Abstract
Purpose of review:
The goal is providing an update to the latest research surrounding optoelectronic devices, highlighting key studies and benefits and limitations of each device.
Recent Findings:
The Argus II demonstrated long-term safety after five-year follow-up. Due to lack of tack fixation, subretinal implants appear to displace over time. PRIMA’s completed primate trial showed initial safety and potential for improved vision, resulting in ongoing clinical trials Bionic Vision Australia developed a new 44-electrode suprachoroidal device currently in a clinical trial. Orion (cortical stimulation) is currently undergoing a clinical trial to demonstrate safety.
Summary:
Devices using external camera for images are unaffected by corneal or lens opacities but disconnect eye movements from image perception, while the opposite is true for implants directly detecting light. Visual acuity provided by devices is more complicated than implant electrode density and new devices aim to target this with innovative approaches.
Keywords: Optoelectronic devices, retinal implants, Argus, OptoEpiret, PRIMA, Bionic Vision Australia
Introduction
Optoelectronic devices are devices that can transduce electrical-to-optical or optical-to-electrical signals[1]. They have a wide array of applications, but one key application is to restore vision in patients with significant retinal diseases (Summary of devices in Table 1). Once there is permanent damage to retinal structures, there are few treatments[2]. Several different retinal prostheses designed over the last few years have the goal of restoring vision to patients with significant outer retinal diseases lacking treatment, such as retinitis pigmentosa and advanced non-exudative macular degeneration with geographic atrophy[3–6]. The majority of optoelectronic devices currently require some degree of inner retina function to transmit signals to the optic nerve. One device circumvents the visual pathways and directly stimulates the occipital lobe[7]. This review will discuss the significance of retinal implants studied in clinical trials over the past few years, focusing on most recent updates, and highlight the key findings and differences between them.
Table 1:
Summary of Optoelectronic Devices
| Device: | Type: | How it works: | Benefits: | Limitations: |
|---|---|---|---|---|
ARGUS II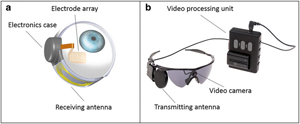 Source: Courtesy of SecondSight |
Epiretinal implant with external camera | External camera records and transmits pulses wirelessly through microelectrode array to inner retina | Unaffected by corneal or lens opacities due to wireless transmission from camera to array; long-term safety has been demonstrated | Visual perception through fixed camera is independent of eye movement; 60 electrodes limits potential visual acuity |
IRIS II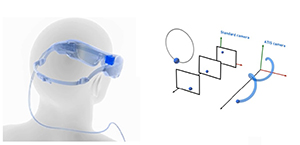 Source: Courtesy of Pixium Vision |
Epiretinal implant with external camera | External camera records and transmits pulses wirelessly through microelectrode array to inner retina | Exchangeable system allows for replacement; unaffected by corneal or lens opacities due to wireless transmission from camera to array | Visual perception through fixed camera is independent of eye movement; 150 electrodes but requires further studies to demonstrate efficacy |
Alpha IMS/AMS (Discontinued)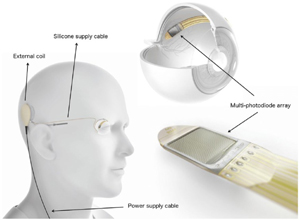 Source: Retina Implant AG, Reutlingen, Germany, used by Bloch et al. 2019, licensed under CC-NY. |
Subretinal implant | Photodiode array in subretinal space that detects light and converts it to electrical signals transmitted to overlying retina | Vision generated corresponds with natural eye movement due to subretinal implant itself directly detecting light | Affected by opacities between cornea and retina due to requiring light to reach the retina; 1500 (IMS) or 1600 (AMS) electrodes but visual acuity significantly below potential |
PRIMA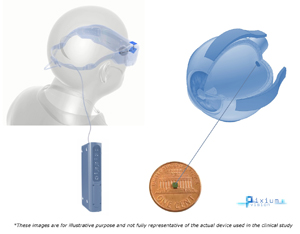 Source: Courtesy of Pixium Vision |
Subretinal implant with external camera | External camera records video that is processed and projected via infrared laser patterns through the pupil to subretinal implant, where photovoltaic array converts the light to electrical signals stimulating bipolar cells | Design of subretinal implant includes ground grid around each stimulating electrode allowing theoretical improvement of resolution | Affected by opacities between cornea and retina due to requiring light to reach the retina; 378 pixels (electrodes) but requires clinical trials to demonstrate efficacy |
Bionic Vision Australia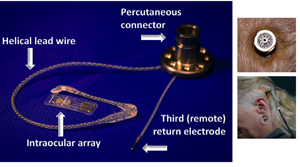 Source: Courtesy of Ayton et al. 2014, originally from Bionic Vision Technologies |
Suprachoroidal implant with external camera | External camera records and transmits pulses wirelessly through microelectrode array through choroid to inner retina | Unaffected by corneal or lens opacities due to wireless transmission from camera to array | Visual perception through fixed camera is independent of eye movement; 44 channels (electrodes) limits potential visual acuity and requires clinical trials to demonstrate efficacy |
Orion Cortical Implant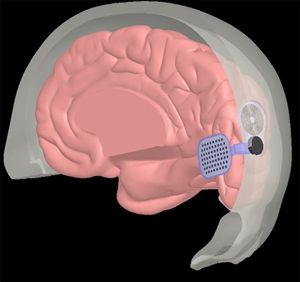 Source: Courtesy of SecondSight |
Cortical implant with external camera | External camera records and transmits pulses wirelessly to microelectrode array to medial occipital lobe | Unaffected by corneal or lens opacities due to wireless transmission from camera to array; potential for use in patients with significant inner retina and/or optic nerve degeneration | Visual perception through fixed camera is independent of eye movement; 60 electrodes limits potential visual acuity; requires clinical trials to demonstrate efficacy and long-term safety |
Epiretinal Implants
Argus II Implant
The Argus II is the first commercially available implant technology allowing patients with retinitis pigmentosa to regain some visual function. The Argus II implant received FDA approval in 2013 for retinitis pigmentosa patients with light perception or worse vision[8]. The Argus II system consists of three major components: an external video camera, external patient-worn system, and epiretinal implant[8]. The video camera is fixed on a pair of glasses worn by the patient. The patient-worn system supplies power to the system electronics and converts camera-captured images to corresponding electrode impulses, which are transmitted to the retina by a microelectrode array. The 2013 build of the epiretinal implant has a 60-channel microelectrode array, although the next generation implant is planned to have 240 electrodes including peripheral electrodes[9].
