Abstract
Hoechst side population (SP) analysis has proven to be a valuable technique for identifying and sorting stem and early progenitor cells in a variety of tissues and species. In this method, the DNA binding dye Hoechst 33342 is loaded into the cell population of interest; stem cells preferentially exclude this dye, and these low-fluorescence cells can be detected by flow cytometry. However, Hoechst SP analysis usually requires a flow cytometer equipped with an ultraviolet laser source for optimal performance. Unfortunately, ultraviolet lasers are expensive and are not common fixtures on flow cytometers. Violet laser diodes emitting in the 395 to 410 nm range are less expensive and have become much more common on flow cytometers, but do not provide optimal excitation of Hoechst 33342. DyeCycle Violet is a cell-permeable DNA binding dye with a chemical structure similar to Hoechst 33342, but with a longer excitation maximum. DyeCycle Violet can substitute for Hoechst 33342 when performing side population analysis on a cytometer with a violet laser source. The procedure for DyeCycle Violet labeling for side population is described, as well as limitations particular to this dye.
Keywords: stem cell, side population, Hoechst 33258, DyeCycle Violet
Introduction
This protocol describes the use of the cell-permeable DNA binding dye DyeCycle Violet (DCV) as a violet-excited alternative for Hoechst 33342 in the side population (SP) technique. The Hoechst SP method was originally described by Goodell et al. (1996), and has proven useful in identifying and sorting stem cells and early progenitors in a variety of species and hematopoetic and non-hematopoetic tissues (see Units 9.18 and 9.23; Challen and Little, 1996). However, good resolution of the Hoechst SP requires an ultraviolet laser, an expensive and uncommon light source on commercial flow cytometers. DyeCycle Violet shares the efflux pump specificity of Hoechst 33342, but possesses an excitation maximum that corresponds more closely to violet laser diodes, now a much more common excitation source on flow cytometers. DCV also generates a side population that corresponds well with stem cell activity, and is a useful for replacement for Hoechst 33342 under violet excitation conditions (Telford et al, 2007).
The labeling conditions for DCV are very similar to those for Hoechst 33342; as for the original technique, some empirical experimentation with incubation conditions and time may be necessary for some cell or tissue types. Optimization of the technique with a cell type expressing a clear SP region (such as mouse bone marrow or human cord blood) is highly recommended prior to attempting with other cell types.
Basic Protocol
Strategic Planning
This Protocol requires a flow cytometer equipped with a violet laser diode. The instrument should have two PMT detectors aligned to the violet laser source. The same filter set appropriate for Hoechst SP analysis can be used for DCV SP. A typical filter set consists of a blue bandpass (i.e. 450/40 nm), a red bandpass filter (i.e. 675/20 nm) and a dichroic mirror to separate the blue and red filters (i.e. a 580 longpass). These filter values are not precise, and some flexibility with regard to filter choice is permitted; generally, filters appropriate for DAPI (blue) and APC/Cy5 (red) will work for this method. The dichroic can be a long-pass or short-pass depending on the instrument, and should be between 560 and 610 nm. An example of a typical filter configuration (BD LSR II) is shown in Figure 9.30.1.
Figure 9.30.1.
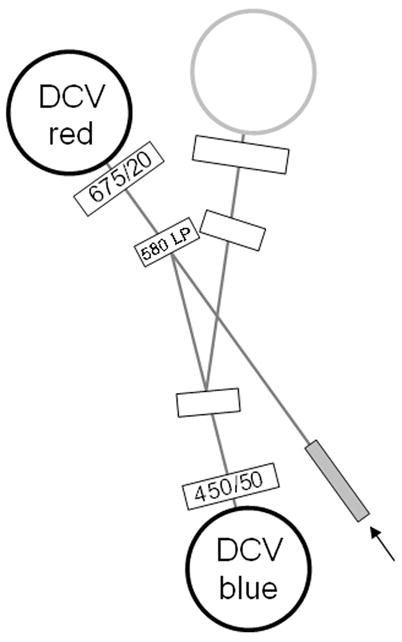
Typical optical configuration for DCV SP analysis (BD Biosciences LSR II), using 450/50 nm and 675/20 nm bandpass filters for blue and red signal detection, and a 580 long-pass dichroic to separate the signals.
