Abstract
Background:
In the STICH trial coronary artery bypass grafting (CABG) reduced all-cause death and hospitalization in a time-to-first-event analysis in patients with and ischemic cardiomyopathy and left ventricular ejection fraction ≤ 35%.
Objectives:
We investigated the impact of CABG on first and recurrent hospitalization in this population.
Methods:
A total of 1212 patients were randomized; 610 to CABG + optimal medical therapy (CABG) and 602 to optimal medical therapy alone (MED) alone and followed for a median 9.8 years. All-cause and cause-specific hospitalizations were analyzed as both time-to-first-event and recurrent event, while controlling for competing risk of death during the latter analysis.
Results:
Of the 1212 patients, 757 died (62/4%) and 732(60.4%) were hospitalized at least once for a total of 2549 total all-cause hospitalizations. Most hospitalizations (66.2%) were for cardiovascular causes of which approximately half (907, or 52.9%) were for heart failure. among 732 (60.4%) patients and 757 (62.4%) deaths during follow up. Of total hospitalizations (1817, or 71.3% the total) were recurrent events. The CABG group experienced fewer all-cause hospitalizations in both the time -to-first-event (349 CABG vs. 383 MED, adjusted HR 0.85, 95% CI 0.74- 0.98, p= 0.03) and recurrent event. analyses (1199 CABG vs. 1350 MED, HR=0.78, 95% CI 0.65 −0.94, p< 0.001). This was driven by fewer CV hospitalizations (744 vs. 968, p < 0.001, adjusted HR 0.66, 95% CI 0.55-0.81, p=0.001); of which the majority of these were for HF (395 vs. 512, p < 0.001, adjusted HR= 0.68, 95% CI 0.52-0.89, p=0.005). We did not observe a difference in non-CV events.
Conclusions:
Over 10 years follow-up, CABG reduced all-cause, CV and HF hospitalizations in both time-to-first-event and recurrent event analyses. With longer follow up, recurrent events become dominant, providing a clearer picture of the overall impact of CABG in this population.
Keywords: Heart failure, ischemic cardiomyopathy, coronary artery bypass grafting, hospitalization, morbidity
Condensed Abstract
We investigated the impact of coronary artery bypass grafting (CABG) on first and recurrent hospitalization in the STICH study.1212 patients were randomized, 610 to CABG + optimal medical therapy (CABG) and 602 to optimal medical therapy (MED) alone. Over a median 9.8 years follow up, 732 patients experienced 2549 total all-cause hospitalizations, of which most (1817 or 75.8% the total) were recurrent events. Fewer patients in the CABG group were hospitalized as compared with the MED group (349 vs. 383, adjusted HR 0.85, 95% CI 0.74- 0.98, p= 0.03). CABG also reduced total all- cause hospitalizations (850 CABG vs. 967 MED, HR=0.78, 95% CI 0.65 −0.94, p= 0.008), total CV hospitalizations (744 vs. 940, p < 0.001, adjusted HR 0.66, 95% CI 0.55-0.81, p=0.001) and HF hospitalizations (395 vs. 512, p < 0.001, adjusted HR= 0.68, 95% CI 0.52-0.89, p=0.005).
Introduction
Hospitalizations are a major cause of the poor health related quality of life experienced by patients with heart failure (HF) (1,2) and exert a large financial and logistical burden on health care systems around the world (3). In clinical trials the true burden of hospitalizations is not captured by conventional time-to-first event analyses, which do not count subsequent events, thus under-representing the total burden experienced by patients (4,5). This is also due in part to the shorter duration of study. Intermediate-term analyses (2-3 years) of several clinical trials show that recurrent hospitalizations account for up to 50% of the total hospitalizations (6-9). With long term follow-up, recurrent event analysis is more likely to fully capture the overall clinical impact of treatment.
Several examples exist where pharmacological treatments reduced recurrent hospitalizations (6-9), but not in a time-to-first-event analysis (4). More recently, percutaneous valvular procedures have recently reported hospitalization (10). Surgical interventions may actually increase adverse events in the short term with potential to decrease subsequent events over longer periods of follow-up (5). For example, the Surgical Treatment for Ischemic Heart Failure (STICH) trial demonstrated an early disadvantage of CABG until approximately 2 years follow up, after which the survival curves crossed. Only with long term follow did we observe an absolute 8% reduction (16% relative reduction) in total mortality. However, with longer, (11,12). Additionally, hospitalization in STICH was not reported independently of the composite outcomes with all-cause mortality. It is reasonable to expect that recurrent event analysis over a longer follow up period would be well suited for evaluation of surgical procedures. We report the effect of coronary artery bypass grafting (CABG) on recurrent and total hospitalization over a 10-year period in patients randomized in the STICH trial. We hypothesized that CABG would reduce time to first, recurrent, and total all-cause hospitalization in patients with chronic ischemic cardiomyopathy and left ventricular (LV) ejection fraction (EF) ≤ 35%.
