Abstract
Purpose:
To evaluate whether loss of ≥5 μm in global retinal nerve fiber layer (RNFL) thickness on spectral-domain optical coherence tomography (SDOCT) between two consecutive visits is specific for glaucoma progression.
Design:
Prospective cohort.
Participants:
92 eyes in 49 controls and 300 eyes in 210 glaucoma subjects.
Methods:
Study subjects completed at least five standard automated perimetry and SDOCT examinations at 6-month intervals over at least 2 years. Eyes were categorized as progressing from glaucoma if the average RNFL declined by ≥5 μm between two consecutive visits. The false positive proportion was estimated by two methods: 1) ≥5 μm loss in controls and 2) ≥5 μm gain in glaucoma. The false positive proportion was subtracted from the cumulative proportion of eyes categorized with glaucoma progression in order to estimate the true progression prevalence.
Main Outcome Measures:
False positive and true progression prevalence of subjects with glaucoma detected as progressing on SDOCT.
Results:
After five years of semi-annual testing, the cumulative proportion of false positives based on ≥5 μm RNFL losses between visits was 24.8% in the controls. While 40.6% of glaucoma eyes were diagnosed with progression at 5 years, only 15.8% would have been considered ‘true’ progression based on the expected false positive ratio from the controls (i.e. 40.6% – 24.8%). The cumulative proportion of an intervisit gain of ≥5 μm at 5 years was 27.4% in glaucoma eyes, suggesting that only 13.2% of eyes with glaucoma had truly progressed (i.e. 40.6% – 27.4%).
Conclusion:
Loss of ≥5 μm in average RNFL between consecutive SDOCT tests is not specific for glaucoma progression. Application of this intervisit “rule of 5” can result in a high cumulative proportion of false positives over time, which could lead to unnecessary interventions in patients whose disease is stable. More specific diagnostic criteria are needed to help clinicians determine whether patients are progressing from glaucoma so that therapy escalation is both timely and appropriate.
PRÉCIS
Application of a “rule of 5” where loss of ≥5 μm in global RNFL thickness between consecutive tests is considered evidence of glaucoma progression leads to a high proportion of false positive tests over time.
Glaucoma is the most common cause of irreversible blindness in the world today.1 Interest in earlier detection of glaucoma and precise monitoring has led to the development of various tools that evaluate structural changes in the retinal nerve fiber layer (RNFL) and optic nerve head.2–4 Since such structural changes often precede and may predict the future development of visual field defects,5 it follows that initiation of appropriate therapy in response to early signs of structural damage may mitigate future functional impairment from glaucoma.
In recent years, spectral domain-optical coherence tomography (SDOCT) has risen to the forefront as the most widely adopted modality for detecting and monitoring structural changes related to glaucoma and other optic neuropathies.4 However, to date there is no agreed-upon criteria for determining whether progression from glaucoma is in fact occurring. Accurate detection of progression depends fundamentally on the ability to distinguish true change from test-retest variability. A change that exceeds the expected test-retest variability is assumed to have a high chance of representing true disease progression rather than noise. Several previous investigations have attempted to establish confidence limits on the variability of measurements obtained by SDOCT. Leung and colleagues conducted repeated testing within a few weeks on a group of 31 normal eyes and concluded that the 95% confidence interval for reproducibility of average RNFL thickness was 4.86 μm for the Cirrus HD-OCT (Carl-Zeiss Meditec, Inc.).6 In a subsequent study, Tan et al. found a value of 4.89 μm for test-retest limits of variability for Cirrus HD-OCT and 4.95 μm for Spectralis SDOCT, also based on repeated testing in normal subjects conducted over a short interval of time.7 These previously identified limits of variability have led to an informal “rule of 5”, which has been widely employed by clinicians for detecting glaucoma progression. According to this rule, when an eye presents with a loss of 5 or more μm in average RNFL between consecutive SDOCT tests, many clinicians consider this quantitative evidence of glaucoma progression.
