Abstract
Purpose
This prospective study presents the results of a new approach in the treatment of primary macronodular adrenal hyperplasia (PMAH), with simultaneous total adrenalectomy of the larger adrenal gland and partial adrenalectomy of the contralateral adrenal gland (adrenal-sparing surgery).
Materials and Methods
We performed a prospective study including 17 patients with PMAH treated surgically with adrenal-sparing surgery in a tertiary referral hospital, with a median follow-up of 41 months. Clinical, hormonal, and genetic parameters were evaluated before surgery and during follow-up. All patients had at least 1 radiological examination before and after the procedure.
Results
Among the 17 patients, all but 1 patient had complete hypercortisolism control, and 12 recovered normal adrenal function after surgery. Significant improvement in clinical parameters was observed: weight loss (P = .004); reduction of both systolic (P = .001) and diastolic (P = .001) blood pressure; and reduction in the number of antihypertensive drugs (P < .001). Intra-, peri-, and postoperative complications were not observed.
Conclusion
Adrenal-sparing surgery is a safe and feasible procedure to treat patients with PMAH, providing a substantial chance of hypercortisolism control without the disadvantages of lifetime corticosteroid replacement.
Keywords: PMAH, partial adrenalectomy, hypercortisolism, adrenal surgery, ARMC5
Primary macronodular adrenal hyperplasia (PMAH) is a rare cause of adrenal Cushing syndrome (CS). In general, PMAH presents bilateral adrenal nodules with variable cortisol production [1-3].
The clinical spectrum of presentation ranges from mild hypercortisolism to overt CS. Recent studies demonstrated an association between germline/somatic mutations in the tumor suppressor gene ARMC5 in approximately 50% of cases [4, 5]. The increased frequency of early-diagnosed familial PMAH rendered subclinical hypercortisolism (SH) a frequent manifestation of this disease, corresponding to a majority of cases in specific populations [6]. Because of uneven presentation and severity of this disease, its treatment is challenging.
Clinical treatments for PMAH have included different steroidogenesis inhibitors (ketoconazole; mitotano) and abnormally expressed antagonists of G protein receptors (β-adrenergic receptor antagonists; long-acting leuprolide acetate); however, these have low efficacy to control hypercortisolism [7-10]. Thus, surgical treatment has been widely used. Bilateral adrenalectomy was the standard option in the past for PMAH associated with CS and bilateral involvement [6]. However, this modality leads to permanent adrenal insufficiency and has the drawback of requiring lifetime hormonal replacement. In addition, patients with this condition have a significantly impaired quality of life and a high risk of adrenal crisis, and mortality ranges from 6% to 8% [11]. To avoid this, unilateral adrenalectomy was proposed as a treatment option for patients with mild CS [12-15]. A retrospective study involving 15 patients with a median follow-up of 60 months demonstrated good results with this procedure, reporting 60% of hypercortisolism remission. Nevertheless, in this same cohort, the authors observed 13% hypercortisolism relapse [16].Therefore, the gold standard treatment for PMAH has not been set yet.
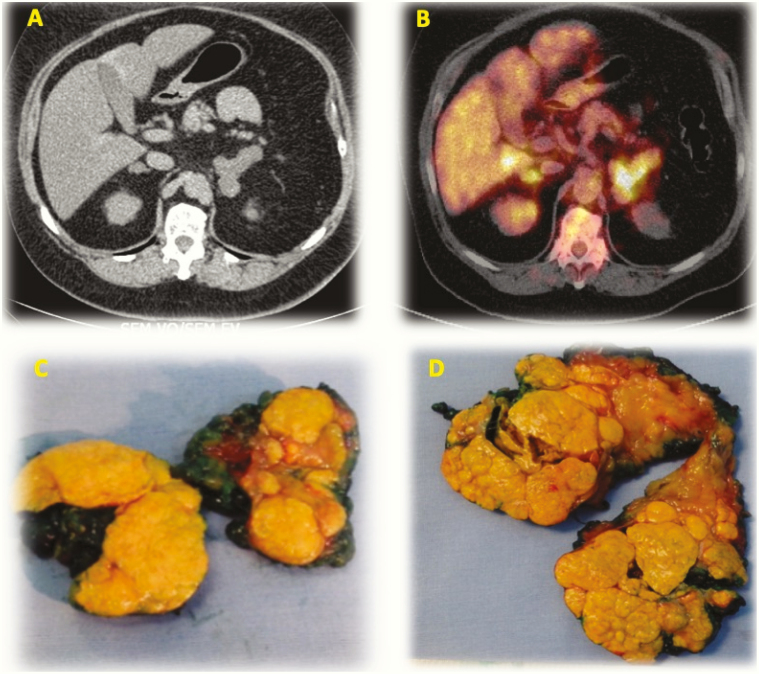
Partial adrenalectomy was described for treatment of diseases affecting both adrenal glands [17]. Current evidence regarding the use of partial adrenalectomy for PMAH is limited, with only a small number of PMAH cases [18-20]. To overcome hormonal replacement caveats while minimizing the risks of hypercortisolism relapse, we performed a series of simultaneous total adrenalectomy of the largest adrenal gland and partial adrenalectomy of the contralateral gland (adrenal-sparing surgery) to treat patients with PMAH. Interestingly, PMAH nodules may exhibit increased uptake of 18F-fluorodeoxyglucose (18F-FDG) (Fig. 1), although lesions are benign [21, 22].
Figure 1.
Enlarged adrenal glands in PMAH and surgical pieces after adrenal sparing surgery. (A) CT image. (B) image in 18F-FDG-PET/CT. (C) opened surgical piece with nankin ink on surface – right partial adrenalectomy. (D) opened surgical piece with nankin ink on surface – left total adrenalectomy.
A strong correlation between 18F-FDG uptake and the largest adrenal diameter was observed in patients with PMAH [21, 22]. Thus, performing this functional examination is not essential for deciding which side to perform total adrenalectomy and a decision based on gland size is feasible.
