Abstract
Introduction:
Maternal obesity increases neonatal risk for obesity and metabolic syndrome later in life. Prior attempts to break this intergenerational obesity cycle by limiting excessive gestational weight gain have failed to reduce neonatal adiposity. Alternatively, pre-conception lifestyle interventions may improve the in utero metabolic milieu during early pregnancy leading to improved fetal outcomes. This randomized controlled trial (RCT) is evaluating whether a lifestyle intervention to reduce weight and improve maternal metabolism in preparation for pregnancy (LIPP) attenuates neonatal adiposity, compared to standard medical advice.
Material and Methods:
Overweight/class 1 obese women after a previous pregnancy, ~12 weeks postpartum, preparing for a subsequent pregnancy, will be block randomized (1:1) to either LIPP or standard of care in a parallel design. Randomization is stratified by lactation status and overweight vs. class 1 obesity. The LIPP program consists of intensive short-term weight loss followed by weight maintenance until conception using supervised exercise and a low glycemic Mediterranean diet.
Primary Outcomes:
Group differences in neonatal adiposity at birth assessed by PEA POD and placental mitochondrial lipid metabolism.
Secondary Outcomes:
Group differences in maternal pregravid and gestational body composition, insulin sensitivity, β-cell function, fasting metabolic and inflammatory biomarkers, and overall quality of life. Exploratory outcomes include umbilical cord blood insulin resistance, lipid profile and inflammation.
Discussion:
This RCT will determine the efficacy of maternal weight loss prior to pregnancy on reducing neonatal adiposity. Findings may change standard obstetrical care by providing Level 1 evidence on lifestyle interventions improving neonatal outcomes for women planning for pregnancy.
Keywords: postpartum, pregnancy, gestational weight gain, maternal obesity, postpartum weight loss, fetal adiposity, lifestyle intervention, Mediterranean Diet
INTRODUCTION
Neonates born to mothers with obesity are at increased risk for obesity[1] and metabolic syndrome[2] during childhood. We hypothesize that maternal weight loss may break this intergenerational obesity cycle; however, the most effective time to implement a lifestyle intervention in mothers with obesity is currently unknown. Several randomized controlled trials have employed lifestyle interventions for women with obesity during pregnancy, yet have failed to provide evidence that this approach improves neonatal outcomes[3–9]. As a result, there is a growing consensus that lifestyle intervention initiated during pregnancy does not decrease fetal overgrowth—even in the presence of maternal improvements, including reduced gestational weight gain and dietary maternal glycemic load, improved physical activity, and body composition[10, 11]. A potential reason for these disappointing conclusions is that placental growth rates and nutrient metabolism may be programmed by maternal metabolic status during early pregnancy, representing a critical time window for placental function to affect fetal growth and development[12]. Thus, initiating a lifestyle intervention during gestation (after the first trimester) may be too late to improve neonatal metabolic health.
Pregravid BMI in obese women is a strong predictor of childhood obesity, independent of maternal gestational diabetes mellitus (GDM) or excessive gestational weight gain (GWG)[13, 14], raising the possibility that lifestyle intervention prior to pregnancy may break the intergenerational obesity cycle. One mechanism whereby this may occur is through optimizing the in utero metabolic milieu as early as the first trimester, resulting in healthy fetal growth and development. A leading physiological candidate for gestational overgrowth is maternal hyperinsulinemia and insulin resistance, which occurs to a larger degree in women with obesity compared to normal weight women[15]. Thus, weight loss in women with obesity may improve pregravid insulin sensitivity and β-cell function by normalizing circulating insulin levels to that of normal-weight women, resulting in a metabolically healthy in utero environment and promoting optimal fetal growth. While in utero modification of maternal metabolism holds promise as an effective strategy to reduce excess fetal adiposity known to contribute to childhood obesity it has yet to be prospectively tested.
The primary objective of this randomized controlled trial (RCT) is to test the efficacy of a lifestyle intervention, initiated prior to conception, on fetal health in women with overweight/class 1 obesity. We will examine how maternal lifestyle intervention after a previous pregnancy, and prior to a subsequent pregnancy, impacts maternal metabolism and subsequent fetal growth/adiposity. In order to gain insight into potential contributing mechanisms, we will assess differences in maternal insulin sensitivity, β-cell function, and inflammatory biomarkers. We will also examine how lifestyle intervention prior to pregnancy affects placental mitochondrial function and lipid oxidation, which potentially alters lipid availability to the fetus. This information would provide a mechanism whereby maternal pre-pregnancy metabolic milieu regulates placental nutrient function, transfer and subsequent fetal growth.
MATERIAL AND METHODS
Study Design and Randomization
The Lifestyle Intervention in Preparation for Pregnancy (LIPP) study is a parallel design multicenter RCT in which overweight and obese postpartum women are prospectively assigned to either LIPP or standard of care (Figure 1). Participants are randomized (1:1) in blocks of 4 according to the following stratifications: (1) BMI 25–30, not breastfeeding at 3 months, (2) BMI 25–30, breastfeeding at 3 months, less than 50% (3) BMI 25–30, breastfeeding > 50% at 3 months, (4) BMI 30–35, not breastfeeding at 3 months, (5) BMI 30–35, breastfeeding at 3 months less than 50%. (6) BMI 30–35, breastfeeding > 50% at 3 months. The study will enroll 200 participants across all sites.