Previous studies on SDOCT variability have been conducted by performing a sequence of tests over a short period of time.6–9 The rationale for this stems from the fact that glaucoma is not expected to progress in a short period of time and, therefore, any observed change can be attributed to test-retest variability. However, it is questionable whether short-term confidence limits of variability apply to long-term test-retest of glaucoma patients. Short-term test-retest studies usually include highly motivated patients who agree to come several times within a few weeks for testing with highly skilled operators. These conditions may differ from those involved in the testing of the vast majority of subjects who are monitored in clinical practice. Increased variability in the long term could result from a variety of factors including changes in the conditions of the testing environment, operators with different skill levels, limitations in patient motivation, and development of media opacities over time. It is also plausible that variability may change over time with advancing disease stage. In addition, application of a short-term variability threshold does not account for the potential impact of normal aging on changes in RNFL over time.10–13 An additional potential limitation of applying the “rule of 5” for detecting progression stands from the possible inflation of type I error from the repeated application of the rule to assess progression as patients are being monitored over the years, leading to a high proportion of eyes being falsely categorized as progressing. It is important to understand the false positive rate of any algorithm used to diagnose progression since escalation of care in patients who are not truly progressing is potentially harmful and may adversely impact their quality of life.14 The purpose of this study was to investigate the specificity of the “rule of 5” for detecting glaucoma progression, by investigating changes detected by applying this rule to a long-term cohort of glaucoma and healthy subjects followed over time.
METHODS
Study subjects were included from a prospective longitudinal cohort study designed to evaluate optic nerve structure and visual function in glaucoma. Written informed consent was provided by all study subjects. Institutional review board approval was obtained for this study, and it was conducted in accordance with the tenets of the Declaration of Helsinki and the Health Insurance Portability and Accountability Act.
All subjects were adults at least 18 years of age and completed a comprehensive annual ophthalmic examination that included measurement of intraocular pressure, pachymetry, and best-corrected visual acuity (BCVA) as well as slit lamp and dilated fundus examination at baseline and annually thereafter. The BCVA of all included subjects was at least 20/40 with a spherical equivalent within ±5.00 diopters and no media opacity. In addition, all subjects had open angles on undilated gonioscopy. Optic disc stereophotographs were obtained each year and reviewed for evidence of glaucomatous optic neuropathy in a masked fashion by at least two graders based on the presence of neuroretinal rim notching, excavation, or thinning, and/or characteristic RNFL defects.15 In addition, both 24–2 Swedish interactive threshold algorithm of the Humphrey Field Analyzer II (HFA-II, Carl Zeiss Meditec, Dublin, CA) and SDOCT of the circumferential retinal nerve fiber layer (Spectralis version 5.4.7.0; Heidelberg Engineering, Heidelberg, Germany) were obtained approximately every 6 months.
Subjects received a diagnosis of glaucoma at the baseline visit if they demonstrated glaucomatous optic neuropathy on optic disc stereographs and/or repeatable visual field defects on SAP using the 24–2 Swedish interactive threshold algorithm standard algorithm on HFA 2. Repeatable visual field defects were defined as at least three consecutive abnormal SAP results in which the pattern standard deviation p-value was <0.05, and/or the glaucoma hemifield test results were outside the range of normal limits. SAP results also had to meet reliability parameters of ≤15% false-positive errors and ≤33% fixation losses or false-negative errors.
Control subjects were recruited from the community and maintained an intraocular pressure less than 22 mmHg. They were excluded if they developed any signs or symptoms of glaucoma on follow-up testing characterized by intraocular pressure greater than or equal to 22 mmHg, or characteristic changes in the optic nerve head or visual field. Any subjects with systemic or ocular comorbidities that could impact the optic nerve or interpretation of the visual field were also excluded.
All included eyes completed at least 5 reliable SDOCT tests of sufficient signal strength (i.e. >15 dB) that were centered on the optic nerve and were free from RNFL segmentation errors or artifacts. The global RNFL thickness was calculated from the average of all RNFL thickness measurements acquired from a 3.45-mm circle centered on the optic disc consisting of 1536 A-scan points.
Data analysis
In the present analysis, we evaluated whether the loss of ≥5 μm in global RNFL between two consecutive visits, i.e. an intervisit “rule of 5”, can be reasonably considered diagnostic of glaucoma progression in RNFL over time. Kaplan-Meier failure estimates of the time to first detection of progression were graphed for the “rule of 5” definition in both the control cohort and in the glaucoma cohort. Flexible parametric models (FPM) of the hazard function were then constructed with restricted cubic splines to smooth the overall curve.16 The FPM models were used to calculate estimates of the cumulative proportion of progression at each annual time-point in both the controls and glaucoma cohorts.