Methods
Patient population
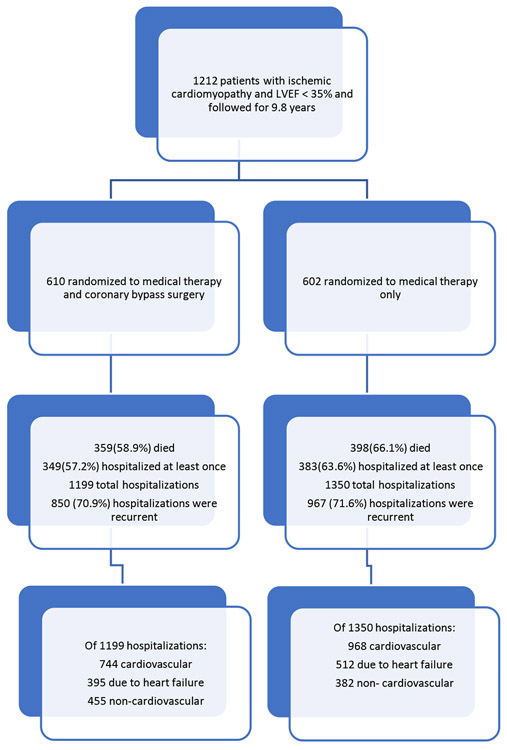
The design and enrollment characteristics of the STICH trial have previously been reported in detail (13). In brief, this study was a multicenter, non-blinded, randomized trial funded by the National Heart, Lung, and Blood Institute (NHLBI) and conducted in 22 countries at 99 sites and included 1212 study subjects followed for a median 9.8 (9.1, 11.0) years. Potentially suitable candidates for surgical myocardial revascularization with ischemic cardiomyopathy and LVEF) ≤ 35% were eligible for enrollment. Exclusion criteria included cardiogenic shock, recent myocardial infarction within 3 months and mitral stenosis or need for aortic valve surgery. All study subjects provided written informed consent and were randomized to receive optimal medical therapy alone (MED) or in combination with CABG (CABG).
Follow up and endpoint analysis
After enrollment, all subjects were followed every 4 months for the first year and every 6 months thereafter for 5 years in total. A study extension for outcome follow-up to a total of 10 years was granted, with overall follow-up of 1212 subjects to that point. Follow-up after 1-year post randomization was maintained via clinic visits or telephone contact by the enrolling investigator or delegate. All deaths were adjudicated by an independent clinical events committee according to the trial charter. As hospitalizations were not adjudicated, investigator-reported cause of hospitalization was used. The outcome of interest in this analysis was all-cause hospitalization, but we also included CV hospitalization, HF hospitalization (a component of CV hospitalization) and non-CV hospitalization as outcomes. For clarity, we refer to ‘first’ hospitalization as those that occurred in a time-to-first-event analysis, which began at randomization. Hospital admission for planned CABG as part of STICH was not considered as a hospitalization for this analysis. ‘Recurrent’ hospitalization was used to describe any hospitalization that occurred after the ‘first’ event. Total hospitalization included both ‘first’ and ‘recurrent’ hospitalization.
Statistical analysis
Baseline clinical characteristics were descriptively reported using mean (standard deviation), median (25%-75% percentiles) for continuous variables and by count and percentage for categorical variables. Group characteristics according to hospitalization were compared using ANOVA for continuous variables and Pearson’s chi square test for categorical variables. Kaplan-Meier curves for time-to-first all-cause hospitalization were constructed to as cumulative risk of hospitalization. Hospitalization rate per 100 person- years was constructed by dividing the total number of hospitalizations in each treatment group by the total number of follow-up years in that group multiplied by 100 (7). Relative risks for hospitalization were expressed as hazard ratios with associated confidence intervals and were derived from multivariable Cox proportional hazards models. To determine factors associated with repeat hospitalization, Andersen-Gill model with robust standard errors was used to account for correlated events within a patient (14). In addition, the cumulative incidence of hospitalization was calculated for each treatment group. In order to mitigate the effect of survivor bias, we used the Ghosh and Lin method (15) to estimate the cumulative incidence of hospitalization by treating death as a competing risk. A Forest plot was used to illustrate treatment differences among pre-specified subgroups. All analyses were performed based on the intention to treat principle only and nominal 0.05 significance level was used for statistical comparisons. Hazard ratios were adjusted according to pre-specified factors including age, gender, history of myocardial infarction, atrial fibrillation, peripheral vascular disease, systolic blood pressure, heart rate, ejection fraction, number of diseased vessels, serum creatinine, plasma hemoglobin, body mass index, end systolic volume index, moderate or severe mitral regurgitation, Canadian Cardiovascular Society angina grade, Duke index, and treatment with statin or ASA. All statistical calculations were performed using version SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
The baseline characteristics of the study population according to hospitalization status are shown in Table 1 (expanded list of characteristics can be found in Supplemental Table 1). In total, 1212 study subjects were followed for a median 9.8 (9.1, 11.0) years (Table 2) during which 757 (62.4%) patients died. A total of 25 patients (3%) were lost to follow-up; 19 subjects could not be contacted and 6 withdrew consent for follow-up.
Table 1.
