Abstract
Aims
To examine perceived infection risk of COVID-19 and the health and related behavior changes among people with diabetes, compared with people without diabetes, and to examine factors associated with self-reported health during the national quarantine period in China.
Methods
The 2020 China COVID-19 Survey is an anonymous 74-item survey administered via social media across China. A national sample of 10,545 adults in all 31 provinces in mainland China provided data on sociodemographic characteristics, awareness, attitudes towards COVID-19, lifestyle factors, and health outcomes during the quarantine. Regression models tested associations among study variables adjusting for covariates.
Results
Among the 9,016 total participants (42.6% men and 57.4% women), 585 reported having diagnosed diabetes and 8,431 had no diabetes. Participants with diabetes perceived themselves to be at higher risk and were more worried about being infected with COVID-19 when compared to non-diabetic individuals (p < 0.001). During the COVID-19 pandemic, participants with diabetes were more likely to experience food and drug shortages and to increase their physical activity, compared to their counterparts. Among diabetic respondents, a high proportion of current smokers (74.1%) and drinkers (68.5%) reported increased amounts of smoking and drinking. People with diabetes were 11% less likely to report excellent or very good health. Having 150 min/week physical activity was positively associated with excellent or very good health (prevalence ratio, PR = 1.14, 95%CI 1.11–1.16).
Conclusions
A high proportion of people with diabetes perceived risk of COVID-19 infection and increased their smoking and drinking during the pandemic.
Keywords: COVID-19, Pandemic, Diabetes, Lifestyle, China
1. Introduction
Since the outbreak of the novel coronavirus disease (COVID-19) was first reported in Wuhan City in China in December 2019 [1], it has quickly become a global pandemic. A novel betacoronavirus, known as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was identified as the pathogen for COVID-19 [2]. China implemented a lockdown of Wuhan in late January 2020 to contain the spread of COVID-19. On March 11, 2020, the World Health Organization (WHO) declared COVID-19, which triggered severe pneumonia and acute, even lethal, lung failure, a pandemic [3]. COVID-19 infection is a double challenge for people with diabetes. It was reported that among COVID-19 patients admitted to hospitals, diabetes was the second most prevalent comorbidity (with a rate of approximately 10–20%) [4], [5], [6]. A growing body of clinical and population studies shows that patients with diabetes, when contracting SARS-CoV-2, are more likely to develop severe symptoms and complications [7], [8], and they have much higher admission rates in intensive care units. The risk of dying from COVID-19 was up to 50% higher for those with diabetes, particularly among elderly type 2 diabetes cases [9] than for people without diabetes [10], [11].
In addition to the direct vulnerability to the worst outcomes of COVID-19, people with diabetes can be affected by limited access to healthcare due to massive population lockdowns. Consequently, their health condition would become worse. Second, the prolonged period of quarantine and social distancing, although effective to contain the spread of the virus, is likely to have undesirable effects on the lifestyle behaviors and health and well-being of chronic disease patients, including those with diabetes. Third, during pandemics such as COVID-19, vulnerable populations, including women, the elderly, and those with chronic diseases, become more vulnerable due to their impaired ability to access and understand health information and make sound, informed decisions.
China has the largest number of diabetes patients in the world, and the number has been continually rising due to the improvement of living standards and increase of obesity. China has an estimated 116 million adults living with diabetes (10.9% total diabetes and 35.7% prediabetes) [12], [13], [14]. So far, it is unknown how Chinese diabetes patients perceived COVID-19 related risks and how they modified their health-related behaviors accordingly during the pandemic.
The objectives of this time-sensitive study are: (1) to examine perceived risk of COVID-19 and levels of worry among people with diabetes, and compare with the perceptions of those without diabetes; (2) to examine the health-related experiences and behaviors of people with diabetes when compared with those without diabetes, and (3) to examine factors associated with self-reported health status during the COVID-19 quarantine period in China.
2. Methods and materials
2.1. Study design and participants
The China COVID-19 Survey is a cross-sectional anonymous survey that was administered via WeChat, China's leading social network. Almost every Chinese adult uses WeChat daily, and many children use it as well. We selected this platform not only because the nation was under quarantine and we could only reach respondents online, but also because WeChat is China's leading social network with more than one billion users, and most Chinese adults use WeChat daily. Based on national usage statistics in 2020, the WeChat’s penetration rate in China is 78% among 16–64-year-olds [15]. Survey data collection was administered between April 25 and early May 11, 2020. We used both snowball and convenience sampling approaches to recruit a diverse sample in China.
The 2020 China COVID-19 Survey questionnaire has 74 items and provides about 150 study variables. It covers 8 comprehensive topics: (1) Awareness, knowledge, attitudes, and practices toward COVID-19, (2) personal experiences and impacts of COVID-19, (3) attitude toward government responses to COVID-19, (4) healthcare-seeking behaviors, (5) demographics characteristics, (6) lifestyle behaviors, (7) psychological well-being, and (8) health outcomes, including obesity and other non-communicable diseases (NCDs) during the COVID-19 pandemic. The Xi'an Jiaotong University Institutional Review Board approved study procedures, and participants provided consent online. Data includes a national sample of 10,545 adults aged>=18 years in 31 province-level administrative units.
