Abstract
Background:
From 1980 to 2017, a fluorochemical manufacturing facility discharged wastewater containing poorly understood per- and polyfluoroalkyl substances (PFAS) to the Cape Fear River, the primary drinking water source for Wilmington, North Carolina, residents. Those PFAS included several fluoroethers including HFPO-DA also known as GenX. Little is known about the bioaccumulation potential of these fluoroethers.
Objective:
We determined levels of fluoroethers and legacy PFAS in serum samples from Wilmington residents.
Methods:
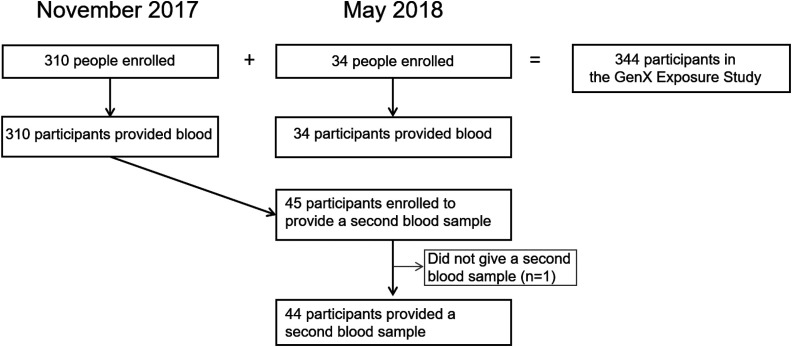
In November 2017 and May 2018, we enrolled 344 Wilmington residents of age into the GenX Exposure Study and collected blood samples. Repeated blood samples were collected from 44 participants 6 months after enrollment. We analyzed serum for 10 fluoroethers and 10 legacy PFAS using liquid chromatography–high-resolution mass spectrometry.
Results:
Participants’ ages ranged from 6 to 86 y, and they lived in the lower Cape Fear Region for 20 y on average (standard deviation: 16 y). Six fluoroethers were detected in serum; Nafion by-product 2 and PFO4DA were detected in of participants. PFO3OA and NVHOS were infrequently detected. Hydro-EVE was present in a subset of samples, but we could not quantify it. GenX was not detected above our analytical method reporting limit (). In participants with repeated samples, the median decrease in fluoroether levels ranged from 34% for Nafion byproduct 2 to 65% for PFO4DA in 6 months due to wastewater discharge control. Four legacy PFAS (PFHxS, PFOA, PFOS, PFNA) were detected in most () participants; these levels were higher than U.S. national levels for the 2015–2016 National Health and Nutrition Examination Survey. The sum concentration of fluoroethers contributed 23% to participants’ summed PFAS (median: ).
Conclusion:
Poorly understood fluoroethers released into the Cape Fear River by a fluorochemical manufacturing facility were detected in blood samples from Wilmington, North Carolina, residents. Health implications of exposure to these novel PFAS have not been well characterized. https://doi.org/10.1289/EHP6837
Introduction
Per- and polyfluoroalkyl substances (PFAS) are a broad class of synthetic chemicals used to manufacture fluoropolymers, stain repellents, paper coatings, and fire-fighting foams (Kissa 2001). In addition to the PFAS produced for commercial purposes, other PFAS can be formed as by-products or impurities of fluorochemical production (Dinglasan et al. 2004; James and Franklin 1966; Liang et al. 1998; Moore et al. 1966). Many PFAS have high aqueous solubility and are persistent in the environment. As a result, PFAS are stable in water and can travel over long distances in freshwater and marine ecosystems (Banzhaf et al. 2017; Möller et al. 2010). PFAS releases into the environment can therefore impact drinking water sources both near and far from the source of contamination (Hu et al. 2016; Ingelido et al. 2018; Mak et al. 2009; Pan et al. 2018; Sharma et al. 2016; Sun et al. 2016).
PFAS are not substantially removed by most conventional drinking-water treatment processes, including coagulation, flocculation, sedimentation, filtration, and disinfection (Rahman et al. 2014). Elevated concentrations of PFAS have been reported in the finished drinking water of community water systems that source water from areas with industrial facilities producing or using PFAS (Graber et al. 2019; Hu et al. 2016). Notably, perfluorooctanoic acid (PFOA) releases from a fluorochemical plant near Parkersburg, West Virginia, resulted in parts-per-billion levels of PFOA in drinking water sourced from contaminated wells; in the community, tap water consumption was a significant predictor of serum PFOA levels (Emmett et al. 2006; Hoffman et al. 2011). Human exposure to PFAS [PFOA and perfluorooctane sulfonate (PFOS) are the most studied to date] has been associated with thyroid disease, ulcerative colitis, elevated cholesterol levels, developmental delays, liver disease, kidney and testicular cancer, and immunosuppression (ATSDR 2018; DeWitt et al. 2009; Steenland et al. 2010; Sunderland et al. 2019).
In North Carolina, a 2,150-acre fluorochemical manufacturing facility (i.e., Fayetteville Works) (Figure 1) discharged process wastewater to the Cape Fear River as early as 1980 (Wagner and Buckland 2017). Several poorly understood PFAS, including hexafluoropropylene oxide dimer acid (HFPO-DA or GenX), have been detected in water samples collected downriver of the facility’s effluent discharge point (Hopkins et al. 2018; McCord and Strynar 2019; McCord et al. 2018; Strynar et al. 2015; Sun et al. 2016; Zhang et al. 2019). These PFAS are collectively referred to as fluoroethers because they have the traditional perfluoroalkyl carbon chains characteristic of legacy PFAS, such as PFOA, but the chains are interrupted by ether oxygen(s) (see Figure S1) (Strynar et al. 2015). The released fluoroethers, including GenX, were generated as by-products of fluoropolymer production at Fayetteville Works facility (Hopkins et al. 2018; McCord and Strynar 2019). Human exposure to by-products of fluorochemical manufacturing has not been studied to date.
Figure 1.
Cape Fear River Basin, North Carolina, United States. Note: PFAS, per- and polyfluoroalkyl substances.
Approximately downriver of Fayetteville Works is the raw water intake for the Cape Fear Public Utility Authority (CFPUA), which provides drinking water to approximately 200,000 people in New Hanover County, home to Wilmington, North Carolina. Raw water concentrations of the fluoroethers were similar to treated water concentrations because the fluoroethers were not measurably removed by CFPUA’s water treatment processes, which included several advanced steps (i.e., raw and settled water ozonation, biofiltration, and ultraviolet light disinfection) (Hopkins et al. 2018). In early June 2017, the public became aware of the presence of GenX in their drinking water (Hagerty 2017). Community concern and subsequent action by the North Carolina Department of Environmental Quality (NC DEQ) resulted in the fluorochemical manufacturer reducing its wastewater discharges to the Cape Fear River on 21 June 2017, and by September 2017, the facility stopped discharging process wastewater containing PFAS into the Cape Fear River (NC DEQ 2017). As a result, the GenX concentration in Wilmington’s drinking water source dropped from approximately before discharge control to approximately 1 week later (Hopkins et al. 2018; Sun et al. 2016; Zhang et al. 2019).
We initiated The GenX Exposure Study in November 2017 to answer community members’ questions about their exposure to GenX and other PFAS. We included in our analysis fluoroethers that were by-products of fluorochemical manufacturing at Fayetteville Works as well as legacy PFAS historically used throughout the Cape Fear River Basin. We report here the initial findings for serum PFAS levels measured in a Wilmington, North Carolina, population.
Methods
Study Population
In November 2017 and May 2018, we recruited individuals from New Hanover County, North Carolina, to participate in the GenX Exposure Study. We partnered with Cape Fear River Watch, a local nongovernmental organization focusing on water quality in the region; the New Hanover County Health Department; the New Hanover County NAACP; and informal community partners to inform the public about the study. Press releases, news stories, public service announcements, recruitment flyers, social media platforms, and the study website (https://genxstudy.ncsu.edu/) were used to promote the study.
CFPUA distributes drinking water to the City of Wilmington and unincorporated areas of New Hanover County not served by privately owned systems. CFPUA operates three treatment plants with separate distribution systems: One plant sources water from the lower Cape Fear River, and the other two from various groundwater sources (CFPUA 2020b). Most (153,200 or 80%) of the 190,500 people served by CFPUA receive water from the lower Cape Fear River (NC Drinking Water Watch 2020). The Richardson and Monterey Heights groundwater treatment plants serve 37,250 people collectively.
Study participants were required to be current residents of New Hanover County, of age, and to have lived in a home served with CFPUA drinking water for at least 12 months prior to November 2017 (the start of enrollment). Up to four individuals per household were allowed to participate. We excluded pregnant women and people who were human immunodeficiency virus- or hepatitis C-positive. Individuals were recruited in both English and Spanish. The majority of our participants were recruited in November 2017, with a smaller, targeted recruitment in May 2018. In November, interested individuals contacted the study office to be screened for eligibility. Eligible individuals were scheduled for a clinic visit at the New Hanover County Health Department during the weekend of 10–12 November 2017. We conducted a second recruitment of participants in May 2018, aimed at increasing participation of African Americans. We joined the annual health fair at the MLK Center in Wilmington, hosted by the New Hanover County NAACP. Recruitment, enrollment, and biological sample collection took place at the MLK Center on 5 May 2018. We also scheduled repeat blood and urine collection from a random sample of the November 2017 participants.
All study participants provided written informed consent to participate. All phases of the study were conducted in compliance with the North Carolina State University Institutional Review Board.
Data Collection
During clinic visits, we consented participants, administered a questionnaire, collected biological samples (blood and urine), and measured height and weight. Study staff administered a questionnaire to each participant at the clinic visit to collect information on demographics, drinking water habits, residential history, health history, and PFAS exposures other than drinking water. Children completed a shortened version of the adult questionnaire. Parents provided the residential history for their children.
