Abstract
Development of improved approaches for HIV-1 prevention will likely be required for a durable end to the global AIDS pandemic. Recent advances in preclinical studies and early phase clinical trials offer renewed promise for immunologic strategies for blocking acquisition of infection. Clinical trials are currently underway to evaluate the efficacy of two vaccine candidates and a broadly neutralizing antibody (bNAb) against HIV-1 infection in humans. However, the vast diversity of HIV-1 represents a major challenge for both active and passive immunization. Here we review current immunologic strategies for HIV-1 prevention, with a focus on current and next generation vaccines and bNAbs.
Keywords: vaccine, broadly neutralizing antibodies, HIV-1, viral diversity, prevention, bNAbs
Introduction
The HIV-1 epidemic continues to threaten global health and economic development, with 1.8 million new HIV-1 infections in 2017 alone (1–3). Behavioral and biomedical interventions have reduced HIV-1 incidence since the height of the global epidemic, but the impact of these interventions has plateaued in recent years. For example, global HIV-1 incidence decreased only 5% between 2016 and 2017, despite nearly $10 billion spent on HIV-1 prevention efforts over this same period (1–3).
Until recently, HIV-1 prevention efforts focused on behavioral interventions, male circumcision, and antiretroviral therapy (ART) for preventing mother-to-child-transmission and post-exposure prophylaxis (4–12). Randomized controlled trials have now established that transmission does not occur between serodiscordant couples if the HIV-infected partner has an undetectable viral load, and that uninfected individuals are far less likely to acquire HIV-1 if they take ART, which is termed pre-exposure prophylaxis, or PrEP (13–28). In particular, studies have demonstrated the efficacy of tenofovir disoproxil fumarate and emtricitabine (TDF-FTC) based PrEP regimens in reducing HIV-1 transmission in men who have sex with men, transgender women, heterosexual men, and injection drug users (20–28). As a result, PrEP has become a major component of HIV-1 prevention efforts, and clinical trials are currently testing long-acting injectable antiretrovirals, implantable devices, and vaginal rings.
There are a number of limitations to treatment-as-prevention and PrEP strategies. Most importantly, access and compliance are major challenges for widespread implementation of PrEP. For example, women in sub-Saharan Africa at risk for HIV-1 infection may have suboptimal access to medical care and may face stigma for possession of antiretroviral pills. Long-term use of TDF-FTC can also be associated with renal toxicity and osteoporosis, and patients need to be screened for co-infection with hepatitis B and C before receiving TDF-FTC due to antiviral drug interactions (22, 29–31). Alternative PrEP agents, such as long-acting injectable integrase inhibitors, will likely improve compliance challenges but may lead to additional toxicity concerns during pregnancy (32). There is also a risk that PrEP might be started in patients who are already acutely infected with HIV, leading to treatment with a suboptimal regimen and the development of resistance.
Given the limitations of current biomedical options for HIV-1 prevention, there is a critical need for new HIV-1 prevention methods. Recent data has generated renewed enthusiasm for immunologic approaches, including both active immunization with vaccines and passive immunization with bNAbs. Five large clinical efficacy trials are currently underway to evaluate both active and passive immunization strategies for HIV-1 (Table 1). These trials are testing the clade C canarypox ALVAC (Env/Gag/Pro)/gp120 vaccine (HVTN 702), the global mosaic Ad26 (Env/Gag/Pol)/gp140 vaccine (HVTN 705/706; HPX 2008/3002), and the broadly neutralizing antibody VRC01 (HVTN 703/704). In this article, we review active and passive immunization strategies for HIV-1 prevention and discuss the challenge of global HIV-1 diversity for these efforts.
Table 1.
Efficacy Trials of Vaccines and bNAbs for HIV-1 Prevention.
| Trial | Phase | Product Type | Product Description | Clinical Trial Number |
|---|---|---|---|---|
| HVTN 703 | Phase 2b | Antibody | VRC01 in sub-Saharan Africa | NCT02568215 |
| HVTN 704 | Phase 2b | Antibody | VRC01 in Americas and Europe | NCT02716675 |
| HVTN 705 HPX2008 |
Phase 2b | Vaccine | Trivalent adenovirus 26 vector expressing mosaic Env/Gag/Pol + clade C gp140 with alum in sub-Saharan Africa | NCT03060629 |
| HVTN 702 | Phase 2b/3 | Vaccine | Canarypox vector expressing clade C Env/Gag/Pro + bivalent clade C gp120 with MF59 in sub-Saharan Africa | NCT02968849 |
| HVTN 706 HPX3002 |
Phase 3 | Vaccine | Tetravalent adenovirus 26 vector expressing mosaic Env/Gag/Pol + bivalent mosaic/clade C gp140 with alum in Americas and Europe | NCT03964415 |
Global HIV-1 Sequence Diversity
The primary target for HIV-specific antibody responses is the surface envelope glycoprotein (Env), which exhibits profound sequence diversity. All HIV-1 proteins are under immune pressure during chronic infection and are highly variable, but the diversity of Env is greater than Gag and Pol, which are good targets for T-cell responses (Fig. 1A). The diversity of HIV-1 results from rapid virus replication, the mutation-prone reverse transcriptase, the propensity for recombination and insertion and deletion events (indels), and serial escape from immune selection pressure over years during chronic infections. During the early expansion of the HIV-1 epidemic in Africa, major clades were established, and based on their phylogenetic relationships, a nomenclature to describe these clades (A-K) was defined (33). These major clades persist, and some clades dominate regionally (e.g. C in southern Africa, B in the United States), while others are very rare; the geographic distribution of these clades in various regions of the world is shown in Fig. 1B.
Figure 1. HIV-1 global diversity and vaccine antigen design.

(A) Coverage of HIV-1 diversity by natural, mosaic, and conserved element vaccine antigens. Sequence database alignments for Gag, Pol, Env and Nef were used to explore the extent of coverage of HIV-1 potential T-cell epitope (PTE) diversity that is achieved by different vaccine antigens. The graphs on the left span each protein, starting with the 9 amino acid peptide fragment (9-mer) beginning at position 1 (positions 1–9), shifting to the 9-mer starting at position 2 (positions 2–10), and so on. The black line tracks the maximum coverage that can be achieved by a bivalent (2-antigen) vaccine, and represents the sum of the 2 most common variants of each 9-mer sequence in each “column” of 9-mers. Given that overlapping 9-mers often have different preferred amino acids in a given position, the upper bound cannot be completely achieved. The height of the colored region at any position shows the extent of the 9-mer coverage that is achieved by each vaccine. The graphs on the right show the same information reordered, such that PTEs with the highest-to-lowest coverage are shown left-to-right in descending order. Here we use 4 examples, one each to illustrate 4 antigen design concepts: i) Single natural proteins, using as an example the antigens used in the Step trial. The Step vaccine spanning Gag, Pol and Nef is shown in gold (75). This vaccine did not confer overall protection, and although the majority of people responded, only a relatively small number of responses were elicited by this vaccine per person, and PTE diversity coverage was often poor and far from optimal. ii) A computationally designed complementary mosaic pair of proteins, that optimizes PTE coverage. The vaccine antigens that were used in the NHP studies that are serving as the basis of the Imbokodo human trial (63, 67), are shown in green. Note that the green area nearly approaches the black line (the reason it does not do this in Pol in the right hand figure is that not all of Pol was included in the vaccine, the coverage is excellent in the parts of Pol that were included, as shown on the left). iii) A conserved region vaccine that includes moderately large fragments of relatively conserved regions spanned by paired mosaics (tHIVconsvX, in blue) (96). Iv) A conserved element vaccine (p24CE), that focused on very highly conserved short fragments of HIV-1 located within p24 (93, 100). (B) Geographical distribution of subtypes and CRFs worldwide (left), in Asia (center) and in Africa (right). Distribution of subtypes is shown as pie-charts with each subtype and CRF represented by a different color shown in the legend. The Geography Search Interface on the Los Alamos HIV-1 Database was used to generate these data (https://www.hiv.lanl.gov/components/sequence/HIV/geo/geo.comp).
In a comparison of HIV-1 Env protein sequences (clades A-D) in the HIV-1 sequence compendium alignment, the within-clade median difference between Env amino acid sequences was 22% (quartiles 20–24%, range 15–29%), while between clades the median number of differences was 27% (quartiles 26–29%, range 22–44%). HIV-1 continues to diversify within clades, and on the time scale of decades the within-clade cross-reactive neutralization potency of sera from natural infection significantly diminishes (34, 35), suggesting the diversity challenge for immunologic responses is gradually worsening over time. HIV-1 evolution is further complicated by recombination. Some interclade recombinants (called circulating recombinant forms, or CRFs (33)) have expanded into major epidemic linages in their own right (36), such as CRF01 (an A/E recombinant) that is dominant in southeast Asia, and CRF02 (an A/G recombinant) that is dominant in west Africa (Fig. 1B). Some inter-subtype recombinants are only involved in local transmission chains, and so they are identified as a CRF as they are circulating, even though they are of limited epidemiological importance, and there are also many recombinants that have unique breakpoints, particularly in geographic regions where multiple clades co-circulate, e.g. A and D clade in Uganda (37).
Inter-subtype variability also means that different subtypes and CRFs have distinctive neutralization profiles, which is an important consideration for both active immunization with vaccines and passive delivery of bNAbs, as the phrase “heterologous breadth” does not necessarily imply global applicability (35, 38). For example, CRF01 strains, which are common in Southeast Asia, are highly resistant to antibodies that target Env variable region 3 (V3), whereas clade B strains, which are common in North America and Europe, often have reduced sensitivity to antibodies that target variable region 2 (V2) (38).
HIV-1 Sequence Diversification During Chronic Infection
HIV-1 evolution within infected individuals begins soon after infection, and given that the observed diversity that arises is due to selection from continuing cycles of immune response and escape (39–44), it is directly relevant to how we think about harnessing immunologic strategies to prevent HIV. Structurally, this diversity is manifested as both variable amino acids and glycans on the HIV-1 Env trimer (Fig. 2A–B). Such variable positions are found in virtually all bNAb target epitopes (Fig. 2A), e.g. bNAbs targeting the CD4 receptor binding site (CD4bs) contact positions in the highly variable V5 loop. Thus, we have previously argued that bNAbs do not derive their breadth from primarily recognizing conserved epitopes, rather that bNAbs have been selected in vivo to tolerate much of the natural diversity, likely resulting from antibody-Env co-evolution (40, 45–47) (Fig. 2B). Recombination is also rampant within chronic infections (48) and in viral rebound upon ART interruption (49, 50), and is an effective way to enable rapid escape from immune selection pressure.
Figure 2. HIV-1 Env diversity.

(A) Amino acid diversity. The top panels show the global amino acid diversity per site mapped onto a trimeric Env crystal structure (PDB: 5FYJ from ref. (172)). Each site on the trimer is color-coded according to the diversity as measured by sequence entropy (173); dark blue indicates highly conserved sites, while red indicates highly variable sites. The regions of high diversity are typically the hypervariable V1, V2, V4 and V5 loops, indicated on the structure (“hyp” = hypervariable). The left panels show the side-view of the Env trimer with the apex on the top and the viral membrane on the bottom, and the right panels show the view looking down the trimer apex. (B) Glycan diversity. The panels show the mapping of variable and conserved glycan sites on the trimer structure (same views as in the top panels). Glycan sites are color-coded according to frequency in M-group: blue indicate >95%, lightblue 80–95%, red 50–80% and pink 20–50%. The hypervariable loops do carry glycans, however given their high sequence and length variability, an alignment and thus numbering of sites in such regions is not meaningful. Thus, glycan sites in the hypervariable loops are ignored. (C) Hypervariable loop diversity. Variation in hypervariable loop characteristics are shown for V1, V2, V4 and V5 from top to bottom. (V3 is relatively conserved and does not have hypervariable region). For each hypervariable loop, the left panel shows length variation, center shows charge and right shows the number of glycans. Variability for each hypervariable loop characteristic is shown as a histogram with the characteristic on the horizontal axis and the number of M-group Envs with a particular value of characteristic on the vertical axis. All analyses in this figure were performed using the 2017 Filtered Web Env reference alignment from the Los Alamos HIV Database (a total of 5398 Env sequences one per individual and spanning all global subtypes and CRFs).