In this prospective study, we evaluate the short-term outcomes of adrenal-sparing surgery for the PMAH treatment, assessing the impact on hypercortisolism control, the improvement of clinical and metabolic parameters, and the recovery of a normal cortisol secretion.
1. Materials and Methods
The ethics committee of the hospital approved the study and written informed consent was received. This study included 17 patients (12 females, age range from 18 to 70 years). All underwent adrenal-sparing surgery with a median follow-up of 41 months after surgery. The choice of which adrenal gland to be completely resected was based on the findings of computed tomography (CT) and 18F-FDG positron emission tomography. PMAH diagnosis was suspected in patients with classical features of CS (n = 7) and in patients with SH associated with incidental bilateral nodular adrenal glands on CT scan (n = 10). Patients with CS were classified according to high clinical index of suspicion for hypercortisolism, such as facial plethora, easy bruising, large violaceous striae, and proximal myopathy. In addition, they presented with at least 2 abnormal results of the following examinations: 24 hour urinary cortisol, midnight salivary cortisol, and nonsuppression of serum cortisol after low-dose dexamethasone suppression test (DST) with 1 mg of dexamethasone overnight (F cut-off >1.8 µg/dL or 50 nmol/L) and with low/suppressed plasmatic levels of adrenocorticotropin (ACTH) [23]. Patients with SH presented with metabolic syndrome (obesity; blood hypertension; glucose intolerance; or diabetes mellitus type 2 and dyslipidemia) and were lacking typical clinical signs of hypercortisolism (facial plethora; proximal muscle weakness; easy bruising; and violaceous striae wider than 1 cm). All patients presented normal values of midnight salivary cortisol and 24-hour urinary cortisol with abnormal serum cortisol levels after low-dose DST with 1 mg of dexamethasone overnight (F cut-off >1.8 µg/dL or 50 nmol/L) [24]. We also evaluated aldosterone and renin or renin plasmatic activity and Na/K, and all were at normal levels without suspicion of primary aldosteronism.
For serum cortisol, we used an antibody from Beckman (RRID:AB_2802133 [25]) (during 2019, we used an antibody from Abbott, RRID:AB_2783639 [26]). For salivary cortisol, we used an antibody from Roche (RRID:AB_2802131 [27]). For ACTH we used RRID:AB_2783634 [28]. To measure the dexamethasone and the urinary cortisol dosage we used liquid chromatography mass spectrometry. The total urinary cortisol dosage, from 2009 to 2015, was measured using a chemiluminescent immunoassay.
Adrenal volume measurement was performed at preoperative CT and 3-month postoperative CT by 2 experienced radiologists. ARMC5 was analyzed from all patients; germline and somatic mutations were evaluated (Table 1) and sited according to the National Institutes of Health recommendation [29]. Histological diagnosis of PMAH was confirmed by an expert pathologist (M.C.N.Z.).
Table 1.
Summary of ARMC5 allelic variants identified
| Subject | ARMC5 germline allelic variant | Location | State | Prediction | ARMC5 Somatic Alteration | Location | State | Prediction |
|---|---|---|---|---|---|---|---|---|
| 1 | normal | NA | ||||||
| 2 | c.476-1G>C | Intron1 | Heterozygous | Pathogenica | c.2053_2055delCTT (p.Leu685del) | Exon 6 | Heterozygous | Uncertain significanceb |
| 3 | c.1181T>C (p.Leu394Prol) | Exon 3 | Heterozygous | Uncertain Significanceb | c.1559delG (p.Gly520Aspfs*24) | Exon 4 | Heterozygous | pathogenicb |
| 4 | c.170dupG (p.Ile58Asnfs*45) | Exon 1 | Heterozygous | Pathogenicb | LOH | |||
| 5 | c.2423A>C (p.His808Pro) | Exon 6 | Heterozygous | Likely pathogenicb | c.283_295delTCGGCCGCGTCGGG (p.Ser96Serfs*2) | Exon 1 | Heterozygous | Likely pathogenicb |
| 6 | c.170dupG (p.Ile58Asnfs*45) | Exon 1 | Heterozygous | Pathogenicb | c.2082_2088delCCCGCTC (p.Pro695Serfs*20) | Exon 6 | Heterozygous | Likely pathogenicb |
| 7 | c.280_281delT (p.Ser94Valfs*8) | Exon 1 | Heterozygous | Likely pathogenicb | Absent | |||
| 8 | Normal | NA | ||||||
| 9 | c.1960C>T (p.Arg654*) | Exon 5 | Heterozygous | Likely pathogenicb | c.1575_1582delCCTGCTGC (p.Leu526Alafs*9) | Exon 4 | Heterozygous | Likely pathogenicb |
| 10 | Normal | NA | ||||||
| 11 | Normal | NA | ||||||
| 12 | c.799C>T (p.Arg267*, rs369721476) | Exon 3 | Heterozygous | Uncertain significanceb | Absent | |||
| 13 | Normal | NA | ||||||
| 14 | Normal | NA | ||||||
| 15 | c.407T>C (p.Leu136Pro, rs771845578) | Exon 1 | Heterozygous | Uncertain significanceb | NA | |||
| 16 | c.1094T>C (p.Leu365Pro, rs587777663) | Exon 3 | Heterozygous | Uncertain Significanceb | c.295G>T (p.Gly99*) | Exon 1 | Heterozygous | Likely pathogenicb |
| 17 | c.1094T>C (p.Leu365Pro, rs587777663) | Exon 3 | Heterozygous | Uncertain Significanceb | LOH |
aPrediction made using the Human splicing finder (http://www.umd.be/HSF/).
bPrediction made using the Varsome The Human Genomics Community (https://varsome.com/).
We used the Charlson comorbidity index (CCI) to evaluate the mortality risk for patients. The CCI is among the best-known and widely used indexes of comorbidity and was originally developed to predict 1-year mortality in a mixed population of internal medicine patients using comorbidity derived from a chart review. The CCI consists of 19 selected conditions that are weighted and summed to an index on a 0 to 33 scale [30, 31].