Trained phlebotomists collected nonfasting blood samples from participants. For participants who were of age, four tubes of blood (two red-top tubes for serum, two ethylenediaminetetraacetic acid (EDTA) tubes for whole blood or plasma) were collected. For children 6–10 years of age, two red-top tubes for serum were collected. Serum tubes were spun at for 10 min in a Sorvall RT 600D centrifuge at room temperature. Serum was aliquoted into transfer tubes. One EDTA tube was processed for plasma; the remainder was saved as whole blood. Spot urine samples were provided by study participants during the clinic visit. Urine and blood samples were stored on dry ice and transported to East Carolina University (Greenville, NC) and stored at . A 2-mL aliquot of serum was shipped on dry ice to the U.S. Environmental Protection Agency (EPA) in Research Triangle Park, North Carolina, where they were stored at until analysis.
PFAS Analysis in Blood
Analytical standards.
Native standards for GenX, perfluorobutanoic acid (PFBA), perfluoropentanoic acid (PFPeA), perfluorohexanoic acid (PFHxA), perfluoroheptanoic acid (PFHpA), PFOA, perfluorononanoic acid (PFNA), perfluorobutane sulfonic acid (PFBS), perfluorohexane sulfonic acid (PFHxS), PFOS, and 6:2 fluorotelomer sulfonate (6:2 FTS) and mass-labeled standards for GenX, PFBA, PFHxA, PFOA, PFNA, PFHxS, PFOS, and 6:2 FTS were purchased dissolved in methanol from Wellington Laboratories (see Table S1). Analytical standards for perfluoro-2-methoxyacetic acid (PFMOAA), perfluoro-2-methoxypropanoic acid, 2,3,3,3-tetrafluoro-2-(pentafluoroethoxy)propanoic acid, perfluoro-2-ethoxypropanoic acid (PEPA), perfluoro-3,5-dioxahexanoic acid (PFO2HxA), perfluoro-3,5,7-trioxaoctanoic acid (PFO3OA), perfluoro-3,5,7,9-tetraoxadecanoic acid (PFO4DA), perfluoro-3,5,7,9,11-pentaoxadodecanoic acid (PFO5DoA), and 1,1,2,2-tetrafluoro-2-(1,2,2,2-tetrafluoroethoxy)ethanesulfonic acid (NVHOS) and for perfluoro-3,6-dioxa-4-methyl-7-octene-1-sulfonic acid (Nafion by-product 1), and perfluoro-2-{[perfluoro-3-(perfluoroethoxy)-2-proanyl]oxy}ethanesulfonic acid (Nafion by-product 2) were acquired as aqueous solutions () from the Chemours Company because there were no commercial sources. The identity of each standard was confirmed by high-resolution mass spectrometry (HRMS). A mixed PFAS standard stock solution was prepared in methanol at .
Sample preparation.
Fifty microliters of serum was transferred into polypropylene tubes and formic acid containing mass-labeled standards () was added to denature serum proteins. Each sample was then vortex mixed and cold () acetonitrile was added to precipitate proteins. The sample was vortex mixed again and centrifuged at for 5 min in an IEC CL31R Multispeed Centrifuge (Thermo Scientific) at room temperature. Finally, a aliquot of the acetonitrile supernatant was placed into a liquid chromatography (LC) vial with ammonium formate buffer (1:1 mixture).
Sample analysis.
Measurements for 20 PFAS, 10 fluoroethers, and 10 legacy PFAS (Table 1) in serum were conducted using LC-HRMS. Each serum sample was analyzed using a Thermo Vanquish ultra-performance liquid chromatograph coupled to a Thermo Orbitrap Fusion mass spectrometer. Using a injection volume, PFAS were separated on an Accucore Vanquish column (, particle diameter). The mobile phases were 95:5% vol/vol water:acetonitrile with ammonium formate (Eluent A) and 5:95% vol/vol water:acetonitrile with ammonium formate (Eluent B), with a flow rate of . The LC method used a 3-min pre-equilibration time at 10% B followed by a linear gradient from 10% to 100% over 10 min with a 3-min hold at 100% B. The mass spectrometer was run in full scan mode with a mass range of 100–700 Da and 120,000 resolving power at m/z 200.
Table 1.
Ten fluoroethers and 10 legacy PFAS measured for in serum samples in the GenX exposure study.
| Short name | U.S. EPA registry name | Formula | CASN (hyperlinked to U.S. EPA Chemicals Dashboarda) | DTXSIDb | Monoisotopic mass, deprotonated | # of fluorinated carbons | Chain lengthc |
|---|---|---|---|---|---|---|---|
| Fluoroethers | |||||||
| HFPO-DA (GenX) | Hexafluoropropylene oxide dimer acid | 13252-13-6 | 70880215 | 328.9677 | 5 | 7 | |
| PMPA | Perfluoro-2-methoxypropanoic acid | 13140-29-9 | 80528474 | 228.9741 | 3 | 5 | |
| PEPA | Perfluoro-2-ethoxypropanoic acid | 267239-61-2 | 60896486 | 278.9709 | 4 | 6 | |
| PFO2HxA | Perfluoro-3,5-dioxahexanoic acid | 39492-88-1 | 50892351 | 244.9691 | 3 | 6 | |
| PFO3OA | Perfluoro-3,5,7-trioxaoctanoic acid | 39492-89-2 | 20892348 | 310.9608 | 4 | 8 | |
| PFO4DA | Perfluoro-3,5,7,9-butaoxadecanoic acid | 39492-90-5 | 90723993 | 376.9525 | 5 | 10 | |
| PFO5DoA | Perfluoro-3,5,7,9,11-pentaoxadodecanoic acid | 39492-91-6 | 50723994 | 442.9442 | 6 | 12 | |
| NVHOS | 1,1,2,2-Tetrafluoro-2-(1,2,2,2-tetrafluoroethoxy)ethanesulfonic acid | 801209-99-4 | 80904754 | 296.9473 | 4 | 6 | |
| Nafion by-product 1 | Perfluoro-3,6-dioxa-4-methyl-7-octene-1-sulfonic acid | 29311-67-9 | 30892354 | 442.9264 | 7 | 10 | |
| Nafion by-product 2 | Perfluoro-2-{[perfluoro-3-(perfluoroethoxy)-2-propanyl]oxy}ethanesulfonic acid | 749836-20-2 | 10892352 | 462.9327 | 7 | 10 | |
| Legacy PFAS | |||||||
| PFBA | Perfluorobutanoic acid | 375-22-4 | 4059916 | 212.9792 | 3 | 4 | |
| PFPeA | Perfluoropentanoic acid | 2706-90-3 | 6062599 | 262.9760 | 4 | 5 | |
| PFHxA | Perfluorohexanoic acid | 307-24-4 | 3031862 | 312.9728 | 5 | 6 | |
| PFHpA | Perfluoroheptanoic acid | 375-85-9 | 1037303 | 362.9696 | 6 | 7 | |
| PFOA | Perfluorooctanoic acid | 335-67-1 | 8031865 | 412.9664 | 7 | 8 | |
| PFNA | Perfluorononanoic acid | 375-95-1 | 8031863 | 462.9632 | 8 | 9 | |
| PFBS | Perfluorobutane sulfonic acid | 375-73-5 | 5030030 | 298.9429 | 4 | 5 | |
| PFHxS | Perfluorohexane sulfonic acid | 355-46-4 | 7040150 | 398.9366 | 6 | 7 | |
| PFOS | Perfluorooctane sulfonic acid | 1763-23-1 | 3031864 | 498.9302 | 8 | 9 | |
| 6:2 FTS | 6:2 fluorotelomer sulfonate | 27619-97-2 | 6067331 | 426.9679 | 6 | 9 | |
Note: CASN, Chemical Abstracts Services Number; EPA, Environmental Protection Agency; GenX, hexafluoropropylene oxide dimer acid.
U.S. EPA CompTox Chemistry Dashboard (https://comptox.epa.gov/dashboard).
DTXSID is a unique substance identifier used in the U.S. EPA CompTox Chemistry Dashboard (Williams et al. 2017).
Includes carbon, oxygen, and sulfur atoms in the fluoroalkyl chain but does not include oxygen atoms in the anionic group (i.e., does not include O in carboxylic acid).
Extracted ion chromatograms for 6:2 FTS () yielded a doublet peak that was selected for follow-up MS/MS investigation with higher-energy C-trap dissociation (HCD) normalized collision energy of 45. Standards of 6:2 FTS (Schultz et al. 2004) and a polyfluoroalkyl ether carboxylic acid 2,2,3,3-tetrafluoro-3-((1,1,1,2,3,3-hexafluoro-3-(1,2,2,2-tetrafluoroethoxy)propan-2-yl)oxy)propanoic acid (known as Hydro-EVE) (Chemical Abstracts Services Number 773804-62-9) (U.S. EPA 2020) were prepared and analyzed by LC-HRMS/MS; annotated MS/MS spectra were compared with spectra collected from 10 serum samples randomly selected from our Wilmington cohort samples.
Calibration standards ranging in concentration from to were prepared in newborn calf serum (ThermoFisher Scientific) by spiking PFAS standard stock solution into the serum; calibration standards were processed using the protocol for human serum samples described above. Compounds were quantified using a relative response ratio of the native standard and isotopically labeled internal standard; the [M-H]− or []− ions were used. Integration of PFAS isomers was consistent with U.S. EPA Method 537.1 (U.S. EPA 2018); that is, for compounds with branched and linear isomers (PFOA, PFOS, PFHxS), peaks for the branched and linear isomers were integrated together to report total concentration.