The minimum violet laser power installed on commercial instrumentation is usually 20 to 25 mW; this power level is sufficient for cuvette cytometers. However, higher power violet lasers (50 to 100 mW) are becoming standard equipment on multilaser cytometers, and will improve SP resolution somewhat. If analysis and/or sorting are carried out on a jet-in-air instrument, higher power levels (at least 50 mW) are essential. We have done successful DCV SP analysis on a BD Bioscience FACSAria and LSR II, a Beckman-Coulter Gallios, a Stratedigm S1000 and a Cytek Development modified FACSCalibur with a violet laser. An ultraviolet laser can also be used to excite DCV if it is available.
A fluorescent alignment verification microsphere standard is highly recommended, used to verify violet laser alignment. Single intensity Rainbow Ultra beads (Spherotech, Liberty, IL) or AlignFlow UV beads (Invitrogen, Carlsbad, CA) both work well. Rainbow 8-peak (Spherotech) or InSpeck Blue (Invitrogen) multi-intensity bead cocktails are even more effective for monitoring laser alignment, since small changes in weak signal resolution can be observed
Materials List
DMEM+ or HBSS+ (see recipe).
Mouse bone marrow or human cord blood.
DyeCycle Violet (supplied as a 10 mM stock solution, Invitrogen, Carlsbad, CA, USA).
Propidium iodide (PI) 1 mg/ml stock solution.
Verapamil (Sigma Chemical Co., St. Louis, MO, USA). Verapamil can be prepared as a stock solution of 10 mM in DMSO, and used at a final concentration of 50 μM (1:200 dilution).
Fumitremorgin C (Sigma Chemical Co.) Fumitremorgin C is a more specific inhibitor of ABC transporters, and can also be used as a DCV inhibitor. It can be prepared as a stock solution of 2 mM in distilled water, and used at a final concentration of 10 μM (1:200 dilution).
37°C waterbath.
Flow cytometer equipped with a violet laser diode, blue and red detection, as described in Strategic Planning, above.
Plasticware required for cell isolation and incubation (5 or 15 ml polypropylene tubes, pipets, etc.). Polypropylene tubes are best for the labeling steps, as cells will often adhere to polystyrene.
Cell preparation
1) Prepare the HBSS+ and DMEM+. See the Reagents and Solutions section for directions.
2) Isolate the cell type of interest. Count the nucleated cells, and adjust the cell volume to between 1 and 5 × 106 cells/ml in DMEM+.
For mouse bone marrow, femurs and tibias from euthanized mice are surgically removed, and the bone marrow extruded into a 50 ml polypropylene tube using a syringe containing HBSS+ and a 27 gauge needle. The resulting bone marrow suspension is allowed to settle for 5 minutes (to remove bone fragments). The suspension is then slowly drawn with a syringe through an 18 gauge needle to disrupt matrix fragments, and the resulting suspension filtered through 70 micron mesh. The suspension is then washed by centrifugation, resuspended in DMEM+ and counted. Human cord blood is similarly filtered and washed by centrifugation prior to resuspension and counting. All cell preparation steps are generally done at room temperature. Multiple aliquots of mouse bone marrow can be frozen and subsequently thawed for use as positive controls.
DCV labeling can be carried out at cell concentrations up to 5 × 106 cell/ml for larger cell numbers.
3) Prewarm the cell suspensions in the 37°C water bath. Transfer an aliquot of the cell suspension to a second tube as the efflux pump inhibitor control.
4) Add verapamil at a final concentration of 50 μM or fumitremorgin C at a final concentration of 10 μM to the inhibitor control sample, and preincubate at 37°C for 15 minutes.
Labeling with DCV
5) Add the DCV to both cell suspensions at a final concentration between 10 μM (1:1000 and 1:500 dilutions). Incubate at 37°C for 90 minutes.
DCV at a final concentration of 10 μM is generally adequate for hematopoetic tissues. Other tissues may require higher or lower concentrations.