A. Surgical treatment
Surgical treatment was indicated in 3 circumstances: (1) presence of CS regardless of age; (2) SH in patients with aged <50 years; (3) SH associated with difficult to control blood hypertension, dyslipidemia, or diabetes mellitus in patients aged between 50 and 70 years.
In cases of symmetric adrenal gland size and uptake, the right side was elected for partial resection. Surgical treatment was performed in 2 sequential steps, as described by Srougi et al. [32]. (1) Partial adrenalectomy: the incision of the adrenal gland was done with the aim of resecting at least two-thirds of the gland parenchyma, removing all visible nodules and preserving the main adrenal vein whenever possible. For hemostasis, 10-mm Hem-o-lock® clips (Morrisville, NC, USA) and monopolar electrocautery were used. (2) Total adrenalectomy: after repositioning the patient, the entire contralateral gland was removed. Whenever possible, the midline laparoscopic port used in partial adrenalectomy was also used for contralateral total adrenalectomy. Intraoperative ultrasound imaging was not used. Resection of the nodules was performed based on CT scan reconstruction planning and intraoperative visual estimation. Specimens were weighted and sent for pathological evaluation. The diagnosis of PMAH was confirmed by pathological analysis performed by the same pathologist. The same experienced urologist proctoring a urology resident operated on all patients. Intra- and postoperative data were recorded, including medical and surgical complications.
B. Treatment outcomes
All patients received 100-mg of hydrocortisone dose in anesthesic induction and, thereafter, 50 mg every 8 hours. After the return of oral ingestion, all patients started oral hydrocortisone replacement (20 mg at 8 am and 10 mg at 2 pm). During follow-up, medical visits were monthly in the first trimester, every 3 months until completion at 1, year, and every 6 months thereafter. Morning serum cortisol was measured before hydrocortisone intake every medical visit. Hydrocortisone dose was adjusted based on serum cortisol measurement and the presence of clinical symptoms. Fludrocortisone replacement (50 μg/day) was prescribed when the patient had either hypotension episodes or low aldosterone levels associated with high levels of plasmatic renin. Glucocorticoid replacement was interrupted when serum cortisol level was >8.8 μg/dL [33]. The cosyntropin test was performed in order to evaluate remnant adrenal response (250-μg bolus; cut-off for response was serum cortisol over 18 μg/dL at 60 minutes). This test was not performed in the whole cohort as it was not available in our institution during most of the study period (Table 2). The endpoint was control of hypercortisolism, defined when the patient presented physiologic levels of serum cortisol (5–25 μg/dL; 0.18-0.90 nmol/L) without needing hormonal replacement. When clinical signs of adrenal insufficiency were detected and/or serum cortisol levels were below 5 μg/dL (8:00 am), additional oral hydrocortisone was replaced with a dose of >0.2 mg/kg/day. Blood pressure, serum glucose, serum triglycerides, serum cholesterol (high-density lipoprotein and low-density lipoprotein ), and body mass index (BMI) were compared before surgery, 12 months after surgery, and at the last visit for each patient. The number of medications to control blood hypertension, diabetes mellitus, and dyslipidemia were recorded and compared.
Table 2.
Results of cosyntropin test
| Post cosyntropin cortisol (μg/dL) | |||
|---|---|---|---|
| Subject | Baseline serum cortisol (μg/dL) | 30 minutes | 60 minutes |
| 9 | 5.9 | 18.8 | 19.9 |
| 12 | 11.9 | N/A | 21.3 |
HPA axis recovery: serum cortisol >18 μg/dL measured 60 minutes after endovenous administration of 250 µg of synthetic cosyntropin.
Remission of hypercortisolism after surgery was assessed and correlated with age, gender, BMI, serum cortisol, plasmatic ACTH, serum cortisol after DST, presence of germline/somatic ARMC5 mutation, and time between PMAH diagnosis and surgical treatment. Preoperative largest adrenal volume and postoperative adrenal volume were also correlated with time to hypothalamic–adrenal–pituitary (HPA) axis recovery.
C. Statistical analysis
Kaplan–Meier survival curves were used to estimate the time for the HPA axis to recover. Curves were compared using the log-rank test, and risk factors were evaluated using the Cox proportional hazards model. In comparisons of clinical and metabolic parameters, the nonparametric Friedman test was used. Whenever a significant difference was observed, the Bonferroni correction was applied. Statistical significance was considered when P < .05. Statistical tests were performed using IBM SPSS® version 24. (IBM, USA).
2. Results
Clinical characteristics at presentation are summarized on Table 3. At the time of surgical treatment, the median age was 50 years (range 41-69 years), and median BMI was 29 (range 31-48 kg/m2). All surgeries were successfully performed via laparoscopy access. Seven patients presented classical CS, while 10 had SH. Two patients from the same family had the same germline ARMC5 mutation.
Table 3.
