Abstract
Introduction
Triplet regimen of carboplatin or cisplatin with pemetrexed and pembrolizumab is a standard treatment for patients with advanced, chemo-naïve, non-squamous non-small cell lung cancer. However, subgroup analysis for patients aged ≥75 years indicated that elderly patients who received the triplet regimen may have had shorter survival times than if they had chemotherapy alone (HR of 2.09). Treatments in the elderly are not always as effective or safe as for non-elderly patients, so there remains concern over whether the triplet regimen can be widely used in the elderly.
Methods and analysis
This is a single-arm, prospective, multicentre phase II study. The primary endpoint is set as the overall response rate according to Response Evaluation Criteria in Solid Tumors V.1.1. Secondary endpoints are progression-free survival, disease control rate and safety. This trial will enrol 22 patients.
Ethics and dissemination
This study was approved by the Wakayama Medical University Central Review Board on 2 December 2019 (approval number: W-32). Patients have been enrolled since February 2020. As the study will complete accrual in January 2022, results will be submitted for publication in peer-reviewed medical journals within 2023 and international scientific meetings. This study will provide significant information on whether the triplet regimens are clinically beneficial to elderly patients.
Trial registration number
Japan Registry of Clinical Trials (jRCTs051190095).
Keywords: chemotherapy, oncology, clinical trials
Strengths and limitations of this study.
This is a prospective, multicentre, single arm phase II study to evaluate the efficacy and safety of triple combination of carboplatin (CBDCA), pemetrexed (PEM) and pembrolizumab in elderly patients (≥75 years) with non-squamous non-small cell lung cancer (non-sq).
First planned prospective study to assess the additional efficacy of immune-checkpoint inhibitors combined with platinum doublet in elderly patients with lung cancer.
Criteria for reduction, postponement and cessation of CBDCA and PEM have been defined, they were not in KEYNOTE 189 trial, and this has enabled us to properly evaluate efficacy and safety through a typical, established method.
Although this is a small sized, single arm, multi-institutional phase II study, results obtained from 22 participants will confer significant information to determine treatment plans for elderly patients with advanced non-sq.
Introduction
The KEYNOTE 189 trial was a randomised phase III trial designed to compare triplet regimen of cisplatin or carboplatin (CBDCA), pemetrexed (PEM) and pembrolizumab with cisplatin or CBDCA and PEM. It elucidated superiority of the triplet regimen in overall survival (OS) and progression-free survival (PFS).1 The triplet regimen is recognised worldwide as standard treatment for patients with advanced, chemo-naïve, non-squamous non-small cell lung cancer (non-sq). However, in the triplet-regimen patients ≥75 years of age, the HR for OS is 2.09 (0.84–5.23) and PFS is 1.73 (0.77–3.90).
In pooled analysis of KEYNOTE 010, KEYNOTE 024 and KEYNOTE 042, pembrolizumab monotherapy significantly prolonged OS compared with chemotherapy with HR 0.76 for programmed death-ligand 1 (PD-L1) ≥1% and HR 0.46 for PD-L1 ≥50% in patients ≥75 years of age.2 Most recently, a CBDCA/PEM combination therapy was reported in a phase III study of non-sq patients ≥75 years with median PFS of 6.4 months and 0.9% treatment-related death.3 These studies clarified that the therapies have no major safety or efficacy issues in an elderly population. Considering this, and that only 17% of deaths in patients aged ≥75 years in KEYNOTE 189 study were treatment-related, the most appropriate reason for the high HR for OS and PFS at ≥75 years is the imbalance of complication-related deaths. However, the efficacy and safety of this triplet regimen for the elderly requires prospective evaluation. The current prospective study could, therefore, have clinical significance in evaluation of the additional efficacy and safety of pembrolizumab when combined with CBDCA and PEM in elderly, chemo-naïve, patients with non-sq. This is the first prospective trial of immune-checkpoint inhibitors combined with chemotherapy that targets the elderly. If additional efficacy and safety of pembrolizumab can be demonstrated in this prospective study, the value of the triplet regimen for elderly patients will be much increased.
