Abstract
Hydrocephalus is the most common neurosurgical disorder worldwide and is characterized by enlargement of the cerebrospinal fluid (CSF)-filled brain ventricles from failed CSF homeostasis. Since the 1840’s, physicians have observed inflammation in the brain and the CSF spaces in both post-haemorrhagic (PHH) and post-infectious hydrocephalus (PIH). Reparative inflammation is an important protective response that eliminates foreign organisms, damaged cells, and physical irritants; however, inappropriately triggered or sustained inflammation can initiate or propagate disease, respectively. Recent data have begun to uncover the molecular mechanisms by which inflammation — driven by toll-like receptor 4 (TLR4)-regulated cytokines, immune cells, and signalling pathways — contributes to the pathogenesis of hydrocephalus. We propose that therapeutic approaches that target inflammatory mediators in both PHH and PIH could address multiple drivers of disease, including choroid plexus CSF hypersecretion, ependymal denudation, and tissue damage and scarring of intraventricular and parenchymal (glia-lymphatic) CSF pathways. Here, we review the evidence for a prominent role of inflammation in the pathogenic mechanism of PHH and PIH, and highlight promising targets for therapeutic intervention. Focusing research efforts on inflammation could shift our view of hydrocephalus from that of a life-long neurosurgical disorder to that of a preventable neuro-inflammatory condition.
Introduction
Historically, hydrocephalus has been defined as the progressive distension of the brain ventricular system that results from inadequate passage of cerebrospinal fluid (CSF) from its main site of production at the choroid plexus epithelium (CPe) to its site(s) of reabsorption into the systemic circulation (for example, the arachnoid granulations)1. This view of hydrocephalus is based on the bulk flow model of CSF circulation and is being modified by the development of hydrodynamic models that account for additional factors such as cardiac pulsatility2,3. Furthermore, emerging data suggest alternative sources of both CSF production and reabsorption, for example, the glia-lymphatic (or “glymphatic”) pathways. In addition, recent genetic analyses indicate that many forms of congenital hydrocephalus, both inherited and spontaneous, are a result of altered regulation of neural stem cell fate4. Regardless of aetiology, hydrocephalus is often characterized by increased intracranial pressure, ventricular enlargement from CSF build-up, and structural brain damage that, if left untreated, can progress to neurological decline, coma and death.5
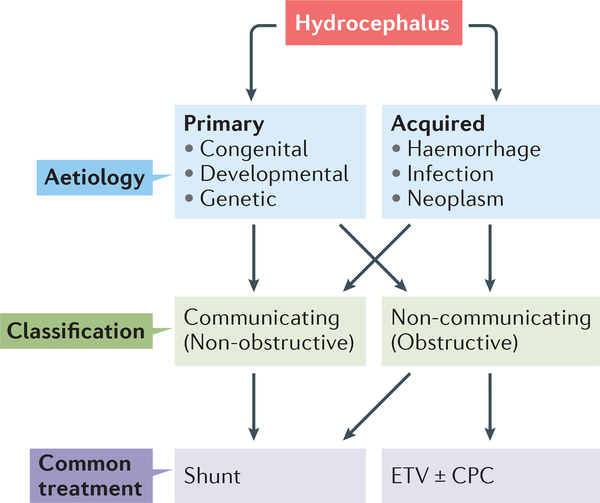
Historically, hydrocephalus has been classified as either primary (congenital, developmental, and/or genetic) or secondary to CNS insults such as haemorrhage, infection, trauma or tumor growth (Fig. 1)5–8. Post-haemorrhagic hydrocephalus (PHH) and post-infectious hydrocephalus (PIH) are two of the most common forms of hydrocephalus worldwide (Supplementary Table 1, 2)5,8, and are traditionally thought to be caused by obstructive mechanisms that prevent CSF reabsorption9,10. However, many patients with PHH or PIH have no discernible physical impediment to CSF flow in the ventricular and subarachnoid spaces. Nevertheless, surgical CSF diversion via implantation of permanent ventricular shunts or endoscopic ventriculostomy remains the mainstay of care for many patients with PHH or PIH. Although these procedures are life-saving, they frequently fail or result in complications11. Additionally, in resource-poor countries, these neurosurgical treatments are often unavailable owing to limited resources and a lack of specialized personnel such as neuro-intensivists and neurosurgeons5.
Figure 1 |. Classification and treatment of hydrocephalus.
Hydrocephalus can be dividied into primary and acquired forms. Hemorrhage and infection arc two of the most common causes of hydrocephalus worldwide. Both primary and acquired forms of hydrocephalus can involve intraventricular obstruction of CSF flow, which can be treated with a ventriculo-peritoneal shunt or endoscopic third ventriculostomy (ETV). ETV can be performed with or without choroid plexus coagulation (CPC). To date, all treatments for hydrocephalus are surgical, and have a high morbidity and failure rate.
One fundamental obstacle to the development of more effective treatments for hydrocephalus, including non-surgical therapies, is our limited knowledge of the molecular physiology of the disease. In this Review, we summarize the existing literature on the epidemiology, aetiology and treatment of PHH and PIH. We highlight the similarities between PHH and PIH, and synthesize recent findings on the contribution of inflammatory mediators, including toll-like receptor-4 (TLR4)-regulated cytokines and immune cells, to the pathogenesis of hydrocephalus. We suggest that in PHH and PIH, two critical functions of the CPe — immune function and CSF secretion —maladaptively engage in an epithelial response to injury that leads to acute inflammation-dependent hypersecretion of CSF. We also speculate that sustained inflammation propagated by ongoing injury to the CPe, ependymal cells and brain tissue is likely to affect CSF resorption. Impaired CSF homeostasis at the chronic stage of PHH or PIH might be a result of intraventricular obstruction via ependymal scar formation, or extraventricular obstruction via arachnoid scar impairment of glymphatic pathways. This model of acute and chronic inflammation might describe better than previous models the pathological changes occuring in PHH and PIH across time (that is, acute versus chronic changes) and space (for example, changes to the CPe, ependyma, aqueduct or glymphatic system). We emphasize that throughout this complex process, acute and chronic inflammation is mediated by specific molecular signals that could provide therapeutic targets. Thus, improved understanding of the shared pathophysiology of PHH and PIH could catalyze the discovery of therpauetic agents for both forms of hydrocephalus.
Global epidemiology of PIH and PHH
The prevalence of hydrocephalus described in the current literature varies four-fold among different reports12, This lack of consistency has prevented reliable estimation of the international prevalence and incidence of the conditions8. However, a recent meta-analysis indicated a global prevalence of hydrocephalus of 88 cases per 100,000 individuals under 18 years of age, 11 cases per 100,000 individuals between 19 and 64 years of age, and 175 cases per 100,000 individuals over 64 years of age12. This meta-analysis controlled for study quality, publication bias, and population heterogeneity. In the same study, the prevalence of hydrocephalus in individuals under 18 years of age was nearly twofold higher in Africa than in North America, indicating a difference in hydrocephalus burden between resource-rich and resource-poor countries. An analysis of global incidence rates of hydrocephalus by Dewan et al,8 also suggested that the epidemiology of PHH and PIH is driven by socio-economic status. According to this analysis, PIH is the predominant form of acquired hydrocephalus in resource-poor countries and PHH is the most common cause of acquired paediatric hydrocephalus in resource-rich, countries8,13.
PHH occurs primarily in preterm neonates with a very low birth weight (< 1500 grams), in whom the condition is secondary to germinal matrix haemorrhage14,15. In these neonates, PHH is often fatal unless adequate prenatal, neonatal intensive, and neurosurgical care is provided16. Accordingly, PHH in infants is underrepresented in countries that lack the resources to provide this care8,13. For example, in East Africa, with sparse neonatal intensive care resources and only 1 neurosurgeon per ∼10,000,000 individuals17, most neonates with a very low birth weight do not survive13. PHH is also a common cause of hydrocephalus in adults14,18,19, in whom the condition is often associated with intraventricular haemorrhage resulting from hypertension, aneurysm rupture, or traumatic brain injury.20
PIH is the most common cause of pediatric hydrocephalus worldwide5 and is most prevalent in Africa, Latin America and Southeast Asia8. As mentioned above, in resource-poor countries, PIH is more common that PHH. This predominace of PIH is likely to result from the increased occurance of peripartum infections in these countries, caused by hygienically challenging neonatal environments and the lack of advanced obstetric care17,21. Within the region known as the African meningitis belt, seasonal increases in meningitis have been linked with PIH22. In areas where tuberculosis is endemic, such as South Africa,23 India,24,25 China,26 and Philippines,27 post-tuberculosis hydrocephalus constitutes a considerable disease burden. Interestingly, congenital Zika virus has been shown to cause severe hydrocephalus in Brazi28. In resource-rich countries, PIH associated with prenatal infection is often caused by Toxoplasma gondii and cytomegalovirus, whereas typical neonatal aetiologies include bacterial sepsis from Escherichia coli, Streptococcus agalactiae, and Listeria monocytogenes.5,29,30 Among adults, the most common causes of PIH include the bacteria Neisseria meningitidis and Streptococcus pneumoniae,31 although viral, fungal, and protozoan infections have been implicated in the development of PIH in patients who are immunocompromised.32,33 Identifying the bacterium responsible for PIH in patients in resource-poor countries has been difficult owing to limited access to advanced clinical microbiological diagnostics30. Additionally, factors such as proximity to farm animals30, access to prenatal care8,30, and seasonal changes in rainfall,34 among other differences in living conditions, can result in a wide variety of bacterial infections, some of which cannot be detected with standard methods.
Current treatments
The current approach to treating hydrocephalus involves either ventriculo-peritoneal shunting of CSF or endoscopic third ventriculostomy (ETV). ETV is often combined with choroid plexus cauterization (ETV/CPC). Shunting is the most common treatment for PHH and PIH across all age groups5,29,35–38. The treatment involves the subcutaneous tunneling of silicone elastomer tubing from the cerebral ventricles to the peritoneal cavity, thus draining excess CSF. An interposed valve is used to prevent retrograde fluid flow or excessive loss of CSF5. However, mechanical obstructions and/or malfunctions, tubing complications, and infections frequently occur in patients with shunts, which substantially decreases their quality of lifes,41–44. In the U.S., >50% of shunts fail within 2 years of insertion and 70% fail within 10 years, making shunt failure the most common medical device failure in the country5,39. The high likelihood of shunt failure means that these patients need life-long access to immediate neurosurgical care40.
ETV/CPC is the alternative to shunting and has been increasingly used worldwide to treat both PHH and PIH in infantS41,42. ETV/CPC involves endoscopic fenestration of the floor of the third ventricle to provide an alternate pathway for CSF reabsorption, coupled with electro-thermal destruction of the CPe which might reduce CSF production. In infants aged <6 months, ETV/CPC results in cognitive development outcomes and brain growth similar to those seen in shunting, although the newer technique reduces ventricular size more slowly and to a smaller degree29,43,5,38. ETV/CPC also has lower long-term failure rates than shunting,40 and is not affected by hardware complications44. However, ETV/CPC requires more advanced technical expertise45,46 and has a higher short-term failure rate than shunting38,40. Furthermore, the long-term effects of ETV/CPC are unknown, and the procedure can negatively affect other functions of the CPe, including immune function, nutrient reabsorption and multiple aspects of neurodevelopment47–49. Recent data indicate that ETV/CPC is the preferred treatment option in resource-poor countires where access to urgent neurosurgical care is limited29.
