Abstract
Objectives
Height loss is associated with vertebral fracture risk and osteoporosis. We assumed that height loss may indicate the risk of falls because the presence of osteoporosis is significantly associated with sarcopenia development. We studied the association of height loss with falls and sarcopenia.
Methods
This study included 610 community-dwelling women. We measured the height, weight, appendicular skeletal muscle mass index (ASMI), grip strength, and gait speed. Additionally, we recorded the individual’s tallest height, and the presence or absence of single or multiple falls during the preceding 12 months. The participants were classified into nonheight loss, 2- to 3-cm height loss, 3- to 4-cm height loss, and over 4-cm height loss groups. The association of height loss with falls and sarcopenia were examined using multiple logistic regression analysis.
Results
We found that 3- to 4-cm height loss and over 4-cm height loss were significantly associated with falls (odds ratio [OR], 1.637; 95% confidence interval [CI], 1.023–2.619; P = 0.04), (OR, 1.742, 95% CI, 1.054–2.877; P = 0.03), respectively. Additionally, over 4-cm height loss was significantly associated with sarcopenia for ASMI calculated by participant’s tallest recalled height squared (OR, 2.676; 95% CI, 1.122–6.284; P = 0.026).
Conclusions
We found that the risk of falls was advanced at 3- to 4-cm height loss and over 4-cm height loss, and sarcopenia started at over 4-cm height loss. Height loss may be a useful indicator of the risk of falls and sarcopenia.
Keywords: Community-dwelling older women, Height loss, Falls, Trunk skeletal muscle mass, Sarcopenia
1. Introduction
Previous studies have suggested that vertebral fracture and osteoporosis are associated with height loss [[1], [2], [3]]. Most osteoporosis and vertebral fractures are asymptomatic [4], but height loss measured by subtracting the current measured height from the tallest recalled height is a predictor for osteoporosis and vertebral fracture risk. A fragility fracture is a major public health issue worldwide. Osteoporosis is not only a major cause of fractures but also ranks high among the diseases that cause people to become bedridden with serious complications [5]. A previous study showed that of the 487,298 fragility fractures, vertebral fractures accounted for the highest percentage at 59.8% (206,053 patients/yr) in Japan [6]. In particular, previous studies have reported that osteoporotic vertebral fracture is associated with increased mortality and decreased health-related quality of life [7,8]. Additionally, a history of vertebral fracture increases the risk for hip and other clinical fractures [9]. Osteoporosis and vertebral fracture occurred 6 times more in women than in men [4,10]. The prevalence of vertebral fracture among women in their 50s, 60s, 70s, and 80s (years) was 2.1%, 9.1%, 20.5%, and 54.2%, respectively, in Japan [11]. Thus, elderly women tend to decrease more in height than men.
Height loss is determined by not only previous vertebral fracture and osteoporosis, but also caused by spinal deformities, involving thoracic kyphosis [2]. Identifying an individual’s height loss is a useful indicator for detecting osteoporosis, vertebral fracture, and thoracic kyphosis risks in women. Additionally, height loss may indicate the risk of falls because osteoporosis, vertebral fracture, and thoracic kyphosis are significantly associated with sarcopenia, involving low muscle mass and motor functions [[12], [13], [14]]. The presence of sarcopenia indicates a higher risk of falls than nonsarcopenia [15,16]. However, there are no reports on the association of height loss and risk of falls and sarcopenia. We hypothesized that height loss is defined based on the risk of falls and sarcopenia. Thus, our study was aimed at clarifying the association between height loss and risk of falls and sarcopenia.
2. Methods
2.1. Study population
Data for this study were obtained from community-dwelling women of Satte City, Japan, who participated in routine osteoporosis examinations. The present study was a cross-sectional analysis of 610 women aged between 60 and 90 years living in the community with the ability to walk independently. Participants were excluded if they had employed a nursing care service in Japan, already had osteoporosis, severe cognitive impairment, or were diagnosed with Parkinson disease or stroke. This study was conducted following the principles of the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study. The study was conducted with the approval of the institutional ethics committee of the Japan University of Health Sciences (No. 3001).