Due to the lack of a perfect independent reference standard to establish glaucoma progression, we used two approaches to gauge insight into the accuracy of the “rule of 5” for detecting progression. In the first approach, we assumed that the cumulative proportion of healthy control eyes showing progression by the “rule of 5” was indicative of the false positive proportion and that the cumulative proportion of eyes that did not show progression approximated the specificity of the test. The true progression prevalence at a given time point was then estimated from the cumulative proportion of eyes in the glaucoma group detected as progressing in excess of the expected false-positives based on the controls, which is a common approach in the literature.17–20 In the second approach, we assessed the cumulative proportion of eyes that exhibited a positive change (i.e., a gain) of at least 5μm between consecutive visits during long-term monitoring. As RNFL thickness measurements are not expected to truly increase, assessing the proportion of eyes exhibiting such a change during follow-up may serve as a proxy for the false-positive rate.21 The true progression prevalence at a given time point was then estimated from the cumulative proportion of eyes in the glaucoma group detected as progressing in excess of those who exhibited gains (i.e. an increase in RNFL thickness) by the “rule of 5”.
All statistical analyses were completed in STATA (version 15.1, StataCorp, College Station, TX).
RESULTS
Study Subject Characteristics
This study included 92 eyes of 49 healthy controls and 300 eyes of 210 glaucoma subjects. Table 1 provides the baseline demographic and clinical characteristics of included subjects. The mean age at baseline was significantly greater in the glaucoma cohort relative to the healthy controls (68.2 ± 10.2 vs. 51.4 ± 15.6 years, respectively, p<0.001). A lower proportion of glaucoma subjects were female or African American (p<0.05). Average baseline SAP mean deviation was −5.2 ± 5.8 dB in eyes with glaucoma and −0.14 ± 1.13 dB in control eyes (p<0.001). Mean baseline average RNFL thickness was significantly thinner in glaucoma (74.8 ± 17 μm) relative to healthy controls (100.2 ± 9.9 μm; p<0.001). The mean follow-up time was 5.4 ± 1.5 years for glaucoma and 4.5 ± 1.9 years for controls. The median number of SDOCT tests completed was 11 (IQR 7–14) in the glaucoma cohort and 9 (IQR 8–11) in the control cohort (p=0.008).
Table 1.
Demographic and Clinical Characteristics.
| Glaucoma N= 300 eyes in 210 subjects | Controls N=92 eyes in 49 subjects | P-value | |
|---|---|---|---|
| Median (IQR) | 69.6 (60.8 – 75.0) | 51.0 (43.3 – 60.4) | |
| Male | 103/210 (49.0%) | 16/49 (32.7%) | |
| Non-African American | 151/210 (71.9%) | 25/49 (51.1%) | |
| Median (IQR) | −3.47 (−6.37 – −1.65) | −0.12 (−1.0 – 0.93) | |
| Median (IQR) | 74.0 (63.0 – 86.0) | 100.0 (93.0 – 108) | |
| Median (IQR) | −0.125 (−1.375 – 0.75) | −0.25 (−1.25 – 0.56) | |
| Median (IQR) | 11 (7 – 14) | 9 (8 – 11) | |
| Median (IQR) | 5.88 (4.51 – 6.63) | 4.83 (2.25 – 5.96) |
IQR = interquartile range; SD = standard deviation; dB = decibels; SDOCT = Spectral domain optical coherence tomography
Wilcoxon rank-sum test
Chi-square test
Cumulative proportion and incidence of progression over time
Approach 1 – False-positives estimated from measurements in healthy controls
Figure 1 compares the cumulative proportion over time of subjects in the glaucoma group that exhibited progression (i.e, an intervisit decline) in RNFL thickness measurements according to the “rule of 5” and controls that were falsely identified as progressing by the same application of the rule. Table 2 presents a summary of the cumulative proportion of progression at annual time points. For example, at 5 years of follow-up, the cumulative proportion of false-positives was 24.8%, resulting in an expected specificity of only 75.2%. From the glaucoma group, 40.6% of the eyes had shown progression in 5 years. From these estimates, the expected “true progression prevalence”, i.e., glaucoma eyes that truly exhibited progression, would be 40.6% − 24.8% = 15.8%. Similar calculations were performed for the other time points (Table 2).
Figure 1.
Flexible Parametric Model Estimates of the Cumulative Proportion of Progression in Global Retinal Nerve Fiber Layer on Spectral-Domain Optical Coherence Tomography over time by the Intervisit Losses of ≥5 μm in Glaucoma Control Cohorts.
Table 2.