6) Centrifuge the cells at 400 × g in a refrigerated centrifuge and decant the supernatant. Resuspend in cold DMEM+, and immediately place on ice. If no additional immunolabeling is required, cells can be analyzed immediately. Add propidium iodide (2 μg/ml final concentration, 1:500 dilution of a 1 mg/ml initial stock) 5 minutes prior to analysis.
Analysis should be carried out within four hours of cell preparation. Cells should be stored at 4°C prior to analysis. Propidium iodide should only be added immediately prior to analysis.
Flow cytometry of DCV labeled cells
7) Verify violet laser alignment prior to analysis using microsphere alignment beads. An example of this is shown in Figure 9.30.2, where premixed Spherotech 8-population Rainbow beads were analyzed through the 450/50 nm bandpass filter detector using a violet laser diode. Although the distribution is not linear, seven of the eight populations are distinguishable, presenting a distinctive pattern that can be tracked for changes.
8) Analyze cells on an instrument equipped with a violet laser diode, and two PMT detectors equipped with 450/50 nm and 675/20 nm filters, using a 560–610 nm long-pass or short-pass filter to separate the signals. A typical DCV SP distribution (in comparison with Hoechst SP) is shown in Figure 9.30.3.
Figure 9.30.2.
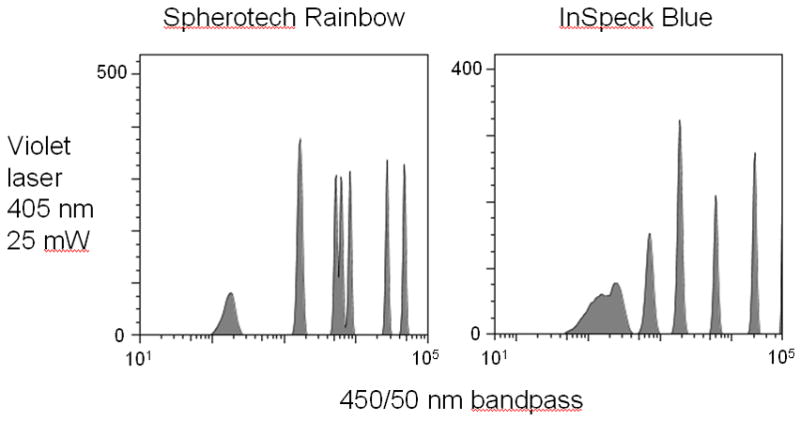
Typical fluorescence distribution of premixed Spherotech 8-peak Rainbow beads and Invitrogen Molecular Probes InSpeck Blue beads, excited with a violet laser diode and detected through a 450/50 nm bandpass filter. Note that all microspheres are not distinguishable in this analysis; although these bead sets are not specifically designed for violet laser verification, their distinctive “signature” can be easily monitored over time and deteriorations in laser performance easily detected. Analysis was carried on a BD Biosciences LSR II (San Jose, CA).
Figure 9.30.3.
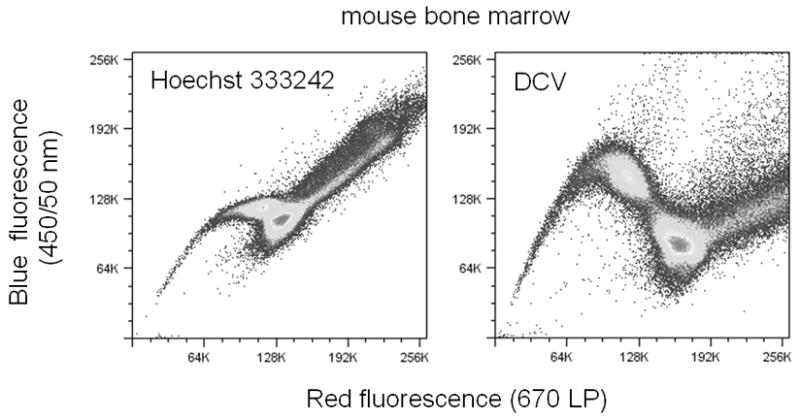
Hoechst and DCV SP analysis of mouse bone marrow, using ultraviolet laser excitation (355 nm, 20 mW). Analysis was carried on on a BD Biosciences LSR II. Note the difference in distribution and orientation of the SP population with DCV in comparison with Hoechst 33342.