Another aspect of Env variability is the immense length and sequence variation of the hypervariable sections of the variable loops V1, V2, V4, and V5 (Fig. 2C). Indels are extremely common in these regions, thus they cannot be readily aligned, even within chronic infections that were initiated by single founding viruses; thus we have advocated for alignment-free characteristics of hypervariable regions, such as their length, net charge and number of glycans when considering the impact of these regions on antibody sensitivity (38). For example, M-group Envs show a median V1 hypervariable loop length of 22 amino acids, though the loop length can actually range from 6 to 58 amino acids. Such hypervariable regions can interact with several bNAbs (e.g. hypervariable V1 with V3 glycan bNAbs, hypervariable V2 with V2 apex bNAbs and hypervariable V5 against CD4bs bNAbs), and they can have a profound impact on bNAb sensitivity (38).
Vaccines that Generate Functional Non-Neutralizing Antibodies
Historically, vaccines have been critical tools for ending viral epidemics (51). The scientific challenges in the development of a prophylactic HIV-1 vaccine, however, are unprecedented, including the vast diversity of global HIV-1 and the inability to induce broadly reactive neutralizing antibodies by vaccination. Accumulating data from clinical and preclinical studies suggest that functional non-neutralizing antibodies may provide at least partial protection against HIV-1 infection (52–54). The RV144 trial of a canarypox vector prime (ALVAC-Env/Gag/Pro), gp120 protein boost strategy in Thailand provided the first clinical evidence of efficacy for any HIV-1 vaccine (55–60). This study demonstrated 31% efficacy, and protection correlated with V1/V2-specific IgG1 and IgG3 responses, antibody-dependent cellular cytotoxicity activity, and decreased IgA responses (57). Non-neutralizing antibody responses have also been shown to correlate with partial protection in nonhuman primate studies (61–64). Using a comprehensive antibody profiling approach known as systems serology, we showed that reduced risk of infection in this model correlated with functional antibody responses, such as antibody-dependent cellular phagocytosis (ADCP), antibody-dependent cellular cytotoxicity (ADCC), and antibody-dependent complement deposition (ADCD) (65, 66). We also observed that an adenovirus serotype 26 (Ad26)-based vector prime, gp140 protein boost strategy afforded 66% protection against acquisition of infection following SHIV-SF162P3 challenge in NHPs, and both non-neutralizing antibody responses and T-cell responses were immune correlates of protection (67).
Based on clinical data with the ALVAC/gp120 vaccine and preclinical data with the Ad26/gp140 vaccine, two parallel vaccine development programs have led to clinical efficacy trials. RV144 involved ALVAC vectors expressing clade B and CRF01_AE antigens boosted with alum-adjuvanted bivalent clade B/E gp120 (ALVAC + gp120 B/E). The current goal of this program is to confirm and extend the RV144 findings using clade C antigens in South Africa (Fig. 1B), a more potent adjuvant (MF59), and an additional boost immunization. The phase 1/2 study of ALVAC + gp120 C/C showed that it was safe and immunogenic; it elicited binding antibodies in 100% of vaccine recipients, although V1V2-specific responses were lower than those observed in RV144 (68). These responses supported moving this vaccine into a phase 2b/3 efficacy trial of 5,200 men and women in South Africa in the Uhambo study (HVTN 702; Table 1). This study is now fully enrolled, and initial efficacy results are expected in 2021.
The second HIV-1 vaccine program currently in clinical efficacy trials involves an Ad26 vector expressing bioinformatically optimized HIV-1 “mosaic” Env/Gag/Pol antigens and boosted with alum-adjuvanted gp140. A prototype Ad26 vector expressing a single clade A HIV-1 Env immunogen (Ad26.ENVA.01) was shown to be safe, well-tolerated, and immunogenic (69–71). Multivalent Ad26 vectors expressing mosaic HIV-1 Env/Gag/Pol immunogens were manufactued with the goal of enhancing cellular immune breadth and functional antibodies against diverse global viruses (63, 72, 73). In a phase 1/2a clinical trial, we explored the safety and immunogenicity of various regimens involving multivalent Ad26.Mos.HIV vector priming with Ad26.Mos.HIV, MVA.Mos.HIV, and/or Env gp140 boosting in 393 subjects in Rwanda, South Africa, Thailand, Uganda, and the US (67). Humoral and cellular immune responses in humans proved comparable to those in nonhuman primates that afforded partial protection, and the mosaic Gag/Pol/Env Ad26 prime, Ad26 plus high-dose gp140 boost vaccine was selected for a phase 2b clinical efficacy trial in 2,600 women in five countries in sub-Saharan Africa called Imbokodo (HVTN 705; HPX 2008; Table 1). Preliminary efficacy results are expected in 2021. A phase 3 trial is currently being launched with a similar vaccine involving the mosaic Gag/Pol/Env Ad26 prime, Ad26 plus high-dose bivalent C+M gp140 boost vaccine in 3,800 men and transgender individuals who have sex with men/transgender individuals in the Americas and Europe (HVTN 706; HPX 3002; Table 1).
Another vaccine efficacy trial that is being planned involves combining PrEP with active vaccination. This PrEPVac study aims to test the combined efficacy of both modalities of HIV-1 prevention, with PrEP provided for the first 6 months of the study when immune responses are being induced by the vaccine. Multiple other vaccine approaches that induce non-neutralizing antibody responses are also being explored in preclinical studies and in early phase clinical trials that are not reviewed here (see www.avac.org/pxrd for a complete database of ongoing HIV-1 vaccine trials).
Vaccines that Generate T-Cell Responses
Vaccine approaches aimed at inducing broad and potent T-cell responses are also being developed. Two vaccine trials that had no efficacy in terms of preventing HIV-1 infection still showed that T-cell responses exerted immune selection pressure in follow-up analyses. The Step trial tested whether T-cell responses elicited by an Ad5 vector expressing natural B-clade Gag, Pol, and Nef could protect against HIV-1 infection or impact viral control following infection. The Step study did not show decreased infection risk (74, 75); instead there was an increase in HIV-1 acquisition among uncircumcised and/or Ad5-seropositive vaccinated study participants (76). Nevertheless, the vaccine led to immunologic pressure at transmission, as viruses from infected vaccinees were genetically further from the vaccine antigens than viruses from placebo recipients, most strikingly within Gag (77, 78). Furthermore, there was an inverse correlation between plasma viremia and the number of vaccine-induced CD8+ T-cell responses (79). These clinical data are consistent with non-human primate SIV challenge studies that have shown that vaccine-elicited CD8+ T-cell responses, particularly those targeting Gag, correlated with improved viral control and survival following infection (63, 80–86).
A second trial, HVTN505, evaluated a DNA prime, Ad5 boost delivery and included HIV-1 B-clade Gag, Pol, and Nef antigens, as well as three diverse gp145 Envs (87). Again, no overall reduction in HIV-1 acquisition or in viral load upon infection was observed in the HVTN505 vaccine group (88). However a subset analysis identified an association between reduced infection risk and CD8+ T-cell vaccine responses (89), as well as an association between reduced infections and IgG responses among those with low T-cell responses (90). The associations between vaccine-elicited CD8+ T-cell responses and reduced rates of infection (89, 90) or reductions in viral load (77) raise the possibility of a vaccine benefit, but given the lack of overall protection in both the STEP and HVTN505 human trials, this will require further study. Moreover, T-cell responses as well as antibody responses correlated with protection in NHPs in the SHIV challenge study leading up to the Imbokodo clinical trial (67).
There are three basic approaches that have been undertaken to attempt to improve the CD8+ T-cell vaccine responses relative to the vaccines used in the Step and HVTN505 trials (Fig. 1A). The first is used in the Imbokodo trial. This vaccine includes essentially complete protein antigens, as did STEP and HVTN505, but instead of natural proteins, complementary pairs of HIV-1 mosaic immunogens are used. As mentioned above, mosaic proteins are computationally designed to provide nearly optimal coverage of potential epitopes circulating in a target population. In this case, two complementary mosaics of each protein are included (Fig. 1A) (72). Mosaics not only optimize epitope diversity coverage, but also minimize epitope redundancy and the inclusion of rare epitopes that would favor type-specific vaccine responses. In NHP studies, mosaics elicited significantly higher numbers of T-cell responses with greater cross-reactivity (73, 91, 92). Mosaic Env proteins also elicited non-neutralizing antibodies that were associated with protection in SHIV challenge models (63, 67). The second vaccine approach focuses on conserved regions with greater cross-reactive potential, excluding the most variable regions while still retaining substantial regions of the HIV-1 proteins. They include a large number of potential epitopes, and a very broad spectrum of human HLA presenting molecules are represented (93–98). Some conserved region strategies are also enriched for epitopes that have been shown to be associated with low viral loads in natural infection (95, 96, 99). Others use complementary mosaic designs, as even relatively conserved regions of HIV-1 are still quite variable, and complementary mosaic protein designs can be used to maximize epitope coverage in such vaccines (96, 97). The third vaccine approach includes only very short regions that span highly conserved sections of HIV, for example the p24 conserved element vaccine, p24CE (Fig. 1A) (93, 100). A proposed alternative to the conserved element approach is to use similarly short regions that, instead of being conserved, are highly networked at the protein level, so are likely to be functionally critical (101). Both strategies may be particularly advantageous as therapeutic vaccines, by redirecting the immune response to protein locations where immune escape would likely come with a high fitness cost (93, 102–104).
A novel approach in the generation of a T-cell-based vaccine involves the induction of nonclassical MHC-E restricted CD8+ T-cell responses by a modified cytomegalovirus (CMV) vector (105–107). Remarkably, in NHP studies, CMV vector-based vaccines led to exquisite virologic control and clearance in approximately half of vaccinated NHPs (107, 108).
Vaccines that Generate Broadly Neutralizing Antibodies
A major unsolved problem in the HIV-1 vaccine field is the development of immunogens capable of inducing bNAbs. Such antibodies have proven extremely difficult to elicit by vaccination, and despite large research efforts, no such immunogens yet exist. Challenges include the high degree of Env sequence diversity as well as the extensive “glycan shield” that protects the Env trimer surface from antibody attack (109). Monoclonal antibodies have been identified in HIV-infected humans with exceptional potency and breadth, but typically these bNAbs emerge only after years of chronic HIV-1 infection and after multiple rounds of antibody-Env coevolution (45). Germline B-cell receptors of such bNAbs show unusual features, such as long third heavy chain complementary determining regions (CDRH3s) and short third light chain complementary determining regions (CDRL3s), which are able to penetrate or negotiate the glycan shield, respectively (110). The maturation of bNAbs also requires substantial somatic hypermutation, which partially explains the long time for development of bNAbs during chronic HIV-1 infection.