Annual Cumulative Proportion Estimates (95% Confidence Interval) of ≥5 μm Loss in Retinal Nerve Fiber Layer on Spectral-Domain Optical Coherence Tomography in Control and Glaucoma Cohorts by Flexible Parametric Models.
| Rule of 5: ≥5 μm Loss | Year 2 | Year 3 | Year 4 | Year 5 | Year 6 |
|---|---|---|---|---|---|
| Glaucoma | 0.160 (0.125 – 0.204) | 0.271 (0.225 – 0.324) | 0.330 (0.280 – 0.386) | 0.406 (0.352 – 0.464) | 0.536 (0.481 – 0.594) |
| Controls | 0.067 (0.034 – 0.129) | 0.124 (0.072 – 0.208) | 0.186 (0.118 – 0.286) | 0.248 (0.165 – 0.361) | 0.308 (0.205 – 0.447) |
| True progression | 0.093 | 0.147 | 0.144 | 0.158 | 0.228 |
Approach 2 – False-positives estimated from positive increase in RNFL thickness measurements
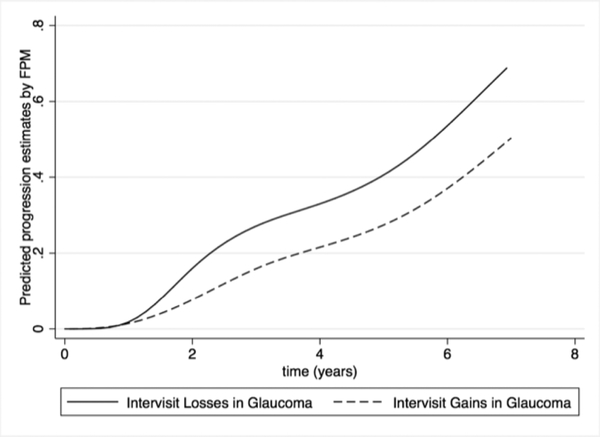
Figure 2 compares the cumulative proportion over time of glaucoma subjects that exhibited a gain (i.e., intervisit increase) in RNFL thickness measurements according to the “rule of 5” versus those that demonstrated glaucoma progression by an intervisit loss of at least 5μm. After 5 years of follow-up, 27.4% of glaucoma eyes had exhibited an intervisit gain of at least 5μm on average RNFL thickness, resulting in an estimated specificity of 72.6% according to this method. As previously described, 40.6% of the eyes had shown progression in 5 years, resulting in an estimated “true progression prevalence” of 13.2%. Table 3 shows similar calculations for other time points.
Figure 2.
Flexible Parametric Model (FPM) Estimates of the Cumulative Proportion of Progression in Global Retinal Nerve Fiber Layer on Spectral-Domain Optical Coherence Tomography over Time by the Intervisit Losses of ≥5 μm in Glaucoma and Intervisit Gains of ≥5 μm in Glaucoma.
Table 3.
Annual Cumulative Proportion Estimates (95% Confidence Interval) of ≥5 μm Loss versus ≥5 μm Gain in Retinal Nerve Fiber Layer on Spectral-Domain Optical Coherence Tomography in Glaucoma Cohort by Flexible Parametric Models.
| Rule of 5 | Year 2 | Year 3 | Year 4 | Year 5 | Year 6 |
|---|---|---|---|---|---|
| ≥5 μm Loss | 0.160 (0.125 – 0.204) | 0.271 (0.225 – 0.324) | 0.330 (0.280 – 0.386) | 0.406 (0.352 – 0.464) | 0.536 (0.481 – 0.594) |
| ≥5 μm Gain | 0.077 (0.053 – 0.111) | 0.159 (0.123 – 0.204) | 0.216 (0.173 – 0.267) | 0.274 (0.227– 0.329) | 0.371 (0.317 – 0.430) |
| True Progression | 0.083 | 0.112 | 0.114 | 0.132 | 0.165 |
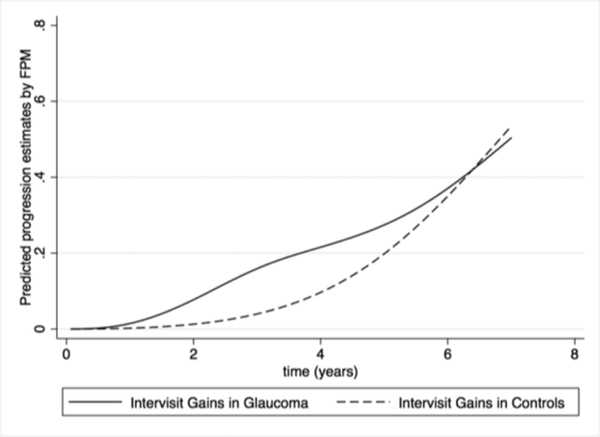
We also investigated gains according to the “rule of 5” in the healthy group. At 5 years of follow-up, the cumulative proportion of healthy eyes exhibiting a positive increase greater than 5μm on RNFL thickness was 19.8% (Table 4). Figure 3 shows the cumulative proportions of eyes in the glaucoma and control cohort that exhibited gains of ≥5 μm in RNFL thickness over time.