SP analysis (both Hoechst and DCV) is very sensitive to instrument alignment, and will be noticeably degraded if the violet laser is not well aligned. Perform frequent quality control checks on your instrument, using a fluorescent microsphere that can be excited with a violet laser source. Establish a quality control system specific to the violet laser to verify instrument alignment prior to analysis.
Run the cells, and set up dotplots for forward scatter (FSC) versus side scatter (SSC), and forward or side scatter versus PI fluorescence (usually the 488 nm excited PE or PE-Cy5 detector). Initial gating can be done on both the FSC/SSC and PI plots, or just on the PI-negative viable cells. The background fluorescence in the PE/PI channel will be somewhat high due to incidental DCV fluorescence, but should be well below the fluorescence of PI-labeled non-viable cells.
Then display the DCV fluorescence as a dotplot, with the DCV red fluorescence on the X-axis, and the DCV blue fluorescence on the Y-axis. The DCV blue and red detectors should be set for linear scaling, and the G0/G1 population of the cell distribution placed roughly in the center of the dotplot, using the appropriate detector voltage controls.
The DCV SP shows a distribution somewhat different than that observed for Hoechst SP (Figure 9.30.3). The upward and downward “arch” of the DCV SP population is more pronounced than that seen for the Hoechst SP, and the overall separation between the G0/G1 population and the SP population is larger. Care should be taken to make sure that the DCV SP population is well above the zero point on the red detector scale.
As with all stem cell analyses, large numbers of cells should be analyzed and saved (a minimum of 500,000).
Simultaneous immunolabeling
9) As with Hoechst SP, cells can be immunolabeled after DCV efflux and prior to analysis. Centrifuge at 4°C, resuspend in HBSS+ at the appropriate concentration, and add the desired conjugated antibodies. Wash with cold HBSS+ and store on ice until analysis.
Unlike Hoechst 33342, DCV is somewhat excited by the 488 nm laser, and has a small emission component in the fluorescein, PE and PE-Cy5 ranges. As a result, fluorescein and PE conjugated antibodies are not recommended for use with DCV, as the background fluorescence will be higher than normal. PE-Cy5.5 and PE-Cy7 can be used, and any red- excited fluorochrome (APC, Cy5, APC-Cy5.5, APC-Cy7) will work as well.
Reagents and solutions
DMEM+
Dulbecco’s Minimal Essential Media (DMEM) containing:
2% fetal bovine serum (FBS)
2 mM HEPES
Store for up to two weeks at 4°C.
HBSS+
Hank’s balanced salt solution (HBSS) containing:
2% fetal bovine serum (FBS)
2 mM HEPES
Store for up to two weeks at 4°C.
Propidium iodide (PI)
Dissolve powdered PI at 1 mg/ml in distilled water and store at 4°C. Solution should be stored in the dark for up to one month.
COMMENTARY
Background information
Since its development more than fifteen years ago, Hoechst SP analysis has proven to be a useful technique for identifying stem cells and early progenitors in both hematopoietic and non-hematopoietic tissues (Goodell et al, 1996; Goodell, 2005). It depends on high expression of the ABCG2 transporter pump (BCRP in mice) in these cell subsets; the DNA binding dye Hoechst 33342 is rapidly effluxed from these cells following loading, and the Hoechst-dim cells can be readily identified by flow cytometry. Although not entirely universal for the stem cell phenotype, the phenomenon is observed in a wide variety of tissues and species, and often correlates well with stem cell activity. The recent focus on cancer stem cells has increased the interest in this technique, since good phenotypic markers for stem cells in tumors are frequently lacking (Hadnagy et al, 2006; Marthew et al, 2009; She et al, 2008).