Serum samples were run in batches of approximately 50 samples. Each batch contained in-house spiked newborn calf serum samples for continuing calibration checks. National Institutes of Standards and Technology (NIST) standard reference material (SRM) 1957 human serum was analyzed for calibration verification (acceptance criteria were difference from consensus value). Mean concentrations of legacy PFAS (PFHpA, PFHxS, PFOA, PFOS, and PFNA) in SRM 1957 were within 10% difference of reference values determined by an interlaboratory analysis (see Table S2). We calculated the precision between replicate analyses by taking the difference divided by the average. Intrarun replicate analysis precision for duplicate analyses was less than 30% for most PFAS (see Table S3). As expected, lower replicate precision was observed at lower concentrations.
The study sera were run in batches across eight analytical runs. Each analyte was assigned a batch-specific method reporting limit (MRL) defined as the first point of the standard curve for which the regression equation yielded a calculated value within 30% of the true value. For analytes with significant background signal in calf serum blanks, the MRL was designated as three times the maximum response in newborn calf serum blanks (i.e., in the standard), if higher than the MRL from the calibration curve. Higher instrument background levels for PFPeA, PFO2HxA, and GenX were observed on some analytical runs and resulted in higher batch-specific MRLs for those PFAS (see Table S4). In addition, the mass spectrometer had a high background response for the mass corresponding to PFMOAA, making it difficult to distinguish PFMOAA standards. We prioritized the method development for PFAS with longer alkyl (ether) chain length (e.g., PFO5DoA), which we suspected were more likely to be detected in blood (Ng and Hungerbühler 2014). Thus, we moved forward without measuring samples for PFMOAA.
Statistical Methods
To calculate summary statistics, we used the first blood sample collected from each participant (i.e., the blood sample collected when the participant was enrolled; that is, the November 2017 sample for most participants and the May 2018 sample for new enrollees in May). We present results for PFAS detected in 60% or more of 344 serum samples. For samples analyzed in duplicate, average values were used in the analyses. Sample results below the MRL were assigned a fill value of the MRL divided by the square root of 2 (Calafat et al. 2007; Daly et al. 2018). However, when we summed the mass concentration of all detectable PFAS to determine total PFAS in serum, we added 0 to the total for PFAS that were below the MRL so that we did not bias the sum upward because of multiple nondetected chemicals. We assessed correlation of PFAS serum concentrations using Spearman correlation coefficients; values greater than or equal to 0.70 were considered highly correlated.
To compare differences between participants served with treated Cape Fear River water or another drinking water source, we used a Wilcoxon rank sum test. Two study participants who were enrolled in the early stages of the recruitment effort and who shared the same residence did not meet the study eligibility criterion of residing in the CFPUA service area. Their residence, however, was in Wilmington, and their drinking water source was not the Cape Fear River. Therefore, we included these two participants as part of the group with drinking water not sourced from the Cape Fear River.
For participants who provided repeat samples, we calculated percentage change over time using serum PFAS concentrations in November 2017 and May 2018. Percentage change was calculated as
| (1) |
We also used a Wilcoxon test for paired samples to evaluate differences in serum PFAS concentrations between November 2017 and May 2018. All statistical analyses were conducted in R (version 3.5.1; R Development Core Team). The significance level for all statistical analyses was .
Comparison Data
To determine whether fluoroethers were detectable in people living remote from the fluorochemical manufacturing site, we analyzed 20 stored serum samples collected in 2008–2009 from 30- to 44-y-old women participating in an unrelated research study, and living in the Raleigh, Durham, and Chapel Hill, North Carolina, area (Crawford et al. 2017) (Figure 1).
Results
Study Population
In November 2017 and May 2018, we enrolled 344 participants, including 289 adults and 55 children; 310 enrolled in November 2017 and 34 enrolled in May 2018. We collected repeat blood samples from 44 participants (Table 2, Figure 2). Participants ranged in age from 6 to 86 y, with a median age of 50 y. The average years lived in the lower Cape Fear Region was 20 y [standard deviation (SD): 16 y], and 72% of participants reported residing in the region for . In 75 of the 231 participating households (32%), at least 2 household members participated in the study. Most participants (97%) had drinking water sourced from the lower Cape Fear River, but 9 participants had another drinking water source.
Table 2.
Demographic characteristics of the 344 Wilmington, North Carolina, GenX exposure study participants.
| Characteristic | November 2017 () [ (%)] | November 2017 (resampled May 2018) () [ (%)] | May 2018 () [ (%)] |
|---|---|---|---|
| Adult/child | |||
| Adult () | 256 (82.6) | 42 (95.5) | 33 (97.1) |
| Child | 54 (17.4) | 2 (4.6) | 1 (2.94) |
| Age group (y)a | |||
| 6–17 | 54 (17.5) | 2 (4.6) | 1 (3.1) |
| 18–29 | 12 (3.9) | 1 (2.3) | 2 (6.3) |
| 30–39 | 37 (12.0) | 4 (9.1) | 2 (6.3) |
| 40–49 | 57 (18.4) | 10 (22.7) | 2 (6.3) |
| 50–59 | 51 (16.5) | 9 (20.5) | 4 (12.5) |
| 60–69 | 62 (20.1) | 9 (20.5) | 13 (40.6) |
| 70–86 | 36 (11.7) | 9 (20.5) | 8 (25.0) |
| Gender | |||
| Female | 189 (61.0) | 28 (63.6) | 27 (79.4) |
| Male | 120 (38.7) | 16 (36.4) | 7 (20.6) |
| Transgender | 1 (0.3) | 0 | 0 |
| Race/ethnicityb | |||
| Black, non–Hispanic | 8 (2.6) | 0 | 27 (79.4) |
| Hispanic, regardless of race | 33 (10.7) | 3 (7.0) | 0 |
| White, non–Hispanic | 261 (84.7) | 40 (93.0) | 4 (11.8) |
| Otherc | 6 (2.0) | 0 | 3 |
| Spanish speaker | 17 (5.5) | 0 | 0 |
| Residence in lower Cape Fear Region (y)d | |||
| 1–9 | 88 (28.5) | 10 (22.7) | 6 (18.8) |
| 10–19 | 112 (36.3) | 18 (40.9) | 7 (21.9) |
| 20–39 | 76 (24.6) | 6 (13.6) | 6 (18.8) |
| 40–49 | 16 (5.2) | 5 (11.4) | 3 (9.4) |
| 50–73 | 17 (5.5) | 5 (11.4) | 10 (31.3) |
| Drinking water sourcee | |||
| CFPUA groundwater | 5 (1.6) | 1 (2.3) | 2 (5.9) |
| CFPUA Cape Fear River | 301 (97.7) | 42 (97.7) | 32 (94.1) |
| Not served by CFPUA | 2 (0.7) | 0 | 0 |
| Number of households | 201 | 35 | 30 |
| Participants per household | |||
| 1 | 130 (64.7) | 28 (80.0) | 26 (86.7) |
| 2 | 46 (22.9) | 6 (17.4) | 4 (13.3) |
| 3 | 12 (6.0) | 0 | 0 |
| 4 | 13 (6.5) | 1 (2.9) | 0 |
Note: CFPUA, Cape Fear Public Utility Authority; GenX, hexafluoropropylene oxide dimer acid.
Missing age for three participants.
Missing race/ethnicity for two participants.
Other includes mixed-race individuals Native American/Pacific Islander, black or African American and Native American/Pacific Islander and white and other, Native American/Pacific Islander and white. May 2018: Other includes: American Indian/Alaska Native and Black or African American, black or African American and Native American/Pacific Islander and white, black or African American and white.
Missing years lived in lower Cape Fear River Region for 1 participant for the November 2017/May 2018 repeaters and 2 participants for the May 2018 new participants.
CFPUA distributes drinking water to New Hanover County, home of the City of Wilmington. Missing water source for 2 participants for November 2017, 1 participant for May 2018 repeaters.
Figure 2.
Study enrollment and blood sample collection in the GenX Exposure Study: Wilmington, North Carolina. Note: GenX, hexafluoropropylene oxide dimer acid.
PFAS Analysis in Blood
Our analytical method was developed to determine concentrations of 10 fluoroethers and 10 legacy PFAS (Table 1; see also Table S1) in the serum of all participants. The choice of which PFAS to include in our analytical method was informed by which PFAS had been reported in the lower Cape Fear River (Strynar et al. 2015; Sun et al. 2016) and for which PFAS analytical standards were available. We detected five fluoroethers (Nafion by-product 2, PFO4DA, PFO5DoA, PFO3OA, and NVHOS) and five legacy PFAS (PFHxS, PFHpA, PFOA, PFOS, and PFNA) in at least 15% of the first blood samples (collected from 310 participants in November 2017 and 34 in May 2018) (Table 3; see also Figure S1). Nafion by-product 2 and PFO4DA were detected in serum from almost all participants (99%). Concentrations of Nafion by-product 2 [, interquartile range , ] were similar to concentrations of PFO4DA (, , ). We detected PFO3OA and NVHOS infrequently, with PFO3OA detected in 28% and NVHOS in 15% of samples. GenX was not detected in sera from our cohort. We did not detect the fluoroethers in serum samples collected in 2008–2009 from 20 women living north of Fayetteville Works, who were not served with drinking water sourced from the lower Cape Fear River (see Table S5). Due to a mass interference in calibration standards analyzed by LC-HRMS that impacted PFO5DoA quantitation, we are not reporting PFO5DoA results in the main manuscript. The erroneous results originally published have been moved to Table S9 for reference.
Table 3.