Patients characteristics at presentation
| N | Age | Sex | BMI (kg/ m2) | Charlson score | Clinic presentation | ARMC5 mutation | MENI | Serum cortisol (μg/dL) | Salivary cortisol (μg/dL) | Urinary cortisol (μg/24 h) | ACTH (pg/ mL) | Dexamethasone suppression test (μg/dL) | Adrenal volume in CT scan (cm3) | Adrenal uptake in PET/CT (SUV) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | |||||||||||||
| 1 | 53 | F | 49 | 1 | SH | – | – | 8.9 | 0.21 | 172 | <5 | 5.0 | 26.4 | 13.2 | 5.0 | 5.1 |
| 2 | 63 | F | 41 | 3 | CS | + | + | 25.9 | 0.48 | 450 | <5 | 32.8 | 54.5 | 19.2 | 8.9 | 7.1 |
| 3 | 49 | F | 27 | 2 | CS | + | – | 27.8 | 0.32 | 364 | <5 | 27.1 | 11.4 | 47.2 | 4.1 | 5.5 |
| 4 | 45 | F | 23 | 1 | CS | + | – | 18.5 | 0.65 | 389 | <5 | 18.7 | 17.4 | 38.1 | 3.4 | 2.7 |
| 5 | 45 | M | 29 | 1 | CS | + | – | 26.6 | 0.75 | 1419 | <5 | 3.9 | 220 | 67 | 4.9 | 3.3 |
| 6 | 45 | F | 31 | 1 | CS | + | – | 12.7 | 0.08 | 78 | <5 | 7.2 | 10.1 | 9.3 | 3.6 | 3.8 |
| 7 | 52 | M | 35 | 4 | CS | + | – | 26.8 | 0.85 | 100 | <5 | 25.1 | 48.6 | 41.2 | 5.6 | 5.0 |
| 8 | 50 | F | 28 | 1 | CS | – | – | 28.6 | 0.98 | 619 | <5 | 29.6 | 2 | - | 4.2 | - |
| 9 | 41 | M | 27 | 0 | SH + INC | + | – | 13.7 | 0.19 | 28 | <5 | 10.5 | 33.5 | 52.8 | 4.4 | 4.9 |
| 10 | 68 | M | 28 | 3 | SH + INC | – | – | 24.9 | 0.03 | 157 | 13.9 | 6.3 | 19.1 | 8.4 | 6.8 | 5.7 |
| 11 | 47 | F | 34 | 2 | SH + INC | – | – | 6 | 0.09 | 90 | <5 | 12.4 | 33.1 | 15.3 | 3.0 | 2.6 |
| 12 | 47 | F | 41 | 1 | SH + INC | + | – | 17 | 0.13 | 213 | <5 | 15.7 | 23 | 18.3 | 4.1 | 4.7 |
| 13 | 49 | F | 21 | 3 | SH + INC | – | + | 16 | 0.11 | 80 | 16.9 | 4.9 | 7.9 | 5 | 4.0 | 4.8 |
| 14 | 69 | F | 26 | 3 | SH + INC | – | – | 10.5 | 0.05 | 34 | 20 | 3.2 | 13.2 | 16.3 | 3.2 | 3.4 |
| 15 | 69 | F | 36 | 2 | SH + INC | + | – | 10.8 | 0.014 | 21 | <5 | 13.9 | 50.5 | 9.8 | 4.7 | 4.3 |
| 16 | 61 | M | 35 | 3 | SH + FAM | + | – | 9.1 | 0.06 | 17 | <5 | 3.7 | 9.5 | 12.6 | 2.3 | 4.1 |
| 17 | 41 | F | 48 | 0 | SH + FAM | + | – | 14.9 | 0.07 | 12 | 16.7 | 7.8 | 4.8 | 5.1 | 4.4 | 4.5 |
Reference ranges: serum cortisol = 5–25 μg/dL; midnight salivary cortisol = 0.274 μg/dL; urinary cortisol = 50–310 μg/24 hours (subjects 1-13 excluding 6); free urinary cortisol = 3.0 at 43 μg/24 hours (subjects 14-17 including 6); ACTH = 7.2–63.3 pg/mL; dexamethasone suppression test = cortisol <1.8 μg/dL.
Abbreviations: CS, Cushing’s syndrome; SH, subclinical hypercortisolism, INC, adrenal incidentaloma; MENI, meningioma; FAM, familial screening.
Right partial adrenalectomy was performed in 11 patients, 3 of whom presented both adrenal glands with similar volume in CT scan and uptake in 18F-FDG PET/CT (Table 4).
Table 4.
Intraoperative characteristics and follow-up
| Surgical specimen weight (g) | Postoperative adrenal volume (cm3) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Surgical time(min) | Anesthesia time (min) | Postoperative ICU | Hospital stay(days) | Partial adrenalectomy side | Left | Right | Left | Right | Hormonal axis recovery | Hormonal reposition (months) | Follow- up(months) |
| 1 | 180 | 280 | – | 4 | R | 40.2 | 9.4 | 0 | 0.6 | + | 40 | 54 |
| 2 | 200 | 270 | + | 23 | R | 158.9 | 15.9 | 0 | 6.2 | + | 32 | 52 |
| 3 | 170 | 285 | – | 9 | L | 50 | (both) | 6.9 | 0 | – | 88 | 88 |
| 4 | 195 | 300 | – | 3 | R | 65.8 | 11.8 | 0 | 8.5 | + | 17 | 40 |
| 5 | 240 | 390 | + | 3 | R | 57 | 22.9 | 0 | 12.1 | – | 52 | 52 |
| 6 | 160 | 390 | + | 7 | R | 32,3 | 23.1 | 0 | 2.1 | + | 18 | 47 |
| 7 | 190 | 510 | + | 5 | R | 65 | 33.2 | 0 | 3.6 | – | 42 | 42 |
| 8 | 125 | 210 | – | 11 | R | – | 18 | 0 | 0.4 | – | 42 | 42 |
| 9 | 240 | 320 | – | 8 | L | 45.9 | 74.2 | 5 | 0 | + | 15 | 41 |
| 10 | 190 | 370 | + | 10 | R | 46.6 | 13.9 | 0 | 3.3 | + | 6 | 38 |
| 11 | 210 | 330 | – | 8 | R | 55.5 | 22.3 | 0 | 0.5 | – | 28 | 28 |
| 12 | 180 | 330 | – | 8 | L | 46.7 | 39.6 | 6.7 | 0 | + | 10 | 26 |
| 13 | 190 | 280 | + | 5 | L | 21.3 | 29.0 | 3 | 0 | + | 14 | 23 |
| 14 | 205 | 250 | – | 10 | L | 17.3 | 31.5 | 3.6 | 0 | + | 4 | 17 |
| 15 | 300 | 385 | + | 5 | R | 90.3 | 20 | 0 | 2 | + | 12 | 15 |
| 16 | 240 | 360 | – | 4 | L | 30.8 | 22 | 3 | 0 | + | 8 | 14 |
| 17 | 180 | 225 | + | 3 | R | 36.7 | 22 | 0 | 1 | + | 6 | 13 |
Abbreviations: R, right; L, left; ICU, intensive care unit; min, minutes.
