Abstract
Background:
Healthy gait dynamics are characterized by the presence of fractal, persistent stride-to-stride variations, which become more random with Parkinson’s disease (PD). Rhythmic auditory stimulation with fractal beat-to-beat variations can change gait dynamics in people with PD toward more persistence.
Research Question:
How does gait in people with PD change when synchronizing steps with fractal melodic metronomes with different step-to-beat ratios, and which stimulus do they prefer?
Methods:
In this cross-sectional study, 15 people with PD and 15 healthy older adults walked over-ground in three conditions: self-paced, paced by a fractal auditory stimulus with a 1:1 step-to-beat ratio (‘metronome’), and fractal auditory stimulus with a 1:2 step-to-beat ratio (‘music’). Gait dynamics were recorded with instrumented insoles, and detrended fluctuation analysis (DFA) was applied to the series of stride time intervals. Stimuli preference was assessed using Likert-like scales and open-ended questions. ANOVAs were used to compare mean, coefficient of variation, α-DFA, and the responses from the continuous Likert scales. Pearson correlations were used to assess the relationship between ‘music’ and ‘metronome’ enjoyment or difficulty with gait outcomes, and to determine the association between baseline α-DFA and changes due to the stimuli.
Results:
Our major findings are that i) stride-to-stride variations were more persistent with the ‘metronome’ compared to baseline for both groups, ii) the effect was greater for people with lower α-DFA at baseline (i.e., more random stride-to-stride variations), and iii) both groups found the ‘metronome’ less difficult to synchronize with.
Significance:
This study showed that people with PD and healthy older adults walk with higher statistical persistence in their stride-to-stride variations when instructed to synchronize their steps with a fractal stimulus. Participants with lower persistence at baseline benefited the most from the fractal ‘metronome’, highlighting the importance to develop patient-centered tests and interventions.
Keywords: fractal fluctuations, gait variability, Parkinson’s disease, rhythmic auditory stimulation
Introduction
Humans spontaneously synchronize their movements to auditory rhythms such as metronomes or music [1]. Rhythmic entrainment can guide step timing in people with Parkinson’s disease (PD), who show deficient internal rhythmicity [2–4]. People with PD synchronizing their steps to metronomes or music tend to increase their stride length and gait speed, and to reduce their stride time variability. Rhythmic auditory stimulations (RAS) are typically based on isochronous signals, with the exact same time interval between tones. However, healthy human gait presents variations that are temporally structured [5–9], which can be measured for example by the scaling exponent α from the detrended fluctuation analysis (α-DFA) [10]. Healthy locomotion is characterized by persistent stride-to-stride variations, i.e., large variations are more likely to be followed by larger variations, and vice-versa. The presence of persistent stride-to-stride variations suggests that the locomotor system preserves a long-term ‘memory’ that extend over hundreds of strides in a scale-free, fractal-like manner.
People with PD present stride-to-stride variations that are less persistent and more random [11–18], with stride-to-stride randomness correlating with disease severity [17]. If fractal variations reflect locomotor system’s health and adaptive capacity, as suggested by theories of complex adaptive systems [19–21], gait rehabilitation should aim to increase the persistence of stride-to-stride variations. Stride-to-stride variations can be manipulated toward more randomness or more persistence when the inter-beat intervals present random or persistent variations, respectively [22–24]. People with PD are able to synchronize with fractal RAS, and to shift their stride-to-stride variations away from randomness toward more persistence. Despite promising results, many questions remain unaddressed to use fractal RAS in PD. Notably, RAS are typically presented in a 1:1 step-to-sound ratio, i.e., participants synchronize steps to every sound they hear. A sub-division benefit has been shown in the context of basic sensorimotor synchronization (finger tapping) [1]: the presence of an additional sound between two beats (i.e., 1:2 step-to-beat ratio) improves synchronization accuracy and reduces movement variability. It is unknown if such an effect may be present in fractal RAS for PD gait. Another limitation of previous studies is that participant’s feedback was rarely collected, but it is important to consider patient-centered outcomes about the stimuli to develop effective interventions.
In the present study, we compared two types of fractal RAS in people with PD and healthy older adults, namely 1:1 step-to-beat ratio (referred to in the following as ‘metronome’), and 1:2 step-to-beat ratio (referred to in the following as ‘music’). We also investigated participants’ perception of enjoyment, difficulty and preference for the two stimuli. Our hypotheses were that both stimuli will increase persistence of stride-to-stride variations, in particular for the PD group with ‘music’, and that higher enjoyment will be reported when synchronizing steps with ‘music’.
