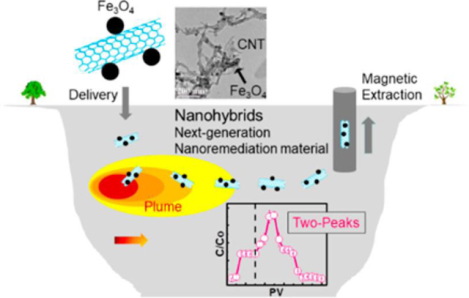
Abstract
Carbon—metal oxide nanohybrids (NHs) are increasingly recognized as the next-generation, promising group of nanomaterials for solving emerging environmental issues and challenges. This research, for the first time, systematically explored the transport and retention of carbon nanotube—magnetite (CNT–Fe3O4) NH aggregates in water-saturated porous media under environmentally relevant conditions. A macromolecule modifier, carboxymethylcellulose (CMC), was employed to stabilize the NHs. Our results show that transport of the magnetic CNT–Fe3O4 NHs was lower than that of nonmagnetic CNT due to larger hydrodynamic sizes of NHs (induced by magnetic attraction) and size-dependent retention in porous media. Classical Derjaguin–Landau–Verwey–Overbeek (DLVO) theory can explain the mobility of NHs under varying experimental conditions. However, in contrast with colloid filtration theory, a novel transport feature—an initial lower and a following sharp—higher peaks occurred frequently in the NHs’ breakthrough curves. The magnitude and location of both transport peaks varied with different experimental conditions, due to the interplay between variability of fluid viscosity and size-selective retention of the NHs. Promisingly, the estimated maximum transport distance of NHs ranged between ~0.38 and 46 m, supporting the feasibility of employing the magnetically recyclable CNT–Fe3O4 NHs for in situ nanoremediation of contaminated soil, aquifer, and groundwater.
Graphical Abstract
Introduction
Nanoscience and nanotechnology are advancing our broad societal goals in various fields including but not limited to medicine (e.g., magnetic resonance imaging),(1) agriculture (e.g., nutrient nanocapsules and nanosensors),(2) energy (e.g., solar cells and supercapacitors),(3) and environmental remediation (e.g., photocatalysts).(4, 5) They are unprecedentedly maximizing human potentials toward many frontiers such as addressing the water–energy–agriculture–environment (WEAE) nexus.(6) Recently, the focus of interest in nanomaterial (NM) research and development has shifted from singular NMs to multicomponent nanohybrids (NHs), which are nano/hierarchical assemblies of multiple NMs conjugated by noncovalent (hydrogen bond, van der Waals (vdW), and electrostatic interactions) or covalent (molecular linkage) bonds.(7–9) The goal of developing NHs is to maximize the existing functionality (e.g., contaminant adsorption and degradation efficiency) and achieve novel functionality that cannot be obtained by manipulating the singular NM system.(7–9) Taking environmental remediation as an example, the carbon nanotube (CNT) individually shows limited adsorption capacity toward metoprolol. When heterohybridized with nanoscale zerovalent iron (nZVI), the conjugated CNT–nZVI NHs exhibit significantly higher and faster adsorption and degradation efficiency for metoprolol (compared to CNT) due to a greater specific surface area (SSA) and Fenton-type catalytic reactions in the CNT-nZVI nanostructures.(10) Promisingly, compared to the single-component NMs, the performance and functionality of NHs are optimized. This is particularly true for carbon-family-based metal oxide NHs. Specifically, the photoelectrocatalytic reactivity and sensitivity of CNT/graphene oxide (GO)—FexOy/TiO2/ZnO/MnO2/SiO2/Ag for removing toxic gases (NOx),(11) organic pollutants (dyes and phenolic compounds),(10, 12) heavy metals (As(III)/As(V), Cd2+, Cr(VI), Pb2+, and Hg2+),(12, 13) and radionuclides (U(VI))(14, 15) are demonstrated to be greater than those of singular NMs. This is attributed to the combinative/synergistic effects within the hybridized nanostructure system.(7–9)
The superior performance and multifunctionality of carbon—metal oxide NHs make them the next-generation, promising candidates for in situ nanoremediation of contaminated air, soil, aquifer, and groundwater. It is also anticipated that increasing production and use of NHs will result in their release to the environment. However, to date, little is known about their fate and transport in the subsurface, which will likely restrict their widespread applications in resolving issues and meeting challenges within WEAE nexus. Over the past decade, the fate and transport of individual NMs such as carbonaceous (CNT(16–19) and graphene(20–22)) and iron-bearing NMs (nZVI(23–25) and magnetite (Fe3O4)(26, 27)) in the subsurface have been well explored. It is noted that colloid science principles, Derjaguin–Landau–Verwey–Overbeek (DLVO) theory(28, 29) and colloid filtration theory (CFT),(30) can describe the colloidal stability and transport of individual NMs in aquatic environments. Nonetheless, deviations between experimental observations and theoretical predictions occur frequently under unfavorable attachment conditions or with varying properties (e.g., irregular shapes and surface modifications) of NMs. For example, under unfavorable conditions, nonexponential retention(31) of NMs was frequently encountered in porous media (e.g., hyperexponential retention for tubular CNT).(16) Surface modifications(32) due to surfactant and polyelectrolyte coatings impart non-DLVO interactions (steric hindrance) and change the rheological properties (viscosity) of fluid in porous media,(33) likely yielding unanticipated transport behaviors for NMs. CFT predicts that transport of colloids in porous media is controlled by random Brownian motion and that interception and gravitational sedimentation start to dominate colloid deposition when the particle diameter is ≥1 μm.(30, 34) Once released to the environment, the transport of individual NMs is dependent on the interplay of various physicochemical conditions including (1) NM-specific properties (e.g., size/shape/hydrophilicity/aggregation state/surface chemistry), (2) medium-specific properties (e.g., grain size/shape/surface chemistry/moisture content), (3) solution chemistries (e.g., pH/ionic strength and composition (IS and IC)/presence of natural organic matter (NOM)), and (4) hydrodynamic conditions (e.g., flow rate/temperature/oxygen content).(35, 36) However, it is unclear whether the colloid science principles used for describing the environmental behaviors of individual NMs hold true for the assembled NHs, e.g., how and to what extent (e.g., qualitatively, semiquantitatively, or quantitatively) the DLVO theory and CFT explain NHs’ transport in the subsurface. In particular, NHs’ new features such as altered hydrophilicity, magnetism, DLVO-type (e.g., vdW attraction) interactions, and enhanced population heterogeneity (e.g., size distribution, morphology, surface defect and roughness, and charge heterogeneity; compared to individual NMs) likely impact their aggregation and transport propensities in the subsurface.(9, 37)
Assembling magnetic iron oxide (e.g., Fe3O4) NMs having high redox potential and contaminant immobilization capacity(38–40) with CNT having superlative mechanical strength and large SSA(41) provides a powerful strategy (e.g., synergistic adsorption and redox degradation) for in situ capturing and decomposing/detoxificating both inorganic and organic pollutants. This study systematically investigated the transport of the multifunctional CNT–Fe3O4 NHs in water-saturated porous media under environmentally relevant conditions. An environmentally friendly and “green” (nontoxic and biodegradable) macromolecule carboxymethylcellulose (CMC) was employed to stabilize the CNT–Fe3O4 NHs as they were highly hydrophobic and easily aggregated. The CMC has long been used to stabilize individual nZVI(23, 42, 43) and Fe3O4 NMs(44) by imparting electrosteric repulsions arising from the hydrophilic, negatively charged carboxymethyl (−CH2–COOH) groups. Critical parameters characterizing the NHs’ mobility in water-saturated porous media were evaluated to shed light on the feasibility of using the recyclable CNT–Fe3O4 NHs for in situ nanoremediation of contaminated sites. DLVO theory and CFT were employed in combination with electrokinetic property and hydrodynamic size of NHs to understand the mechanisms behind their transport and retention within the porous media. Significant efforts were devoted to explore how DLVO theory and CFT explain the variability in NHs’ transport under varying experimental conditions. Finally, potential limitations and future research directions of DLVO theory/CFT in describing NHs’ transport in the subsurface were also discussed.
Materials and Methods
Preparation of CNT–Fe3O4 NH Influents and Solution Chemistries
Carbon nanotube (purity > 99.9%, outer diameter = 8–15 nm, inside diameter = 3–5 nm, length = 10–50 μm, and carboxyl group content = 1.85%) was purchased from the Cheap Tubes Inc. (Grafton, VT). The CNT–Fe3O4 NHs were synthesized in house, and their physicochemical properties including those of purchased CNT were characterized using X-ray diffraction (XRD), transmission electron microscopy (TEM), Raman spectroscopy, and thermogravimetric analysis (TGA) (see Supporting Information (SI) S1). The synthesized CNT–Fe3O4 NHs were highly hydrophobic because most hydrophilic carboxyl groups on the CNT surfaces were used for anchoring CNT and magnetite during synthesis.
