Highlights
-
•
We retrospectively studied the prevalence and clinical characterization of cancer patients with asymptomatic SARS-CoV-2 infection history in the only cancer hospital in Wuhan.
-
•
Serosurvey of SARS-CoV-2 among hospital visitors from two hospitals, one at the epicenter in Wuhan, and one outside the epicenter, in Guangzhou.
-
•
Cancer patients are more likely to develop asymptomatic COVID-19 infection than persons with similar exposure history.
-
•
Transmissibility of asymptomatic cases is relatively low, even in vulnerable populations such as cancer patients.
Dear Editor,
We read with the interest the recent paper by Minotti and colleagues who described a systematic review of literature about immunosuppressive status affecting children and adults in SARS-CoV-2 infection,1 which reported patients with cancer may have milder forms of COVID19 infection. There have also some articles reported that cancer patients may be at increased risk of COVID-19 along with poorer prognosis.2 , 3 However, no comparison has been made to date between asymptomatic, RNA-negative cancer patients with positive viral serum antibodies versus caregivers living in a similar environment. In this letter, we aimed to examine the prevalence and clinical characteristics of asymptomatic COVID-19 in cancer patients versus caregivers with a similar COVID-19 exposure history from the experience of the only cancer hospital in Wuhan, China.
A total of 3261 consecutive individuals who visited Hubei Cancer Hospital from March 9, 2020 to April 7, 2020 (comprising 2094 cancer patients and 1167 caregivers) were required to undergo chest computed tomography (CT) and routine bloodwork as well as viral serum antibodies against SARS-CoV-2 by the colloidal gold immunoassay. All patients with positive SARS-CoV-2 antibodies underwent SARS-CoV-2 nucleic acid testing by RT-PCR. To illustrate the situation outside the epicenter, a serosurvey from the First Affiliated Hospital of Guangzhou Medical University (Guangzhou, China) was also reviewed. This investigation was approved by the institutional ethics board of Hubei Cancer Hospital.
Confirmation of COVID-19 infection was defined per positive nucleic acid testing or SARS-CoV-2 antibody testing, based on the criteria published by the COVID-19 Diagnosis and Treatment Plan (Provisional 7th Edition) from the National Health Commission of China.4 Asymptomatic SARS-CoV-2 infections herein were defined as the presence of SARS-CoV-2 antibody (without positive nucleic acid testing) and showing no COVID-19 related symptoms upon clinical evaluation.5 Categorical variables were presented with counts (%); continuous variables were presented as mean (SD) if normally distributed, or median (IQR) if not. A Chi-square test and a Mann-Whitney U test/Student t-test were used to compare the difference between categorical and continuous variables respectively. All statistical analyses were performed using SPSS 23.0 (SPSS Inc., Chicago, IL, USA).
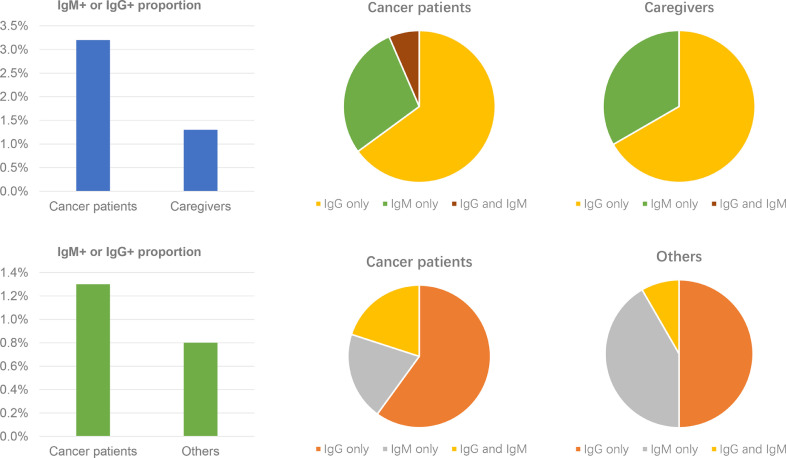
67 (3.2%) of the cancer patients with positive for serum IgM and/or IgG antibodies (17 IgG- IgM+, 45 IgG+ IgM-, and 5 IgG+ IgM+) were significantly more than the 15 (1.3%) of the caregivers (5 IgG- IgM+, 10 IgG+ IgM-) (P<0.001). The result was similar to the Guangzhou cohort, 1.3% (5/375) of cancer patients and 0.8% (12/1469) of caregivers, respectively (Table 1 and Fig. 1 ).
Table 1.