2.2. Measures
Body composition measurements, such as height, body weight, body mass index (BMI), percent body fat (BF%), and trunk and appendicular skeletal muscle mass (SMM) were recorded. Trunk and appendicular SMM and BF% were evaluated by bioimpedance analysis (TANITA, MC-780, Japan). Appendicular SMM was the total of the muscle masses of the 4 limbs. Appendicular skeletal muscle index (ASMI), involving sarcopenia was calculated by dividing appendicular SMM by the squares of the current measured height and the participant’s maximum height (kg/m2). Bone mineral density (BMD) of the distal nondominant forearm was measured using single-energy X-ray absorptiometry (Hitachi Aloca, DCS-600EXV, Japan). The BMD was calculated as the young adult mean (YAM) score (%) to unify the value scales. We defined the YAM score of less than 80% as suspected osteoporosis because it is considered as a candidate for osteoporosis and a close examination is recommended in Japan. Participants were interviewed regarding their history of falling and were asked the following question: “Have you fallen over the past 12 months, and if yes, did you experience falls?” We defined fallers as participants who had experienced single or multiple falls during the past 12 months before this study; all other participants were defined as non-fallers. According to a previous study on falls [17], a fall is defined as a sudden, unintentional change in the position of an individual to the floor or ground or falling and hitting an object like a chair or stair. We evaluated the data from the three-point scale non-fallers, single faller and multiple fallers. Moreover, participants were interviewed about their age and individual tallest recalled height. We calculated the height loss by subtracting the current measured height from the participant’s tallest recalled height [[1], [2], [3]]. The height loss is self-reported, and participants were classified into the following groups: <2-cm height loss, nonheight loss; ≥2 cm, <3 cm height loss (2- to 3-cm height loss); ≥3 cm, <4 cm height loss (3- to 4-cm height loss); and ≥4-cm height loss. Sarcopenia was based on the criteria outlined in the Asia Working Group for Sarcopenia [18]. We used the criteria to identify sarcopenia in Japanese women on the basis of ASMI <5.8, grip strength <19.3 kg, and gait speed 1.19 m/s [19,20].
Grip strength was assessed once on the dominant hand in a standing position using a grip dynamometer (T.K.K.5401, Takei Corp., Niigata, Japan). The participants were instructed to extend their elbows at 180° to the forearms, and the highest measure was recorded (kg of force).
To measure gait speed, the participants were asked to walk a straight 6-m course at their normal pace, and the time required to complete the course was recorded. Gait speed was calculated by dividing the distance by gait time (m/s).
2.3. Statistical analysis
Differences in measurements among the age groups (60–64, 65–69, 70–74, 75–79, and over 80 years) were examined by 1-way analysis of variance (ANOVA) and the Tukey-Kramer test; nonparametric data were analyzed using the Kruskal-Wallis test. Differences in measurements among the nonheight loss, 2–3 cm, 3–4 cm, and over 4-cm height loss groups were examined by ANOVA and the Tukey-Kramer test, and nonparametric data were examined using the Kruskal-Wallis test.
Multiple logistic regression analysis was used to determine the association of height loss with falls and sarcopenia. We compared with 2- to 3-cm height loss and nonheight loss group using multiple logistic analysis as model 1. Additionally, 3- to 4-cm height loss and over 4-cm height loss groups were compared with the nonheight loss group using multiple logistic analysis as models 2 and 3, respectively. We adjusted by calculating the association of age, weight, BMD and fall as independent variables (Table 1). Further, using multiple logistic regression analysis, we adjusted for age, weight, BMD and sarcopenia for ASMI calculated by the participant’s tallest recalled height squared as independent variables (Table 2). The data were analyzed using IBM SPSS version 24.0 (IBM Corp., Armonk, NY, USA).
Table 1.