The NHs were easily aggregated due to strong vdW and magnetic attractions (magnetism was confirmed using a magnet). Various surfactants (sodium dodecyl sulfate (SDS), Triton X-100, and Tween 20), polyelectrolytes (poly(acrylic acid) (PAA), poly(vinyl alcohol) (PVA), polyvinylpyrrolidone (PVP)), and macromolecules (CMC; nominal molecular weight = 90 000 g/mol; Sigma-Aldrich) were tested to investigate the NHs’ colloidal stability. By monitoring the UV–vis response of absorbance for the NHs under different surfactants, polyelectrolytes, and macromolecules, we found that only the CMC at a concentration of 2% well stabilized the NHs (see SI S2). Consequently, 2% CMC was chosen as the “modifier” to stabilize the CNT–Fe3O4 NHs for column experiments (described below).
Environmentally relevant solution chemistries were selected to evaluate the mobility of CNT–Fe3O4 NHs in porous media including monovalent (0, 1, 10, and 50 mM NaCl) and divalent electrolytes (0, 0.33, 1.67, and 3.33 mM CaCl2; note IS of 0, 0.33, 1.67, and 3.33 mM CaCl2 is 0, 1, 5, and 10 mM, respectively), the presence of NOMs (0, 1, and 10 mg C/L Suwannee River humic acid (SRHA) and fulvic acid (SRFA)) (details on preparing SRHA/SRFA stock suspensions were given in SI S3), pHs (4.0, 7.3, and 10), and particle concentrations (10, 25, and 50 mg/L) (Table 1).(45) Two percent CMC-stabilized CNT–Fe3O4 NH influents (via adding a predetermined volume of 5% CMC stock suspension; S3) at the desired solution chemistries were freshly prepared via ultrasonication at 100 W and 42 kHz for 1 h (Branson 3510R-DTH sonicator). Changes in concentration and average hydrodynamic radius (rh) of CNT–Fe3O4 NHs in the influent suspensions were monitored over the time frame of influent injection experiments (described below) to investigate their colloidal stability (aggregation propensity). The influent concentration changes of NHs were determined spectrophotometrically at 218 nm (SI Figure S1). Time-resolved dynamic light scattering (DLS) was used to monitor the rh change of NHs in influents using the Zetasizer Nano-ZS ZEN3600 analyzer (Malvern Instruments Inc.) at a scattering angle of 173° and 25 °C (see SI S4).
Table 1.
Physicochemical Properties of Column Transport Experiments and Mass Recoveries of CNT–Fe3O4 NHs (in 2% CMC) under Different Experimental Conditionsa
| mass recovery (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| NaCl (mM) | CaCl2 (mM) | SRHA (mg C/L) | SRFA (mg C/L) | pH | particle conc (mg/L) | sand size (μm) | Meff | Mret | Mtot |
| 0 | 0 | 0 | 0 | 7.3 | 10 | 360 | 88.3 ± 0.02 | 12.8 ± 0.25 | 101 ± 0.23 |
| 1 | 0 | 0 | 0 | 7.3 | 10 | 360 | 79.9 ± 2.4 | 25.0 ± 0.49 | 105 ± 2.9 |
| 10 | 0 | 0 | 0 | 7.3 | 10 | 360 | 37.8 ± 0.67 | 62.2 ± 1.1 | 100 ± 0.41 |
| 50 | 0 | 0 | 0 | 7.3 | 10 | 360 | 20.3 ± 0.88 | 84.2 ± 0.18 | 104 ± 1.1 |
| 0 | 0.33 | 0 | 0 | 7.3 | 10 | 360 | 57.4 ± 2.0 | 44.2 ± 2.2 | 102 ± 0.29 |
| 0 | 1.67 | 0 | 0 | 7.3 | 10 | 360 | 22.7 ± 4.4 | 73.1 ± 0.9 | 95.8 ± 3.5 |
| 0 | 3.33 | 0 | 0 | 7.3 | 10 | 360 | 16.2 ± 0.84 | 86.9 ± 1.4 | 103 ± 2.3 |
| 1 | 0 | 1 | 0 | 7.3 | 10 | 360 | 86.9 ± 1.5 | 14.2 ± 0.28 | 101 ± 1.2 |
| 1 | 0 | 10 | 0 | 7.3 | 10 | 360 | 97.6 ± 3.8 | 8.0 ± 0.28 | 106 ± 4.1 |
| 1 | 0 | 0 | 1 | 7.3 | 10 | 360 | 83.1 ± 2.6 | 14.9 ± 0.72 | 98.0 ± 1.9 |
| 1 | 0 | 0 | 10 | 7.3 | 10 | 360 | 92.2 ± 1.2 | 9.7 ± 0.77 | 102 ± 0.41 |
| 1 | 0 | 0 | 0 | 4.0 | 10 | 360 | 35.0 ± 0.58 | 66.1 ± 1.8 | 101 ± 2.4 |
| 1 | 0 | 0 | 0 | 10 | 10 | 360 | 89.5 ± 0.67 | 16.1 ± 0.49 | 106 ± 1.2 |
| 0 | 0 | 0 | 0 | 7.3 | 25 | 360 | 98.5 ± 1.2 | 8.0 ± 0.36 | 107 ± 1.5 |
| 0 | 0 | 0 | 0 | 7.3 | 50 | 360 | 104 ± 0.49 | 4.0 ± 0.30 | 108 ± 0.19 |
| 1 | 0 | 0 | 0 | 7.3 | 10 | 280 | 28.6 ± 1.8 | 64.9 ± 0.89 | 93.5 ± 2.7 |
| 1 | 0 | 0 | 0 | 7.3 | 10 | 230 | 97.4 ± 0.36 | 6.1 ± 0.42 | 104 ± 0.79 |
SRHA and SRFA are Suwannee River humic acid and Suwannee River fulvic acid, respectively. Conc is concentration. Meff, Mret, and Mtot are mass percentages of CNT–Fe3O4 NHs recovered from effluent, retentate, and total column, respectively. Other column transport parameters such as porosity, pore–water velocity, and dispersivity are shown in SI Table S1.
Porous Media
Ottawa sands (U.S. Silica, Berkeley Springs, WV) were chosen as representative aquifer materials for column experiments. The sands were presifted through 40, 50, 60, and 70 mesh sieves (U.S. Standard Testing Sieves), and the fractions captured by 40–50, 50–60, and 60–70 mesh sieves (average grain sizes of 360, 280, and 230 μm, respectively) were used. Before use, the sieved sands were cleaned using 1 M HCl and washed thoroughly with deionized (DI) water (Nanopure Diamond D11911, Barnstead International, Dubuque, IA). The colloidal particles from pulverized, cleaned sands were used as surrogates of sand grains for electrophoretic mobility (EPM) measurements in triplicate on the Zetasizer analyzer at 25 °C. Experimentally determined EPM values were converted to apparent zeta (ζ) potentials for the sand grains using the Smoluchowski equation.(46)
Column Experiments
Column experiments were conducted using glass chromatography columns (1.7 cm i.d. × 10 cm long).(47) Each column was dry packed using the cleaned 360, 280, or 230 μm sands. The packed column was then purged with CO2 gas to remove any air remaining during column packing processes and to maximize the water accessibility of the column. After purging, the column was immediately saturated with DI water slowly in an upward mode. Following the saturation step, a nonreactive tracer experiment was performed to assess the hydrodynamic properties of column (see SI S5 and Table S1).
After completion of the tracer experiment, the column was pre-equilibrated with the desired background solution (Table 1). Two percent CMC was not coinjected during the pre-equilibration procedure because CMC alters the column hydromechanical (rheological) properties due to its high viscosity(33) (described below). A two-step transport experiment was then initiated by injecting 3 PVs of CNT–Fe3O4 NH influents (in 2% CMC) at the desired solution chemistries (designated as phase 1), followed by elution with 7 PVs of NH-free background electrolyte solution (without CMC; phase 2). Column experiments with different collector sizes (360, 280, and 230 μm, respectively) were also performed to examine the role of straining(48, 49) on NHs’ retention. Previous studies suggest that, under unfavorable conditions, secondary minimum starts to capture colloids when solution IS is ≥10 mM NaCl(50) or ≥1 mM CaCl2.(45) To identify whether secondary minimum is also involved in NHs’ retention, two three-step transport experiments were run at the highest NaCl (50 mM) and CaCl2 concentrations (3.33 mM) investigated in this study, i.e., eluting the column with 8 PVs of DI water in phase 3 after completing the two-step transport experiments. Darcy velocity was maintained at 0.44 cm/min for all experiments, mimicking typical fluid velocities in coarse aquifers(51) or in forced gradient in situ remediation.(52) Column effluents were collected via a fraction collector. Following completion of each transport experiment, the spatial distribution of CNT–Fe3O4 NHs retained in the column (dissection experiments) was determined (SI S6). Column transport and dissection experiments were also performed for CNT alone (in 2% CMC) to compare the mobility between NHs and parent NMs.