Figure 1 Caption.
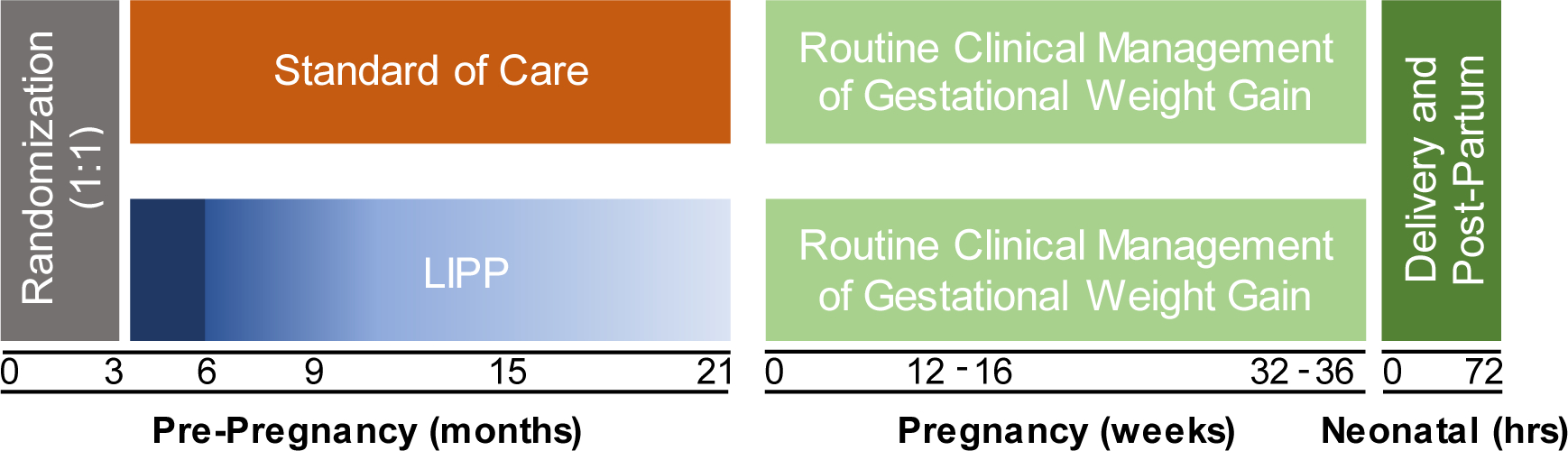
The lifestyle intervention in preparation for pregnancy (LIPP) study design. 200 women approximately 12 weeks postpartum, classified as overweight or class I obesity prior to their most recent pregnancy, will be prospectively randomized (1:1) in blocks of 4 to receive either the LIPP or standard of care treatment. The LIPP program consists of 3 months of intensive weight loss, followed by weight loss maintenance lasting until their subsequent pregnancy. Maternal outcome assessments will occur at 3, 6, 9, 15, and 21 months postpartum. Both LIPP and standard of care participants will receive routine clinical management for maternal and gestational health during their subsequent pregnancy. Maternal outcome assessments will occur at 12–16 and 32–36 weeks of gestation. Neonatal body composition will be determined within 72 hours of birth upon delivery.
Screening, Enrollment, and Recruitment
The participating institutions include Tufts Medical Center, Pennington Biomedical Research Center, MetroHealth Medical Center, and Cleveland Clinic. Eligible participants will be enrolled into the study at approximately three months postpartum (+/− 3 weeks). Participants must have been overweight or obese (BMI: 25–35) prior to their most recent pregnancy, between the ages of 18–40, and planning on a future pregnancy within the following 1–2 years. Inclusion and exclusion criteria are presented in Table 1. Prior to enrollment, participants are screened for eligibility and medical clearance in order to enroll in the study. Group assignment occurs after the confirmation of eligibility criteria. This study is using a centralized Institutional Review Board at Tufts Medical Center. All participants must provide written informed consent prior to participation.
TABLE 1.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Participant recruitment is achieved by face-to-face recruitment efforts in clinical settings, which is supported by partnerships with various local hospitals and birthing centers. Interested participants are provided with general advertising materials, including study website and flyers. Social media advertisements and digital campaigns are also used to generate public awareness, and the study team regularly attends community events for new and expecting mothers.
Intervention
Lifestyle Intervention in Preparation for Pregnancy (LIPP) Group:
This assignment involves sequential phases of personalized weight loss and subsequent weight maintenance. Each of the phases differ by the degree of supervision and participant independence.