Soluble trimers that mimic the native Env spike have recently been developed. BG505 SOSIP.664 is a soluble protein that links Env gp120 and gp41 subunits with a disulfide bond and is stabilized with an I559P substitution in the pre-fusion state (109, 111–114). The BG505 SOSIP.664 gp140 vaccine has been shown to elicit potent autologous NAb responses to BG505 virus in animal models with very limited breadth (112–116) and that target an immunodominant “hole” in the glycan shield of this virus (110, 113), although other epitopes are also targeted (112). Some differences between SOSIP trimers and virion-expressed Env spikes have also been described (117), and approaches that utilize structural biology to design improved SOSIP immunogens are being actively pursued by multiple laboratories.
It is likely that multiple immunogens will be required to induce bNAbs (41, 118, 119). Studies of B-cell ontogeny leading to the development of bNAbs in HIV-infected humans has led to the concept of sequential immunization to recapitulate antigenic exposure in chronic HIV-1 infection by a sequence of immunogens. This approach involves priming with an immunogen that can stimulate particular germline antibody genes, followed by boosting with a series of intermediate constructs to coax B-cell development along the specific pathways that lead to bNAbs (43, 120). Stimulation of appropriate antibody germline B-cells may be required for vaccine induction of some bNAbs, since such germline B cells have the rare CDR configurations that are likely prerequisites for the development of bNAbs. However, such germline B cells typically show no cross-reactivity with natural Envs, and thus germline-targeting immunogens may be required. For example, eOD-GT8 is a nanoparticle-based engineered immunogen that was designed to stimulate precursor B cells for VRC01, which targets the CD4 binding site (121–123), and is currently being explored in a phase 1 clinical trial. Sequential immunogens have also been designed that aim to induce V3-specific NAbs (124, 125). However, none of these immunogens or combinations of immunogens have yet elicited bNAbs in wildtype animal models or in humans.
Epitope-targeted immunogen design strategies represent an alternative approach for inducing bNAbs. Examples of this approach include fusion peptide (FP) and V3-glycopeptide based immunogens, which present minimal epitope fragments as a means to avoid distracting off-target responses targeting other regions of the full Env spike (126–129). Another approach is the use of signature-based epitope targeted (SET) vaccine. We recently reported the design and testing of V2-SET immunogens that aim to increase induction of V2-specific NAbs using neutralization signatures from large virus panels to optimize exposure and diversity of the V2 epitope on the Env surface (38). Both the FP targeted and V2-SET vaccines have induced NAb responses with moderate breadth in small animals, and also in NHPs for the former (38, 126, 128).
Passive Immunization with bNAbs for HIV-1 Prevention
While passive transfer of monoclonal antibodies for HIV-1 prevention is not a novel concept, there is renewed interest in this approach as a result of the discovery of bNAbs with impressive in vitro neutralization breadth and potency. The Antibody Mediated Prevention (AMP) trials are two phase 2b clinical studies (HVTN 703/704; Table 1) that are assessing the protective efficacy of the CD4bs-specific bNAb VRC01 against HIV-1 acquisition in humans (130). These studies are evaluating VRC01 in heterosexual women in sub-Saharan Africa and in men and transgender persons who have sex with men in the Americas and in Europe. Enrollment in these trials is complete and results are anticipated in 2020.
Studies of bNAbs for HIV-1 prevention have two main goals. First, these studies will help define the neutralization titers required for protection, which will inform the development of next generation vaccines that aim to induce neutralizing antibody responses. Second, passive immunization with bNAbs may also be a clinically relevant HIV-1 prevention strategy. In particular, long-acting bNAbs may provide a viable alternative to antiretroviral drugs as PrEP. It is possible that bNAbs will have fewer adverse effects than antiretroviral drugs (particularly during pregnancy and adolescence), and efforts to extend the half-life of bNAbs could lead to less frequent administration than current long-acting antiretroviral drugs. Antibodies have been engineered for enhanced breadth and potency (131–134), and incorporation of “LS” mutations that improve the affinity between antibody Fc and the neonatal Fc-receptor (FcRn) have been shown to extend in vivo half-life (135).
A critical goal of the AMP trials is to define the degree of neutralization activity that is required to achieve high-level protection in humans. Multiple studies have shown the ability of passively transferred bNAbs to protect against simian-human immunodeficiency virus (SHIV) challenge in NHPs (132, 136–143). Serum neutralization titers required for protection ranged from inhibitory dilution for 50% neutralization (ID50) of approximately 30 to 1000, with most studies finding ID50 titers of approximately 100 to be protective (143); these ID50 values correspond to approximate ID80 titers of 7.5, 250, and 25, respectively (144, 145). Recently, a meta-analysis of bNAb passive transfer studies against high-dose SHIV challenges demonstrated a significant correlation between serum neutralization titer and protection, with ID80 titers of 1.7 (95% CI: 0.1–20) and 170 (95% CI: 15–1889) required for 50% and 95% protection, respectively (Pegu et al., submitted).
We have also analyzed two preclinical studies involving two different biological settings: a vaccine study that used high-dose SHIV challenges, and a passive bNAb infusion study that used low-dose SHIV challenges. The first study involved an adjuvanted BG505 SOSIP.664 gp140 protein vaccine against repeated high-dose SHIV-BG505 challenges (146). The baseline rate of infection in this study was 80% per challenge in control animals (Fig. 3A), which is slightly lower than the 100% per challenge infection rate in the meta-analysis of high-dose SHIV challenge studies by Pegu et al. Nevertheless, the ID80 titer requirements of 33 and 230 for protection levels of 50% and 95%, respectively, were comparable (Fig. 3A).
Figure 3. Potency of neutralization required for in vivo success.
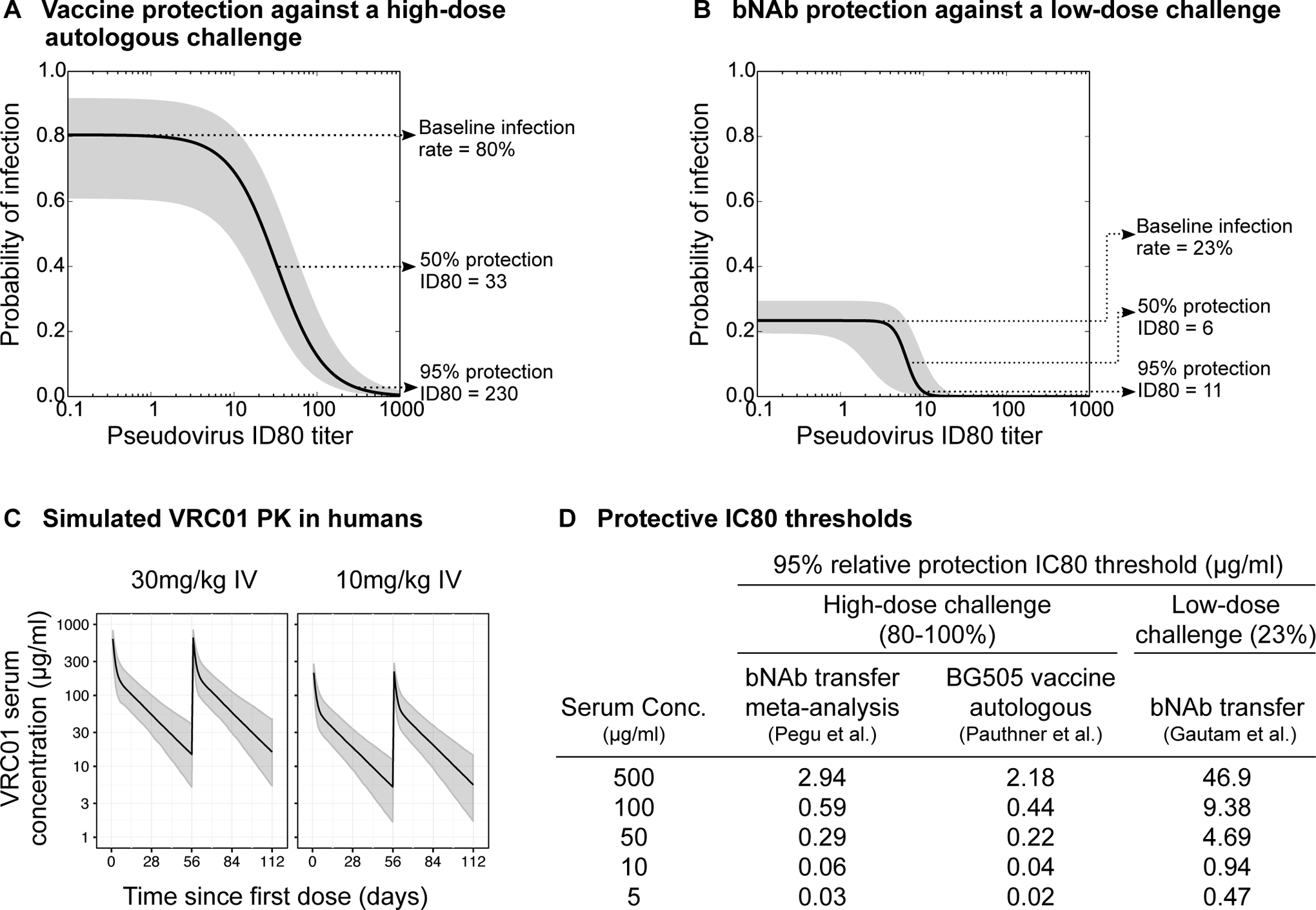
(A-B) Modeling of protection in macaque SHIV challenge studies as a function of serum neutralization ID80 titers using two models of neutralization-based protection. Panel (A) shows protection conferred by BG505 SOSIP vaccine-induced neutralization against an autologous high dose challenge, as modeled in Pauthner et al. (174). Panel (B) shows the protection conferred by passively transferred bNAbs against a repeated low-dose SHIV challenge in unvaccinated macaques, modeled by Wagh et al. (157) using data from Gautam et al. (139). The grey shaded areas are approximate 95% confidence intervals for each model. See supplemental methods for details of calculation. (C) Simulated VRC01 pharmacokinetic profiles in the two dosing groups matching those in AMP trials, reproduced with permission from Huang et al. (149). (D) Protective IC80 thresholds for 95% or higher relative protection are shown based on the two protection models above, as well as the protection model for bNAb passive transfer against a high-dose SHIV challenge from the meta-analysis by Pegu et al.
The second study involved a passive bNAb transfer study with repeated low-dose SHIV-AD8-EO challenges (139). In this study, a single intravenous infusion of 20 mg/kg of various bNAbs was administered, and animals were challenged weekly with low doses of virus that resulted in a 23% per challenge infection rate in controls. It took a median of 3 (range 2–6) challenges to infect the control animals, and thus this low-dose model may be more representative of human HIV-1 exposures than the high-dose challenge models. Of note, HIV-1 acquisition risk in humans has been estimated to be approximately 138 per 10,000 exposures for receptive anal intercourse, and 8 per 10,000 receptive penile-vaginal exposure (147), markedly lower than even the low dose challenge in NHPs. We applied a modified logistic regression model to the serum neutralizing activity at the time of each challenge and whether or not the challenge resulted in infection, similar to the above protective models (148) (Fig. 3B; see Supplemental Methods). This low-dose challenge model predicted that ID80 titers of 6 and 11 were required for 50% and 95% protection, respectively, substantially lower than the ID80 titers required for protection against high-dose SHIV challenge. The high-dose challenge contains more infectious virions than the low-dose challenge, and thus a higher fraction of the inoculum presumably needs to be neutralized to achieve complete protection. Other factors may also contribute to these differences, including different characteristics of the various SHIVs used, differences between monoclonal and polyclonal antibodies, and experimental specifics such as the use of exogenous human bNAbs in the passive transfer studies compared with elicited rhesus NAbs in the active vaccine study.