As the patient moves his/her head, the camera moves with it, changing the view accordingly. Because the camera is external to the eye, its movement is not correlated with eye movement. The images captured by the video camera are sent to the video processing unit, where they are converted to electrical stimulation pulses, which are transmitted wirelessly to the epiretinal implant. Receiving the signals, the implant emits small pulses of electricity to stimulate the cells of the inner retinal layer, bypassing the photoreceptors, and travelling up the optic nerve. The patient then perceives patterns of light that roughly correspond to the image[8]. Significant post-implantation training needs to take place to learn how to interpret the stimulation.
At this point, Argus II has been implanted in over 300 cases globally[3]. Best reported visual acuity is logMAR 1.8 (20/1260)[10]. When the patients were tested with the system on versus off in the grating visual acuity test, patients performed better with the system on, although most patients reported visual precepts that appeared as moving shadows[11]. Based on self-report questionnaires, patients noticed significant improvement in orientation and mobility tasks like finding a door or following a line as well as mild improvements in activities of daily living and quality of life[9].
Since Argus II is the first commercially available implant, numerous studies have examined its long-term effects. A recent one-year follow-up of a cohort of patients showed that the serious adverse events (SAE) after surgery were conjunctival erosion, hypotony, and retinal detachment, consistent with the SAEs reported during the original Argus II clinical trial, with no new SAEs reported[12]. Vision function testing was similarly consistent with original clinical trial data. These findings are consistent with five-year follow-up from clinical trial and while endophthalmitis was reported early in the clinical trial, since the introduction of IV antibiotics to the surgical protocol, there have been no further cases[13]. Histopathologic assessment of the optic nerve and retina from patient with chronically implanted Argus II showed more optic nerve atrophy in the implanted eye, but no additional damage in the temporal quadrant corresponding to the location of the array[14]. There was no evidence of additional tissue damage in the implanted eye at the nontack locations or histologic evidence of inflammatory reactions, which supports the long-term safety of the device, although this study only examined a single patient. For the first time, simultaneous explantation and implantation of Argus II was completed in a patient, requiring a technically challenging surgery with potential for hypotony, hemorrhage, and fibrosis complicating the procedure, although demonstrated feasibility of an exchange[15].
Overall, Argus II has shown to be reasonably safe over long-term, with the majority of adverse events occurring shortly after implantation and few new events afterwards[13,16]. However, while questionnaires have demonstrated mild improvements in ADLs and quality of life, visual acuity with only 60 electrodes remains very limited. Further, due to reliance on an external camera, the visual field only responds to changes in head position instead of eye position, greatly differing from the patient’s natural vision and therefore requires extensive training[17]. Despite these limitations there is an ongoing study in Europe for patients with geographic atrophy with macular degeneration[18]. The entry criteria for visual acuity in this study is hand motion of worse.
Intelligent Retinal Implant System (IRIS II)
The IRIS II was developed from IRIS after Pixium Vision acquired it in 2007[19]. It is an epiretinal implant with a 150-electrode stimulating array that is exchangeable. It consists of the same three major components as the Argus II, and similarly, it relies on an external camera attached to a pair of glasses for the video feed. The images captured by the camera are processed by an external patient-worn device and the resulting electrical pulses are transmitted to the implant, which generates stimulation currents to stimulate the remaining neurons of the degenerated retina. There is a novel event-based image sensor that detects changes directly during image capture stage. Unlike standard cameras which capture full images of the scene at a specified rate, event cameras monitor for brightness changes in the scene independently for every pixel[20]. Once a certain threshold of change in brightness is reached, an event is sent to the camera to change the pixel display. This offers several advantages including elimination of motion blur and lower latency and should better mimic human eye function.