However, the instrumentation requirements for Hoechst SP have considerably limited its accessibility. Hoechst 33342 has an excitation maximum well in the ultraviolet range (λEX = 351 nm), and an ultraviolet laser source is usually required for good Hoechst SP resolution. Ultraviolet lasers have traditionally been expensive and difficult to maintain on flow cytometers. Until recently, a water cooled gas laser system (argon or krypton) was required to produce ultraviolet laser light for cytometry. In addition to being expensive and maintenance-intensive, these lasers were large and could only be accommodated on large-frame cells sorters like the BD FACSVantage, Beckman-Coulter Altra or Beckman-Coulter (formerly Dako) MoFlo. These large cell sorters are difficult to use for analysis-only applications, and are almost entirely dependant on a jet-in-air delivery of the lasers to the laser. Jet-in-air cell presentation systems provide less optical sensitivity than cuvette systems (i.e. the benchtop FACSCalibur), and may produce poorer Hoechst SP resolution. More recently, a new generation of solid state diode, Nd:YAG and Nd:YVO4 ultraviolet lasers have been developed that are smaller, easier to maintain and can be more readily integrated into benchtop analyzers and cell sorting systems. However, these lasers are even more expensive than their water-cooled predecessors. Taken together, these issues made Hoechst SP difficult to carry out and accessible to only a small group of laboratories.
Due to the difficulties surrounding ultraviolet laser light in flow cytometry, several investigators have since attempted to use longer wavelength violet laser sources for Hoechst 33342 excitation. Violet laser diodes are far less expensive than ultraviolet sources, and are now common fixtures on flow cytometers (Telford et al, 2003; Telford et al, 2006). They have achieved relatively high power levels, and are used for a variety of flow cytometric applications, including phenotyping (i.e. Cascade Blue, Pacific Blue and quantum nanoparticles). Violet laser diodes emit from 395 to 415 nm, with the most common peak at approximately 405 nm. While several laboratories have reported success in using violet lasers for Hoechst 33342, the majority of studies have shown poorer Hoechst SP resolution at this wavelength (Telford et al, 2004). This is not surprising considering the excitation curve for Hoechst 33342; only 2–3% of maximal excitation of the dye would be expected at this excitation wavelength. A SP region IS in fact observed using 405 nm excitation, but it is diffuse and often difficult to distinguish from other non-stem effluxing cells, as well as dead cells and debris (Telford et al, 2004). We maintain that good SP resolution is critical for good stem cell identification, especially for more complex non-hematopoetic tissues that might show multiple efflux populations and larger dead cell and debris components. Suboptimal dye excitation should therefore be avoided when carrying out this technique.
DyeCycle Violet (DCV) was therefore proposed as a replacement for Hoechst 33342 when an ultraviolet laser was not available (Telford et al, 2007). DCV is structurally similar to the Hoechst dyes, but possesses an excitation maximum more than 20 nm longer than Hoechst 33342 (λEX = 375 nm). DCV shows 20–25% of maximum excitation at 405 nm, a considerable improvement over Hoechst 33342. Mouse bone marrow and human cord blood give a distinctive SP region when labeled with DCV and analyzed on a flow cytometer equipped with either an ultraviolet or violet source. In addition, the SP regions are very similar when analyzed with either ultraviolet or violet lasers; violet excitation shows a very small deterioration in resolution, but a much smaller loss than that seen with Hoechst 33342 (Figure 9.30.4). DCV SP analysis can be carried out using violet lasers at relatively low power levels (~25 mW), although higher laser powers are now typically installed on modern flow cytometers, and are more desirable. Ultraviolet and near-ultraviolet sources (i.e. 375 nm UV laser diodes) used for Hoechst SP will also work for DCV SP analysis.
Figure 9.30.4.
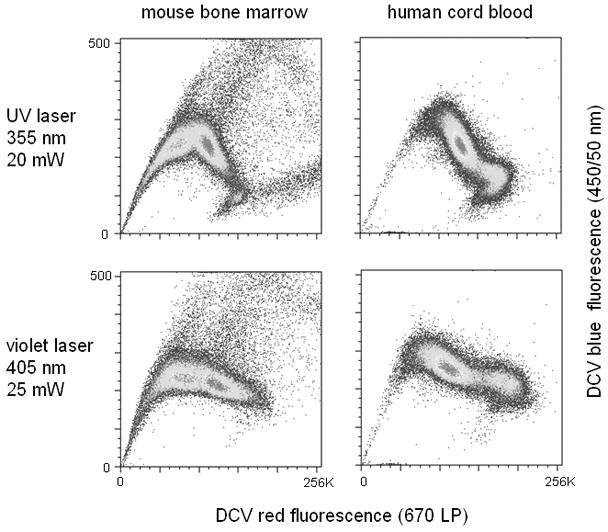
DCV SP analysis of mouse bone marrow and human cord blood, using either ultraviolet (355 nm, 20 mW) or violet laser diode excitation (405 nm, 25 mW).