Patients operated on showed significant improvement in clinical parameters after surgery: reductions in weight (88-82 kg; P = .004), in systolic (145-121 mmHg; P = .001) and diastolic blood pressure (91-78 mmHg; P =0.001), in the number of hypertension medications (2.8-1.1; P < .001), and in the number of antidiabetic drugs (1-0; P = .021). These differences were noted between preoperative and both postoperative measures (12 months and last visit) (Fig. 2). Other metabolic parameters did not change significantly (Table 3).
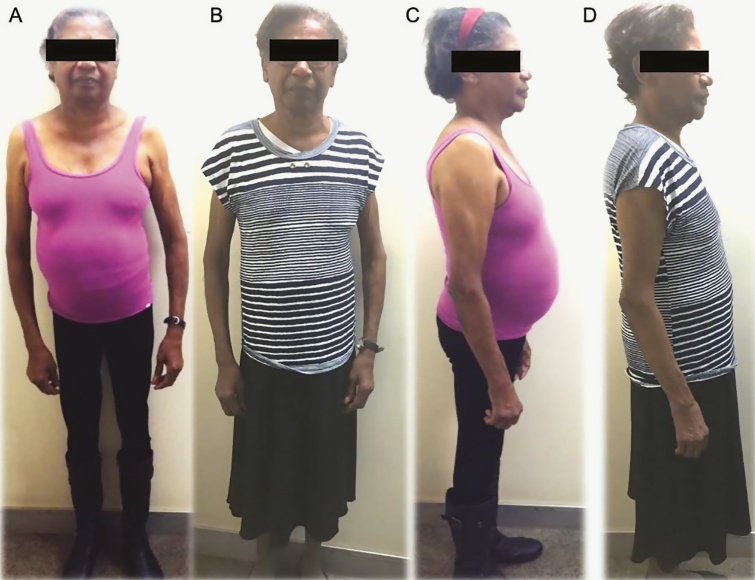
Figure 2.
Patient with PMAH. Before (A,C) and after surgical treatment (B,D). Patient consented to the use of images.
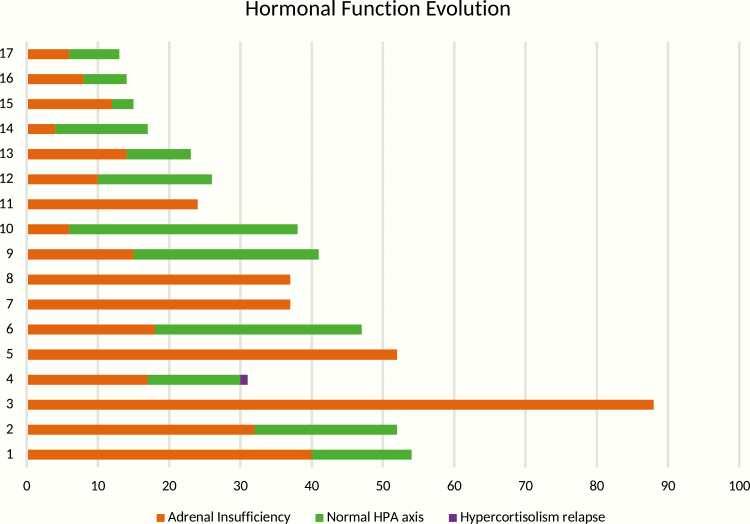
In a median follow-up of 41 months, 12 patients achieved control of hypercortisolism, while the other 5 still had adrenal insufficiency (Fig. 3). The mean time to adrenal function recovery was 32.4 months (median 17 month; range 6-84). At postoperative CT, the mean adrenal volume was 4.07 cm3 (range 0.6-12.1). As expected, we observed that normal ACTH before surgery was a predictor for faster recovery of the physiological HPA axis. None of the studied variables was significant for determining the maintenance of postoperative adrenal insufficiency (Tables 5 and 6). One patient (subject 4) developed hypercortisolism recurrence, which was diagnosed because of abnormal elevation in serum cortisol after DST (Fig. 2). This patient underwent a second surgical procedure.
Figure 3.
Hormonal function evolution. Patient × time (months).
Table 5.