Effective pharmacological treatments for PHH and PIH have yet to be developed. Clinical trials for furosemide (a loop diuretic) and acetazolamide (carbonic anhydrase inhibitor) aimed to decrease CSF production by inhibiting ion flux across the basolateral and apical membranes of the CPe that provides the the osmotic gradient for water transport50. These drugs were not effective in treating PHH caused by neonatal germinal matrix hemorrhages51–53 and were associated with higher rates of shunt placement and worse neurological outcomes than placebo51. This lack of efficacy is likely to reflect systemic administration and the poor blood-brain barrier permeability of acetazolamide and loop diuretics54–57. The International Posthaemorrhagic Ventricular Dilatation Drug Trial Group, which carried out one of the trials, concluded that these drugs could not be recommended as therapeutics51.
The classical model of pathogenesis
That PHH and PIH result from intraventricular CSF accumulation owing to failed CSF homeostasis is widely accepted. According to the classical model of CSF dynamics, PHH and PIH result from obstruction of intraventricular CSF flow and/or dysfunction of extraventricular arachnoid granulations, which causes a decrease in CSF reabsorption10,16. This model is supported by some PHH case series that reported occlusion of the fourth ventricular outflow tracts by fibrous thickening of the leptomeninges, known as “tetra-ventricular” PHH58,59. Evidence from other PHH case series suggests that blood and its breakdown products acutely obstruct narrow CSF passages such as the cerebral aqueduct60,61. Some authors have implicated the arachnoid granulations in post- intraventricular haemorrhage communicating hydrocephalus, suggesting that microthrombi and debris from intraventricular haemorrhage can plug arachnoid villi and impair CSF reabsorption, and that inflammation and scarring of the arachnoid at the posterior fossa might hinder CSF flow62. Indeed, obstruction of CSF flow by an intraventricular blood clot, or a scarred-over aqueduct are apparent and almost certainly causative in some cases of PIH and PHH. Combinations of all of the above mechanisms are likely to contribute to development of hydrocephalus, especially in the chronic stage.
Despite these reports, the classical model is supported by sparse experimental evidence,7,14 neglects the potential role of increased CSF secretion6,7,63, and overlooks clinical and pre-clinical evidence of CPe inflammation in PHH and PIH7,64–67. In addition, this model fails to acknowledge that development of arachnoid granulations, which are believed to reabsorb much of adult CSF, is gradual68,69. This gradual development means that arachnoid granulations are not yet present in human infants or in most animal models of PHH, suggesting that arachnoid scarring does not have a central role in the pathophysiology of PHH. The classical model does not account for other sites of CSF reabsorption, such as the ventricular ependyma, perineural space, leptomeninges, glymphatics, and nasal mucosa,62,68,70–72 or the role of ependymal ciliary beating on CSF bulk flow73. Moreover, CSF hypersecretion caused by CPe villous hyperplasia or choroid plexus tumors is sufficient to cause non-obstructive hydrocephalus6. Finally, intracerebroventricular injection of blood metabolites causes CPe inflammation7,64–66 and hydrocephalus74 in animal models. Collectively, these observations suggest that the classical model is unable to adequately explain PHH and PIH, and that alternative or complementary pathogenic mechanism(s) need to be considered.
The pathogenic role of inflammation
Evidence from humans
The possibility of a role for inflammation in the pathogenesis of human PHH and PIH was discussed in the scientific literature as early as 184075–79 and is supported by several lines of clinical evidence. For example, in infants and adults who have had an infection or brain haemorrhage, levels of IL-6, IL-4, TNF-α, TGF-β1, and other inflammatory markers in the CSF and the peripheral blood correlate with the likelihood of subsequently developing hydrocephalus, and with the severity of the condition80–83. In addition, neuropathological examination of brain tissue from fetuses and infants with PHH or PIH shows signs of neuroinflammation, such as microglial activation and reactive gliosis58,84,85. Although these laboratory and neuropatho logical studies have identified correlations between inflammation and ventriculomegaly, the strongest evidence for the pathogenic role of inflammation in hydrocephalus comes from a randomized, double-blind, placebo-controlled trial of the corticosteroid dexamethasone in 545 adults with tuberculous meningitis86. Intriguingly, dexamethasone administration decreased the frequency of hydrocephalus in these individuals. These findings are consistent with an earlier retrospective study, in which higher dexamethasone doses were associated with decreased frequency of hydrocephalus following aneurysmal subarachnoid haemorrhage87. Altogether, the evidence discussed in this section suggests that reducing inflammation could be a powerful approach for treatingPHH and PIH.
Evidence from animal models
Evidence from pre-clinical animal models provides further support for the pathogenic role of inflammation in PHH and PIH. As in patients, haemorrhage or infection in rabbit and rodent models results in upregulated expression of inflammatory markers as well as activation of TLR4-dependent inflammatory signalling pathways64–66,88. In particular, inflammation of the choroid plexus and ependymal layer of the lateral ventricles was observed in an animal model of PHH66. Histological studies of brain tissue showed leukocytic infiltration, microglial activation and reactive gliosis in rodent models of PIH and PHH89–91.
Experimental manipulation of inflammatory pathways provides strong evidence that acute inflammation is both necessary and sufficient to induce PHH and PIH. In animal models, pharmacological inhibition or genetic ablation of TLR4 signalling attenuates molecular and histological correlates of inflammation as well as ventricular dilation7,92–94. Furthermore, pharmacological or genetic hyperactivation of inflammation causes ventricular dilation95–97. Interestingly, in rabbit and dog studies, steroid administration decreased CPe-mediated CSF production98–100, which is reflected in the benefits of steroid administration observed in patients with PHH and PIH86,87.
These preclinical findings show that inflammation is a shared pathogenic event of PHH and PIH, thus supporting use of anti-inflammatory agents such as glucocorticoids to treat patients with these conditions.
The immune function of the CPe
The CPe is located within the cerebral ventricles and consists of a single cell layer of polarized cuboidal epithelial cells surrounding fenestrated capillaries. These epithelial cells actively secrete sodium (Na+), potassium (K+), chloride (C1−) and bicarbonate (HCO3−) ions, amongst others, resulting in an osmotic gradient that drives transport of water from the blood to the ventricular space. The CPe is responsible for ∼80% of CSF production in rodentS3,7,50,101 and probably contributes a similar percentage in humans. The remainder of the CSF is likely to be derived from bulk flow of brain interstitial fluid3. The CPe is the most actively secreting epithelium in the human body, producing CSF at a rate of ∼400–500 mL per day50. As such, the CPe receives more blood flow per gram of tissue than any other tissue in the body50,102 and metabolizes more ATP than any other epithelium103. CSF secretion by the CPe is subject to strict regulation and can be modified by multiple neuro-humoral mechanisms 50.
Epithelial barrier cells are constantly exposed to microbes and other environmental insults that can compromise tissue function, either by excessive activation of inflammation or by direct cell damage. The major challenge facing the immune system is to neutralize foreign invaders and resolve injury without inflicting the collateral damage that perpetuates a chronic inflammatory cycle. Maintaining immune homeostasis is particularly challenging at barrier sites where constant exposure to immunogenic agents can induce destructive inflammation. Although the function of the innate immune system at barrier epithelia in the intestine, skin, and respiratory tract has been well studied, the immune functions of the blood-CSF barrier (CPe) and the brainCSF barrier (ependyma) have received less attention.
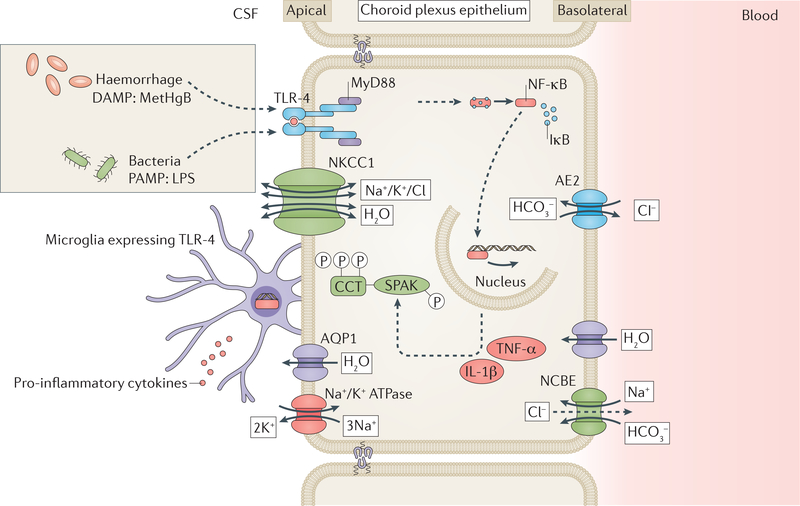
The CPe functions as a tightly regulated gate that separates the blood and CSF, but allows circulating immune cells to enter the brain for defense and repair104. Like other epithelial cells, the cells of the CPe express toll-like receptors (TLRs) on their surface. Pathogen-associated molecular patterns (PAMPs) in the CSF bind to these TLRs, resulting in activation of nonspecific innate immune responses105,106. Several different TLRs64,107, including TLR47, are highly expressed in the CPe and are regulated in specific ways by different pro-inflammatory stimuli7,64,65. The gram-negative bacterial cell wall component lipopolysaccharide, which is common in patients with PIH in Western countries, is a classic PAMP and canonical TLR4 ligand that activates NF-κB-dependent cytokine production and immune cell recruitment106,108 (figure 1).
TLRs also recognize damage-associated molecular patterns (DAMPs), or “alarmins”, which are released from tissue in response to injury and are interpreted by adjacent cells as foreign danger signals109. DAMPs that bind to TLRs include heat shock proteins,110 matrix degradation products111–113, the S100A8-S100A9 protein complex114, lysophosphatidic acid115, and intraventricular haemorrhage-derived blood-breakdown products such as methemoglobin (metHgb) and iron74,110,111,116 (figure 1)
TLR4-dependent CPe hypersecretion in PHH
The CPe is one of the first brain structures to encounter extravasated blood after intraventricular haemorrhage 48,64,117,118. Recent studies in animal models have shown that intracerebroventricular injection of autologous blood into the lateral ventricles is sufficient to cause ventriculomegaly, as well as NF-κB activation and cytokine production in CPe cells7,66. In rabbit pups and human infants with intraventricular haemorrhage, the level of metHgb in CSF strongly correlates with that of the TLR4-dependent cytokine TNF-α64,65. Furthermore, at physiological concentrations, intracerebroventricular delivery of metHgb activates TLR4 homodimers or TLR4/2 heterodimers,119,120 promoting nuclear translocation of NF-κB and TNF-α and IL-1β secretion,116,119,120 and is sufficient to cause ventriculomegaly64,65.