1. Methods
We recruited 15 people with PD and 15 healthy older adults (OA). Based on a previous study [13], a power analysis indicated that 12 subjects per group were needed to detect statistically significant differences between groups, with an alpha value of 0.05 at 80 % power. The study was approved by the University of Nebraska Medical Center’s Institutional Review Board and all participants provided written informed consent prior to participation.
Participants with PD were instructed to visit the laboratory during their self-reported ‘on-state’. All subjects were able to walk independently and unassisted for 15 minutes continuously at their own preferred walking speed. Exclusion from participation resulted if individuals had any known neurological disease (other than PD), orthopedic disease, lower extremity vascular disease, or cardiac disease determined by self-report. Other exclusion criteria included lower extremity injury or surgery within the past six months, experience of at least one fall or at least three slips or trips within the past 12 months, being legally deaf or blind, or scoring below 24 on the Mini-Mental State Examination (MMSE). In addition, PD participants were excluded if they had a deep-brain stimulator, a Hoehn & Yahr (H&Y) score greater than four, or if they scored above 16 on the Freezing of Gait Questionnaire (FoG-Q).
We first conducted a series of self-reported questionnaires that included a medical history, to confirm that eligibility criteria were met; Modified Falls Efficacy Scale (MFES), used to assess fear of falling, Geriatric Depression Scale (GDS), to assess mood state, and MMSE, to assess cognition status. We also conducted functional assessments including the Fullerton Advanced Balance scale (FAB), to test the multiple dimensions of balance; and Timed Up and Go test (TUG), to assess mobility. Demographics and results from these tests can be found in Table 1.
Table 1.
Demographics and results from questionnaires and tests.
| PD (N=14) | OA (N=14) | |
|---|---|---|
| Number of men/women | 10/4 | 7/7 |
| Age (years) | 68.00 ± 9.55 | 68.93 ± 11.24 |
| Height (cm) | 170.39 ± 12.48 | 171.23 ± 11.52 |
| Weight (kg) | 80.36 ± 13.57 | 74.87 ± 14.96 |
| Fullerton Advanced Balance scale | 34.64 ± 3.99* | 38.00 ± 2.25 |
| Timed Up and Go (s) | 9.47 ± 1.89 | 10.00 ± 2.24 |
| Mini-Mental State Examination | 28.62 ± 1.71 | 28.50 ± 1.56 |
| Geriatric Depression Scale | 1.42 ± 1.31* | 0.00 ± 0.00 |
| Modified Falls Efficacy Scale | 9.52 ± 0.68* | 10.00 ± 0.00 |
| Freezing of Gait score | 5.14 ± 4.20 | --- |
| Hoehn and Yahr scale | 1.71 ± 0.58 | --- |
Mean ± standard deviations for: PD, patients with Parkinson’s disease; OA, older adults.
p<0.05
After performing the questionnaires and assessments, pressure sensitive insoles (Noraxon USA Inc.; 1500 Hz) were placed in participants shoes. Participants were then given headphones and an iPod, which was fixed around the participants waist using a waist pack. Participants then performed three conditions of 15 minutes over-ground walking on a 200-meter indoor track. The conditions consisted of Baseline walking (no auditory stimulus), ‘Metronome’ walking (fractally structured melodic metronome with a 1:1 step-to-beat ratio), and ‘Music’ walking (fractally structured melodic metronome with a 1:1 step-to-beat ratio). Baseline walking was always performed first, during which participants wore the headphones with no stimuli, and were instructed to walk at their own preferred pace. During this condition, participating personnel counted the number of steps during 30 seconds at four minutes and again at 12 minutes. The two 30-second step counts were summed to obtain an estimate of steps per minutes over the full trial (i.e., stepping frequency).
The next two conditions, Metronome and Music walking, were presented in random order to prevent any learning effects. The stimuli were created as follows: first, a time series of step intervals from a healthy young participant (α-DFA = 1.00, CV=2%) was used as a reference. The Fur Elise melody was modified so that each tone presents the same duration of 200 ms. The melody was repeated until the stimulus lasts 15 min. The timing of the tones was then matched to the timing of the reference time series. This resulted in an ‘altered’ Für Elise melody with tones of equal length and fractal fluctuations in the inter-tone intervals (i.e., ‘fractal music’, α-DFA = 1.00). The fractal ‘metronome’ was simply built by removing one tone every two tones (α-DFA = 1.02). The average inter-tone intervals for the fractal ‘metronome’ matched participant’s individual inter-step intervals (determined from Baseline) to facilitate rhythmic entrainment to every tone. Similarly, the average inter-tone intervals for the fractal ‘music’ was two times faster than each participant’s individual inter-step intervals, to facilitate rhythmic entrainment to every two tones. Short samples of the auditory stimuli are available in Supplementary Data 1 and 2.