For the present study, the study sample included 9,016 participants. Of the total, 8,432 have neither diabetes nor other NCDs (hypertension, heart disease, stroke, cancer, asthma, chronic lung disease, etc.) (n = 8,432; referred to as “people without diabetes” hereafter) and 585 people have been diagnosed as having diabetes (n = 585; referred to as “people with diabetes” hereafter). Those with other NCDs but not diabetes were excluded in the analysis. We focused on the following study variables: demographic characteristics, awareness and attitudes toward COVID-19, lifestyle behaviors, sleep, and quality of life.
3. Measurements
3.1. Demographic variables
Data on demographic characteristics, including gender, education, income, residence, and occupation were collected and then included as covariates in analysis models. For this study, we adapted the related questions used in the China Chronic Disease and Risk Factor (CCDRF) Survey [16] developed by the China Center for Disease Control Chronic Disease Control Center ().
3.2. Awareness and attitude toward COVID-19
Awareness and attitudes towards the COVID-19 were assessed using items adapted from a recent COVID-19 awareness, attitude, and action questionnaire [17]. Two questions asked participants to rate the likelihood they perceived that they or someone in the family might get infected with COVID-19 and levels of worry about getting coronavirus: (1) “How worried are you about being infected with COVID-19?” Responses include a 4-point scale: not worried at all, a little worried, somewhat worried, and very worried; and (2) “How likely do you think it is that you or someone in your family may get infected with the coronavirus this year?” Responses include a 4-point scale: very likely, somewhat likely, not that likely, and not at all likely.
3.3. Health-related experiences and behavior changes during COVID-19
We adopted the questionnaire developed by Conway and colleagues [18]. Two items, drug shortage and food shortage due to COVID-19, were used to measure coronavirus experiences. Measures of health-related behaviors, including changes in lifestyle behaviors (diet, physical activity, and sleep), were assessed using items from the Kadoorie Study of Chronic Disease in China (KSCDC) [19] and the CCDRF Survey [16]. These surveys were validated in the Chinese population. Body mass index (BMI) was calculated using self-reported weight and height. We used BMI>=24 to define overweight/obese [20]. Physical activity during COVID-19 was measured with items adapted from the International Physical Activity Questionnaire (IPAQ) [21]. We then calculated participants’ weekly physical activity minutes and whether they meet the 150 min weekly physical activity recommendation. Participants’ self-rated health [22] was measured by the most commonly used item from Patient-Reported Outcomes Measurement Information System (PROMIS) item with responses from poor to excellent.
3.4. Diabetes status
To assess chronic disease status, participants completed questions regarding the following questions: “Did they currently have a chronic disease? If so, they were to identify specific diseases or health conditions.” Healthy individuals who self-identified of having no existing chronic diseases were defined as “healthy people without diabetes,” those who self-identified as having diabetes was defined as “people with diabetes.” Those with other chronic diseases but no diabetes was excluded in the analysis.
3.5. Statistical analyses
We first described the distribution of sample characteristics including age, gender, education, income (low, medium, high based on tertiles), residence (city, town, and rural), smoking (non-smoker, current smoker, ex-smoker), and alcohol drinking (non-drinker, current drinker, ex-drinker), body mass index (BMI, based on reported weight and height), and weight status (classified based on BMI>=24 for overweight and >=28 for obesity, which are the Chinese national BMI cut points), and analysed these by the subjects’ diabetes status. Chi square test was used to compare differences between groups for categorical variables and ANOVA for continuous variables.
Second, we graphically presented the distributions of frequency of physical activity, change in smoking and alcohol drinking by perceived COVID-19 infection risk with bar charts. Then we performed a multivariable Poisson regression with robust variance to calculate prevalence ratio (PR) [23], to assess the association between diabetes status and lifestyle behaviour changes during COVID-19 pandemic with self-reported health (excellent/very good vs. others), while controlling for sociodemographic variables. It is usually preferable to use PRs instead of odds ratios (ORs) in cross-sectional studies when the prevalence of outcome measures is above 10% [24].
All the analyses were performed using STATA 16.1 (Stata Corporation, College Station, TX, USA). Statistical significance was considered when p < 0.05 (two-sided).
4. Results
4.1. Sample demographic characteristics
The sample characteristics by diabetes status are summarized in Table 1 . A total of 9,016 participants (3,839 men and 5,177 women) were included in the present study, of which 585 (6.5%) reported having diabetes. Compared to participants without diabetes, more participants with diabetes were men (60.3%) and older. They were more likely to live in cities (68.7%), had a lower level of education, a high level of income (47.4%), and were overweight (23.8%) or obese (10%). Notably, those with diabetes were more likely to be current smokers (43.6% vs. 11.8%, p < 0.001) and current alcohol drinkers (40.2% vs. 19.7%, p < 0.001), compared to those without diabetes.
Table 1.
Sample characteristics by diabetes status among participants of 2020 China COVID 19 online survey (N = 9,016).