Baseline characteristics according to number of all-cause hospitalizations post discharge
| Baseline Characteristics | All participants (N=1212) |
No all- cause hospitalizations (N=480) |
1 all- cause hospitalization (N=242) |
2 or more all- cause hospitalizations (N=490) |
P value |
|---|---|---|---|---|---|
| Age (years) | 59.7 (53.6, 67.2) | 57.4 (52.8, 65.0) | 60.6 (53.9, 67.9) | 61.1 (54.4, 68.8) | <.0001 |
| Male | 1064(87.8%) | 429(89.4%) | 213(88.0%) | 422(86.1%) | 0.1219 |
| Minority | 421(34.7%) | 252(52.5%) | 64(26.4%) | 105(21.4%) | <.0001 |
| BMI (kg/m2) | 26.8 (24.0, 29.8) | 26.1 (23.6, 29.4) | 26.7 (24.1, 30.0) | 27.5 (24.4, 30.2) | 0.0002 |
| Hypertension | 728(60.1%) | 286(59.6%) | 143(59.1%) | 299(61.0%) | 0.6467 |
| Hyperlipidemia | 730(60.3%) | 254(52.9%) | 147(60.7%) | 329(67.4%) | <.0001 |
| Diabetes | 478(39.4%) | 156(32.5%) | 93(38.4%) | 229(46.7%) | <.0001 |
| Previous stroke | 92(7.6%) | 31(6.5%) | 19(7.9%) | 42(8.6%) | 0.2146 |
| Peripheral vascular disease | 184(15.2%) | 68(14.2%) | 28(11.6%) | 88(18.0%) | 0.0983 |
| Chronic renal insufficiency | 94(7.8%) | 27(5.6%) | 14(5.8%) | 53(10.8%) | 0.0024 |
| Atrial fibrillation/flutter | 153(12.6%) | 57(11.9%) | 28(11.6%) | 68(13.9%) | 0.3467 |
| Previous PCI | 156(12.9%) | 37(7.7%) | 33(13.6%) | 86(17.6%) | <.0001 |
| Previous CABG | 36(3.0%) | 17(3.5%) | 5(2.1%) | 14(2.9%) | 0.5331 |
| Current NYHA | 0.6166 | ||||
| I | 139(11.5%) | 49(10.2%) | 28(11.6%) | 62(12.7%) | |
| II | 626(51.7%) | 250(52.1%) | 128(52.9%) | 248(50.6%) | |
| III | 412(34.0%) | 170(35.4%) | 76(31.4%) | 166(33.9%) | |
| IV | 35(2.9%) | 11(2.3%) | 10(4.1%) | 14(2.9%) | |
| Heart rate (bpm) | 74.0 (66.0, 82.0) | 76.0 (69.0, 84.0) | 72.0 (64.0, 80.0) | 72.0 (64.0, 80.0) | <.0001 |
| Systolic BP, (mmHg) | 120.0 (110.0, 130.0) | 120.0 (110.0, 130.0) | 120.0 (110.0, 130.0) | 120.0 (110.0, 130.0) | 0.0294 |
| Diastolic BP, (mmHg) | 78.0 (70.0, 80.0) | 80.0 (70.0, 80.0) | 76.5 (70.0, 80.0) | 75.0 (68.0, 80.0) | <.0001 |
| Plasma hemoglobin (g/dL) | 13.9 (12.7, 14.9) | 14.0 (12.9, 15.0) | 13.8 (12.6, 14.8) | 13.8 (12.4, 14.8) | 0.0082 |
| Baseline EF (%) | 28.0 (22.0, 34.0) | 28.0 (23.0, 34.0) | 28.0 (22.8, 34.0) | 27.5 (22.0, 33.3) | 0.5038 |
Minority- Any patient whose ethnicity is Hispanic or Latino, or whose race is non-white. Continuous variables expressed as median and 25% - 75% interquartile range; MED- Randomized to best medical therapy; CABG- Randomized to coronary artery bypass surgery plus best medical therapy; kg- kilogram; NHYA- New York Heart Association; bpm- beats per minute; BMI- body mass index; BP- blood pressure; PCI- percutaneous coronary intervention; CABG- coronary artery bypass surgery; ESLVI- end systolic left ventricular volume index; EDVI- end diastolic left ventricular volume index. LAD- Left anterior descending artery.
Table 2.
Number of patients hospitalized and number of hospitalizations in STICH
| Rehospitalization Characteristics | All patients (N=1212) |
Medical Treatment Only (N=602) |
CABG + Medical Treatment (N=610) |
|---|---|---|---|
| Duration of follow-up (years) | |||
| n | 1212 | 602 | 610 |
| Median (25th, 75th) | 6.7 (2.9, 9.3) | 6.2 (2.6, 9.1) | 7.3 (3.2, 9.4) |
| All-cause mortality | 757(62.5%) | 398(66.1%) | 359(58.9%) |
| Number of all-cause rehospitalizations | 2549 | 1350 | 1199 |
| 0 | 480(39.6%) | 219(36.4%) | 261(42.8%) |
| 1 | 242(20.0%) | 120(19.9%) | 122(20.0%) |
| 2 | 162(13.4%) | 79(13.1%) | 83(13.6%) |
| 3 or more all-cause hospitalizations | 328 (27.0%) | 184 (30.5) | 144 (23.6%) |
| Total number of patients with at least 1 all-cause rehospitalization | 732(60.4%) | 383(63.6%) | 349(57.2%) |