In the Metronome condition, participants were instructed to match their steps with each tone as best as possible. In the Music condition, they were instructed to match their steps to every other tone (Figure 1). Participants were given approximately 30 seconds of practice before each trial, to ensure they could hear the stimulus and understood the instructions. Participants were given at least 5 minutes rest to sit on a bench and to drink water. After the three conditions were performed, participants completed a questionnaire to assess their perception of different aspects of the stimulations.
Figure 1.

Illustration of the protocol for only a few steps. Participants were instructed to step in time with every sound in the Metronome condition (middle panel) or with every other sound of the Music condition (upper panel). The inter-tone intervals were not periodic but presented fractal, persistent fluctuations. Short samples of the auditory stimuli are available in Supplementary Data 1 and 2.
Data processing
Stride time intervals were extracted from the pressure sensitive insoles and identified as the difference between two consecutive stride times with custom Matlab©R2018a scripts. The first 50 stride intervals were removed to ensure further analyses were applied on steady-state walking periods. Further analyses were applied on time series of 450 stride intervals, which corresponded to the minimal longest time series from all participants in all conditions (Supplementary Data 3) [13–14, 25–26]. From each time series, we determined the mean, coefficient of variation (CV) and the scaling exponent (α-DFA). The scaling exponent was determined using the evenly-spaced DFA algorithm [27], which provides information about the degree of statistical persistence. For stationary time series, variations are persistent for 0.5 < α < 1 anti-persistent for 0 < α < 0.5, and random for α = 0.5. In this study, we used window sizes ranging from 10 to N/8, where N is the time series length.
Statistical analysis
Two-way (2 group x 3 conditions) ANOVAs were used to compare mean, CV and α-DFA. Two-way (2 group x 2 conditions) ANOVAs were used to compare the responses from the continuous Likert scales. Chi-Square test of association was use to compare the percentages of stimuli preference between groups. When a significant interaction was found, a Tukey post-hoc was used, and paired samples t-tests were used to differentiate the effect of experimental conditions. Greenhouse-Geisser correction were used when the assumption of sphericity was violated. Pearson-r correlations were used to assess the relationship between ‘music’ and ‘metronome’ enjoyment or difficulty with mean stride time, CV and α-DFA. To determine the association between baseline α-DFA and change due to the stimuli, we performed correlations between changes in α-DFA from baseline (in both conditions) and baseline α-DFA.
2. Results
All participants were able to complete the study protocol, but technical difficulties led to discarding data collected from the left pressure sensor for most participants. Data from the right sensor were also discarded for two participants (one PD and one OA). This led us to analyze data from the right sensor for 14 participants in each group.
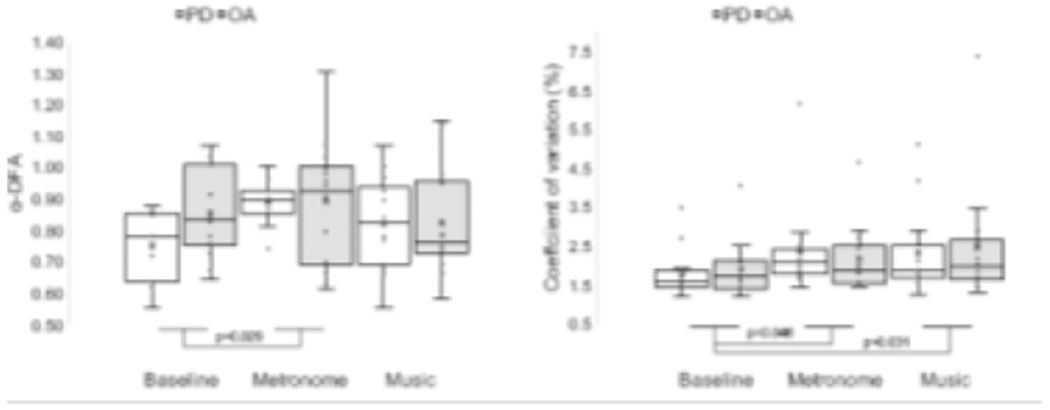
There were no significant differences between groups for mean, CV nor α-DFA (p>0.05; Table 3). There was a main effect of conditions for α-DFA (F (1.496,38.905)=4.069, p=0.035, η2=0.135) and CV (F (2,52)=3.689, p=0.032, η2=0.124). The α-DFA scaling exponents were significantly higher (t(27)=−2.308, p=0.029) with the ‘metronome’ as compared to baseline (Figure 2). The CV significantly increased from baseline with ‘metronome’ (t(27)=−2.096, p=0.046) and ‘music’ (t(27)=−2.282, p=0.031).