Interestingly, many secretory epithelia respond to pro-inflammatory stimuli by increasing fluid secretion121, which helps maintain homeostasis by clearing pathogens or debris from the epithelial surface122,123. However, inappropriately initiated or sustained inflammation of secretory epithelia can lead to disease 124,125. For example, dysregulated epithelial inflammation and associated fluid hypersecretion can be observed in several conditions, including chemical, autoimmune or infectious forms of pleuritis, colitis and pancreatitis13,. In addition, chronic inflammation can cause tissue damage, which propagates and amplifies the initial inflammatory response via the release of host-derived DAMPs.
Until recently, the impact of inflammation on the secretory capacity of the CPe has been difficult to study, reflecting a paucity of techniques that can adequately measure and manipulate rates of CSF secretion in vivo. However, a recently developed microneurosurgical technique that enables the real-time measurement of CSF secretion rate in live rats 126 has facilitated several novel observations of CSF dynamics in experimental PHH7. Infusion of autologous blood into the right lateral ventricle provoked a TLR4-NF-κB-dependent CPe inflammatory response that was associated with a >3-fold increase in CSF secretion. This increase in secretion was detected from 24h to at least 7 days after experimental intraventricular haemorrhage and could be inhibited by administration of the NKCC1 transporter inhibitor bumetanide. At the 7 day timepoint ventriculomegaly was also osberved7. CPe inflammation was characterized by greatly up-regulated phosphorylation of NF-κB, production of TNF-α and IL-1β, as well as infiltration of activated ED-1+ microglia and macrophages (Figure 2)7. The same study also found that this intraventricular haemorrhage-induced CSF hypersecretion resulted from TLR4-dependent activation of the NF-κB-regulated STE20/SPS1-related, proline-alanine-rich kinase (SPAK).
Figure 2. Proposed mechanism of CSF hypersecretion in PHH and PIH.
Host-derived danger-associated molecular patterns (DAMPs) such as methemoglobin (metHgB) enter the CSF during intraventricular haemorrhage and pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS) enter the CSF during bacterial meningitis. These DAMPs and PAMPs are thought to bind toll-like receptor 4 (TLR4) on the surface of the choroid plexus epithelium (CPe). This binding stimulates a TLR-4-MyD88 signalling cascade leading to nuclear translocation of nuclear factorkB (NF-κB). Nuclear NF-κB stimulates production of pro-inflammatory cytokines, for example, tumour necrosis factor-α (TNF-α) and interleukin 1β (IL-1β), which increase activity of Ste20-type stress kinase (SPAK). SPAK phosphorylates its canonical substrate, the Na+/K+/2Cl ion co-transporter- (NKCC1), and probably also phosphorylates other ion transporter targets. NKCC1 phosphorylation increases activity of the transporter, which results in a net increase in cerebrospinal fluid (CSF) production by the CPe. DAMPs and PAMPs in the CSF also bind to TLR-4 on the surface of microglial cells that are resident on the choroid plexus. This binding results in the production of pro-inflammatory cytokines by the microglia. These cytokines can bind receptors on the CPe and likely progogate CPe inflammation and CSF hypersecretion. AQP1, AE2 and NCBE are some additional transporter proteins that facilitate the passage of water (AQP1) and ions (AE2 and NCBE) across the plasma membrane. Cl−
SPAK integrates and transduces environmental stress signals, including NF-κB-regulated inflammatory cytokines 127–130 such as TNF-α, IL-1β, and IFN-γ. In addition, NF-κB is itself a transcriptional regulator of SPAK130. Interestingly, in models of colitis,131 IgA nephropathy, 132 and hypoxic lung injury133, TNF-α130 and IFN-γ134 have been shown to stimulate SPAK in an NF-κB-dependent manner, indicating that positive feedback occurs. SPAK directly interacts with the TNFα receptor RELT to activate downstream stress response pathways mediated by p38 and JNK1/2 signalling135. When activated, SPAK binds, phosphorylates, and stimulates the cotransporter NKCC1 at the CPe apical membrane7,136,137. NKCC1 accounts for >50% of the total CSF production by the CPe, and SPAK is the most important regulator of NKCCl7,101. However, SPAK also binds and/or regulates multiple other ion transporters138, for example, the CPe basolateral membrane transporters Na+-dependent Cl−/HCO3− exchanger (NCBE) and (in the presence of scaffolding protein spinophilin) Na+-independent CI-/HCO3− exchanger (AE2)139. Interestingly, stimulatory phosphorylation of NCBE140 was recently implicated in CSF hypersecretion in neonatal PHH, suggesting that SPAK might be responsible for inflammation-induced up-regulation of NCBE140. Therefore, SPAK seems to be a crucial link between TLR4-dependent CPe inflammation and CSF hypersecretion. In an animal model of PHH, genetic inhibition of TLR4 or SPAK returned CSF secretion to healthy levels and prevented hydrocephalus by decreasing intraventricular haemorrhage-induced phosphorylation of NKCC1, as did treatment with inhibitors of TLR4-NF-κB or SPAK-NKCC1 before intraventricular haemorrhage7. These data suggest that the ability of CPe CSF production capacity to be dynamically regulated in response to inflammation, known as ‘immuno-secretory plasticity’, is important in the pathogenesis of acute PHH, and that pharmacological targeting of TLR4 or SPAK could be a promising treatment approach.
TLR activation in PIH
Bacterial CNS infection is probably the most common cause of PIH5,8. Bacteria can cross the blood-brain barrier (BBB) and CPe to gain entry to the CNS141. Once in the CNS, bacteria replicate within subarachnoid and ventricular CSF spaces, and cause intense CPe, ependymal, and CSF inflammation by releasing cell wall fragments that are highly immunogenic142. The robust acute inflammatory response associated with PIH has been assumed to cause noncommunicating (obstructive) hydrocephalus via blockage of the aqueduct, the 4th ventricle outlets, or the basal subarachnoid spaces around the 4th ventricle. However, the acute onset of PIH, often within 12 hours of infection, precedes the expected onset of post-inflammatory scarring and aqueductal obstructions.
PAMPs contained in PIH-causing organisms promote local inflammation through recognition by antigen-presenting cells, for example, microglia and CPe cells, that express cell surface patternrecognition receptors, for example, TLRs144. TLR2, TLR4 and TLR5 are all expressed by the CPe64,107, and exhibit exhibit ligand-specific regulation in response to pro-inflammatory stimuli from PIH-associated microorganisms. TLR4 recognises lipopolysaccharide found on gram-negative bacterial cell walls7,30,64,65 and S. pneumoniae-derived pneumolys145, and TLR4 activation by these stimuli causes the CPe to produce inflammatory cytokines such as TNF-α, IL-1β and IL-6146–148, which results in recruitment of additional immune cells into the CNS from across the BBB and CPe149. TLR2 recognizes lipoteichoic acids from S. pneumoniae,150 L. monocytogenes,151–153 and S. agalactiae,154 whereas TLR5 recognizes flagellin of flagellated bacteria155. There is some evidence that activation of TLR2156 in the CPe leads to chemotaxis and leukocyte infiltration; however, additional work is needed to elucidate the function of the TLRs in the CPe.
The role of the ependymal epithelium
The pathogenesis of hydrocephalus after hemorrhage or infection is certainly not limited to CSF hypersecretion from the CPe. The ependymal epithelium, ventricular zone and subventricular zone also contribute to the development of PHH in neonates; these structures are likely involved in PIH,157–159 however, more research directly investigating this is required. The ventricular zone is a single layer of mono-ciliated neural stem cells that lines the embryonic ventricular system. In utero, these cells develop into the multi-ciliated ependymal ventricular wall that separates the CSF-filled venrticles from underlying brain parenchyma160,161. The subventricular zone lies adjacent to the ventricular zone and is a region of densely populated neural progenitor cells 160,161. Together, the ventricular zone and subventricular zone are critical regions for the birth of newborn neurons and glia during perinatal development161. Postmortem histological analysis of frontal and subcortical brain regions showed that, compared with controls, infants with intraventricular haemorrhage had a loss of neural stem cells in the subventricular zone, reduced numbers of multi-ciliated ependymal cells, cytoplasmic relocation of N-cadherin (the adhesion protein connecting the cells of the ventricular zone), periventricular heterotopia, and abnormal invasion of astrocytes into areas affected by hemorrhage162. These findings were associated with abnormal brain development, altered CSF dynamics, and the development of ventriculomegaly162,163. The histological findings were supported by findings from in vitro models of mouse intraventricular haemorrhage163. Together, the clinical and preclinical data suggest that intraventricular haemorrhage leads to loss of developing and mature ciliated epithelial cells, resulting in disrupted ciliary beating and abnormal CSF flow. This disruption, in combination with developmental abnormalities such as periventricular heterotopias and glial activation, could contribute to the pathogenesis of PHH and associated neurodevelopmental sequelae.
PHH and PIH: shared therapies?
Given that TLRs recognize both DAMPs and PAMPs, PIH and PHH might share common pathogenic mechanisms driven by PAMP and DAMP-triggered innate immune responses, raising the possibility that anti-inflammatory treatments could modulate development of hydrocephalus in both conditions. Before permanent CSF shunting, many patients with PIH and PHH require urgent placement of temporary CSF diversion devices such as external ventricular drains, implanted access reservoirs, or lumbar drains. In patients with these devices, intraventricular administration of medications targeting TLR4-dependent inflammation seems particularly attractive. Such medications could include anti-inflammatory agents that target the TLR4-NF-κB pathway, for example, the TLR4 inhibitor Tak242, pyrrolidine dithiocarbamate164 or melatonin165,166. Data from an animal model suggest that systemic administration of Tak242 is effective in treating PHH7. Additionally, systemic administration of Tak242 was tested in a clinical trial in patients with sepsis167. Although Tak242 administration did not significantly alter mortality rate or suppress cytokine levels, the authors noted that gram-status of bacteria was not an inclusion criteria. Reterospective analysis showed that only 40% of patients in the study had gram-negative infection, which would be more likely to respond to TLR-4 inhibition167 than gram-positive or fungal infection. Agents that sequester DAMPs, PAMPs or cytokines, for example, neutralizing antibodies or decoy receptor "sponges" 168,169, might also be effective treatments for PIH and PHH, and could be more specific than corticosteroids, a more general anti-inflammatory agent. Obviously, each of these potential agents will require experimental validation in models of PIH and PHH.
Given the importance of NKCC1 in CSF secretion101 and intraventricular haemorrhage-induced CSF hypersecretion7, the NKCC1 inhibitor, bumetanide could reduce the CSF secretion induced by acute inflammation in PIH and PHH. Systemic bumetanide was found to reduce the symptoms of autism in children170–172; however the drug and its derivatives show a low level of CNS penetration that indicates that systemic administration of these agents to treat neurological disorders might not always be effective7,55. Therefore, intracerebroventricular delivery of bumetanide via the CSF diversion devices discussed above might be a more suitable method of delivery. However, bumetanide has been associated with hearing loss when added to phenobarbital for treatment of seizures in neonates173, suggesting that bumetanide should not be administered to infants at this age.
SPAK might be preferable to NKCC1 as a therapeutic target for PIH and PHH, as SPAK is more highly expressed in CPe than in any other epithelial tissue, is an amplifier of the TLR4-dependent inflammatory reaction and cytokine production? and is a master regulator of multiple ion transporters136. Regardless of the potential of targeting SPAK, we propose that targeting inflammation as opposed to CPe ion transport is the most promising therapeutic approach for PIH and PHH, because, in addition to driving the initial CSF hypersecretory response, inflammation is likely to contribute to the ensuing tissue damage and release of DAMPs that ultimately leads to sustained hydrocepahlus. Additionally, it might be beneficial to preserve the acute CSF hypersecretion response as it could clear debris from the CPe and ependymal surface.
