.05). Amongst the 433 subjects, 103 reached the endpoint of DHFA. In univariate Cox models adjusted for multiple comparisons by a modified Bonferroni correction based on the principal components explaining >95% of the variability in measured species, increased concentrations of ceramides 16:0, 18:0 and 18:1 were associated with increased risk of DHFA (Figure 1A). When further adjusted for the MAGGIC risk score (the time of heart failure diagnosis was not available, and excluded from the score), increased concentrations of ceramides 16:0 and 18:0 were associated with the risk of DHFA (Figure 1B). Amongst these, ceramide 16:0 exhibited the most significant association (HR 1.35 95% CI 1.13–1.63, P=0.0011). Both ceramide 16:0 and 18:0 were also associated with all-cause death. When participants were stratified by tertiles of ceramide 16:0, we found no significant differences in clinical variables including age, gender, race, history of myocardial infarction, coronary revascularization, chronic obstructive pulmonary disease, hypertension, diabetes, atrial fibrillation, smoking, body mass index, use of insulin, aspirin, statins, beta blockers, renin-angiotensin-aldosterone system inhibitors, estimated glomerular filtration rate, hematocrit, or natriuretic peptide levels. We did not observe significant interactions between baseline ceramide 16:0 levels and randomization to spironolactone therapy (P = 0.60), statin therapy, or BMI for the outcome of DHFA.
Figure 1. Associations between sphingolipids and outcomes in TOPCAT.
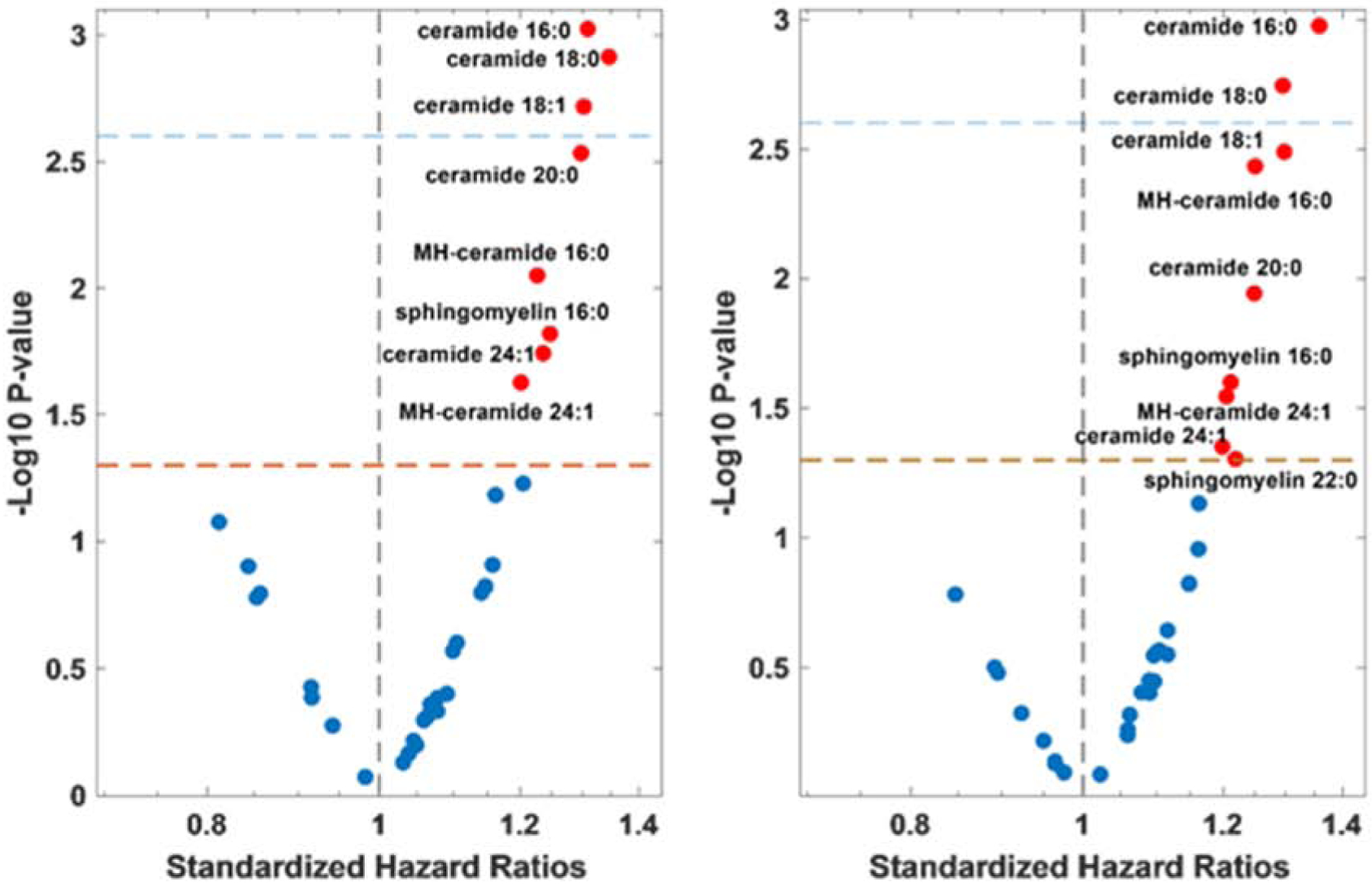
A) Univariate standardized hazard ratios and B) MAGGIC risk score adjusted standardized hazard ratios (x-axis) versus p-values (y-axis). The red-dotted lines indicate p = 0.05, while the blue-dotted lines indicate statistical significance after correction for multiple comparisons (p = 0.0025).