A single-arm, open-label, prospective European trial is currently ongoing with 10 participants to evaluate the safety and effectiveness of the IRIS II[19]. All participants had entry visual acuity of 2.3 logMAR or worse in both eyes using FrACT test (bare light perception or worse Snellen equivalent). The eye with worse vision was implanted and at 4 weeks after implantation, the device was switched on for the first time and participants began intensive training in the utilization of the device. In the square localization test, mean error was lower in on vs off testing at the 3 and 6-month timepoints. Goldman visual field testing showed 5/9 participants having a measurable field with the device on while none had a measurable field with device off. The picture recognition test also showed better performance in 7/9 participants with device on. However, the study is currently underpowered to evaluate efficacy and visual acuity was not evaluated. At six months, the adverse events that have been reported include ocular hypotony (2/10 pts), persistent pain in implanted eye (1/10), and vitreoretinal preretinal traction in implanted eye (1/10).
Overall, the IRIS II has demonstrated reasonable safety at 6 months with a comparable adverse effect profile compared to the Argus II implant, although long-term safety still needs to be assessed. Given the increased number of electrodes compared to Argus II, there is a potential for better visual acuity compared to the Argus II implant, however, future studies will be needed to further elucidate that. As an epiretinal implant that relies on external camera like the Argus II, the visual field similarly responds only to patient head position instead of eye position, differing from natural vision, and therefore requires extensive training[17].
OptoEpiret
OptoEpiret is the newly developed epiretinal implant that combines the group’s former projects EpiRet3 and large stimulating array (VLARS), while adding an integrated circuit-based optical capturing system[21]. VLARS is a large multielectrode array that covers a wider area of the retina than other implants, allowing for increased visual angle[22]. Array sizes for prior implants were limited by the size of the wound needed to insert the array, however the VLARS is flexible enough to be implanted in a folded state, allowing for a larger array than other epiretinal devices. The array of photodiodes on the anterior surface of the integrated circuit records the images and the resulting optical information is converted by the circuit into stimulation pulses forwarded to electrodes on the posterior surface[23]. Therefore, the 9mm array can capture visual information, perform visual processing, and stimulate the ganglion layer. Unlike other epiretinal implants, the OptoEpiret system does not require an external camera, although it does need an external device powering the system.
This device has been tested for surgical feasibility in six rabbit eyes, through implantation of inactive devices. The main adverse events were intravitreal hemorrhage, retinal tearing, and detachment of the array. Its predecessor, the EpiRet3, had undergone clinical trial with six patients and was able to elicit visual precepts in patients, but visual acuity was not assessed. The OptoEpiret is still in the early stages and requires studies of feasibility and efficacy in humans. The integrated circuit-based optical capturing system makes the implant work similarly to subretinal implants described below and shares similar benefits and drawbacks. Light is required to hit the implant before electrical stimulation on the retina is produced. In doing so, this allows for artificial vision that mimics natural vision, changing the image via eye movement[24,25]. It supports normal saccades and pursuit eye movements and prevents image fading through microsaccades. However, it requires corneal and lenticular clarity, as any blockage in the path of light before it reaches the retina will obstruct vision, as in natural vision.
Subretinal Implants
Alpha IMS and AMS
Alpha-IMS is a 1500-electrode subretinal implant that converts light to electrical stimulation within the eye[24]. One major issue affecting the alpha-IMS was its very short longevity, lasting on average 0.6 years due to corrosion of the hermetic seal[26]. The alpha-AMS is the improved 1600-electrode version, designed to address some of the issues that plagued the alpha-IMS[5]. It has biphasic (alternating current) pulse instead of monophasic (direct current), a slightly wider polyimide foil to accommodate the slightly larger chip, and is coated to avoid corrosion of the electrical insulation[27]. There are three main components: external power supply box carried by the patient, power transducer implanted subdermally, and subretinal silicone microchip coated with electrodes on one side. Power starts from the handheld control unit (power supply box) and wirelessly charges the power transducer[28]. The power then reaches the chip via polyimide foil which is connected to the subperiosteal induction coil by a silicone cable. The alpha IMS/AMS works by converting light into electrical stimulation directly in the eye, which has benefits and drawbacks as described previously (OptoEpiret section).
A clinical trial has been performed with alpha AMS with a cohort of 15 patients with follow-up up to 12 months[29]. Vision function prior to implantation was light perception without projection (14/15) or no light perception (1/15). Implant-mediated visual perception was seen in 13/15 patients, with the other two patients having damaged implants. In the visual tasks performed, detection (how many) and localization (where) of geometric shapes or table objects were improved with the device on, although identification of objects did not show significant improvement. While light perception was possible for most of the patients, only about half were able to localize light correctly. At 12 months, detection of grating orientation was improved in patients with implant on versus off, although at several prior timepoints, there was no statistical significance. Visual acuity was assessed in two patients with Landolt C-rings with measurements of 20/1111 and 20/546 Snellen visual acuity. Landolt C-rings consist of rings each with a gap facing varying directions and test the patient’s ability to determine the orientation of the gap at different distances.
