Abstract
Infection by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is responsible for the second pandemic of the XXI century after influenza A in 2009. As of mid-June 2020, more than 4,40,000 fatal cases of SARS-CoV-2-related disease (COVID-19) have occurred worldwide. Besides its prominent expression at the level of the respiratory apparatus, COVID-19 is also characterized by a substantial degree of cardiovascular involvement, both in terms of deterioration of pre-existing conditions, and as the effect of inflammation-facilitated acute events. They include ischemic/inflammatory heart disease, ventricular arrhythmias, conduction disturbances, thrombotic events at the level of the lungs, and systemic activation of the coagulation cascade, configuring the scenario of disseminated intravascular coagulation. Herein, we summarize the main COVID-19 features of relevance for the clinicians in the cardiovascular field. The rationale, concerns, and possible side effects of specific therapeutic measures, including anticoagulants, renin-angiotensin-aldosterone system inhibitors, and anti-inflammatory/antiviral medications applied to the treatment of COVID-19 are also discussed.
Keywords: COVID-19, Cardiovascular diseases, Renin-angiotensin-aldosterone system inhibitors, Coagulation, Electrocardiography
Background
Rhinolophus hipposideros (commonly known as “horseshoe bats”) are habitual hosts of coronaviruses. The Rhinolophus affinis, in particular, is very common in the wide Chinese province of Yunnan [1], and it is believed to represent the natural reservoir host for the progenitor of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [2] that is responsible for the second pandemic of the XXI century after that of influenza A (H1N1) in 2009 [3]. Anthropization of wild territories, as well as low food safety standards, are the most likely conditions that allowed the spillover of the wild virus to humans in Wuhan, the capital city of the Hubei province [4]. In fact, the large wholesale market of the city is considered the plausible scenario for animal-to-human transmission of the virus [5]. Whatever the exact contingency of human infection, the outbreak of SARS-CoV-2-related disease (defined as COVID-19) has quickly reached the proportion of a pandemic, an effect facilitated by viral contagiousness, late information sharing, inadequate preparedness for a new disease, and jeopardized readiness to face the health emergency worldwide [6]. More than 8,000,000 cases, including over 430,000 deaths, have occurred as of mid-June 2020 worldwide [7]. Patients with COVID-19 and either cardiovascular risk factors or established cardiovascular disease represent a particularly vulnerable population [8].
Search methodology
For the purpose of this narrative mini-review, a comprehensive search of major electronic databases (PubMed, EMBASE) was conducted for articles in the English language published in medical journals from inception through June 2020. The search used the terms “COVID-19”, “SARS-CoV-2”, “cardiovascular disease(s)”, “hypertension”, “ECG”, “arrhythmia”, “QT interval”, “embolism”, “thrombosis”, “coagulation”, and “renin-angiotensin-aldosterone system inhibitors” in several combinations. The search results were accessed, and relevant references were made for the purpose of this review.
Cardiovascular disease and COVID-19
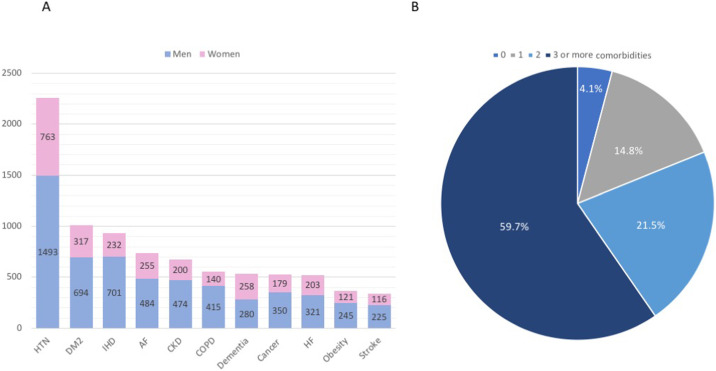
Fatal COVID-19 cases are significantly more common among elderly individuals and in the presence of comorbidities [9]. Of 3335 Italian patients dying in-hospital as of June 4th and for whom it was possible to analyze clinic charts, only 136 deceased patients (4.1%) had no pre-existing pathologies, while 493 (14.8%), 716 (21.5%), and 1990 (59.7%) reported having one, two, or at least three chronic diseases, respectively, with hypertension being the most frequently reported comorbidity, followed by diabetes and ischemic heart disease (Fig. 1 A and B) [9], [10]. Although mortality was much higher in men, the mean number of pre-existing diseases was similar between genders (median ± standard deviation 3 ± 1.9) [10]. In keeping with these data, an analysis of 44,672 confirmed COVID-19 cases from Wuhan, China indicated increased case-fatality rates in the presence of cardiovascular diseases (10.5%) and hypertension (6.0%) (overall case-fatality rate: 2.3%) [11]. In a retrospective case series from Italy, hypertension was the most common comorbidity among COVID-19 patients referred to the Intensive Care Unit (ICU) irrespective of age, with a global prevalence of 49%, followed by cardiovascular diseases (21%) and hypercholesterolemia (18%) [12]. Also, the prevalence of hypertension was higher among critically ill patients who died in the ICU compared with individuals discharged from the ICU (+23%, p < 0.001) [12].