Abbreviations: MH-ceramide- monohexosylceramide
Although increased ceramide 24:0 levels, the most abundant very long-chain ceramide species, were previously associated with reductions in all-cause mortality (3), we found no association between ceramide 24:0 and DHFA. Moreover, increased ceramide 16:0 was associated with DHFA in a model adjusted for ceramide 24:0 (Standardized HR = 1.35; 95%CI = 1.12–1.61; P = 0.0012).
In this study, we identify that increased circulating concentrations ceramide 16:0 and 18:0 are associated with DHFA in HFpEF, consistent with the growing body of literature that long-chain ceramides are associated with cardiovascular outcomes (3,4). In contradistinction to prior reports suggesting very-long chain ceramides are associated with reduced risk(2), we did not observe any associations between increased ceramide species and reduced DHFA risk. Our findings are consistent with the recent report by Lemaitre et al. who found that increased ceramide 16:0 is associated with incident HF (4).
Because palmitate is the most abundant fatty acid in the Western diet and we have measured predominantly palmitate-derived ceramides, our studies are consistent with the possibility that that long-chain ceramides are potential mediator of the link between poor diet and poor health outcomes. Our samples were not necessarily obtained in the fasting state, which could have confounded our results. Regardless of diet, carbon chain lengths of ceramide species are also dependent on particular ceramide synthases. Ceramide synthase 5 (CerS5), for example, is involved in the synthesis of ceramide 16:0 species. In murine models of high fat feeding, genetic ablation of CerS5 leads to decrease in ceramide 16:0 and 18:0 species in multiple tissues, decreased weight gain, and reduced white adipose tissue inflammation (5). In summary, ceramide 16:0 concentrations are associated with risk of incident heart failure, DHFA in HFpEF, and metabolic dysfunction and inflammation in animal models.
Acknowledgments:
We acknowledge the VCU Lipidomics/Metabolomics Core, the NIH-NCI Cancer Center Support Grant P30 CA016059 to the VCU Massey Cancer Center, as well as a shared resource grant (S10RR031535) from the National Institutes of Health.
Funding: This study was supported by NIH grants P30DK056341, R56HL-124073-01A1 (J.A.C), R01 HL 121510-01A1 (J.A.C), 5-R21-AG-043802-02 (J.A.C) and a VISN-4 research
grant from the department of Veterans Affairs (J.A.C). AJ is supported by K08HL138262-01.
Disclosures: Dr. Chirinos is supported by NIH grants R01-HL 121510-01A1, R61-HL-146390, R01-AG058969, 1R01-HL104106, P01-HL094307, R03-HL146874-01 and R56-HL136730. He has received consulting honoraria from Sanifit, Microsoft, Fukuda-Denshi, Bristol-Myers Squibb, OPKO Healthcare, Ironwood Pharmaceuticals, Pfizer, Akros Pharma, Merck, Edwards Lifesciences, Bayer and JNJ. He has received research grants from the NIH, Microsoft, Fukuda-Denshi and Bristol-Myers Squibb. He is named as inventor in an UPenn patent for the use of inorganic nitrates/nitrites for the treatment of HF and Preserved Ejection Fraction and a patent application for the use of novel neoepitope biomarkers of tissue fibrosis in heart failure. Dr. Javaheri is supported by K08HL138262-01 and a named co-inventor on the patent application for the use of fusion protein nanodiscs for the treatment of heart failure.
Other authors have no disclosures.
Abbreviations:
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- BNP
B-type natriuretic peptide
- DHFA
death or heart failure admission
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Summers SA. Could Ceramides Become the New Cholesterol? Cell Metab 2018;27:276–280. [DOI] [PubMed] [Google Scholar]
- 2.Peterson LR, Xanthakis V, Duncan MS et al. Ceramide Remodeling and Risk of Cardiovascular Events and Mortality. J Am Heart Assoc 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laaksonen R, Ekroos K, Sysi-Aho M et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur heart J.2016;37:1967–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemaitre RN, Jensen PN, Hoofnagle A et al. Plasma Ceramides and Sphingomyelins in Relation to Heart Failure Risk. Circ Heart Fail 2019;12:e005708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gosejacob D, Jager PS, Vom Dorp K et al. Ceramide Synthase 5 Is Essential to Maintain C16:0-Ceramide Pools and Contributes to the Development of Diet-induced Obesity. J Biol Chem 2016;291:6989–7003. [DOI] [PMC free article] [PubMed] [Google Scholar]