Despite reaching a best visual acuity of 20/546 (1.43 logMAR equivalent), the alpha AMS still falls short of its expected potential visual acuity of 20/200 (1.0 logMAR equivalent) given the 1600-electrode array[5]. Previously, the alpha IMS was analyzed and its reported best visual acuity of 1.69 logMAR was found to correspond to 0.816 phosphenes per degree[30]. However, the alpha-IMS implant has 2.98 electrodes per degree and would therefore be expected to have a much better visual acuity[31]. The modifications to the alpha AMS improved the theoretical longevity of the device compared to the alpha IMS but did not significantly change the visual acuity.
In the cohort of 15 patients, eight SAEs were experienced in four patients, including conjunctival dehiscence (2 patients), postoperative movement of implant (2 patients), partial loss of silicone tamponade (1 patient), and pain in region of implant (1 patient)[29]. A recent study examining 27 alpha IMS implants and 8 alpha AMS implants found that 15/27 alpha IMS devices (56%) had displacement of the subretinal chip relative to the optic disc and 7/8 alpha AMS devices (87%) displaced[32]. The mean displacement in both cohorts was 0.66mm. This is likely attributable to the lack of fixation by a tack, unlike epiretinal implants. Functionally, displacement from the foveal region can result in decreased visual function, although the displacement observed is relatively small (less than 20% of the size of the chip).
Overall, the alpha AMS has a reasonable safety profile based on the trials, comparable to other retinal implants. The alpha AMS offers the advantage of allowing the patient to utilize natural eye movements, including pursuits and saccades, therefore requiring less extensive patient training. However, while it has demonstrated modest improvements in visual function, the visual acuity fell short of its theoretical potential. Unfortunately, due to patient dissatisfaction with the resulting visual performance of the implant, the company Retinal Implant AG dissolved in 2019 and alpha IMS and AMS are now discontinued[33].
PRIMA - Photovoltaic Retinal Prosthesis
PRIMA is a novel system developed by Pixium Vision aimed at improving visual acuity compared to other retinal prostheses by utilizing several advancements, including an architecture that allows for a ground grid to surround each stimulating electrode[6]. It is thought that one of the limiting factors preventing other implanted devices from attaining expected visual acuity based on pixel number is that the electrical current generated by the electrodes must travel to a distal ground, resulting in poor confinement of the electrical field[34,35]. The PRIMA system consists of an external camera mounted on a pair of glasses, a digital projector, a pocket computer, and a 378-pixel subretinal photovoltaic array.
The external camera on the glasses captures visual scenes in the environment and sends them to the pocket computer equipped with artificial intelligence, which processes and simplifies the images, extracting the useful information. These simplified images are sent back to the glasses where the digital projector projects the images using pulses of near-infrared radiation (NIR) onto the subretinal implant. Based on the signals received from the image analysis system of the computer, a specific laser pattern is projected onto the implant. This results in specific stimulation of photovoltaic cells in the implant, which converts the optical information into electrical stimulation to activate bipolar cells. The pulses of light are in the near-infrared spectrum (880–915nm) to prevent photophobic effects[36]. Since the initial capturing of the visual stimulus is by the external camera, the visual field responds only to patient head position instead of eye position, differing from natural vision, and therefore requires extensive training. Since the light is ultimately projected onto the subretinal implant, the patient requires cornea clarity and cataract removal for artificial vision.
In vitro, it was demonstrated that using NIR at the shortest stimulus (1ms) can activate retinal ganglion cells indirectly (presumably through bipolar cells), which was confirmed by blocking the synapse during stimulation which subsequently blocked retinal ganglion cell activation[6]. In rodents, this system showed potential in yielding high spatial resolutions[36], so a trial in non-human primates was conducted. In three macaques, implants were placed subretinally, after damage of the retinal photoreceptors using a vibratome[6]. This study tested the implants through direct stimulation using NIR light activation. Prior to implantation, the macaques were trained to perform a saccade detection task entailing gaze fixation on central spot followed by peripheral stimulation introduced within 9ms of removal of central target. After implantation, in 2/3 macaques, there were able to generate repeated successful saccades in response to NIR stimulations. Two years after implantation, the implant-induced responses were still present without change in function. In the third macaque, there was lack of any behavioral response to implant stimulation and further testing showed that it was not possible to activate that implant. Since this study tested the implant itself, without testing the entire system from the external camera to image processing and projection onto the implant, the efficacy of the entire system remains to be tested. While the findings in the study show potential for the device to assist with functional vision, it is difficult to extrapolate the findings to visual function or acuity in humans.
PRIMA is a novel system developed with the goal of attaining better visual acuity than current retinal implants can achieve, using a ground grid architecture surrounding each electrode and NIR activation of photovoltaic electrodes. Since it relies on direct stimulation of the implant by the NIR light, it requires a clear cornea, lens, and vitreous. Safety and feasibility of the implant in humans remains to be established, although the clinical trials are currently ongoing for both the original feasibility study in France and new trials in Pittsburgh to assess this. Further studies beyond the initial feasibility trials will be required to demonstrate efficacy in improving visual function and acuity.