| Number of cardiovascular rehospitalizations | 1688 | 944 | 744 |
| 0 | 594(49.0%) | 260(43.2%) | 334(54.8%) |
| 1 | 240(19.8%) | 126(20.9%) | 114(18.7%) |
| 2 | 143(11.8%) | 83(13.8%) | 60(9.8%) |
| 3 or more cardiovascular hospitalizations | 235 (19.4%) | 133 (22.1%) | 102 (16.7%) |
| Total number of patients with ≥1 cardiovascular rehospitalization | 618(51.0%) | 342(56.8%) | 276(45.2%) |
| Number of heart failure rehospitalizations | 907 | 512 | 395 |
| 0 | 855(70.5%) | 401(66.6%) | 454(74.4%) |
| 1 | 118(9.7%) | 67(11.1%) | 51(8.4%) |
| 2 | 119(9.8%) | 68(11.3%) | 51(8.4%) |
| 3 or more heart failure hospitalizations | 120 (10.0%) | 66 (10.9%) | 54 (8.9%) |
| Total number of patients with ≥1 heart failure rehospitalization | 357(29.5%) | 201(33.4%) | 156(25.6%) |
| Number of non-cardiovascular rehospitalizations | 871 | 410 | 461 |
| 0 | 847(69.9%) | 430(71.4%) | 417(68.4%) |
| 1 | 63(5.2%) | 23(3.8%) | 40(6.6%) |
| 2 | 69(5.7%) | 27(4.5%) | 42(6.9%) |
| 3 or more non-cardiovascular hospitalizations | 233 (19.2%) | 122 (20.2%) | 111 (18.1%) |
| Total number of patients with at least 1 non-cardiovascular rehospitalization | 365(30.1%) | 172(28.6%) | 193(31.6%) |
Time to first event analysis
There were 732, ‘first’ hospitalizations or 28.7% of total hospitalizations; 618 (84.4%) of these were for a CV reason and 357 (48.7% of total hospitalizations) were due to HF (Tables 2 and 3). In the time-to-first event analysis, CABG reduced all-cause hospitalizations (349 vs. 383 patients, adjusted HR 0.85, 95% CI 0.74- 0.98, p= 0.03) (Table 3), CV hospitalizations (276 CABG vs. 342 MED, adjusted HR 0.71, 95%CI 0.60- 0.83, p< 0.001) and HF hospitalizations (156 CABG vs. 201 MED, adjusted HR 0.71, 95% CI0.57, 0.89, p= 0.003). We did not observe a difference in non-CV hospitalizations for CABG vs. MED alone (193 vs. 172, adjusted HR 1.18 (0.95, 1.47, p=0.27).
Table 3 –
Impact of coronary artery bypass grafting on risk of first, recurrent and total hospitalization.
| Outcome | CABG | MED | Adjusted Hazard Ratio |
95% Confidence Interval |
p value |
|---|---|---|---|---|---|
| Time to first event analysis | |||||
| All-cause hospitalization (n= 732) | 349(57.2%) | 383(63.6%) | 0.85 | 0.74 – 0.98 | 0.03 |
| Cardiovascular hospitalization (n= 618) | 276(45.2%) | 342(56.8%) | 0.71 | 0.60 – 0.83 | 0.001 |
| Heart failure hospitalization (n= 357) | 156(25.6%) | 201(33.4%) | 0.71 | 0.57 – 0.89 | 0.003 |
| Non-cardiovascular hospitalization (n= 365) | 193(31.6%) | 172(28.6%) | 1.18 | 0.95 – 1.47 | 0.27 |
| Total hospitalization analysis. | |||||
| All-cause hospitalization (n= 2549) | 1199 | 1350 | 0.78 | 0.65 – 0.94 | < 0.001 |
| Cardiovascular hospitalization (n= 1712) | 744 | 968 | 0.66 | 0.55 – 0.81 | 0.001 |
| Heart failure hospitalization (n= 907) | 395 | 512 | 0.68 | 0.52 – 0.89 | 0.005 |
| Non-cardiovascular hospitalization (n= 837) | 455 | 382 | 1.05 | 0.78 – 1.40 | 0.76 |
CABG- coronary artery bypass surgery; HF – heart failure; MED- optimal medical therapy
Recurrent and total hospitalization
Overall, there were 2549 all-cause hospitalizations and most of these 1817 (71.3%) were recurrent (i.e. not first events during follow-up) (Table 3). The number of recurrent all-cause hospitalizations in individual patients ranged from 1-28 (Figure 1). More than 27% of patients experienced 3 or more hospitalizations; 51 patients experienced ≥10 hospitalizations (654 occurrences, 26% of events).
Figure 1:
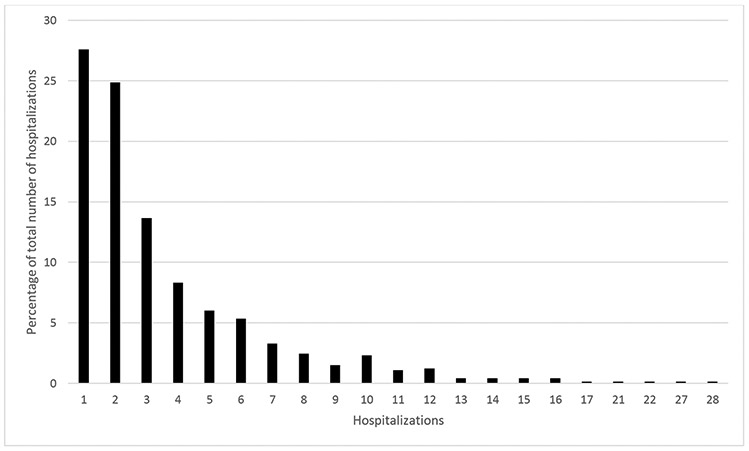
Frequency distribution of all-cause hospitalizations during follow- p.