Pre- and postoperative clinical and metabolic parameters
| Multiple Comparison | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Average | Median | Standard deviation | IQR | P* | Moment | adjusted P** |
| Weight (kg) | |||||||
| Initial | 87.9 | 86 | 23.7 | 42.0 | .004 | 1–2 | .003 |
| 12 months | 81.5 | 85 | 19.9 | 33.7 | 1–3 | .31 | |
| Last visit | 83.3 | 88 | 20.3 | 36.3 | 2–3 | .31 | |
| Body mass index (kg/m 2) | |||||||
| Initial | 32.8 | 30.5 | 8.2 | 11.4 | .051 | ||
| 12 months | 30.5 | 29.0 | 6.3 | 9.5 | |||
| Last visit | 29.9 | 30.0 | 5.6 | 9.5 | |||
| Systolic blood pressure (mmHg) | |||||||
| Initial | 145.2 | 140 | 23.9 | 30.0 | .001 | 1–2 | .002 |
| 12 months | 121.6 | 120 | 17.9 | 20.0 | 1–3 | .018 | |
| Last visit | 125.9 | 130 | 9.5 | 10.0 | 2–3 | 1 | |
| Diastolic blood pressure (mmHg) | |||||||
| Initial | 91.1 | 90 | 10.9 | 20.00 | .001 | 1–2 | .024 |
| 12 months | 79.4 | 80 | 11.9 | 20.00 | 1–3 | .008 | |
| Last visit | 78.8 | 80 | 6.0 | 5.00 | 2–3 | 1 | |
| Drugs: dyslipidemia (n) | |||||||
| Initial | 0.58 | 1 | NA | 1.00 | .368 | ||
| 12 months | 0.41 | 0 | NA | 1.00 | |||
| Last visit | 0.41 | 0 | NA | 1.00 | |||
| Drugs- diabetes (n) | |||||||
| Initial | 0.9 | 1 | NA | 2.00 | .021 | 1–2 | .368 |
| 12 months | 0.5 | 0 | NA | 1.00 | 1–3 | .595 | |
| Last visit | 0.6 | 0 | NA | 1.00 | 2–3 | 1 | |
| Drugs: hypertension (n) | |||||||
| Initial | 2.8 | 3 | NA | 2.00 | <.0001 | 1–2 | .006 |
| 12 months | 1.1 | 1 | NA | 2.00 | 1–3 | .002 | |
| Last visit | 1.0 | 1 | NA | 2.00 | 2–3 | 1 | |
| Glucose (mg/dL) | |||||||
| Initial | 116.4 | 108,0 | 38.4 | 40.50 | .138 | ||
| 12 months | 99.5 | 98,0 | 20.9 | 21.50 | |||
| Last visit | 97.6 | 90,0 | 21.3 | 24.00 | |||
| Glycated hemoglobin (%) | |||||||
| Initial | 6.6 | 6,2 | 1.2 | 1.44 | .42 | ||
| 12 months | 6.2 | 5,9 | 0.9 | 1.00 | |||
| Last visit | 6.0 | 5,9 | 0.6 | 0.95 | |||
| Cholesterol (mg/dL) | |||||||
| Initial | 207.0 | 199,0 | 38.8 | 69.00 | .092 | ||
| 12 months | 185.1 | 179,0 | 38.7 | 54.00 | |||
| Last visit | 184.8 | 181,0 | 32.9 | 56.00 | |||
| Low-density lipoprotein(mg/dL) | |||||||
| Initial | 117.4 | 110,0 | 30.7 | 47,50 | .664 | ||
| 12 months | 105.8 | 112,0 | 34.2 | 53,50 | |||
| Last visit | 111.5 | 113,0 | 39.8 | 68,00 | |||
| High-density lipoprotein (mg/dL) | |||||||
| Initial | 50.2 | 50,0 | 12.9 | 27.00 | .884 | ||
| 12 months | 50.2 | 48,0 | 14.5 | 25.00 | |||
| Last visit | 49.0 | 48,0 | 11.7 | 19.00 | |||
| Triglycerides (mg/dL) | |||||||
| Initial | 180.5 | 156,0 | 92.6 | 111.50 | .356 | ||
| 12 months | 160.3 | 148,0 | 60.7 | 96.00 | |||
| Last visit | 174.7 | 176,0 | 75.5 | 104.50 | |||
IQR, interquartile ranges.
Table 6.
Influence in time to recover HPA axis function
| CI (95%) for RR | ||||
|---|---|---|---|---|
| Variable | P | RR | Inferior | Superior |
| ACTH (measurable) | .003 | 12.15 | 2.366 | 62.38 |
| Serum basal cortisol | .077 | 0.936 | 0.870 | 1.007 |
| DST cortisol | .104 | 0.949 | 0.892 | 1.011 |
| Bigger adrenal volume | .150 | 0.980 | 0.954 | 1.007 |
| SUVMAX | .822 | 0.952 | 0.622 | 1.458 |
| SUVMAX (>3,1) | NA | |||
| ARMC5 mutation | .972 | 0.979 | 0.294 | 3.256 |
| Meningioma | .672 | 1.393 | 0.300 | 6.482 |
| Gender | .799 | 1.186 | 0.320 | 4.398 |
| Age | .063 | 1.069 | 0.996 | 1.146 |
| BMI | .747 | 1.012 | 0.943 | 1.085 |
| BMI (>30) | .655 | 1.302 | 0.409 | 4.152 |
| Disease time | .103 | 1.011 | 0.998 | 1.025 |
| Postoperative adrenal volume | .486 | 0.942 | 0.797 | 1.114 |
Abbreviations: ACTH, adrenocorticotropin; BMI, body mass index; DST, dexamethasone suppression test; RR, relative risk; CI, confidence interval.
3. Discussion
Adrenal-sparing surgery exhibited good results, achieving satisfactory hypercortisolism remission and improvement of clinical parameters. In our cohort, 95% and 70.5% of the patients achieved normocortisolemia and recovery of the physiologic HPA axis, respectively. Moreover, this surgical approach was safe, with an acceptable rate of complications in a cohort of patients with important clinical comorbidities associated with metabolic syndrome.
Lowery et al. assessed a cohort of 23 patients with CS or SH harboring bilateral adrenal disease caused by different pathologies, treated with simultaneous total adrenalectomy and contralateral partial adrenalectomy. Corticosteroid supplementation was needed in 78% of the patients and mean time of hormonal reposition was 32 months [20]. In the present study, all patients needed corticosteroid replacement after surgery, and median time of hormonal reposition was 17 months (range 4-84). In our analysis, patients with preoperative mild hypercortisolism presented a better recovery of the HPA axis. Recently, Di Dalmazi et al. published a systematic review involving 28 studies, with 248 patients operated on due to SH and 377 patients operated on due to overt CS [34]. The prevalence of adrenal insufficiency immediately after surgery was 65.3% in the SH group and 99.7% in the CS group, while the time to HPA axis recovery was 6.5 and 11.2 months, respectively [34].
In our analysis, patients with mild hypercortisolism more commonly presented HPA axis recovery; this is in agreement with an important previous study published in 2014. Prete et al., in a recent retrospective analysis, observed that patients with primary adrenal overt CS treated with unilateral adrenalectomy had a median period of adrenal insufficiency of 18.5 months, compared with 6 months in patients with primary adrenal SH treated the same way [35].