The concentrations of CNT–Fe3O4 NHs and CNT in the effluents and retentates (retained colloids collected from dissection experiments) were determined spectrophotometrically at 218 nm (SI Figure S1). To eliminate the interferences of CMC in NHs (and CNT) measurements, two-step column transport experiments were also performed for 2% CMC alone under experimental conditions identical to those for the transport of CNT–Fe3O4 NHs (Table 1). The actual concentration of CNT–Fe3O4 NHs (and CNT) in the effluents was obtained by subtracting the spectrophotometric response (at 218 nm) of CMC from that of the NHs (and CNT) at the identical PV. Furthermore, to minimize the potential interferences of particle aggregation and sedimentation, spectrophotometric measurements for all NHs, CNT, and CMC in the effluents were controlled to be completed within 1.5 h after collecting from the fraction collector. Additionally, selected column effluents of NHs were digested in 2.6 M HNO3 for 3 days at 25 °C and then filtered through 0.2 μm cellulose acetate membranes. Total Fe concentrations in the filtered digestion solutions were determined using inductively coupled-plasma optical emission spectroscopy (ICP-OES; PerkinElmer Optima 3300DV, Norwalk, CT) to verify the reliability of spectrophotometric measurements of the NHs. Selected column effluents of NHs were also measured using ICP-OES (effluents after HNO3 digestion for total Fe measurements) to identify whether Fe3O4 NMs detach from the NHs or dissolve under acidic conditions (pH 4.0). Our measurements (Fe content < detection limit, meaning no Fe3O4 NMs detach or dissolve) substantiate the integrity of the NHs during porous media transport.
Electrokinetic Properties and Hydrodynamic Radii of CNT–Fe3O4 NHs in the Influents
Electrophoretic mobility (EPM) and ζ-potential of CNT–Fe3O4 NHs (and CNT) in various influents (Table 1) were measured using the Zetasizer analyzer at 25 °C. The ζ-potentials of CNT–Fe3O4 NHs and sand grains were then used to calculate their interaction energy using DLVO theory that includes vdW and electrostatic double-layer (EDL) interactions. It is impossible to determine Hamaker constant (A) of the hydrophobic CNT–Fe3O4 NHs (without CMC modification) in aqueous solutions experimentally. Thus, three Hamaker constants, i.e., Hamaker constants of CNT (ACNT = 6.00 × 10–20 J)(53, 54) and Fe3O4 NMs (AFe3O4 = 33.0 × 10–20 J)(55) and an estimated Hamaker constant of the NHs (ACNT–Fe3O4 = 17.6 × 10–20 J) based on the Hamaker constants of parent NMs and NHs’ elemental (C and Fe) contents, were employed to calculate the interaction energy between NHs and sand grains.(56, 57) Details on the DLVO interaction energy calculation including the inherent limitation of this approach are given in SI S7.
The average hydrodynamic radius (rh) of CNT–Fe3O4 NHs (and CNT) in various influents and the hydrodynamic particle size distribution of CNT–Fe3O4 NHs in the influents at different NaCl and CaCl2 concentrations were determined using DLS (described above). Particle size distribution of 2% CMC was also determined to investigate the role of CMC on the transport of CNT–Fe3O4 NHs. The CONTIN algorithm was used to convert intensity autocorrelation functions to intensity-weighted rh using the Stokes–Einstein equation for spherical particles.(58) Given that the NHs, CNT, and CMC are nonspherical, DLS provides the diameter of a sphere that has the same average translational diffusion coefficient as the particle being measured. For selected column experiments, the rh of NHs and CNT in the effluents and retentates (retained in the column inlet 0–1 cm) was measured using DLS to understand size-dependent retention in saturated sand.
Data Analyses
Parameters characterizing the mobility of CNT–Fe3O4 NHs in water-saturated porous media including single-collector contact efficiency (η), attachment efficiency (α), deposition rate coefficient (kd), and maximum transport distance (Lmax) were calculated using the well-defined Tufenkji–Elimelech (TE) equation (SI S8).(34)
Results and Discussion
Electrokinetic Properties and Hydrodynamic Radii of CNT–Fe3O4 NHs in the Influents and Electrokinetic Properties of Sand Grains
Physicochemical properties of CNT–Fe3O4 NHs and CNT were characterized using XRD, TEM, Raman spectroscopy, and TGA (Figure 1). A strong peak occurred at 26.5° for both NHs and CNT in the XRD spectra, which corresponds to the d spacing of the [002] plane of graphite (CNT; Figure 1a).(59) In contrast, the characteristic reflections of 30.0° [220], 35.6° [311], and 43.4° [400] of Fe3O4 (JCPDS No. 19–0629) occurred only for the NHs, confirming the successful hybridization of CNT and Fe3O4 NMs. TEM images showed that the Fe3O4 NMs (20–30 nm size) heterogeneously deposited on CNT’s surface as dense aggregates (Figure 1b). This is due to the large SSA and surface reactivity and strong magnetic attraction of the Fe3O4 NMs that promote partial aggregation on the CNT’s surface (see SI Figure S5). The Fe3O4 NMs were strongly anchored on the CNT’s surface even after ultrasonication treatment (100 W and 42 kHz for 1 h) since no Fe was released from the NHs (via ICP-OES measurements). This could be due to the strong covalent interaction between CNT (e.g., carboxyl (1.85% in the pristine CNT) and carbonyl groups) and Fe3O4 NMs.(7) The Raman spectrum of CNT exhibited three absorption bands at 1343, 1571, and 2688 cm–1 (Figure 1c), which are assigned to the D, G′, and G′ bands, respectively.(60) However, these three bands shifted to higher wavenumbers of 1357, 1582, and 2710 cm–1, respectively, for the NHs, yielding a larger D/G-band intensity ratio of ID/IG = 0.326 (ID/IG = 0.316 for CNT). This indicates a smaller average size of the sp2 domains of carbon for the NHs,(60) likely due to the enhanced aggregation of NHs (more compressed structure) induced by magnetic attraction (see S1 for more discussion). The TGA profiles showed that Fe accounted for 42.8% of the total elemental mass because CNT was decomposed completely when the temperature was >930 °C (Figure 1d). The C and Fe elemental composition information was used to calculate the Hamaker constant and density of the NHs for DLVO interaction energy and transport parameter calculations (described below).
Figure 1.

XRD patterns (a), TEM images (b), Raman spectra (c), and TGA profiles (d) of the synthesized carbon nanotube–magnetite nanohybrids (CNT–Fe3O4 NHs) and parent CNT component.
The EPMs and ζ-potentials of CNT–Fe3O4 NHs in the influents (in 2% CMC) and sand grains under different experimental conditions are shown in Table S2. Both NHs and sand grains were negatively charged, suggesting unfavorable conditions for column experiments. As mentioned above, the NHs without CMC modification were highly hydrophobic, making EPM (and DLS) measurements impossible in aqueous solutions. However, in 2% CMC, the ζ-potentials of NHs in the influents were highly negative (−39.4 to −58.7 mV) due mainly to the adsorption of hydrophilic, negatively charged carboxymethyl groups of CMC (e.g., ζ-potential of 2% CMC alone = −49.3 mV at 1 mM NaCl and pH 7.3) onto NHs’ surface. The ζ-potential of NHs in the influents (in 2% CMC) was more negative than that of 2% CMC alone at a specified solution chemistry, e.g., −53.4 > −49.3 mV at 1 mM NaCl and pH 7.3 (Table S2). This discrepancy could be due to the deprotonation of carboxyl and carbonyl groups of the NHs, although most carboxyl groups are expected to be used for anchoring CNT and magnetite NMs (described above). Consistent with reported results for individual NMs and sand grains,(61–63) decreasing IS and increasing NOM concentration and pH increased (more negative) the ζ-potential of both NHs and sand grains. The divalent Ca2+ decreased the ζ-potentials of NHs and sand grains more than monovalent Na+ at equivalent ISs by greater charge screening (both Na+ and Ca2+) and neutralization (Ca2+ only) effects.(46)
Table S2 also shows the variability in rh of CNT–Fe3O4 NHs in the influents (2% CMC) with varying experimental conditions. Greater aggregation (larger rh) of the NHs was associated with the lower ζ-potentials due to less electrostatic repulsions according to the DLVO interaction prediction (described below). Similar findings have been documented for individual NMs.(61, 62) The measured hydrodynamic diameter (2rh) of NHs and CNT (Table S2) lied within the diameter (8–15 nm) and length (10–50 μm) of the CNT, consistent with the results reported previously.(19) This is likely because, for nonspherical NHs and CNT, DLS gives the diameter of a sphere that has the same average translational diffusion coefficient as the particle (NHs and CNT) being measured (described above).
Mobility Comparison between CNT–Fe3O4 NHs and Individual CNT
Breakthrough curves (BTCs) and retention profiles (RPs) of CNT–Fe3O4 NHs and CNT (both in 2% CMC) under the identical transport condition (1 mM NaCl and pH 7.3) are presented in Figure 2. Total mass recoveries (Mtot) of NHs in the effluents (Meff) and retentates (Mret) under different experimental conditions are given in Table 1, which confirms a high degree of confidence in our experimental measurements because virtually all NHs were recovered from column experiments (Mtot–NHs = 93.5–108% and Mtot–CNT = 99.4%). The ICP-OES results for effluent Fe analyses matched (standard errors < 5%) the NHs’ BTC data, further demonstrating the reliability of the UV–vis spectrophotometric method for the NHs’ quantification. No physical nonequilibrium processes(64) occurred in the column because of the symmetrical and no-tailing BTC of the tracer (S4) with the exception of the one in 230 -μm sand (described below).
Figure 2.