| Total (n = 9,016) n (%) | No Diabetes (n = 8,431) n (%) | Diabetes (n = 585) n (%) | p-value | |
|---|---|---|---|---|
| Sex | <0.001 | |||
| Men | 3,839 (42.6) | 3486(41.4) | 353(60.3) | |
| Women | 5,177 (57.4) | 4945(58.7) | 232(39.7) | |
| Age group (years) | <0.001 | |||
| 18-29 | 4,407 (48.9) | 4,119 (48.9) | 288 (49.2) | |
| 30-39 | 3,011 (33.4) | 2,844 (33.7) | 167 (28.5) | |
| 40-49 | 1,082 (12.0) | 1,016 (12.1) | 66 (11.3) | |
| 50-59 | 437 (4.8) | 388 (4.6) | 49 (8.4) | |
| 60-80 | 79 (0.9) | 64 (0.8) | 15 (2.6) | |
| Education | <0.001 | |||
| Elementary school and below | 132 (1.5) | 95 (1.1) | 37 (6.3) | |
| Junior high/high school diploma | 3,204 (35.5) | 2,924 (34.7) | 280 (47.9) | |
| Bachelor’s degree or above | 5,680 (63.0) | 5,412 (64.2) | 268 (45.8) | |
| Residence | <0.001 | |||
| City | 5,550 (61.6) | 5,148 (61.1) | 402 (68.7) | |
| Town | 2,076 (23.0) | 1,980 (23.5) | 96 (16.4) | |
| Rural | 1,390 (15.4) | 1,303 (15.5) | 87 (14.9) | |
| Income levels (tertile) | <0.001 | |||
| Low | 3,081 (43.1) | 2,920 (43.9) | 161 (32.7) | |
| Medium | 1,846 (25.8) | 1,748 (26.3) | 98 (19.9) | |
| High | 2,222 (31.1) | 1,989 (29.9) | 233 (47.4) | |
| Occupation | <0.001 | |||
| Student | 1,876 (20.8) | 1,766 (20.9) | 110 (18.8) | |
| Employed | 5,997 (66.5) | 5,630 (66.8) | 367 (62.7) | |
| Unemployed | 930 (10.3) | 854 (10.1) | 76 (13.0) | |
| Retired | 213 (2.4) | 181 (2.1) | 32 (5.5) | |
| Smoking | <0.001 | |||
| Current smoker | 1,248 (13.8) | 993 (11.8) | 255 (43.6) | |
| None smoker | 7,150 (79.3) | 6,879 (81.6) | 271 (46.3) | |
| Ex-smoker | 618 (6.9) | 559 (6.6) | 59 (10.1) | |
| Alcohol drinking | <0.001 | |||
| Current drinker | 1,899 (21.1) | 1,664 (19.7) | 235 (40.2) | |
| None drinker | 6,294 (69.8) | 6,028 (71.5) | 266 (45.5) | |
| Ex-drinker | 823 (9.1) | 739 (8.8) | 84 (14.4) | |
| Body Mass Index | <0.001 | |||
| Underweight | 955 (10.7) | 878 (10.5) | 77 (14.0) | |
| Normal | 5,644 (63.3) | 5,357 (64.0) | 287 (52.2) | |
| Overweight | 1,705 (19.1) | 1,574 (18.8) | 131 (23.8) | |
| Obese | 614 (6.9) | 559 (6.7) | 55 (10.0) |
4.2. Perceived COVID-19 risk and worry, health-related experiences, and lifestyle behavior changes during COVID-19 pandemic
Table 2 describes participants’ self-perceived COVID-19 risk and worry, as well as their health-related experiences and lifestyle changes during COVID-19 pandemic by diabetes status. Participants with diabetes had a substantially increased likelihood of perceiving themselves to be “very likely” of becoming infected (40% in participants with diabetes vs. 8% in participants without diabetes, p < 0.001). Correspondingly, they were very worried about being infected with COVID-19 (54.5% in participants with diabetes vs 36% in participants without diabetes, p < 0.001). Slightly more than half (52.1%) of the subjects with diabetes reported their general health as “excellent,” while the prevalence was 41.0% among those without diabetes. During the COVID-19 pandemic, participants with diabetes were more likely to: experience food shortages (59.8%) and drug shortages (59.7%), increase in their physical activity over what it was prior to the pandemic (44.6%), have a higher proportion (73.8% vs. 58.1%, p < 0.001) of meeting weekly 150 min physical activity recommendations, report that COVID-19 had a high impact on their diet (43.1% vs. 10.7%, P < 0.001), and sleep<6 h per day (25.4% vs. 10.9%, p < 0.001), when compared to their counterparts. Among smokers, a high proportion reported significantly increased smoking during the pandemic (61.6% in participants with diabetes and 22.2% in participants without diabetes). A similar pattern was observed in the increase of alcohol drinking: 54% diabetic and 10.2% non-diabetic participants reported significant increases in drinking. Overall, 77.1% of the participants with diabetes and 82.1% of the participants without diabetes reported their health to be excellent/very good.
Table 2.
Self-perceived covid-19 risk and worry, health related experiences and lifestyle changes during COVID-19 pandemic, by diabetes status (n = 9,016).