Table 3.
Mean (±SD) of α-DFA, coefficient of variation (CV), and mean of stride time series.
| PD | OA | |||||
|---|---|---|---|---|---|---|
| Baseline | Metronome | Music | Baseline | Metronome | Music | |
| Mean (s) | 1.08±0.09 | 1.10±0.10 | 1.08±0.09 | 1.08±0.14 | 1.07±0.15 | 1.07±0.14 |
| CV (%) | 1.79±0.61 | 2.34±1.16 | 2.31±1.09 | 1.90±0.74 | 2.19±0.85 | 2.46±1.55 |
| α-DFA | 0.76±0.11 | 0.89±0.07 | 0.82±0.14 | 0.86±0.14 | 0.89±0.20 | 0.82±0.17 |
Figure 2.
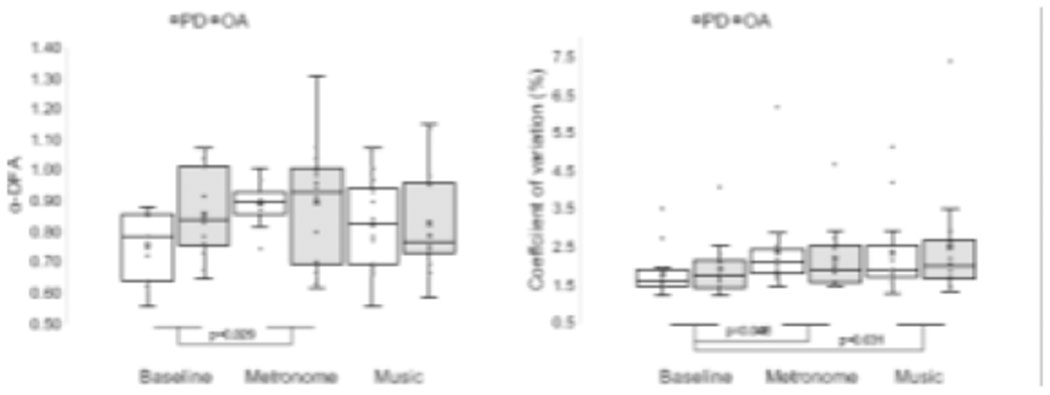
Effects of groups and conditions on α-DFA and coefficient of variations. Boxplots (exclusive median method) represent the following: ‘X’ the mean; line inside box the median; lower and upper box boundaries 25th and 75th percentiles, respectively; lower and upper error lines local minimum and maximum, respectively; values outside the box outliers, defined as lying 1.5 times the interquartile range from either end of the box.
Self-reported difficulty to synchronize steps to the stimuli was significantly higher in the Music condition (F (1,26)=14.025, p=0.001, η2=0.350). There was no difference between stimuli for the enjoyment items (F (1,26)= 1.761, p=0.196, η2=0.063). The relationship between stimulus preference and groups was not statistically significant (X2(1)=0.144, p=0.705): 50% of the OA group and 57.1% of the PD group preferred the ‘metronome’. There were no statistically significant correlations between ‘music’ and ‘metronome’ enjoyment nor difficulty and mean stride time, nor α-DFA, in any conditions. The only statistically significant correlation was observed between self-reported difficulty in the Metronome condition and stride-to-stride CV in the Metronome condition for the PD group (r(12)=0.690, p=0.006). There was also a significant negative correlation (Figure 3) between Baseline α-DFA and the difference between Metronome α-DFA and Baseline α-DFA for both groups (rPD(12)=−0.801, p<0.001; rOA(12)=−0.648, p<0.001). There were no correlations between Baseline α-DFA and the difference between Music α-DFA and Baseline α-DFA.
Figure 3.