Baseline Characteristics of All Asymptomatic SARS-CoV-2 Infections.
| Total (N = 82) | Cancer patients (n = 67) (%) | Caregivers (n = 15) (%) | P Value | Paired Cancer patients (n = 51) (%) | Paired Caregivers (n = 51) (%) | P Value | |
|---|---|---|---|---|---|---|---|
| Age, median (IQR), y | 58 | 58 | 56 | 0.490 | 58 (50–64) | 53 (43–63) | 0.063 |
| Age, y | 0.616 | 0.537 | |||||
| ≤60 | 51 | 40 (59.7) | 11 (73.3) | 31 (60.8) | 34 (66.7) | ||
| >60 | 31 | 27 (40.3) | 4 (26.7) | 20 (39.2) | 17 (33.3) | ||
| Sex | 0.917 | 0.303 | |||||
| Female | 54 | 45 (67.2) | 9 (60.0) | 35 (68.6) | 30 (58.8) | ||
| Male | 28 | 22 (32.8) | 6 (40.0) | 16 (31.4) | 21 (41.2) | ||
| Clinical manifestations (in last 3 months) | |||||||
| Fever | 3 | 2 (3.0) | 1 (6.7) | 0.492 | 2 (3.9) | 0 (0.0) | 0.153 |
| Cough | 1 | 1 (1.5) | 0 (0.0) | 0.634 | 1 (2.0) | 0 (0.0) | 0.315 |
| Chest pain | 1 | 1 (1.5) | 0 (0.0) | 0.634 | 0 (0.0) | 0 (0.0) | 1.000 |
| Chronic diseases | |||||||
| Hypertension | 13 | 12 (17.9) | 1 (6.7) | 0.281 | 12 (23.5) | 3 (5.9) | 0.012 |
| Diabetes | 4 | 3 (4.5) | 1 (6.7) | 0.722 | 4 (7.8) | 1 (2.0) | 0.169 |
| Cardiovascular disease | 1 | 1 (1.5) | 0 (0.0) | 0.634 | 1 (2.0) | 0 (0.0) | 1.000 |
| COPD | 2 | 2 (3.0) | 0 (0.0) | 0.488 | 2 (3.9) | 0 (0.0) | 0.153 |
| Chronic kidney disease | 2 | 2 (3.0) | 0 (0.0) | 0.488 | 1 (2.0) | 0 (0.0) | 0.315 |
| Chronic liver disease | 3 | 2 (3.0) | 1 (6.7) | 0.492 | 2 (3.9) | 1 (2.0) | 0.558 |
| Leucocytes (× 10⁹ per L; normal range 4.0–10.0) | 0.625 | 0.125 | |||||
| Increased | 2 | 1 (1.5) | 1 (6.7) | 2 (3.9) | 0 (0.0) | ||
| Decreased | 3 | 3 (4.5) | 0 (0.0) | 2 (3.9) | 0 (0.0) | ||
| Lymphocytes (× 10⁹ per L; normal range 1•1–3•2) | 0.706 | 0.213 | |||||
| Increased | 1 | 1 (1.5) | 0 (0.0) | 1 (2.0) | 0 (0.0) | ||
| Decreased | 2 | 2 (3.0) | 0 (0.0) | 2 (3.9) | 0 (0.0) | ||
| Chest CT findings | 0.371 | 0.012 | |||||
| Suspicious | 8 | 8 (11.9) | 0 (0.0) | 6 (11.8) | 0 (0.0) | ||
| Unsuspicious | 74 | 59 (88.1) | 15 (100.0) | 45 (88.2) | 51 (100.0) |
Note: IQR: interquartile range; COPD: chronic obstructive pulmonary disease; P<0.05 indicates that the difference was statistically significant.
Fig. 1.
Positive rate of SARS-CoV-2 IgM/IgG and proportion of IgM+, IgG+ and IgM+ IgG+ in different groups in Wuhan and Guangzhou.
In the 82 infected persons, the median age was 58 years (range: 25–75) and 54 (65.9%) patients were female as well as 77 (93.9%) had no obvious clinical manifestations and 5 cases had self-limiting symptoms in the last three months (three with fever, one with dry cough, and one with chest pain). Leukocytes were below the normal range in three (3.7%) patients and above the normal range in 2 (2.4%) patients. Only two (2.4%) patients had lymphopenia. According to chest CT results, 8 (9.8%) patients were suspected of having manifestations of SARS-CoV-2 infection (Table 1). Of the 67 infected cancer patients, breast cancer was the most frequent type (17 [25.4%]), followed by lung cancer (16 [23.4%]). 27 [40.3%] of 67 infected cancer patients had a history of anticancer therapy (e.g. surgery and/or chemotherapy) within the last six months.