Phase 1:
Intense Weight Loss Phase (12 weeks). The initial phase of the intervention consists of a closely supervised exercise and diet approach. The exercise component consists of 60-minute sessions at 80–85% HRmax, occurring at a frequency of 3–5 days per week. Exercise mode varies, and includes treadmill, cycle, and/or elliptical machines. Exercise sessions are supervised by a trained exercise physiologist or qualified research staff. Exercise intensity is monitored using Polar heart rate monitors. Exercise compliance is based on exercise session attendance, which is recorded in individual training logs. Additionally, participants are counseled to maintain a daily step count of >10,000 steps per day and receive an electronic scale (Aria, FitBit, California, USA) and fitness watch (Fitbit, California, USA) for additional support and monitoring. Compliance to physical activity recommendations is monitored using FitBit tracking. The dietary component involves behavioral counseling by a registered dietitian or trained research staff on reducing caloric intake to achieve weight loss through adherence to a low glycemic Mediterranean Diet. One-on-one nutrition counseling sessions will occur on a weekly basis. Dietary compliance is monitored using a Mediterranean diet adherence questionnaire (modified from the MEDAS) [16]. Additionally, quantitative nutrition data is collected via 3-day dietary records and 24-hr dietary recalls every 4 weeks. Participants are provided with extra virgin olive oil imported from Sicily. The desired weight loss target is approximately 5–7% weight loss from pregravid body weight, and additional weight loss is acceptable.
Phase 2:
Sustained Weight Loss and Maintenance Phase. Phase 2 is divided into three periods that progressively reduce in the degree of supervision, and eventually transition to participant independence. During the first 12+ weeks (Phase 2A), participants will continue to exercise 3–5 days per week, but will be supervised for 2–3 of these exercise sessions. Exercise mode may additionally consist of structured exercise classes and participants are counseled to maintain additional unsupervised exercise for 60 minutes per day, up to a total of 5 exercise sessions per week at 85% HRmax and a step count of >10,000 steps per day. Exercise compliance will be assessed as exercise session attendance per week, including supervised and unsupervised sessions. Physical activity compliance will be monitored using FitBit tracking. The dietary approach uses individualized strategies to promote weight loss or weight maintenance through continued counseling on the low glycemic Mediterranean dietary pattern. Dietary compliance during phase two will be assessed using the Mediterranean adherence questionnaire, 3-day dietary records, and 24-hr dietary recalls collected at 6, 9, 15, and 21-months postpartum.
If the desired weight loss is maintained, participants move into a more independent and less supervised phase (Phase 2B) for the next 24 weeks. The dietary component consists of individualized behavioral therapy to promote weight maintenance and adherence to the low glycemic Mediterranean dietary pattern. The exercise component shifts to 1 supervised exercise session per week, lasting 60 minutes in duration. Exercise mode may additionally consist of structured exercise classes. Participants are counseled to continue to achieve additional unsupervised exercise up to 5 days per week and a step count of >10,000 steps per day.
If the desired weight loss is maintained after this 24-week period, and the participant has not yet become pregnant, participants are no longer required to attend supervised exercise sessions (Phase 2C). Participants are counseled to maintain up to 5 exercise sessions per week and to continue to follow a low glycemic Mediterranean Diet supplemented with study-provided extra virgin olive oil. Participants are monitored by bodyweight and weekly step count via electronic scale (Aria, FitBit, California, USA) and fitness watch (FitBit, California, USA). Participants remain in this phase up until they become pregnant. Thus, the time duration spent in this phase will vary across participants. Participants will be contacted once a month and encouraged to adhere to the protocol, as well as report on pregnancy status. If at any time, weight loss or weight maintenance is not achieved (i.e., weight regain occurs), participants receive additional counseling and supervision until target bodyweight is sustained.
Standard of Care:
Participants randomized to standard of care are provided with behavioral counseling from a registered dietitian or trained research staff and receive handouts describing healthy behaviors including physical activity guidelines from the Department of Health and Human Services and US Department of Agriculture Dietary Guidelines. Participants continue to receive advice and recommendations from their primary healthcare providers. Participants who are not pregnant at the end of the 18-month pre-pregnancy study period (21 months postpartum) are followed by study staff with a monthly telephone call encouraging them to follow the plan they were assigned at randomization and inquire if they are pregnant. If pregnant, participants are scheduled for their early pregnancy metabolic assessment.
Subsequent Pregnancy and Delivery:
Participation in the treatment allocation ceases upon confirmation of pregnancy. All participants are asked to follow a diet supporting healthy weight gain during pregnancy and to maintain moderate physical activity based upon their level of physical activity before pregnancy, per the subject’s primary obstetrical caregiver and American College of Obstetricians and Gynecologists (ACOG) guidelines. Study staff maintain contact with participants during the perinatal period and through delivery.
STUDY ENDPOINTS
The primary outcomes are differences in neonatal adiposity at birth and placental lipid oxidation. We hypothesize that women who initiate a lifestyle intervention prior to pregnancy will deliver a baby with lower fat accretion and enhanced placental mitochondrial function. Secondary outcomes are the mother’s pregravid metabolic health. We hypothesize that a lifestyle intervention initiated before pregnancy will lower maternal insulin resistance, inflammation, body weight, adiposity, resting energy expenditure, and improve exercise capacity compared to standard of care. The rationale for these endpoints is that maternal pre-pregnancy BMI and neonatal adiposity at birth are risk factors for childhood adiposity and metabolic dysfunction[13]. A timeline of neonatal and maternal primary and secondary outcome assessments is presented in Table 2.