Reparative vs. damaging inflammation
Although recent studies have begun to identify inflammatory mediators of PHH,7,64–66,116,174 numerous gaps in our understanding remain. For example, we do not know the identities of the intraventricular haemorrhage-induced metabolite(s) that bind to TLR4, although metHgb has already been identified as a TLR4 ligand116. In addition, further work is needed to identify the components of the TLR4 signalling cascade induced by acute or chronic intraventricular haemorrhage, the dynamic spectra and profiles of TLR4-dependent cytokines and immune cells, and the contribution of additional inflammation-dependent mechanisms (for example, those resulting from accompanying tissue injury) to PHH. Establishing the duration of inflammation-induced CPe hypersecretion and whether TLR4 inhibition after intraventricular haemorrhage can prevent PHH will also be important.
It seems likely that a CSF hypersecretory response from an inflamed CPe contributes to development of acute hydrocephalus, which occurs before chronic inflammation can lead to scarring. However, additional TLR-dependent or innate immune mechanisms, for example, activation of microglial NOD-like receptors 175,176, can be triggered by inflammation-induced tissue damage of CNS barrier epithelia (the CPe and ependyma) and associated DAMP release. Activation of these additional mechanisms could propagate and sustain the neuro-inflammatory reaction in the CPe, and affect other CSF homeostatic pathways such as the recently characterized and still controversial glymphatic system (Figure 2). Neuroimaging studies of patients with idiopathic normal pressure hydrocephalus revealed significant suppression of glymphatic clearance in these individuals177,178. This observation is significant, because the glymphatic system is a possible alternative pathway for CSF efflux.
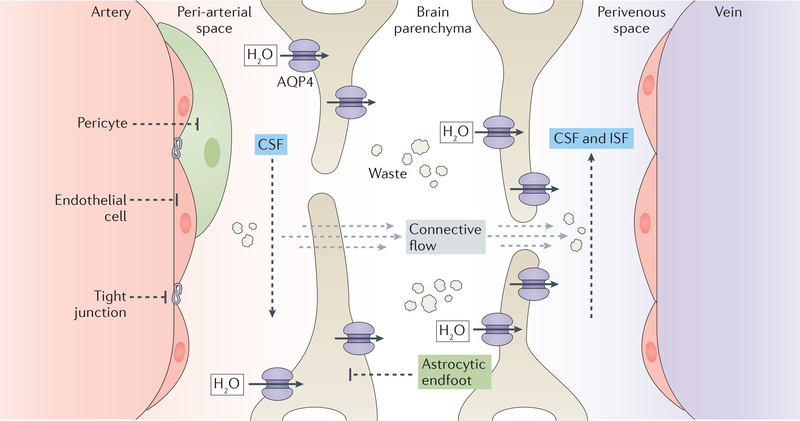
In the glymphatic model of CSF circulation, arterial pulsatility drives CSF influx into the periarterial space. A combination of arterial pulsatility and high levels of the water channel aquaporin-4 in the vascular endfeet of astrocytes then facilitates movement of CSF from the periarterial space to the brain parenchyman, where the CSF mixes with interstitial fluid (ISF). This mixture of CSF and ISF is drained via the perivascular spaces surrounding the deep veins, as well as by cranial and spinal nerves, and is collected by meningeal and cervical lymphatic vessels179. The recent identification of the glymphatic system raises the question of whether inflammation-dependent impairment of glymphatic fluid efflux contributes to PHH or PIH. In a recent animal study, germinal matrix haemorrhage resulted in impaired glymphatic transport. In this study, inhibition of reactive astrogliosis after haemorrhage improved glymphatic function and attenuated progression of PHH180. Impaired glymphatic fluid transport was also noted in mouse models of subarachnoid bleeding, traumatic brain injury, and inflammation181. However, some experimental evidence does not support a major role for the glymphatic pathway in CSF homeostasis182–187.
After injury, cytokines derived from microglia and epithelia induce the production of additional cytokines and growth factors by underlying connective tissue fibroblasts, promoting epithelial proliferation and repair188. As in the intestine and respiratory tract189, chronic tissue damage or inflammation in the CPe and ependyma, such as that associated with extensive intraventricular haemorrhage (for example, grade IV germinal matrix haemorrhage) and with partially treated ventriculitis, might drive conversion of activated connective tissue fibroblasts to extracellular matrix-producing myofibroblasts. This conversion could lead to fibrosis, recruitment of inflammatory cells, and excessive production of inflammatory mediators, thus driving pathological inflammation that exacerbates tissue damage. In support of this theory, damage to the CPe, intraventricular fibrosis and septation, and friable ependyma have all been observed in individuals with chronic PIH or PHH10,190,191. In addition, ependymal denudation and ventricular zone disruption were identified in patients with chronic PHH and in animal models of the condition162.
Some studies have shown reduced CSF secretion in chronic hydrocephalus192–194. Silverberg et al.,193 suggest that this reduced CSF production results from prolonged elevation in intracranial pressure. Kosteljanetz et al.,194 noted great variation (within and among patients) in the rate of CSF production after subarachnoid haemorrhage, though measurements from different individuals were taken at different times after the initial bleeding event. In our proposed model of the contribution of inflammation to hydrocephalus (Figure 3), CSF hypersecretion occurs acutely (1–7 days) after infection or haemorrhage and is then followed by scarring, inflammation (fibrosis), and CSF malabsorption. The latter phase would be accompanied by normal or even decreased levels CSF production as the CPe becomes chronically scarred and fibrotic.
Figure 3. Glymphatic CSF transport.
The glymphatic system is a perivascular cerebrospinal fliud (CSF) and interstitial fluid (ISF) exchange network that mediates waste clearance and CSF efflux from the brain to outlets such as the cervical or meningeal lymphatic system and the major draining venous sinuses. In the glymphatic system, as arteries on the surface of the cortex penetrate the brain, CSF enters the parenchyma alongside the vessels, ensheathed by astroglial endfeet. Driven by cardiac-driven arteriole pulsations, and facilitated by the high expression of aquaporin 4 (AQP4) in the astroglial endfeet, CSF exits the perivascular space and mixes with the brain’s ISF. Either by bulk flow or diffusion, the mixture of CSF and ISF flows through the parenchyma into either perivenous or perineural spaces (perineural spaces not illustrated). The fluid then travels along the perivenous or perineural spaces until it drains into to the dural sinuses or lymphatic vessels on the way to the general circulation for clearance.
At present, our ability to investigate CSF circulation in individuals with hydrocephalus is limited by a paucity of appropriate non-invasive molecular imaging tools. CSF circulation is thought to occur either according to the bulk flow model or the hydrodynamic model. In the bulk flow model, CSF is secreted by the CPe and enters the venous system via arachnoid granulations. According to this model, hydrocephalus results from the obstruction of CSF as it flows from the ventricles to the arachnoid granulations5. In the hydrodynamic model, arterial systolic pressure waves entering the brain are transmitted to the subarachnoid spaces, venous capacitance vessels, and interact with intraventricular pulsations transmitted by the choroid plexus195,196. The intraventricular pulsations facilitate CSF egress through the ventricular outlet foraminas. In this model, hydrocephalus is caused by decreased elasticity (secondary to increased ICP or hypertension) of these pulsation absorbing structures, contributing to abnormally high pulsation amplitudes that result in ventricular expansion. Elements of both models are likely to be operative in both normal CSF homeostasis and hydrocephalus, with possible contributions from glymphatic and lymphatic pathways.
Imaging modalities such as contrast magnetic resonance imaging have been used to assess CSF flow in the Sylvian aqueduct between the third and fourth ventricles. In some cases of communicating hydrocephalus, these imaging measurements have revealed up to 6-fold increased retrograde fluid flow through the aqueduct.197 Other non-invasive imaging modalities such as time-spatial labelling inversion pulse imaging198,199 enable measurement of CSF movement in real-time and could be applied to the study of hydrocephalus. Innovative imaging reagents such as bis-5-hydroxy-tryptamide-diethylenetriaminepentaacetate gadolinium with cross-linked iron oxide nanoparticles200 or europium-doped very small superparamagnetic iron oxide particles201 seem to be particularly sensitive detectors of CPe neuroinflammation. However, these reagents have not yet been applied to the study of inflammatory hydrocephalus.
Conclusions
Emerging data have identified inflammatory pathways involving TLR4-regulated CSF cytokines and immune cells, that are likely to be important for the pathogenesis of hydrocephalus, suggesting that pharmacological prevention of PIH and PHH is feasible. Nonetheless, much additional work is needed before any of the potential treatment strategies discussed in this Review can be tested in clinical trials. This work will involve the continued identification of specific inflammatory mechanisms that contribute to the pathogenesis of PHH and PIH; development of pharmacological agents that modulate these targets; and pre-clinical trials of these agents in relevant experimental models. A therapeutic approach that addresses neuroinflammation might not only prevent shunt-dependence, but might also ameliorate the neurodevelopmental sequelae of PIH and PHH that are not addressed by surgical CSF diversion, for example, inflammation-induced tissue damage and resultant cerebral palsy. Such an approach would reduce the lifelong morbidity and economic burden associated with hydrocephalus surgery, and could be life-saving in regions with limited access to neurosurgical care.
Supplementary Material
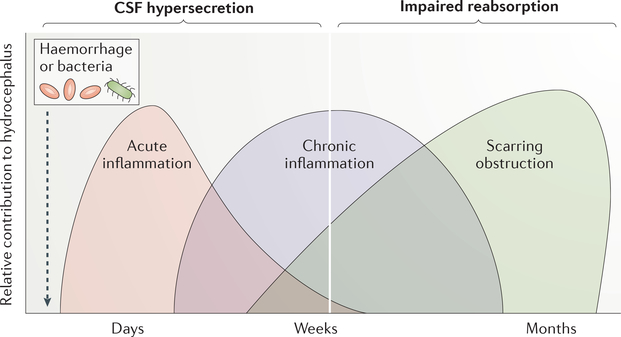
Figure 4: Proposed inflammatory contributors to PHH and PIH.
Illustration showing the relative contribution of inflammation to hydrocephalus in the days week and months following haemorrhage or bacterial infection. CNS exposure to foreign pathogen-derived damage-associated molecular patterns (PAMPs), for example bacterial cell wall fragments, or host-derived damage-associated molecular patterns (DAMPs), for example blood-breakdown products, leads to an acute inflammatory response (red) in the CSF that takes place in the days to weeks after haemorrhage or infection. This response is characterized by recruitment of immune cells (e.g. microglia) and TLR4-dependent CSF hypersecretion by the CPe. Tissue damage, including friability and denudation at CSFbrain (ependyma) and CSF-blood (CPe) barrier sites, is likely to propagate and sustain the initial infectious or traumatic insult via release of other DAMPs, resulting in a transition from acute reparative inflammation (red) to chronic pathological inflammation (blue). This chronic inflammation is likely to result in scarring and obstruction of CSF drainage pathways (e.g., brain parenchymal glymphatics and meningeal lymphatics; green), which would impair CSF reabsorption. Early modulation of TLR4 activity in post-infectious and post-haemorrhagic hydrocephalus could reduce the acute CPe hypersecretory response, and prevent chronic inflammation-induced scarring. In addition, anti-inflammatory therapies offer the potential advantage of preventing the need for surgical CSF diversion, and alleviating inflammation-induced brain damage that contributes to poor long-term neurodevelopmental outcomes.