Concentrations of fluoroethers and legacy PFAS in first serum sample collected from 344 Wilmington, North Carolina, residents (289 adults and 55 children). The PFAS detected in more than 60% of samples are shown.
| PFAS | a (%) | Concentration (ng/mL) | ||||
|---|---|---|---|---|---|---|
| 10th percentile | 25th percentile | Median | 75th percentile | 95th percentile | ||
| Fluoroethers | ||||||
| Nafion by-product 2 | ||||||
| Adults | 286 (99) | 1.0 | 1.8 | 3.2 | 5.1 | 8.5 |
| Children | 55 (100) | 0.6 | 1.1 | 1.6 | 2.2 | 3.8 |
| Overall | 341 (99) | 0.8 | 1.5 | 2.7 | 4.6 | 8.4 |
| PFO4DA | ||||||
| Adults | 284 (98) | 0.4 | 0.8 | 2.3 | 5.7 | 13.7 |
| Children | 55 (100) | 0.7 | 1.3 | 2.6 | 4.8 | 8.9 |
| Overall | 339 (99) | 0.4 | 0.9 | 2.5 | 5.5 | 12.8 |
| Legacy PFAS | ||||||
| PFOS | ||||||
| Adults | 287 (99) | 3.8 | 5.5 | 9.4 | 14.5 | 28.2 |
| Children | 55 (100) | 2.8 | 3.0 | 5.1 | 7.8 | 11.5 |
| Overall | 342 (99) | 3.3 | 5 | 8.6 | 13.6 | 26.8 |
| PFOA | ||||||
| Adults | 288 (99.7) | 1.7 | 2.9 | 4.8 | 7.2 | 11.3 |
| Children | 55 (100) | 1.9 | 2.3 | 3.0 | 4.1 | 6.5 |
| Overall | 343 (99.7) | 1.7 | 2.7 | 4.3 | 6.9 | 11.0 |
| PFHxS | ||||||
| Adults | 282 (98) | 1.2 | 2.2 | 3.5 | 5.5 | 8.6 |
| Children | 54 (98) | 1.2 | 1.4 | 1.9 | 2.6 | 4.7 |
| Overall | 336 (98) | 1.2 | 1.8 | 3.2 | 5.2 | 8.5 |
| PFNA | ||||||
| Adults | 280 (97) | 0.6 | 0.9 | 1.3 | 2.2 | 3.6 |
| Children | 54 (98) | 0.4 | 0.5 | 0.8 | 1.0 | 1.5 |
| Overall | 334 (97) | 0.5 | 0.8 | 1.2 | 2.0 | 3.3 |
| PFHpA | ||||||
| Adults | 170 (59) | 0.1 | 0.2 | 0.2 | 0.5 | 1.4 |
| Children | 45 (82) | 0.2 | 0.2 | 0.4 | 0.7 | 1.0 |
| Overall | 215 (63) | 0.1 | 0.2 | 0.3 | 0.6 | 1.3 |
Note: MRL, method reporting limit; Nafion by-product 2, perfluoro-2-{[perfluoro-3-(perfluoroethoxy)-2-propanyl]oxy}ethanesulfonic acid; PFAS, per- and polyfluoroalkyl substances; PFHpA, perfluoroheptanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluoroctanoic acid; PFOS, Perfluorooctane sulfonic acid; PFO4DA, perfluoro-3,5,7,9-butaoxadecanoic acid; PFO5DoA, perfluoro3,5,7,9,11-pentaoxadecanoic acid butaoxadecanoic acid.
The MRL was for Nafion by-product 2, PFO4DA, and PFO5DoA (range: for PFOS; for PFOA; for PFHxS; for PFNA, and for PFHpA.
Apart from the fluoroethers, we detected four legacy PFAS in sera from most () participants. PFOS was dominant, with a median serum concentration of (, ), followed by PFOA (, ), PFHxS (, , ) and PFNA (, , ). PFHpA was detected in 63% of participants’ serum (, , ). Summed PFAS concentrations ranged from 1.5 to , with a median PFAS level of in adults and in children (Table 4). The median summed mass concentration of four fluoroethers (NVHOS, PFO3OA, PFO4DA, and Nafion by-product 2) was (, ). These fluoroethers accounted for 23% of the summed mass concentration of PFAS detected in serum; the percentage was slightly higher for children (25.9%) than for adults (21.6%).
Table 4.
Summed mass concentrations of fluoroethers (NVHOS, PFO3OA, PFO4DA, Nafion by-product 2) and legacy PFAS (PFOS, PFOA, PFHxS, PFNA, and PFHpA) in serum from 344 Wilmington, North Carolina, residents (289 adults and 55 children).
| Category | Concentration [ng/mL (percentage of total PFAS)]a | ||||
|---|---|---|---|---|---|
| 10th percentile | 25th percentile | Median | 75th percentile | 95th percentile | |
| fluoroethers | |||||
| Adults | 1.5 (14.0) | 3.2 (19.5) | 5.9 (21.6) | 10.9 (26.7) | 19.7 (32.6) |
| Children | 1.7 (18.9) | 2.8 (22.2) | 4.4 (25.9) | 7.4 (33.0) | 13.5 (38.5) |
| Overall | 1.5 (14.6) | 3.0 (19.6) | 5.7 (22.8) | 10.2 (26.4) | 19.0 (31.6) |
| legacy PFAS | |||||
| Adults | 8.0 (74.8) | 12.2 (74.4) | 20.8 (76.2) | 29.8 (72.9) | 47.8 (79.1) |
| Children | 6.8 (75.6) | 8.1 (64.3) | 11.3 (66.5) | 16.4 (73.2) | 24.0 (68.4) |
| Overall | 7.6 (73.8) | 11.1 (72.5) | 18.8 (75.2) | 28.7 (74.2) | 47.1 (78.2) |
| all PFAS | |||||
| Adults | 10.7 | 16.4 | 27.3 | 40.9 | 60.4 |
| Children | 9.0 | 12.6 | 17.0 | 22.4 | 35.1 |
| Overall | 10.3 | 15.3 | 25.0 | 38.7 | 60.2 |
Note: Nafion by-product 2, perfluoro-2-{[perfluoro-3-(perfluoroethoxy)-2-propanyl]oxy}ethanesulfonic acid; NVHOS, 1,1,2,2-tetrafluoro-2-(1,2,2,2-tetrafluoroethoxy)ethanesulfonic acid; PFAS, per- and polyfluoroalkyl substances; PFHpA, perfluoroheptanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; PFO3OA, perfluoro-3,5,7,9-butaoxadecanoic acid; PFO4DA, perfluoro-3,5,7-trioxaoctanoic acid.
Percentage of total PFAS concentration (the sum of fluoroethers and legacy PFAS analyzed for in this study) is shown in parentheses.
The concentrations of fluoroethers and legacy PFAS were highly correlated for some, but not all, PFAS in sera collected in November 2017 (Table 5). Nafion by-product 2 was highly correlated the legacy PFOA (). PFO4DA was highly correlated only with PFHpA. The correlations for PFAS were similar in sera collected in May 2018 (see Table S6). The nine participants whose residences were not served with drinking water sourced from the lower Cape Fear River water had significantly lower serum levels of all detected fluoroethers and PFOA, but not PFOS, PFHxS, PFNA, or PFHpA, than the 333 participants with drinking water sourced from the lower Cape Fear River (Table 6).
Table 5.
Spearman’s correlation coefficients greater than 0.70 between PFAS concentrations in 310 participants who provided blood samples in November 2017.
| PFAS | Correlated with | Correlation coefficient |
|---|---|---|
| Nafion by-product 2 | ||
| PFOA | 0.74 | |
| PFO4DA | PFHpA | 0.75 |
| PFHxS | ||
| PFOA | 0.86 | |
| PFOS | 0.73 | |
| PFNA | 0.78 | |
| PFHpA | PFO4DA | 0.75 |
| PFOA | Nafion by-product 2 | 0.74 |
| PFHxS | 0.86 | |
| PFOS | 0.70 | |
| PFNA | 0.86 | |
| PFOS | PFHxS | 0.73 |
| PFOA | 0.70 | |
| PFNA | 0.84 | |
| PFNA | ||
| PFHxS | 0.78 | |
| PFOA | 0.86 | |
| PFOS | 0.84 | |
Note: Correlation was limited to November due to changing serum concentrations between November 2017 and May 2018. Note: Nafion by-product 2, perfluoro-2-{[perfluoro-3-(perfluoroethoxy)-2-propanyl]oxy}ethanesulfonic acid; PFAS, per- and polyfluoroalkyl substances; PFHpA, perfluoroheptanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluoroctanoic acid; PFOS, perfluorooctane sulfonic acid; PFO4DA, perfluoro-3,5,7,9-butaoxadecanoic acid.
Table 6.
Comparison of serum PFAS concentrations in participants from residences served with treated Cape Fear River water and from residences served by another drinking water source.
| PFAS | Median concentration [range (ng/mL)] | ||
|---|---|---|---|
| Served by Cape Fear River sourced drinking water () | Served by another drinking water source () | Wilcoxon test for difference (-value) | |
| Fluoroethers | |||
| Nafion by-product 2 | 2.8 (0.07–16.9) | 0.6 (0.07–2.2) | 0.0003 |
| PFO4DA | 2.5 (0.07–51.2) | 0.6 (0.07–6.0) | 0.0098 |
| Legacy PFAS | |||
| PFOS | 8.6 (0.18–62.6) | 5.5 (0.4–18.1) | 0.09 |
| PFOA | 4.4 (0.07–20.2) | 2.2 (1.5–6.0) | 0.01 |
| PFHxS | 3.2 (0.2–15.2) | 1.9 (0.6–5.1) | 0.02 |
| PFNA | 1.2 (0.07–7.5) | 0.7 (0.3–2.1) | 0.10 |
| PFHpA | 0.3 (0.07–4.5) | 0.2 (0.07–0.9) | 0.47 |
Note: Nafion by-product 2, perfluoro-2-{[perfluoro-3-(perfluoroethoxy)-2-propanyl]oxy}ethanesulfonic acid; PFAS, per- and polyfluoroalkyl substances; PFHpA, perfluoroheptanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluoroctanoic acid; PFOS, perfluorooctane sulfonic acid; PFO4DA, perfluoro-3,5,7,9-butaoxadecanoic acid.