Impact of CABG upon recurrent and total hospitalization
There were fewer total all-cause hospitalizations in the CABG group (1199[57.2%] vs. 1350 [63.6%], p < 0.001, adjusted HR=0.78, 95% CI 0.65 −0.94, p= 0.008) (Table 3, Figure 2). This was driven by fewer total CV hospitalizations (744 [45.2%] vs. 968 [56/8%], p < 0.001, adjusted HR 0.66, 95% CI 0.55-0.81, p=0.001), and its subset of total hospitalizations for HF (395 [25/6%] vs. 512 [33.4%], p < 0.001, adjusted HR= 0.68 95%, CI 0.52-0.89, p=0.005) (Table 3). There was no difference in non-HF CV hospitalizations. The CABG group experienced 117 fewer recurrent all- cause admissions and 158 fewer recurrent CV admissions, of which number 72 fewer occurred for HF. Differences in hospitalization rates were consistent using both the Cumulative Incidence and Ghosh and Lin methods, which accounts for the competing risk of all-cause death. All-cause hospitalization curves began to diverge within 30 days of randomization (Figures 3 and 4) and translated into a 6% lower annual cumulative rate of hospitalization at 10 years follow-up (39% vs. 45%, p< 0.001). A very small proportion of hospitalizations (100 or 4.0%) were due to myocardial infarction while 70 (2.7%) were due to stroke. No difference in either outcome was observed according to randomized therapy.
Figure 2:
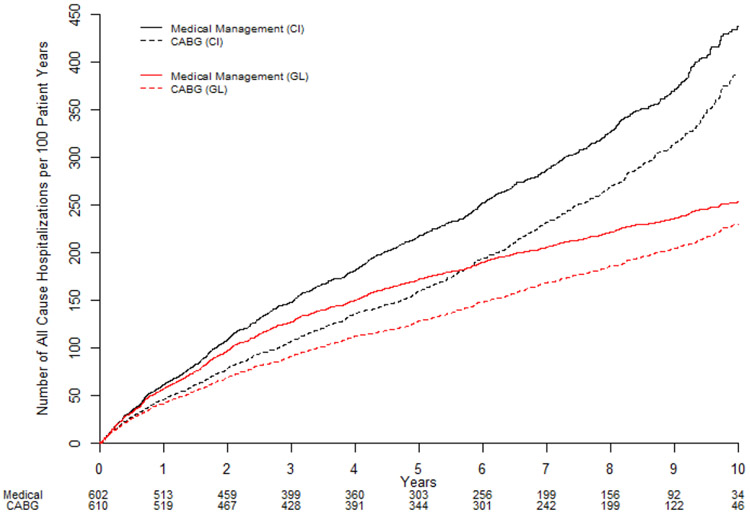
All-cause hospitalization expressed as estimated rate per 100 patients as: Cumulative incidence (black lines) and Ghosh and Lin estimates accounting for the competing risk of all-cause death (red lines). CABG = coronary artery bypass surgery plus optimal medical therapy; MED = optimal medical therapy alone.
Figure 3:
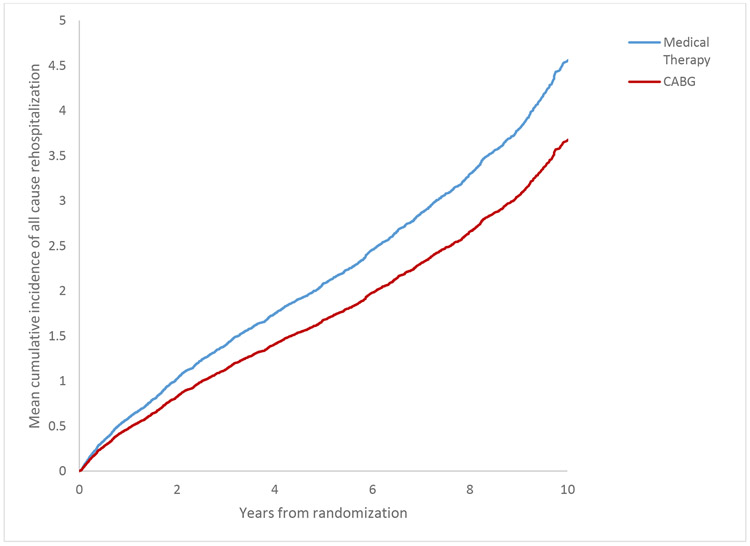
Mean cumulative incidence of all-cause hospitalization during the entire 9.8 year follow up period. CABG = coronary artery bypass surgery plus optimal medical therapy; MED = optimal medical therapy alone.
Figure 4:
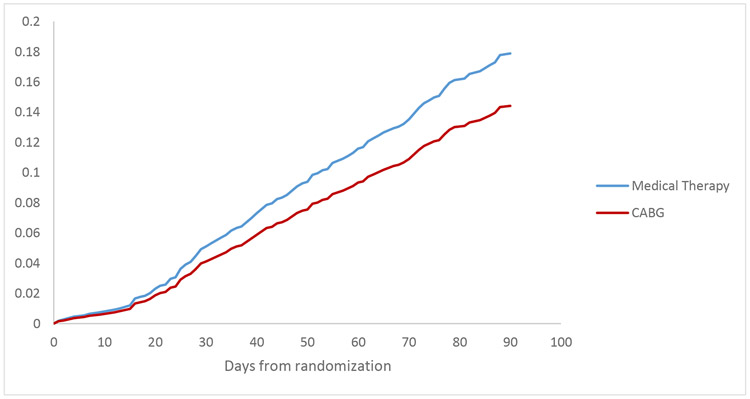
Mean cumulative incidence of all-cause hospitalization during the first 90 days post randomization. CABG = coronary artery bypass surgery plus optimal medical therapy; MED = optimal medical therapy alone.