Fig. 1.
Chronic comorbidities among 3335 deceased COVID-19 patients in Italy as of June 4th, 2020. (A) Comorbidities by gender. (B) Proportion of patients with 0, 1, 2, or at least 3 comorbidities: 4.1%, 14.8%, 21.5%, and 59.7%, respectively. HTN, hypertension; DM2, diabetes mellitus type 2; IHD, ischemic heart disease; AF, atrial fibrillation; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; HF, heart failure.
As the most common pre-existing conditions, cardiovascular diseases might have potentially exacerbated the severity of COVID-19. COVID-19, in fact, is mostly a lung disease, where initial local damage can be followed by an intense and relatively late cytokine storm originating from imbalance of T cells activation with dysregulated release of interleukin (IL)-6, IL-17, and other cytokines (Fig. 2 ) [13]. In parallel, COVID-19 can also begin with signs of a severe coronary artery disease or myocarditis in the absence of a history of cardiovascular diseases, or in the presence of isolated cardiovascular risk factors [14], [15]. Myocardial damage can be caused by direct myocardial injury related to upregulation of angiotensin-converting enzyme 2 (ACE2) in the heart and coronary vessels, but it can be also secondary to molecular mimicry following the activation of adaptive auto-immune type mechanisms, or be exacerbated by hypoxia in the context of respiratory failure [8]. Severe respiratory distress can be the expression of both pulmonary and cardiogenic edema, independent of pre-existing cardiovascular disease, a possibility that conditionates the choice of specific supportive therapies and the clinical outcome [16]. In patients with established cardiovascular diseases, COVID-19 contributes to precipitate hardly balanced conditions, as well as those previously well controlled by therapy [15]. A possible explanation is the immune dysregulation occurring in the course of the viral infection, similarly to what was previously described for other pathogens [17], but the role of pre-existing age-related immunologic dysfunction should not be discarded [18], [53]. The destabilizing effect of pulmonary hypertension on chronic heart failure, as well as the impact of COVID-19 emergency on the clinical course, including the fast triage, of patients with pre-existing cardiovascular conditions have been also pointed out [16]. Therefore, a reciprocal influence is evident between COVID-19 and cardiovascular diseases, with pre-existing pathologies exacerbating the infectious disease, and SARS-CoV-2 favoring or inducing acute cardiovascular events. The possible interference of antiviral drugs with statins, the risk of cardiotoxicity induced by anti-inflammatory agents such as hydroxychloroquine and chloroquine, and the effects of corticosteroids, such as methylprednisolone, on water retention and ionic imbalance deserve appropriate consideration [16]. The long-term effects of COVID-19-related inflammatory burst on cardiovascular health are unknown, but appropriate monitoring has been suggested [8].