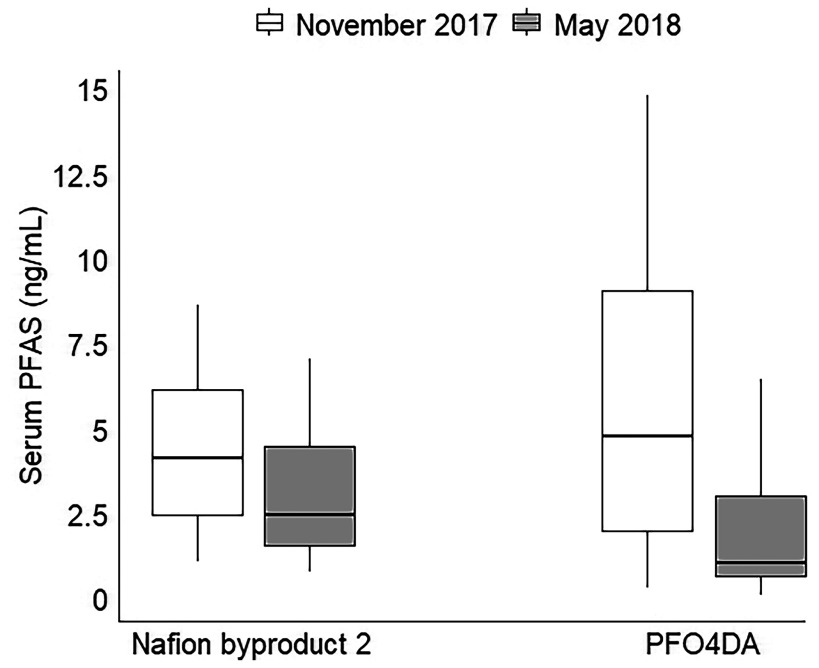
We evaluated change over 6 months for serum PFAS levels using results from 44 participants (42 adults and 2 children) who provided samples in both November 2017 and May 2018. Levels of the two fluoroethers (Nafion by-product 2 and PFO4DA) decreased significantly in the 6 months between sampling (Figure 3; see also Table S7). For the fluoroethers, the median percentage decrease across the 44 participants was 34% [95% confidence interval (CI): 30, 38%] for Nafion by-product 2 and 65% (95% CI: 53, 76%). In contrast, the median percentage decrease for the four legacy PFAS detected in most participants (PFOA, PFOS, PFHxS, and PFNA) ranged between 0% and 13%.
Figure 3.
Box and whisker plot of Nafion by-product 2 and PFO4DA concentrations (ng/mL) in serum from 44 Wilmington, North Carolina, residents (42 adults and 2 children) who provided blood samples in November 2017 and May 2018. Boxes show median concentrations and the 25th and 75th percentiles; 5th and 95th percentiles are indicated by the whiskers. The MRL was . See Table S7 for corresponding numeric data. Note: MRL, method reporting limit; Nafion by-product 2, perfluoro-2-{[perfluoro-3-(perfluoroethoxy)-2-propanyl]oxy}ethanesulfonic acid; PFAS, per- and polyfluoroalkyl substances; PFO4DA, perfluoro-3,5,7,9-butaoxadecanoic acid.
In addition to the PFAS we targeted, we identified another fluorinated chemical with similar accurate mass [M-H] and retention time as the fluorotelomer sulfonate 6:2 FTS. The MS/MS fragmentation pattern for the unknown chemical was consistent with a polyfluoroalkyl ether carboxylic acid known as Hydro-EVE. Hydro-EVE is the carboxylate form of Nafion by-product 2 and was first identified in the Cape Fear River downstream of Fayetteville Works in 2017 (McCord and Strynar 2019). Targeted MS/MS on 10 serum samples randomly selected from our Wilmington cohort samples revealed diagnostic fragments of Hydro-EVE (i.e., 184.9840 Da corresponding to ). In some samples, diagnostic fragments of 6:2 FTS (i.e., 80.9652 Da corresponding to ) were also present, indicating the presence of co-eluting 6:2FTS and Hydro-EVE in at least some of the serum samples. The overlap of precursor mass ( difference) and retention time prevented us from confidently resolving Hydro-EVE and 6:2 FTS using our current analytical method.
Discussion
To our knowledge, we are reporting the first measurements of five fluoroethers (Nafion by-product 2, PFO4DA, PFO5DoA, PFO3OA, and NVHOS) in humans. A sixth fluoroether (Hydro-EVE) was detected in a subset of serum samples, but we could not determine concentrations because we lacked an analytical standard at the time of method development. We detected Nafion by-product 2 and PFO4DA in the sera of almost all participants, including children as young as 6 years of age. Our findings suggest a nearly universal presence of the fluoroethers at nanograms per milliliter (i.e., parts per billion) levels in Wilmington, North Carolina, residents 5 months after discharge control was implemented at Fayetteville Works facility. Moreover, the fluoroethers were important contributors to participants’ total PFAS serum levels. In nearly half of our cohort, the sum concentration of five fluoroethers contributed 25% or more to total PFAS serum levels. We are likely underestimating the fluoroether contribution to total PFAS given that we could not quantify Hydro-EVE and, thus, could not include it in the calculation.
We suspect that the primary route of exposure to GenX and other fluoroethers was through consumption of drinking water sourced from the lower Cape Fear River. All of the fluoroethers we detected in serum have been detected in water samples from the lower Cape Fear River at some point since 2012 (Hopkins et al. 2018; McCord and Strynar 2019; McCord et al. 2018; Strynar et al. 2015; Sun et al. 2016; Zhang et al. 2019). There were significantly lower fluoroether levels in participants whose drinking water was not sourced from the Cape Fear River, and we did not detect the fluoroethers in a North Carolina reference population who lived approximately north of Fayetteville Works and whose drinking water was not sourced from the Cape Fear River. Moreover, there was a decrease in serum fluoroether levels in the 6 months following wastewater discharge control to the river. That decrease in serum levels was likely related to the fact that fluoroether concentrations in drinking water at the time of blood collection had dropped substantially compared with historical drinking water concentrations (Hopkins et al. 2018), and news of GenX contamination of drinking water may have prompted people to stop consuming tap water altogether. We expect that exposure to poorly understood fluoroethers was not limited to Wilmington residents. Other public water systems rely on the lower Cape Fear River as a source of drinking water, and we estimate approximately 280,000 residents in New Hanover, Brunswick, and Pender counties were impacted. Overall, our results highlight that additional research is needed to characterize human exposure to poorly understood PFAS; researchers have reported substantial amounts of unidentified organic fluorine in blood samples from populations in Germany and China (Miaz et al. 2020; Miyake et al. 2007; Yeung and Mabury 2016; Yeung et al. 2008).
The motivation for our study was to answer community members’ questions about their exposure to GenX. Much of the public’s attention has focused on GenX despite the fact that other fluoroethers were present in Wilmington’s drinking water. We did not detect GenX in serum samples even though it was still detectable in drinking water [at (CFPUA 2020a)] in November 2017, when most participants provided their first blood sample. Participants had likely been exposed to much higher GenX concentrations in the 20 y (on average) that they had lived in the lower Cape Fear Region. Before discharge control was implemented at Fayetteville Works, GenX concentrations in CFPUA’s raw water were several hundred nanograms per liter. Sun et al. (2016) reported the average GenX concentration was (range: 55–) in river samples collected in 2013. In 2014, the GenX concentration was (Zhang et al. 2019). Similarly, in a study of 30 people whose private wells were contaminated with GenX at levels above the North Carolina provisional health goal of (NC DHHS 2017) (and as high as ), the NC Department of Health and Human Services did not detect GenX in serum or urine (Pritchett et al. 2019).
No human data exist to estimate the serum half-life for GenX. For an estimate of potential GenX half-life, we considered PFHxA, a six-carbon PFAS that is structurally similar to GenX and has estimates of half-life in humans of about 1 month [geometric mean (GM): 32 d; min: 14 d, max: 49 d] (Russell et al. 2013). It is possible that we did not detect GenX in serum because serum levels dropped below our MRL in the 5 months (or five half-lives, if similar to PFHxA) between when discharge control was implemented and when we collected our first round of samples.
Little is known about the bioaccumulation potential of fluoroethers. It was unclear whether fluoroethers would behave similarly to legacy PFAS, for which longer perfluoroalkyl chain length has been associated with higher bioaccumulation potential (Ng and Hungerbühler 2014). In serum, the highest detection frequencies among the 10 fluoroethers we targeted were for Nafion by-product 2 and PFO4DA; these chemicals are 9–12 atoms long when counting the carbon atoms, ether oxygen atoms, and sulfur atom (if a sulfonate group was present). Therefore, the detected fluoroethers can be considered long-chain compounds even though they do not fit the commonly accepted definition for long-chain PFAS (OECD 2018) and, consequently, their detection in serum was not unexpected. Chain length alone is not sufficient to explain bioaccumulation potential for all compounds. For example, PFHpA, which contains seven carbon atoms in its chain, was detected in 61% of participant sera, whereas GenX (also 7 atoms long when counting the ether oxygen) was not detected. Overall, our data suggest that, once exposure has stopped, the fluoroethers are eliminated from the body faster than legacy PFAS with matching chain lengths, for which human serum half-lives are known. However, only 44 participants provided two biological measurements (6 months apart) for us to evaluate change over time. Another round of biological sample collection from our cohort and/or expanding the study cohort would be informative for half-life calculations.