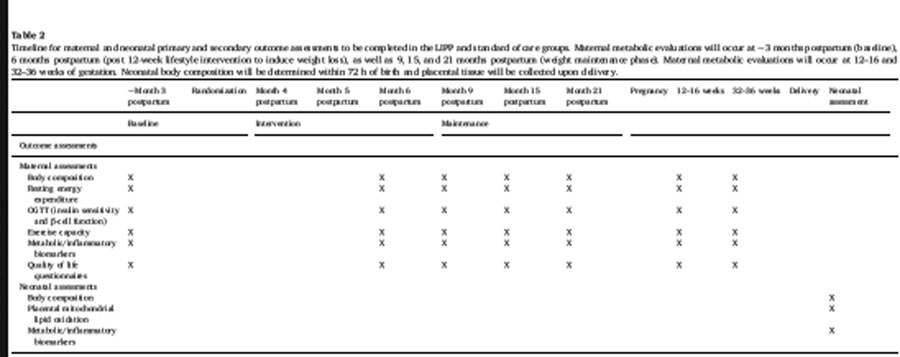
|
OUTCOME ASSESSMENTS
Neonatal Assessments
Body Composition:
Neonatal adiposity will be assessed at the time of birth using air displacement plethysmography (PEA POD, Cosmed, Rome Italy). Additionally, validated neonatal anthropometrics will be assessed including skinfold measures. Neonatal weight will be assessed using a calibrated scale, and length will be measured to the nearest 1.0 cm.
Placental Mitochondrial Lipid Oxidation:
The placenta will also be collected and processed for assessments of mitochondrial lipid oxidation as described previously[17]. Briefly, freshly isolated placental explants will be incubated in the presence of 100 μM cold palmitate and 3H-palmitate for 18 hrs. At the end of the incubation period, media will be collected to quantify the FAO rate by detection of 3H2O using the vapor phase equilibration method. Esterification into total lipids will be determined by homogenizing the treated explants in acetone and incubating with agitation at room temperature overnight. An aliquot of the acetone-extract lipid suspension will be used to determine the radioactive content by liquid scintillation counting. Oxidation and esterification rates will be defined as nmol palmitate/mg tissue/hr.
Metabolic Status:
Venous umbilical cord blood will be obtained for measurement of glucose, insulin, C-peptide, lipid profiling, and inflammatory markers, including C-reactive protein (CRP), interleukin (IL)-6 and leptin. Neonatal insulin resistance will be determined by Homeostatic Model Assessment of Insulin Resistance (HOMA-IR).
Maternal Assessments
Metabolic testing will be performed at baseline, approximately 3 months postpartum and after the initial fully supervised weight loss phase, and then at the subsequent 6, 9, 15, and 21-month time points. During the subsequent pregnancy, assessments will be conducted at 12–16 and 32–36 weeks of gestation. Maternal metabolic assessments include body composition, resting energy expenditure, exercise capacity, insulin sensitivity and β-cell function, as well as metabolic and inflammatory biomarkers.
Body Composition:
Anthropometry measurements include height, weight, and hip and waist circumference measured to the nearest 1.0 cm (pre-pregnancy). Body composition will be assessed by whole body air plethysmography (Bod Pod; Cosmed, Rome, Italy)[18] using a hydration constant of 76% for fat free mass during late pregnancy[19].
Resting Energy Expenditure:
Indirect calorimetry will be performed in the fasted state with the subject laying supine. Expired air is continuously sampled for 30 minutes using an automated system (reference system/s) in a semi-darkened, thermoneutral (22 ± 1°C) environment under a ventilated hood. Oxidative and non-oxidative glucose metabolism is estimated and urine samples are obtained before and after the measure in order to calculate non-protein RQ (NPRQ).
Exercise Capacity:
An incremental graded treadmill test is performed to assess maximal oxygen consumption. Valid tests must achieve three of the following four criteria: oxygen consumption plateau (< 150 ml/min), heart rate within 15 beats of age-predicted HRmax, respiratory exchange ratio > 1.15, and/or volitional fatigue, as previously described[20–23].
Insulin Sensitivity and β-Cell Function:
A 75-gram oral glucose tolerance test (OGTT) is used to assess insulin sensitivity and β-cell function. After an overnight fast, blood samples are drawn at −10, 0, 30, 60, 90, 120, and 180 minutes after the time of glucose ingestion. Plasma glucose is measured using the glucose oxidase method (YSI; Yellow Springs, OH). Insulin will be measured by radioimmunoassay (Millipore, Billerica, MA). Diagnosis of gestational diabetes mellitus (GDM) is made using criteria recommended by ACOG[24].
Metabolic and Inflammatory Biomarkers:
At the baseline visit, fasting blood samples are obtained to measure complete blood count (CBC), thyroid stimulating hormone (TSH), HbA1C, lipid panel (triglycerides, total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol), liver function, renal function and total free fatty acids (FFA). At the baseline visit and all other study visits, fasting blood samples are obtained to measure circulating inflammatory markers by ELISA (R&D Systems, Minneapolis, MN), including: adiponectin, leptin, interleukin-6, interleukin-8, TNF-α, and hsCRP. Longitudinal samples from individual participants will be stored at −80°C and run in the same assay in duplicate at completion to decrease variability.