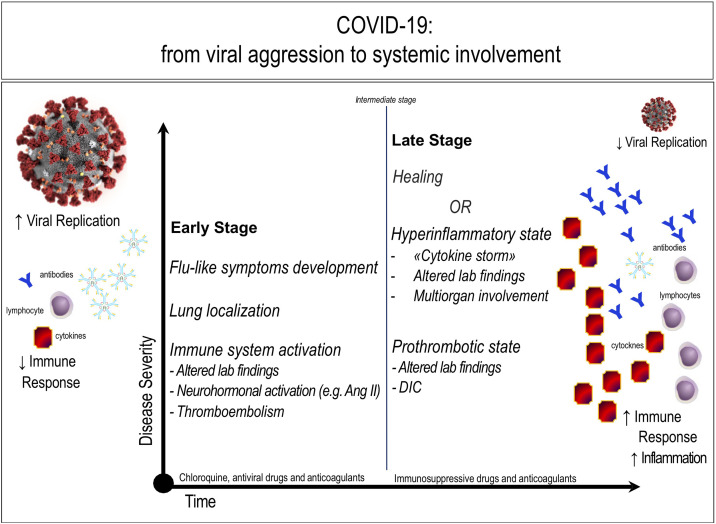
Fig. 2.
COVID-19: from viral aggression to systemic involvement. In the early phase, COVID-19 affects the sites of replication, primarily represented by the lungs: the immune system begins to generate antibodies and cytokines, while specific virus-dependent mechanisms limit the lymphocytic response. In a second phase, healing or worsening of the disease can occur. Specifically, a cytokine syndrome (“cytokine storm”) can develop, leading to a marked increase in the intensity of local and systemic inflammation, leading to multiorgan insufficiency. Hypercoagulation is a feature of both the early and the late stage of the disease, potentially leading to thrombosis and/or disseminated intravascular coagulation (DIC).
The increase in the levels of angiotensin II (Ang II), caused by the binding of SARS-CoV-2 with ACE2, is present since the early stage and participates in the induction of damage. The figure is original from the author (CF).
Hypercoagulability in COVID-19
Consistent data from case series indicate that massive, inappropriate activation of the coagulation cascade can occur in COVID-19 patients. For instance, among 94 patients with documented viral infection, circulating antithrombin III was significantly reduced, while D-dimer and fibrinogen were elevated, compared with 40 healthy volunteers [19]. Further, the first-month mortality rate was double among 449 patients with COVID-19 compared with 104 individuals with non-COVID pneumonia (29.8% versus 15.4%, respectively; p = 0.003), and COVID-19 patients had significantly higher platelet count than non-COVID-19 individuals (215 ± 100 × 109/L versus 188 ± 98 × 109/L, p = 0.015), indicating more intense inflammation in the former [19]. The use of heparin seemed to improve their prognosis, especially in COVID-19 patients with circulating D-dimer >3.0 μg/mL (first-month mortality rate: 32.8% versus 52.4%, p = 0.017) [20]. Among 183 patients consecutively hospitalized for COVID-19, whose mortality rate was strongly associated with higher circulating D-dimer and fibrin degradation products (FDP) (p < 0.05), 71.4% of deceased patients versus 0.6% of survivors (i.e. a single patient) met the diagnostic criteria for disseminated intravascular coagulation (DIC) according to the International Society of Thrombosis and Haemostasis [21]. Time from hospitalization to the onset of DIC was very short (median: 4 days, range: 1–12 days) [21]. The same authors demonstrated that low molecular weight heparin (or other anticoagulant) administered for at least one week improved the prognosis in 449 patients with severe COVID-19 and a sepsis induced coagulopathy (SIC) score ≥4 (mortality rate 40.0% versus 64.2%, p = 0.029), or with circulating D-dimer at least 6-fold higher than the normal value (mortality rate 32.8% versus 52.4%, p = 0.017) [22]. Of note, clinical deterioration with hemodynamic instability might occur in critically ill COVID-19 patients as the expression of localized thrombotic disease of the lung, as well as thromboembolic disease originating from lower limbs.