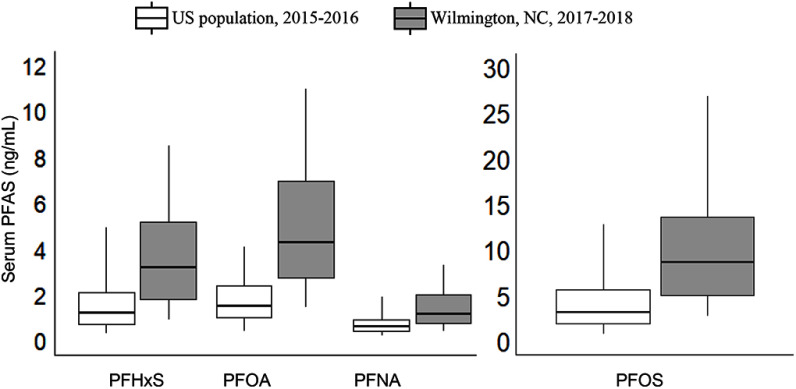
The serum levels of four legacy PFAS (PFOA, PFOS, PFHxS, and PFNA) in our study cohort exceeded the GMs for the U.S. population as defined by the National Health and Nutrition Examination Survey (NHANES) results for survey years 2015–2016 (CDC 2019). The median serum concentration for PFOA in our cohort () exceeded the 95th percentile for the U.S. population () (Figure 4). We suspect that drinking water sourced from the lower Cape Fear River is the reason for the elevated PFOA serum concentrations in our cohort for the following reasons. First, the nine participants not served with lower Cape Fear River water had significantly lower PFOA serum levels (median of ) than the 333 participants with Cape Fear River water (). The levels of legacy PFAS in women from the Raleigh, Durham, Chapel Hill area (our North Carolina reference population) were not elevated relative to U.S.-wide estimates for females based on NHANES 2007–2008, which is comparable to when those blood samples were collected (Kato et al. 2011). Second, serum PFOA in our cohort was highly correlated with serum levels of Nafion by-, to which our cohort was primarily exposed through consumption of drinking water.
Figure 4.
Box and whisker plot of legacy PFAS concentrations (ng/mL) in sera from 344 Wilmington, North Carolina residents and the U.S. population based on NHANES data from the 2015–2016 survey year (CDC 2015–2016). Concentrations of Linear PFOA and linear PFOS were used for the U.S. population. Boxes show median concentrations and 25th and 75th percentiles; 5th and 95th percentiles are indicated by the whiskers. In the analysis of Wilmington residents’ sera, the median MRL for PFHxS, PFOA, and PFNA was , and PFOS was . For NHANES, the MRL was for the PFAS. See Table S8 for corresponding numeric data. PFHpA data are not presented because PFHpA is seldom detected in NHANES participants. For NHANES 2013–2014, which is the most recent PFHpA data available, the median was less than the MRL of . Note: MRL, method reporting limit; NHANES, National Health and Nutrition Examination Survey; PFAS, per- and polyfluoroalkyl substances; PFHpA, perfluoroheptanoic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluoroctanoic acid; PFOS, perfluorooctane sulfonic acid.
An important question is whether the elevated PFOA in participant serum samples is because of their exposure to PFOA concentrations in the Cape Fear River currently or because of their exposure to much higher PFOA concentrations in the river historically. A few studies with water samples collected as early as 2006 have provided data on legacy PFAS concentrations in the Cape Fear River Basin. PFOA was detected in surface water samples collected throughout the Basin in 2006 ( on average) (Nakayama et al. 2007). In 2013, PFOA was detected in 9 of 34 water samples from the lower Cape Fear River, at the drinking water intake point for the CFPUA’s treatment plant (; ) (Sun et al. 2016). However, it was not detected in finished drinking water samples collected in 2013–2015 for the U.S. EPA’s Third Unregulated Contaminant Monitoring Rule (UCMR 3) likely due to a relatively high MRL of PFOA (U.S. EPA 2017). Given that PFOA has been found throughout the Cape Fear River basin, PFOA sources to the river are likely from multiple contributors.
To assess the potential contribution of drinking water to serum PFOA levels, we used a pharmacokinetic model of serum PFOA (Bartell 2017). If we assume that consumption of drinking water is the predominant pathway for participants’ PFOA exposure (Vestergren and Cousins 2009), we would expect a serum level of after 20 y of exposure to drinking water containing PFOA (20 y was, on average, the number of years lived in New Hanover County across our participants). The GM serum level of across our participants is close to this prediction. In addition, the 23 children in our study aged 6 through 11 y had elevated serum concentrations (GM: PFOA) relative to children (aged 6 through 11 y) in the United States (GM: ) (Ye et al. 2018). The available data suggest that PFOA concentrations in Wilmington’s drinking water from 2006 to 2017 (Nakayama et al. 2007; Sun et al. 2016), which covers the time period our child participants would have lived in Wilmington, were below the U.S. EPA’s health advisory level of for combined PFOA and PFOS. Altogether, our data suggest that ongoing exposure to PFOA concentrations currently in the lower Cape Fear River has contributed to the elevated serum PFOA levels, although we cannot rule out that exposure to higher PFOA levels in the river may have occurred at some point.
It is not known whether Wilmington residents’ exposure to PFAS has or will result in adverse health effects. Recent toxicology papers focusing on PFO4DA provide the first insights into the health effects of this chemical: Similar health outcomes were reported for PFO4DA as PFOA, namely, hepatotoxicity in mice (Guo et al. 2019; Sun et al. 2019). Another recent study, in zebrafish, reported developmental effects from PFO3OA, PFO4DA, and PFO5DoA (Wang et al. 2020). The available animal data suggest that GenX exposure induces similar health outcomes as PFOA, but at higher external doses (Gannon et al. 2016; Hoke et al. 2016; Rae et al. 2015; Rushing et al. 2017; Wang et al. 2017). In recent in vitro studies, GenX exposure produced toxic effects on rat thyroid cells (Coperchini et al. 2020) and human liver carcinoma cells (Wen et al. 2020). Adverse health effects of exposure to multiple PFAS in mixtures have largely been understudied.
Our study has some limitations and some unique strengths. Study participants were volunteers and may not be representative of the general Wilmington population. Despite that our participants were volunteers, our study cohort was diverse with respect to age, gender, race, and number of years lived in the lower Cape Fear River Region and included children 6–17 years of age. Children are not consistently included in NHANES surveys and data on PFAS concentrations in children are limited (Ye et al. 2018). By recruiting volunteers, we assembled a cohort at a specific point in time and collected blood samples to assess PFAS levels within 5 months of the source of PFAS exposure changing. Recent research suggests other biological matrices (e.g., whole blood, urine) may be better for detection of PFAS with short biological half-lives (Calafat et al. 2019; Poothong et al. 2017), although the half-lives of the fluoroethers presented are unknown. We chose serum biomonitoring as the first step to assess exposure to PFAS because serum is considered a gold standard for assessing human exposure to chemicals (Calafat et al. 2019). We collected one biological measurement for most participants; as such, we cannot use our study samples to assess historic exposure levels. Future PFAS analysis of urine samples provided by study participants may be informative in distinguishing between recent and historic exposures. The identification and analysis of archived serum samples collected before discharge control, which was implemented in June 2017, will provide more information regarding historical serum levels of fluoroethers, a class of chemically unique and poorly understood PFAS, in the lower Cape Fear River Region.
Conclusions
People with drinking water sourced from the lower Cape Fear River were exposed to fluoroethers in wastewater from a fluorochemical manufacturing facility. We detected six fluoroethers in participant sera 5 months after fluorochemical discharge to the Cape Fear River stopped. The median summed mass concentration of four fluoroethers (NVHOS, PFO3OA, PFO4DA, and Nafion by-product 2) in serum was (, ), and these fluoroethers accounted for 23% of the overall summed mass concentration of PFAS in the sera. The median decrease in individual fluoroether levels was 28% or more in 6 months as a result of discharge control at Fayetteville Works. Further, our findings suggest that consumption of water sourced from the lower Cape Fear River resulted in elevated levels of some legacy PFAS in serum relative to U.S. national averages.
Supplementary Material
Acknowledgments
We thank A. Boomershine for help with recruitment of Spanish-speaking participants and D. Maxwell of the New Hanover County NAACP for help with recruiting African American participants. We thank our New Hanover County Community Science Advisory Board (D. Dixon, V. Guidry, D. Howard, Z. Moore, K. Tribié Reid, J. Rinsky, D. Sargent, E. Uzcategui, L. Wallace, and S. Webb) for helpful discussions. We thank A. Robuck for creating the map in Figure 1. We thank A. Steiner for providing comparison samples collected under National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development grants R21 HD060229 and R01 HD067683. We thank K. Steenland for helpful input in study design. This research was funded by the National Institute of Environmental Health Sciences (NIEHS; 1R21ES029353), the Center for Human Health and the Environment at North Carolina State University (P30ES025128), and the North Carolina Policy Collaboratory. A.A.W. was funded in part by a training grant from the NIEHS (T32 ES007018). This article was reviewed in accordance with the policy of the National Exposure Research Laboratory, U.S. Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the view and policies of the agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
References
- ATSDR (Agency for Toxic Substances and Disease Registry). 2018. Toxicological Profile for Perfluoroalkyls (Draft for Public Comment). https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=1117&tid=237 [accessed 13 December 2019].