Association of self-reported height loss with falls.
| Model | OR (95% CI) | P-value |
| Model 1: 2- to 3-cm height loss; presence = 1 | ||
| Age, +1 yr | 1.146 (1.093–1.203) | <0.001 |
| Body weight, +1 kg | 1.005 (0.978–1.032) | 0.726 |
| BMD, +1 %YAM | 0.276 (0.013–5.937) | 0.411 |
| Falls, +1 time | 1.079 (0.669–1.741) | 0.754 |
| Model 2: 3- to 4-cm height loss; presence = 1 | ||
| Age, +1 yr | 1.152 (1.091–1.216) | <0.001 |
| Body weight, +1 kg | 0.991 (0.96–1.022) | 0.559 |
| BMD, +1 %YAM | 1.331 (0.039–45.311) | 0.874 |
| Falls, +1 time | 1.637 (1.023–2.619) | 0.04 |
| Model 3: Over 4-cm height loss; presence = 1 | ||
| Age, +1 yr | 1.233 (1.164–1.307) | <0.001 |
| Body weight, +1 kg | 1.016 (0.981–1.052) | 0.373 |
| BMD, +1 %YAM | 0.005 (<0.001–0.212) | 0.006 |
| Falls, +1 time | 1.742 (1.054–2.877) | 0.03 |
OR, odds ratio; CI, confidence interval; BF%, body fat percentage; SMM, skeletal muscle mass; ASMI, appendicular skeletal muscle index; BMD, body mineral bone density; YAM, young adult meanModel 1 was adjusted using multiple logistic regression analysis with age, body weight, BMD, and falls as independent variables, and 2- to 3-cm height loss compared with nonheight loss as explanatory variable. Model 2 was adjusted using multiple logistic regression analysis with age, body weight, BMD, and falls as independent variables, and 3- to 4-cm height loss compared with nonheight loss as explanatory variable. Model 3 was adjusted using multiple logistic regression analysis with age, body weight, BMD, and falls as independent variables, and over 4-cm height loss compared with nonheight loss as explanatory variable.
Table 2.
Association of self-reported height loss with sarcopenia for ASMI calculated by participant’s tallest recalled height squared.
| Model | OR (95% CI) | P-value | |
|---|---|---|---|
| Model 1: 2- to 3-cm height loss; presence = 1 | |||
| Age, +1 yr | 1.147 (1.093–1.203) | <0.001 | |
| Body weight, +1 kg | 1.011 (0.984–1.039) | 0.436 | |
| BMD, +1 %YAM | 0.292 (0.016–5.371) | 0.407 | |
| Sarcopenia for ASMI calculated by participant’s tallest recalled height squared, +1; presence = 1 | 1.666 (0.751–3.696) | 0.209 | |
| Model 2: 3- to 4-cm height loss; presence = 1 | |||
| Age, +1 yr | 1.151 (1.091–1.214) | <0.001 | |
| Body weight, +1 kg | 1 (0.969–1.032) | 0.993 | |
| BMD, +1 %YAM | 0.54 (0.019–15.208) | 0.718 | |
| Sarcopenia for ASMI calculated by participant’s tallest recalled height squared, +1; presence = 1 | 2.032 (0.882–4.68) | 0.096 | |
| Model 3: Over 4-cm height loss; presence = 1 | |||
| Age, +1 yr | 1.238 (1.168–1.312) | <0.001 | |
| Body weight, +1 kg | 1.024 (0.989–1.061) | 0.185 | |
| BMD, +1 %YAM | 0.009 (0–0.287) | 0.008 | |
| Sarcopenia for ASMI calculated by participant’s tallest recalled height squared, +1; presence = 1 | 2.676 (1.122–6.384) | 0.026 | |
OR, odds ratio; CI, confidence interval; BF%, body fat percentage; SMM, skeletal muscle mass; ASMI, appendicular skeletal muscle index; BMD, body mineral bone density; YAM, young adult mean.Model 1 was adjusted using multiple logistic regression analysis with age, body weight, BMD, and sarcopenia for ASMI calculated by participant’s tallest recalled height squared as independent variables, and 2- to 3-cm height loss compared with nonheight loss as explanatory variable. Model 2 was adjusted using multiple logistic regression analysis with age, body weight, BMD, and sarcopenia for ASMI calculated by participant’s tallest recalled height squared as independent variables, and 3- to 4-cm height loss compared with nonheight loss as explanatory variable. Model 3 was adjusted using multiple logistic regression analysis with age, body weight, BMD, and sarcopenia for ASMI calculated by participant’s tallest recalled height squared as independent variables, and over 4-cm height loss compared with nonheight loss as explanatory variable.