Aim of the study
The main objective of this study is to evaluate additional efficacy by adding pembrolizumab to the CBDCA/PEM combination in elderly patients (≥75 years) that have non-sq as a first line setting.
Study design
This study is a single-arm, prospective, multicentre phase II study conducted in four facilities in Japan. The protocol scheme is shown in figure 1. Study duration will be 3 years.
Figure 1.
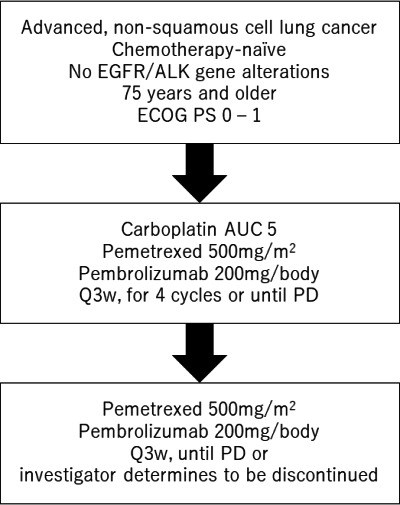
Protocol scheme of this study. AUC, area under the curve; ALK, anaplastic lymphoma kinase; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; PD, progressive disease; PS, performance status.
Endpoints
Primary endpoint is set as the overall response rate using the Response Evaluation Criteria in Solid Tumors (RECIST) V.1.1. Secondary endpoints are PFS, OS and safety.
Eligibility criteria of the participants
Inclusion criteria
Patients are required to fulfil all the following criteria:
Have histologically or cytologically confirmed non-sq. When pathologically mixed, non-squamous cell type must be dominant.
Be in advanced stage (IIIB, C and IV) or recurrence after surgical resection and not adaptable for curative radiotherapy.
Be negative for both of epidermal growth factor receptor gene mutations and anaplastic lymphoma kinase (ALK) gene alterations.
Negative or unknown for ROS1 and BRAF gene alterations.
Have no previous systemic anticancer treatment (neo-adjuvant or adjuvant chemotherapy) is allowed when final administration was more than 6 months before.
Have adequate renal function (a creatinine clearance of ≥45 mL/min using Cockcroft-Gault equation).
Have measurable lesions according to evaluation by an investigator or radiologist based on RECIST V.1.1. Previously irradiated target lesions are considered measurable if disease progression is confirmed.
Are expected to survive for longer than 3 months.
Provide written informed consent to participate in the study.
Exclusion criteria
Patients are excluded if they meet any of all the following criteria:
Obvious interstitial pneumonia confirmed by CT.
Have received radiotherapy to the lungs exceeding 30 Gy within 6 months before registration.
Underwent palliative radiation therapy within 7 days before registration.
Have symptomatic brain metastases.
Have active double cancer (within 2 years).
Have active hepatitis B or C.
Have history of HIV.
Have uncontrolled pleural effusion or ascites.
Aspirin or other non-steroidal anti-inflammatory drugs (NSAIDs) cannot be suspended (except when using aspirin of ≤1.3 g/day) for 5 days (8 days for long-acting drugs, such as piroxicam).
Have active autoimmune disease (patients who need systemic administration of corticosteroids in excess of 10 mg/day in terms of prednisolone, or treatment with an immunosuppressant).
Cannot, or do not receive folic acid or vitamin B12 replacement therapy.
Discontinuation criteria
Interventions are discontinued if patients meet any of all the following criteria:
Disease progression or death.
Grade 3 or higher adverse event deemed by the investigator to be unacceptable.
Interstitial lung disease/pneumonitis grade 2 or higher.
If adverse events cannot be controlled by hormone replacement therapy or insulin replacement.
The administration criteria of pembrolizumab are not satisfied on day 106 after the discontinuation of chemotherapy on day 43 (see below).
Investigator determines that protocol treatment cannot be continued in consideration of safety.
Background data
Patient background data are collected prospectively for all patients including sex, age, performance status, comorbidities, smoking history, allergies, gene alterations, PD-L1 expression on tumours and history of treatments for cancer.