Taken together, a paramount cardiovascular impairment occurs during COVID-19, both in terms of deterioration of a pre-existing condition during viral infection and as a clinical sign of disease expression. In addition, COVID-19 determines a significant activation of the coagulation cascade, mainly expressed as thrombotic events in the lungs, in the absence of predisposing risk factors and/or history of lower limb thrombophlebitis [15]. Coagulation activation and endothelial dysfunction could be the expression of sustained inflammatory response associated with vascular inflammation. In this context, the postulated pleiotropic properties of heparin and its derivatives, including their anti-inflammatory effect, represent an additional rationale for their use in severe COVID-19 [23]. Low molecular weight heparin or fondaparinux prescription has been recommended in hospitalized COVID-19 patients [24]. A similar recommendation was made by the World Health Organization to prevent incident thrombophlebitis in these patients [25]. However, it must be noted that the site of thrombosis during COVID-19 is atypical, as it occurs rather in the lungs than in lower limbs, even in the absence of specific risk factors and/or history of thromboembolism [26]. Therefore, given the elevated risk of localized thrombotic events and/or DIC, coagulation parameters should be carefully monitored in COVID-19 patients, particularly in those with severe disease. The impact of antiviral drugs on the metabolism of anticoagulants and antiplatelets agents is another issue deserving appropriate consideration [16].
COVID-19 and renin-angiotensin-aldosterone system inhibitors
Finally, two additional aspects in the relationship of COVID-19 with cardiovascular diseases deserve to be briefly explored.
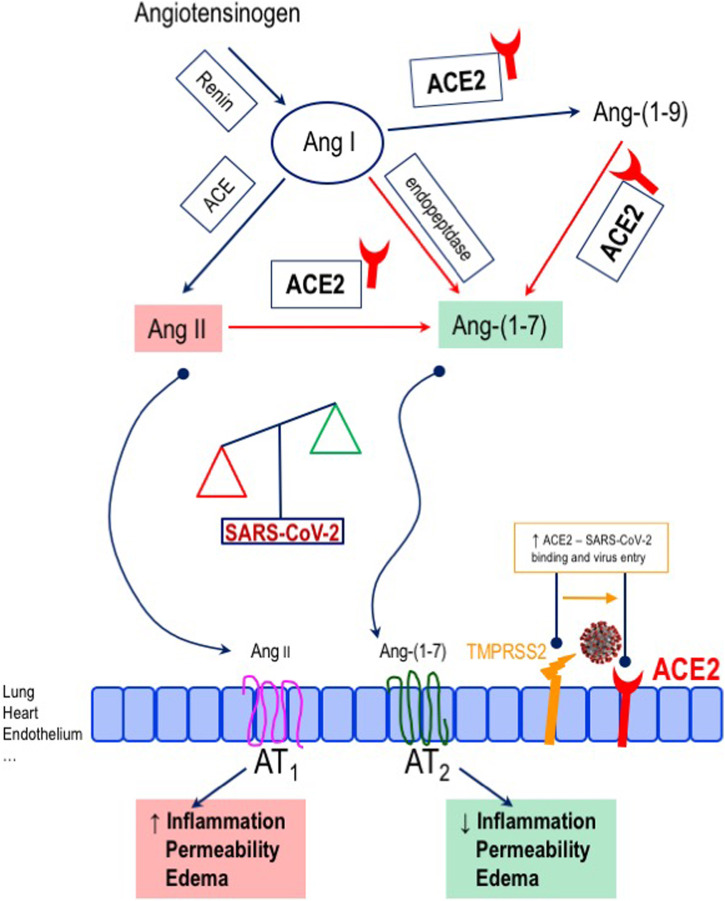
First, there has been concern regarding the potential favoring role of ACE inhibitors and angiotensin-receptor blockers (ARBs) in terms of viral infection. In fact, ACE inhibitors and sartans promote the expression of ACE2, i.e. the enzyme that converts the vasoconstrictor angiotensin II (Ang II) into the vasodilator, anti-inflammatory Ang-(1–7) and that works as the functional receptor for the entry of SARS-CoV2 into the cell, with a facilitating effect mediated by the type II transmembrane serine protease TMPRSS2 (Fig. 3 ) [27], [28], [29], [30]. However, experimental data do not fully support this hypothesis [31], [32]. Indeed, ACE2 appears to play a protective role against pulmonary inflammation [33], [34]. In accordance with this, for COVID-19 as for SARS [35], AT1 receptor blockade and the subsequent increase in ACE2 activity/availability might be protective against the pulmonary involvement related to the infection [36], [37]. Therefore, the concern on the use of renin-angiotensin-aldosterone system (RAAS) inhibitors during the coronavirus outbreak is currently not supported by causal evidence. According to this, first reports from China do not indicate a harmful effect of ACE inhibitors or sartans in COVID-19 patients [38]. Meta-analytic data indicate that ACE inhibitor/ARB exposure was not associated with a higher risk of SARS-CoV-2 infection, nor with increased risk of severe COVID-19 [39], [40], [41]. Indeed, treatment with RAAS inhibitors was associated with reduced risk of mortality compared to non-treatment among over 19,000 COVID-19 cases (OR 0.48, 95% CI, 0.29–0.81; p = 0.006) [39]. Similar findings were described for patients with hypertension and COVID-19 infection (OR 0.57, 