Abstract
Aims
Diabetes mellitus has been reported to be one of the most prevalent comorbidity in patients with Coronavirus Disease 2019 (COVID-19). We aimed to assess the association of comorbid diabetes with COVID-19 severity or mortality in China.
Methods
We performed a systematic literature search from six electronic databases on diabetes and COVID-19. The outcome of interest was disease severity or mortality. Heterogeneity among the studies was assessed by the Cochran Q test and the I2 statistic. A random effects model was applied to calculate the pooled risk ratio (RR) with 95% confidence interval (CI).
Results
Nine studies from different provinces/cities were identified according to the predefined inclusion and exclusion criteria. There were a total of 1070 patients with diabetes, out of the 8807 COVID-19 cases. The majority of the cases were derived from Hubei Province. A low degree of heterogeneity in the risk estimates was observed in the included studies. Meta-analysis showed that there was a significant association of preexisting diabetes with disease severity or death. The pooled RR was 2.96 (95% CI: 2.31–3.79; p < 0.001). Sensitivity analysis demonstrated no significant changes in the pooled estimates.
Conclusions
Comorbid diabetes was associated with an increased risk of disease severity or death in Chinese COVID-19 patients.
Keywords: Diabetes, COVID-19, Comorbidity, Severity, Death
1. Introduction
An unprecedented Coronavirus Disease 2019 (COVID-19), caused by a novel beta-coronavirus Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) [1], was first reported in China in December 2019, and has now rapidly evolved as a global pandemic [2]. WHO has declared COVID-19 a public health emergency of international concern [3]. By May 30, 2020, there have been 5,819,962 confirmed cases and 362,786 deaths reported worldwide [3].
The COVID-19 patients experience a wide spectrum of clinical severity, ranging from mild to severe or fatal [4]. A growing body of evidence suggests that the variability in clinical patterns could partly be attributable to their underlying comorbidity [5]. Studies from China [4], [6], [7], [8] found that diabetes mellitus was the second most prevalent comorbidity of COVID-19, and people with diabetes appeared to be likely to have worse clinical outcomes than those without the condition. A recent study observed that the overall case-fatality rate was 7.3% among those patients with preexisting comorbid diabetes [9].
To date, there are several meta-analyses [10], [11], [12], [13], [14] exploring the association of comorbidity including diabetes with disease severity or death. However, none of these meta-analyses calculated the risk ratio of severity or mortality between patients with diabetes versus those without diabetes. More importantly, some studies included in the meta-analyses were size-limited reports predominantly from Hubei Province and potential overlapping of patients across the included studies was not considered, which could have biased the estimates. Along with the latest studies published, especially those outside Hubei Province, we are able to perform an updated analysis to better examine the association of comorbid diabetes with COVID-19 severity or mortality in China.
2. Methods
2.1. Search strategy
We performed a systematic literature search through databases PubMed, Web of Knowledge, medRxiv, and bioRxiv for English-language literatures, the China National Knowledge infrastructure (CNKI) and the Wanfang database for Chinese-language publications. The Medical Subject Heading (MeSH) terms and/or key words included “COVID-19” OR “SARS-CoV-2” OR “2019-nCoV” AND “Diabetes” AND “China” (or each name of the 31 provinces/ municipalities/ autonomous regions in mainland China). Titles and abstracts of the resulting literatures were screened for further review. The references of identified literatures were checked as well if needed. Only original studies were considered for the review. Duplicate and overlapping publications were excluded. Two authors (YZ and CW) independently did the literature search. Any disagreements were resolved after discussion. This study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline.
2.2. Eligibility criteria
We included all research articles in adult patients diagnosed with COVID-19; with direct or indirect information on the outcome of disease severity or mortality grouped by comorbid diabetes; conducted in Mainland China and published between January 01 and May 30, 2020. The following types of article were excluded: total number of cases below 30; articles other than original research (review articles, letters, or commentaries); case reports and series; articles on pediatric populations; geographically covered by/overlapped with other studies.