Table 4.
Annual Cumulative Proportion Estimates (95% Confidence Interval) of ≥5 μm Gain in Retinal Nerve Fiber Layer on Spectral-Domain Optical Coherence Tomography in Glaucoma and Control Cohorts by Flexible Parametric Models.
| Rule of 5: ≥5 μm Gains | Year 2 | Year 3 | Year 4 | Year 5 | Year 6 |
|---|---|---|---|---|---|
| Glaucoma | 0.077 (0.053 – 0.111) | 0.159 (0.123 – 0.204) | 0.216 (0.173 – 0.267) | 0.274 (0.227– 0.329) | 0.371 (0.317 – 0.430) |
| Controls | 0.013 (0.003 – 0.051) | 0.040 (0.016 – 0.094) | 0.096 (0.050 – 0.181) | 0.198 (0.125 – 0.307) | 0.350 (0.242 – 0.488) |
Figure 3.
Flexible Parametric Model (FPM) Estimates of the Cumulative Proportion of Intervisit Gains ≥5 μm in Global Retinal Nerve Fiber Layer on Spectral-Domain Optical Coherence Tomography over Time in Glaucoma and Controls.
DISCUSSION
Accurate detection of progression is central to the proper management of glaucoma. An inaccurate diagnosis of progression can have adverse ramifications if additional or more invasive treatments are pursued in patients whose disease is stable. This study demonstrates that use of a ≥5 μm decline in global RNFL between consecutive SDOCT tests as a criterion for diagnosing glaucoma progression could lead to a very high proportion of patients being falsely identified as progressing from glaucoma. After 5 years, approximately one-quarter of control eyes had demonstrated at least one event of RNFL loss ≥5 μm, while one-fifth had demonstrated a ≥5 μm gain between visits. This suggests that the losses in RNFL observed in the controls were more likely fluctuations due to test-retest variability rather than the result of normal aging. Our study demonstrates that a ≥5 μm loss in RNFL between visits is not specific for glaucoma and can lead to a high proportion of false positive tests over time. The “rule of 5” is based on the assumption that a decline in peripapillary RNFL on SDOCT that exceeds the expected short-term test-retest variability is suspicious for glaucomatous progression. We found that application of this intervisit “rule of 5” to a longitudinal cohort led to a substantially greater proportion of eyes being falsely classified as progressing over time than would have been expected based on its original derivation.6, 7, 9 Several factors may explain this phenomenon. First, inflation of the type 1 error rate by repeated testing over time poses a significant challenge to clinicians when they are faced with the task of interpreting changes on SDOCT in routine clinical practice. As a patient is reassessed at each visit, the probability that the test is a false positive due to chance will increase with each successive test obtained. Even though we only evaluated the proportion of patients that had one event of progression by the “rule of 5”, we estimated that nearly one-quarter would be falsely categorized as progressing by five years of follow-up. Moreover, we expect that as patients are followed over a lifetime, use of the intervisit “rule of 5” could lead to an even higher rate of detection of false positives than reported in this study since each patient can potentially accumulate multiple episodes of ≥5 μm losses from variability.
Variability on SDOCT may also differ depending on whether or not a subject has glaucoma, and, moreover, the stage of glaucoma disease severity. The short-term variability thresholds of RNFL on SDOCT were determined using normal healthy subjects in some studies.7 Other groups included both controls and glaucoma subjects,6, 8 but did not directly report on whether the variability of the test differed for diseased and healthy eyes. It is possible that not only short-term but also long-term variability in RNFL measurements on SDOCT may be greater in subjects with glaucoma relative to controls. At five years, the glaucoma cohort in our study showed a greater proportion of intervisit gains than the controls, with 27.4% showing gains in glaucoma while only 19.8% showed gains in the controls. If the proportion of gains reasonably reflects variability, as we infer, then variability in glaucoma eyes may be greater than that of control eyes over time. Accurate segmentation may be more difficult in glaucoma due to the fact that the RNFL is thinner, leading to more errors and variability. This finding calls into question whether controls should be used to define the specificity of SDOCT for glaucoma progression in clinical studies, and point to the need for better criteria for distinguishing progressors from non-progressors among glaucoma subjects.