| Total | No diabetes | Diabetes | p-value | |
|---|---|---|---|---|
| N = 9,016, n (%) | N = 8,431, n (%) | N = 585, n (%) | ||
| Self-perceived risk of infection and level of worry | ||||
| How likely do you think it is that you or someone in your family may get infected? | <0.001 | |||
| Very likely | 907 (10.1) | 673 (8.0) | 234 (40.0) | |
| Somewhat likely | 2,130 (23.6) | 1,972 (23.4) | 158 (27.0) | |
| Not that likely | 4,200 (46.6) | 4,065 (48.2) | 135 (23.1) | |
| Not at all likely | 1,779 (19.7) | 1,721 (20.4) | 58 (9.9) | |
| How worried are you about being infected with COVID-19? | <0.001 | |||
| Very worried | 3,352 (37.2) | 3,033 (36.0) | 319 (54.5) | |
| Somewhat worried | 3,157 (35.0) | 3,021 (35.8) | 136 (23.2) | |
| A little worried | 2,107 (23.4) | 1,997 (23.7) | 110 (18.8) | |
| Not worried at all | 400 (4.4) | 380 (4.5) | 20 (3.4) | |
| Self-reported overall health | ||||
| Excellent | 3,693 (41.0) | 3,388 (40.2) | 305 (52.1) | |
| Very good | 3,679 (40.8) | 3,533 (41.9) | 146 (25.0) | |
| Good | 1,442 (16.0) | 1,326 (15.7) | 116 (19.8) | |
| Fair | 187 (2.1) | 172 (2.0) | 15 (2.6) | |
| Poor | 15 (0.2) | 12 (0.1) | 3 (0.5) | |
| Food shortages | <0.001 | |||
| Yes | 2,937 (26.9) | 2,487 (25.0) | 350 (59.8) | |
| No | 7,708 (73.1) | 7,473 (75.0) | 235 (40.2) | |
| Drug shortages | <0.001 | |||
| Yes | 3,173 (30.1) | 2,824 (28.4) | 349 (59.7) | |
| No | 7,372 (69.9) | 7,136 (71.6) | 236 (40.3) | |
| Lifestyle behavior changes | ||||
| During COVID-19 epidemic, did you usually have more or less physical activity in a week than before? | <0.001 | |||
| Increased a lot | 1,608 (17.8) | 1,347 (16.0) | 261 (44.6) | |
| Increased a little | 2,257 (25.0) | 2,122 (25.2) | 135 (23.1) | |
| No change | 2,220 (24.6) | 2,106 (25.0) | 114 (19.5) | |
| Decreased a little | 1,559 (17.3) | 1,511 (17.9) | 48 (8.2) | |
| Decreased a lot | 1,372 (15.2) | 1,345 (16.0) | 27 (4.6) | |
| During COVID-19 epidemic, about how many hours on average per day did you do physical activity? , (in hours) | 1.2 (1.5) | 1.1 (1.4) | 2.0 (2.0) | <0.001 |
| PA 150 min/week | 5,333 (59.2) | 4,901 (58.1) | 432 (73.8) | <0.001 |
| How has your diet been impacted during COVID-19 epidemic? | <0.001 | |||
| High impact | 1,154 (12.8) | 902 (10.7) | 252 (43.1) | |
| Some impact | 2,967 (32.9) | 2,808 (33.3) | 159 (27.2) | |
| Average impact | 2,041 (22.6) | 1,936 (23.0) | 105 (17.9) | |
| Minimal impact | 2,044 (22.7) | 1,990 (23.6) | 54 (9.2) | |
| No impact at all | 810 (9.0) | 795 (9.4) | 15 (2.6) | |
| During COVID-19, how many hours of sleep did you get each day on average? | 7.8 (1.2) | 7.9 (1.2) | 7.6 (1.6) | <0.001 |
| Sleep duration | <0.001 | |||
| <=6 h | 1,026 (11.7) | 897 (10.9) | 129 (25.4) | |
| 6 – 8 h | 5,787 (66.1) | 5,523 (66.9) | 264 (52.1) | |
| >8 h | 1,944 (22.2) | 1,830 (22.2) | 114 (22.5) | |