2.3. Data extraction
Data were extracted using a standardized data collection form. Information was extracted from each included article, including the first author, inclusion date of patients, study location, study design, gender, age, sample size, comorbid diabetes, and disease severity/mortality. Severe COVID-19 cases were defined if patients had indication of respiratory rate >30 breaths/min, or SpO2 % ≤ 93% on room air, or PaO2/FiO2 ≤ 300 mmHg, or critical complication (respiratory failure, septic shock, and or multiple organ dysfunction/failure) [15].
2.4. Statistical analysis
Meta-analysis was conducted in order to evaluate the association of comorbid diabetes with disease severity/mortality. Heterogeneity among the studies was assessed by the Cochran Q test and the I 2 statistic. An I 2 value ≤25% indicates a low degree of heterogeneity [16]. The pooled risk ratio (RR) with 95% CI in the forest plot was analyzed using a random effects model (Mantel-Haenszel method) irrespective of the heterogeneity. Begg’s funnel-plot analysis was performed to qualitatively assess the risk of publication bias [17]. In addition, a sensitivity analysis was performed using the sequential omission of individual studies to assess the quality and consistency of the results. All analyses were performed using R (version 3.6.2, www.r-project.org). A two-tailed p value <0.05 was considered statistically significant in all analyses.
3. Results
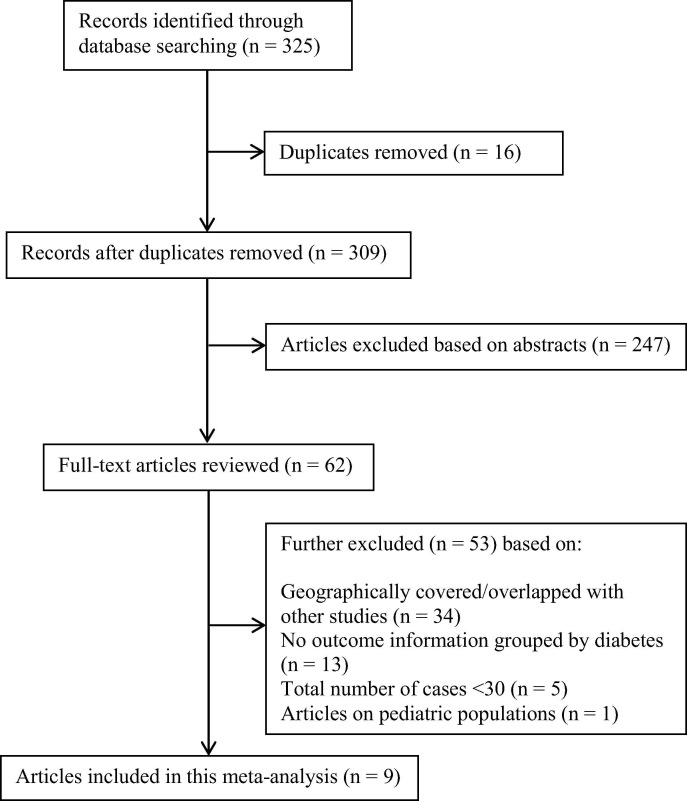
Initial search yields 325 records, and 309 records remained after the removal of duplicates. After screening the abstracts 247 records were excluded. After evaluating 62 full-text articles for eligibility, 53 full-text articles were further excluded. Finally, 9 studies from different provinces/cities [6], [18], [19], [20], [21], [22], [23], [24], [25] were included in the meta-analysis (Fig. 1 ).
Fig. 1.
Flow diagram depicting the literature search and selection strategy. After applying the inclusion and exclusion criteria, a total of nine studies were included in the final meta-analysis.
The basic characteristics of the patients in the identified 9 studies are presented in Table 1 . Overall, the patients were enrolled between Dec.30, 2019 and Mar.20, 2020. Men accounted for 48.1% of the included cases. The median age was 44–61 years. Death information grouped by diabetes status was only available in one study from Hubei, whereas disease severity as the outcome was used in the other eight studies. Moreover, 83.3% of the cases were derived from Hubei Province, which is proportional to the latest national data (82.1%) [26].
Table 1.