Correlation between Baseline α-DFA and the difference between Metronome α-DFA and Baseline α-DFA for the PD group (left) and the OA group (right). Positive values on the y-axis indicate that α-DFA was higher with the fractal metronome compared to Baseline for a given individual. Note that for 6 OA, the values are negative, indicating that α-DFA was lower (i.e., more random) when walking with the fractal ‘metronome’
To the question ‘How would you make the stimulus more enjoyable?’, a recurrent answer was related to using ‘real’ or ‘better’ music, using music that participants enjoy, and more variety of music (Table 2). To the question ‘How would you change the stimulus if you had to walk to it every day for 15 minutes?’, many participants in both groups highlighted the need to change the music not only between days but also within day. Another important aspect was related to using real music, with a strong beat and of their own choice. A final aspect was related to the presence of a strong beat.
Table 2.
Self-report from participants about the stimuli. Questions 1 to 4 were rated on a continuous Likert scale which consisted in a horizontal line of 133 mm, where left corresponded to ‘No difficulty’ (Question 1 and 2) or ‘Not enjoyable’ (Question 3 and 4), and right corresponded to ‘Very difficult’ or ‘Very enjoyable’. The marks were converted into scores that corresponded to the length (in mm) from the left side. Questions 5 to 8 were open ended. The bullet points summarize the main answers.
| Music | Metronome | |
|---|---|---|
| Q1 & Q2: How difficult was synchronizing your steps to… |
PD: 64.07±29.83 [19-112] OA: 57.14±32.93 [6-100] |
PD: 36.14±34.06 [5-114] OA: 32.36±29.98 [2-88] |
| Q3 & Q4: How enjoyable was walking to… |
PD: 80.50±30.21 [19-127] OA: 86.57±26.49 [32-118] |
PD: 78.00±31.40 [22-117] OA: 76.07±30.61 [4-125] |
| Q5: Which stimulus did you prefer? |
PD: 6 (43%) OA: 7 (50%) |
PD: 8 (57%) OA: 7 (50%) |
| Q6: How would you make the stimuli more enjoyable? | • Less repeating/More variety of music (x3) • Real music (x3) • Little bit quicker (x2) • Better music (x2) • Steady pace (x2) • Familiar music (x2) • More music • More modern music • Full orchestra • Step up the beat |
|
| Q7: How would you change the stimuli if you had to walk to it every day for 15 minutes? | • Variety of music/Change everyday to avoid boredom(x7) • Real music (x5) • Make stimulus easier to synchronize with/Beat would be drums/Step up the beat (x3) • Wouldn’t/Like the pace (x2) • Faster • Up to date tunes • No music • More repeating tones • Steady pace • Song with words • Personal choice of music |
|
| Q8: Other comments? | • Good experience (x3) • Research team very helpful (x5) • Enjoyed learning about the process/ Very interesting (x2) • Would like to know how to improve walking based on personal data |
|
3. Discussion
In this study, we analyzed gait dynamics of people with Parkinson’s disease and older adults walking while synchronizing their steps with either a ‘metronome’ (i.e., 1:1 step-to-tone ratio) or a ‘music’ (i.e., 1:2 ratio) that presented fractal fluctuations in the inter-beat intervals. We were also interested in participants’ perception of the different stimuli. Our major findings are that i) stride-to-stride variations were more persistent with the ‘metronome’, but not with the ‘music’, compared to baseline for both groups, ii) the effect was greater for people with lower α-DFA at baseline (i.e., more random stride-to-stride variations), and iii) both groups found the ‘metronome’ less difficult to synchronize with. Overall, the results of this work show that improvements in stride-to-stride variations from the ‘metronome’ may be because it was easy to synchronize with, but participants would prefer to follow music that has a strong beat, and that they can choose.
We did not find any statistically significant differences between PD and OA groups for mean, CV nor α-DFA of stride time intervals. Previous research evidenced higher CV and lower α-DFA in PD patients [11–18]. While between-group differences from the ANOVA were not statistically significant, an ad-hoc t-test showed that α-DFA at Baseline was lower in the PD group compared to the OA group (t(27)= −2.1540, p=0.041), similar to another study from our group [13],
Our first hypothesis was not confirmed: α-DFA was greater than Baseline only in the Metronome condition. This result goes against our predictions that synchronizing steps with a fractal RAS would show a sub-division benefit. Based on participants reports, it is possible that the music stimulus did not present a strong enough beat, so they were not able to rely on a clear external source of timing. While we did not collect the step-to-tone synchronization, it is possible that synchronization was less accurate during Music compared to Metronome condition. Both stimuli increased stride time CV in both groups: CV is often defined as a measure of gait stability, and is typically higher in PD compared to controls [3]. Therefore, at first glance our results may indicate that both stimuli led to more instability. However, it is important to stress that in our study, stride time CV in the PD group was not different from the OA group (and in fact, was slightly lower). During Metronome and Music conditions, stride time CV increased but was still below 2.4%, which has been reported as the upper threshold to discriminate PD from controls [28–29]. Future studies should also investigate the effect of fractal stimuli on gait speed and cognitive load, which have been associated to increased CV of stride time.