95% CI 0.38–0.84, p = 0.004) [40]. A meta-analysis of 16 studies that involved 24,676 COVID-19 patients indicated that the combined risk of death and/or critical disease was reduced by about 23% among RAAS users, with ACE inhibitors, but not ARBs alone, explaining the observed protective effect [41]. In addition, findings from an Italian cohort of 133 consecutive SARS-CoV-2 positive hypertensive subjects presenting with acute respiratory symptoms and/or fever, who were grouped according to prior antihypertensive therapy (ACE inhibitor, N = 40; ARBs, N = 42; not on RAAS inhibitors, N = 51), indicate no difference between ACE inhibitors and ARBs groups in terms of hospital admission rate, oxygen therapy, and need for non-invasive ventilation [42].
Fig. 3.
Effects of SARS-CoV-2 binding to angiotensin-converting enzyme (ACE) 2. ACE2, the functional receptor for SARS-CoV-2, converts angiotensin II (Ang II) into angiotensin (1–7) [Ang-(1–7)], which exerts vasodilator, anti-inflammatory effects. The binding of SARS-CoV-2 to the enzyme, facilitated by the type II transmembrane serine proteases (TMPRSS2), determines an unbalance in Ang II and Ang-(1–7), with a decrease in the latter. The figure is original from the authors.
In analogy with adults, there is no evidence at present that children with hypertension, cardiovascular diseases, or chronic kidney disease, whether or not on RAAS inhibitors, are at increased risk for SARS-CoV-2 infection or severe COVID-19 [43]. Indeed, the benign clinical course of COVID-19 in children compared with adults triggered the question as to whether any differences in the Ang-(1–7) pathway, also impacting on the effects of RAAS inhibitors, might depend on age [43].
On the other hand, whether infection by coronaviruses might affect ACE2 function adversely, thus potentially contributing to the disease burden, is a possibility that deserves careful consideration. In fact, a consistent activation of the RAAS, possible expression of the decreased degradation of Ang II to Ang-(1–7) after virus binding to ACE2, has been described, with possible pro-inflammatory effects and prognostically negative consequences [44]. Elevated Ang II in COVID-19 patients has been linked to vascular hyperpermeability and severe acute lung injury [44], [45], as well as to hypokalemia [46] as a possible sign of hyperaldosteronism [47]. Previous evidence supported a detrimental role of elevated Ang II in association with decreased Ang-(1–7) in left ventricular remodeling in response to myocardial infarction [48]. A postulated possibility for increased cardiovascular risk in the context of COVID-19 is also the reduced cardioprotection due to Ang-(1–7) depletion and the lack of its anti-inflammatory, antioxidant, and vasodilating properties. Before the results of specifically designed studies become available (more than 20 trials on this topic are currently registered in ClinicalTrials.gov), a report on the association between in-hospital use of ACE inhibitors/ARBs and all-cause mortality in 1128 COVID-19 patients with hypertension indicated that such risk was 37% to 30% lower among ACE inhibitors/ARBs users than in non-users after controlling for potential confounders [49]. Since ACE inhibitors prevent the formation of Ang II and, therefore, that of Ang-(1–7), while ARBs determine an accumulation of Ang II that serves as substrate to ACE2 for conversion into Ang-(1–7) [27], it is possible that different molecular events occur with the use of one or the other drug class in the course of COVID-19.