Suprachoroidal Implants
Bionic Vision Australia
Bionic Vision Australia created a novel suprachoroidal retinal prosthesis. The implant is placed between the sclera and choroid, meaning that the surgery doesn’t breach retinal tissue and therefore doesn’t require vitrectomy or incisions into retina. This location was also chosen to produce a more anatomically stable location for implantation. However, as a result of its location, the electrodes are 250–400uM further from target retinal ganglion cells than in an epiretinal implant[37]. Initial testing showed that the electrode array, even at that distance, was still able to produce cortical responses[38]. The setup used in the first-in-human trial consists of a head-mounted video camera and the 20-stimulating electrode implant, which is connected to two large return electrodes and a third (remote) electrode via helical lead wire and percutaneous connector implanted behind the ear[39]. In this trial, light localization testing using four quadrants was improved with device on versus off and Landolt-C task gave estimated 2.62 LogMAR visual acuity on average (20/8397 Snellen equivalent). All three patients developed combined subretinal and suprachoroidal hemorrhages after procedure. In the subsequent 12-month monitoring period, all three electrode arrays remained functional.
Given the promising results from the prototype implant, Bionic Vision Australia developed an upgraded 44-channel suprachoroidal retinal prosthesis to provide a wider field of view[40]. Testing of the new implant was initially performed in 10 felines with four cases of surgical or stability complications and eight demonstrating safe electrode array insertion, which enabled continuation to clinical trial. Four patients with light-perception only were implanted and showed improved ability to discriminate phosphenes in central vs peripheral locations with the device on vs off as well as improved touch precision in square localization task in two of the patients[4]. The trial is still ongoing, so long-term safety of the implant and visual function are to be determined.
Overall, Bionic Vision Australia has demonstrated that the suprachoroidal implant is feasible. Utilizing an external camera for images sent to the implant means that, like some existing epiretinal implants, vision is controlled by head movement instead of natural eye movement, requiring extensive patient training[8]. The initial device with only 20 stimulating electrodes had a very low ceiling for potential visual acuity, and while 44 channels is an improvement, it will still likely limit the potential visual acuity. Further trials will need to be conducted to evaluate safety and efficacy of the device.
Cortical implants
Orion Cortical Implant
Orion, created by Second Sight (developers of Argus II), is a subdural implant that directly stimulates the visual cortex[7]. It includes the same camera, video processing unit, and transmitter coil as the Argus II. The implantable component consists of a receiver coil and internal circuit that sits on the skull as well as the subdural electrode grid with 60 electrodes placed on the medial occipital lobe. In theory, this setup will work similarly as the Argus II, except the electrical pulses from the video processing unit will be transmitted to the cortex instead of retina. This should allow for broader application in patients with significant inner retina and/or optic nerve degeneration.
The implant was initially tested in a single blind patient using a neurostimulator with no clinical complications and phosphenes were able to be elicited by stimulation. After safety was demonstrated in that case, an ongoing follow-up study was initiated with implantations in five blind patients, all of whom reported perceptions of phosphenes when implants were stimulated afterwards. The external components of the system have not yet been tested with the implant in patients. Like the Argus II, usage of external camera for vision will utilize head movement instead of natural eye movement and require significant patient training in its use. The Orion cortical implant has potential for use in a broader scope of application than existing retinal implants but is in early stages of clinical testing and still requires long-term safety and efficacy to be established in clinical trials.
Conclusion
The epiretinal implants were the first retinal implants tested and rely on an external camera to deliver the electrical signals to the implant. A similar approach is used in the suprachoroidal implant. In doing so, the cornea and lens are bypassed, so pathology causing opacities anterior to the retina do not affect the artificial vision. However, since the camera is mounted on a pair of glasses, the image only changes with head movement instead of natural eye movement[17,41]. On the other hand, implants that are activated through direct light activation (alpha IMS/AMS) require light to reach the implant for electrical stimulation, so any opacities anterior to the retina can block the implant stimulus. In doing so, patient’s natural eye movements can be used, such as pursuits and saccades, including microsaccades to prevent image fading[24,25]. The PRIMA implant uses an external camera, so it requires head movement, but the glasses project near-infrared radiation to the subretinal implant, so any opacities anterior to the retina can still block the implant stimulus.
There is still much to be understood about how the specifics of an implant affect the resulting visual acuity and the alpha IMS/AMS project has demonstrated that the solution is not as simple as maximizing the number of electrodes. Ongoing clinical trials will answer many of these questions, particularly in how changing the architecture of the chip to include surrounding ground grid around electrodes will affect visual acuity. As the implants continue to improve, visual acuity achieved in patients will improve, and additional visual functions can be examined. In fact, groups have already started studying ways to modify the Argus II camera to provide additional functions, such as thermal-sensing capabilities[42] or using two head-mounted cameras to enable depth discrimination[43].
Retinal prostheses have the potential to restore vision in patients with otherwise irreversible loss of vision. While the requirement for functional retinal ganglion cells means certain conditions like glaucoma or retinal artery occlusion cannot currently be managed with most of these systems, prostheses that target more upstream pathways such as the optic nerve, lateral genicular nucleus, or visual cortex may someday be able to treat those conditions[44,45]. There is a current trial for the Orion implant which provides direct occipital lobe stimulation. Optoelectronic devices in their application for vision restoration are already helping patients regain visual function and this technology is still in its infancy. With time, they will likely be able to improve vision across many etiologies of vision loss and provide enhanced visual function and acuity.