The volume of remnant adrenal gland seems to have a pivotal role in the outcome of adrenal-sparing surgery for PMAH. One may infer that patients treated with this approach will wait longer for recurrence due to the smaller volume of remnant adrenal gland compared with patients treated with unilateral adrenalectomy. It has been suggested that 15% to 30% of remnant adrenal gland would be sufficient to maintain physiologic adrenal hormonal production when performing partial adrenalectomy for most adrenal diseases [36]. However, this proportion might be different when dealing with PMAH, which affects the entire gland. In addition, PMAH is frequently associated with germline mutations, considerably increasing the risk of recurrence. It should be emphasized that adrenal glands afflicted by PMAH have inefficient steroidogenesis. Thus, theoretically more remnant adrenal tissue should be kept compared with other adrenal pathologies, with the aim of recovery of the HPA axis.
Debillon et al. [16] described the treatment of PMAH with unilateral adrenalectomy in 15 patients, reporting 60% of hypercortisolism remission. However, in the same cohort the rate of disease recurrence was 13% after 7 years of follow-up [16]. More recently, Osswald et al. [37] reported that up to 68% of patients with PMAH treated with unilateral adrenalectomy sustain or present hypercortisolism recurrence. In our series, we observed 1 case of hypercortisolism recurrence, within 41 months of follow-up [37]. Considering the heterogenous presentation of PMAH, there are probably patients in which unilateral adrenalectomy is more suitable, while in others the same approach may lead to early hypercortisolism recurrence. We hypothesize that the ideal surgical treatment for PMAH should be chosen according to each specific PMAH presentation.
This study has inherent limitations, namely the small number of patients and absence of a control group. However, this is the first prospective series addressing PMAH treatment with adrenal-sparing surgery. In addition, we are the first to evaluate the use of pre- and postoperative adrenal volume measurement in PMAH. Another important issue is that as family cases of patients with germline mutations in ARMC5 are screened more cases of PMAH will be diagnosed at an early stage, and the precise indication of each surgical procedure will be challenging.
To the best of our knowledge, surgical treatment should be indicated in strictly selected patients and the choice between unilateral, bilateral, and adrenal-sparing surgery tailored for each case.
In conclusion, adrenal-sparing surgery is a safe and feasible procedure for PMAH, providing hypercortisolism remission while potentially avoiding the drawbacks of corticosteroid replacement. Additionally, patients undergoing this treatment exhibited substantial improvement of clinical parameters. Within a short follow-up, adrenal-sparing surgery proved to be an effective alternative. Further studies with a higher level of evidence and comprising larger cohorts are necessary to validate the role of this therapeutic modality.
Acknowledgments
We thank the all the medical residents of the Urology, Endocrinology, and Pathology Departments for performing the operations, taking care of the patients during hospital stay, and for pathological analysis. We also thank Vinicius Calsavara (statistician) for his contribution in our analysis.
Financial Support: this work was supported in part by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) grants to M.C.B.V.F. (2015/50192-9).
Glossary
Abbreviations
- ACTH
adrenocorticotropin
- CCI
Charlson comorbidity index
- CS
Cushing syndrome
- CT
computed tomography
- HPA
hypothalamic–adrenal–pituitary
- PMAH
primary macronodular adrenal hyperplasia
- SH
subclinical hypercortisolism
Contributor Information
Fabio Yoshiaki Tanno, Email: fabio.tanno@hc.fm.usp.br.
Maria Candida Barisson Villares Fragoso, Email: maria.villares@hc.fm.usp.br.
Additional Information
Disclosure Summary: The authors have nothing to disclosure.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Kirschner MA, Powell RD, Lipsett MB. Cushing’s syndrome: nodular cortical hyperplasia of adrenal glands with clinical and pathological features suggesting adrenocortical tumor. J Clin Endocrinol Metab. 1964;24:947-955. [DOI] [PubMed] [Google Scholar]
- 2. Lacroix A, Baldacchino V, Bourdeau I, Hamet P, Tremblay J. Cushing’s syndrome variants secondary to aberrant hormone receptors. Trends Endocrinol Metab. 2004;15(8):375-382. [DOI] [PubMed] [Google Scholar]
- 3. Newell-Price J, Bertagna X, Grossman AB, Nieman LK. Cushing’s syndrome. Lancet. 2006;367(9522):1605-1617. [DOI] [PubMed] [Google Scholar]
- 4. Alencar GA, Lerario AM, Nishi MY, et al. ARMC5 mutations are a frequent cause of primary macronodular adrenal hyperplasia. J Clin Endocrinol Metab. 2014;99(8):E1501-E1509. [DOI] [PubMed] [Google Scholar]
- 5. Assié G, Libé R, Espiard S, et al. ARMC5 mutations in macronodular adrenal hyperplasia with Cushing’s syndrome. N Engl J Med. 2013;369(22):2105-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lacroix A. ACTH-independent macronodular adrenal hyperplasia. Best Pract Res Clin Endocrinol Metab. 2009;23(2):245-259. [DOI] [PubMed] [Google Scholar]
- 7. Lacroix A, N’Diaye N, Mircescu H, Tremblay J, Hamet P. The diversity of abnormal hormone receptors in adrenal Cushing’s syndrome allows novel pharmacological therapies. Braz J Med Biol Res. 2000;33(10):1201-1209. [DOI] [PubMed] [Google Scholar]
- 8. Nagai M, Narita I, Omori K, Komura S, Arakawa M. Adrenocorticotropic hormone-independent bilateral adrenocortical macronodular hyperplasia treated with mitotane. Intern Med. 1999;38(12):969-973. [DOI] [PubMed] [Google Scholar]