Baseline characteristics and hospitalization
The mean age of patients was higher in those who experienced 1 or more hospitalization for any cause (Table 1). The prevalence of comorbidities was higher in those who had 1 or more hospitalizations but the proportion that had CCS class II or more angina was lower. Baseline NYHA class and LVEF were similar in the groups with no hospitalization, 1 hospitalization and those with 2 or more hospitalizations. Similar patterns were observed irrespective of hospitalization for CV causes, HF or non-CV causes (Supplemental tables 2 and 3).
Discussion
In this report of hospitalization from the STICH trial over a 10-year follow-up, several important findings can be stated. To our knowledge, this is the first report of a beneficial effect of CABG on first, and recurrent hospitalization for patients with ischemic cardiomyopathy- an effect that increased as follow up duration lengthened. The effect is seen primarily via a reduction in CV hospitalization (predominantly its subset of HF hospitalization) rather than prevention of nonfatal myocardial infarction or stroke. With long term follow up we observe recurrent hospitalization to be responsible for over 70% of hospitalization events.
HF and hospitalization: A lifelong risk
Most observational HF studies suggest hospitalization is responsible for ≥70% of the total disease-related cost of care (4,6,8). Repeated hospitalization is associated with shorter life expectancy, and worse quality of life and symptoms, even in patients initially presenting with mild symptoms (9,16). Previously published clinical trial reports of recurrent hospitalization in patients with HFrEF, involving medication or disease management interventions, have examined events over a shorter time periods (i.e. 1-4 years) and have indicated that recurrent hospitalization accounts for approximately 30- 50% of all events(6,7,17). Cardiac resynchronization therapy, eplerenone, and sacubitril/valsartan confer a reduction in recurrent hospitalization in addition to the demonstrated benefits using time-to-first-event analysis (6,10,17-19). Due to the short follow-up in these studies (2-3 years), the lifelong impact of these therapies on hospitalization is not known. Long-term analyses of medical interventions have been reported, such as with the Study of Left Ventricular Dysfunction (SOLVD), although only mortality was collected and reported (20). In our study, the median duration of follow-up of 9.8 years was much longer than in other trials of medical or device therapy in HF, with an overall mortality (62.4%) nearly two-thirds of the study population (4,6,8). Consequently, we have been able to describe the burden of hospitalizations. We found that nearly 75%of hospitalizations were recurrent, and that more than 3 hospitalizations (2549) recorded for each death (757). These findings underscore the notion that long-term follow-up allows for a more complete understanding of the health implications of effective HF interventions.
Early and sustained benefit of CABG
Several published studies have shown an early increase in risk of mortality with CABG. Not surprisingly, we observed an initial increase in mortality (5.1%) following CABG (21). Consequently, the survival curves did not cross until 1 year following randomization (11). However, hospitalization did not follow this pattern, instead demonstrating divergence of event rates throughout the duration of the study. The absence of early increased hospital admission in the CABG arm might be explained by the fact that following randomization the surgical patients were not exposed to rehospitalization during the phase of in hospital post-surgical care. The reduction in total hospitalizations over the entire time horizon of the STICH trial remained even when hospitalizations during the first month or first year following randomization were excluded. We also observed fewer recurrent admissions in the CABG group vs. MED alone (800 vs. 967) indicating an increasing benefit of CABG with longer follow-up. Non-CV hospitalizations (either time-to-first event or recurrent event analyses) were reassuringly not increased by CABG. Unlike iterative interventions such as medications and disease management programs, costs associated with CABG mostly occur upfront (22). Thus, similar to other one-time interventions such as implantable cardioverter defibrillator therapy, the cost benefit of effective CABG will be expected improve over time (23).
The value proposition of CABG for ischemic cardiomyopathy must be considered in context of both short- and long-term outcomes. In addition to the early increase in mortality, patients in the STICH trial who underwent CABG experienced a 23% likelihood of significant complications such as acute kidney injury, ventricular arrhythmias and cardiac arrest (21). Long-term results of the STICH trial offer critical data to inform the decision to undertake CABG for suitable patients.
Reduction of CV hospitalization and mechanism of benefit
In STICH, CABG led to fewer CV hospitalizations, nearly half of which were due to HF. This finding is consistent with other trials, such as EMPHASIS HF, in which the reduction in CV hospitalization was driven by fewer HF hospitalizations (8). It is noteworthy that reduction of mortality in this study included both sudden death (HR: 0.73; 95% CI: 0.54 to 0.99; p = 0.041) and fatal pump failure events (HR: 0.64; 95% CI: 0.41 to 1.00; p = 0.05) (24). The lack of reduction in hospitalization due to myocardial infarction indicates that CABG may confer an effect beyond reduction in myocardial infarction, although we did previously report a reduction in fatal myocardial infarction (HR: 0.07; 95% CI: 0.01 to 0.57) (24). In addition, reduction of subclinical ischemia or other non-HF-specific CV events (83 fewer events), such as acute coronary syndrome or other conditions, is possible. Low levels of circulating troponin, thought to represent cardiomyocyte injury, are associated with increased risk in patients with chronic HF. Recent studies have shown that interventions which improve outcomes also associate with lower levels of circulating troponin(25,26). A reasonable extension of this concept, that restoration of coronary blood flow via revascularization may improve the local myocyte supply/demand ratio and lead to reduced circulating troponins. While unproven, this remains a plausible explanation for the mechanism of CABG benefit in ischemic cardiomyopathy and warrants further investigation.