Funding
This research is funded in part by NIH Center Core Grants P30 EY001319 (Bethesda, Maryland), Research to Prevent Blindness Unrestricted Grant (New York, New York).
Ajay E. Kuriyan reports a grant from Second Sight for a clinical trial, a grant from Genentech, and personal fees from Allergen, Genentech, Regeneron, Bausch Health, and Alimera Sciences.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest
Victor Wang declares no potential conflicts of interest.
Human and animal rights:
This article does not contain any studies with human or animal subjects performed by any of the authors
References
- [1].Dowling J Current and future prospects for optoelectronic retinal prostheses. Eye 2009;23:1999–2005. 10.1038/eye.2008.385. [DOI] [PubMed] [Google Scholar]
- [2].Wang AL, Knight DK, Vu TT, Mehta MC. Retinitis Pigmentosa: Review of Current Treatment. Int Ophthalmol Clin 2019;59:263–80. 10.1097/IIO.0000000000000256. [DOI] [PubMed] [Google Scholar]
- [3].Frontiers | The Argus-II Retinal Prosthesis Implantation; From the Global to Local Successful Experience | Neuroscience n.d https://www.frontiersin.org/articles/10.3389/fnins.2018.00584/full (accessed February 14, 2020). [DOI] [PMC free article] [PubMed]
- [4].Petoe MA, Titchener SA, Shivdasani MN, Nayagam DA, Epp SB, Villalobos J, et al. A 44 channel suprachoroidal retinal prosthesis: initial psychophysical results. Invest Ophthalmol Vis Sci 2019;60:4993–4993. [Google Scholar]
- [5].Assessment of the Electronic Retinal Implant Alpha AMS in Restoring Vision to Blind Patients with End-Stage Retinitis Pigmentosa n.d https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5818267/ (accessed February 16, 2020). [DOI] [PMC free article] [PubMed]
- [6]. Prévot P-H, Gehere K, Arcizet F, Akolkar H, Khoei MA, Blaize K, et al. Behavioural responses to a photovoltaic subretinal prosthesis implanted in non-human primates. Nat Biomed Eng 2019. 10.1038/s41551-019-0484-2. [DOI] [PubMed] [Google Scholar]; This study demonstrated safety and potential improvement in visual function of PRIMA in non-human primates, up to two years after implantation. In doing so, it paved the way for initial clinical trials to be performed.
- [7].Niketeghad S, Pouratian N. Brain Machine Interfaces for Vision Restoration: The Current State of Cortical Visual Prosthetics. Neurotherapeutics 2019;16:134–43. 10.1007/s13311-018-0660-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Markowitz M, Rankin M, Mongy M, Patino BE, Manusow J, Devenyi RG, et al. Rehabilitation of lost functional vision with the Argus II retinal prosthesis. Can J Ophthalmol 2018;53:14–22. 10.1016/j.jcjo.2017.12.001. [DOI] [PubMed] [Google Scholar]
- [9].Duncan JL, Richards TP, Arditi A, da Cruz L, Dagnelie G, Dorn JD, et al. Improvements in vision-related quality of life in blind patients implanted with the Argus II Epiretinal Prosthesis. Clin Exp Optom 2017;100:144–50. 10.1111/cxo.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Humayun MS, Dorn JD, Cruz L da, Dagnelie G, Sahel J-A, Stanga PE, et al. Interim Results from the International Trial of Second Sight’s Visual Prosthesis. Ophthalmology 2012;119:779–88. 10.1016/j.ophtha.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dagnelie G, Jeter PE, Adeyemo K, Rozanski C, Nkodo A-F, Massof RW. Psychometric properties of the PLoVR ultra-low vision (ULV) questionnaire. Invest Ophthalmol Vis Sci 2014;55:2150–2150. [Google Scholar]
- [12].One-Year Safetyand Performance Assessment of the Argus II Retinal Prosthesis: A Postapproval Study | Medical Devices and Equipment | JAMA Ophthalmology | JAMA Network n.d https://jamanetwork.com/journals/jamaophthalmology/article-abstract/2734219 (accessed February 15, 2020). [DOI] [PMC free article] [PubMed]
- [13].da Cruz L, Dorn JD, Humayun MS, Dagnelie G, Handa J, Barale P-O, et al. Five-year safety and performance results from the Argus II Retinal Prosthesis System clinical trial. Ophthalmology 2016;123:2248–54. 10.1016/j.ophtha.2016.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Lin T-C, Wang L-C, Yue L, Zhang Y, Falabella P, Zhu D, et al. Histopathologic Assessment of Optic Nerves and Retina From a Patient With Chronically Implanted Argus II Retinal Prosthesis System. Transl Vis Sci Technol 2019;8:31–31. 10.1167/tvst.8.3.31. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study examined the retinal histology of an Argus II-implanted patient, finding increased optic nerve atrophy, but no increased damage in the location of the retina corresponding to the array itself, consistent with long-term safety.