Interventions
Patients receive CBDCA (area under the curve (AUC) 5.0), PEM (500 mg/m2) and pembrolizumab (200 mg/body) every 3 weeks. After four cycles, PEM and pembrolizumab will be continued until disease progression or the investigator determines that protocol treatment should be discontinued. AUC is calculated based on the Calvert formula.
Starting criteria for the first and after second courses are shown in table 1. Administration criteria for chemotherapy (CBDCA/PEM) and pembrolizumab after the second course are independent. If the criteria are not met, investigators are required to repeat the test and confirm that the criteria are met before starting treatment. Even if the starting criteria are met, the investigator may postpone any or all of CBDCA, PEM and pembrolizumab when postponement is considered necessary for safety by a principal investigator. For pembrolizumab, the treatment can be paused if patients require tapering of the steroid used to treat the adverse event until the steroid dose is reduced to ≤10 mg/day in prednisone.
Table 1.
Starting criteria for first and after second cycle of carboplatin, pemetrexed and pembrolizumab
| First cycle | After second cycle | ||
| Treatment | All | CBDCA, PEM | Pembrolizumab |
| ECOG PS | 0–1 | 0–2 | 0–2 |
| Neutrophils (/mm3) | ≥1500 | ≥1500 | N/A |
| Haemoglobin (g/L) | 0.8 | 0.8 | N/A |
| Platelet (/L) | ≥100×109 | ≥100 ×109 | N/A |
| AST, ALT | ≤100 (≤150 when with liver metastasis) | ≤100 (≤150 when with liver metastasis) | N/A |
| Total bilirubin (mg/dL) | ≤2 | ≤2 | N/A |
| Creatinine clearance | ≥45 mL/min | ≥45 mL/min | N/A |
| Constipation, appetite loss, nausea, vomit, oral mucositis, fatigue, phlebitis | N/A | ≥Grade 2 | N/A |
| Other medication related non-haematological adverse events except for hyponatraemia, alopecia, weight loss | N/A | ≥Grade 1 | N/A |
| Pneumonitis | N/A | Grade 0 | See below |
| Immune-related adverse events (eg, pneumonitis, hepatitis, colitis, endocrine disorder*, eye disorder, myocarditis, neuropathy, etc.) | N/A | N/A | ≤Grade 1 |
| Amylase increase, hyperglycaemic, skin disorder | N/A | N/A | ≤Grade 2 |
| Immune-related cerebromeningitis | N/A | N/A | Grade 0 |
| Non-haematological adverse events | N/A | N/A | ≤Grade 2 |
*Hypothyroidism, hypopituitarism (ACTH secretion deficiency) and type 1 diabetes that are stable with hormone replacement therapy can be administered.
ACTH, adrenocorticotropic hormone; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; CBDCA, carboplatin; ECOG, Eastern Cooperative Oncology Group; N/A, not applicable; PEM, pemetrexed; PS, performance status.
If an adverse event corresponding to the dose reduction criteria (table 2) is observed within the treatment course, the dose of CBDCA and/or PEM should be reduced at the start of the next course according to the dose reduction method shown in table 3. If the dose reduction criteria are met two or more times, the dose is reduced based on the dose at the time when the dose reduction criteria are met. If a three-stage dose reduction from the initial dose is required (two dose reductions and further dose reductions are required), treatment is discontinued. If the investigator or coinvestigator determines that toxicity is clearly causal to one of the two chemotherapy agents, it is appropriate to reduce the dose of that drug alone and not the other drugs. If the investigator determines that the toxicity is causally related to both chemotherapeutic agents, the dose is reduced for both agents according to the recommended dose adjustment shown in table 3.
Table 2.