We also observed a positive correlation between stride time CV in the Metronome condition and perceived difficulty in the same condition, only for the PD group. In contrast, the OA group showed a negative correlation between stride time CV and perceived difficulty, in both Metronome and Music conditions. While we do not have enough data to address this difference between groups, it is possible that participants in the OA group adopted a ‘posture-first’ strategy to increase postural stability (e.g., reducing stride time CV) when facing a challenging situation. Participants in the PD group may show a different strategy, by walking with increased instability in challenging conditions.
The ‘metronome’ did not affect all participants equally: PD participants with lower Baseline α-DFA benefited the most from the ‘metronome’. This is evidenced by the negative correlation between Baseline α-DFA and the change in α-DFA from Baseline to Metronome. The OA group also showed a similar (but slightly lower) correlation. However, six out the 14 OA participants also showed a decrease in α-DFA during Metronome condition, while only one PD participant showed a decrease in α-DFA from Baseline to Metronome (and this participant already had a high DFA value of 0.86). This result is important, because participants with lower α-DFA (i.e., more random stride-to-stride variations) are more likely to experience balance impairments leading to falls [17]. This result also shows that it is possible to impact stride-to-stride variations with the use of RAS even in the most affected patients. Similarly, it suggests that fractal RAS may not be useful for participants with high Baseline α-DFA. This is in line with other studies supporting the importance to develop patient-centered interventions aimed at changing stride-to-stride variations in people with PD (e.g., treadmill walking [18]).
Our second hypothesis was not confirmed: both groups found the ‘metronome’ and the music equally enjoyable to walk with. What is remarkable is the very large range of answers, once again suggesting that it is crucial to take the patient’s perspective into account when developing interventions. In contrast, both groups found the ‘metronome’ less difficult to synchronize with, likely because it presented a more salient beat compared to our music stimulus, as discussed earlier. Importantly, across all participants, there was a positive correlation between enjoyability for the ‘metronome’ and enjoyability for the music (r(26)=0.615, p<0.001). This suggests that participants who liked (or disliked) one stimulus also liked (or disliked) the other stimulus. The aggregated results from the open-ended questions are in line with previous research [30] suggesting that participants would prefer auditory stimuli characterized by a variety of music (as opposed to ‘metronome’), with a strong beat, and that they are familiar with.
This study presents several limitations. A major outcome missing from this study is a measure of step-to-tone synchronization, needed to confirm that participants were able to step in time with the stimuli. Despite this limitation, our results clearly show that the Metronome condition significantly impacted gait variability. Another limitation is related to the nature of the auditory stimuli: we chose an altered version of the Fur Elise melody, based on previous studies from our group, but this melody lacks a strong beat which may have limited rhythmic entrainment, in particular during the Music condition. Finally, we did not compare the fractal RAS to isochronous RAS (i.e., with no variations) or random RAS (i.e., with uncorrelated variations). However, fractal analysis of gait variability requires around 500 gait cycles [25–26], which takes about 10-15 min to collect. Participants already walk three times 15 minutes, and it would not be reasonable to add more conditions without inducing potential fatigue effects or reduced effect of medications for the PD group.
4. Conclusion
This study showed that PD patients and age-matched controls walked with higher statistical persistence in their stride-to-stride variations when synchronizing their steps with a fractal ‘metronome’. Participants with lower persistence at baseline benefited the most from the fractal ‘metronome’, highlighting the importance to develop patient-centered tests and interventions. Future studies are needed to determine the long-term effects of fractal metronomes on stride-to-stride variations, to determine the relationship between stride-to-stride variations and adaptive behaviors, and to determine the potential neural changes occurring during and after synchronization with fractal metronomes.
Supplementary Material
Highlights.