Breakthrough curves (BTCs; a) and retention profiles (RPs; b) of CNT–Fe3O4 NHs and individual CNT, respectively, in water-saturated sand columns at 1 mM NaCl and pH 7.3 (in 2% CMC). Breakthrough curve describes the normalized effluent concentration of NMs, C/Co (where Co is the initial influent concentration of CNT–Fe3O4 NHs or CNT) as a function of pore volume (PV), and retention profile shows the normalized solid-phase retention concentration of NMs, S/Co (where S is retention amount of CNT–Fe3O4 NHs or CNT per gram dry sand) as a function of distance from the column inlet. For column transport experiments, 3 PVs (marked by dashed line in a) of CNT–Fe3O4 NH or CNT influent were injected in the column followed by elution with 7 PVs of NM-free background electrolyte solution (without CMC). Other column transport parameters were the same including CNT–Fe3O4 NH or CNT influent concentration = 10 mg/L, influent pH = 7.3, background electrolyte = 1 mM NaCl, average sand grain size = 360 μm, and Darcy velocity = 0.44 cm/min. (c) For comparison, the breakthrough curve of 2% CMC alone under experimental conditions (1 mM NaCl and pH 7.3) identical to that of NHs/CNT is shown. (d) Average hydrodynamic radius (rh) of CNT–Fe3O4 NHs and CNT in the effluents (collected from a). Error bars represent the standard deviations from duplicate experiments. Note different y-axis scales in figure.
The mobility of CNT–Fe3O4 NHs was lower than that of CNT with less transport (Meff = 79.9% vs 95.1%) and greater retention (Mret = 25.0% vs 4.3%) (Figure 2a and 2b). Interestingly, transport of both NHs and CNT reached a lower peak initially (at 1.5 PV) and maintained the peak plateau for 2 PVs (designated as “peak 1”) and then increased sharply to achieve the second higher peak (“peak 2”) upon switching background electrolyte solution (without CMC) into the column (at 3 PV; Figure 2a). The two-peak transport feature has never been reported previously and is anticipated to be associated with the transport behavior of the “modifier”—CMC. The BTC of 2% CMC alone at the identical transport condition is shown in Figure 2c, which replicated the transport characteristics of NHs/CNT with two transport peaks and long-term tailings. While previous studies frequently employed CMC to stabilize individual NMs, the impact of CMC on the transport behavior of NMs was insignificant due to the low applied CMC concentrations (≤1%). The observed two-peak transport feature of 2% CMC (and NHs and CNT) is due primarily to the viscosity difference between CMC and water. Specifically, the viscosity of CMC (90 000 g/mol) is at least 2 orders of magnitude higher than that of water (~300 vs 0.89 mPa.s at 25 °C),(33) so the transport velocity of CMC is much lower than that of water in porous media (higher viscosity, lower transport velocity), which explains the sharp peak 2 and long-term tailing of CMC when replacing CMC with water (at 3 PV). The early breakthrough (peak 1) of CMC indicates that heterogeneity occurred in the CMC populations that exhibited varying transport velocities. Indeed, heterogeneities in molecular weight and crystallite,(65) configuration,(66) aggregation state,(33) and particle size are expected to affect the rheological property (Newtonian behavior) and thus transport velocity of CMC (see SI S9 for more discussion). The hydrodynamic particle size distribution of 2% CMC (1 mM NaCl and pH 7.3) is presented in SI Figure S4b, which shows the size heterogeneity in the CMC populations: 36.2% (area 1) and 63.8% (area 2) CMC particles have sizes of 0.40 ± 0.11 and 4.6 ± 1.4 nm, respectively. The smaller CMC particles having lower viscosity are likely to breakthrough earlier, yielding peak 1. Nonetheless, most CMC particles have high viscosity (greater than that of water), and thus, most CMCs exhibit more retarded breakthrough (peak 2). Clearly, the early breakthrough and long-term tailing of 2% CMC alone suggest viscosity heterogeneity among CMC populations.
The transport pattern of both NHs and CNT replicated the breakthrough behavior of CMC, highlighting CMC-mediated transport of NHs/CNT. The normalized effluent values (C/Co) of both peaks and peak locations of the peak 2 varied with different degrees of tailing. Specifically, the C/Co values of both peaks followed the identical order NHs < CNT < CMC (Figure 2a and 2c), which is opposite to the order of their rh values in the influents (1632 > 1526 > 3.4 nm) (Table S2 and Figure S4). This is due primarily to the greater interception efficiency (ηI) and overall single-collector contact efficiency (ηo) at larger rh, because ηI and ηo increase monotonically with colloid size when the diameter is ≥1 μm.(34) To better understand the two-peak transport feature, rh of NHs and CNT in the influents, effluents, and retentates was determined (Figure 2d and Table 2). The rh of NHs was in the order effluent < influent < retentate (e.g., 1432 < 1632 < 2001 nm in 1 mM NaCl and pH 7.3; Table 2), indicating size-selective retention in porous media. The size-selective retention that larger particles preferentially retain in the column (retentates) due likely to greater ηI and ηo and smaller ones elute out (effluents) has been reported in colloid transport studies.(47) Furthermore, for the effluents, the rh of both NHs and CNT increased progressively with PV (Figure 2d), again strongly substantiating the size-selective retention. It is thus logical to anticipate that smaller NHs/CNT associated with lower viscous CMCs breakthrough earlier (yielding peak 1), whereas larger NHs/CNT coated with higher viscous CMCs are eluted out more retarded (i.e., peak 2). The peak location of peak 2 was earlier for CNT than that of NHs (4.5 vs 5 PV; Figure 2a), further supporting that larger cumbersome NHs (effluent rh: CNT < NHs; Figure 2d) have a lower transport velocity (i.e., more retarded breakthrough). Greater retention of NHs observed in Figure 2b is due to greater rh (greater ηI and ηo) in the influents and effluents (Figure 2d). In summary, the above results underscore the interplay of viscosity variability of fluid and size-selective retention in the transport of NMs (in 2% CMC) in water-saturated porous media.
Table 2.
Average Hydrodynamic Radius (rh) of CNT–Fe3O4 NHs in the Influents (in 2% CMC), Effluents, and Retentates at Different NaCl and CaCl2 Concentrations
| rh (nm) | |||||||
|---|---|---|---|---|---|---|---|
| NaCl (mM) | CaCl2 (mM) | sand grain diameter (μm) | influent | effluenta | retentateb | Δrhc | rh/r50d (−) |
| 0 | 0 | 360 | 994 ± 226 | 836 ± 48 | 1096 ± 41 | 260 | 5.52 × 10–3 |
| 1 | 0 | 360 | 1632 ± 143 | 1432 ± 152 | 2001 ± 182 | 569 | 9.07 × 10–3 |
| 10 | 0 | 360 | 1687 ± 167 | 1455 ± 182 | 2285 ± 234 | 830 | 9.37 × 10–3 |
| 50 | 0 | 360 | 2025 ± 290 | 1734 ± 324 | 2789 ± 188 | 1055 | 1.13 × 10–2 |
| 0 | 0.33 | 360 | 1988 ± 237 | 1691 ± 182 | 2338 ± 146 | 647 | 1.10 × 10–2 |
| 0 | 1.67 | 360 | 2238 ± 399 | 1825 ± 297 | 2875 ± 221 | 1050 | 1.24 × 10–2 |
| 0 | 3.33 | 360 | 2728 ± 349 | 1958 ± 350 | 3568 ± 438 | 1610 | 1.52 × 10–2 |
Retentates retained at the column inlet (0–1 cm) were used for rh measurements.
rh difference of NHs in the effluents and retentates.
r50 is the average sand grain radius (180 μm). The rh/r50 values were calculated to test the effect of straining on the retention of CNT–Fe3O4 NHs in column experiments.
Mobility of CNT–Fe3O4 NHs under Varying Physicochemical Conditions
Environmentally relevant physicochemical conditions were chosen to investigate the mobility of CNT–Fe3O4 NHs (in 2% CMC) including NaCl (Figure 3a and 3b), CaCl2 (Figure 3c and 3d), SRHA (Figure 3e and 3f), and SRFA concentrations (Figure 3g and 3h), pHs (Figure S6a and S6b), particle concentrations (Figure S6c and S6d), and collector sizes (Figure S6e and S6f). The DLVO interaction energies between CNT–Fe3O4 NHs and sand grains under different experimental conditions are shown in SI Figure S7 and Table S3. Before interpreting the mobility of NHs under different experimental conditions, it is important to understand the colloidal stability of NH influents. In 2% CMC, the NH influents were stable (influent concentration and rh stayed unchanged; Figure S2) over the time frame of influent injection experiments. Recalling that without CMC partial aggregation of NHs was pronounced due to magnetic attraction (Figure S5), our findings emphasize the critical role of 2% CMC in stabilizing the NH aggregates (e.g., NHs exist as stable aggregates because rh varies with solution chemistries). No further aggregation and gravitational sedimentation of NHs occurred in the influents during influent injection experiments, again confirming the strong stabilizing effect of 2% CMC. Nonetheless, both CFT and TE equation predict that gravitational sedimentation is appreciable when colloid diameter is ≥1 μm.(30, 34) This is likely because highly viscous and negatively charged CMCs (described above) counterbalance the role of gravity in causing particle sedimentation.