Basic characteristics of included studies in China.
| Study | Inclusion date of patients | Region | Number of patients | Male (%) | Age (median (interquartile range)/ mean (SD), years) | Diabetes (%) | Outcome |
|---|---|---|---|---|---|---|---|
| Zhu et al. [6] | Dec.30-Mar.20 | Hubei | 7337 | 3477 (47.4%) | 54 (42–64) | 952 (13.0%) | Mortality |
| Zheng et al. [19] | Jan.17-Feb.07 | Changsha | 161 | 80 (49.7%) | 45 (34–57) | 7 (4.3%) | Severity |
| Yuan et al. [25] | Jan.24-Feb.23 | Chongqing | 223 | 106 (47.5%) | 46.5 (16.1) | 18 (8.1%) | Severity |
| Huang et al. [24] | Jan.22-Feb.10 | Jiangsu | 202 | 116 (57.4%) | 44 (33–54) | 19 (9.4%) | Severity |
| Wei et al. [20] | Not available | Anhui | 167 | 95 (56.9%) | 42.3 (15.3) | 11 (6.6%) | Severity |
| Cao et al. [21] | Jan.20-Feb.15 | Shanghai | 198 | 101 (51.0%) | 50.1 (16.3) | 15 (7.6%) | Severity |
| Cai et al. [18] | Jan.11-Feb.16 | Shenzhen | 383 | 183 (47.8%) | 45 (34–57) (Non-severe group) 61 (52–65) (Severe group) |
22 (5.7%) | Severity |
| Fan et al. [22] | Jan.20-Mar.15 | Liaoning | 55 | 30 (54.5%) | 46.8 | 8 (14.5%) | Severity |
| Liao et al. [23] | Jan.16-Mar.15 | Sichuan | 81 | 51 (63.0%) | 50 (39–65) | 18 (22.2%) | Severity |
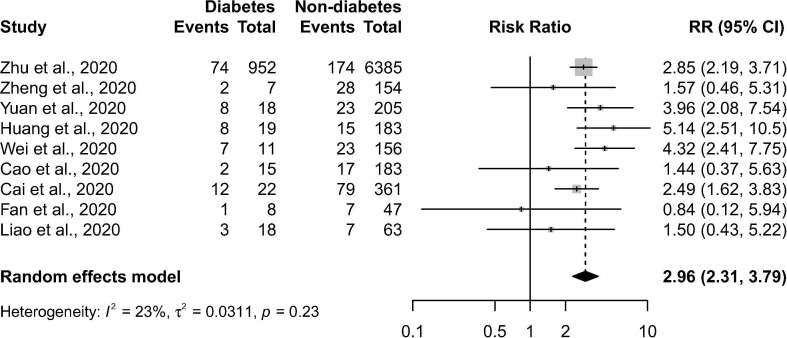
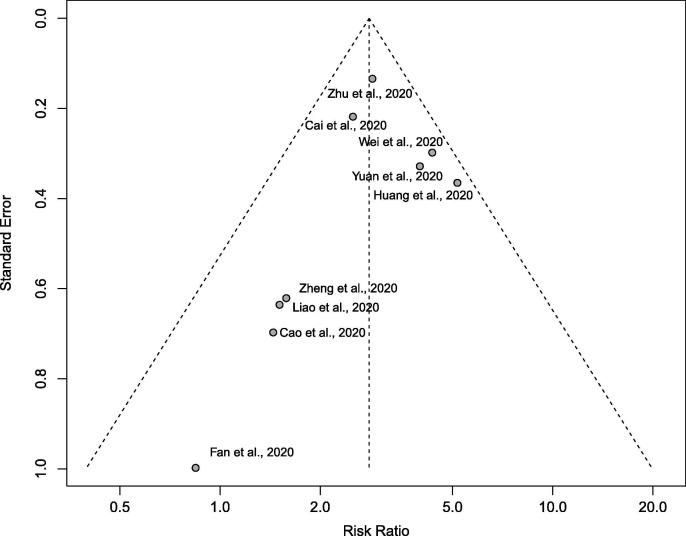
There were a total of 1070 patients with diabetes, out of the 8807 COVID-19 cases. There was a low degree of heterogeneity (p = 0.235 in Cochran’s Q test, I 2 = 23%) in the risk estimates in the included studies. As shown in Fig. 2 , comorbid diabetes was found to be significantly associated with disease severity or death of COVID-19 (pooled RR = 2.96 (95% CI: 2.31–3.79; p < 0.001)). The shapes of Begg’s funnel plot did not reveal evidence of obvious publication bias (Fig. 3 ). During the sensitivity analysis, the omission of any individual study did not show a significant change in the pooled estimates (RR ranged from 2.80 to 3.07).