- Banzhaf S, Filipovic M, Lewis J, Sparrenbom CJ, Barthel R. 2017. A review of contamination of surface-, ground-, and drinking water in Sweden by perfluoroalkyl and polyfluoroalkyl substances (PFASs). Ambio 46(3):335–346, PMID: , 10.1007/s13280-016-0848-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell SM. 2017. Online serum PFOA calculator for adults. Environ Health Perspect 125(10):104502, PMID: , 10.1289/EHP2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Kato K, Hubbard K, Jia T, Botelho JC, Wong L-Y. 2019. Legacy and alternative per-and polyfluoroalkyl substances in the U.S. general population: paired serum-urine data from the 2013–2014 National Health and Nutrition Examination Survey. Environ Int 131:105048, PMID: , 10.1016/j.envint.2019.105048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Wong L-Y, Kuklenyik Z, Reidy JA, Needham LL. 2007. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ Health Perspect 115(11):1596–1602, PMID: , 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2019. Fourth National Report on Human Exposure to Environmental Chemicals. Updated Tables, January 2019, Volume One. Atlanta, GA: Centers for Disease Control and Prevention. https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Jan2019-508.pdf [accessed 1 July 2020]. [Google Scholar]
- CFPUA (Cape Fear Public Utility Authority). 2020a. Emerging contaminants. https://www.cfpua.org/761/Emerging-Compounds [accessed 15 January 2020].
- CFPUA. 2020b. Water treatment. https://www.cfpua.org/257/Water-Treatment [accessed 15 January 2020].
- Coperchini F, Croce L, Denegri M, Pignatti P, Agozzino M, Netti GS, et al. 2020. Adverse effects of in vitro GenX exposure on rat thyroid cell viability, DNA integrity and thyroid-related genes expression. Environ Pollut 264:114778, PMID: , 10.1016/j.envpol.2020.114778. [DOI] [PubMed] [Google Scholar]
- Crawford NM, Fenton SE, Strynar M, Hines EP, Pritchard DA, Steiner AZ. 2017. Effects of perfluorinated chemicals on thyroid function, markers of ovarian reserve, and natural fertility. Reprod Toxicol 69:53–59, PMID: , 10.1016/j.reprotox.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly ER, Chan BP, Talbot EA, Nassif J, Bean C, Cavallo SJ, et al. 2018. Per-and polyfluoroalkyl substance (PFAS) exposure assessment in a community exposed to contaminated drinking water, New Hampshire, 2015. Int J Hyg Environ Health 221(3):569–577, PMID: , 10.1016/j.ijheh.2018.02.007. [DOI] [PubMed] [Google Scholar]
- DeWitt JC, Shnyra A, Badr MZ, Loveless SE, Hoban D, Frame SR, et al. 2009. Immunotoxicity of perfluorooctanoic acid and perfluorooctane sulfonate and the role of peroxisome proliferator-activated receptor alpha. Crit Rev Toxicol 39(1):76–94, PMID: , 10.1080/10408440802209804. [DOI] [PubMed] [Google Scholar]
- Dinglasan MJA, Ye Y, Edwards EA, Mabury SA. 2004. Fluorotelomer alcohol biodegradation yields poly- and perfluorinated acids. Environ Sci Technol 38(10):2857–2864, PMID: , 10.1021/es0350177. [DOI] [PubMed] [Google Scholar]
- Emmett EA, Shofer FS, Zhang H, Freeman D, Desai C, Shaw LM. 2006. Community exposure to perfluorooctanoate: relationships between serum concentrations and exposure sources. J Occup Environ Med 48(8):759–770, PMID: , 10.1097/01.jom.0000232486.07658.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon SA, Fasano WJ, Mawn MP, Nabb DL, Buck RC, Buxton LW, et al. 2016. Absorption, distribution, metabolism, excretion, and kinetics of 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoic acid ammonium salt following a single dose in rat, mouse, and cynomolgus monkey. Toxicology 340:1–9, PMID: , 10.1016/j.tox.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Graber JM, Alexander C, Laumbach RJ, Black K, Strickland PO, Georgopoulos PG, et al. 2019. Per and polyfluoroalkyl substances (PFAS) blood levels after contamination of a community water supply and comparison with 2013–2014 NHANES. J Expo Sci Environ Epidemiol 29(2):172–182, PMID: , 10.1038/s41370-018-0096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Wang J, Yao J, Sun S, Sheng N, Zhang X, et al. 2019. Comparative hepatotoxicity of novel PFOA alternatives (perfluoropolyether carboxylic acids) on male mice. Environ Sci Technol 53(7):3929–3937, PMID: , 10.1021/acs.est.9b00148. [DOI] [PubMed] [Google Scholar]
- Hagerty V. 2017. Toxin taints CFPUA drinking water. Wilmington Star News. 7 June 2017. https://www.starnewsonline.com/news/20170607/toxin-taints-cfpua-drinking-water/1 [accessed 13 December 2019].
- Hoffman K, Webster TF, Bartell SM, Weisskopf MG, Fletcher T, Vieira VM. 2011. Private drinking water wells as a source of exposure to perfluorooctanoic acid (PFOA) in communities surrounding a fluoropolymer production facility. Environ Health Perspect 119(1):92–97, PMID: , 10.1289/ehp.1002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoke RA, Ferrell BD, Sloman TL, Buck RC, Buxton LW. 2016. Aquatic hazard, bioaccumulation and screening risk assessment for ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate. Chemosphere 149:336–342, PMID: , 10.1016/j.chemosphere.2016.01.009. [DOI] [PubMed] [Google Scholar]
- Hopkins ZR, Sun M, DeWitt JC, Knappe DRU. 2018. Recently detected drinking water contaminants: GenX and other per‐and polyfluoroalkyl ether acids. J Am Water Works Assoc 110(7):13–28, 10.1002/awwa.1073. [DOI] [Google Scholar]
- Hu XC, Andrews DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, et al. 2016. Detection of poly-and perfluoroalkyl substances (PFASs) in U.S. drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environ Sci Technol Lett 3(10):344–350, PMID: , 10.1021/acs.estlett.6b00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingelido AM, Abballe A, Gemma S, Dellatte E, Iacovella N, De Angelis G, et al. 2018. Biomonitoring of perfluorinated compounds in adults exposed to contaminated drinking water in the Veneto Region, Italy. Environ Int 110:149–159, PMID: , 10.1016/j.envint.2017.10.026. [DOI] [PubMed] [Google Scholar]
- James CD, Franklin GW. 1966. Fluorocarbon vinyl ether polymers. Patent Number 3,282,875.
- Kato K, Wong L-Y, Jia LT, Kuklenyik Z, Calafat AM. 2011. Trends in exposure to polyfluoroalkyl chemicals in the U.S. population: 1999–2008. Environ Sci Technol 45(19):8037–8045, PMID: , 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- Kissa E. 2001. Fluorinated Surfactants and Repellents. 2nd ed. New York, NY: Dekker. [Google Scholar]
- Liang B, Hung M-H, Resnick PR. 1998. Co-production of perfluoromethyl perfluorovinyl ether and perfluoroethyl perfluorovinyl ether. Patent Number 5,777,179.
- Mak YL, Taniyasu S, Yeung LW, Lu G, Jin L, Yang Y, et al. 2009. Perfluorinated compounds in tap water from China and several other countries. Environ Sci Technol 43(13):4824–4829, PMID: , 10.1021/es900637a. [DOI] [PubMed] [Google Scholar]
- McCord J, Newton S, Strynar M. 2018. Validation of quantitative measurements and semi-quantitative estimates of emerging perfluoroethercarboxylic acids (PFECAs) and hexfluoroprolyene oxide acids (HFPOAs). J Chromatogr A 1551:52–58, PMID: , 10.1016/j.chroma.2018.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J, Strynar M. 2019. Identification of per-and polyfluoroalkyl substances in the Cape Fear River by high resolution mass spectrometry and nontargeted screening. Environ Sci Technol 53(9):4717–4727, PMID: , 10.1021/acs.est.8b06017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaz LT, Plassmann MM, Gyllenhammar I, Bignert A, Sandblom O, Lignell S, et al. 2020. Temporal trends of suspect- and target-per/polyfluoroalkyl substances (PFAS), extractable organic fluorine (EOF) and total fluorine (TF) in pooled serum from first-time mothers in Uppsala, Sweden, 1996–2017. Environ Sci Processes Impacts 22(4):1071–1083, PMID: , 10.1039/C9EM00502A. [DOI] [PubMed] [Google Scholar]
- Miyake Y, Yamashita N, So MK, Rostkowski P, Taniyasu S, Lam PKS, et al. 2007. Trace analysis of total fluorine in human blood using combustion ion chromatography for fluorine: a mass balance approach for the determination of known and unknown organofluorine compounds. J Chromatogr A 1154(1–2):214–221, PMID: , 10.1016/j.chroma.2007.03.084. [DOI] [PubMed] [Google Scholar]
- Möller A, Ahrens L, Surm R, Westerveld J, van der Wielen F, Ebinghaus R, et al. 2010. Distribution and sources of polyfluoroalkyl substances (PFAS) in the River Rhine watershed. Environ Pollut 158(10):3243–3250, PMID: , 10.1016/j.envpol.2010.07.019. [DOI] [PubMed] [Google Scholar]
- Moore JEP, Milian JAS, Sousa EH. 1966. Fluorocarbon ethers derived from hexafluoropropylene epoxide. Patent Number 3,250,808.
- Nakayama S, Strynar MJ, Helfant L, Egeghy P, Ye X, Lindstrom AB. 2007. Perfluorinated compounds in the Cape Fear drainage basin in North Carolina. Environ Sci Technol 41(15):5271–5276, PMID: , 10.1021/es070792y. [DOI] [PubMed] [Google Scholar]
- NC DEQ (NC Department of Environmental Quality). 2017. State orders Chemours to stop chemical releases, begins legal action and steps to suspend permit. https://deq.nc.gov/state-orders-chemours-stop-chemical-releases-begins-legal-action-and-steps-suspend-permit [accessed 13 December 2019].