It remains to be determined which of these NHP models will best predict protection against HIV-1 infection in humans. They may underestimate the impact of a bNAb because the baseline rate of infection, by experimental necessity, is much higher in NHPs. On the other hand, these models may overestimate the impact of a bNAb because NHP studies typically utilize a single SHIV challenge strain, whereas HIV-1 exposure in humans exhibits far greater diversity at both the individual and population levels.
In the AMP trials, VRC01 is infused every 8 weeks, and the decay of serum bNAb concentrations was modeled as a bi-phasic exponential decline (149). For example, an infusion of VRC01 at 30 mg/kg leads to peak serum antibody concentration of ~600 ug/ml, which then declines rapidly over 3 days to ~223 ug/ml (Fig. 3C). This is followed by a slower second phase of decline with a half-life of ~14 days, such that serum antibody concentrations are ~12 ug/ml at the end of each 8-week cycle. In predictive models, this ~170-fold decline in bNAb concentration would be expected to result in decreased levels of protection (Fig. 3D). Using the least stringent of the protective preclinical models (i.e., the low-dose challenge where an ID80 of 10.66 is sufficient for 95% relative protection; Fig. 3B), a serum VRC01 concentration of 500 μg/ml would be predicted to protect against viruses with IC80 < 46.9 μg/ml (=500/10.66), which corresponds to 78% of global circulating viruses. However, towards the end of the infusion cycle, a serum VRC01 concentration of 10 μg/ml would only be predicted to protect against viruses with IC80 < 0.94 μg/ml (=10/10.66), which corresponds to only 34% of global viruses.
The decline in plasma bNAb concentrations highlights the importance of quantifying the decay in protective efficacy with serum neutralizing activity. Quantification of development of bNAb resistance can also be obtained from phase 1 clinical studies of the therapeutic use of bNAbs in HIV-infected individuals who are viremic or undergoing analytical treatment interruptions; in such cases the selection of resistant variants has been clearly demonstrated (150–154). In these studies, most individuals exhibited viral rebound with resistant virus despite high serum bNAb concentrations. For VRC01 and 3BNC117, rebound viruses showed a median increase of 3- to 12-fold in IC80 titers, respectively. For 10–1074, all rebound viruses showed complete neutralization resistance (Fig. 4). Looking at the viral escape pathways, the CD4bs-specific bNAbs appear to select for varied resistance mutations that typically lead to less potent, but not complete loss of, neutralization (150–154). In contrast, the resistance to the V3 glycan bNAb 10–1074 showed two escape pathways that were repeated across hosts: the loss of the glycan at N332 (often a glycan shift from N332 to N334) or a mutation at position 325 in the GDIR motif (HXB2 positions 324–327); both can confer complete 10–1074 resistance (153).
Figure 4. Levels of bNAb resistance that developed in published clinical studies of passively transferred bNAbs (VRC01 (150, 151), 3BNC117 (152, 154), and 10-1074 (153)).

Only participants with documented viral escape from bNAbs in the primary publications were used. The top panels show the pre- and post-infusion sensitivity (x-axis) of viruses isolated from each participant (y-axis). Blue dots indicate median IC80 titers pre-infusion, and light blue bars the inter-quartile range. Red dots indicate median IC80 titers post-infusion, and pink bars the inter-quartile range. The bottom panels show the cumulative distributions of pre-infusion titers (blue curve) and post-infusion titers (red curve), analogous to the breadth-potency plots of in vitro neutralization efficacy of bNAbs against pseudovirus panels. Some studies analyzed bNAb passive transfer in viremic individuals, while others were analytic treatment interruption (ATI) studies. Participants from ATI studies are labeled in green. Pseudovirus neutralization data was usually available and was chosen to facilitate comparison with typical in vitro neutralization panel data. However, for a few participants only viral isolate neutralization data was reported, and these are indicated by boxes around their PTIDs. Of note, viral isolates typically show more resistant neutralization profiles relative to matched pseudoviruses (175).
Limitations of bNAbs for Prevention: Clade-specific Neutralization Resistance
A major challenge facing the development of bNAbs for HIV-1 prevention is the prevalence of viral clades that are resistant to any one particular antibody (38, 148). A striking example is the lack of activity of V3 glycan-dependent bNAbs, such as 10–1074 and PGT121, against CRF01 viruses, the major circulating lineage in Southeast Asia. The reason for this is that a critical Env N-linked glycosylation site N332 in V3 is lost in this clade (38). Similarly, V2 glycan-dependent antibodies, such as PGDM1400 and CAP256, have less breadth and potency for clade B viruses than clade C viruses (38, 155). There is also reduced potency of all bNAbs against clade D viruses, although the number of subtype D viruses available for testing is limited (148). Because most geographical regions are enriched for specific clades (Fig. 1B), the subtype-specific resistance profiles of bNAbs should be considered in decisions to test bNAbs in particular regions.
A clear solution to overcome these issues is to use combinations of bNAbs for prevention. We showed preclinical proof-of-concept of the benefit of bNAb combinations against a mixed SHIV challenge in NHPs (141). These data are consistent with in vitro studies that have shown that bNAb combinations have significantly improved potency and breadth compared to individual bNAbs (148, 156, 157).
Limitations of bNAbs for Prevention: Within-Host Minority Resistant Variants
For a bNAb to block HIV-1 infection, two conditions are intuitively critical: (1) sufficient bNAb titers and (2) sensitivity of the challenge virus to the bNAb. With this simple model, the predicted efficacy of an individual bNAb would be a function of how likely an incoming challenge virus is to be sufficiently sensitive to the bNAb. However, it is likely that an individual will not be exposed to a single virus sequence, but rather to a swarm of highly diverse quasi-species from chronically infected individuals. Population-level molecular epidemiology (Fig. 1B) and neutralization of panels of defined pseudoviruses do not address this complexity. Additionally, it is likely that bNAbs do not work exclusively by blocking virions at the mucosal portal of entry. We showed that PGT121 mediated protection against SHIV-SF162P3 challenge in NHPs can include systemic clearance of distal foci of virus for up to 7 days following challenge (158). Thus, it is theoretically possible that the efficacy of bNAb based protection may be reduced by minor resistant variants within a challenge swarm, which could be selected with bNAb pressure, although experimental support for this concern is currently lacking.
Examples of how within-host diversity can impact bNAb sensitivity are shown in Figure 5. Neutralization data for viruses from 3 chronically infected viremic individuals (153) show that for each individual, there are 1–2 virus variants that are resistant to at least one bNAb, despite other virus variants being sensitive (Fig. 5A). Thus, bNAb combinations that neutralize within-host virus variants with at least 2 active bNAbs may be necessary to avoid escape. An analysis of within-host sequences from 6 chronically individuals similarly shows that each individual harbors frequent minor variants that are resistant to bNAbs from each class, even if the bulk population is sensitive (Fig. 5B). Such resistance signatures can be single amino acids or glycans located in key antibody epitope sites (Fig. 5B), or variable loop characteristics that are significantly associated with bNAb sensitivity levels (Fig. 5B–C).
Figure 5. Natural within-host diversity leads to heterogenous bNAb profiles.

(A) Neutralization IC80 titers for pre-infusion viral variants in Caskey et al. (153). Data from 3 of 11 participants (1HC1, 1HC2, 1HD6K) were selected for display here as examples that illustrate resistance variants comingling with majority sensitive viruses for representative bNAbs in the clinical pipeline (V2 apex, PGDM1400; CD4bs, 3BNC117; and V3, 10–1017). For each participant, viral isolates were obtained from pre-infusion samples and tested against 3 bNAbs. These data are shown as heatmaps where viruses are represented as rows and bNAbs are columns, and each cell shows IC80 titers using the color-coding in the legend. Black cells indicate no detected response, with IC80 > 50μg/ml. (B) HIV-1 bNAb signature amino acids frequencies in the global population and within HIV-infected individuals. Amino acids that are significantly associated with bNAb sensitivity (blue) or resistance (red) across bNAb classes for CD4bs, V2 apex, and V3 glycan bNAbs, are illustrated by LOGO plots indicating the frequency of the amino acids by their relative height in relevant positions. LOGOs were made for the M group Env viruses in the Los Alamos HIV database (5,420 viruses including just one sequence per infected individual, top LOGO). To illustrate how subsets of these signature resistance mutations are commonly sampled in natural infections, LOGOS were made representing distinct sequences from six HIV-1 infections. Subjects were selected simply on the basis of being sampled over a period of several years, and having extensive Env sequences available. The sites displayed here are the subset of significant sensitivity or resistance signatures defined in Bricault et al. (38) that are either structurally defined as antibody contact residues or shown to be relevant for neutralization by mutational analysis or emergence of resistance (3BNC117 and VRC01 for CD4bs bNAbs (152, 154, 176–178), PG9 and PGDM1400 for V2 apex bNAbs (179–182), and PGT121 and 10–1074 for V3 glycan bNAbs (183–187). Hypervariable region characteristics are highly associated with bNAb sensitivity, and patterns are often shared across antibodies within a class (38). Hypervariable regions rapidly evolve during the course of natural infections by insertions and deletions (indels); generally significant evolution has occurred in these regions within the first year of infection. To illustrate in detail how such characteristics vary, we provide an example of the V1 + V2 hypervariable length variation found at the population level in the M group alignment, as well as the variation within the 6 individuals included here, to the right of the LOGOs. The V1 + V2 hypervariable regions are bounded by HXB2 positions 132–152 (V1h) + 185–190 (V2h); outside of these regions most viruses are readily aligned, but within these regions alignments are chaotic due to length variation and minimal retention of shared motifs. Long V1+ V2 hypervariable regions are highly associated with bNAb resistance for many bNAbs, including three major classes of antibodies: CD4bs, V2 apex, and V3 glycan. The association is often stronger when consider V1h + V2h combined than for considering V1h or V2h separately. (C) Examples illustrating the relationship between variable loop characteristics and bNAb sensitivity, and the extent of the diversity sampled during natural infections. The relationship between pseudovirus bNAb sensitivity, represented here as an average IC80 score for a given bNAb/pseudovirus combination using all available M group viral data obtained from the Los Alamos HIV immunology database (www.hiv.lanl.gov/components/sequence/HIV/neutralization/index.html), and loop characteristics is displayed in the 3 black scatterplots. The plot on the left shows the correlation between net charge of the V2 loop (HXB2 positions 159–197), which contains a hypervariable stretch (HXB2 positions 185–10), with PGDM1400 IC80 titers; viruses with more positive V2 loops are generally much more sensitive to V2 apex binding antibodies (38). (This is true for both the hypervariable region within the V2 loop, and the entire V2 loop; the data for the full V2 loop is shown here). The plot in the center shows how the length of the V5 loop is correlated with 3BNC117 sensitivity. The V5 loop (HXB2 positions 459–470) contains a hypervariable region (V5h, HXB2 460–465), the length of this region as well as the number of PNGS sites encompassed in the region are strongly inversely correlated with CD4bs antibody activity (38). The V5 hypervariable region is embedded in the CD4bs contact surface, and of note, slight shifts in length can change the relative position of glycans in the V5 loops, and impact developing antibody sensitivity (188). To develop breadth, CD4bs antibodies have to be selected to tolerate the natural diversity in the V5 region. On the right is shown an example of an association between 10–1074 and V1h+V2h length. As mentioned above, V3 glycan, V2 Apex, and CD4bs (38) bNAbs are all generally more potent against viruses with shorter V1h+V2h lengths. In all three plots, only the positive viruses are shown, as variable region characteristics tend to modulate levels of bNAb sensitivity, but not to completely block activity. Above each of the 3 scatter plots, using the same x-axis, is a display that shows the median and range of loop characteristic of interest in the 6 subjects used to explore within-subject diversity. Within these subjects, there is always variation in the characteristics sampled, and in some cases nearly the full range of global diversity of these loop characteristics can be recapitulated in a single infection.