Figure 3.

Breakthrough curves (a, c, e, and g) and retention profiles (b, d, f, and h) of CNT–Fe3O4 NHs in water-saturated sand columns under different concentrations of NaCl (0, 1, 10, and 50 mM; a and b), CaCl2 (0, 0.33, 1.67, and 3.33 mM; c and d), SRHA (0, 1, and 10 mg C/L; e and f), and SRFA (0, 1, and 10 mg C/L; g and h), respectively, in 2% CMC. Other column transport parameters include injection volumes of CNT–Fe3O4 NH influent = 3 PVs (marked by dashed lines in a, c, e, and g), influent concentration = 10 mg/L, influent pH = 7.3, average sand grain size = 360 μm, and Darcy velocity = 0.44 cm/min (see Table 1). Error bars represent the standard deviations from duplicate experiments.
Similarly, the two-peak transport feature occurred frequently in the NHs’ BTCs with altered C/Co values and peak locations (Figures 3 and S6). Decreasing IS and increasing NOM concentration, pH, particle concentration, and collector size (with the exception of 230 μm sand; Figure S6e and S6f) increased the mobility of NHs (see Meff and Mret in Table 1), consistent with DLVO theory prediction (Figure S7 and Table S3). The lower mobility of NHs under different experimental conditions was again associated with less negative ζ-potential and larger rh due to greater ηI and ηo. The size-selective retention also occurred during NHs transport (rh order effluent < influent < retentate; Table 2), and this effect became more pronounced (increased Δrh) at higher NaCl/CaCl2 concentrations. To better unravel the size-selective retention of NHs in porous media, the hydrodynamic particle size distribution of NHs in the influents (in 2% CMC) was determined (SI Figure S8). Our results indicate a greater degree of particle size heterogeneity (wider particle size distribution) among NHs populations, likely accounting for the greater size-selective retention of NHs at higher NaCl/CaCl2 concentrations. Other potential explanations for the greater size-selective retention at higher NaCl/CaCl2 concentrations include deeper attractive secondary minimum (Table S3) and more pronounced straining (Table 2) given that hyperexponential RPs occurred at high NaCl/CaCl2 concentrations (Figure 3b and 3d). The fact that retained NHs were released upon injecting DI water during the three-step transport experiments (SI Figure S9a) substantiates the important role of secondary minimum in NHs’ retention. However, the extent of NHs’ release was much less in Ca2+ than in Na+ due likely to more pronounced straining due to the larger rh of NHs in the influents, effluents, and retentates (Table 2 and Figure S9b), i.e., Ca2+ bridges the −CH2COOH and −COOH groups of CMC-coated CNT–Fe3O4 NHs. Straining is demonstrated to play a critical role in colloid retention and cause hyperexponential RPs when the diameter ratio of colloid vs collector is ≥0.008.(49) Our calculations show that when the size ratio of NHs vs collector was ≥0.00937 (≥10 mM NaCl and ≥1.67 mM CaCl2; Table 2), straining started to yield the hyperexponential RPs (Figure 3b and 3d). The slight difference (0.008 < 0.00937) of the threshold for straining between our and previous work(49) is due again to the strong stabilizing effect of CMC used (described above). Moreover, straining likely narrows down the pore structure due to particle retention, particularly near the column inlet, further aggravating the hyperexponential retention (e.g., at higher NaCl/CaCl2 concentrations).(21) Please note that while no aggregation of NHs occurred in the influents (described above), particle aggregation was observed in the column system particularly at higher ISs (see retentate vs influent size; Table 2 and Figure S8) due primarily to greater ηI, ηo, and straining within the porous media structures. The unexpected higher mobility and longer tailing in 230 μm sand compared to those in 280 and 360 μm sands (Figure S6e and S6f) are due to the higher dispersivity (0.23 vs 0.16 cm; Table S1) of the NHs in the 230 μm sand (tracer’s BTC also exhibited a longer tailing; data not shown). Consistent with previous findings for individual NMs,(18, 61) the reduced retention of NHs at higher particle concentrations (Figure S6c and S6d) is owing to greater degrees of blocking of retention sites on the sand grains under unfavorable conditions (Table S3).
Variability in C/Co value of both peaks and shift of peak 2 location under different experimental conditions is again due to the coupling of change in fluid viscosity and size-dependent transport in porous media. For example, the C/Co value of peak 1 changed with solution chemistries (IS, NOM, and pH; Figures 3 and S6a and S6b), e.g., lower C/Co value was generally coupled with larger rh due to greater ηI and ηo. However, it stayed unchanged with varying particle concentration and collector size (Figure S6c–f) likely because particles in peak 1 (relatively smaller particles associated with lower viscous CMCs, described above) are insensitive to influent concentration and pore structure in porous media. Introducing NOM in the influent or increasing influent pH expedited the early breakthrough of peak 2 (peak location evolved from 5 to 4.5 PV; Figures 3e, 3g, and S6a) because the transport velocity of NOM is much higher than that of viscous CMC in water and that NOM (and high pH) promotes the dispersion of colloids/NMs due to electrosteric repulsion effect (see ζ-potential, rh, and DLVO interaction energy data in Tables S2 and S3). In certain cases, interestingly, peak 2 disappeared completely, e.g., at high ISs (10–50 mM NaCl and 1.67–3.33 mM CaCl2), at low pH (4.0), and in 280 μm sand or shortened (0.33 mM CaCl2) with limited tailing. This is mainly because the breakthrough of CMC was inhibited to a similar extent, i.e., 2% CMC alone exhibited similar BTCs (peak 1 or two peaks with shortened peak 2; data not shown) under these experimental conditions. High ISs decreased the ζ-potential (less negative) and reduced the viscosity (smaller difference in viscosity among CMC populations)(33) of CMC, yielding lower mobility of CMC with less retardation of peak 2 (or complete disappearance). These findings are again indicative of CMC-mediated transport of NHs. NOM (1–10 mg C/L; Figure 3e–g) promoting the long-distance transport of NHs as evidenced by the long-term tailing and very limited retention. Also, variability in the transient viscosity of pore–water when injecting background electrolyte in phase 2 would influence the C/Co value and location of peak 2 due to the presence of free CMC in porous media, which likely alters the properties of both NHs and collectors.
Exponential and hyperexponential RPs occurred for the NHs (Figures 3 and S6), and straining was responsible for the hyperexponential RPs (described above). Very recently, Goldberg et al.(67) employed a novel decision tree method to quantitatively identify the influence of physicochemical conditions on the retention of NMs in water-saturated porous media and highlighted that high advective flows and influent concentrations masked the impact of other physicochemical conditions, yielding exponential RPs. This well explains the observed exponential RPs of NHs under different particle concentrations (Figure S6d). They also emphasized the dominant role of straining in causing hyperexponential RPs at high aspect ratios of particle vs collector.(67) This supports the critical role of straining in yielding the hyperexponential RPs at higher NaCl/CaCl2 concentrations (larger rh and thus higher aspect ratio of particle vs collector).
Parameters Characterizing the Mobility of CNT–Fe3O4 NHs
The overall single-collector contact efficiency (ηo) that reflects the contribution of individual transport mechanisms of Brownian diffusion (ηD), interception (ηI), and gravitational sedimentation (ηG) was calculated to unravel the mechanisms controlling NHs’ transport and retention (Table S4). Regardless of Hamaker constant, diffusion (ηD) dominated the ηo and gravity (ηG) and interception (ηI) started to play more important roles at higher ISs or lower pHs, consistent with the trends of rh under varying physicochemical conditions (described above). A higher Hamaker constant yielded larger ηD, ηI, ηG, and thus ηo due to a greater contribution of vdW attraction (eqs 12 and 13 in S8 S8). However, some ηo values were determined to be ≥1.0, especially when using the highest Hamaker constant of AFe3O4 = 33.0 × 10–20 J for the calculation, which is inconsistent with the prediction of the TE equation.(34) Potential discrepancies in ηo between our and Tufenkji and Elimelech’s work(34) are due to the larger Hamaker constants (6.00–33.0 × 10–20 vs 0.30–4.00 × 10–20 J) and densities (1.74–5.15 vs 1.00–1.80 g/cm3) of the CNT–Fe3O4 NHs and lower porosities (0.33–0.34 vs 0.36–0.40) of the packed column used here. The estimated α of NHs under different experimental conditions ranged between 3.97 × 10–3 and 6.91 × 10–3 (Table S4), which is smaller than those of individual CNT (0.05–1.0),(19) fullerene (0.01–1.0),(68) and nZVI (2.00 × 10–3–1.0)(24) NMs for similar solution chemistries. The higher mobility of the NHs in 2% CMC (Table 1) and larger ηo of the packed columns (eq 15 in S8 for α calculation) yielded the smaller α in this study.