Thus, any discontinuation of drugs with proven cardiovascular benefit, such as ACE inhibitors and ARBs, should be discouraged until otherwise proven, due to the foreseeable risk of adverse cardiovascular outcomes if this treatment is negated to patients with appropriate indication [50]. Specific studies are awaited to clarify this issue.
COVID-19 and electrocardiography
Last, but not least, the effect on the QT interval of drugs used for COVID-19 deserve mention as one of the possible cardiovascular implications of the viral infection, especially in patients who have a baseline abnormality [51]. In particular, hydroxychloroquine, azithromycin, lopinavir, and ritonavir can all interfere with the IKr (rapid delayed rectifier current) and prolong the QT interval. Hypokalemia secondary to diarrhea could represent another potential mechanism favoring QT prolongation [15]. Lopinavir and ritonavir may result also in PR interval prolongation. The role of hypoxia and neurohormonal imbalance related to lung infection and systemic inflammation must be also considered as a cause of arrhythmias during COVID-19. These mechanisms contribute to explain the particularly high incidence of arrhythmias among infected patients treated in intensive care units [52]. Some anti-inflammatory agents, such as hydroxychloroquine or chloroquine, can increase the risk of torsade des pointes in patients with electrolyte abnormalities or with concomitant use of QT prolonging agents, or decrease the metabolism of beta-blockers, with impact on heat rate [16]. In summary, besides the large number of patients with COVID-19 and pre-existing atrial fibrillation (Fig. 1 A), the therapy used to treat the viral infection, as well as some clinical manifestations of the disease, can trigger the development of ventricular tachyarrhythmias or conduction disturbances. Therefore, monitoring ECG parameters becomes of particular relevance in the context of the SARS-CoV-2 pandemic [51].
Conclusions
COVID-19 is mainly a lung disease. However, a hyperergic immune reaction, or “cytokine storm”, with a dramatic systemic impact can be also observed in a later stage of the disease (Fig. 2). In addition, COVID-19 prognosis can be deteriorated by pre-existing cardiovascular diseases, or acute cardiovascular events can be induced by COVID-19 even in the absence of a related history. They include the possibility of thrombotic manifestations in the form of local (i.e. pulmonary) or systemic (DIC) events, as well as QT interval prolongation. Finally, in the lack of evidence regarding the postulated harmful effect of ACE inhibitors and sartans in terms of SARS-CoV-2 virulence, these drugs should not be discontinued. Indeed, whether infection by coronaviruses might affect ACE2 function adversely, thus potentially contributing to infectious disease burden in selected patients, is a possibility that deserves investigation.
Funding source
This research received no grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosures
The authors declare that there is no conflict of interest.
References
- 1.Li X., Zai J., Zhao Q., Nie Q., Li Y., Foley B.T., et al. Evolutionary history, potential intermediate animal host, and cross-species analyses of SARS-CoV-2. J Med Virol. 2020;92:602–611. doi: 10.1002/jmv.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye Z.-W., Yuan S., Yuen K.-S., Fung S.-Y., Chan C.-P., Jin D.-Y. Zoonotic origins of human coronaviruses. Int J Biol Sci. 2020;16:1686–1697. doi: 10.7150/ijbs.45472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bambra C., Riordan R., Ford J., Matthews F. The COVID-19 pandemic and health inequalities. J Epidemiol Community Health. 2020 doi: 10.1136/jech-2020-214401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The, Lancet COVID-19: too little, too late? Lancet. 2020;395:755. doi: 10.1016/S0140-6736(20)30522-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Situation update worldwide, as of 23 April 2020. European Centre for Disease Prevention and Control [Internet]. Available from: https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases [cited 16.06.20].
- 8.ESC guidance for the diagnosis and management of CV disease during the COVID-19 pandemic. Available from: https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance [cited 16.06.20].