| During COVID-19, did your smoke more or less than before? | <0.001 | |||
| Increase a lot | 377 (30.2) | 220 (22.2) | 157 (61.6) | |
| Increase some | 237 (19.0) | 205 (20.6) | 32 (12.5) | |
| No change | 356 (28.5) | 306 (30.8) | 50 (19.6) | |
| Decrease some | 185 (14.8) | 177 (17.8) | 8 (3.1) | |
| Decrease a lot | 93 (7.5) | 85 (8.6) | 8 (3.1) | |
| How do you compare your alcohol consumptions during COVID-19 compared to your ty | <0.001 | |||
| Increase a lot | 296 (15.6) | 169 (10.2) | 127 (54.0) | |
| Increase some | 289 (15.2) | 255 (15.3) | 34 (14.5) | |
| No change | 632 (33.3) | 586 (35.2) | 46 (19.6) | |
| Decrease some | 326 (17.2) | 315 (18.9) | 11 (4.7) | |
| Decrease a lot | 356 (18.7) | 339 (20.4) | 17 (7.2) |
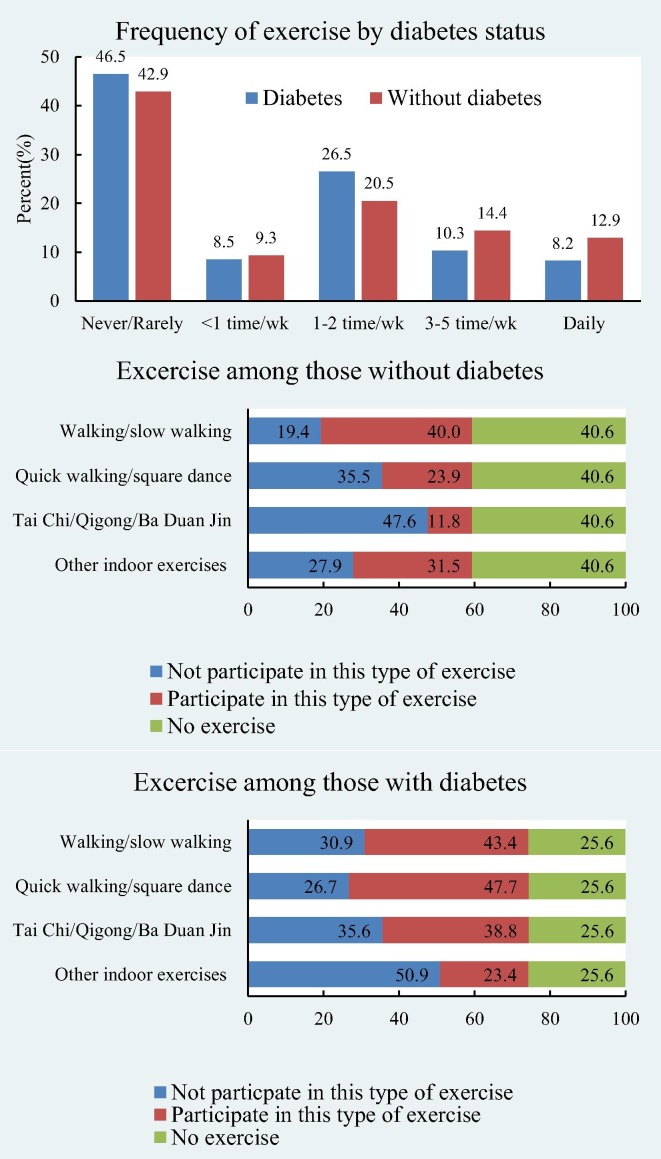
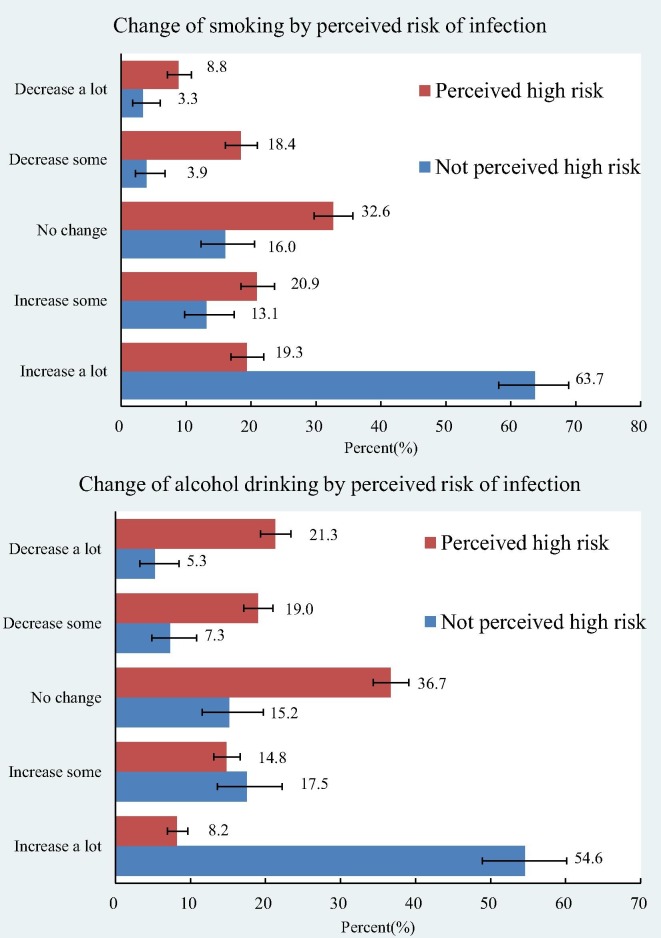
The prevalence of daily exercise was 12.9% in participants with diabetes vs. 8.2% in participants without diabetes (Fig. 1 ). When asked about participating in certain types of exercise, people with diabetes (when participated in physical activity) were more likely to do Tai Chi and/or Qigong than the others (38.8% vs 11.8%). Perceived high COVID-19 infection risk was associated with increases in smoking and drinking (Fig. 2 ). Among those who perceived high infection risk, 63.7% of the smokers increased smoking a lot and 54.8% drinkers increased drinking a lot. The corresponding figures were only 19.3% and 8.2% among those individuals that did not perceive themselves to be at a higher risk of infection.
Fig. 1.
Frequency and types of exercise during COVID-19 pandemic by diabetes status.
Fig. 2.
Perceived COVID-19 infection risk and change in smoking and drinking.
4.3. Multivariate analyses of associations between diabetes and lifestyle behavior changes during COVID-19 pandemic with self-reported health
Findings (Table 3 ) from the multivariable Poisson regression model showed associations between diabetes and lifestyle behavior changes during COVID-19 pandemic with self-reported health, controlling for demographic characteristic. The model showed that those with diabetes were 11% less likely to report having excellent/very good health. Obtaining 150 min/week physical activity was positively associated with excellent/very good health (PR 1.14, 95%CI 1.11–1.16). Compared with those who reported no change of PA, those individuals who increased PA levels were more likely to report excellent/very good health. Compared with those who obtained 6–8 h of sleep per day, participants who got less than 6 h of sleep were 12% less likely to report excellent/very good health. Changes in drinking were not associated with self-reported health outcomes. Surprisingly, those who reported increases in smoking were more likely to have excellent/good health.
Table 3.