Another significant drawback of using short-term variability thresholds for diagnosis of progression, is that this approach fails to account for losses in RNFL that may occur due to normal aging. The mean estimate for age-related change in RNFL on SDOCT has ranged from −0.44 to − 0.54 μm per year among healthy controls, depending on the study population and instrument used.11, 12, 22 Changes in RNFL due to normal aging also vary with the refraction of the patient.23 Nevertheless, aging is unlikely to account for a 5-micron loss in a 6-month interval; therefore, age-related changes are unlikely to play a significant role in our application of the “rule of 5” for detection of progression between visits. Trend-based analyses may be better able to adjust for such expected age-related losses compared to event-based analyses. For example, one study showed that a lower proportion of eyes were diagnosed with glaucoma progression after accounting for age-related losses by using the lower 95% confidence interval of mean age-related change in control eyes.10 Nevertheless, a recent paper by Wu et al. used computer simulations to illustrate that even when different adjustments are made to trend-based analysis to account for aging, a high proportion of false positives can accumulate over time.12 By applying increasingly stringent definitions for age-related loss, Wu and colleagues suggested that the specificity of SDOCT can be improved, but at the potential expense of sensitivity. Determining the appropriate balance in the specificity and sensitivity of tests for glaucoma progression will require a better understanding of the expected functional losses that may result from under- or over-treatment of glaucoma based on structural changes.24
It is possible that requiring the “rule of 5” to be reproducible on a subsequent test could improve its specificity. If one were to develop a “rule of 5 repeated”, in which loss of ≥5 μm between two tests is confirmed by persistence of that loss on the subsequent test, then a lower proportion of tests would be classified with progression. For example, after 5 years, only 18.1% of ≥5 μm losses would be repeated in the glaucoma cohort, suggesting that approximately 22.5% of the losses flagged as progression by the simple “rule of 5” had been false positives. Similarly, in the controls, only 14.6% of losses would be repeated (Supplemental Figure 4, available at http://www.ophthalmologyglaucoma.org). Of note, the proportion of false positives for the simple “rule of 5” identified by this approach is consistent with the proportion of false positives identified by the other two approaches described in this paper. While requiring change identified by the “rule of 5” to be reproducible may improve the specificity of the rule, this is not the original derivation or application of the “rule of 5” as it has been applied in clinical practice.
This study has limitations. We used two different approaches to estimate the specificity and proportion of false positives detected by the intervisit “rule of 5”: control subjects with ≥5 μm losses in global RNFL, and glaucoma subjects with ≥5 μm gains in global RNFL. We also assumed that the true progression prevalence could be reasonably estimated by subtracting the false positive proportion from the total proportion detected as progressing from glaucoma, which is commonly done,12,17 but which may not reflect the absolute truth. Unfortunately, any proposed method for evaluating the accuracy of SDOCT for glaucoma progression is going to be limited by the lack of a true gold-standard. Nevertheless, both of our approaches lead to the same conclusion, with nearly one quarter of subjects falsely detected as progressing after 5 years when the intervisit “rule of 5” was applied.
Irregular patient follow-up may be an additional source of variability in clinical practice that was not observed in this cohort study since follow-up intervals were regularly spaced. Patients in a clinical study may also be better test-takers and may be more cooperative with SDOCT imaging and thus provide superior quality images than patients in a general clinical setting. Operator-dependent factors may also impact the quality and reliability of imaging as different technicians acquire the images over time. Only high-quality SDOCT images that were free from artifacts or segmentation errors were included in this study and the number of technicians acquiring imaging was limited. Thus, in a clinical setting additional sources of variability may lead to an even higher proportion of false positives than was observed in this observational cohort.
The definition of progression used in this analysis was based on the global RNFL rather than specific sectors. However, since glaucoma is characterized by particular RNFL defects, loss of RNFL in specific regions of interest may be more sensitive for glaucoma progression than loss of the global average RNFL.25 This may be especially true at earlier stages of the disease before the floor effect is reached.26, 27 Since the short-term variability of the global RNFL was used to derive the “rule of 5”, it would not be appropriate to apply it to the sectors. Derivation of similar rules for specific sectors of RNFL on SDOCT based on the published short-term variability thresholds is possible but would be of little service since testing of multiple sectors over time would further compound the inflation of the type I error rate. Future studies that evaluate the accuracy of specific sectors of RNFL for progression may consider trend-based analyses that evaluate the superior and inferior quadrants where glaucomatous changes are typically first observed.