- 9.Palmieri L., Vanacore N., Donfrancesco C., Lo Noce C., Canevelli M., Punzo O., et al. Clinical characteristics of hospitalized individuals dying with COVID-19 by age group in Italy. J Gerontol A Biol Sci Med Sci. 2020 doi: 10.1093/gerona/glaa146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Italian National Institute of Health COVID-19 mortality group. Characteristics of SARS-CoV-2 patients dying in Italy. Report based on available data on June 4th, 2020. Available from: https://www.epicentro.iss.it/en/coronavirus/bollettino/Report-COVID-2019_4_june_2020.pdf [cited 0.6.06.20].
- 11.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 12.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clerkin K.J., Fried J.A., Raikhelkar J., Sayer G., Griffin J.M., Masoumi A., et al. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 15.Fried J.A., Ramasubbu K., Bhatt R., Topkara V.K., Clerkin K.J., Horn E., et al. The variety of cardiovascular presentations of COVID-19. Circulation. 2020;141:1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Bondi-Zoccai G., et al. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) Pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pietropaoli D., Del Pinto R., Ferri C., Ortu E., Monaco A. Definition of hypertension-associated oral pathogens in NHANES. J Periodontol. 2019;90:866–876. doi: 10.1002/JPER.19-0046. [DOI] [PubMed] [Google Scholar]
- 18.Del Pinto R., Ferri C. Inflammation-accelerated senescence and the cardiovascular system: mechanisms and perspectives. Int J Mol Sci. 2018;19:3701. doi: 10.3390/ijms19123701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han H., Yang L., Liu R., Liu F., Wu K.-L., Li J., et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58:1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 20.Yin S., Huang M., Li D., Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis. 2020 doi: 10.1007/s11239-020-02105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mousavi S., Moradi M., Khorshidahmad T., Motamedi M. Anti-inflammatory effects of heparin and its derivatives: a systematic review. Adv Pharmacol Sci. 2015;2015:507151. doi: 10.1155/2015/507151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin L., Lu L., Cao W., Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection – a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9:727–732. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. Interim Guidance; 13 March 2020. Available from: https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf [cited 16.06.20].
- 26.Danzi G.B., Loffi M., Galeazzi G., Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J. 2020;41:1858. doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A., et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 28.Ishiyama Y., Gallagher P.E., Averill D.B., Tallant E.A., Brosnihan K.B., Ferrario C.M. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43:970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- 29.Jessup J.A., Gallagher P.E., Averill D.B., Brosnihan K.B., Tallant E.A., Chappell M.C., et al. Effect of angiotensin II blockade on a new congenic model of hypertension derived from transgenic Ren-2 rats. Am J Physiol Heart Circ Physiol. 2006;291:H2166–H2172. doi: 10.1152/ajpheart.00061.2006. [DOI] [PubMed] [Google Scholar]
- 30.Heurich A., Hofmann-Winkler H., Gierer S., Liepold T., Jahn O., Pöhlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol. 2014;88:1293–1307. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burchill L.J., Velkoska E., Dean R.G., Griggs K., Patel S.K., Burrell L.M. Combination renin-angiotensin system blockade and angiotensin-converting enzyme 2 in experimental myocardial infarction: implications for future therapeutic directions. Clin Sci. 2012;123:649–658. doi: 10.1042/CS20120162. [DOI] [PubMed] [Google Scholar]
- 32.Walters T.E., Kalman J.M., Patel S.K., Mearns M., Velkoska E., Burrell L.M. Angiotensin converting enzyme 2 activity and human atrial fibrillation: increased plasma angiotensin converting enzyme 2 activity is associated with atrial fibrillation and more advanced left atrial structural remodelling. Europace. 2017;19:1280–1287. doi: 10.1093/europace/euw246. [DOI] [PubMed] [Google Scholar]