Figure 4 and Table S4 show the variability in kd and Lmax under varying physicochemical conditions. For a specified experimental condition, the calculated kd and Lmax values were the same, regardless of Hamaker constant, because the overall contribution of Hamaker constant was counterbalanced in the term of αηo in eqs 16 and 17 in S8. Consequently, while the Hamaker constant of CNT–Fe3O4 NHs was not determined experimentally, the calculated kd and Lmax values were reliable. The kd was smaller and Lmax was larger in Na+ than in Ca2+ at the equivalent IS (1 and 10 mM; Figure 4), implying that Ca2+ inhibited NHs’ mobility to a greater extent than that of Na+. The extent of kd and Lmax change (Δkd and ΔLmax) vs IS became smoother at higher ISs (both Na+ and Ca2+), likely due to the stabilizing effect of CMC coating on NHs’ surface that cushions the impact of electrolyte. Notably, Lmax elevated significantly with environmentally relevant concentrations (10 mg C/L) of NOMs particularly for SRHA (~28 m), signifying that the NHs would be highly mobile in the subsurface rich in NOMs. Over the range of physicochemical conditions investigated, kd and Lmax ranged between 3.27 × 10–5 and 3.93 × 10–3 s–1 and 0.38 and 46 m, respectively, which are smaller (for kd) and larger (for Lmax) than those previously reported for individual NMs(19, 24, 68) under similar experimental conditions due, again, to the strong stabilizing effect of CMC.
Figure 4.

Calculated deposition rate coefficient (kd; left y axis) and maximum transport distance (Lmax; right y axis) of CNT–Fe3O4 NHs at varying concentrations of NaCl (0, 1, 10, and 50 mM; a), CaCl2 (0, 0.33, 1.67, and 3.33 mM; a; note that IS of 0, 0.33, 1.67, and 3.33 mM CaCl2 is 0, 1, 5, and 10 mM, respectively), SRHA (0, 1, and 10 mg C/L; b), and SRFA (0, 1, and 10 mg C/L; b), respectively, using the Tufenkji–Elimelech (TE) equation.(34) It is important to point out that the kd and Lmax values were calculated to be the same regardless of the Hamaker constants used for the calculations (see SI Table S4). Note different y-axis scales in the figure.
The TE equation is a well-defined semiempirical correlation equation commonly used for predicting ηD, ηI, ηG, and ηo, regardless of favorable/unfavorable conditions, and it has some assumptions. For example, the TE equation assumes spherically shaped colloids and constant viscous fluid. The CNT–Fe3O4 NHs used, however, were nonspherical, and fluid viscosity varied when flushing the column with background solution in column transport experiments. Thus, the TE equation calculation should be considered as estimation of anticipated changes in ηD, ηI, ηG, and ηo with varying experimental conditions and viewed as an approximation of kd and Lmax. Future field studies should be implemented to test the applicability of the TE equation for kd and Lmax evaluation.
Potential Limitations of DLVO Theory and CFT in Describing NHs’ Transport
DLVO theory and CFT have been used to describe the transport of colloids/NMs in porous media, but both strategies have assumptions and potential limitations. Specifically, DLVO theory calculations consider average ζ-potentials for both colloids and collector grains and assume geometrically spherical colloids. Nevertheless, the CNT–Fe3O4 NHs were nanoheteroaggregates of tubular CNT anchored by spherical Fe3O4 NMs (Figure 1b), and the measured ζ-potentials of NHs were the overall surface charges of the nanoassemblies. Moreover, the Hamaker constant of CNT–Fe3O4 NHs cannot be accurately quantified in aqueous solution. Thus, the DLVO calculations should be viewed as an estimation of expected changes in the average interaction energy between NHs and collectors with varying experimental conditions (Table S3). CFT predicts an exponential decrease in colloid retention with transport distance and functions satisfactorily under favorable conditions. However, deviations between experimental data and theory predictions occur under unfavorable conditions including the encountered hyperexponential RPs for CNT–Fe3O4 NHs. This is due primarily to the interplay of size-selective retention and straining in column systems. While NHs’ transport behaviors under different experimental conditions can be described by DLVO theory/CFT semiquantitatively, future studies should focus on developing more rigorous approaches (e.g., modified DLVO theory/CFT) for quantitatively describing the transport of NHs taking into account, for example, variability of fluid viscosity and NHs’ shape, morphology, magnesium, surface chemistry, and aggregation state.
Environmental Implications
Our findings provide valuable knowledge into in situ nanoremediation of contaminated soil, aquifer, and groundwater using the next-generation multifunctional carbon–metal oxide NHs. The rationale of employing the magnetically recyclable CNT–Fe3O4 NHs relies on the fact that the assembled nanostructures of CNT and Fe3O4 have much higher SSA and reactivity (compared to singular NMs), where (in)organic pollutants can be adsorbed and then detoxicated/degraded in situ. Nonetheless, the major challenges arise from the strong hydrophobicity and magnetism of the CNT–Fe3O4 NHs that promote particle aggregation, which deteriorates the overall performance of material. Our results show that 2% CMC can significantly increase the CNT–Fe3O4 NHs’ hydrophilicity, colloidal stability, and mobility in aqueous solutions, reaching the optimal transport ranges (several to dozens of meters) required for in situ remediation.(38) Other crucial benefits of utilizing the “green” and low-cost CMC include (1) the efficacy of NHs would not be compromised by the CMC coating, as demonstrated by Bhattacharjee et al.(43) in the degradation of trichloroethylene using CMC-coated nZVI, (2) no adverse impact would occur during remediation because the CMC is biodegradable, and particularly (3) CMC and its biodegraded intermediates (primarily celluloses) can act as a carbon source for microbes during microbial bioremediation, making the in situ, combined “nanoremediation–bioremediation” technology most promising. Due to the distinct viscosity of CMC and water, the transport of CMC-modified NHs to the targeted contaminant plumes would be retarded, depending on the applied CMC concentration and hydrogeological properties (e.g., flow velocity, IS, pH, and NOM) of the aquifers or groundwater. This will likely extend the operational time of in situ nanoremediation. Even if the aquifer or groundwater has a high IS of 50 mM NaCl or 3.33 mM CaCl2, the NHs are still expected to be mobile (Lmax = 0.43 and 0.38 m, respectively; Table S4). In particular, the Lmax increased to ~3.7–28 m under environmentally relevant concentrations (1–10 mg C/L) of NOMs and reached up to ~46 m at a particle concentration of 50 mg/L, supporting the feasibility of employing the CNT–Fe3O4 NHs for in situ contaminated site remediation. Currently, the synthesis methods of CNT–metal oxide NHs are mature, as have been showcased in ref 37 (our recent book chapter). Nonetheless, the major obstacle of employing NHs for remediation purposes arises from the relatively high cost of the NHs. If the mass production of commercial inexpensive NHs is achieved, we can envision that the multifunctional NHs are highly likely to be employed as the next-generation candidates for in situ nanoremediation of contaminated sites.
Supplementary Material
Acknowledgment
This research was funded by the U.S. EPA. This article has been reviewed in accordance with U.S. EPA policy and approved for publication. However, the research results do not necessarily reflect the views or policies of EPA, and no official endorsement should be inferred. We appreciate Courtney Taylor, Eric Daiber, Michael Brooks, and Junqi Huang for laboratory assistance and technical review. Comments and suggestions from five peer reviewers and Associate Editor Dr. Martin Scheringer helped us improve the quality of this paper.