- 9. Obata Y, Yamada Y, Baden MY, et al. Long-term efficacy of trilostane for Cushing’s syndrome due to adrenocorticotropin-independent bilateral macronodular adrenocortical hyperplasia. Intern Med. 2011;50(21):2621-2625. [DOI] [PubMed] [Google Scholar]
- 10. Omori N, Nomura K, Omori K, Takano K, Obara T. Rational, effective metyrapone treatment of ACTH-independent bilateral macronodular adrenocortical hyperplasia (AIMAH). Endocr J. 2001;48(6):665-669. [DOI] [PubMed] [Google Scholar]
- 11. Rushworth RL, Torpy DJ, Falhammar H. Adrenal Crisis. N Engl J Med. 2019;381(9):852-861. [DOI] [PubMed] [Google Scholar]
- 12. Albiger NM, Ceccato F, Zilio M, et al. An analysis of different therapeutic options in patients with Cushing’s syndrome due to bilateral macronodular adrenal hyperplasia: a single-centre experience. Clin Endocrinol (Oxf). 2015;82(6):808-815. [DOI] [PubMed] [Google Scholar]
- 13. Iacobone M, Albiger N, Scaroni C, et al. The role of unilateral adrenalectomy in ACTH-independent macronodular adrenal hyperplasia (AIMAH). World J Surg. 2008;32(5):882-889. [DOI] [PubMed] [Google Scholar]
- 14. Mazzuco TL, Chaffanjon P, Martinie M, Sturm N, Chabre O. Adrenal Cushing’s syndrome due to bilateral macronodular adrenal hyperplasia: prediction of the efficacy of beta-blockade therapy and interest of unilateral adrenalectomy. Endocr J. 2009;56(7):867-877. [DOI] [PubMed] [Google Scholar]
- 15. Erichsen MM, Løvås K, Fougner KJ, et al. Normal overall mortality rate in Addison’s disease, but young patients are at risk of premature death. Eur J Endocrinol. 2009;160(2):233-237. [DOI] [PubMed] [Google Scholar]
- 16. Debillon E, Velayoudom-Cephise FL, Salenave S, et al. Unilateral Adrenalectomy as a first-line treatment of Cushing’s syndrome in patients with primary bilateral macronodular adrenal hyperplasia. J Clin Endocrinol Metab. 2015;100(12):4417-4424. [DOI] [PubMed] [Google Scholar]
- 17. Nagaraja V, Eslick GD, Edirimanne S. Recurrence and functional outcomes of partial adrenalectomy: a systematic review and meta-analysis. Int J Surg. 2015;16(Pt A):7-13. [DOI] [PubMed] [Google Scholar]
- 18. Kaye DR, Storey BB, Pacak K, Pinto PA, Linehan WM, Bratslavsky G. Partial adrenalectomy: underused first line therapy for small adrenal tumors. J Urol. 2010;184(1):18-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Y, Li H. Classification and surgical treatment for 180 cases of adrenocortical hyperplastic disease. Int J Clin Exp Med. 2015;8(10):19311-19317. [PMC free article] [PubMed] [Google Scholar]
- 20. Lowery AJ, Seeliger B, Alesina PF, Walz MK. Posterior retroperitoneoscopic adrenal surgery for clinical and subclinical Cushing’s syndrome in patients with bilateral adrenal disease. Langenbecks Arch Surg. 2017;402(5):775-785. [DOI] [PubMed] [Google Scholar]
- 21. Alencar GA, Fragoso MC, Yamaga LY, Lerario AM, Mendonca BB. (18)F-FDG-PET/CT imaging of ACTH-independent macronodular adrenocortical hyperplasia (AIMAH) demonstrating increased (18)F-FDG uptake. J Clin Endocrinol Metab. 2011;96(11):3300-3301. [DOI] [PubMed] [Google Scholar]
- 22. Cavalcante IP, Nogueira Zerbini MC, Alencar GA, et al. High 18F-FDG uptake in PMAH correlated with normal expression of Glut1, HK1, HK2, and HK3. Acta Radiologica. 2016;57(3):370-377. [DOI] [PubMed] [Google Scholar]
- 23. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(5):1526-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tabarin A. Do the diagnostic criteria for subclinical hypercortisolism exist? Ann Endocrinol (Paris). 2018;79(3):146-148. [DOI] [PubMed] [Google Scholar]
- 25. RRID:AB_2802133 https://scicrunch.org/resolver/AB_2802133.
- 26. RRID:AB_2783639 https://scicrunch.org/resolver/AB_2783639.
- 27. RRID:AB_2802131 https://scicrunch.org/resolver/AB_2802131.
- 28. RRID:AB_2783634 https://scicrunch.org/resolver/AB_2783634.
- 29. https://www.ncbi.nlm.nih.gov/nuccore/NM_024742.2.
- 30. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. [DOI] [PubMed] [Google Scholar]
- 31. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245-1251. [DOI] [PubMed] [Google Scholar]
- 32. Srougi V, Rocha BA, Tanno FY, et al. The use of three-dimensional printers for partial Adrenalectomy: estimating the resection limits. Urology. 2016;90:217-220. [DOI] [PubMed] [Google Scholar]
- 33. Leong SH, Shander S, Ratnasingam J. Predicting recovery of the hypothalamic-pituitary-adrenal axis after prolonged Glucocorticoid use. Endocr Pract. 2018;24(1):14-20. [DOI] [PubMed] [Google Scholar]
- 34. Di Dalmazi G, Berr CM, Fassnacht M, Beuschlein F, Reincke M. Adrenal function after adrenalectomy for subclinical hypercortisolism and Cushing’s syndrome: a systematic review of the literature. J Clin Endocrinol Metab. 2014;99(8):2637-2645. [DOI] [PubMed] [Google Scholar]
- 35. Prete A, Paragliola RM, Bottiglieri F, et al. Factors predicting the duration of adrenal insufficiency in patients successfully treated for Cushing disease and nonmalignant primary adrenal Cushing syndrome. Endocrine. 2017;55(3):969-980. [DOI] [PubMed] [Google Scholar]
- 36. Brauckhoff M, Gimm O, Thanh PN, et al. Critical size of residual adrenal tissue and recovery from impaired early postoperative adrenocortical function after subtotal bilateral adrenalectomy. Surgery. 2003;134(6):1020-1027; discussion 1027. [DOI] [PubMed] [Google Scholar]
- 37. Osswald A, Quinkler M, Di Dalmazi G, et al. Long-term outcome of primary bilateral Macronodular Adrenocortical hyperplasia After Unilateral Adrenalectomy. J Clin Endocrinol Metab. 2019;104(7):2985-2993. [DOI] [PubMed] [Google Scholar]