Correlation between the Hoechst SP and DCV SP is precise. In mouse bone marrow, Hoechst SP correlates almost exactly with the cell surface phenotype Sca-1+ c-kit+ Lin -. This has also been shown to be true for DCV SP. The drug efflux pump specificity for Hoechst 33342 and DCV have also been shown to be identical. However, there are several critical differences between Hoechst SP and DCV SP that should be noted. First, the overall form of the SP population differs somewhat between the two techniques. Several investigators have commented on the spectral properties of Hoechst 33342 that make it applicable for efflux determination. When analyzing Hoechst SP, we measure fluorescence at two wavelengths, and plot the cells against these two parameters. Single wavelength analysis would be insufficient to see good resolution between the G0/G1 population and the SP. This is largely due to the spectral shift in Hoechst 33342 when either bound or unbound to chromatin. Hoechst 33342 retains some of its blue fluorescence emission when not bound to chromatin, but loses almost all of its red fluorescence in the unbound state; the decrease we observe in Hoechst 33342 fluorescence in the SP (unbound) population is largely due to the shift in red fluorescence. With DCV this shift is even more pronounced; either DCV is brighter in the bound state, or much dimmer in the unbound state. As a result, the DCV SP shows an even greater shift than Hoechst 33342, resulting in a SP population that is well-separated from the rest of the population. This characteristic may make DCV particularly useful for identifying SPs in tissue types that normally display poor Hoechst SP resolution, such as non-hematopoietic tissues that do not dissociate well to single cell suspensions. Unfortunately, the differences between Hoechst 33342 and DCV are not simply in their UV or violet excited blue or red emissions. DCV is also somewhat excited at 488 nm, and has a small emission component in the green to orange range. As a result, it emits into the fluorescein and phycoerythrin channels on a multilaser flow cytometer, elevating the fluorescence backgrounds in these detectors. Simultaneous immunolabeling using fluorescein, PE or PE-Cy5 is complicated by these high backgrounds and it is therefore not recommended.
Longer wavelength 488 nm dyes (PE-Cy5.5, PE-Cy7) can be used, as well as all the red-excited fluorochromes (APC, Cy5, APC-Cy5.5, APC-Cy7, etc.). DCV is not excited by red lasers and no such background is observed. This is not as great a limitation as it was even a few years ago, as antibodies relevant to stem cell analysis (CD34, CD38, CD133 in human, Sca-1, c-kit in mouse) are now readily available using these more unusual fluorochromes.
Critical Parameters and Troubleshooting
As with Hoechst SP, technique difficulties are almost always related to (1) changes to the labeling procedure, (2) the cell sample, or (3) instrumentation problems. The protocol should be followed precisely; changes in buffer constituents can produce aberrant results, as will alterations in incubation times and temperatures. Some modification in the incubation conditions may be required for untested non-hematopoietic tissue types. DCV is provided as a solution in water, with storage at 4°C; freezing may alter the properties of the dye. DCV should be stored in the dark and has a shelf life of approximately six months.
A parallel positive control sample is highly desirable when using either Hoechst or DCV for SP analysis. Mouse bone marrow can be frozen in multiple aliquots using a standard 90% FBS/10% DMSO freezing procedure, and a single aliquot thawed for simultaneous analysis with other samples. This will verify both the technique and instrument operation. Some tissues, particularly those of non-hematopoetic origin, can present a very poor or unusual SP profiles, particularly with regard to the separation of the SP population from the non-effluxed cells.
These samples cannot be used to verify technique or instrument performance. A positive control sample is therefore critical in this situation. An inhibitor control (verapamil, fumetrimorgin C or other membrane pump inhibitor) is also important for estimating the percentage of SP cells.