Quality of Life Questionnaire:
The Short Form Health Survey (SF-36) is being used to assess health-related quality of life[25, 26]. These data will provide a measure of physical and mental health through assessment of physical functioning, bodily pain, limitations due to physical, personal, or emotional problems and well-being, energy/fatigue, and general health perceptions.
STATISTICAL ANALYSIS PLAN
Sample Size and Power
The primary analysis for maternal metabolic outcomes is based on an intent-to-treat comparison between LIPP and standard of care. Group comparisons among study outcomes will be performed using a two-sample test, α=0.05. Covariates will be adjusted using linear regression models. Based on our previous findings from 1-year postpartum mothers, it is estimated that we will have 90% power to detect an absolute or covariate-adjusted improvement in insulin sensitivity of 30%, and an 80% power to detect an improvement as small as 25% in the LIPP relative to standard care[27, 28]. Corresponding 95% confidence intervals for absolute or covariate-adjusted differences or percent improvements in insulin sensitivity between groups will be reported. It is estimated that the standard deviation of change in BMI from randomization until subsequent pregnancy is 5.1 kg/m2. We will have 90% power to detect an absolute or covariate-adjusted difference in BMI of 2.6 kg/m2 and 80% power to detect a difference of 2.26 kg/m2 and 90% power to detect an absolute or covariate-adjusted difference in fat mass of 5.9 kg and 80% power to detect a difference of 5.1 kg between groups.
The primary analysis for differences in neonatal adiposity is also based on an intent-to-treat comparison. Group comparisons among study outcomes will be performed using a two-sample test, α=0.05. Linear regression will be performed, which will include weight (body composition measures) of the participants’ index baby as a covariate. Should any imbalance of confounding factors (for example gestational age) in groups be recognized, linear regression models will be used to perform a covariate adjustment. Based on our preliminary data, we estimate a standard deviation of neonatal mass between groups to be no more than 225 grams. With at least 50 women in each group (assuming a 50% dropout), the t-test or linear regression will have 90% power to detect an absolute or covariate-adjusted difference of 146 gram fat mass between groups. We have 80% power to detect an absolute or co-variate adjusted as small as 126 g fat mass between groups. Corresponding 95% confidence intervals for the absolute or covariate-adjusted difference in neonatal fat mass between groups will be reported.
We will also determine the effect of lifestyle intervention prior to pregnancy on placental mitochondrial fatty acid oxidation at term. We will conduct an intent-to-treat analysis using two sample t-test or non-parametric Wilcoxon rank-sum test to assess differences between groups with α level=0.05. Regression analyses will be used to assess the associations of placental β-oxidation and enzyme activity with maternal inflammatory cytokine levels and insulin resistance in early pregnancy, along with neonatal fat mass with adjustment for gestational age and gender. Based on our previous studies of placental mitochondrial β-oxidation in obese women (38 ± 14 nmol/mg/hr)[17] a sample size of n=18 per group achieved 80% power to detect a difference of 25% between groups using a two-sample t-test.
The same statistical analyses procedures will be applied to secondary outcomes, which will also follow an intent-to-treat approach. Based on our preliminary data, we estimate the standard deviation of birth weight to be 700 grams; with 50 neonates in each group we will have 90% power to detect an absolute or covariate-adjusted difference of 455 grams in birth weight and 80% power to detect a 393 g difference between groups. Additional secondary analyses will be performed on umbilical cord cytokines. Comparisons will be performed using a two-sample t-test at a significance level of α=0.05. Mann-Whitney U tests or log transformations will be employed if data are non-normally distributed. Linear regression models, including confounding factors will be used to perform covariate adjustments. Based on our previous findings[29], it is estimated that the standard deviation of umbilical cord IL-6 and CRP will be 3.4 pg/mL and 7,900 ng/mL, respectively. With 50 women in each group, we will have 90% power to detect an improvement in IL-6 and CRP levels of 50% and 42%, and 80% power to detect an improvement of 42% and 36%, respectively.
DISCUSSION
The LIPP trial is a proof-of-principle study evaluating whether a lifestyle intervention to reduce weight in preparation for pregnancy will reduce neonatal adiposity at birth, compared to a group who received standard medical advice, which we refer to as the standard of care group. Several studies have employed lifestyle interventions in pregnant women but have failed to provide evidence that this approach leads to significant differences in birthweight or more specifically reduced neonatal adiposity. For example, implementation of a lifestyle intervention initiated between 10–14 weeks of gestation resulted in decreased gestational weight gain, yet a paradoxical increase in neonatal body weight at birth[30]. Other RCTs report that lifestyle interventions do not reduce the incidence of large for gestational age babies, despite improved maternal metabolic health[10, 11]. In further agreement, as many as five meta-analyses conclude that lifestyle intervention initiated during pregnancy does not decrease fetal overgrowth[3–7].