Key points.
Hydrocephalus (enlarged brain ventricles associated with failed cerebrospinal fluid [CSF] homeostasis) is the most common neurosurgical disorder and is treated mainly by neurosurgical CSF diversion procedures with high rates of morbidity and failure.
Post-hemorrhagic hydrocephalus (PHH) and post-infectious hydrocephalus (PIH), the most common causes of hydrocephalus, are both characterized by inflammation in the brain tissue and CSF space.
Recent data have begun to uncover the molecular mechanisms by which inflammation, driven by activation of to 11-like receptor-4 (TLR4), contributes to the pathogenesis of hydrocephalus.
Pharmacotherapeutic approaches that target inflammation have the potential to address multiple drivers of PHH and PIH, for example, acute hypersecretion of CSF by the choroid plexus epithelium and scarring of CSF drainage pathways.
Acknowledgements
K.T.K. is supported by NIH grants NRCDP K12-228168, 1RO1NS109358, and R01 NS11102901 Al; the Hydrocephalus Association; the Rudy Schulte Research Institute; and the Simons Foundation. J.K.K. is supported by the Howard Hughes Medical Institute. M.N. is supported by NIH 1R01NS100366 and RF1 AG057575-01. P.Q.D. is supported by NIH Medical Scientist Training Program Training Grant T32GM007205. B.C.W. is supported by NIH grants 1R01HD096693 and 7R01HD085853 and NIH Director’s Pioneer Award 5DP1HD086071. S.J.S. is supported by NIH Director’s Pioneer Award, NIH Director’s Transformative Award 1R01AI145057, and NIH grants 1R01HD096693 and 7R01HD085853. D.D.L. is supported by NIH Director’s Pioneer Award 5DP1HD086071, the Patient Centered Outcomes Research Institute (PCORI 1503-29700), the Hydrocephalus Association, and the Rudy Schulte Research Institute. D.D.L. also receives research support through Microbot Medical, Inc. J.M.S. is supported by grants from the Department of Veterans Affairs (I01BX002889), the Department of Defense (SCI170199), the National Heart, Lung and Blood Institute (R01HL082517) and the National Institute of Neurological Disorders and Stroke (R01NS060801; R01NS102589; R01NS105633). The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Glossary
- Time-spatial labelling inversion pulse imaging
A non-contrast magnetic resonance imaging (MRI) technique using cerebrospinal fluid (CSF) as a tracer to measure CSF flow.
- Bulk flow model
Movement of cerebrospinal fluid (CSF) from the choroid plexus through the cerebro-ventricles and cisterns to the subarachnoid space, where reabsorbtion through the arachnoid granulations occurs.
- Neuro-humoral mechanisms
Sympathetic and hormonal regulation of CSF production. Periventricular heterotopia: Bilateral nodules of grey matter lining the lateral ventricles consisting of neurons that failed to migrate during fetal development.
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria
We searched PubMed for articles in all year ranges with multiple combinations of search terms including, “post-haemorrhagic hydrocephalus”, “post-infectious hydrocephalus”, “worldwide”, “epidemiology”, “ETV/CPC”, “VP Shunt”, “NKCC1”, “SPAK”, “Toll-like receptors” “inflammation”, “obstruction”, “impaired reabsorption”, “CSF hypersecretion”, “cerebrospinal fluid”. There were no language exclusions and articles chosen were based on relevance to topics covered in this Review.
Peer review information
Nature Reviews Neurology thanks [Referee# 1 name], [Referee#2 name] and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
References
- 1.Rekate HL A contemporary definition and classification of hydrocephalus. Seminars in pediatric neurology 16, 9–15 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Benveniste H, Lee H & Volkow ND The Glymphatic Pathway: Waste Removal from the CNS via Cerebrospinal Fluid Transport. Neuroscientist 23, 454–465 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinker T, Stopa E, Morrison J & Klinge P A new look at cerebrospinal fluid circulation. Fluids and barriers of the CNS 11, 10 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furey CG, et al. De novo mutation in genes regulating neural stem cell fate in human congenital hydrocephalus. Neuron 99, 302–314e304 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahle KT, Kulkarni AV, Limbrick DD Jr. & Warf BC Hydrocephalus in children. Lancet 387, 788–799 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Karimy JK, et al. Cerebrospinal fluid hypersecretion in pediatric hydrocephalus. Neurosurg Focus 41, E10 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Karimy JK, et al. Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nature Medicine (2017). [DOI] [PubMed] [Google Scholar]
- 8.Dewan MC, et al. Global hydrocephalus epidemiology and incidence: systematic review and meta-analysis. Journal of neurosurgery, 1–15 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Cherian S, Whitelaw A, Thoresen M & Love S The pathogenesis of neonatal posthemorrhagic hydrocephalus. Brain Pathol 14, 305–311 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strahle J, et al. Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage. Transl. Stroke Res 3, 25–38 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddy GK, Bollam P & Caldito G Long-term outcomes of ventriculoperitoneal shunt surgery in patients with hydrocephalus. World neurosurgery 81, 404–410 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Isaacs AM, et al. Age-specific global epidemiology of hydrocephalus: Systematic review, metanalysis and global birth surveillance. PLoS One 13, e0204926 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warf BC, Campbell JW & Riddle E Initial experience with combined endoscopic third ventriculostomy and choroid plexus cauterization for post-hemorrhagic hydrocephalus of prematurity: the importance of prepontine cistern status and the predictive value of FIESTA MRI imaging. Child’s nervous system: ChNS: official journal of the International Society for Pediatric Neurosurgery 27, 1063–1071 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Chen Q, et al. Post-hemorrhagic hydrocephalus: Recent advances and new therapeutic insights. Journal of the neurological sciences 375, 220–230 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Tsitouras V & Sgouros S Infantile posthemorrhagic hydrocephalus. Child’s nervous system: ChNS: official journal of the International Society for Pediatric Neurosurgery 27,1595–1608 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Murphy BP, et al. Posthaemorrhagic ventricular dilatation in the premature infant: natural history and predictors of outcome. Archives of disease in childhood. Fetal and neonatal edition 87, F37–41 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warf BC East African Neurosurgical Research, C. Pediatric hydrocephalus in East Africa: prevalence, causes, treatments, and strategies for the future. World neurosurgery 73,296–300(2010). [DOI] [PubMed] [Google Scholar]
- 18.Bir SC, et al. Epidemiology of adult-onset hydrocephalus: institutional experience with 2001 patients. Neurosurg Focus 41, E5 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Chahlavi A, El-Babaa SK & Luciano MG Adult-onset hydrocephalus. Neurosurgery clinics of North America 12, 753–760, ix (2001). [PubMed] [Google Scholar]
- 20.Cioca A, Gheban D, Perju-Dumbrava D, Chiroban O & Mera M Sudden death from ruptured choroid plexus arteriovenous malformation. The American journal of forensic medicine and pathology 35, 100–102 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Muir RT, Wang S & Warf BC Global surgery for pediatric hydrocephalus in the developing world: a review of the history, challenges, and future directions. Neurosurg Focus 41, E11 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Aziz IA Hydrocephalus in the Sudan. J R Coll Surg Edinb 21, 222–224 (1976). [PubMed] [Google Scholar]
- 23.Kamat AS, Gretschel A, Vlok AJ & Solomons R CSF protein concentration associated with ventriculoperitoneal shunt obstruction in tuberculous meningitis. The international journal of tuberculosis and lung disease: the official journal of the International Union against Tuberculosis and Lung Disease 22, 788–792 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Aranha A, Choudhary A, Bhaskar S & Gupta LN A Randomized Study Comparing Endoscopic Third Ventriculostomy versus Ventriculoperitoneal Shunt in the Management of Hydrocephalus Due to Tuberculous Meningitis. Asian journal of neurosurgery 13, 1140–1147(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajshekhar V Management of hydrocephalus in patients with tuberculous meningitis. Neurology India 57, 368–374 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Li K, et al. Clinical features, long-term clinical outcomes, and prognostic factors of tuberculous meningitis in West China: a multivariate analysis of 154 adults. Expert review of anti-infective therapy 15, 629–635 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Lee LV Neurotuberculosis among Filipino children: an 11 years experience at the Philippine Children’s Medical Center. Brain Dev 22, 469–474 (2000). [DOI] [PubMed] [Google Scholar]
- 28.van der Linden V, et al. Association of Severe Hydrocephalus With Congenital Zika Syndrome. JAMAneurology (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kulkarni AV, et al. Endoscopic Treatment versus Shunting for Infant Hydrocephalus in Uganda. The New England journal of medicine 377, 2456–2464 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li L, et al. Association of bacteria with hydrocephalus in Ugandan infants. Journal of neurosurgery. Pediatrics 7, 73–87 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Thigpen MC, et al. Bacterial meningitis in the United States, 1998–2007. The New England journal of medicine 364, 2016–2025 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Pyrgos V, Seitz AE, Steiner CA, Prevots DR & Williamson PR Epidemiology of cryptococcal meningitis in the US: 1997–2009. PLoS One 8, e56269 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, et al. Ventriculoperitoneal shunts in non-HIV cryptococcal meningitis. BMC neurology 18, 58 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schiff SJ, Ranjeva SL, Sauer TD & Warf BC Rainfall drives hydrocephalus in East Africa. Journal of neurosurgery. Pediatrics 10, 161–167 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Warf BC Comparison of endoscopic third ventriculostomy alone and combined with choroid plexus cauterization in infants younger than 1 year of age: a prospective study in 550 African children. Journal of neurosurgery 103, 475–481 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Warf BC Hydrocephalus in Uganda: the predominance of infectious origin and primary management with endoscopic third ventriculostomy. Journal of neurosurgery 102, 1–15 (2005). [DOI] [PubMed] [Google Scholar]
- 37.Stagno V, Navarrete EA, Mirone G & Esposito F Management of hydrocephalus around the world. World neurosurgery 79, S23e17–20 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Kulkarni AV First Treatment in Infants With Hydrocephalus: The Case for Shunt. Neurosurgery 63 Suppl 1, 73–77 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Kulkarni AV, et al. Outcomes of CSF shunting in children: comparison of Hydrocephalus Clinical Research Network cohort with historical controls: clinical article. Journal of neurosurgery. Pediatrics 12, 334–338 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Kulkarni AV, et al. Endoscopic third ventriculostomy vs cerebrospinal fluid shunt in the treatment of hydrocephalus in children: a propensity score-adjusted analysis. Neurosurgery 67, 588–593 (2010). [DOI] [PubMed] [Google Scholar]