While CABG did not reduce early post-operative LV volumes or increase EF, it is possible that CABG prevented late LV remodeling or fibrosis and thus, disease progression. Unfortunately, we do not have long-term information for LV remodeling, or measures of congestion (natriuretic peptides) or myocardial damage (high sensitivity troponin) to support these hypotheses. Improved coronary flow could produce many beneficial myocardial effects that lead to a reduction on CV and HF hospitalization. Salutary benefits of CABG have also been reported in the STICH trial, all of which are associated with reduction of hospitalization and add to validity of the results. For example, published sub-analyses of the STICH trial have shown a modest improvement in quality of life as scored by the Kansas City Cardiomyopathy Questionnaire (KCCQ). This effect size is similar to that observed with exercise training as reported in the HF-ACTION trial. It is noteworthy that there were several shared baseline characteristics associated with risk of both all-cause and CV hospitalization. These factors (i.e. serum creatinine, LV volume, atrial fibrillation, anemia) are known to associate with risk of CV and HF hospitalization in HF populations. Unlike cardiac resynchronization therapy, there was no effect of CABG on non-CV outcomes (27). Our data are consistent with those from trials of other efficacious HF therapies (ARB, eplerenone, sacubitril/valsartan) where the major clinical benefit is both in mortality and HF hospitalization.
Limitations
Several limitations in this study deserve consideration. The STICH study enrolled a highly selected patient population with ischemic cardiomyopathy considered acceptable for CABG. Treatment allocation was not blinded in this study, which may have led to ascertainment bias, or difference in clinician decisions based upon whether surgery had been performed. However, it is not known whether this bias might have led to increased or decreased likelihood of a clinical decision to admit to hospital. As previously mentioned, study subjects who underwent CABG while still in hospital (thus prolonging stay) may have experienced less exposure to the risk of recurrent hospitalization. It is important to note that crossover CABG events were counted as hospitalizations. The lack of adjudication for cause-specific hospitalization may have introduced increased error in categorization of hospitalization (but not categorization of recurrent hospitalization). We attempted to account for the competing risk of death, and, although residual confounding may have occurred, the risk of death was lower in the CABG arm and so would not overestimate the impact of CABG on hospitalization. However, Mogensen, et al. recently reported that the reduction of total and recurrent hospitalization observed with sacubitril/valsartan therapy was nearly identical irrespective of the statistical method employed (17). Similarly, apart from study visits, usual care, such as setting of care or intensity/quality of medical therapy may have differed between the two groups and impacted rate of hospitalization. Finally, study subjects were followed by either telephone or visit after 1 year post randomization. As such, the ascertainment of hospitalization may have been less accurate.
Conclusion
In conclusion, the STICH trial showed that CABG reduces all-cause, CV and HF hospitalizations in patients with ischemic cardiomyopathy and reduced LVEF in both time-to-first-event and recurrent event analysis (and more so for recurrent events). This is the first report of reduction in long-term risk of hospitalization for a surgical CV procedure in patients with HF.
Supplementary Material
Central Illustration:
Top panel: Patient disposition over the follow-up period of the Surgical Treatment for Ischemic Heart Failure (STICH) trial. LVEF= left ventricular ejection fraction. Bottom panel: Mean cumulative incidence of all-cause hospitalization during the entire 9.8 year follow up period. CABG = coronary artery bypass surgery plus optimal medical therapy; MED = optimal medical therapy alone.
Perspectives.
Clinical Competency in Medical Knowledge:
CABG reduces initial, recurrent and total hospitalization for patients with ischemic cardiomyopathy and LVEF less than 35%. The reduction is due to lower CV (and its subset of HF hospitalization) hospitalization, occurs early and increases over a 10-year time horizon.
Clinical Competency in Patient Care:
Patients with chronic ischemic cardiomyopathy and LVEF ≤ 35% who are acceptable candidates for CABG should be counseled that in addition to previously known risks and benefits, CABG may further reduce long-term hospitalizations, specifically those for CV reasons.
Translational Outlook 1:
Long-term follow up of clinical trials, such as the STICH trial, provides a more complete understanding of the clinical benefits of CABG, specifically the importance of recurrent events, which increasingly outnumber first events as follow-up lengthens.
Translational Outlook 2:
The inclusion of recurrent events in a clinical trial may help to better model the later economic implications of efficacious therapies.
Acknowledgments
Sources of Funding: This work was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute grants U01 HL-69015, U01 HL-69013, and R01 HL-105853. This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or National Institutes of Health.