These consistent findings highlight the need to test pregravid metabolic health as a treatment target for improving neonatal adiposity. Pregravid maternal BMI is the strongest predictor of childhood obesity, independent of maternal gestational diabetes mellitus (GDM) or excessive gestational weight gain (GWG)[13, 14]. In addition, interpregnancy weight reduction is associated with reduced LGA, while interpregnancy weight gain is associated with increased LGA[31]. A potential explanation for these relationships is that maternal metabolism during early pregnancy, rather than late pregnancy, may be the dominant driver of placental growth, development, and nutrient metabolism[12]. In support, obesity-associated hyperinsulinemia and insulin resistance during early pregnancy negatively impacts placental development by disrupting cholesterol homeostasis and mitochondrial function[32]. Thus, strategies that normalize pregravid hyperinsulinemia and insulin resistance may promote healthy fetal growth and development. Lifestyle interventions utilizing exercise and dietary approaches are feasible, safe and effective for reducing body weight in postpartum women even during lactation[33–38], and thus may be a valuable tool to reduce pregravid BMI, and break this vicious intergenerational obesity cycle. From the current study design, it is not possible to disentangle which specific constituents of the lifestyle intervention (e.g. diet, exercise, or the combination) are driving maternal and neonatal improvements. However, longitudinal assessments of maternal weight loss as well as maternal metabolic assessments will inform our understanding of the relationship between maternal and neonatal metabolic health. The LIPP study is one of the first of its kind, as we aim to improve maternal metabolic status prior to conception, rather than during the time of gestation, to affect fetal growth and potentially long-term metabolic function. On-going studies are evaluating the effects of pre-pregnancy lifestyle interventions as a means to intervene on health outcomes, including gestational diabetes re-occurrence (NCT: NCT02763150), reduced pre-pregnancy weight gain (NCT: NCT02346162), and fetal morphometry (NCT: NCT02541487). This underscores that pre-conception health is gaining momentum as a treatment target.
A primary strength of the LIPP study is that we will evaluate the effects of the intervention at several points pre-pregnancy and during gestation, which will ultimately contribute to a comprehensive physiological and mechanistic viewpoint. We will assess changes in pregravid maternal health, as well as subsequent changes in neonatal metabolic health at the time of birth. These data will be supported by assessments of placental lipid metabolism, providing mechanistic insight into the maternal-fetal intergenerational obesity cycle.
Clinical Implications
Findings from the LIPP study have the potential to provide Level 1 evidence for a 2009 IOM “Guidelines for gestational weight gain in pregnancy” objective. Research recommendation S-7 suggests that by providing pre-conceptual services to women with obesity, including diet and exercise, represents a radical change to the care provided to overweight and obese women of childbearing age[39]. This lifestyle intervention has minimal risk, and thus, has potential for widespread adoption in other settings. If successful, the next step is to determine a cost effective, large-scale implementation approach.
CONCLUSION
This is one of the first randomized controlled trials to test whether lifestyle intervention in preparation for pregnancy can break the vicious intergenerational obesity cycle. We will gain insight into how maternal metabolic health status is related to the fetus via the in utero environment. Specifically, we will evaluate whether a lifestyle intervention initiated prior to conception improves maternal insulin sensitivity, thereby normalizing maternal hyperinsulinemia and insulin resistance during early pregnancy, thus promoting a healthy in utero metabolic milieu, consequently resulting in healthy neonatal growth. Taken together, these data will provide the option to develop strategies to break the vicious cycle of obesity, starting prior to conception. Findings from the LIPP study have the potential to result in a paradigm shift in obstetrical medical practice by changing the way clinical care is provided to women planning to become pregnant.
ACKNOWLEDGEMENTS:
We would like to thank all research staff, clinical partners, and study participants for their time and dedication to this study. This trial is funded by Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD): R01-HD088061.