Dose reduction criteria for carboplatin and pemetrexed
| Platelet (/L) | Neutrophil (/mm3) | CBDCA | PEM |
| Haematological adverse events | |||
| ≥50×109 | ≥500 | No reduction | No reduction |
| ≥50×109 | <500 | 1 dose down | 1 dose down |
| <50×109 without bleeding | Any | 1 dose down | 1 dose down |
| <50×109 with bleeding | Any | 2 dose down | 2 dose down |
| Any | <1000 and fever (≥38.5°C) | 2 dose down | 1 dose down |
| Non-haematological adverse events | |||
| Nausea, vomit, appetite loss | Grade 4 | 1 dose down | 1 dose down |
| Diarrhoea | Grade 3 or 4 | No reduction | 1 dose down |
| Enanthema | Grade 3 or 4 | No reduction | 2 dose down |
| Neurological toxicity | Grade 3 or 4 | 1 dose down | 1 dose down |
| AST/ALT | Grade 3 | 1 dose down | 1 dose down |
| Grade 4 | Discontinue | Discontinue | |
| Others | Grade 4 | 1 dose down | 1 dose down |
ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; CBDCA, carboplatin; PEM, pemetrexed.
Table 3.
Dose reduction method for carboplatin and pemetrexed
| 1 dose down | 2 dose down | 3 dose down | |
| CBDCA (AUC) | AUC 3.75 | AUC 2.5 | Discontinue |
| PEM (500 mg/m2) | 375 | 250 | Discontinue |
AUC, area under the curve; CBDCA, carboplatin; PEM, pemetrexed.
CBDCA, PEM and pembrolizumab will be discontinued when the discontinuation criteria shown in table 4 are met. Discontinuation of CBDCA, PEM and pembrolizumab is independently determined. In addition, if CBDCA and PEM require a third dose adjustment according to dose reduction criteria shown in table 3, or if the criteria for chemotherapy administration for the second and subsequent courses are not met until day 43 (day 1 is a day when the previous course started), CBDCA and/or PEM will be discontinued. If the criteria for pembrolizumab administration for the second and subsequent courses are not met until day 106, pembrolizumab will be discontinued. Any treatment will be discontinued if the investigator or research investigator determines it necessary. The detailed schedule for study participants is presented in figure 2.
Table 4.
Treatment cessation criteria for CBDCA, PEM and pembrolizumab
| CBDCA | PEM | Pembrolizumab | |
| Immune-related adverse events (hepatitis, eye disorder, myocarditis, neuropathy) | N/A | N/A | ≥Grade 3 |
| Immune-related adverse events (diarrhoea, colitis, panhypopituitarism, pancreatitis, skin disorders) | N/A | N/A | ≥Grade 4 |
| Treatment related adverse events (pneumonitis) | ≥Grade 1 | ≥Grade 1 | ≥Grade 2 |
| Immune-related cerebromeningitis | N/A | N/A | ≥Grade 1 |
| Allergic reaction | ≥Grade 3 | ≥Grade 3 | ≥Grade 3 |
CBDCA, carboplatin; PEM, pemetrexed; N/A, not applicable.
Figure 2.
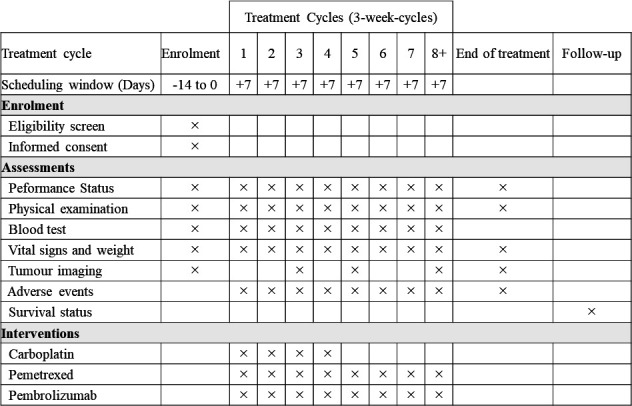
The schedule of enrolment, interventions and assessments. Tumour imaging is scheduled at 6, 12 weeks and then every 9 weeks (±14 days).
Supportive therapy
There is no specified supportive therapy in this study. While patients are recommended to avoid NSAIDs for 5 days from 2 days before the day of administration of PEM to 2 days after the administration, they are not excluded, even if NSAIDs are used.