- [15]. Seuthe AM, Haus A, Januschowski K, Szurman P. First Simultaneous Explantation and Re-Implantation of an Argus II Retinal Prosthesis System. Ophthalmic Surg Lasers Imaging Retina 2019;50:462–5. 10.3928/23258160-20190703-10. [DOI] [PubMed] [Google Scholar]; This study demonstrated the feasibility of exchanging an epiretinal implant (Argus II) as well as the extensive surgical procedure and risks involved in doing so.
- [16].Rizzo S, Barale P-O, Ayello-Scheer S, Devenyi RG, Delyfer M-N, Korobelnik J-F, et al. ADVERSE EVENTS OF THE ARGUS II RETINAL PROSTHESIS: Incidence, Causes, and Best Practices for Managing and Preventing Conjunctival Erosion. Retina Phila Pa 2020;40:303–11. 10.1097/IAE.0000000000002394. [DOI] [PubMed] [Google Scholar]
- [17].Sommerhalder J, Pérez Fornos A. Prospects and Limitations of Spatial Resolution In: Gabel VP, editor. Artif. Vis. Clin. Guide, Cham: Springer International Publishing; 2017, p. 29–45. 10.1007/978-3-319-41876-6_4. [DOI] [Google Scholar]
- [18].Argus II Retinal Prosthesis System Dry AMD Feasibility Study Protocol - Full Text View - ClinicalTrials.gov n.d. https://clinicaltrials.gov/ct2/show/NCT02227498 (accessed February 25, 2020).
- [19]. Muqit MMK, Velikay-Parel M, Weber M, Dupeyron G, Audemard D, Corcostegui B, et al. Six-Month Safety and Efficacy of the Intelligent Retinal Implant System II Device in Retinitis Pigmentosa. Ophthalmology 2019;126:637–9. 10.1016/j.ophtha.2018.11.010. [DOI] [PubMed] [Google Scholar]; This clinical trial of IRIS II demonstrated reasonable safety at 6 months with improved visual function in several metrics, although follow-up studies will need to be performed to demonstrate improvements in visual acuity.
- [20].Gallego G, Delbruck T, Orchard G, Bartolozzi C, Taba B, Censi A, et al. Event-based Vision: A Survey. ArXiv190408405 Cs 2019. [DOI] [PubMed] [Google Scholar]
- [21].Surgical feasibility and biocompatibility of the OptoEpiret retinal stimulator | IOVS | ARVO Journals n.d https://iovs.arvojournals.org/article.aspx?articleid=2744395 (accessed February 17, 2020).
- [22].Waschkowski F, Hesse S, Rieck AC, Lohmann T, Brockmann C, Laube T, et al. Development of very large electrode arrays for epiretinal stimulation (VLARS). Biomed Eng OnLine 2014;13:11 10.1186/1475-925X-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schaffrath K, Kokozinski R, Waschkowski F, Viga R, Mokwa W, Raffelberg P, et al. Biocompatibility of photodiode structures used for epiretinal prosthesis extended by an integrated epiretinal recording (OPTO-EPIRET). IOVS n.d [Google Scholar]
- [24].Zrenner E, Bartz-Schmidt KU, Besch D, Gekeler F, Koitschev A, Sachs HG, et al. The Subretinal Implant ALPHA: Implantation and Functional Results In: Gabel VP, editor. Artif. Vis. Clin. Guide, Cham: Springer International Publishing; 2017, p. 65–83. 10.1007/978-3-319-41876-6_6. [DOI] [Google Scholar]
- [25].Hafed ZM, Stingl K, Bartz-Schmidt K-U, Gekeler F, Zrenner E. Oculomotor behavior of blind patients seeing with a subretinal visual implant. Vision Res 2016;118:119–31. 10.1016/j.visres.2015.04.006. [DOI] [PubMed] [Google Scholar]
- [26].Stingl K, Bartz-Schmidt KU, Besch D, Braun A, Bruckmann A, Gekeler F, et al. Artificial vision with wirelessly powered subretinal electronic implant alpha-IMS. Proc Biol Sci 2013;280:20130077 10.1098/rspb.2013.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Daschner R, Greppmaier U, Kokelmann M, Rudorf S, Rudorf R, Schleehauf S, et al. Laboratory and clinical reliability of conformally coated subretinal implants. Biomed Microdevices 2017;19:7 10.1007/s10544-017-0147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shepherd RK, Shivdasani MN, Nayagam DAX, Williams CE, Blamey PJ. Visual prostheses for the blind. Trends Biotechnol 2013;31:562–71. 10.1016/j.tibtech.2013.07.001. [DOI] [PubMed] [Google Scholar]
- [29]. Stingl K, Schippert R, Bartz-Schmidt KU, Besch D, Cottriall CL, Edwards TL, et al. Interim Results of a Multicenter Trial with the New Electronic Subretinal Implant Alpha AMS in 15 Patients Blind from Inherited Retinal Degenerations. Front Neurosci 2017;11 10.3389/fnins.2017.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]; This clinical trial testing alpha AMS demonstrated that while there were improvements to vision function and measurable visual acuity, the device did not meet expectations. Ultimately, the results from this trial are what led to the discontinuation of the alpha IMS/AMS devices.