3. Results
Table 3 shows the age, height, body weight, BMI, BF%, trunk SMM, ASMI calculated by the current measured height and participant’s tallest recalled height squared, BMD, presence of suspected osteoporosis, height loss, presence of sarcopenia for ASMI calculated by the current measured height and participant’s tallest recalled height squared, grip strength, and gait speed among the different age strata. The prevalence of 2- to 3-cm height loss among women in 60–64, 65–69, 70–74, 75–79, and over 80 years was 9.5%, 18.6%, 21.7%, 27.9%, and 26.3%, the prevalence of 3- to 4-cm height loss was 6.6%, 13.3%, 15.5%, 17,6%, and 31.5%, respectively. Additionally, the prevalence of over 4-cm height loss was 7.3%, 4.9%, 13.6%, 27.9%, and 42.1%, respectively. There were no significant differences among the age strata regarding body weight, BMI, BF%, and trunk SMM. Grip strength and gait speed were lower in the group aged ≥75 years and older than in the group aged 60–64 years. Height, BMD, presence of suspected osteoporosis, and ASMI calculated by the participant’s tallest recalled height squared were significantly lower in the group aged ≥70 years than in the group aged 60–64 years. There was no association between falls and age strata.
Table 3.
Comparison of the characteristics of the participants by age (n = 610).
| Characteristic | 60–64 Years (n = 138) | 65–69 Years (n = 225) | 70–74 Years (n = 159) | 75–79 Years (n = 68) | ≥80 Years (n = 20) |
|---|---|---|---|---|---|
| Age, yr | 62.3 ± 1.4 | 67.2 ± 1.4a | 71.5 ± 1.4a,b | 76.4 ± 1.3a,b,c | 82.2 ± 3.0a,b,c,d |
| Height, cm | 154.6 ± 5.3 | 153.0 ± 8.4 | 151.1 ± 8.9a | 149.9 ± 5.3a,b | 148.7 ± 4.7a |
| Body weight, kg | 53.2 ± 8.7 | 52.4 ± 8.9 | 51.6 ± 7.6 | 51.4 ± 8.3 | 48.0 ± 6.0 |
| Body mass index, kg/m2 | 22.3 ± 3.3 | 22.2 ± 3.5 | 22.5 ± 3.3 | 22.8 ± 3.4 | 21.6 ± 2.8 |
| BF%, % | 28.8 ± 7.5 | 28.9 ± 8.1 | 28.8 ± 8.0 | 29.6 ± 7.9 | 28.3 ± 6.6 |
| Trunk SMM, kg | 20.1 ± 1.7 | 19.8 ± 1.5 | 19.8 ± 1.7 | 19.6 ± 2.0 | 19.2 ± 1.7 |
| ASMI calculated by the individual’s current measured height squared, kg/m2 | 6.3 ± 0.7 | 6.3 ± 0.7 | 6.3 ± 0.7 | 6.3 ± 0.7 | 5.8 ± 0.6a,b,c,d |
| ASMI calculated by the individual’s tallest recalled height squared, kg/m2 | 6.3 ± 0.6 | 6.2 ± 0.7a | 6.2 ± 0.7 | 6.1 ± 0.8 | 5.5 ± 0.6a,b,c,d |
| BMD, g/m2 | 0.53 ± 0.08 | 0.50 ± 0.07a | 0.49 ± 0.08a | 0.47 ± 0.08a,b | 0.47 ± 0.06a |
| BMD, %YAM score | 81.4 ± 12.7 | 76.6 ± 10.9a | 75.4 ± 11.9a | 71.4 ± 10.6a,b,c | 71.8 ± 7.9a |
| Presence of suspected osteoporosis | 62 (39.5) | 143 (63.9)a | 105 (64.3)a | 51 (77.3)a | 15 (81.0)a,b,c |
| Height loss, cm | 1.1 ± 1.4 | 1.5 ± 1.5 | 2.0 ± 1.9a,b | 2.7 ± 1.9a,b,c | 2.9 ± 1.7a,b |
| Presence of 2–3 cm height loss | 13 (9.5) | 42 (18.6) | 35 (21.7) | 19 (27.9) | 5 (26.3) |
| Presence of 3–4 cm height loss | 9 (6.6) | 30 (13.3) | 25 (15.5) | 12 (17.6) | 6 (31.5) |
| Presence of over 4 cm height loss | 10 (7.3) | 11 (4.9) | 22 (13.6) | 19 (27.9) | 8 (42.1) |
| Presence of single fall over the past 12 months | 19 (13.4) | 30 (13.2) | 17 (10.7) | 17 (25.4) | 3 (17.6) |
| Presence of multiple falls over the past 12 months | 3 (2.2) | 4 (1.8) | 6 (3.8) | 3 (4.4) | 2 (10.0) |
| Sarcopenia for ASMI calculated by the current measured height squared | 5 (3.6) | 17 (7.5) | 6 (3.7) | 4 (5.9) | 8 (40.0) |
| Sarcopenia for ASMI calculated by the participant’s tallest recalled height squared | 5 (3.6) | 22 (9.8) | 14 (8.8) | 9 (13.2) | 9 (45.0) |
| Grip strength, kg | 23.5 ± 6.3 | 22.8 ± 5.3 | 22.9 ± 5.1 | 21.1 ± 5.4a | 19.7 ± 2.2a |
| Gait speed, m/s | 1.38 ± 0.21 | 1.37 ± 0.23 | 1.36 ± 0.21 | 1.25 ± 0.20a,b,c | 1.19 ± 0.30a,b,c |
Values are presented as mean ± standard deviation or number (%).