FUNDING: This work is supported by the National Institutes of Health (R01HD088061), T32DK064584 (MLE), T32AT004094 (JTM), and US Department of Agriculture, Agreement N. 58-1950-4-003 (RAF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CLINICAL TRIAL REGISTRATION: NCT03146156
REFERENCES
- [1].Whitaker RC, Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy, Pediatrics 114(1) (2004) e29–36. [DOI] [PubMed] [Google Scholar]
- [2].Boney CM, Verma A, Tucker R, Vohr BR, Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus, Pediatrics 115(3) (2005) e290–6. [DOI] [PubMed] [Google Scholar]
- [3].Dodd JM, Grivell RM, Crowther CA, Robinson JS, Antenatal interventions for overweight or obese pregnant women: a systematic review of randomised trials, Bjog 117(11) (2010) 1316–26. [DOI] [PubMed] [Google Scholar]
- [4].Tanentsapf I, Heitmann BL, Adegboye AR, Systematic review of clinical trials on dietary interventions to prevent excessive weight gain during pregnancy among normal weight, overweight and obese women, BMC Pregnancy Childbirth 11 (2011) 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Quinlivan JA, Julania S, Lam L, Antenatal dietary interventions in obese pregnant women to restrict gestational weight gain to Institute of Medicine recommendations: a meta-analysis, Obstet Gynecol 118(6) (2011) 1395–401. [DOI] [PubMed] [Google Scholar]
- [6].Thangaratinam S, Jolly K, Obesity in pregnancy: a review of reviews on the effectiveness of interventions, Bjog 117(11) (2010) 1309–12. [DOI] [PubMed] [Google Scholar]
- [7].Thangaratinam S, Rogozinska E, Jolly K, Glinkowski S, Duda W, Borowiack E, Roseboom T, Tomlinson J, Walczak J, Kunz R, Mol BW, Coomarasamy A, Khan KS, Interventions to reduce or prevent obesity in pregnant women: a systematic review, Health Technol Assess 16(31) (2012) iii-iv, 1–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Peaceman AM, Clifton RG, Phelan S, Gallagher D, Evans M, Redman LM, Knowler WC, Joshipura K, Haire-Joshu D, Yanovski SZ, Couch KA, Drews KL, Franks PW, Klein S, Martin CK, Pi-Sunyer X, Thom EA, Van Horn L, Wing RR, Cahill AG, Lifestyle Interventions Limit Gestational Weight Gain in Women with Overweight or Obesity: LIFE-Moms Prospective Meta-Analysis, Obesity (Silver Spring) 26(9) (2018) 1396–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Phelan S, Clifton RG, Haire-Joshu D, Redman LM, Van Horn L, Evans M, Joshipura K, Couch KA, Arteaga SS, Cahill AG, Drews KL, Franks PW, Gallagher D, Josefson JL, Klein S, Knowler WC, Martin CK, Peaceman AM, Thom EA, Wing RR, Yanovski SZ, Pi-Sunyer X, One-year postpartum anthropometric outcomes in mothers and children in the LIFE-Moms lifestyle intervention clinical trials, Int J Obes (Lond) 44(1) (2020) 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Poston L, Bell R, Croker H, Flynn AC, Godfrey KM, Goff L, Hayes L, Khazaezadeh N, Nelson SM, Oteng-Ntim E, Pasupathy D, Patel N, Robson SC, Sandall J, Sanders TA, Sattar N, Seed PT, Wardle J, Whitworth MK, Briley AL, Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial, Lancet Diabetes Endocrinol 3(10) (2015) 767–77. [DOI] [PubMed] [Google Scholar]
- [11].Sagedal LR, Overby NC, Bere E, Torstveit MK, Lohne-Seiler H, Smastuen M, Hillesund ER, Henriksen T, Vistad I, Lifestyle intervention to limit gestational weight gain: the Norwegian Fit for Delivery randomised controlled trial, Bjog 124(1) (2017) 97–109. [DOI] [PubMed] [Google Scholar]
- [12].O’Tierney-Ginn P, Presley L, Myers S, Catalano P, Placental growth response to maternal insulin in early pregnancy, J Clin Endocrinol Metab 100(1) (2015) 159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, de Mouzon SH, Amini SB, Perinatal risk factors for childhood obesity and metabolic dysregulation, Am J Clin Nutr 90(5) (2009) 1303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lowe WL Jr., Scholtens DM, Lowe LP, Kuang A, Nodzenski M, Talbot O, Catalano PM, Linder B, Brickman WJ, Clayton P, Deerochanawong C, Hamilton J, Josefson JL, Lashley M, Lawrence JM, Lebenthal Y, Ma R, Maresh M, McCance D, Tam WH, Sacks DA, Dyer AR, Metzger BE, Association of Gestational Diabetes With Maternal Disorders of Glucose Metabolism and Childhood Adiposity, Jama 320(10) (2018) 1005–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kalkhoff RK, Impact of maternal fuels and nutritional state on fetal growth, Diabetes 40 Suppl 2 (1991) 61–5. [DOI] [PubMed] [Google Scholar]
- [16].Ros E, The PREDIMED study, Endocrinol Diabetes Nutr 64(2) (2017) 63–66. [DOI] [PubMed] [Google Scholar]
- [17].Calabuig-Navarro V, Haghiac M, Minium J, Glazebrook P, Ranasinghe GC, Hoppel C, Hauguel de-Mouzon S, Catalano P, O’Tierney-Ginn P, Effect of Maternal Obesity on Placental Lipid Metabolism, Endocrinology 158(8) (2017) 2543–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Siri WE, Body composition from fluid spaces and density: analysis of methods. 1961, Nutrition 9(5) (1993) 480–91; discussion 480, 492. [PubMed] [Google Scholar]
- [19].Catalano PM, Wong WW, Drago NM, Amini SB, Estimating body composition in late gestation: a new hydration constant for body density and total body water, Am J Physiol 268(1 Pt 1) (1995) E153–8. [DOI] [PubMed] [Google Scholar]