Factors associated with self-reported excellent/very good health among adults during COVID-19 pandemic.
| PR | 95%CI | |
|---|---|---|
| Having diabetes | 0.89 | 0.84–0.93 |
| Age groups | ||
| 18-29 | 1.00 | |
| 30-39 | 0.99 | 0.97–1.01 |
| 40-49 | 0.96 | 0.93–1.00 |
| 50-59 | 0.95 | 0.90–1.00 |
| 60-80 | 0.86 | 0.73–1.00 |
| Gender | ||
| Men | 1.00 | |
| Women | 0.97 | 0.95–1.00 |
| Residence | ||
| City | 1.00 | |
| Town | 1.03 | 1.00–1.05 |
| Rural | 0.97 | 0.95–1.00 |
| Education | ||
| Elementary school and below | 1.00 | |
| Junior high/high school diploma | 1.02 | 0.93–1.12 |
| Bachelor’s degree or above | 0.98 | 0.89–1.08 |
| Change of smoking behavior | ||
| No change (smokers) | 1.00 | |
| Increased a lot | 1.08 | 1.00–1.16 |
| Increased a little | 0.97 | 0.89–1.05 |
| Decreased a little | 1.00 | 0.92–1.08 |
| Decreased a lot | 1.07 | 0.97–1.18 |
| None smoker | 1.03 | 0.97–1.08 |
| Change in drinking behavior | ||
| No change (smokers) | 1.00 | |
| Increased a lot | 0.97 | 0.90–1.04 |
| Increased a little | 0.97 | 0.91–1.03 |
| Decreased a little | 1.03 | 0.97–1.09 |
| Decreased a lot | 0.97 | 0.91–1.04 |
| None drinkers | 0.99 | 0.95–1.03 |
| PA 150 min/week | 1.14 | 1.11–1.16 |
| Change in PA | ||
| No change | 1.00 | |
| Increased a lot | 1.11 | 1.08–1.14 |
| Increased a little | 1.07 | 1.04–1.10 |
| Decreased a little | 0.99 | 0.95–1.02 |
| Decreased a lot | 0.87 | 0.83–0.90 |
| Sleep duration | ||
| 6-8 h | 1.00 | |
| <6 h | 0.88 | 0.84–0.91 |
| >8 h | 1.00 | 0.98–1.02 |
| BMI categories | ||
| Normal | 1.00 | |
| Underweight | 0.96 | 0.93–0.99 |
| Overweight | 1.01 | 0.98–1.03 |
| Obese | 0.94 | 0.90–0.98 |
Note: PA = physical activity; all the variables in the table were mutually adjusted for in models.
5. Discussion
Risk factor assessments based on pandemic data from multiple countries, although still notably limited [7], [8], [9], [10], [11], [25], indicate that those at higher risk for severe illness from COVID-19 include older males, and people with obesity and related comorbidities such type 2 diabetes. As China has the highest number of obese and diabetic patients worldwide (about 46% of adults are obese or overweight) [26], [27], and 116 million adults living with diabetes (10.9% total diabetes and 35.7% prediabetes [12]), it is critical to understand how COVID-19 impacts Chinese adults, especially those with chronic conditions like diabetes and overweight/obesity, which likely coexist. To our knowledge, this study is the first sizeable nationwide survey to examine Chinese diabetics and healthy non-diabetic residents’ attitudes towards COVID-19 risks and the impact of COVID-19 on health-related behavior changes, and self-related health during the home quarantine period in China. The survey respondents were from all the provinces, municipal cities, and special administrative regions of China.
Our study shows that Chinese adults with diabetes were more likely to perceive a high risk of COVID-19 infection and high level of worry than those without diabetes. Literature has shown that food insecurity is an important risk factor for type 2 diabetes.[28] People with diabetes are more dependent on the regularity of food and medicine and therefore this may have caused the perceived risk of food and drug shortage. Concurrently, a higher proportion of individuals with diabetes reported an increase in smoking and drinking than those without diabetes in our sample. We attempted to investigate the association between self-reported health and the alcohol drinking and smoking. We found that those who increased smoking were more likely to have excellent/good health. Although this cross-section data does not allow us to draw a causal conclusion, we propose two explanations for future studies to validate. First, increased alcohol consumption and smoking may be related to elevated anxiety and stress due to prolonged isolation and uncertainly during the COVID-19 pandemic. When people’s lives are stressful, alcohol consumption and tobacco smoking may become a coping approach for their stress. Second, the controversial, unproven but popular social media claims that [29] tobacco or nicotine could reduce the risks of COVID-19 may play a role in shaping at-risk individuals’ behaviors. With around 314 million adult smokers, and accounts for nearly one-third (30%) of smokers worldwide in China [30], the use of tobacco products is of particular concern during the COVID-19 pandemic. We advocate for more research to examine the role of smoking in the contraction, transmission, and mortality rate of COVID-19.
Previous research suggest that alcohol consumption is a potential risk factor for type 2 diabetes [31], [32]. Regardless of the amount of alcohol a person consumes, alcohol consumption can weaken the immune systems [33]. People with problematic drinking behaviors can be among the most vulnerable populations for COVID-19 infection [34]. Given the high rates of smoking and drinking among those with diabetes, countries should allocate resources to health stimulus packages, scientific research, and health promotion campaigns to further reduce alcohol drinking and smoking rates.
The COVID-19 pandemic casts new light on some old problems: medication access, drug shortages, and food shortages. Our findings showed that diabetic patients were at higher risk for drug and food shortages. Although some global supply disruptions and shortages cannot be predicted or prevented, government agencies and healthcare providers can provide telemedicine, mail order pharmacy, home delivery, and drive-thru or curbside pick-up of groceries or medications in order to mitigate the shortages while enhancing social distancing.