The problem of within-host diversity is driven by the fact that chronically infected individuals can develop NAbs that target similar epitopes as the bNAbs considered for passive transfer and vaccines. bNAb epitopes are in regions of vulnerability, and thus are relatively frequently targeted in natural infection (143, 159). Thus, bNAb-resistant variants naturally arise during in vivo escape either from autologous neutralizing antibodies targeting the bNAb epitope, even if the host’s antibody lineage does not acquire breadth, or as part of the intricate process of antibody-virus co-evolution that gives rise to neutralization breadth. The latter process involves multiple rounds of viral escape followed by antibody evolution to recognize escaped viruses, and ultimately, most within-host viruses escape matured bNAbs in each infection regardless of their neutralization breadth and potency for heterologous viruses (40, 43, 160). Since bNAbs targeting similar epitopes show similar neutralization profiles and resistance mutations (38), chronically infected individuals who develop bNAbs targeting a particular epitope will often harbor viruses that are relatively resistant to clinically used bNAbs of a similar epitope class. For example, individuals who are known to have developed V2 apex, CD4bs, and V3 glycan bNAbs, respectively, also typically show resistance mutations in the corresponding bNAb signature sites. Studies of cross-sectional cohorts have established that ~50% individuals develop ~50% serum breadth (35, 161). In such individuals, 12–14% develop V2 apex, 21–36% develop V3 glycan and 5–26% develop CD4bs bNAbs, depending on the cohort (162, 163).
Extensive characterization of within-host diversity is currently only possible for a handful of individuals. We therefore cannot assess the coverage of bNAbs against the swarm of viral variants that are present in most chronically HIV-infected individuals. Timing of infection may also be important, as transmissions are most common during acute infection when the within-host viruses are more homogeneous (164–167). Nevertheless, data to date raise substantial concern that within-host minor resistant variants may reduce the projected efficacy of bNAbs in preventing HIV-1 infection. The use of combinations of 3 or more bNAbs can alleviate this concern. If most of the within-host viruses are sensitive to at least 2 bNAbs in the combination, the development of minority variants that are simultaneously resistant to all the bNAbs is less likely during within-host evolution. However, if most viruses are sensitive to only one bNAb in the combination, then there is a higher chance of existence of minority variants that are completely resistant to the combination.
How Many bNAbs Are Required for HIV-1 Prevention?
A combination of bNAbs is clearly required for HIV-1 treatment (150, 152, 153, 168, 169). For the reasons discussed above, a combination of bNAbs will also likely be required for high-level HIV-1 prevention, e.g., to prevent breakthroughs of resistant viral variants transmitted from someone with the diverse viruses that are the hallmark of chronic infection (170). A cocktail of bNAbs that target different epitopes may be effective for both prevention and treatment of HIV-1 infection. Here we argue that a combination of at least 3 bNAbs may be needed for optimal protection against HIV-1 acquisition in diverse populations. We compared the neutralization coverage of monotherapy with VRC01, the dual bNAb combination 3BNC117 + 10–1074, and the triple bNAb combination VRC07–523LS + PGT121 + PGDM1400 (Fig. 6). These regimens are all currently being explored in clinical trials. The dual bNAb combination provides significantly higher breadth and potency compared to VRC01 monotherapy, and this was further improved by the triple bNAb combination (Fig. 6A–B). For example, the geometric mean IC80 is 2.64 μg/ml, 0.48 μg/ml and 0.09 μg/ml for VRC01 monotherapy, the dual bNAb combination, and the triple bNAb combination, respectively. Similarly, the breadth of neutralization at IC80 < 10 μg/ml for viruses with at least one antibody active increases from 76% to 88% to 99%, respectively. Moreover, if it proves necessary to have two or more antibodies active for improved coverage of within-host diversity as argued above, then the triple bNAb combination still has 82% coverage compared with only 41% for the dual bNAb combination with 2 or more bNAbs active at single bNAb IC80 < 10 μg/ml (p = 2.2 × 10−31, Fisher’s exact test).
Figure 6. Inter-subtype variability of bNAb potency and combination bNAb neutralization profiles.

(A) Breadth-potency curves for single bNAbs. Each curve shows the cumulative coverage of viruses (y-axis) with IC80 less than or equal to a given value shown on the x-axis. The full neutralization dataset (n=374, all major subtypes and circulating recombinant forms included) was used. The neutralization data for individual bNAbs was extracted from CATNAP using all viruses that had IC50 and IC80 titers reported for all bNAbs analyzed. (B) Neutralization breadth and potency of bNAb combinations. Left figure shows the breadth-potency curves for the dual combination of 3BNC117 and 10–1074, the triple combination of VRC07–523LS, PGT121 and PGDM1400 and for comparison the single bNAb VRC01, used in the ongoing AMP trial to explore its potential to prevent HIV-1 infection (130). The center figure shows the breadth-potency curves modified to consider as “covered” only those viruses that are simultaneously neutralized by 2 or more bNAbs in the combination at individual bNAb IC80 < 10μg/ml threshold. This threshold is based on the bNAb passive transfer model of protection (Fig. 2B) and an average serum bNAb concentration of ~100 ug/ml. The right figure shows the coverage by the dual and triple combinations for each major subtype analyzed. The percentage of viruses neutralized by 1, 2 or 3 bNAbs in the combination are shown by the colors indicated in the legend below. The clade definition, number of viruses and geographical regions are shown in the bottom left of panel (C). IC80 titers for bNAb combinations were predicted using the webtool CombiNAber (157) on the individual bNAb titers (see supplemental methods for details). Each bNAb is assumed to be at equal concentration in the combination and the combination IC80 titers reported are the sum of concentrations of all bNAbs (e.g. if 3BNC117 + 10–1074 IC80 is 1μg/ml then each bNAb is present at 0.5μg/ml). (C) Subtype-specific distributions of IC80 titers for bNAbs and combinations. IC80 titers are shown as heatmaps for VRC01 (left), 3BNC117, 10–1074 and their combination (center), and VRC07–523LS, PGDM1400, PGT121 and their combination (right). In each heatmap viruses are represented as rows and bNAbs/combinations as columns. Each cell shows IC80 titer for each bNAb or combination for each virus and is color-coded according to the legend in bottom right. Cells colored grey indicate IC80 between 10μg/ml and 50μg/ml, a range of weak neutralization that may not be adequate to provide a beneficial effect, and those colored black indicate IC80 above the highest concentration tested (50μg/ml). Within each grouping of bNAb/combinations, separate heatmaps are shown for each major subtype. Circulating recombinant forms (CRFs) that are major epidemic lineages and that are similar to a single subtype in Env are grouped with the corresponding subtype: CRF02, an important lineage in Western Africa, is subtype A in Env, so is grouped with subtype A; and similarly, CRF07 and CRF08, important lineages in China, are mostly subtype C in Env so grouped with subtype C. The number of viruses in each group are shown in bottom left. The numbers below the heatmaps indicate the percent of viruses in each subtype that were simultaneously neutralized by 1, 2, 3 and 2–3 bNAbs in the combination, using the single bNAb IC80 < 10μg/ml threshold. As having at least 2 antibodies active may be important for success, percentages of viruses with two or more bNAbs active are highlighted in blue.
To further highlight the improved performance of the triple bNAb combination, we focused on each of the major HIV-1 subtypes (Fig. 6C). These patterns result in dramatic differences in coverage by the triple compared with the dual bNAb combination. For CRF01 viruses, no viruses are covered by both bNAbs in the dual bNAb combination, because CRF01 viruses are completely resistant to 10–1074. For clade C viruses, only 37% of viruses would be covered by both viruses in the dual bNAb combination. On the other hand, both 3BNC117 and 10–1074 have good coverage of clade B viruses, with 77% of subtype B viruses covered by both bNAbs, suggesting that the combination of 3BNC117 and 10–1074 would likely perform better against clade B viruses than against clade C or CRF01 viruses. In contrast, the triple bNAb combination has high coverage with at least two bNAbs in the cocktail for all subtypes (77–88%) except for subtype D (55%). This high coverage reflects the fact that the V2-specific bNAb PGDM1400 and the V3-specific bNAb PGT121 have complementary neutralization patterns (141), and the CD4bs-specific bNAb VRC07–523LS itself has exceptional breadth.
A parallel strategy to building bNAb cocktails is to generate bi- or tri-specific antibodies. One such example is the 10E8.4-iMab bispecific antibody that targets the membrane proximal external region (MPER) regions of the virus and host CD4 (131). Another example is the VRC01-PGDM1400–10E8.4 trispecific antibody that simultaneously targets three epitopes on the virus (171). Such multi-specific antibodies offer the possibility of extremely robust breadth with a single product and are currently in early phase clinical trials.
Conclusions
We are at a crossroads in HIV-1 prevention research. There are currently five large phase 2b and phase 3 clinical trials that will provide substantial data on the clinical efficacy of two vaccine candidates and a bNAb in preventing HIV-1 acquisition in humans over the next few years. Meanwhile, next generation vaccines aimed at inducing neutralizing antibodies and combinations of bNAbs targeting multiple epitopes are being developed. Active and passive immunization efforts will need to address the challenges of within-host, population-level, and global HIV-1 diversity to achieve optimal efficacy.
Supplementary Material
Literature Cited
- 1.UNAIDS. 2018. UNAIDS Data 2018.