In summary, this study demonstrates that a high proportion of patients will receive a false positive interpretation if loss of ≥5 μm in global RNFL between consecutive tests is considered diagnostic of progression on SDOCT. Such changes in RNFL between consecutive tests are more likely to represent fluctuation from the variability of the test itself rather than true glaucomatous progression. As tests are repeated over time as part of clinical follow-up, the likelihood of obtaining a false positive test result is further compounded. These findings are important because false positive test results can precipitate additional testing and additional unnecessary therapeutic interventions. Trend-based analyses that account for age-related changes may prove more specific for progression on SDOCT, especially if specific regions of interest can be assessed.
Supplementary Material
Supplemental Figure 4: Flexible Parametric Model (FPM) Estimates of the Cumulative Proportion of Intervisit Losses ≥5 μm in Global Retinal Nerve Fiber Layer on Spectral-Domain Optical Coherence Tomography over Time that were reproducible in Glaucoma and Controls.
Financial Support:
Supported in part by National Institutes of Health/National Eye Institute grant EY027651 (FAM), EY025056 (FAM), EY021818 (FAM), Conselho Nacional de Desenvolvimento Científico e Tecnológico (AAJ) and the Heed Foundation (ACT). The funding organizations had no role in the design or conduct of this research.
Abbreviations:
- BCVA
Best-Corrected Visual Acuity
- FPM
Flexible Parametric Models
- HFA
Humphrey Field Analyzer
- RNFL
Retinal Nerve Fiber Layer
- SAP
Standard Automated Perimetry
- SDOCT
Spectral-Domain Optical Coherence Tomography
Footnotes
Conflict of Interest:
ACT: none. AAJ: none. FAM: Alcon Laboratories (C, F, R), Allergan (C, F), Bausch&Lomb (F), Carl Zeiss Meditec (C, F, R), Heidelberg Engineering (F), Merck (F), nGoggle Inc. (F), Sensimed (C), Topcon (C), Reichert (C, R).
Meeting Presentation: American Glaucoma Society Annual Meeting 2019
REFERENCES
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. British Journal of Ophthalmology. 2006;90(3):262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medeiros FA, Zangwill LM, Alencar LM, et al. Rates of progressive retinal nerve fiber layer loss in glaucoma measured by scanning laser polarimetry. Am J Ophthalmol. 2010;149(6):908–15. [DOI] [PubMed] [Google Scholar]
- 3.Chauhan BC, Hutchison DM, Artes PH, et al. Optic disc progression in glaucoma: comparison of confocal scanning laser tomography to optic disc photographs in a prospective study. Invest Ophthalmol Vis Sci. 2009;50(4):1682–91. [DOI] [PubMed] [Google Scholar]
- 4.Bussel II, Wollstein G, Schuman JS. OCT for glaucoma diagnosis, screening and detection of glaucoma progression. Br J Ophthalmol. 2014;98 Suppl 2:ii15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medeiros FA, Alencar LM, Zangwill LM, et al. Prediction of functional loss in glaucoma from progressive optic disc damage. Arch Ophthalmol. 2009;127(10): 1250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leung CK, Cheung CY, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a variability and diagnostic performance study. Ophthalmology. 2009;116(7):1257–63, 63 e1–2. [DOI] [PubMed] [Google Scholar]
- 7.Tan BB, Natividad M, Chua KC, Yip LW. Comparison of retinal nerve fiber layer measurement between 2 spectral domain OCT instruments. J Glaucoma. 2012;21(4):266–73. [DOI] [PubMed] [Google Scholar]
- 8.Ghasia FF, El-Dairi M, Freedman SF, et al. Reproducibility of spectral-domain optical coherence tomography measurements in adult and pediatric glaucoma. J Glaucoma. 2015;24(1):55–63. [DOI] [PubMed] [Google Scholar]
- 9.Mwanza JC, Chang RT, Budenz DL, et al. Reproducibility of peripapillary retinal nerve fiber layer thickness and optic nerve head parameters measured with cirrus HD-OCT in glaucomatous eyes. Invest Ophthalmol Vis Sci. 2010;51(11):5724–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung CK, Ye C, Weinreb RN, et al. Impact of age-related change of retinal nerve fiber layer and macular thicknesses on evaluation of glaucoma progression. Ophthalmology. 2013;120(12):2485–92. [DOI] [PubMed] [Google Scholar]