Abbreviations:
- CABG
coronary artery bypass grafting
- CV
cardiovascular
- EF
ejection fraction
- HF
heart failure
- HFrEF
heart failure with reduced ejection fraction
- LV
left ventricular
- MED
optimal medical therapy
- NHLBI
National Heart, Lung, and Blood Institute
- STICH
Surgical Treatment for Ischemic Heart Failure
Footnotes
Clinical Trial Registration: Clinicaltrials.gov; Identifier: NCT00023595
Relationship with Industry and Other Entities: Dr. Anderson reports consultancy agreements with Novartis, OrionPharma, and Servier. Dr. Velazquez reports research grants (significant) with Novartis, Amgen, NHLBI, Pfizer and Alnylam and consultant/advisory board agreements (modest) with Novartis, Amgen, and Philips. There are no other disclosures to report.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chun S, Tu JV, Wijeysundera HC et al. Lifetime analysis of hospitalizations and survival of patients newly admitted with heart failure. Circ Heart Fail 2012;5:414–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tran DT, Ohinmaa A, Thanh NX et al. The current and future financial burden of hospital admissions for heart failure in Canada: a cost analysis. CMAJ Open 2016;4:E365–E370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen L, Jhund PS, Mogensen UM et al. Re-Examination of the BEST Trial Using Composite Outcomes, Including Emergency Department Visits. JACC Heart Fail 2017;5:591–599. [DOI] [PubMed] [Google Scholar]
- 5.Skali H, Pfeffer MA, Lubsen J, Solomon SD. Variable impact of combining fatal and nonfatal end points in heart failure trials. Circulation 2006;114:2298–303. [DOI] [PubMed] [Google Scholar]
- 6.Goldenberg I, Hall WJ, Beck CA et al. Reduction of the risk of recurring heart failure events with cardiac resynchronization therapy: MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy). J Am Coll Cardiol 2011;58:729–37. [DOI] [PubMed] [Google Scholar]
- 7.Rogers JK, Jhund PS, Perez AC et al. Effect of rosuvastatin on repeat heart failure hospitalizations: the CORONA Trial (Controlled Rosuvastatin Multinational Trial in Heart Failure). JACC Heart Fail 2014;2:289–97. [DOI] [PubMed] [Google Scholar]
- 8.Rogers JK, McMurray JJ, Pocock SJ et al. Eplerenone in patients with systolic heart failure and mild symptoms: analysis of repeat hospitalizations. Circulation 2012;126:2317–23. [DOI] [PubMed] [Google Scholar]
- 9.Solomon SD, Dobson J, Pocock S et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation 2007;116:1482–7. [DOI] [PubMed] [Google Scholar]
- 10.Stone GW, Lindenfeld J, Abraham WT et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N Engl J Med 2018. [DOI] [PubMed] [Google Scholar]
- 11.Velazquez EJ, Lee KL, Deja MA et al. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med 2011;364:1607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Velazquez EJ, Lee KL, Jones RH et al. Coronary-Artery Bypass Surgery in Patients with Ischemic Cardiomyopathy. N Engl J Med 2016;374:1511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Velazquez EJ, Lee KL, O'Connor CM et al. The rationale and design of the Surgical Treatment for Ischemic Heart Failure (STICH) trial. J Thorac Cardiovasc Surg 2007;134:1540–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin DY, Wei LJ, Ying Z. Model-checking techniques based on cumulative residuals. Biometrics 2002;58:1–12. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh D, Lin DY. Nonparametric analysis of recurrent events and death. Biometrics 2000;56:554–62. [DOI] [PubMed] [Google Scholar]
- 16.Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J 2007;154:260–6. [DOI] [PubMed] [Google Scholar]
- 17.Mogensen UM, Gong J, Jhund PS et al. Effect of sacubitril/valsartan on recurrent events in the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail 2018;20:760–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anand IS, Carson P, Galle E et al. Cardiac resynchronization therapy reduces the risk of hospitalizations in patients with advanced heart failure: results from the Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure (COMPANION) trial. Circulation 2009;119:969–77. [DOI] [PubMed] [Google Scholar]
- 19.Solomon SD, Wang D, Finn P et al. Effect of candesartan on cause-specific mortality in heart failure patients: the Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation 2004;110:2180–3. [DOI] [PubMed] [Google Scholar]
- 20.Jong P, Yusuf S, Rousseau MF, Ahn SA, Bangdiwala SI. Effect of enalapril on 12-year survival and life expectancy in patients with left ventricular systolic dysfunction: a follow-up study. Lancet 2003;361:1843–8. [DOI] [PubMed] [Google Scholar]
- 21.Wrobel K, Stevens SR, Jones RH et al. Influence of Baseline Characteristics, Operative Conduct, and Postoperative Course on 30-Day Outcomes of Coronary Artery Bypass Grafting Among Patients With Left Ventricular Dysfunction: Results From the Surgical Treatment for Ischemic Heart Failure (STICH) Trial. Circulation 2015;132:720–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Violette PD, Filion KB, Haider S, Pilote L, Eisenberg MJ. A cost analysis of nonelective coronary artery bypass graft surgery. J Card Surg 2006;21:621–7. [DOI] [PubMed] [Google Scholar]
- 23.Deniz HB, Ward A, Jaime Caro J, Alvarez P, Sadri H. Cost-benefit analysis of primary prevention of sudden cardiac death with an implantable cardioverter defibrillator versus amiodarone in Canada. Curr Med Res Opin 2009;25:617–26. [DOI] [PubMed] [Google Scholar]
- 24.Carson P, Wertheimer J, Miller A et al. The STICH trial (Surgical Treatment for Ischemic Heart Failure): mode-of-death results. JACC Heart Fail 2013;1:400–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Januzzi JL Jr., Butler J, Jarolim P et al. Effects of Canagliflozin on Cardiovascular Biomarkers in Older Adults With Type 2 Diabetes. J Am Coll Cardiol 2017;70:704–712. [DOI] [PubMed] [Google Scholar]
- 26.McMurray JJ, Packer M, Solomon SD. Neprilysin inhibition for heart failure. N Engl J Med 2014;371:2336–7. [DOI] [PubMed] [Google Scholar]
- 27.Tang AS, Wells GA, Talajic M et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med 2010;363:2385–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.