- 2.UNAIDS. 2015. UNAIDS 2016–2021 Strategy: On the Fast-Track to end AIDS
- 3.Global Burden of Disease Health Financing Collaborator N. 2018. Spending on health and HIV/AIDS: domestic health spending and development assistance in 188 countries, 1995–2015. Lancet 391: 1799–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weller S, Davis K. 2002. Condom effectiveness in reducing heterosexual HIV transmission. The Cochrane Database of Systematic Reviews: CD003255. [DOI] [PubMed] [Google Scholar]
- 5.Holmes KK, Levine R, Weaver M. 2004. Effectiveness of condoms in preventing sexually transmitted infections. Bulletin of the World Health Organization 82: 454–61 [PMC free article] [PubMed] [Google Scholar]
- 6.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. 2005. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Medicine 2: e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailey RC, Moses S, Parker CB, Agot K, Maclean I, et al. 2007. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet 369: 643–56 [DOI] [PubMed] [Google Scholar]
- 8.Gray RH, Kigozi G, Serwadda D, Makumbi F, Watya S, et al. 2007. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet 369: 657–66 [DOI] [PubMed] [Google Scholar]
- 9.Scott-Sheldon LAJ, Huedo-Medina TB, Warren MR, Johnson BT, Carey MP. 2011. Efficacy of behavioral interventions to increase condom use and reduce sexually transmitted infections: a meta-analysis, 1991 to 2010. J Acquir Immune Defic Syndr 58: 489–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eaton LA, Huedo-Medina TB, Kalichman SC, Pellowski JA, Sagherian MJ, et al. 2012. Meta-analysis of single-session behavioral interventions to prevent sexually transmitted infections: implications for bundling prevention packages. American Journal of Public Health 102: e34–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. 2015. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. [PubMed]
- 12.Centers for Disease Control and Prevention. 2016. Announcement: Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV - United States, 2016. Morb Mortal Wkly Rep, pp. 458. [DOI] [PubMed] [Google Scholar]
- 13.LeMessurier J, Traversy G, Varsaneux O, Weekes M, Avey MT, et al. 2018. Risk of sexual transmission of human immunodeficiency virus with antiretroviral therapy, suppressed viral load and condom use: a systematic review. Canadian Medical Association Journal 190: E1350–E60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bavinton BR, Pinto AN, Phanuphak N, Grinsztejn B, Prestage GP, et al. 2018. Viral suppression and HIV transmission in serodiscordant male couples: an international, prospective, observational, cohort study. Lancet HIV 5: e438–e47 [DOI] [PubMed] [Google Scholar]
- 15.Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, et al. 2019. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, et al. 2016. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 375: 830–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, et al. 2011. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 365: 493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanser F, Bärnighausen T, Grapsa E, Zaidi J, Newell M-L. 2013. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science 339: 966–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, et al. 2016. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA 316: 171–81 [DOI] [PubMed] [Google Scholar]
- 20.McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, et al. 2016. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 387: 53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, et al. 2012. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 367: 399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, et al. 2012. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 367: 423–34 [DOI] [PubMed] [Google Scholar]
- 23.Molina J-M, Charreau I, Spire B, Cotte L, Chas J, et al. 2017. Efficacy, safety, and effect on sexual behaviour of on-demand pre-exposure prophylaxis for HIV in men who have sex with men: an observational cohort study. Lancet HIV 4: e402–e10 [DOI] [PubMed] [Google Scholar]
- 24.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, et al. 2010. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 363: 2587–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molina J-M, Capitant C, Spire B, Pialoux G, Cotte L, et al. 2015. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 373: 2237–46 [DOI] [PubMed] [Google Scholar]
- 26.Deutsch MB, Glidden DV, Sevelius J, Keatley J, McMahan V, et al. 2015. HIV pre-exposure prophylaxis in transgender women: a subgroup analysis of the iPrEx trial. Lancet HIV 2: e512–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, et al. 2013. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 381: 2083–90 [DOI] [PubMed] [Google Scholar]
- 28.Karim SSA. 2013. HIV pre-exposure prophylaxis in injecting drug users. Lancet 381: 2060–2 [DOI] [PubMed] [Google Scholar]
- 29.Mulligan K, Glidden DV, Anderson PL, Liu A, McMahan V, et al. 2015. Effects of emtricitabine/tenofovir on bone mineral density in HIV-negative persons in a randomized, double-blind, placebo-controlled trial. Clin Infect Dis 61: 572–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yacoub R, Nadkarni GN, Weikum D, Konstantinidis I, Boueilh A, et al. 2016. Elevations in serum creatinine with tenofovir-based HIV pre-exposure prophylaxis: a meta-analysis of randomized placebo-controlled trials. J Acquir Immune Defic Syndr 71: e115–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. 2018. US Public Health Service: Preexposure prophylaxis for the prevention of HIV infection in the United States—2017 Update: a clinical practice guideline.
- 32.Zash R, Makhema J, Shapiro RL. 2018. Neural-tube defects with dolutegravir treatment from the time of conception. N Engl J Med 379: 979–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robertson DL, Anderson JP, Bradac JA, Carr JK, Foley B, et al. 2000. HIV-1 nomenclature proposal. Science 288: 55–6 [DOI] [PubMed] [Google Scholar]
- 34.Rademeyer C, Korber B, Seaman MS, Giorgi EE, Thebus R, et al. 2016. Features of recently transmitted HIV-1 clade c viruses that impact antibody recognition: implications for active and passive immunization. PLoS Pathog 12: e1005742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hraber P, Korber BT, Lapedes AS, Bailer RT, Seaman MS, et al. 2014. Impact of clade, geography, and age of the epidemic on HIV-1 neutralization by antibodies. J Virol 88: 12623–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang M, Foley B, Schultz AK, Macke JP, Bulla I, et al. 2010. The role of recombination in the emergence of a complex and dynamic HIV epidemic. Retrovirology 7: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee GQ, Bangsberg DR, Mo T, Lachowski C, Brumme CJ, et al. 2017. Prevalence and clinical impacts of HIV-1 intersubtype recombinants in Uganda revealed by near-full-genome population and deep sequencing approaches. AIDS 31: 2345–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bricault CA, Yusim K, Seaman MS, Yoon H, Theiler J, et al. 2019. HIV-1 neutralizing antibody signatures and application to epitope-targeted vaccine design. Cell Host Microbe 25: 59–72 e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bar KJ, Tsao CY, Iyer SS, Decker JM, Yang Y, et al. 2012. Early low-titer neutralizing antibodies impede HIV-1 replication and select for virus escape. PLoS Pathog 8: e1002721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonsignori M, Kreider EF, Fera D, Meyerhoff RR, Bradley T, et al. 2017. Staged induction of HIV-1 glycan-dependent broadly neutralizing antibodies. Sci Transl Med 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonsignori M, Zhou T, Sheng Z, Chen L, Gao F, et al. 2016. Maturation pathway from germline to broad HIV-1 neutralizer of a CD4-mimic antibody. Cell 165: 449–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goonetilleke N, Liu MK, Salazar-Gonzalez JF, Ferrari G, Giorgi E, et al. 2009. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J Exp Med 206: 1253–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao HX, Lynch R, Zhou T, Gao F, Alam SM, et al. 2013. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature 496: 469–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu MK, Hawkins N, Ritchie AJ, Ganusov VV, Whale V, et al. 2013. Vertical T cell immunodominance and epitope entropy determine HIV-1 escape. J Clin Invest 123: 380–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonsignori M, Liao HX, Gao F, Williams WB, Alam SM, Montefiori DC, Haynes BF. 2017. Antibody-virus co-evolution in HIV infection: paths for HIV vaccine development. Immunol Rev 275: 145–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liao HX, Bonsignori M, Alam SM, McLellan JS, Tomaras GD, et al. 2013. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity 38: 176–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Korber B, Hraber P, Wagh K, Hahn BH. 2017. Polyvalent vaccine approaches to combat HIV-1 diversity. Immunol Rev 275: 230–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song H, Giorgi EE, Ganusov VV, Cai F, Athreya G, et al. 2018. Tracking HIV-1 recombination to resolve its contribution to HIV-1 evolution in natural infection. Nat Commun 9: 1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu CL, Pai JA, Nogueira L, Mendoza P, Gruell H, et al. 2018. Relationship between intact HIV-1 proviruses in circulating CD4(+) T cells and rebound viruses emerging during treatment interruption. Proc Natl Acad Sci U S A 115: E11341–E8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen YZ, Lorenzi JCC, Krassnig L, Barton JP, Burke L, et al. 2018. Relationship between latent and rebound viruses in a clinical trial of anti-HIV-1 antibody 3BNC117. J Exp Med 215: 2311–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wadman M, You J. 2017. The vaccine wars. Science 356: 364–5 [DOI] [PubMed] [Google Scholar]
- 52.Kim JH, Excler JL, Michael NL. 2015. Lessons from the RV144 Thai phase III HIV-1 vaccine trial and the search for correlates of protection. Annu Rev Med 66: 423–37 [DOI] [PubMed] [Google Scholar]
- 53.Lynch RM, Yamamoto T, McDermott AB. 2013. HIV vaccine research and discovery in the nonhuman primates model: a unified theory in acquisition prevention and control of SIV infection. Curr Opin HIV AIDS 8: 288–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Butler AL, Fischinger S, Alter G. 2019. The Antibodiome-mapping the humoral immune response to HIV. Curr HIV/AIDS Rep 16: 169–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, et al. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 361: 2209–20 [DOI] [PubMed] [Google Scholar]
- 56.Gottardo R, Bailer RT, Korber BT, Gnanakaran S, Phillips J, et al. 2013. Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial. PLoS ONE 8: e75665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, et al. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366: 1275–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karasavvas N, Billings E, Rao M, Williams C, Zolla-Pazner S, et al. 2012. The Thai phase III HIV type 1 vaccine trial (RV144) regimen induces antibodies that target conserved regions within the V2 loop of gp120. AIDS Res Hum Retroviruses 28: 1444–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Montefiori DC, Karnasuta C, Huang Y, Ahmed H, Gilbert P, et al. 2012. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J Infect Dis 206: 431–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rolland M, Edlefsen PT, Larsen BB, Tovanabutra S, Sanders-Buell E, et al. 2012. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature 490: 417–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barouch DH, Alter G, Broge T, Linde C, Ackerman ME, et al. 2015. Protective efficacy of adenovirus-protein vaccines against SIV challenges in rhesus monkeys. Science: aab3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, et al. 2012. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 482: 89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barouch DH, Stephenson KE, Borducchi EN, Smith K, Stanley K, et al. 2013. Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell 155: 531–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roederer M, Keele BF, Schmidt SD, Mason RD, Welles HC, et al. 2014. Immunological and virological mechanisms of vaccine-mediated protection against SIV and HIV. Nature 505: 502–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barouch DH, Alter G, Broge T, Linde C, Ackerman ME, et al. 2015. Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys. Science 349: 320–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chung AW, Kumar MP, Arnold KB, Yu WH, Schoen MK, et al. 2015. Dissecting polyclonal vaccine-induced humoral immunity against HIV using systems serology. Cell 163: 988–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barouch DH, Tomaka FL, Wegmann F, Stieh DJ, Alter G, et al. 2018. Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13–19). Lancet 392: 232–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bekker LG, Moodie Z, Grunenberg N, Laher F, Tomaras GD, et al. 2018. Subtype C ALVAC-HIV and bivalent subtype C gp120/MF59 HIV-1 vaccine in low-risk, HIV-uninfected, South African adults: a phase 1/2 trial. Lancet HIV 5: e366–e78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baden LR, Karita E, Mutua G, Bekker LG, Gray G, et al. 2016. Assessment of the safety and immunogenicity of 2 novel vaccine platforms for HIV-1 prevention: a randomized trial. Ann Intern Med 164: 313–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baden LR, Liu J, Li H, Johnson JA, Walsh SR, et al. 2015. Induction of HIV-1-specific mucosal immune responses following intramuscular recombinant adenovirus serotype 26 HIV-1 vaccination of humans. J Infect Dis 211: 518–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baden LR, Walsh SR, Seaman MS, Tucker RP, Krause KH, et al. 2013. First-in-human evaluation of the safety and immunogenicity of a recombinant adenovirus serotype 26 HIV-1 Env vaccine (IPCAVD 001). J Infect Dis 207: 240–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fischer W, Perkins S, Theiler J, Bhattacharya T, Yusim K, et al. 2007. Polyvalent vaccines for optimal coverage of potential T-cell epitopes in global HIV-1 variants. Nat Med 13: 100–6 [DOI] [PubMed] [Google Scholar]