Of 67 infected cancer patients, 51 presented with “paired” caregivers living in the same environment. None of the paired caregivers had positive antibodies against SARS-CoV-2. The 51 infected cancer patients showed a larger proportion of hypertension (23.5% vs. 5.9%, P = 0.012) and abnormal CT manifestations (11.8% vs. 0%, P = 0.012) (Table 1). Additionally, the paired cancer patients of all 15 asymptomatic SARS-CoV-2 infected caregivers also remained uninfected.
Nucleic acid testing for SARS-CoV-2 was the standard for COVID-19 diagnosis at the beginning of the epidemic. However, this has many limitations: (1) RT-PCR testing generally takes several hours for results. (2) RT-PCR requires certified laboratories, expensive equipment, and trained technicians to operate. (3) RT-PCR can cause false negatives for COVID-19. Therefore, given the urgent need for a rapid, simple, sensitive, and accurate test to quickly identify infected patients, antibody testing has been largely implemented for these purposes.6
In our study, 82(2.5%) asymptomatic SARS-CoV-2 infections were detected in 3261 individuals who visited Hubei Cancer Hospital, which is higher than the 1.2% asymptomatic COVID-19 cases from the report by the China CDC.7 This is likely related to the high (64.2%) of cancer patients herein, which is consistent with literature showing that the infection rate tends to be higher in cancer patients as compared to non-cancer patients.2 We extend the results of prior studies by showing that the rate of asymptomatic disease in cancer patients is higher than that of persons with similar history of exposure (e.g. caregivers).
Though asymptomatic COVID-19 cases with potential to spread the virus have been reported,8 , 9 we found that paired caregivers of 51 asymptomatic SARS-CoV-2 infected cancer patients were not infected as well as paired cancer patients of 15 asymptomatic SARS-CoV-2 infected caregivers. Thus, asymptomatic infected patients should ideally be treated similar to those with subclinical infection given the very weak transmission ability.
This study has several limitations. First, we did not collect a clear exposure history to COVID-19, so it was difficult to clarify how they got infected. Second, according to the updated COVID-19 Diagnostic Criteria (7th Edition),4 the viral serum antibody is indeed valid for diagnosis; however, false positives and false negatives can still occur. Third, we cannot address the effect of oncologic therapy on re-activation of the virus (or lack thereof).
In conclusion, this experience from the only cancer hospital in Wuhan, China, shows that asymptomatic manifestations of COVID-19 are more likely to occur in cancer patients as compared to non-cancer-afflicted caregivers located in a similar exposure environment. However, transmission of asymptomatic COVID-19 is relatively weak, which is novel information of utility for other nations in preparation for the “second wave” of the pandemic thought to occur later this year.
Declaration of Competing Interest
All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We thank all cancer patients and their caregivers involved in the study. The authors acknowledge the MSD China Holding co., Ltd. Wuhan New World Center, Wuhan, China for reviewing and proofreading.
Funding
None.
References
- 1.Minotti C., Tirelli F., Barbieri E., Giaquinto C., Donà D. How is immunosuppressive status affecting children and adults in SARS-CoV-2 infection? A systematic review. J Infect. 2020;81(1):e61–e66. doi: 10.1016/j.jinf.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang L., Zhu F., Xie L. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020 Mar 26 doi: 10.1016/j.annonc.2020.03.296. pii: S0923-7534(20)36383-3[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020 doi: 10.1016/S1470-2045(20)30096-6. published online Feb 14http://dx.doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Health Commission of China. New coronavirus pneumonia prevention and control program (7th edn). Mar 3, 2020. http://www.gov.cn/zhengce/zhengceku/2020-03/04/5486705/files/ae61004f930d47598711a0d4cbf874a9.pdf(in Chinese).
- 5.Meng H., Xiong R., He R. CT imaging and clinical course of asymptomatic cases with COVID-19 pneumonia at admission in Wuhan, China. J Infect. 2020 Apr 12 doi: 10.1016/j.jinf.2020.04.004. pii: S0163-4453(20)30211-5[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Z., Yi Y., Luo X. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol. 2020 Feb 27 doi: 10.1002/jmv.25727. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145–151. [Google Scholar]
- 8.Du Z.W., Xu X.K., Wu Y., Wang L., Cowling B.J., Meyers L.A. Serial Interval of COVID-19 among Publicly Reported Confirmed Cases. Emerg Infect Dis. 2020 Mar 19;26(6) doi: 10.3201/eid2606.200357. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q., Guan X., Wu P. Early transmission dynamics in wuhan, china, of novel coronavirus-infected pneumonia. N Engl J Med. 2020 Mar 26;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. Epub 2020 Jan 29. [DOI] [PMC free article] [PubMed] [Google Scholar]