While China is slowly beginning to reopen since lifting the lockdown in mid-May, it is of interest to understand how people coped with stress during the time of social distancing and home quarantine in China. Our findings indicate that having an adequate amount of physical activity (i.e., engaging in weekly physical activity for 150 min or more) and increasing physical activity during pandemic were positively associated with self-reported excellent/very good health. Insufficient sleep was more common in people with diabetes than people without diabetes and was related to poor health. These findings further highlight the importance of the promotion of health and wellness during COVID-19.
This study has some strengths and limitations. To our knowledge, no studies to date have reported findings related to perceived infection risks and the impacts of COVID-19 on a large sample of people with diabetes on health-related outcomes and lifestyle behavior changes in China, where COVID-19 was first reported, and many vigorous nationwide measures were taken to control its spread. Our findings may shed light on how to target or manage risk factors (i.e., drinking and smoking) for the diabetic population during the pandemic. Given the anonymous design of the online survey, such an approach can work better to collect people's honest answers, especially about sensitive information; and it is more feasible in special situations like COVID-19, where face-to-face interviews are difficult.
This study has some limitations. First, potential self-selection bias is common in survey data, including ours due to the use of convenience and snowball sampling method. Our sample may also have bias related to age or education since 45.8% of diabetic adults have a bachelor's degree in comparison to 64% of non-diabetic adults. However, some health indicator rates from our data, such as rates of overweight/obesity and diabetes, were consistent with those from other national survey data [27], [35]. Second, the sample was relatively young (48.9% of participants were 18–29 years old and 17.7% 40 years old and above). Therefore, the observations are limited and cannot be extrapolated to older individuals who likely have different perceptions of the COVID-19 pandemic. Third, the survey questionnaire did not ask participants to identify the specific type of diabetes that they had been diagnosed. Although we did not attempt to distinguish type 1 diabetes from type 2 by self-report, the vast majority of diabetes cases in China are currently type 2 [13]. Fourth, the self-reporting scale used for changes in smoking and drinking was crude and subjective (changed a lot, change a little, et al). We did not collect the baseline data for tobacco or alcohol consumptions prior to COVID-19 and cannot really quantify the changes. In addition to collecting data on the self-reported changes in smoking and alcohol consumption for the current diabetic smokers or drinkers during COVID-19 as this study did, it is also important to know the prevalence of initiation of smoking/drinking during the COVID-19 pandemic. Fifth, the number and/or types of medications taken as well as duration of diabetes were not collected in the survey. This was because stratified analysis by number of medications taken by participants would require a much larger sample size. This was beyond the scope of the current investigation. Lastly, a review by Dryhurst and colleagues [36] pointed out that many rapid-response studies almost exclusively rely on single-item measures of risk perception, selectively tapping into either cognitive or emotional dimensions. This study’s two-item measures of risk perception share the above-mentioned limitation. Risk perception is a subjective psychological construct that is influenced by cognitive, emotional, social, cultural, and individual variation both between individuals and between different countries [36]. We encourage future studies to include theory-based measures of risk perception that involve multidimension determinants of risk perception.
In conclusion, our results showed that Chinese adults with diabetes perceived themselves at high risk for COVID-19 infection and were more worried about being infected when compared to individuals without diabetes. They reported experiences with shortages of food and medication and exhibited adverse lifestyle behaviors and declined health. Even though we cannot identify precisely what caused the fear, it may be hypothesized that imprecise information and instructions of being at higher risk for COVID-19 and death in people with diabetes by media and government, without necessary follow-up and support structures in place, created the anxiety. This is indeed a wake-up call, a tocsin, to the world on the importance of primary prevention and providing good support to chronic disease patients, including those with diabetes.
Acknowledgments
Acknowledgements
The authors would like to thank the study participants, collaborators, and staff members who contributed to this study. In particular, we would like to thank Professors Shiyong Liu, Peng Ni and Qianli Xue, and Mrs. Lihua Yan.
Author contributions
A.F.Y, Y.W, Z.S. contributed to the study design, data collection, and drafting the report. All authors contributed to interpretation of the data, commented on and revised the report, and approved the final version for publication.
Sources of Funding
The project is supported in part by research grants from the China Medical Board (Grant number: 16-262) and Xi’an Jiaotong University Global Health Institute.
Declaration of Competing Interest
None.
References
- 1.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. The Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 3.Gupta R., Ghosh A., Singh A.K., Misra A. Clinical considerations for patients with diabetes in times of COVID-19 epidemic. Diab Metab Synd. 2020;14:211–212. doi: 10.1016/j.dsx.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England). 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Resp Med. 2020;8 doi: 10.1016/S2213-2600(20)30116-8. E21-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y., Li H., Zhang J., Cao Y., Zhao X., Yu N. Observational Study in Wuhan; Diabetes Obes Metab: 2020. The Clinical Characteristics and Outcomes of Diabetes Mellitus and Secondary Hyperglycaemia Patients with Coronavirus Disease 2019: a Single-center, Retrospective. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bornstein S.R., Rubino F., Khunti K., Mingrone G., Hopkins D., Birkenfeld A.L. Practical recommendations for the management of diabetes in patients with COVID-19. The Lancet Diab Endocrinol. 2020;8:546–550. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan Y., Yang Y., Wang F., Ren H., Zhang S., Shi X. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diab Res Care. 2020;8 doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Diabetes Association. How COVID-19 Impacts People With Diabetes; 2020.