People with and without Parkinson’s disease synchronized steps with audio cues
The step-to-tone ratio was either 1:1 (metronome) or 1:2 (music)
The audio cues presented fractal inter-tone variations
Stride time variations were more fractal for both groups with the metronome
Changes were greater for people with more random stride time variations at baseline
Acknowledgments
The authors thank Connor Wicks for his help during data collection. This work was supported by the UNMC Skate-a-thon for Parkinson’s fund, the NASA Nebraska Space Grant Fellowship, and the Center for Research in Human Movement Variability of the University of Nebraska at Omaha, NIH(P20GM109090). The study sponsors were not involved in the collection, analysis and interpretation of data, in the writing of the manuscript, nor in the decision to submit the manuscript for publication. The views expressed are those of the authors and not necessarily those of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declared no potential conflict of interest with respect to the research, authorship, and publication of this article. This manuscript has not been published and is not under consideration for publication elsewhere.
References
- 1.Repp BH, Su Y (2013). Sensorimotor synchronization: A review of recent research (2006–2012). Psychon Bull Rev 20, 403–452. 10.3758/s13423-012-0371-2 [DOI] [PubMed] [Google Scholar]
- 2.Ashoori A, Eagleman DM, Jankovic J (2015). Effects of Auditory Rhythm and Music on Gait Disturbances in Parkinson’s Disease. Front Neurol, 6, 234 10.3389/fneur.2015.00234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghai S, Ghai I, Schmitz G, Effenberg AO (2018). Effect of rhythmic auditory cueing on parkinsonian gait: A systematic review and meta-analysis. Sci Rep, 8(1), 506 10.1038/s41598-017-16232-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calabrò RS, Naro A, Filoni S, Pullia M, Billeri L, Tomasello P, et al. (2019). Walking to your right music: a randomized controlled trial on the novel use of treadmill plus music in Parkinson’s disease. J Neuroeng Rehabil, 16(1), 68 10.1186/s12984-019-0533-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hausdorff JM, Peng CK, Ladin Z, Wei JY, and Goldberger AL (1995). Is walking a random walk? Evidence for long-range correlations in the stride interval of human gait. J. Appl. Physiol. 78, 349–358. doi: 10.1152/jappl.1995.78.1.349 [DOI] [PubMed] [Google Scholar]
- 6.Dingwell JB, Cusumano JP (2010). Re-interpreting detrended fluctuation analyses of stride-to-stride variability in human walking. Gait Posture 32, 348–353. doi: 10.1016/j.gaitpost.2010.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terrier P, Turner V, Schutz Y (2005). GPS analysis of human locomotion: Further evidence for long-range correlations in stride-to-stride fluctuations of gait parameters. Hum Mov Sci, 24, 97–115. doi: 10.1016/j.humov.2005.03.002 [DOI] [PubMed] [Google Scholar]
- 8.Pierrynowski MR, Gross A, Miles M, Galea V, McLaughlin L, McPhee C (2005). Reliability of the long-range power-law correlations obtained from the bilateral stride intervals in asymptomatic volunteers whilst treadmill walking. Gait Posture 22, 46–50. doi: 10.1016/j.gaitpost.2004.06.007 [DOI] [PubMed] [Google Scholar]
- 9.Marmelat V, Duncan A, Meltz S (2019). Effect of sampling frequency on fractal fluctuations during treadmill walking. PloS ONE, 14(11), e0218908 10.1371/journal.pone.0218908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng CK, Mietus J, Hausdorff JM, Havlin S, Stanley HE, Goldberger AL (1993). Long-range anticorrelations and non-Gaussian behavior of the heartbeat. Phys Rev Lett, 70, 1343–1346. doi: 10.1103/PhysRevLett.70.1343 [DOI] [PubMed] [Google Scholar]
- 11.Hausdorff JM (2007). Gait dynamics, fractals and falls: Finding meaning in the stride-tostride fluctuations of human walking. Hum Mov Sci 26, 555–589. doi: 10.1016/j.humov.2007.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hove MJ, Suzuki K, Uchitomi H, Orimo S, Miyake Y (2012). Interactive rhythmic auditory stimulation reinstates natural 1/f timing in gait of Parkinson’s patients. PLoS ONE 7(3), e32600. doi: 10.1371/journal.pone.0032600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marmelat V, Reynolds NR, Hellman A (2018). Gait dynamics in Parkinson’s disease: short gait trials “stitched” together provide different fractal fluctuations compared to longer trials. Front Physiol 9:861. doi: 10.3389/fphys.2018.00861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marmelat V, Meidinger RL (2019). Fractal analysis of gait in people with Parkinson’s disease: three minutes is not enough. Gait Posture, 70, 229–234. 