- 41.Drake JM, Kulkarni AV & Kestle J Endoscopic third ventriculostomy versus ventriculoperitoneal shunt in pediatric patients: a decision analysis. Child’s nervous system: ChNS: official journal of the International Society for Pediatric Neurosurgery 25, 467–472 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Kulkarni AV, et al. Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus. The Journal of pediatrics 155, 254–259e251 (2009). [DOI] [PubMed] [Google Scholar]
- 43.Limbrick DD Jr., Baird LC, Klimo P Jr., Riva-Cambrin J & Flannery AM Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 4: Cerebrospinal fluid shunt or endoscopic third ventriculostomy for the treatment of hydrocephalus in children. Journal of neurosurgery. Pediatrics 14 Suppl 1, 30–34 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Pindrik J, Jallo GI & Ahn ES Complications and subsequent removal of retained shunt hardware after endoscopic third ventriculostomy: case series. Journal of neurosurgery. Pediatrics 11, 722–726 (2013). [DOI] [PubMed] [Google Scholar]
- 45.Baird LC First Treatment in Infants With Hydrocephalus: The Case for Endoscopic Third Ventriculostomy/Choroid Plexus Cauterization. Neurosurgery 63 Suppl 1, 78–82 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Kulkarni AV, et al. Endoscopic third ventriculostomy and choroid plexus cauterization in infants with hydrocephalus: a retrospective Hydrocephalus Clinical Research Network study. Journal of neurosurgery. Pediatrics 14, 224–229 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Marques F, et al. The choroid plexus in health and in disease: dialogues into and out of the brain. Neurobiology of disease 107, 32–40 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Ghersi-Egea JF, et al. Molecular anatomy and functions of the choroidal blood-cerebrospinal fluid barrier in health and disease. Acta Neuropathol 135, 337–361 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Lauer AN, Tenenbaum T, Schroten H & Schwerk C The diverse cellular responses of the choroid plexus during infection of the central nervous system. American journal of physiology. Cell physiology 314, C152–C165 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Damkier HH, Brown PD & Praetorius J Cerebrospinal fluid secretion by the choroid plexus. Physiol Rev 93, 1847–1892 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Group IPDT International randomised controlled trial of acetazolamide and furosemide in posthaemorrhagic ventricular dilatation in infancy. International PHVD Drug Trial Group. Lancet 352, 433–440 (1998). [PubMed] [Google Scholar]
- 52.Whitelaw A, Kennedy CR & Brion LP Diuretic therapy for newborn infants with posthemorrhagic ventricular dilatation. The Cochrane database of systematic reviews, Cd002270 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Libenson MH, Kaye EM, Rosman NP & Gilmore HE Acetazolamide and furosemide for posthemorrhagic hydrocephalus of the newborn. Pediatric neurology 20, 185–191 (1999). [DOI] [PubMed] [Google Scholar]
- 54.Teppema LJ & Dahan A Acetazolamide and breathing. Does a clinical dose alter peripheral and central CO(2) sensitivity? American journal of respiratory and critical care medicine 160, 1592–1597 (1999). [DOI] [PubMed] [Google Scholar]
- 55.Erker T, et al. The bumetanide prodrug BUM5, but not bumetanide, potentiates the antiseizure effect of phenobarbital in adult epileptic mice. Epilepsia (2016). [DOI] [PubMed] [Google Scholar]
- 56.Tollner K, et al. A novel prodrug-based strategy to increase effects of bumetanide in epilepsy. Annals of neurology 75, 550–562 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Seelig A, Gottschlich R & Devant RM A method to determine the ability of drugs to diffuse through the blood-brain barrier. Proceedings of the National Academy of Sciences of the United States of America 91, 68–72 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Larroche JC Post-haemorrhagic hydrocephalus in infancy. Anatomical study. Biology of the neonate 20, 287–299 (1972). [DOI] [PubMed] [Google Scholar]
- 59.Omar AT, Bagnas MAC, Del Rosario-Blasco KAR, Diestro JDB, Khu KJO Shunt surgery for neurocutaneous melanosis with hydrocephalus: case report and review of the literature. World neurosurgery (2018). [DOI] [PubMed] [Google Scholar]
- 60.Whitelaw A Intraventricular haemorrhage and posthaemorrhagic hydrocephalus: pathogenesis, prevention and future interventions. Seminars in neonatology: SN 6, 135–146. (2001). [DOI] [PubMed] [Google Scholar]
- 61.Lategan B, Chodirker BN, Del Bigio MR Fetal hydrocephalus caused by cryptic intraventricular hemorrhage. Brain Pathol 20, 391–398 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hill A, Shackelford GD & Volpe J J. A potential mechanism of pathogenesis for early posthemorrhagic hydrocephalus in the premature newborn. Pediatrics 73, 19–21 (1984). [PubMed] [Google Scholar]
- 63.Milhorat TH, Hammock MK, Davis DA & Fenstermacher JD Choroid plexus papilloma. I. Proof of cerebrospinal fluid overproduction. Childs Brain 2, 273–289 (1976). [PubMed] [Google Scholar]
- 64.Gram M, et al. Extracellular hemoglobin - mediator of inflammation and cell death in the choroid plexus following preterm intraventricular hemorrhage. Journal of neuroinflammation 11, 200 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gram M, et al. Hemoglobin induces inflammation after preterm intraventricular hemorrhage by methemoglobin formation. Journal of neuroinflammation 10, 100 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simard PF, et al. Inflammation of the choroid plexus and ependymal layer of the ventricle following intraventricular hemorrhage. Transl. Stroke Res 2, 227–231 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barichello T, et al. Pathophysiology of neonatal acute bacterial meningitis. Journal of medical microbiology 62, 1781–1789 (2013). [DOI] [PubMed] [Google Scholar]
- 68.Bateman GA & Brown KM The measurement of CSF flow through the aqueduct in normal and hydrocephalic children: from where does it come, to where does it go? Child’s nervous system: ChNS: official journal of the International Society for Pediatric Neurosurgery 28, 55–63 (2012). [DOI] [PubMed] [Google Scholar]
- 69.Oi S & Di Rocco C Proposal of "evolution theory in cerebrospinal fluid dynamics" and minor pathway hydrocephalus in developing immature brain. Child’s nervous system: ChNS: official journal of the International Society for Pediatric Neurosurgery 22, 662–669.(2006). [DOI] [PubMed] [Google Scholar]
- 70.Oreskovic D, Rados M & Klarica M Role of choroid plexus in cerebrospinal fluid hydrodynamics. Neuroscience 354, 69–87 (2017). [DOI] [PubMed] [Google Scholar]
- 71.Miyajima M & Arai H Evaluation of the Production and Absorption of Cerebrospinal Fluid. Neurologia medico-chirurgica 55, 647–656 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lohrberg M & Wilting J The lymphatic vascular system of the mouse head. Cell Tissue Res 366, 667–677 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Olstad EW, et al. Ciliary Beating Compartmentalizes Cerebrospinal Fluid Flow in the Brain and Regulates Ventricular Development. Current biology: CB 29, 229–24le226 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gao C, et al. Role of red blood cell lysis and iron in hydrocephalus after intraventricular hemorrhage. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism 34, 1070–1075 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Polis B, Polis L & Nowoslawska E Surgical treatment of post-inflammatory hydrocephalus. Analysis of 101 cases. Child’s nervous system: ChNS: official journal of the International Society for Pediatric Neurosurgery 35, 237–243 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raouf A, Zidan I & Mohamed E Endoscopic third ventriculostomy for post-inflammatory hydrocephalus in pediatric patients: is it worth a try? Neurosurg Rev 38, 149–155; discussion 155 (2015). [DOI] [PubMed] [Google Scholar]
- 77.Acute Hydrocephalus, or Water in the Head, an Inflammatory Disease, and Curable Equally and by the Same Means with Other Diseases of Inflammation. The British and foreign medical review 11, 151–158 (1841). [PMC free article] [PubMed] [Google Scholar]
- 78.Davis DD Acute Hydrocephalus, Or, Water in the Head: An Inflammatory Disease, and Curable Equally by the Same Means with Other Diseases of Inflammation, (Taylor & Walton, 1840). [PMC free article] [PubMed] [Google Scholar]
- 79.Hydrocephalus Reconsidered; and its Relations to Inflammation and Irritation of the Brain defined, with Cases from Hospital and Private Practice, &c. Provincial Medical and Surgical Journal s1–15, 16–17 (1851). [Google Scholar]
- 80.Sharma S, et al. Cytokines do play a role in pathogenesis of tuberculous meningitis: A prospective study from a tertiary care center in India. Journal of the neurological sciences 379, 131–136 (2017). [DOI] [PubMed] [Google Scholar]
- 81.Chaudhry SR, et al. Elevated Systemic IL-6 Levels in Patients with Aneurysmal Subarachnoid Hemorrhage Is an Unspecific Marker for Post-SAH Complications. International journal of molecular sciences 18(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kitazawa K & Tada T Elevation of transforming growth factor-beta 1 level in cerebrospinal fluid of patients with communicating hydrocephalus after subarachnoid hemorrhage. Stroke 25, 1400–1404 (1994). [DOI] [PubMed] [Google Scholar]
- 83.Whitelaw A, Christie S & Pople I Transforming growth factor-betal: a possible signal molecule for posthemorrhagic hydrocephalus? Pediatr. Res 46, 576–580 (1999). [DOI] [PubMed] [Google Scholar]
- 84.Mlakar J, et al. Zika Virus Associated with Microcephaly. The New England journal of medicine 374, 951–958 (2016). [DOI] [PubMed] [Google Scholar]
- 85.Ulfig N, Bohl J, Neudorfer F & Rezaie P Brain macrophages and microglia in human fetal hydrocephalus. Brain Dev 26, 307–315 (2004). [DOI] [PubMed] [Google Scholar]
- 86.Thwaites GE, et al. Serial MRI to determine the effect of dexamethasone on the cerebral pathology of tuberculous meningitis: an observational study. Lancet Neurol 6, 230–236 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schurkamper M, Medele R, Zausinger S, Schmid-Elsaesser R & Steiger HJ Dexamethasone in the treatment of subarachnoid hemorrhage revisited: a comparative analysis of the effect of the total dose on complications and outcome. J Clin Neurosci 11, 20–24 (2004). [DOI] [PubMed] [Google Scholar]
- 88.Gutierrez-Murgas YM, Skar G, Ramirez D, Beaver M & Snowden JN IL-10 plays an important role in the control of inflammation but not in the bacterial burden in S. epidermidis CNS catheter infection. Journal of neuroinflammation 13, 271 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hausler M, et al. Murine gammaherpesvirus-68 infection of mice: A new model for human cerebral Epstein-Barr virus infection. Annals of neurology 57, 600–603 (2005). [DOI] [PubMed] [Google Scholar]
- 90.Zhu W, et al. Mouse models of intracerebral hemorrhage in ventricle, cortex, and hippocampus by injections of autologous blood or collagenase. PLoS One 9, e97423 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Harada T, Takamoto M, Jin DH, Tada T & Sugane K Young C3H mice infected with Toxoplasma gondii are a novel experimental model of communicating hydrocephalus. Neurological research 29, 615–621 (2007). [DOI] [PubMed] [Google Scholar]