- [30].Subretinal electronic chips allow blind patients to read letters and combine them to words | Proceedings of the Royal Society B: Biological Sciences n.d https://royalsocietypublishing.org/doi/full/10.1098/rspb.2010.1747 (accessed February 16, 2020). [DOI] [PMC free article] [PubMed]
- [31].Eiber CD, Lovell NH, Suaning GJ. Attaining higher resolution visual prosthetics: a review of the factors and limitations. J Neural Eng 2013;10:011002 10.1088/1741-2560/10/1/011002. [DOI] [PubMed] [Google Scholar]
- [32]. Kuehlewein L, Troelenberg N, Stingl K, Schleehauf S, Kusnyerik A, Jackson TL, et al. Changes in microchip position after implantation of a subretinal vision prosthesis in humans. Acta Ophthalmol (Copenh) 2019;97:e871–6. 10.1111/aos.14077. [DOI] [PubMed] [Google Scholar]; This study demonstrated a potential downside to subretinal implants compared to epiretinal implants. The lack of tack fixation in subretinal implants allows for displacement to occur over time, which may affect the long-term visual function and acuity of patients with these implants.
- [33].Retina Implant - Your Expert for retinitis pigmentosa - Retina Implant n.d https://www.retina-implant.de/en/ (accessed February 16, 2020).
- [34].Improved Focalization of Electrical Microstimulation Using Microelectrode Arrays: A Modeling Study n.d https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0004828 (accessed February 16, 2020). [DOI] [PMC free article] [PubMed]
- [35].Bendali A, Rousseau L, Lissorgues G, Scorsone E, Djilas M, Dégardin J, et al. Synthetic 3D diamond-based electrodes for flexible retinal neuroprostheses: Model, production and in vivo biocompatibility. Biomaterials 2015;67:73–83. 10.1016/j.biomaterials.2015.07.018. [DOI] [PubMed] [Google Scholar]
- [36].Lorach H, Goetz G, Smith R, Lei X, Mandel Y, Kamins T, et al. Photovoltaic restoration of sight with high visual acuity. Nat Med 2015;21:476–82. 10.1038/nm.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ayton LN, Guymer RH, Luu CD. Choroidal thickness profiles in retinitis pigmentosa. Clin Experiment Ophthalmol 2013;41:396–403. 10.1111/j.1442-9071.2012.02867.x. [DOI] [PubMed] [Google Scholar]
- [38].Cicione R, Shivdasani MN, Fallon JB, Luu CD, Allen PJ, Rathbone GD, et al. Visual cortex responses to suprachoroidal electrical stimulation of the retina: effects of electrode return configuration. J Neural Eng 2012;9:036009 10.1088/1741-2560/9/3/036009. [DOI] [PubMed] [Google Scholar]
- [39].Ayton LN, Blamey PJ, Guymer RH, Luu CD, Nayagam DAX, Sinclair NC, et al. First-in-Human Trial of a Novel Suprachoroidal Retinal Prosthesis. PLoS ONE 2014;9 10.1371/journal.pone.0115239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Abbott CJ, Nayagam DAX, Luu CD, Epp SB, Williams RA, Salinas-LaRosa CM, et al. Safety Studies for a 44-Channel Suprachoroidal Retinal Prosthesis: A Chronic Passive Study. Invest Ophthalmol Vis Sci 2018;59:1410–24. 10.1167/iovs.17-23086. [DOI] [PubMed] [Google Scholar]; This study demonstrated the safety of implantation for the novel Bionic Vision Australia suprachoroidal implant in felines and was a necessary step towards starting the current ongoing clinical trials.
- [41].Retinotopic to Spatiotopic Mapping in Blind Patients Implanted With the Argus II Retinal Prosthesis. - PubMed - NCBI n.d https://www.ncbi.nlm.nih.gov/pubmed/28114567 (accessed February 17, 2020). [DOI] [PubMed]
- [42].He Y, Huang NT, Caspi A, Roy A, Montezuma SR. Trade-Off Between Field-of-View and Resolution in the Thermal-Integrated Argus II System. Transl Vis Sci Technol 2019;8:29–29. 10.1167/tvst.8.4.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sadeghi R, Barry M, Gibson P, Caspi A, Roy A, Dagnelie G. Depth discrimination in Argus II wearers using a stereo sensor based on two head-mounted cameras. Invest Ophthalmol Vis Sci 2019;60:4975–4975. [Google Scholar]
- [44].Pezaris JS, Reid RC. Demonstration of artificial visual percepts generated through thalamic microstimulation. Proc Natl Acad Sci U S A 2007;104:7670–5. 10.1073/pnas.0608563104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Brelén ME, De Potter P, Gersdorff M, Cosnard G, Veraart C, Delbeke J. Intraorbital implantation of a stimulating electrode for an optic nerve visual prosthesis. Case report. J Neurosurg 2006;104:593–7. 10.3171/jns.2006.104.4.593. [DOI] [PubMed] [Google Scholar]