Fig. 2.
Forest plot showing the association of preexisting diabetes with disease severity or death in COVID-19 patients. The horizontal lines indicate the lower and upper limits of the 95% CI, and the size of the grey squares reflects the relative weight of each study in the meta-analysis. RR: risk ratio.
Fig. 3.
Funnel plots for detecting publication bias. The X-axis represents the point estimate of risk ratio; the Y-axis represents the standard error.
4. Discussion
In this meta-analysis including nine original studies from different provinces/cities in China, we observed a positive association of diabetes with COVID-19 severity or mortality. It indicated that patients with preexisting diabetes were at higher risk of having worse outcome including severe COVID-19 infection or death, compared to those without diabetes. A low level of statistical heterogeneity (I 2 = 23%) and the results of sensitivity analysis showed consistency across studies.
Our findings are basically in agreement with a previous meta-analysis [12], with similar estimated effect sizes. Notably, only one study [19] were included in both of the meta-analyses. It indicates the necessity of our study due to the quick update in literature regarding diabetes and COVID-19 during the ongoing pandemic. There were also several meta-analyses [10], [27], [28], [29], [30], [31] available regarding the diabetes and disease severity. However, they did not specifically focus on diabetes as a risk indicator, which could affect the eligibility criteria and therefore studies ought to be included. In addition, a nationwide data from the early phase of the epidemic in China [4] reported that patients with diabetes had a 59% increased risk of reaching to composite endpoints including admission to ICU, or invasive ventilation, or death, after adjustment for age and smoking status. The calculated RR based on a study from Wuhan [32] was 1.94 (95% CI: 1.10–3.39) comparing the mortality between diabetes patients and their sex- and age-matched controls. It is understandable that the adjusted estimate is lower than what we calculated, which indicates the presence of confounding factors. Furthermore, our findings from China are essentially consistent with the recent global data [33].
Indeed, patients with diabetes mellitus, especially type 2 diabetes, are more likely than others to develop severe infection. A higher risk of hospitalization and ICU admission after pandemic influenza A (H1N1) infection has been reported [34]. Notably, diabetes is also a major contributor to worse prognosis in the previous epidemic coronavirus infections. Those with preexisting diabetes had a higher risk of death than those without the condition in patients with Middle East respiratory syndrome coronavirus (MERS-CoV) [35] and Severe Acute Respiratory Syndrome (SARS) [36].
A variety of mechanisms have been suggested for increased risk of disease severity or death associated with comorbid diabetes [37]. Poor glycemic control leading to immune dysfunction especially impaired T-cell response is believed to play a pivotal role [38]. Other important mechanisms may include chronic low-grade inflammation, downregulation of angiotensin converting enzyme 2 (ACE2), and related comorbidities such as obesity, cardiovascular disease and hypertension [5], [39], [40]. In addition, whether dipeptidyl peptidase-4 (DPP-4) enzyme is involved in the link between diabetes and the severity of COVID-19 remains unknown [2], and further work is needed.
The major strength of this study is the inclusion of geographically diverse samples to avoid potential overlapping in patients. Moreover, we considered eligible studies in both English and Chinese languages, which minimized the possibility of missing any important findings relevant to this topic in China. By integrating the currently best available data from the country, our study provides more reliable estimates. However, the true association of diabetes with the risk of disease severity or death has probably been overestimated. Due to lack of information including demographic and clinical data, we cannot examine to what extent diabetes independently contributes to the increased risk. Ideally, a comprehensive analysis of COVID-19 patients by diabetes status using nationwide original data with adequate consideration of confounding should be conducted in order to better understand the association.