- 73.Barouch DH, O’Brien KL, Simmons NL, King SL, Abbink P, et al. 2010. Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nat Med 16: 319–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, et al. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372: 1881–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, et al. 2008. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet 372: 1894–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duerr A, Huang Y, Buchbinder S, Coombs RW, Sanchez J, et al. 2012. Extended follow-up confirms early vaccine-enhanced risk of HIV acquisition and demonstrates waning effect over time among participants in a randomized trial of recombinant adenovirus HIV vaccine (Step Study). J Infect Dis 206: 258–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rolland M, Tovanabutra S, deCamp AC, Frahm N, Gilbert PB, et al. 2011. Genetic impact of vaccination on breakthrough HIV-1 sequences from the STEP trial. Nat Med 17: 366–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hertz T, Logan MG, Rolland M, Magaret CA, Rademeyer C, et al. 2016. A study of vaccine-induced immune pressure on breakthrough infections in the Phambili phase 2b HIV-1 vaccine efficacy trial. Vaccine 34: 5792–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Janes H, Friedrich DP, Krambrink A, Smith RJ, Kallas EG, et al. 2013. Vaccine-induced gag-specific T cells are associated with reduced viremia after HIV-1 infection. J Infect Dis 208: 1231–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu J, O’Brien KL, Lynch DM, Simmons NL, La Porte A, et al. 2009. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 457: 87–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nehete PN, Nehete BP, Hill L, Manuri PR, Baladandayuthapani V, et al. 2008. Selective induction of cell-mediated immunity and protection of rhesus macaques from chronic SHIV(KU2) infection by prophylactic vaccination with a conserved HIV-1 envelope peptide-cocktail. Virology 370: 130–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tuyishime S, Haut LH, Kurupati RK, Billingsley JM, Carnathan D, et al. 2018. Correlates of protection against SIVmac251 infection in rhesus macaques immunized with chimpanzee-derived adenovirus vectors. EBioMedicine 31: 25–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Genesca M, McChesney MB, Miller CJ. 2009. Antiviral CD8+ T cells in the genital tract control viral replication and delay progression to AIDS after vaginal SIV challenge in rhesus macaques immunized with virulence attenuated SHIV 89.6. J Intern Med 265: 67–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wilson NA, Keele BF, Reed JS, Piaskowski SM, MacNair CE, et al. 2009. Vaccine-induced cellular responses control simian immunodeficiency virus replication after heterologous challenge. J Virol 83: 6508–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yamamoto T, Johnson MJ, Price DA, Wolinsky DI, Almeida JR, et al. 2012. Virus inhibition activity of effector memory CD8(+) T cells determines simian immunodeficiency virus load in vaccinated monkeys after vaccine breakthrough infection. J Virol 86: 5877–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stephenson KE, Li H, Walker BD, Michael NL, Barouch DH. 2012. Gag-specific cellular immunity determines in vitro viral inhibition and in vivo virologic control following simian immunodeficiency virus challenges of vaccinated rhesus monkeys. J Virol 86: 9583–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Graham BS, Koup RA, Roederer M, Bailer RT, Enama ME, et al. 2006. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 DNA candidate vaccine. J Infect Dis 194: 1650–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hammer SM, Sobieszczyk ME, Janes H, Karuna ST, Mulligan MJ, et al. 2013. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med 369: 2083–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Janes HE, Cohen KW, Frahm N, De Rosa SC, Sanchez B, et al. 2017. Higher T-cell responses induced by DNA/rAd5 HIV-1 preventive vaccine are associated with lower HIV-1 infection risk in an efficacy trial. J Infect Dis 215: 1376–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fong Y, Shen X, Ashley VC, Deal A, Seaton KE, et al. 2018. Modification of the association between T-cell immune responses and human immunodeficiency virus type 1 infection risk by vaccine-induced antibody responses in the HVTN 505 trial. J Infect Dis 217: 1280–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Santra S, Liao HX, Zhang R, Muldoon M, Watson S, et al. 2010. Mosaic vaccines elicit CD8+ T lymphocyte responses that confer enhanced immune coverage of diverse HIV strains in monkeys. Nat Med 16: 324–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stephenson KE, SanMiguel A, Simmons NL, Smith K, Lewis MG, et al. 2012. Full-length HIV-1 immunogens induce greater magnitude and comparable breadth of T lymphocyte responses to conserved HIV-1 regions compared with conserved-region-only HIV-1 immunogens in rhesus monkeys. J Virol 86: 11434–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kulkarni V, Valentin A, Rosati M, Rolland M, Mullins JI, Pavlakis GN, Felber BK. 2014. HIV-1 conserved elements p24CE DNA vaccine induces humoral immune responses with broad epitope recognition in macaques. PLoS One 9: e111085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Letourneau S, Im EJ, Mashishi T, Brereton C, Bridgeman A, et al. 2007. Design and preclinical evaluation of a universal HIV-1 vaccine. PLoS ONE 2: e984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mothe B, Hu X, Llano A, Rosati M, Olvera A, et al. 2015. A human immune data-informed vaccine concept elicits strong and broad T-cell specificities associated with HIV-1 control in mice and macaques. J Transl Med 13: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ondondo B, Murakoshi H, Clutton G, Abdul-Jawad S, Wee EG, et al. 2016. Novel conserved-region t-cell mosaic vaccine with high global HIV-1 coverage is recognized by protective responses in untreated infection. Mol Ther 24: 832–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang OO, Ali A, Kasahara N, Faure-Kumar E, Bae JY, Picker LJ, Park H. 2015. Short conserved sequences of HIV-1 are highly immunogenic and shift immunodominance. J Virol 89: 1195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Abdul-Jawad S, Ondondo B, van Hateren A, Gardner A, Elliott T, Korber B, Hanke T. 2016. Increased Valency of Conserved-mosaic Vaccines Enhances the Breadth and Depth of Epitope Recognition. Mol Ther 24: 375–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zou C, Murakoshi H, Kuse N, Akahoshi T, Chikata T, et al. 2019. Effective suppression of hiv-1 replication by cytotoxic t lymphocytes specific for pol epitopes in conserved mosaic vaccine immunogens. J Virol 93: e02142–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kulkarni V, Rosati M, Valentin A, Ganneru B, Singh AK, et al. 2013. HIV-1 p24(gag) derived conserved element DNA vaccine increases the breadth of immune response in mice. PLoS ONE 8: e60245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gaiha GD, Rossin EJ, Urbach J, Landeros C, Collins DR, et al. 2019. Structural topology defines protective CD8(+) T cell epitopes in the HIV proteome. Science 364: 480–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Borthwick N, Ahmed T, Ondondo B, Hayes P, Rose A, et al. 2014. Vaccine-elicited human T cells recognizing conserved protein regions inhibit HIV-1. Mol Ther 22: 464–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mothe B, Llano A, Ibarrondo J, Daniels M, Miranda C, et al. 2011. Definition of the viral targets of protective HIV-1-specific T cell responses. J Transl Med 9: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mothe B, Manzardo C, Sanchez-Bernabeu A, Coll P, Morón-López S, et al. 2019. Therapeutic vaccination refocuses T-cell responses towards conserved 1 regions of HIV-1 in 2 early treated individuals (BCN 01 study). eClinicalMedicine in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, et al. 2011. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473: 523–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hansen SG, Piatak M Jr., Ventura AB, Hughes CM, Gilbride RM, et al. 2013. Immune clearance of highly pathogenic SIV infection. Nature 502: 100–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hansen SG, Sacha JB, Hughes CM, Ford JC, Burwitz BJ, et al. 2013. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science 340: 1237874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hansen SG, Wu HL, Burwitz BJ, Hughes CM, Hammond KB, et al. 2016. Broadly targeted CD8(+) T cell responses restricted by major histocompatibility complex E. Science 351: 714–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ward AB, Wilson IA. 2017. The HIV-1 envelope glycoprotein structure: nailing down a moving target. Immunological Reviews 275: 21–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wagh K, Kreider EF, Li Y, Barbian HJ, Learn GH, et al. 2018. Completeness of HIV-1 envelope glycan shield at transmission determines neutralization breadth. Cell Reports 25: 893–908.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.de Taeye SW, Ozorowski G, Torrents de la Peña A, Guttman M, Julien J-P, et al. 2015. Immunogenicity of stabilized HIV-1 envelope trimers with reduced exposure of non-neutralizing epitopes. Cell 163: 1702–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Klasse PJ, LaBranche CC, Ketas TJ, Ozorowski G, Cupo A, et al. 2016. Sequential and simultaneous immunization of rabbits with HIV-1 envelope glycoprotein SOSIP.664 trimers from clades A, B and C. PLoS Pathog 12: e1005864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McCoy LE, van Gils MJ, Ozorowski G, Messmer T, Briney B, et al. 2016. Holes in the glycan shield of the native HIV envelope are a target of trimer-elicited neutralizing antibodies. Cell Rep 16: 2327–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sanders RW, van Gils MJ, Derking R, Sok D, Ketas TJ, et al. 2015. HIV-1 Vaccines. HIV-1 neutralizing antibodies induced by native-like envelope trimers. Science 349: aac4223–aac [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Voss JE, Andrabi R, McCoy LE, de Val N, Fuller RP, et al. 2017. Elicitation of neutralizing antibodies targeting the V2 apex of the HIV envelope trimer in a wild-type animal model. Cell Reports 21: 222–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sok D, Le KM, Vadnais M, Saye-Francisco KL, Jardine JG, et al. 2017. Rapid elicitation of broadly neutralizing antibodies to HIV by immunization in cows. Nature 548: 108–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lu M, Ma X, Castillo-Menendez LR, Gorman J, Alsahafi N, et al. 2019. Associating HIV-1 envelope glycoprotein structures with states on the virus observed by smFRET. Nature 568: 415–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Doria-Rose NA, Schramm CA, Gorman J, Moore PL, Bhiman JN, et al. 2014. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature 509: 55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bhiman JN, Anthony C, Doria-Rose NA, Karimanzira O, Schramm CA, et al. 2015. Viral variants that initiate and drive maturation of V1V2-directed HIV-1 broadly neutralizing antibodies. Nat Med 21: 1332–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Andrabi R, Bhiman JN, Burton DR. 2018. Strategies for a multi-stage neutralizing antibody-based HIV vaccine. Curr Opin Immunol 53: 143–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sok D, Briney B, Jardine JG, Kulp DW, Menis S, et al. 2016. Priming HIV-1 broadly neutralizing antibody precursors in human Ig loci transgenic mice. Science 353: 1557–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jardine JG, Ota T, Sok D, Pauthner M, Kulp DW, et al. 2015. HIV-1 Vaccines. Priming a broadly neutralizing antibody response to HIV-1 using a germline-targeting immunogen. Science 349: 156–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Briney B, Sok D, Jardine JG, Kulp DW, Skog P, Menis S, et al. 2016. Tailored immunogens direct affinity maturation toward HIV neutralizing antibodies. Cell 166: 1459–70.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Steichen JM, Kulp DW, Tokatlian T, Escolano A, Dosenovic P, et al. 2016. HIV vaccine design to target germline precursors of glycan-dependent broadly neutralizing antibodies. Immunity 45: 483–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Escolano A, Steichen JM, Dosenovic P, Kulp DW, Golijanin J, et al. 2016. Sequential immunization elicits broadly neutralizing anti-HIV-1 antibodies in Ig knockin mice. Cell 166: 1445–58.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kong R, Xu K, Zhou T, Acharya P, Lemmin T, et al. 2016. Fusion peptide of HIV-1 as a site of vulnerability to neutralizing antibody. Science 352: 828–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cai H, Orwenyo J, Giddens JP, Yang Q, Zhang R, et al. 2017. Synthetic three-component HIV-1 V3 glycopeptide immunogens induce glycan-dependent antibody responses. Cell Chemical Biology 24: 1513–22.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xu K, Acharya P, Kong R, Cheng C, Chuang GY, et al. 2018. Epitope-based vaccine design yields fusion peptide-directed antibodies that neutralize diverse strains of HIV-1. Nat Med 24: 857–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Escolano A, Gristick HB, Abernathy ME, Merkenschlager J, Gautam R, et al. 2019. Immunization expands B cells specific to HIV-1 V3 glycan in mice and macaques. Nature 570: 468–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gilbert PB, Juraska M, deCamp AC, Karuna S, Edupuganti S, et al. 2017. Basis and statistical design of the passive HIV-1 antibody mediated prevention (AMP) test-of-concept efficacy trials. Stat Commun Infect Dis 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huang Y, Yu J, Lanzi A, Yao X, Andrews CD, et al. 2016. Engineered bispecific antibodies with exquisite HIV-1-neutralizing activity. Cell 165: 1621–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xu L, Pegu A, Rao E, Doria-Rose N, Beninga J, et al. 2017. Trispecific broadly neutralizing HIV antibodies mediate potent SHIV protection in macaques. Science 358: 85–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fetzer I, Gardner MR, Davis-Gardner ME, Prasad NR, Alfant B, Weber JA, Farzan M. 2018. eCD4-Ig variants that more potently neutralize HIV-1. J Virol 92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rudicell RS, Kwon YD, Ko SY, Pegu A, Louder MK, et al. 2014. Enhanced potency of a broadly neutralizing HIV-1 antibody in vitro improves protection against lentiviral infection in vivo. J Virol 88: 12669–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gaudinski MR, Coates EE, Houser KV, Chen GL, Yamshchikov G, et al. 2018. Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: A Phase 1 open-label clinical trial in healthy adults. PLoS Med 15: e1002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, et al. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med 6: 207–10 [DOI] [PubMed] [Google Scholar]