BF%, body fat percentage; SMM, skeletal muscle mass; ASMI, appendicular skeletal muscle index; BMD, body mineral bone density; YAM, young adult mean. Data on the presence of 2–3 cm height loss, 3–4 cm height loss, over 4 cm height loss, falls over the past 12 months, and sarcopenia for ASMI calculated by the current height and participant’s tallest recalled height squared are presented as number and percentage of participants.
Significant difference (P < 0.05) compared with the group aged 60–64 years.
Significant difference (P < 0.05) compared with the group aged 65–69 years.
Significant difference (P < 0.05) compared with the group aged 70–74 years.
Significant difference (P < 0.05) compared with the group aged 75–79 years.
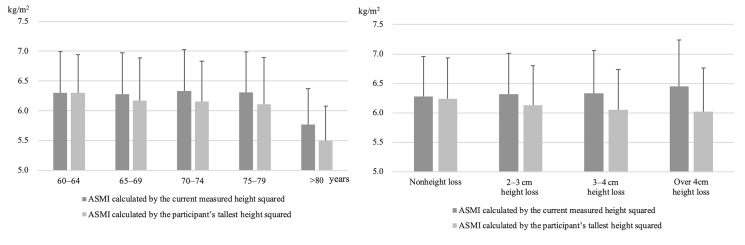
Table 4 shows the age, height, body weight, BMI, BF%, trunk SMM, ASMI calculated by the current measured height and participant’s tallest recalled height squared, BMD, presence of suspected osteoporosis, height loss, presence of sarcopenia for ASMI calculated by the current measured height and participant’s tallest recalled height squared, grip strength, and gait speed among height loss group. ASMI calculated by participant’s tallest recalled height squared, BMD, and presence of suspected osteoporosis, height loss were significantly lower in the over 4-cm height loss group than in the nonheight loss group. Trunk SMM, grip strength, and gait speed were significantly lower in the 3- to 4-cm height loss group than in the nonheight loss group. The trend in ASMI calculated by the current measured height squared increased in the over 4-cm height loss group (Fig. 1).
Table 4.
Comparison of women in the nonheight, 2– to 3- cm, 3– to 4- cm, and over 4- cm height loss groups (n = 610).