Laser alignment is critical for both Hoechst and DCV SP analysis. The SP population is quite dim, and small changes in instrument alignment can produce large changes in SP resolution. Use alignment microspheres to verify instrument alignment. Spherotech 8-peak Rainbow and Invitrogen Molecular Probes InSpeck Blue bead arrays both have distinctive distributions in the blue emission range when excited by a violet (or ultraviolet) laser (Figure 9.30.2), and small changes in alignment can be readily detected. Violet laser diodes also have a small but documented failure rate; they can gradually lose power over time, or change their beam geometries with age, causing a loss of alignment and sensitivity. If simple beam alignment does not resolve an issue, verify laser performance.
Time considerations
Approximately 3 hours should be allotted for DCV SP sample preparation, from obtaining the cells until the start of analysis. Since large numbers of cells should be analyzed to obtain a statistically significant SP percentage value, sufficient time should also be allotted for the flow cytometry.
Anticipated Results
DCV SP analysis should identify the same population of stem cells and progenitors as Hoechst SP analysis using a violet laser source.
Literature cited
- Challen GA, Little MH. A side order of stem cells: The SP phenotype. Stem Cells. 2006;24:3–12. doi: 10.1634/stemcells.2005-0116. Comprehensive review of Hoechst SP analysis. [DOI] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Connor AS, Mulligan RC. Isolation and functional properties of murime hematopoetic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. The original reference for Hoechst SP analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell MA. Current Protocols in Cytometry. John Wiley and Sons; NY, NY: 2005. Stem cell identification and sorting using the Hoechst 33342 side population (SP) pp. 9.18.1–9.18.11. Detailed Hoechst SP method. [DOI] [PubMed] [Google Scholar]
- Hadnagy A, Gaboury L, Beaulieu R, Balicki D. SP analysis may be used to identify cancer stem cell populations. Experimental Cell Research. 2006;312:3701–3710. doi: 10.1016/j.yexcr.2006.08.030. A review of Hoechst SP analysis in cancer stem cells. [DOI] [PubMed] [Google Scholar]
- Mathew G, Timm EA, Jr, Sotomayor P, Godoy A, Montecinos VP, Smith GJ, Huss WJ. ABCG2-mediated DyeCycle Violet efflux defined side population in benign and malignant prostate. Cell Cycle. 2009;8:1053–1061. doi: 10.4161/cc.8.7.8043. DCV SP analysis in cancer stem cell identification. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She JJ, Zhang PG, Wang ZM, Gan WM, Che XM. Identification of side population cells from bladder cancer cells by DyeCycle Violet staining. Cancer Biol Ther. 2008;7:1663–1668. doi: 10.4161/cbt.7.10.6637. DCV SP analysis in cancer stem cell identification. [DOI] [PubMed] [Google Scholar]
- Telford WG, Kapoor V, Jackson J, Burgess W, Buller G, Hawley T, Hawley R. Violet laser diodes in flow cytometry: an update. Cytometry. 2006;69:1153–60. doi: 10.1002/cyto.a.20340. [DOI] [PubMed] [Google Scholar]
- Telford WG, Hawley TS, Hawley RG. Analysis of violet-excited fluorochromes by flow cytometry using a violet laser diode. Cytometry. 2003;54A:48–55. doi: 10.1002/cyto.a.10046. Use of violet laser diodes for flow cytometry. [DOI] [PubMed] [Google Scholar]
- Telford WG, Frolova EG. Discrimination of Hoechst side population in mouse bone marrow with violet and near-UV laser diodes. Cytometry. 2004;57A:45–52. doi: 10.1002/cyto.a.10109. Suboptimal excitation of Hoechst 33342 with violet laser diodes. [DOI] [PubMed] [Google Scholar]
- Telford WG, Bradford J, Godfrey W, Robey RW, Bates SE. Side population analysis using a violet-excited cell permeable DNA binding dye. Stem Cells. 2007;25:1029–1036. doi: 10.1634/stemcells.2006-0567. First use of DCV for SP analysis. [DOI] [PubMed] [Google Scholar]