- 137.Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, et al. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med 6: 200–6 [DOI] [PubMed] [Google Scholar]
- 138.Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, et al. 2009. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med 15: 951–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gautam R, Nishimura Y, Pegu A, Nason MC, Klein F, et al. 2016. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature 533: 105–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Moldt B, Rakasz EG, Schultz N, Chan-Hui P-Y, Swiderek K, et al. 2012. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci U S A 109: 18921–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Julg B, Liu PT, Wagh K, Fischer WM, Abbink P, et al. 2017. Protection against a mixed SHIV challenge by a broadly neutralizing antibody cocktail. Sci Transl Med 9: eaao4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Julg B, Tartaglia LJ, Keele BF, Wagh K, Pegu A, et al. 2017. Broadly neutralizing antibodies targeting the HIV-1 envelope V2 apex confer protection against a clade C SHIV challenge. Sci Transl Med 9: eaal1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sok D, Burton DR. 2018. Recent progress in broadly neutralizing antibodies to HIV. Nat Immunol 19: 1179–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Webb NE, Montefiori DC, Lee B. 2015. Dose-response curve slope helps predict therapeutic potency and breadth of HIV broadly neutralizing antibodies. Nat Commun 6: 8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McCoy LE, Falkowska E, Doores KJ, Le K, Sok D, et al. 2015. Incomplete neutralization and deviation from sigmoidal neutralization curves for HIV broadly neutralizing monoclonal antibodies. PLoS Pathog 11: e1005110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pauthner MG, Nkolola JP, Havenar-Daughton C, Murrell B, Reiss SM, et al. 2019. Vaccine-induced protection from homologous tier 2 SHIV challenge in nonhuman primates depends on serum-neutralizing antibody titers. Immunity 50: 241–52 e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. 2014. Estimating per-act HIV transmission risk: a systematic review. AIDS 28: 1509–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wagh K, Seaman MS, Zingg M, Fitzsimons T, Barouch DH, et al. 2018. Potential of conventional & bispecific broadly neutralizing antibodies for prevention of HIV-1 subtype A, C & D infections. PLoS Pathog 14: e1006860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Huang Y, Zhang L, Ledgerwood J, Grunenberg N, Bailer R, et al. 2017. Population pharmacokinetics analysis of VRC01, an HIV-1 broadly neutralizing monoclonal antibody, in healthy adults. MAbs: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bar KJ, Sneller MC, Harrison LJ, Justement JS, Overton ET, et al. 2016. Effect of HIV antibody VRC01 on viral rebound after treatment interruption. N Engl J Med 375: 2037–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lynch RM, Boritz E, Coates EE, DeZure A, Madden P, et al. 2015. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med 7: 319ra206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Caskey M, Klein F, Lorenzi JC, Seaman MS, West AP, et al. 2015. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 522: 487–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Caskey M, Schoofs T, Gruell H, Settler A, Karagounis T, et al. 2017. Antibody 10–1074 suppresses viremia in HIV-1-infected individuals. Nat Med 23: 185–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Scheid JF, Horwitz JA, Bar-On Y, Kreider EF, Lu CL, et al. 2016. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature 535: 556–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Doria-Rose NA, Bhiman JN, Roark RS, Schramm CA, Gorman J, et al. 2015. New member of the V1V2-directed CAP256-VRC26 lineage that shows increased breadth and exceptional potency. J Virol 90: 76–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kong R, Louder MK, Wagh K, Bailer RT, deCamp A, et al. 2015. Improving neutralization potency and breadth by combining broadly reactive HIV-1 antibodies targeting major neutralization epitopes. J Virol 89: 2659–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wagh K, Bhattacharya T, Williamson C, Robles A, Bayne M, et al. 2016. Optimal combinations of broadly neutralizing antibodies for prevention and treatment of HIV-1 clade C infection. PLoS Pathog 12: e1005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu J, Ghneim K, Sok D, Bosche WJ, Li Y, et al. 2016. Antibody-mediated protection against SHIV challenge includes systemic clearance of distal virus. Science 353: 1045–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kwong PD, Mascola JR. 2012. Human antibodies that neutralize HIV-1: identification, structures, and B cell ontogenies. Immunity 37: 412–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gao F, Bonsignori M, Liao HX, Kumar A, Xia SM, et al. 2014. Cooperation of B cell lineages in induction of HIV-1-broadly neutralizing antibodies. Cell 158: 481–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hraber P, Seaman MS, Bailer RT, Mascola JR, Montefiori DC, Korber BT. 2014. Prevalence of broadly neutralizing antibody responses during chronic HIV-1 infection. AIDS 28: 163–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rusert P, Kouyos RD, Kadelka C, Ebner H, Schanz M, et al. 2016. Determinants of HIV-1 broadly neutralizing antibody induction. Nat Med 22: 1260–7 [DOI] [PubMed] [Google Scholar]
- 163.Landais E, Huang X, Havenar-Daughton C, Murrell B, Price MA, et al. 2016. Broadly neutralizing antibody responses in a large longitudinal sub-Saharan HIV primary infection cohort. PLoS Pathog 12: e1005369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Brenner BG, Roger M, Routy JP, Moisi D, Ntemgwa M, et al. 2007. High rates of forward transmission events after acute/early HIV-1 infection. J Infect Dis 195: 951–9 [DOI] [PubMed] [Google Scholar]
- 165.Volz EM, Ionides E, Romero-Severson EO, Brandt MG, Mokotoff E, Koopman JS. 2013. HIV-1 transmission during early infection in men who have sex with men: a phylodynamic analysis. PLoS Med 10: e1001568; discussion e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Powers KA, Ghani AC, Miller WC, Hoffman IF, Pettifor AE, et al. 2011. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet 378: 256–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pilcher CD, Tien HC, Eron JJ, Vernazza PL, Leu SY, et al. 2004. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J Infect Dis 189: 1785–92 [DOI] [PubMed] [Google Scholar]
- 168.Bar-On Y, Gruell H, Schoofs T, Pai JA, Nogueira L, et al. 2018. Safety and antiviral activity of combination HIV-1 broadly neutralizing antibodies in viremic individuals. Nat Med 24:1701–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mendoza P, Gruell H, Nogueira L, Pai JA, Butler AL, et al. 2018. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature 561: 479–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hauser A, Kusejko K, Johnson LF, Wandeler G, Riou J, et al. 2019. Bridging the gap between HIV epidemiology and antiretroviral resistance evolution: Modelling the spread of resistance in South Africa. PLoS Comput Biol 15: e1007083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Xu L, Pegu A, Rao E, Doria-Rose N, Beninga J, et al. 2017. Trispecific broadly neutralizing HIV antibodies mediate potent SHIV protection in macaques. Science 358: 85–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Stewart-Jones GB, Soto C, Lemmin T, Chuang GY, Druz A, et al. 2016. Trimeric HIV-1-env structures define glycan shields from clades A, B, and G. Cell 165: 813–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Korber BT, Kunstman KJ, Patterson BK, Furtado M, McEvilly MM, Levy R, Wolinsky SM. 1994. Genetic differences between blood- and brain-derived viral sequences from human immunodeficiency virus type 1-infected patients: evidence of conserved elements in the V3 region of the envelope protein of brain-derived sequences. J Virol 68: 7467–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pauthner M, Havenar-Daughton C, Sok D, Nkolola JP, Bastidas R, et al. 2017. Elicitation of robust tier 2 neutralizing antibody responses in nonhuman primates by HIV envelope trimer immunization using optimized approaches. Immunity 46: 1073–88 e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cohen YZ, Lorenzi JCC, Seaman MS, Nogueira L, Schoofs T, et al. 2018. Neutralizing activity of broadly neutralizing anti-HIV-1 antibodies against clade B clinical isolates produced in peripheral blood mononuclear cells. J Virol 92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Huang J, Kang BH, Ishida E, Zhou T, Griesman T, et al. 2016. Identification of a CD4-binding-site antibody to HIV that evolved near-pan neutralization breadth. Immunity 45: 1108–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhou T, Zhu J, Wu X, Moquin S, Zhang B, et al. 2013. Multidonor analysis reveals structural elements, genetic determinants, and maturation pathway for HIV-1 neutralization by VRC01-class antibodies. Immunity 39: 245–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Schoofs T, Klein F, Braunschweig M, Kreider EF, Feldmann A, et al. 2016. HIV-1 therapy with monoclonal antibody 3BNC117 elicits host immune responses against HIV-1. Science 352: 997–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Andrabi R, Voss JE, Liang CH, Briney B, McCoy LE, et al. 2015. Identification of common features in prototype broadly neutralizing antibodies to HIV envelope v2 apex to facilitate vaccine design. Immunity 43: 959–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.McLellan JS, Pancera M, Carrico C, Gorman J, Julien JP, et al. 2011. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature 480: 336–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sok D, van Gils MJ, Pauthner M, Julien JP, Saye-Francisco KL, et al. 2014. Recombinant HIV envelope trimer selects for quaternary-dependent antibodies targeting the trimer apex. Proc Natl Acad Sci U S A 111: 17624–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.O’Rourke SM, Schweighardt B, Phung P, Mesa KA, Vollrath AL, Tatsuno GP, et al. 2012. Sequences in glycoprotein gp41, the CD4 binding site, and the V2 domain regulate sensitivity and resistance of HIV-1 to broadly neutralizing antibodies. J Virol 86: 12105–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Barnes CO, Gristick HB, Freund NT, Escolano A, Lyubimov AY, et al. 2018. Structural characterization of a highly-potent V3-glycan broadly neutralizing antibody bound to natively-glycosylated HIV-1 envelope. Nat Commun 9: 1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Alam SM, Aussedat B, Vohra Y, Meyerhoff RR, Cale EM, et al. 2017. Mimicry of an HIV broadly neutralizing antibody epitope with a synthetic glycopeptide. Sci Transl Med 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gristick HB, von Boehmer L, West AP, Schamber M, Gazumyan A, et al. 2016. Natively glycosylated HIV-1 Env structure reveals new mode for antibody recognition of the CD4-binding site. Nat Struct Mol Biol 23: 906–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ferguson AL, Falkowska E, Walker LM, Seaman MS, Burton DR, Chakraborty AK. 2013. Computational prediction of broadly neutralizing HIV-1 antibody epitopes from neutralization activity data. PLoS One 8: e80562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, et al. 2011. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477: 466–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fera D, Schmidt AG, Haynes BF, Gao F, Liao HX, Kepler TB, Harrison SC. 2014. Affinity maturation in an HIV broadly neutralizing B-cell lineage through reorientation of variable domains. Proc Natl Acad Sci U S A 111: 10275–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