References
- 1.Kircher MF; de la Zerda A; Jokerst JV; Zavaleta CL; Kempen PJ; Mittra E; Pitter K; Huang R; Campos C; Habte F; Sinclair R; Brennan CW; Mellinghoff IK; Holland EC; Gambhir SS A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle Nat. Med. 2012, 18 (5) 829–834 DOI: 10.1038/nm.2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sozer N; Kokini JL Nanotechnology and its applications in the food sector Trends Biotechnol. 2009, 27 (2) 82–89 DOI: 10.1016/j.tibtech.2008.10.010 [DOI] [PubMed] [Google Scholar]
- 3.Zhang Q; Uchaker E; Candelaria SL; Cao G Nanomaterials for energy conversion and storage Chem. Soc. Rev 2013, 42 (7) 3127–3171 DOI: 10.1039/c3cs00009e [DOI] [PubMed] [Google Scholar]
- 4.Khin MM; Nair AS; Babu VJ; Murugan R; Ramakrishna S A review on nanomaterials for environmental remediation Energy Environ. Sci. 2012, 5 (8) 8075–8109 DOI: 10.1039/c2ee21818f [DOI] [Google Scholar]
- 5.Klaine SJ; Alvarez PJJ; Batley GE; Fernandes TF; Handy RD; Lyon DY; Mahendra S; McLaughlin MJ; Lead JR Nanomaterials in the environment: Behavior, fate, bioavailability, and effects Environ. Toxicol. Chem 2008, 27 (9) 1825–1851 DOI: 10.1897/08-090.1 [DOI] [PubMed] [Google Scholar]
- 6.Bursten JR; Roco MC; Yang W; Zhao YL; Chen CY; Savolainen K; Gerber C; Kataoka K; Krishnan Y; Bayley H; Nazar L; Milana S; Vandersypen L; Weiss PS; Schummer J Nano on reflection Nat. Nanotechnol. 2016, 11 (10) 828–834 DOI: 10.1038/nnano.2016.232 [DOI] [PubMed] [Google Scholar]
- 7.Eder D Carbon nanotube-inorganic hybrids Chem. Rev. 2010, 110 (3) 1348–1385 DOI: 10.1021/cr800433kr [DOI] [PubMed] [Google Scholar]
- 8.Banin U; Ben-Shahar Y; Vinokurov K Hybrid semiconductor–metal nanoparticles: From architecture to function Chem. Mater. 2014, 26 (1) 97–110 DOI: 10.1021/cm402131nr [DOI] [Google Scholar]
- 9.Aich N; Plazas-Tuttle JR; Lead JR; Saleh NB A critical review of nanohybrids: Synthesis, applications and environmental implications Environ. Chem. 2014, 11 (6) 609–623 DOI: 10.1071/EN14127 [DOI] [Google Scholar]
- 10.Yanez HJ; Wang Z; Lege S; Obst M; Roehler S; Burkhardt CJ; Zwiener C Application and characterization of electroactive membranes based on carbon nanotubes and zerovalent iron nanoparticles Water Res. 2017, 108, 78–85 DOI: 10.1016/j.watres.2016.10.055 [DOI] [PubMed] [Google Scholar]
- 11.Woan K; Pyrgiotakis G; Sigmund W Photocatalytic carbon-nanotube-TiO2 composites Adv. Mater. 2009, 21 (21) 2233–2239 DOI: 10.1002/adma.200802738 [DOI] [Google Scholar]
- 12.Chowdhury S; Balasubramanian R Recent advances in the use of graphene-family nanoadsorbents for removal of toxic pollutants from wastewater Adv. Colloid Interface Sci. 2014, 204, 35–56 DOI: 10.1016/j.cis.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 13.Zhang W; Shi X; Zhang Y; Gu W; Li B; Xian Y Synthesis of water-soluble magnetic graphene nanocomposites for recyclable removal of heavy metal ion J. Mater. Chem. A 2013, 1 (5) 1745–1753 DOI: 10.1039/C2TA00294A [DOI] [Google Scholar]
- 14.Zong P; Wang S; Zhao Y; Wang H; Pan H; He C Synthesis and application of magnetic graphene/iron oxides composite for the removal of U(VI) from aqueous solutions Chem. Eng. J 2013, 220, 45–52 DOI: 10.1016/j.cej.2013.01.038 [DOI] [Google Scholar]
- 15.Li ZJ; Huang ZW; Guo WL; Wang L; Zheng LR; Chai ZF; Shi WQ Enhanced photocatalytic removal of uranium(VI) from aqueous solution by magnetic TiO2/Fe3O4 and its graphene composite Environ. Sci. Technol 2017, 51 (10) 5666–5674 DOI: 10.1021/acs.est.6b05313r [DOI] [PubMed] [Google Scholar]
- 16.Kasel D; Bradford SA; Simunek J; Heggen M; Vereecken H; Klumpp E Transport and retention of multi-walled carbon nanotubes in saturated porous media: Effects of input concentration and grain size Water Res. 2013, 47 (2) 933–944 DOI: 10.1016/j.watres.2012.11.019 [DOI] [PubMed] [Google Scholar]
- 17.Liu X; O’Carroll DM; Petersen EJ; Huang Q; Anderson CL Mobility of multiwalled carbon nanotubes in porous media Environ. Sci. Technol 2009, 43 (21) 8153–8158 DOI: 10.1021/es901340dr [DOI] [PubMed] [Google Scholar]
- 18.Zhang M; Bradford SA; Simunek J; Vereecken H; Klumpp E Do goethite surfaces really control the transport and retention of multi-walled carbon nanotubes in chemically heterogeneous porous media? Environ. Sci. Technol 2016, 50 (23) 12713–12721 DOI: 10.1021/acs.est.6b03285r [DOI] [PubMed] [Google Scholar]
- 19.Jaisi DP; Saleh NB; Blake RE; Elimelech M Transport of single-walled carbon nanotubes in porous media: Filtration mechanisms and reversibility Environ. Sci. Technol 2008, 42 (22) 8317–8323 DOI: 10.1021/es801641vr [DOI] [PubMed] [Google Scholar]
- 20.He JZ; Li CC; Wang DJ; Zhou DM Biofilms and extracellular polymeric substances mediate the transport of graphene oxide nanoparticles in saturated porous media J. Hazard. Mater 2015, 300, 467–474 DOI: 10.1016/j.jhazmat.2015.07.026 [DOI] [PubMed] [Google Scholar]
- 21.Wang D; Shen C; Jin Y; Su C; Chu L; Zhou D Role of solution chemistry in the retention and release of graphene oxide nanomaterials in uncoated and iron oxide-coated sand Sci. Total Environ. 2017, 579, 776–785 DOI: 10.1016/j.scitotenv.2016.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xia T; Qi Y; Liu J; Qi Z; Chen W; Wiesner MR Cation-inhibited transport of graphene oxide nanomaterials in saturated porous media: The Hofmeister effects Environ. Sci. Technol 2017, 51 (2) 828–837 DOI: 10.1021/acs.est.6b05007r [DOI] [PubMed] [Google Scholar]
- 23.He F; Zhao D; Liu J; Roberts CB Stabilization of Fe-Pd nanoparticles with sodium carboxymethyl cellulose for enhanced transport and dechlorination of trichloroethylene in soil and groundwater Ind. Eng. Chem. Res 2007, 46 (1) 29–34 DOI: 10.1021/ie0610896r [DOI] [Google Scholar]
- 24.Saleh N; Kim HJ; Phenrat T; Matyjaszewski K; Tilton RD; Lowry GV Ionic strength and composition affect the mobility of surface-modified Fe0 nanoparticles in water-saturated sand columns Environ. Sci. Technol 2008, 42 (9) 3349–3355 DOI: 10.1021/es071936br [DOI] [PubMed] [Google Scholar]
- 25.Bossa N; Carpenter A; Kumar N; de Lannoy CFP; Wiesner MR Cellulose nanocrystal-zero valent iron nanocomposites for groundwater remediation Environ. Sci.: Nano 2017, 4 (6) 1294–1303 DOI: 10.1039/C6EN00572A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becker MD; Wang Y; Paulsen JL; Song YQ; Abriola LM; Pennell KD In situ measurement and simulation of nano-magnetite mobility in porous media subject to transient salinity Nanoscale 2015, 7 (3) 1047–1057 DOI: 10.1039/C4NR05088F [DOI] [PubMed] [Google Scholar]
- 27.Ersenkal DA; Ziylan A; Ince NH; Acar HY; Demirer M; Copty NK Impact of dilution on the transport of poly(acrylic acid) supported magnetite nanoparticles in porous media J. Contam. Hydrol 2011, 126 (3–4) 248–257 DOI: 10.1016/j.jconhyd.2011.09.005 [DOI] [PubMed] [Google Scholar]
- 28.Derjaguin BV; Landau LD Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes Acta Physicochim. URSS 1941, 14, 733–762 [Google Scholar]
- 29.Verwey EJW; Overbeek JTG Theory of the stability of lyophobic colloids; Elsevier: Amsterdam, The Netherlands, 1948. [Google Scholar]
- 30.Yao KM; Habibian MT; O’Melia CR Water and waste water filtration: Concepts and applications Environ. Sci. Technol 1971, 5 (11) 1105–1112 DOI: 10.1021/es60058a005r [DOI] [Google Scholar]
- 31.Goldberg E; Scheringer M; Bucheli TD; Hungerbühler K Critical assessment of models for transport of engineered nanoparticles in saturated porous media Environ. Sci. Technol 2014, 48 (12) 12732–12741 DOI: 10.1021/es502044kr [DOI] [PubMed] [Google Scholar]
- 32.Phenrat T; Saleh N; Sirk K; Kim HJ; Tilton RD; Lowry GV Stabilization of aqueous nanoscale zerovalent iron dispersions by anionic polyelectrolytes: Adsorbed anionic polyelectrolyte layer properties and their effect on aggregation and sedimentation J. Nanopart. Res 2008, 10 (5) 795–814 DOI: 10.1007/s11051-007-9315-6 [DOI] [Google Scholar]
- 33.Yang XH; Zhu WL Viscosity properties of sodium carboxymethylcellulose solutions Cellulose 2007, 14 (5) 409–417 DOI: 10.1007/s10570-007-9137-9 [DOI] [Google Scholar]