- 92.Guo J, et al. Minocycline-induced attenuation of iron overload and brain injury after experimental germinal matrix hemorrhage. Brain research 1594, 115–124 (2015). [DOI] [PubMed] [Google Scholar]
- 93.Sansing LH, et al. Toll-like receptor 4 contributes to poor outcome after intracerebral hemorrhage. Annals of neurology 70, 646–656 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang YC, et al. Toll-like receptor 4 antagonist attenuates intracerebral hemorrhageinduced brain injury. Stroke 44, 2545–2552 (2013). [DOI] [PubMed] [Google Scholar]
- 95.Lattke M, Magnutzki A, Walther P, Wirth T & Baumann B Nuclear factor kappaB activation impairs ependymal ciliogenesis and links neuroinflammation to hydrocephalus formation. The Journal of neuroscience: the official journal of the Society for Neuroscience 32, 11511–11523 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Galbreath E, Kim SJ, Park K, Brenner M & Messing A Overexpression of TGFbeta 1 in the central nervous system of transgenic mice results in hydrocephalus. Journal of neuropathology and experimental neurology 54, 339–349 (1995). [DOI] [PubMed] [Google Scholar]
- 97.Tada T, Kanaji M & Kobayashi S Induction of communicating hydrocephalus in mice by intrathecal injection of human recombinant transforming growth factor-beta 1. Journal of neuroimmunology 50, 153–158 (1994). [DOI] [PubMed] [Google Scholar]
- 98.Lindvall-Axelsson M, Hedner P & Owman C Corticosteroid action on choroid plexus: reduction in Na+-K+-ATPase activity, choline transport capacity, and rate of CSF formation. Exp Brain Res 77, 605–610 (1989). [DOI] [PubMed] [Google Scholar]
- 99.Weiss MH & Nulsen FE The effect of glucocorticoids on CSF flow in dogs. Journal of neurosurgery 32, 452–458 (1970). [DOI] [PubMed] [Google Scholar]
- 100.Sato O, Hara M, Asai T, Tsugane R & Kageyama N The effect of dexamethasone phosphate on the production rate of cerebrospinal fluid in the spinal subarachnoid space of dogs. Journal of neurosurgery 39, 480–484 (1973). [DOI] [PubMed] [Google Scholar]
- 101.Steffensen AB, et al. Cotransporter-mediated water transport underlying cerebrospinal fluid formation. Nature communications 9, 2167 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Keep RF & Jones HC A morphometric study on the development of the lateral ventricle choroid plexus, choroid plexus capillaries and ventricular ependyma in the rat. Brain research. Developmental brain research 56, 47–53 (1990). [DOI] [PubMed] [Google Scholar]
- 103.Praetorius J Water and solute secretion by the choroid plexus. Pflugers Arch 454, 1–18 (2007). [DOI] [PubMed] [Google Scholar]
- 104.Praetorius J & Damkier HH Transport across the choroid plexus epithelium. American journal of physiology. Cell physiology 312, C673–c686 (2017). [DOI] [PubMed] [Google Scholar]
- 105.Medzhitov R TFR-mediated innate immune recognition. Seminars in immunology 19, 1–2. (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Coorens M, et al. Cathelicidins Inhibit Escherichia coli-Induced TFR2 and TFR4 Activation in a Viability-Dependent Manner. Journal of immunology (Baltimore, Md.: 1950) 199, 1418–1428 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Skipor J, Szczepkowska A, Kowalewska M, Herman AP & Fisiewski P Profile of toll-like receptor mRNA expression in the choroid plexus in adult ewes. Acta veterinaria Hungarica 63, 69–78 (2015). [DOI] [PubMed] [Google Scholar]
- 108.Rivest S Molecular insights on the cerebral innate immune system. Brain, behavior, and immunity 17, 13–19 (2003). [DOI] [PubMed] [Google Scholar]
- 109.Miyake K Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Seminars in immunology 19, 3–10 (2007). [DOI] [PubMed] [Google Scholar]
- 110.Fang H, et al. Toll-like receptor 4 (TFR4) is essential for Hsp70-like protein 1 (HSP70F1) to activate dendritic cells and induce Thl response. The Journal of biological chemistry 286, 30393–30400 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsan MF & Gao B Endogenous ligands of Toll-like receptors. Journal of leukocyte biology 76, 514–519(2004). [DOI] [PubMed] [Google Scholar]
- 112.Chen S, Luo J, Reis C, Manaenko A & Zhang J Hydrocephalus after Subarachnoid Hemorrhage: Pathophysiology, Diagnosis, and Treatment. BioMed research international 2017, 8584753 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Okamoto T, et al. Matrix metalloproteinases in infants with posthemorrhagic hydrocephalus. Early Hum. Dev 84, 137–139 (2008). [DOI] [PubMed] [Google Scholar]
- 114.Ehrchen JM, Sunderkotter C, Foell D, Vogl T & Roth J The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. Journal of leukocyte biology 86, 557–566 (2009). [DOI] [PubMed] [Google Scholar]
- 115.Yang B, Zhou Z, Li X & Niu J The effect of lysophosphatidic acid on Toll-like receptor 4 expression and the nuclear factor-KB signaling pathway in THP-1 cells. Mol Cell Biochem 422, 41–49 (2016). [DOI] [PubMed] [Google Scholar]
- 116.Kwon MS, et al. Methemoglobin is an endogenous toll-like receptor 4 ligand-relevance to subarachnoid hemorrhage. International journal of molecular sciences 16, 5028–5046 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Demeestere D, Libert C & Vandenbroucke RE Clinical implications of leukocyte infiltration at the choroid plexus in (neuro)inflammatory disorders. Drug discovery today 20,928–941 (2015). [DOI] [PubMed] [Google Scholar]
- 118.Kleine TO & Benes L Immune surveillance of the human central nervous system (CNS): different migration pathways of immune cells through the blood-brain barrier and blood-cerebrospinal fluid barrier in healthy persons. Cytometry. Part A: the journal of the International Society for Analytical Cytology 69, 147–151 (2006). [DOI] [PubMed] [Google Scholar]
- 119.Wang YC, et al. Toll-like receptor 2/4 heterodimer mediates inflammatory injury in intracerebral hemorrhage. Annals of neurology 75, 876–889 (2014). [DOI] [PubMed] [Google Scholar]
- 120.Cox KH, Cox ME, Woo-Rasberry V & Hasty DL Pathways involved in the synergistic activation of macrophages by lipoteichoic acid and hemoglobin. PLoS One 7, e47333 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Berkes J, Viswanathan VK, Savkovic SD & Hecht G Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut 52, 439–451 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wilson R, et al. Upper respiratory tract viral infection and mucociliary clearance. European journal of respiratory diseases 70, 272–279 (1987). [PubMed] [Google Scholar]
- 123.Doyle WJ, et al. Nasal and otologic effects of experimental influenza A virus infection. The Annals of otology, rhinology, and laryngology 103, 59–69 (1994). [DOI] [PubMed] [Google Scholar]
- 124.Kotas ME & Medzhitov R Homeostasis, inflammation, and disease susceptibility. Cell 160,816–827(2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nowarski R, Jackson R & Flavell RA The Stromal Intervention: Regulation of Immunity and Inflammation at the Epithelial-Mesenchymal Barrier. Cell 168, 362–375 (2017). [DOI] [PubMed] [Google Scholar]
- 126.Karimy JK, et al. A novel method to study cerebrospinal fluid dynamics in rats. J. Neurosci. Methods 241, 78–84 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Piechotta K, Garbarini N, England R & Delpire E Characterization of the interaction of the stress kinase SPAK with the Na+−K+−2Cl− cotransporter in the nervous system: evidence for a scaffolding role of the kinase. The Journal of biological chemistry 278, 52848–52856 (2003). [DOI] [PubMed] [Google Scholar]
- 128.Shekarabi M, et al. WNK Kinase Signaling in Ion Homeostasis and Human Disease. Cell metabolism 25, 285–299 (2017). [DOI] [PubMed] [Google Scholar]
- 129.Yan Y & Merlin D Ste20-related proline/alanine-rich kinase: a novel regulator of intestinal inflammation. World journal of gastroenterology 14, 6115–6121 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yan Y, et al. Nuclear factor-kappaB is a critical mediator of Ste20-like proline-/alaninerich kinase regulation in intestinal inflammation. The American journal of pathology 173, 1013–1028 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Thiagarajah JR, Donowitz M & Verkman AS Secretory diarrhoea: mechanisms and emerging therapies. Nat. Rev. Gastroenterol. Hepatol 12, 446–457 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lin TJ, et al. SPAK plays a pathogenic role in IgA nephropathy through the activation of NF-kappaB/MAPKs signaling pathway. Free radical biology & medicine 99, 214–224 (2016). [DOI] [PubMed] [Google Scholar]
- 133.Lan CC, et al. Inhibition of Na-K-Cl cotransporter isoform 1 reduces lung injury induced by ischemia-reperfusion. The Journal of thoracic and cardiovascular surgery 153,206–215 (2017). [DOI] [PubMed] [Google Scholar]
- 134.Yan Y, Nguyen H, Dalmasso G, Sitaraman SV & Merlin D Cloning and characterization of a new intestinal inflammation-associated colonic epithelial Ste20-related protein kinase isoform. Biochimica et biophysica acta 1769, 106–116 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Polek TC, Talpaz M, Spivak-Kroizman T The TNF receptor, RELT, binds SPAK and uses it to mediate p38 and JNK activation. Biochemical and biophysical research communications 343, 125–134 (2006). [DOI] [PubMed] [Google Scholar]
- 136.Alessi DR, et al. The WNK-SPAK/OSR1 pathway: master regulator of cation-chloride cotransporters. Science signaling 7, re3 (2014). [DOI] [PubMed] [Google Scholar]
- 137.Thastrup JO, et al. SPAK/OSR1 regulate NKCC1 and WNK activity: analysis of WNK isoform interactions and activation by T-loop trans-autophosphorylation. Biochem. J 441, 325–337 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.de Los HP, et al. The WNK-regulated SPAK/OSR1 kinases directly phosphorylate and inhibit the K+−Cl- co-transporters. Biochem. J 458, 559–573 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 139.Lee D, Lee SA, Shin DM & Hong JH Chloride Influx of Anion Exchanger 2 Was Modulated by Calcium-Dependent Spinophilin in Submandibular Glands. Front Physiol 9,889 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li Q, et al. Targeting Germinal Matrix Hemorrhage-Induced Overexpression of Sodium-Coupled Bicarbonate Exchanger Reduces Posthemorrhagic Hydrocephalus Formation in Neonatal Rats. J Am Heart Assoc 7(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kim KS Mechanisms of microbial traversal of the blood-brain barrier. Nature reviews. Microbiology 6, 625–634 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Koedel U, Klein M & Pfister H-W New understandings on the pathophysiology of bacterial meningitis. Curr Opin Infect Dis 23, 217–223 (2010). [DOI] [PubMed] [Google Scholar]
- 143.Deopujari CE, Padayachy L, Azmi A, Figaji A & Samantray SK Neuroendoscopy for post-infective hydrocephalus in children. Child’s nervous system: ChNS: official journal of the International Society for Pediatric Neurosurgery 34, 1905–1914 (2018). [DOI] [PubMed] [Google Scholar]
- 144.Sellner J, Tauber MG & Leib SL Pathogenesis and pathophysiology of bacterial CNS infections. Handbook of clinical neurology 96, 1–16 (2010). [DOI] [PubMed] [Google Scholar]
- 145.Malley R„ et al. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proceedings of the National Academy of Sciences of the United States of America 100, 1966–1971 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lahrtz F, Piali L, Spanaus KS, Seebach J & Fontana A Chemokines and chemotaxis of leukocytes in infectious meningitis. Journal of neuroimmunology 85, 33–43. (1998). [DOI] [PubMed] [Google Scholar]