- [20].Yassine HN, Marchetti CM, Krishnan RK, Vrobel TR, Gonzalez F, Kirwan JP, Effects of exercise and caloric restriction on insulin resistance and cardiometabolic risk factors in older obese adults--a randomized clinical trial, J Gerontol A Biol Sci Med Sci 64(1) (2009) 90–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Solomon TP, Haus JM, Kelly KR, Rocco M, Kashyap SR, Kirwan JP, Improved pancreatic beta-cell function in type 2 diabetic patients after lifestyle-induced weight loss is related to glucose-dependent insulinotropic polypeptide, Diabetes Care 33(7) (2010) 1561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Solomon TP, Haus JM, Kelly KR, Cook MD, Riccardi M, Rocco M, Kashyap SR, Barkoukis H, Kirwan JP, Randomized trial on the effects of a 7-d low-glycemic diet and exercise intervention on insulin resistance in older obese humans, Am J Clin Nutr 90(5) (2009) 1222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sady SP, Carpenter MW, Sady MA, Haydon B, Hoegsberg B, Cullinane EM, Thompson PD, Coustan DR, Prediction of VO2max during cycle exercise in pregnant women, J Appl Physiol (1985) 65(2) (1988) 657–61. [DOI] [PubMed] [Google Scholar]
- [24].Carpenter MW, Coustan DR, Criteria for screening tests for gestational diabetes, Am J Obstet Gynecol 144(7) (1982) 768–73. [DOI] [PubMed] [Google Scholar]
- [25].Jacobson AM, de Groot M, Samson JA, The evaluation of two measures of quality of life in patients with type I and type II diabetes, Diabetes Care 17(4) (1994) 267–74. [DOI] [PubMed] [Google Scholar]
- [26].Ware JE Jr., Gandek B, Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project, J Clin Epidemiol 51(11) (1998) 903–12. [DOI] [PubMed] [Google Scholar]
- [27].Kirwan JP, Varastehpour A, Jing M, Presley L, Shao J, Friedman JE, Catalano PM, Reversal of insulin resistance postpartum is linked to enhanced skeletal muscle insulin signaling, J Clin Endocrinol Metab 89(9) (2004) 4678–84. [DOI] [PubMed] [Google Scholar]
- [28].Friedman JE, Kirwan JP, Jing M, Presley L, Catalano PM, Increased skeletal muscle tumor necrosis factor-alpha and impaired insulin signaling persist in obese women with gestational diabetes mellitus 1 year postpartum, Diabetes 57(3) (2008) 606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S, Fetuses of obese mothers develop insulin resistance in utero, Diabetes Care 32(6) (2009) 1076–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Vinter CA, Jensen DM, Ovesen P, Beck-Nielsen H, Jorgensen JS, The LiP (Lifestyle in Pregnancy) study: a randomized controlled trial of lifestyle intervention in 360 obese pregnant women, Diabetes Care 34(12) (2011) 2502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jain AP, Gavard JA, Rice JJ, Catanzaro RB, Artal R, Hopkins SA, The impact of interpregnancy weight change on birthweight in obese women, Am J Obstet Gynecol 208(3) (2013) 205.e1–7. [DOI] [PubMed] [Google Scholar]
- [32].Lassance L, Haghiac M, Leahy P, Basu S, Minium J, Zhou J, Reider M, Catalano PM, Hauguel-de Mouzon S, Identification of early transcriptome signatures in placenta exposed to insulin and obesity, Am J Obstet Gynecol 212(5) (2015) 647.e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lovelady CA, Garner KE, Moreno KL, Williams JP, The effect of weight loss in overweight, lactating women on the growth of their infants, N Engl J Med 342(7) (2000) 449–53. [DOI] [PubMed] [Google Scholar]
- [34].Colleran HL, Lovelady CA, Use of MyPyramid Menu Planner for Moms in a weight-loss intervention during lactation, J Acad Nutr Diet 112(4) (2012) 553–8. [DOI] [PubMed] [Google Scholar]
- [35].Stendell-Hollis NR, Thompson PA, West JL, Wertheim BC, Thomson CA, A comparison of Mediterranean-style and MyPyramid diets on weight loss and inflammatory biomarkers in postpartum breastfeeding women, J Womens Health (Larchmt) 22(1) (2013) 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].O’Toole ML, Sawicki MA, Artal R, Structured diet and physical activity prevent postpartum weight retention, J Womens Health (Larchmt) 12(10) (2003) 991–8. [DOI] [PubMed] [Google Scholar]
- [37].Nascimento SL, Pudwell J, Surita FG, Adamo KB, Smith GN, The effect of physical exercise strategies on weight loss in postpartum women: a systematic review and meta-analysis, Int J Obes (Lond) 38(5) (2014) 626–35. [DOI] [PubMed] [Google Scholar]
- [38].Rono K, Stach-Lempinen B, Klemetti MM, Kaaja RJ, Poyhonen-Alho M, Eriksson JG, Koivusalo SB, Prevention of gestational diabetes through lifestyle intervention: study design and methods of a Finnish randomized controlled multicenter trial (RADIEL), BMC Pregnancy Childbirth 14 (2014) 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Institute of M, National IOMPWG Research Council Committee to Reexamine, The National Academies Collection: Reports funded by National Institutes of Health, in: Rasmussen KM, Yaktine AL (Eds.), Weight Gain During Pregnancy: Reexamining the Guidelines, National Academies Press (US) National Academy of Sciences, Washington (DC), 2009. [PubMed] [Google Scholar]