- 11.Leung CK, Yu M, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a prospective analysis of age-related loss. Ophthalmology. 2012;119(4):731–7. [DOI] [PubMed] [Google Scholar]
- 12.Wu Z, Saunders LJ, Zangwill LM, et al. Impact of Normal Aging and Progression Definitions on the Specificity of Detecting Retinal Nerve Fiber Layer Thinning. Am J Ophthalmol. 2017;181:106–13. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Francis BA, Dastiridou A, et al. Longitudinal and Cross-Sectional Analyses of Age Effects on Retinal Nerve Fiber Layer and Ganglion Cell Complex Thickness by Fourier-Domain OCT. Transl Vis Sci Technol. 2016;5(2):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janz NK, Wren PA, Lichter PR, et al. The Collaborative Initial Glaucoma Treatment Study: interim quality of life findings after initial medical or surgical treatment of glaucoma. Ophthalmology. 2001;108(11):1954–65. [DOI] [PubMed] [Google Scholar]
- 15.Sample PA, Girkin CA, Zangwill LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol. 2009; 127(9): 1136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Royston P, Parmar MK. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002;21(15):2175–97. [DOI] [PubMed] [Google Scholar]
- 17.Abe RY, Diniz-Filho A, Zangwill LM, et al. The Relative Odds of Progressing by Structural and Functional Tests in Glaucoma. Invest Ophthalmol Vis Sci. 2016;57(9):OCT421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medeiros FA, Zangwill LM, Anderson DR, et al. Estimating the rate of retinal ganglion cell loss in glaucoma. Am J Ophthalmol. 2012;154(5):814–24 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Z, Medeiros FA, Weinreb RN, Zangwill LM. Performance of the 10–2 and 24–2 Visual Field Tests for Detecting Central Visual Field Abnormalities in Glaucoma. Am J Ophthalmol. 2018;196:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Z, Weng DSD, Rajshekhar R, et al. Evaluation of a Qualitative Approach for Detecting Glaucomatous Progression Using Wide-Field Optical Coherence Tomography Scans. Transl Vis Sci Technol. 2018;7(3):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leung CK, Cheung CY, Weinreb RN, et al. Evaluation of Retinal Nerve Fiber Layer Progression in Glaucoma: A Study on Optical Coherence Tomography Guided Progression Analysis. IOVS 2010. January;51(1):217–222. [DOI] [PubMed] [Google Scholar]
- 22.Vianna JR, Danthurebandara VM, Sharpe GP, et al. Importance of Normal Aging in Estimating the Rate of Glaucomatous Neuroretinal Rim and Retinal Nerve Fiber Layer Loss. Ophthalmology. 2015;122(12):2392–8. [DOI] [PubMed] [Google Scholar]
- 23.Lee MW, Kim JM, Shin YI, Jo YG, Kim JY. Longitudinal Changes in Peripapillary Retinal Nerve Fiber Layer Thickness in High Myopia: A Prospective Observational Study. Ophthalmology. 2019. April;126(4):522–528. [DOI] [PubMed] [Google Scholar]
- 24.Saunders LJ, Medeiros FA, Weinreb RN, Zangwill LM. What rates of glaucoma progression are clinically significant? Expert Rev Ophthalmol. 2016;11(3):227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Z, Thenappan A, Weng DSD, et al. Detecting Glaucomatous Progression With a Region-of-Interest Approach on Optical Coherence Tomography: A Signal-to-Noise Evaluation. Transl Vis Sci Technol. 2018;7(1): 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sihota R, Sony P, Gupta V, et al. Diagnostic capability of optical coherence tomography in evaluating the degree of glaucomatous retinal nerve fiber damage. Invest Ophthalmol Vis Sci. 2006;47(5):2006–10. [DOI] [PubMed] [Google Scholar]
- 27.Mwanza JC, Kim HY, Budenz DL, et al. Residual and Dynamic Range of Retinal Nerve Fiber Layer Thickness in Glaucoma: Comparison of Three OCT Platforms. Invest Ophthalmol Vis Sci. 2015;56(11):6344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 4: Flexible Parametric Model (FPM) Estimates of the Cumulative Proportion of Intervisit Losses ≥5 μm in Global Retinal Nerve Fiber Layer on Spectral-Domain Optical Coherence Tomography over Time that were reproducible in Glaucoma and Controls.