10.1016/j.gaitpost.2019.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ota L, Uchitomi H, Ogawa KI, Orimo S, Miyake Y (2014). Relationship between Neural Rhythm Generation Disorders and Physical Disabilities in Parkinson’s Disease Patients’ Walking. PLoS ONE 9(11), e112952. doi: 10.1371/journal.pone.0112952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uchitomi H, Ota L, Ogawa KI, Orimo S, Miyake Y (2013). Interactive rhythmic cue facilitates gait relearning in patients with Parkinson’s disease. PLoS ONE 8, e72176. doi: 10.1371/journal.pone.0072176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warlop T, Detrembleur C, Bollens B, Stoquart G, Crevecoeur F, Jeanjean A, et al. (2016). Temporal organization of stride duration variability as a marker of gait instability in Parkinson’s disease. J Rehab Med, 48, 865–871. doi: 10.2340/16501977-2158 [DOI] [PubMed] [Google Scholar]
- 18.Warlop T, Detrembleur C, Stoquart G, Jeanjean A (2018). Gait complexity and regularity are differently modulated by treadmill walking in Parkinson’s disease and healthy population. Front Physiol 9:68. doi: 10.3389/fphys.2018.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delignières D, Marmelat V (2013). Degeneracy and long-range correlation. Chaos, 23(4), 043109. [DOI] [PubMed] [Google Scholar]
- 20.Goldberger AL, Amaral LAN., Hausdorff JM, Ivanov PC, Peng CK, Stanley HE. (2002). Fractal dynamics in physiology: alterations with disease and aging. Proc Natl Acad Sci, USA. 99, 2466–2472. doi: 10.1073/pnas.012579499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marmelat V, Delignières D (2011). Complexity, coordination, and health: avoiding pitfalls and erroneous interpretations in fractal analyses. Medicina (Kaunas), 47(7), 393–398. [PubMed] [Google Scholar]
- 22.Roerdink M, Daffertshofer A, Marmelat V, Beek PJ (2015). How to sync to the beat of a persistent fractal metronome without falling off the treadmill? PLoS ONE 10(7): e0134148. doi:10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marmelat V, Torre K, Beek PJ, Daffertshofer A (2014). Persistent fluctuations in stride intervals under fractal auditory stimulation. PLoS ONE 9(3): e91949. doi: 10.1371/journal.pone.0091949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dotov DG, Bayard S, Cochen de Cock V, Geny C, Driss V, Garrigue G, Bardy B, Dalla Bella S. (2017). Biologically-variable rhythmic auditory cues are superior to isochronous cues in fostering natural gait variability in Parkinson’s disease. Gait Posture, 51, 64–69. 10.1016/j.gaitpost.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 25.Delignières D, Ramdani S, Lemoine L, Torre K, Fortes M, Ninot G (2006). Fractal analysis for short time series: a reassessement of classical methods. J Math Psychol, 50, 525–544. doi: 10.1016/j.jmp.2006.07.004 [DOI] [Google Scholar]
- 26.Warlop T, Bollens B, Detrembleur C, Stoquart G, Lejeune T, Crevecoeur F (2018). Impact of series length on statistical precision and sensitivity of autocorrelation assessment in human locomotion. Hum Mov Sci 55, 31–42. 10.1016/j.humov.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 27.Almurad ZMH, Delignières D (2016). Evenly spacing in Detrended Fluctuation Analysis. Physica A, 451, 63–69. 10.1016/j.physa.2015.12.155 [DOI] [Google Scholar]
- 28.König N, Singh NB, Baumann CR, Taylor WR (2016). Can Gait Signatures Provide Quantitative Measures for Aiding Clinical Decision-Making? A Systematic Meta-Analysis of Gait Variability Behavior in Patients with Parkinson’s Disease. Front Hum Neurosci, 10, 319 10.3389/fnhum.2016.00319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravi DK, Gwerder M, Ignasiak NK, Baumann CR, Uhl M, van Dieën JH, et al. (2020). Revealing the optimal thresholds for movement performance: A systematic review and meta-analysis to benchmark pathological walking behavior. Neurosci Biobehav Rev, 108, 24–33. 10.1016/j.neubiorev.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Bella SD, Dotov D, Bardy B, de Cock VC (2018), Individualization of music‐based rhythmic auditory cueing in Parkinson’s disease. Ann N.Y. Acad Sci, 1423: 308–317. doi: 10.1111/nyas.13859 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