To summarize, in this meta-analysis, we observed that comorbid diabetes was associated with a nearly twofold increased risk of having a greater disease severity or mortality among COVID-19 patients in China. Our findings provide new evidence that careful attention and medical intervention should be given to patients with diabetes when facing the COVID-19 pandemic.
Authors’ contributions
LG extracted and analyzed the data, and drafted the manuscript; ZS, HZ and AH conceptualized the paper; YZ and CW did the literature search; ZS, NM, HZ and AH provided critical review and revision; and all authors approved the final manuscript.
Funding
None.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bornstein S.R., Rubino F., Khunti K., Mingrone G., Hopkins D., Birkenfeld A.L., et al. Practical recommendations for the management of diabetes in patients with COVID-19. The Lancet Diabetes Endocrinol. 2020;8:546–550. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Coronavirus disease (COVID-19) outbreak. https://www.who.int.
- 4.Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M., et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: A Nationwide Analysis. Eur Respir J. 2020 doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hussain A, Bhowmik B, do Vale Moreira NC. COVID-19 and diabetes: Knowledge in progress. Diabetes Res Clin Pract 2020; 162: 108142. [DOI] [PMC free article] [PubMed]
- 6.Zhu L., She Z.G., Cheng X., Qin J.J., Zhang X.J., Cai J., et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020 doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng Y., Ling Y., Bai T., Xie Y., Huang J., Li J., et al. COVID-19 with different severity: a multi-center study of clinical features. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lian J., Jin X., Hao S., Cai H., Zhang S., Zheng L., et al. Analysis of epidemiological and clinical features in older patients with corona virus disease 2019 (COVID-19) out of Wuhan. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 10.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., et al. Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: A systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Wang S, Sun L, Qin G. Prevalence of diabetes mellitus in 2019 novel coronavirus: a Meta-analysis. Diabetes Res Clin Pract. 2020: 108200. [DOI] [PMC free article] [PubMed]
- 12.Kumar A., Arora A., Sharma P., Anikhindi S.A., Bansal N., Singla V., et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr. 2020;14:535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia - A systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14:395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roncon L., Zuin M., Rigatelli G., Zuliani G. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Health Commission of China. Diagnosis and treatment of pneumonia caused by novel coronavirus (trial version 5). Febrary 11, 2020.
- 16.Ioannidis J.P., Patsopoulos N.A., Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. 2007;335:914–916. doi: 10.1136/bmj.39343.408449.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterne J.A., Sutton A.J., Ioannidis J.P., Terrin N., Jones D.R., Lau J., et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343 doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 18.Cai Q., Chen F., Wang T., Luo F., Liu X., Wu Q., et al. Obesity and COVID-19 Severity in a Designated Hospital in Shenzhen, China. Diabetes Care. 2020 doi: 10.2337/dc20-0576. [DOI] [PubMed] [Google Scholar]
- 19.Zheng F., Tang W., Li H., Huang Y.X., Xie Y.L., Zhou Z.G. Clinical characteristics of 161 cases of corona virus disease 2019 (COVID-19) in Changsha. Eur Rev Med Pharmacol Sci. 2020;24:3404–3410. doi: 10.26355/eurrev_202003_20711. [DOI] [PubMed] [Google Scholar]
- 20.Wei Y.Y., Wang R.R., Zhang D.W., Tu Y.H., Chen C.S., Ji S., et al. Risk factors for severe COVID-19: Evidence from 167 hospitalized patients in Anhui, China. J Infect. 2020 doi: 10.1016/j.jinf.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao M., Zhang D., Wang Y., Lu Y., Zhu X., Li Y., et al. Clinical Features of Patients Infected with the 2019 Novel Coronavirus (COVID-19) in Shanghai, China. medRxiv. 2020;2020(03) [Google Scholar]