- 147.Krebs VL, Okay TS, Okay Y & Vaz FA Tumor necrosis factor-alpha, interleukinlbeta and interleukin-6 in the cerebrospinal fluid of newborn with meningitis. Arquivos de neuro-psiquiatria 63, 7–13 (2005). [DOI] [PubMed] [Google Scholar]
- 148.van Furth AM, Roord JJ & van Furth R Roles of proinflammatory and anti-inflammatory cytokines in pathophysiology of bacterial meningitis and effect of adjunctive therapy. Infection and immunity 64, 4883–4890 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Grandgirard D & Leib SL Meningitis in neonates: bench to bedside. Clinics in perinatology 37, 655–676 (2010). [DOI] [PubMed] [Google Scholar]
- 150.Dessing MC, et al. Role played by Toll-like receptors 2 and 4 in lipoteichoic acidinduced lung inflammation and coagulation. The Journal of infectious diseases 197, 245–252. (2008). [DOI] [PubMed] [Google Scholar]
- 151.Flo TH, et al. Human toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. Journal of immunology (Baltimore, Md.: 1950) 164, 2064–2069 (2000). [DOI] [PubMed] [Google Scholar]
- 152.Janot L, et al. CD14 works with toll-like receptor 2 to contribute to recognition and control of Listeria monocytogenes infection. The Journal of infectious diseases 198, 115–124. (2008). [DOI] [PubMed] [Google Scholar]
- 153.Seki E, et al. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. Journal of immunology (Baltimore, Md.: 1950) 169 3863–3868 (2002). [DOI] [PubMed] [Google Scholar]
- 154.Mook-Kanamori BB, Geldhoff M, van der Poll T & van de Beek D Pathogenesis and pathophysiology of pneumococcal meningitis. Clinical microbiology reviews 24, 557–591 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hayashi F, et al. The innate immune response to bacterial flagellin is mediated by Tolllike receptor 5. Nature 410, 1099–1103 (2001). [DOI] [PubMed] [Google Scholar]
- 156.Mottahedin A, Joakim Ek C, Truve K, Hagberg H & Mallard C Choroid plexus transcriptome and ultrastructure analysis reveals a TLR2-specific chemotaxis signature and cytoskeleton remodeling in leukocyte trafficking. Brain, behavior, and immunity 79, 216–227(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jimenez AJ, Dominguez-Pinos MD, Guerra MM, Femandez-Llebrez P, PerezFigares JM Structure and function of the ependymal barrier and diseases associated with ependyma disruption. Tissue barriers 2, e28426 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Guerra MM, et al. Cell Junction Pathology of Neural Stem Cells Is Associated With Ventricular Zone Disruption, Hydrocephalus, and Abnormal Neurogenesis. Journal of neuropathology and experimental neurology 74, 653–671 (2015). [DOI] [PubMed] [Google Scholar]
- 159.Yung YC, et al. Lysophosphatidic acid signaling may initiate fetal hydrocephalus. Sci Transl Med 3, 99ra87 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bayatti N, et al. A molecular neuroanatomical study of the developing human neocortex from 8 to 17 postconceptional weeks revealing the early differentiation of the subplate and subventricular zone. Cerebral cortex (New York, N.Y.: 1991) 18, 1536–1548 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rodriguez EM, et al. A cell junction pathology of neural stem cells leads to abnormal neurogenesis and hydrocephalus. Biological research 45, 231–242 (2012). [DOI] [PubMed] [Google Scholar]
- 162.McAllister JP, et al. Ventricular Zone Disruption in Human Neonates With Intraventricular Hemorrhage. Journal of neuropathology and experimental neurology 76, 358–375 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Castaneyra-Ruiz L, et al. Blood Exposure Causes Ventricular Zone Disruption and Glial Activation In Vitro. Journal of neuropathology and experimental neurology 77, 803–813 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liu SF, Ye X & Malik AB Inhibition of NF- B Activation by Pyrrolidine Dithiocarbamate Prevents In Vivo Expression of Proinflammatory Genes. Circulation 100,1330–1337(1999). [DOI] [PubMed] [Google Scholar]
- 165.Hu Y, et al. Melatonin protects against blood-brain barrier damage by inhibiting the TLR4/ NF-kB signaling pathway after LPS treatment in neonatal rats. Oncotarget 8, 31638–31654(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Robinson S, et al. Extended combined neonatal treatment with erythropoietin plus melatonin prevents posthemorrhagic hydrocephalus of prematurity in rats. Front Cell Neurosci 12, 322 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rice TW, et al. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Critical care medicine 38, 1685–1694 (2010). [DOI] [PubMed] [Google Scholar]
- 168.Allette YM, et al. Decoy peptide targeted to Toll-IL-IR domain inhibits LPS and TLR4-active metabolite morphine-3 glucuronide sensitization of sensory neurons. Scientific reports 7, 3741 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jung K, et al. Toll-like receptor 4 decoy, TOY, attenuates gram-negative bacterial sepsis. PLoS One 4, e7403 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lemonnier E, et al. A randomised controlled trial of bumetanide in the treatment of autism in children. Transl. Psychiatry 2, e202 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lemonnier E & Ben-Ari Y The diuretic bumetanide decreases autistic behaviour in five infants treated during 3 months with no side effects. Acta Paediatr 99, 1885–1888 (2010). [DOI] [PubMed] [Google Scholar]
- 172.Lemonnier E, et al. Effects of bumetanide on neurobehavioral function in children and adolescents with autism spectrum disorders. Translational psychiatry 7, e1056 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pressler RM, et al. Bumetanide for the treatment of seizures in newborn babies with hypoxic ischaemic encephalopathy (NEMO): an open-label, dose finding, and feasibility phase 1/2 trial. Lancet Neurology 14, 469–477 (2015). [DOI] [PubMed] [Google Scholar]
- 174.Sveinsdottir S, et al. Altered expression of aquaporin 1 and 5 in the choroid plexus following preterm intraventricular hemorrhage. Developmental neuroscience 36, 542–551 (2014). [DOI] [PubMed] [Google Scholar]
- 175.Gharagozloo M, et al. NLR-Dependent Regulation of Inflammation in Multiple Sclerosis. Frontiers in immunology 8, 2012 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.White CS, Lawrence CB, Brough D & Rivers-Auty J Inflammasomes as therapeutic targets for Alzheimer’s disease. Brain Pathol 27, 223–234 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ringstad G, Vatnehol SAS & Eide PK Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain: a journal of neurology 140, 2691–2705 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ringstad G, et al. Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. JCI insight 3(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nedergaard M Neuroscience. Garbage truck of the brain. Science 340, 1529–1530 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ding Y, et al. Astrogliosis inhibition attenuates hydrocephalus by increasing cerebrospinal fluid reabsorption through the glymphatic system after germinal matrix hemorrhage. Exp Neurol 320, 113003 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Plog BA & Nedergaard M The Glymphatic System in Central Nervous System Health and Disease: Past, Present, and Future. Annual review of pathology 13, 379–394 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jin BJ, Smith AJ & Verkman AS Spatial model of convective solute transport in brain extracellular space does not support a "glymphatic" mechanism. The Journal of general physiology 148, 489–501 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Smith AJ, Yao X, Dix JA, Jin BJ & Verkman AS Test of the ’glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. Elife 6(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Smith AJ & Verkman AS The "glymphatic" mechanism for solute clearance in Alzheimer’s disease: game changer or unproven speculation? FASEB journal: official publication of the Federation of American Societies for Experimental Biology 32, 543–551. (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Iliff J & Simon M CrossTalk proposal: The glymphatic system supports convective exchange of cerebrospinal fluid and brain interstitial fluid that is mediated by perivascular aquaporin-4. The Journal of physiology 597, 4417–4419 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Smith AJ & Verkman AS Rebuttal from Alex J. Smith and Alan S. Verkman. The Journal of physiology 597, 4427–4428 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Simon M & Iliff J Rebuttal from Matthew Simon and Jeffrey Iliff. The Journal of physiology 597, 4425–4426 (2019). [DOI] [PubMed] [Google Scholar]
- 188.Eming SA, Hammerschmidt M, Krieg T & Roers A Interrelation of immunity and tissue repair or regeneration. Semin Cell Dev Biol 20, 517–527 (2009). [DOI] [PubMed] [Google Scholar]
- 189.Klingberg F, Hinz B & White ES The myofibroblast matrix: implications for tissue repair and fibrosis. The Journal of pathology 229, 298–309 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sarnat HB Ependymal reactions to injury. A review. Journal of neuropathology and experimental neurology 54, 1–15 (1995). [DOI] [PubMed] [Google Scholar]
- 191.Fukumizu M, Takashima S & Becker LE Neonatal posthemorrhagic hydrocephalus: neuropathologic and immunohistochemical studies. Pediatric neurology 13, 230–234 (1995). [DOI] [PubMed] [Google Scholar]
- 192.Marlin AE, Wald A, Hochwald GM & Malhan C Kaolin-induced hydrocephalus impairs CSF secretion by the choroid plexus. Neurology 28, 945–949 (1978). [DOI] [PubMed] [Google Scholar]
- 193.Silverberg GD, et al. Downregulation of cerebrospinal fluid production in patients with chronic hydrocephalus. Journal of neurosurgery 97, 1271–1275 (2002). [DOI] [PubMed] [Google Scholar]
- 194.Kosteljanetz M Cerebrospinal fluid production in subarachnoid haemorrhage. Br J Neurosurg 2, 161–167 (1988). [DOI] [PubMed] [Google Scholar]
- 195.Mestre H, et al. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nature communications 9, 4878 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wagshul ME, Eide PK & Madsen JR The pulsating brain: A review of experimental and clinical studies of intracranial pulsatility. Fluids and barriers of the CNS 8, 5 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hladky SB & Barrand MA Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids and barriers of the CNS 11, 26 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Shibukawa S, et al. Time-spatial Fabeling Inversion Pulse (Time-SFIP) with Pencil Beam Pulse: A Selective Fabeling Technique for Observing Cerebrospinal Fluid Flow Dynamics. Magn Reson Med Sci 17, 259–264 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yamada S, et al. Visualization of cerebrospinal fluid movement with spin labeling at MR imaging: preliminary results in normal and pathophysiologic conditions. Radiology 249,644–652(2008). [DOI] [PubMed] [Google Scholar]
- 200.Hoffmann A, et al. MRI of Iron Oxide Nanoparticles and Myeloperoxidase Activity Links Inflammation to Brain Edema in Experimental Cerebral Malaria. Radiology 290, 359–367 (2019). [DOI] [PubMed] [Google Scholar]
- 201.Millward JM, et al. Application of Europium-Doped Very Small Iron Oxide Nanoparticles to Visualize Neuroinflammation with MRI and Fluorescence Microscopy. Neuroscience (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.