- 34.Tufenkji N; Elimelech M Correlation equation for predicting single-collector efficiency in physicochemical filtration in saturated porous media Environ. Sci. Technol 2004, 38 (2) 529–536 DOI: 10.1021/es034049rr [DOI] [PubMed] [Google Scholar]
- 35.Petosa AR; Jaisi DP; Quevedo IR; Elimelech M; Tufenkji N Aggregation and deposition of engineered nanomaterials in aquatic environments: Role of physicochemical interactions Environ. Sci. Technol 2010, 44 (17) 6532–6549 DOI: 10.1021/es100598hr [DOI] [PubMed] [Google Scholar]
- 36.Wang M; Gao B; Tang D Review of key factors controlling engineered nanoparticle transport in porous media J. Hazard. Mater 2016, 318, 233–246 DOI: 10.1016/j.jhazmat.2016.06.065 [DOI] [PubMed] [Google Scholar]
- 37.Wang D; Sun W; Su C Environmental Applications and Implications of Carbon Nanotube-Metal Oxide Nanocomposites In Metal Oxide Nanocomposites: Synthesis and Applications; Visakh PM, Ed.; John Wiley & Sons, 2017; Chapter 8. [Google Scholar]
- 38.White AF; Peterson ML Reduction of aqueous transition metal species on the surfaces of Fe(II)-containing oxides Geochim. Cosmochim. Acta 1996, 60 (20) 3799–3814 DOI: 10.1016/0016-7037(96)00213-X [DOI] [Google Scholar]
- 39.Pang SC; Chin SF; Anderson MA Redox equilibria of iron oxides in aqueous-based magnetite dispersions: Effect of pH and redox potential J. Colloid Interface Sci. 2007, 311 (1) 94–101 DOI: 10.1016/j.jcis.2007.02.058 [DOI] [PubMed] [Google Scholar]
- 40.Chun CL; Hozalski RM; Arnold WA Degradation of drinking water disinfection byproducts by synthetic goethite and magnetite Environ. Sci. Technol 2005, 39 (21) 8525–8532 DOI: 10.1021/es051044gr [DOI] [PubMed] [Google Scholar]
- 41.Coleman JN; Khan U; Blau WJ; Gun’ko YK Small but strong: A review of the mechanical properties of carbon nanotube-polymer composites Carbon 2006, 44 (9) 1624–1652 DOI: 10.1016/j.carbon.2006.02.038 [DOI] [Google Scholar]
- 42.He F; Zhao D Manipulating the size and dispersibility of zerovalent iron nanoparticles by use of carboxymethyl cellulose stabilizers Environ. Sci. Technol 2007, 41 (17) 6216–6221 DOI: 10.1021/es0705543r [DOI] [PubMed] [Google Scholar]
- 43.Bhattacharjee S; Basnet M; Tufenkji N; Ghoshal S Effects of rhamnolipid and carboxymethylcellulose coatings on reactivity of palladium-doped nanoscale zerovalent iron particles Environ. Sci. Technol 2016, 50 (4) 1812–1820 DOI: 10.1021/acs.est.5b05074r [DOI] [PubMed] [Google Scholar]
- 44.Si S; Kotal A; Mandal T; Giri S; Nakamura H; Kohara T Size-controlled synthesis of magnetite nanoparticles in the presence of polyelectrolytes Chem. Mater. 2004, 16 (18) 3489–3496 DOI: 10.1021/cm049205nr [DOI] [Google Scholar]
- 45.Stumm W; Morgan JJ Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, 3rd ed.; John Wiley & Sons, 1995. [Google Scholar]
- 46.Elimelech M; Gregory J; Jia X; Williams RA Particle Deposition and Aggregation: Measurement, Modeling and Simulation; Butterworth-Heinemann: Oxford, U.K., 1995. [Google Scholar]
- 47.Wang D; Jin Y; Jaisi DP Effect of size-selective retention on the cotransport of hydroxyapatite and goethite nanoparticles in saturated porous media Environ. Sci. Technol 2015, 49 (14) 8461–8470 DOI: 10.1021/acs.est.5b01210r [DOI] [PubMed] [Google Scholar]
- 48.Bradford SA; Yates SR; Bettahar M; Simunek J Physical factors affecting the transport and fate of colloids in saturated porous media Water Resour. Res. 2002, 38 (12) 63–1–63–12 DOI: 10.1029/2002WR001340 [DOI] [Google Scholar]
- 49.Xu S; Gao B; Saiers JE Straining of colloidal particles in saturated porous media. Water Resour. Res 2006, 42 (12), DOI: 10.1029/2006WR004948. [DOI] [Google Scholar]
- 50.Tufenkji N; Elimelech M Deviation from the classical colloid filtration theory in the presence of repulsive DLVO interactions Langmuir 2004, 20 (25) 10818–10828 DOI: 10.1021/la0486638r [DOI] [PubMed] [Google Scholar]
- 51.Harter T; Wagner S; Atwill ER Colloid transport and filtration of Cryptosporidium parvum in sandy soils and aquifer sediments Environ. Sci. Technol 2000, 34 (1) 62–70 DOI: 10.1021/es990132wr [DOI] [Google Scholar]
- 52.Su C; Puls RW; Krug TA; Watling MT; O’Hara SK; Quinn JW; Ruiz NE A two and half-year-performance evaluation of a field test on treatment of source zone tetrachloroethene and its chlorinated daughter products using emulsified zero valent iron nanoparticles Water Res. 2012, 46 (16) 5071–5084 DOI: 10.1016/j.watres.2012.06.051 [DOI] [PubMed] [Google Scholar]
- 53.Qu L; Dai L; Stone M; Xia Z; Wang ZL Carbon nanotube arrays with strong shear binding-on and easy normal lifting-off Science 2008, 322 (5899) 238–242 DOI: 10.1126/science.1159503 [DOI] [PubMed] [Google Scholar]
- 54.Leckband D; Israelachvili J Intermolecular forces in biology Q. Rev. Biophys 2001, 34 (2) 105–267 DOI: 10.1017/S0033583501003687 [DOI] [PubMed] [Google Scholar]
- 55.Faure B; Salazar-Alvarez G; Bergstrom L Hamaker constants of iron oxide nanoparticles Langmuir 2011, 27 (14) 8659–8664 DOI: 10.1021/la201387dr [DOI] [PubMed] [Google Scholar]
- 56.Hua Z; Zhang J; Bai X; Ye Z; Tang Z; Liang L; Liu Y Aggregation of TiO2–graphene nanocomposites in aqueous environment: Influence of environmental factors and UV irradiation Sci. Total Environ. 2016, 539, 196–205 DOI: 10.1016/j.scitotenv.2015.08.143 [DOI] [PubMed] [Google Scholar]
- 57.Israelachvili JN Intermolecular and Surface Forces; Academic Press: San Diego, CA, 1992. [Google Scholar]
- 58.French RA; Jacobson AR; Kim B; Isley SL; Penn RL; Baveye PC Influence of ionic strength, pH, and cation valence on aggregation kinetics of titanium dioxide nanoparticles Environ. Sci. Technol 2009, 43 (5) 1354–1359 DOI: 10.1021/es802628nr [DOI] [PubMed] [Google Scholar]
- 59.Yan XB; Tay BK; Yang Y; Po WYK Fabrication of three-dimensional ZnO–carbon nanotube (CNT) hybrids using self-assembled CNT micropatterns as framework J. Phys. Chem. C 2007, 111 (46) 17254–17259 DOI: 10.1021/jp076064er [DOI] [Google Scholar]
- 60.Dresselhaus MS; Dresselhaus G; Saito R; Jorio A Raman spectroscopy of carbon nanotubes Phys. Rep. 2005, 409 (2) 47–99 DOI: 10.1016/j.physrep.2004.10.006 [DOI] [Google Scholar]
- 61.Wang D; Ge L; He J; Zhang W; Jaisi DP; Zhou D Hyperexponential and nonmonotonic retention of polyvinylpyrrolidone-coated silver nanoparticles in an Ultisol J. Contam. Hydrol 2014, 164, 35–48 DOI: 10.1016/j.jconhyd.2014.05.007 [DOI] [PubMed] [Google Scholar]
- 62.Keller AA; Wang H; Zhou D; Lenihan HS; Cherr G; Cardinale BJ; Miller R; Ji Z Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices Environ. Sci. Technol 2010, 44 (6) 1962–1967 DOI: 10.1021/es902987dr [DOI] [PubMed] [Google Scholar]
- 63.Chen G; Liu X; Su C Distinct effects of humic acid on transport and retention of TiO2 rutile nanoparticles in saturated sand columns Environ. Sci. Technol 2012, 46 (13) 7142–7150 DOI: 10.1021/es204010gr [DOI] [PubMed] [Google Scholar]
- 64.Wang Y; Li Y; Fortner JD; Hughes JB; Abriola LM; Pennell KD Transport and retention of nanoscale C60 aggregates in water-saturated porous media Environ. Sci. Technol 2008, 42 (10) 3588–3594 DOI: 10.1021/es800128mr [DOI] [PubMed] [Google Scholar]
- 65.Kulicke WM; Kull AH; Kull W; Thielking H Characterization of aqueous carboymethylcellulose solutions in terms of their molecular structure and its influence on rheological behaviour Polymer 1996, 37 (13) 2723–2731 DOI: 10.1016/0032-3861(96)87634-8 [DOI] [Google Scholar]
- 66.Prusova SM; Ryabinina IV; Prusov AN Rheological properties and structure of aqueous solutions of polysaccharides: Solutions of sodium carboxymethylcellulose fractions of different molar mass Fibre Chem. 2002, 34 (3) 177–180 DOI: 10.1023/A:1020562930015 [DOI] [Google Scholar]
- 67.Goldberg E; McNew C; Scheringer M; Bucheli TD; Nelson P; Hungerbuhler K What factors determine the retention behavior of engineered nanomaterials in saturated porous media? Environ. Sci. Technol 2017, 51 (5) 2729–2737 DOI: 10.1021/acs.est.6b05217r [DOI] [PubMed] [Google Scholar]
- 68.Espinasse B; Hotze EM; Wiesner MR Transport and retention of colloidal aggregates of C60 in porous media: Effects of organic macromolecules, ionic composition, and preparation method Environ. Sci. Technol 2007, 41 (21) 7396–7402 DOI: 10.1021/es0708767r [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