- NC DHHS (NC Department of Health and Human Services). 2017. Questions and answers regarding North Carolina Department of Health and Human Services updated risk assessment for GenX (perfluoro-2-propoxypropanoic acid). https://files.nc.gov/ncdeq/GenX/NC%20DHHS%20Risk%20Assessment%20FAQ%20Final%20Clean%20071417%20PM.pdf [accessed 12 December 2019].
- NC Drinking Water Watch. 2020. Drinking water branch. https://www.pwss.enr.state.nc.us/NCDWW2/Maps/Map_Template.jsp [accessed 15 January 2020].
- Ng CA, Hungerbühler K. 2014. Bioaccumulation of perfluorinated alkyl acids: observations and models. Environ Sci Technol 48(9):4637–4648, PMID: , 10.1021/es404008g. [DOI] [PubMed] [Google Scholar]
- OECD (Organization for Economic Co-operation and Development). 2018. Toward A New Comprehensive Global Database of Per- and Polyfluoroalkyl Substances (PFASs): Summary Report on Updating the OECD 2007 List of Per- and Polyfluoroalkyl Substances (PFASs). http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV-JM-MONO(2018)7&doclanguage=en [accessed 1 July 2020].
- Pan Y, Zhang H, Cui Q, Sheng N, Yeung LWY, Sun Y, et al. 2018. Worldwide distribution of novel perfluoroether carboxylic and sulfonic acids in surface water. Environ Sci Technol 52(14):7621–7629, PMID: , 10.1021/acs.est.8b00829. [DOI] [PubMed] [Google Scholar]
- Poothong S, Thomsen C, Padilla-Sanchez JA, Papadopoulou E, Haug LS. 2017. Distribution of novel and well-known poly-and perfluoroalkyl substances (PFASs) in human serum, plasma, and whole blood. Environ Sci Technol 51(22):13388–13396, PMID: , 10.1021/acs.est.7b03299. [DOI] [PubMed] [Google Scholar]
- Pritchett JR, Rinsky JL, Dittman B, Christensen A, Langley R, Moore Z, et al. 2019. Notes from the field: targeted biomonitoring for GenX and other per- and polyfluoroalkyl substances following detection of drinking water contamination—North Carolina, 2018. MMWR Morb Mortal Wkly Rep 2019 68(29):647–648, PMID: , 10.15585/mmwr.mm6829a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae JMC, Craig L, Slone TW, Frame SR, Buxton LW, Kennedy GL. 2015. Evaluation of chronic toxicity and carcinogenicity of ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate in Sprague–Dawley rats. Toxicol Rep 2:939–949, PMID: , 10.1016/j.toxrep.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MF, Peldszus S, Anderson WB. 2014. Behaviour and fate of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in drinking water treatment: a review. Water Res 50:318–340, PMID: , 10.1016/j.watres.2013.10.045. [DOI] [PubMed] [Google Scholar]
- Rushing BR, Hu Q, Franklin JN, McMahen RL, Dagnino S, Higgins CP, et al. 2017. Evaluation of the immunomodulatory effects of 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate in C57BL/6 mice. Toxicol Sci 156(1):179–189, PMID: , 10.1093/toxsci/kfw251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell MH, Nilsson H, Buck RC. 2013. Elimination kinetics of perfluorohexanoic acid in humans and comparison with mouse, rat and monkey. Chemosphere 93(10):2419–2425, PMID: , 10.1016/j.chemosphere.2013.08.060. [DOI] [PubMed] [Google Scholar]
- Schultz MM, Barofsky DF, Field JA. 2004. Quantitative determination of fluorotelomer sulfonates in groundwater by LC MS/MS. Environ Sci Technol 38(6):1828–1835, PMID: , 10.1021/es035031j. [DOI] [PubMed] [Google Scholar]
- Sharma BM, Bharat GK, Tayal S, Larssen T, Bečanová J, Karásková P, et al. 2016. Perfluoroalkyl substances (PFAS) in river and ground/drinking water of the Ganges River basin: emissions and implications for human exposure. Environ Pollut 208(Pt B):704–713, PMID: , 10.1016/j.envpol.2015.10.050. [DOI] [PubMed] [Google Scholar]
- Steenland K, Fletcher T, Savitz DA. 2010. Epidemiologic evidence on the health effects of perfluorooctanoic acid (PFOA). Environ Health Perspect 118(8):1100–1108, PMID: , 10.1289/ehp.0901827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strynar M, Dagnino S, McMahen R, Liang S, Lindstrom A, Andersen E, et al. 2015. Identification of novel perfluoroalkyl ether carboxylic acids (PFECAs) and sulfonic acids (PFESAs) in natural waters using accurate mass time-of-flight mass spectrometry (TOFMS). Environ Sci Technol 49(19):11622–11630, PMID: , 10.1021/acs.est.5b01215. [DOI] [PubMed] [Google Scholar]
- Sun M, Arevalo E, Strynar M, Lindstrom A, Richardson M, Kearns B, et al. 2016. Legacy and emerging perfluoroalkyl substances are important drinking water contaminants in the Cape Fear River Watershed of North Carolina. Environ Sci Technol Lett 3(12):415–419, 10.1021/acs.estlett.6b00398. [DOI] [Google Scholar]
- Sun S, Guo H, Wang J, Dai J. 2019. Hepatotoxicity of perfluorooctanoic acid and two emerging alternatives based on a 3D spheroid model. Environ Pollut 246:955–962, PMID: , 10.1016/j.envpol.2018.12.065. [DOI] [PubMed] [Google Scholar]
- Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. 2019. A review of the pathways of human exposure to poly-and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol 29(2):131–147, PMID: , 10.1038/s41370-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). 2017. Occurrence data for the third unregulated contaminant monitoring rule. 2013–2015. https://www.epa.gov/dwucmr/occurrence-data-unregulated-contaminant-monitoring-rule [accessed 13 December 2019].
- U.S. EPA. 2018. Method 537.1: Determination of Selected Per- and Polyfluorinated Alkyl Substances in Drinking Water by Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS). Washington, DC: U.S. EPA, Office of Research and Development, National Center for Environmenal Assessment. [Google Scholar]
- U.S. EPA. 2020. CompTox Chemistry Dashboard. 2,2,3,3-Tetrafluoro-3-{[1,1,1,2,3,3-hexafluoro-3-(1,2,2,2-tetrafluoroethoxy)propan-2-yl]oxy}propanoic acid. https://comptox.epa.gov/dashboard/DTXSID60904459 [accessed 21 April 2020].
- Vestergren R, Cousins IT. 2009. Tracking the pathways of human exposure to perfluorocarboxylates. Environ Sci Technol 43(15):5565–5575, PMID: , 10.1021/es900228k. [DOI] [PubMed] [Google Scholar]
- Wagner A, Buckland T. 2017. Chemours: GenX polluting the Cape Fear since 1980. StarNews Online. 15 June 2017. Updated 16 June 2017. https://www.starnewsonline.com/news/20170615/chemours-genx-polluting-cape-fear-since-1980 [accessed 12 December 2019].
- Wang J, Shi G, Yao J, Sheng N, Cui R, Su Z, et al. 2020. Perfluoropolyether carboxylic acids (novel alternatives to PFOA) impair zebrafish posterior swim bladder development via thyroid hormone disruption. Environ Int 134:105317, PMID: , 10.1016/j.envint.2019.105317. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang X, Sheng N, Zhou X, Cui R, Zhang H, et al. 2017. RNA‐sequencing analysis reveals the hepatotoxic mechanism of perfluoroalkyl alternatives, HFPO2 and HFPO4, following exposure in mice. J Appl Toxicol 37(4):436–444, 10.1002/jat.3376. [DOI] [PubMed] [Google Scholar]
- Wen Y, Mirji N, Irudayaraj J. 2020. Epigenetic toxicity of PFOA and GenX in HepG2 cells and their role in lipid metabolism. Toxicol In Vitro 65:104797, PMID: , 10.1016/j.tiv.2020.104797. [DOI] [PubMed] [Google Scholar]
- Williams AJ, Grulke CM, Edwards J, McEachran AD, Mansouri K, Baker NC, et al. 2017. The CompTox Chemistry Dashboard: a community data resource for environmental chemistry. J Cheminform 9(1):61, PMID: , 10.1186/s13321-017-0247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Kato K, Wong L-Y, Jia T, Kalathil A, Latremouille J, et al. 2018. Per-and polyfluoroalkyl substances in sera from children 3 to 11 years of age participating in the National Health and Nutrition Examination Survey 2013–2014. Int J Hyg Environ Health 221(1):9–16, PMID: , 10.1016/j.ijheh.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung LWY, Mabury SA. 2016. Are humans exposed to increasing amounts of unidentified organofluorine? Environ Chem 13(1):102–110, 10.1071/EN15041. [DOI] [Google Scholar]
- Yeung LWY, Miyake Y, Taniyasu S, Wang Y, Yu H, So MK, et al. 2008. Perfluorinated compounds and total and extractable organic fluorine in human blood samples from China. Environ Sci Technol 42(21):8140–8145, PMID: , 10.1021/es800631n. [DOI] [PubMed] [Google Scholar]
- Zhang C, Hopkins ZR, McCord J, Strynar MJ, Knappe DRU. 2019. Fate of per-and polyfluoroalkyl ether acids in the total oxidizable precursor assay and implications for the analysis of impacted water. Environ Sci Technol Lett 6(11):662–668, PMID: , 10.1021/acs.estlett.9b00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.