- 22.Fan L, Liu C, Li N, Liu H, Gu Y, Liu Y, et al. Medical treatment of 55 patients with COVID-19 from seven cities in northeast China who fully recovered: a single-center, retrospective, observational study. medRxiv 2020: 2020.03.28.20045955. [DOI] [PMC free article] [PubMed]
- 23.Liao X, Chen H, Wang B, Li Z, Zhang Z, Li W, et al. Critical Care for Severe COVID-19: A Population-based Study from a Province with Low Case-fatality Rate in China. medRxiv. 2020:2020.03.22.20041277. [DOI] [PMC free article] [PubMed]
- 24.Huang R., Zhu L., Xue L., Liu L., Yan X., Wang J., et al. Clinical findings of patients with coronavirus disease 2019 in Jiangsu province, China: A retrospective, multi-center study. PLoS Negl Trop Dis. 2020;14 doi: 10.1371/journal.pntd.0008280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jing Yuan Y.S., Zuo Yujie, et al. Analysis of clinical characteristics of 223 patients with new coronavirus pneumonia in Chongqing city. Xi Nan Da Xue Xue Bao (Natural Science Edition) 2020;42:17–24. [Google Scholar]
- 26.National Health Commission of the People's Republic of China. http://www.nhc.gov.cn/xcs/xxgzbd/gzbd_index.shtml, May 30, 2020. [DOI] [PMC free article] [PubMed]
- 27.Wang X., Fang X., Cai Z., Wu X., Gao X., Min J., et al. Comorbid chronic diseases and acute organ injuries are strongly correlated with disease severity and mortality among COVID-19 patients: a systemic review and meta-analysis. Research (Wash D C). 2020;2020:2402961. doi: 10.34133/2020/2402961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, Gong X, Wang L, Guo J. Effects of hypertension, diabetes and coronary heart disease on COVID-19 diseases severity: a systematic review and meta-analysis. medRxiv 2020:2020.03.25.20043133.
- 29.Wang B., Li R., Lu Z., Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY). 2020;12:6049–6057. doi: 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li B., Yang J., Zhao F., Zhi L., Wang X., Liu L., et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu Y., Sun J., Dai Z., Deng H., Li X., Huang Q., et al. Prevalence and severity of corona virus disease 2019 (COVID-19): A systematic review and meta-analysis. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi Q., Zhang X., Jiang F., Zhang X., Hu N., Bimu C., et al. Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China: a two-center, retrospective study. Diabetes Care. 2020 doi: 10.2337/dc20-0598. [DOI] [PubMed] [Google Scholar]
- 33.Singh A.K., Gillies C.L., Singh R., Singh A., Chudasama Y., Coles B., et al. Prevalence of comorbidities and their association with mortality in patients with COVID-19: A Systematic Review and Meta-analysis. Diabetes Obes Metab. 2020 doi: 10.1111/dom.14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allard R., Leclerc P., Tremblay C., Tannenbaum T.N. Diabetes and the severity of pandemic influenza A (H1N1) infection. Diabetes Care. 2010;33:1491–1493. doi: 10.2337/dc09-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmadzadeh J., Mobaraki K., Mousavi S.J., Aghazadeh-Attari J., Mirza-Aghazadeh-Attari M., Mohebbi I. The risk factors associated with MERS-CoV patient fatality: A global survey. Diagn Microbiol Infect Dis. 2020;96 doi: 10.1016/j.diagmicrobio.2019.114876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang J.K., Feng Y., Yuan M.Y., Yuan S.Y., Fu H.J., Wu B.Y., et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23:623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 37.Gupta R., Hussain A., Misra A. Diabetes and COVID-19: evidence, current status and unanswered research questions. Eur J Clin Nutr. 2020 doi: 10.1038/s41430-020-0652-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Critchley J.A., Carey I.M., Harris T., DeWilde S., Hosking F.J., Cook D.G. Glycemic control and risk of infections among people with type 1 or type 2 diabetes in a large primary care cohort study. Diabetes Care. 2018;41:2127–2135. doi: 10.2337/dc18-0287. [DOI] [PubMed] [Google Scholar]
- 39.Marhl M., Grubelnik V., Magdic M., Markovic R. Diabetes and metabolic syndrome as risk factors for COVID-19. Diabetes Metab Syndr. 2020;14:671–677. doi: 10.1016/j.dsx.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muniyappa R., Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol-Endocrinol Metabolism. 2020;318:E736–E741. doi: 10.1152/ajpendo.00124.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]