Follow-up and assessment
Evaluations will be conducted every 6 weeks (±2 weeks) until the end of four courses, and once every 9 weeks (±2 weeks) after that. For follow-up of patients who discontinued protocol treatment, examination and efficacy evaluation will be performed at the discretion of each investigator. If the protocol treatment is discontinued for reasons other than disease progression; however, the prescribed evaluation of the treatment efficacy should be performed every three (±2) weeks until confirmation of disease progression, start of post-treatment, or the end of the follow-up period. Evaluations will be confirmed by an expert committee. All adverse events are assessed with Common Terminology Criteria for Adverse Events V.4.0.
Statistical analysis
The primary endpoint is the overall response rate. Calculate point to be estimated are those of response rate and two-sided 90% CI. We expect that the response rate in the elderly will be about 45% in point estimates based on KEYNOTE 189. An accurate CI based on the binomial distribution is used for the interval estimation of the response rate.
Secondary endpoints are OS, PFS and safety. Median values for OS and PFS are estimated using the Kaplan-Meier method, and 95% CIs are calculated using the Greenwood method. For safety, the frequency of all adverse events and the worst grade are calculated. Summary of statistics for the most abnormally low value or the most abnormally high value of each item of the haematological test, and biochemical test data are collected.
Sample size calculation
The response rate of CBDCA/PEM was 18.9% in KEYNOTE 189 (95% CI 13.8% to 25.0%)1 and 22.6% (95% CI 12.9% to 35.0%) in other phase II trial which treated only elderly (≥70).4 The response rate of CBDCA/PEM combined with pembrolizumab was 47.6% (95% CI 42.6% to 52.5%) in KEYNOTE 189.1 In line with these, the threshold was defined as 20% and the expectation is defined as 45%. With alpha error of 0.1 (both sides) and power of 0.90, 19 cases are calculated to be necessary. Considering deviation cases, 22 cases were set as target registration numbers.
Patients and public involvement
Neither patients, nor members of the public were directly involved in construction of this study. However, we believe assessment of benefits of pembrolizumab in addition to CBDCA and PEM in patients with ≥75 years is publicly important.
Informed consent
All participants will be informed of the purpose of the study, the plan of intervention, the benefits and the possible risks to the participants, after which they will sign the patient consent form. All candidates will be given sufficient time to consider whether they would like to participate in this study. Patients participating in the study will be allowed, at any stage, to withdraw from the study without restrictions.
Participating institutions
Wakayama Medical University Hospital, Wakayama National Hospital, National Hospital Organization Minami Wakayama Medical Center and Naga Municipal Hospital.
Data storage and security data
Data are collected via electronic data capturing system hosted by the Wakayama Medical University Clinical Trial Center with a secure web interface. Study investigators or those designated as such use strictly controlled personal electronic signatures (IDs and passwords) to login to the electorical data capture (EDC) system. The accuracy of all data input is confirmed by study investigators. Hard copy will be stored in a cabinet with a lock in a secure location. Access to records and data will be limited to study related persons.
Monitoring
This trial will employ centralised monitoring. Monitoring will be performed annually by each dataset collected via the EDC system to evaluate and improve study progress and quality. Monitoring consists of checking data as necessary, and individual facilities may be contacted to enter or add any missing data.
Discussion
Elderly people, usually defined as ≥70 years or ≥75 years, are thought to require different treatment strategies to non-elderly people. In this population, combination of gemcitabine and vinorelbine cannot surpass each individual agent in its antitumour effect,5 and significant superiority of the combination of docetaxel and cisplatin over docetaxel alone could not be shown. Subsequently, phase III studies showed that combination of CBDCA and paclitaxel significantly prolonged survival compared with gemcitabine or vinorelbine alone,6 but treatment-related deaths when patients were treated by CBDCA and paclitaxel were as frequent as 4.4% in this study. Considering these data about double agents combined regimens, there are remaining concerns about the clinical benefits of the regimen of combined triple agents in elderly patients.