- 12.Wang L., Gao P., Zhang M., Huang Z., Zhang D., Deng Q. Prevalence and Ethnic Pattern of Diabetes and Prediabetes in China in 2013. JAMA. 2017;317:2515–2523. doi: 10.1001/jama.2017.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu C., Jia W. Diabetes in China: Epidemiology and Genetic Risk Factors and Their Clinical Utility in Personalized Medication. Diabetes. 2018;67:3. doi: 10.2337/dbi17-0013. [DOI] [PubMed] [Google Scholar]
- 14.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Research and Clinical Practice. 2019;157. [DOI] [PubMed]
- 15.Iqbal M. WeChat Revenue and Usage Statistics Business of Apps; 2020.
- 16.Yao Y., Liu G., Wang L., Zhao H., Zhao Z., Zhang M. Disease and disparity in China: a view from stroke and MI disease. Int J Equity Health. 2019;18:85. doi: 10.1186/s12939-019-0986-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf M.S., Serper M., Opsasnick L., O'Conor R.M., Curtis L.M., Benavente J.Y. Awareness, Attitudes, and Actions Related to COVID-19 Among Adults With Chronic Conditions at the Onset of the U.S. Outbreak. Ann Intern Med. 2020 doi: 10.7326/M20-1239. 0:null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conway L.G., Woodard Shailee R., Zubrod A. Impacts, and Experiences Questionnaires. National Institute of Health; Government Response: 2020. Social Psychological Measurements of COVID-19: Coronavirus Perceived Threat. [Google Scholar]
- 19.Chen Z., Lee L., Chen J., Collins R., Wu F., Guo Y. Cohort Profile: The Kadoorie Study of Chronic Disease in China (KSCDC) Int J Epidemiol. 2005;34:1243–1249. doi: 10.1093/ije/dyi174. [DOI] [PubMed] [Google Scholar]
- 20.Zhou B. Predictive values of body mass index and waist circumference to risk factors of related diseases in Chinese adult population. Zhonghua Liu Xing Bing Xue Za Zhi. 2002;23:5–10. [PubMed] [Google Scholar]
- 21.Lee P.H., Macfarlane D.J., Lam T.H., Stewart S.M. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act. 2011;8:115. doi: 10.1186/1479-5868-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hays R.D., Spritzer K.L., Thompson W.W., Cella D.U.S. General Population Estimate for “Excellent” to “Poor” Self-Rated Health Item. J Gen Intern Med. 2015;30:1511–1516. doi: 10.1007/s11606-015-3290-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barros A.J.D., Hirakata V.N. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Method. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen M.R., Deddens J.A. A comparison of two methods for estimating prevalence ratios. BMC Med Res Method. 2008;8:9. doi: 10.1186/1471-2288-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamara A., Tahapary D.L. Obesity as a predictor for a poor prognosis of COVID-19: A systematic review. Diab Metab Syndr. 2020;14:655–659. doi: 10.1016/j.dsx.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y., Wang L., Qu W. New national data show alarming increase in obesity and noncommunicable chronic diseases in China. Eur J Clin Nutr. 2017;71:149–150. doi: 10.1038/ejcn.2016.171. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y., Xue H., Sun M., Zhu X., Zhao L., Yang Y. Prevention and control of obesity in China. Lancet Glob Health. 2019;7:e1166–e1167. doi: 10.1016/S2214-109X(19)30276-1. [DOI] [PubMed] [Google Scholar]
- 28.Najibi N., Firoozi R., Shahrezaee S., Eshraghian M., Daneshi-Maskooni M., Dorosty-Motlagh A. Food insecurity is an important risk factor for type 2 diabetes: a case-control study of new referrals to the University clinics, Shiraz, Southern Iran. BMC Public Health. 2019;19:885. doi: 10.1186/s12889-019-7236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engin A.B., Engin E.D., Engin A. Two important controversial risk factors in SARS-CoV-2 infection: Obesity and smoking. Environ Toxicol Pharmacol. 2020;78 doi: 10.1016/j.etap.2020.103411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parascandola M., Xiao L. Tobacco and the lung cancer epidemic in China. Transl Lung Cancer Res. 2019;8:S21–S30. doi: 10.21037/tlcr.2019.03.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pietraszek A., Gregersen S., Hermansen K. Alcohol and type 2 diabetes. A review. Nutr, Metabolism Cardiovasc Diseases. 2010;20:366–375. doi: 10.1016/j.numecd.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Cullmann M., Hilding A., Östenson C.-G. Alcohol consumption and risk of pre-diabetes and type 2 diabetes development in a Swedish population. Diabet Med. 2012;29:441–452. doi: 10.1111/j.1464-5491.2011.03450.x. [DOI] [PubMed] [Google Scholar]
- 33.Sarkar D., Jung M.K., Wang H.J. Alcohol and the Immune System. Alcohol Res: Curr Rev. 2015;37:153–155. [Google Scholar]
- 34.Da B.L., Im G.Y., Schiano T.D. COVID-19 Hangover: A Rising Tide of Alcohol Use Disorder and Alcohol-Associated Liver Disease. Hepatology. 2020 doi: 10.1002/hep.31307. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y.F., Sun M.X., Xue H., Zhao W.H., Yang X.G., Zhu X.Y. Understanding the China Blue Paper on Obesity Prevention and Control and policy implications and recommendations for obesity prevention and control in China. Zhonghua Yu Fang Yi Xue Za Zhi. 2019;53:875–884. doi: 10.3760/cma.j.issn.0253-9624.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Dryhurst S., Schneider C.R., Kerr J., Freeman A.L.J., Recchia G., van der Bles A.M. Risk perceptions of COVID-19 around the world. J Risk Res. 2020;1–13 [Google Scholar]