- 33.Imai Y., Kuba K., Penninger J.M. Angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Cell Mol Life Sci. 2007;64:2006–2012. doi: 10.1007/s00018-007-6228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Supé S., Kohse F., Gembardt F., Kuebler W.M., Walther T. Therapeutic time window for angiotensin-(1–7) in acute lung injury. Br J Pharmacol. 2016;173:1618–1628. doi: 10.1111/bph.13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuba K., Imai Y., Penninger J.M. Angiotensin-converting enzyme 2 in lung diseases. Curr Opin Pharmacol. 2006;6:271–276. doi: 10.1016/j.coph.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng H., Wang Y., Wang G.-Q. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol. 2020;92:726–730. doi: 10.1002/jmv.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020 doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meng J., Xiao G., Zhang J., He X., Ou M., Bi J., et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9:757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X., Yu J., Pan L.-Y., Jiang H.-Y. ACEI/ARB use and risk of infection or severity or mortality of COVID-19: a systematic review and meta-analysis. Pharmacol Res. 2020;158:104927. doi: 10.1016/j.phrs.2020.104927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo X., Zhu Y., Hong Y. Decreased mortality of COVID-19 with renin-angiotensin-aldosterone system inhibitors therapy in patients with hypertension: a meta-analysis. Hypertension. 2020 doi: 10.1161/HYPERTENSIONAHA.120.15572. [DOI] [PubMed] [Google Scholar]
- 41.Pirola C.J., Sookoian S. Estimation of renin-angiotensin-aldosterone-system (RAAS)-inhibitor effect on COVID-19 outcome: a meta-analysis. J Infect. 2020 doi: 10.1016/j.jinf.2020.05.052. S0163-4453(20)30329-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Felice C., Nardin C., Di Tanna G.L., Grossi U., Bernardi E., Scaldaferri L., et al. Use of RAAS inhibitors and risk of clinical deterioration in COVID-19: results from an Italian cohort of 133 hypertensives. Am J Hypertens. 2020 doi: 10.1093/ajh/hpaa096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.South A.M., Brady T.M., Flynn J.T. ACE2 (angiotensin-converting enzyme 2), COVID-19, and ACE inhibitor and Ang II (angiotensin II) receptor blocker use during the pandemic: the pediatric perspective. Hypertension. 2020;76:16–22. doi: 10.1161/HYPERTENSIONAHA.120.15291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen D., Jr., Li X., Song Q., Sr., Hu C., Jr., Su F., Dai J. Hypokalemia and clinical implications in patients with coronavirus disease 2019 (COVID-19), Infectious Diseases (except HIV/AIDS) medRxiv. 2020 doi: 10.1101/2020.02.27.20028530. [DOI] [Google Scholar]
- 47.Murray E., Tomaszewski M., Guzik T.J. Binding of SARS-CoV-2 and angiotensin-converting enzyme 2: clinical implications. Cardiovasc Res. 2020;116:e87–e89. doi: 10.1093/cvr/cvaa096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kassiri Z., Zhong J., Guo D., Basu R., Wang X., Liu P.P., et al. Loss of angiotensin-converting enzyme 2 accelerates maladaptive left ventricular remodeling in response to myocardial infarction. Circ Heart Fail. 2009;2:446–455. doi: 10.1161/CIRCHEARTFAILURE.108.840124. [DOI] [PubMed] [Google Scholar]
- 49.Zhang P., Zhu L., Cai J., Lei F., Qin J.-J., Xie J., et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126:1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.European Society of Cardiology. Position statement of the ESC Council on Hypertension on ACE-inhibitors and angiotensin receptor blockers. ESC; 2020. Available from: https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang.
- 51.Wu C.-I., Postema P.G., Arbelo E., Behr E.R., Bezzina C.R., Napolitano C., et al. SARS-CoV-2, COVID-19, and inherited arrhythmia syndromes. Heart Rhythm. 2020 doi: 10.1016/j.hrthm.2020.03.024. S1547-5271(20)30285-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Del Pinto Rita, Wright Jackson T., Monaco Annalisa, Pietropaoli Davide, Ferri Claudio. Vitamin D and blood pressure control among hypertensive adults: results from NHANES 2001-2014. Int J Hypertens. 2020;38(1):150–158. doi: 10.1097/HJH.0000000000002231. In this issue. [DOI] [PubMed] [Google Scholar]