In this study, with reference to the KEYNOTE 189 study protocol, the criteria for dose reduction, cessation, postponement and resumption of CBDCA and PEM were specified, which was left to the discretion of the attending physician in the KEYNOTE 189 study protocol. We believe that assessing efficacy and safety in the elderly in a well-established and typical way is useful for a fair assessment.
A limitation of this study is the small number of cases. The main purpose of this study; however, is to evaluate the efficacy of adding pembrolizumab to the combination of CBDCA and PEM, and we hypothesised increase of response rate from 20% to 45%, which are referred from the KEYNOTE 189 trial, and calculated number of enrolments. We therefore believe that this number of cases is sufficient to achieve the main purpose.
Taken together, if the current study reaches its primary endpoint, it will suggest that pembrolizumab can add additive efficacy when combined with CBDCA and PEM in elderly patients. Safety information concerning prospectively enrolled and periodically evaluated elderly patients will be highly valuable.
Ethics and dissemination
The study is designed and implemented according to good clinical practice and conducted in accordance with the Declaration of Helsinki. It was approved by the Wakayama Medical University Central Review Board on 2 December 2019 (approval number: W-32). Patients have been enrolled since February 2020. As the study will complete accrual in January 2022, results will be published within 2023. This study will provide important information whether triplet regimens of pembrolizumab, CBDCA and PEM would be clinically beneficial in elderly patients. For participants, results of this study will be announced by their physicians.
Supplementary Material
Acknowledgments
We deliver our thanks to Dr Masahiro Katsuda for constructive advice. We also thank Ms Keiko Fujimura, Ms Michiyo Fukaumi and all the staff for their valuable support in participant recruitment. Patient advisers and public were not involved in this study. We acknowledge English proofreading by Benjamin Phillis at the Clinical Study Support Center, Wakayama Medical University.
Footnotes
Contributors: YO drafted the manuscript. YO and TS designed the protocol. TY performed the statistical analysis. YA, YH, KK and NY further aided in assessment and revision of the protocol and revised the manuscript. All authors have read and approved the final version (version 1.0) of the protocol.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/coi_disclosure.pdf and declare no support from any organisation for the submitted work; YO has received personal fees from MSD K.K. and Eli Lilly, YH received personal fees from Bristle-Myers Squibb, NY received personal fees from MSD K.K. and Eli Lilly, Bristle-Myers Squibb, and Nichi-iko Pharmaceutical; no other relationships or activities that could appear to have influenced the submitted work.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018;378:2078–92. 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 2.Nosaki K, Saka H, Hosomi Y, et al. Safety and efficacy of pembrolizumab monotherapy in elderly patients with PD-L1-positive advanced non-small-cell lung cancer: pooled analysis from the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 studies. Lung Cancer 2019;135:188–95. 10.1016/j.lungcan.2019.07.004 [DOI] [PubMed] [Google Scholar]
- 3.Okamoto I, Nokihara H, Yoh K, et al. Randomized phase III study comparing carboplatin plus pemetrexed followed by pemetrexed versus docetaxel in elderly patients with advanced non-squamous non-small-cell lung cancer (JCOG1210/WJOG7813L). J Clin Oncol 2019;37:9031–31. 10.1200/JCO.2019.37.15_suppl.9031 [DOI] [Google Scholar]
- 4.Gervais R, Robinet G, Clément-Duchêne C, et al. Pemetrexed and carboplatin, an active option in first-line treatment of elderly patients with advanced non-small cell lung cancer (NSCLC): a phase II trial. Lung Cancer 2013;80:185–90. 10.1016/j.lungcan.2013.01.008 [DOI] [PubMed] [Google Scholar]
- 5.Gridelli C, Perrone F, Gallo C, et al. Chemotherapy for elderly patients with advanced non-small-cell lung cancer: the multicenter Italian lung cancer in the elderly study (Miles) phase III randomized trial. J Natl Cancer Inst 2003;95:362–72. 10.1093/jnci/95.5.362 [DOI] [PubMed] [Google Scholar]
- 6.Quoix E, Zalcman G, Oster J-P, et al. Carboplatin and Weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet 2011;378:1079–88. 10.1016/S0140-6736(11)60780-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