| Variable | Nonheight loss group (n = 344) | 2– to 3- cm height loss group (n = 114) | 3– to 4- cm height loss group (n = 82) | Over 4-cm height loss group (n = 70) |
|---|---|---|---|---|
| Age, yr | 67.1 ± 4.2 | 70.2 ± 4.9a | 70.4 ± 5.4a | 72.7 ± 6.3a,b,c |
| Height, cm | 154.1 ± 7.5 | 152.0 ± 5.2a | 150.2 ± 4.9a | 149.1 ± 4.9a,b |
| Body weight, kg | 52.7 ± 8.5 | 52.3 ± 8.2 | 51.5 ± 8.9 | 51.2 ± 9.1 |
| Body mass index, kg/m2 | 22.1 ± 3.3 | 22.7 ± 3.1 | 22.8 ± 3.7 | 23.1 ± 3.6 |
| BF%, % | 28.4 ± 7.5 | 29.3 ± 7.5 | 30.3 ± 8.2 | 30.5 ± 8.2 |
| Trunk SMM, kg | 20.1 ± 1.6 | 19.9 ± 1.6 | 19.2 ± 1.6a,b | 19.1 ± 1.9a,b |
| ASMI calculated by the individual’s current measured height squared, kg/m2 | 6.3 ± 0.7 | 6.3 ± 0.7 | 6.3 ± 0.7 | 6.5 ± 0.8 |
| ASMI calculated by the individual’s tallest recalled height squared, kg/m2 | 6.3 ± 0.7 | 6.1 ± 0.7 | 6.1 ± 0.7 | 6.0 ± 0.7a |
| BMD, g/m2 | 0.51 ± 0.07 | 0.49 ± 0.07 | 0.49 ± 0.10 | 0.47 ± 0.09a |
| BMD, %YAM score | 78.3 ± 11.2 | 75.8 ± 10.5 | 75.8 ± 14.9 | 71.9 ± 13.3a |
| Presence of suspected osteoporosis | 198 (57.6) | 70 (61.4) | 51 (62.2) | 52 (74.3)a |
| Height loss, cm | 0.8 ± 0.6 | 2.4 ± 0.3a | 3.2 ± 0.7a,b | 5.2 ± 1.8a,b,c |
| Presence of single fall over the past 12 months | 40 (11.7) | 16 (14.2) | 16 (20.8)a | 12 (17.6)a |
| Presence of multiple falls over the past 12 months | 5 (1.5) | 3 (2.6) | 4 (4.9) | 6 (8.6)a |
| Sarcopenia for ASMI calculated by the current measured height squared | 17 (4.9) | 7 (6.1) | 9 (11.0) | 7 (10.0) |
| Sarcopenia for ASMI calculated by the participant’s tallest recalled height squared | 20 (5.8) | 13 (11.4) | 12 (14.6) | 14 (20.0)a, |
| Grip strength, kg | 24.0 ± 4.0 | 23.2 ± 3.5 | 22.4 ± 4.3a | 21.3 ± 3.7a,b |
| Gait speed, m/s | 1.39 ± 0.22 | 1.34 ± 0.19 | 1.32 ± 0.24a | 1.24 ± 0.24a,b |
Values are presented as mean ± standard deviation or number (%).
BF%, body fat percentage; SMM, skeletal muscle mass; ASMI, appendicular skeletal muscle index; BMD, body mineral bone density; YAM, young adult mean. Data of presence of the current back pain treatment, sarcopenia for ASMI calculated by the individual’s tallest recalled height squared and ASMI calculated by the current measured height squared are presented number and percentage of participants.
Significant difference (P < 0.05) compared with the nonheight loss group.
Significant difference (P < 0.05) compared with the 2–to 3-cm height loss group.
Significant difference (P < 0.05) compared with the 3– to 4-cm height loss group.
Fig. 1.
Comparison of the ASMI calculated by the current measured height and participant’s tallest recalled height squared according to age and height loss. ASMI, appendicular skeletal muscle index.
Table 1, Table 2 show the results for models 1–3. No relationship was observed between 2- to 3-cm height loss and falls in model 1 (Table 1). Age and falls were significantly associated with 3- to 4-cm height loss and over 4-cm height loss in models 2 and 3, respectively (Table 1). Additionally, sarcopenia for ASMI calculated by the individual’s tallest recalled height, BMD, and BF% were significantly associated with over 4-cm height loss in model 3 (Table 2).
4. Discussion
We attempted to clarify the association of height loss with falls and sarcopenia using multiple logistic regression analysis. Consequently, there was an association between height loss and falls; additionally, we found that height loss was also associated with sarcopenia for ASMI calculated by participant’s tallest recalled height squared and BMD.
Self-reported height loss is important in women aged ≥60 years who have undetected fracture risks. Previous studies reported that height loss was associated with osteoporosis and was a predictor of vertebral fracture [1,2]. Huang et al. [3] found that women with at least one incidence of vertebral fracture lost an average of 2.1 cm in height, whereas the average height loss among those without incident fractures was only 0.4 cm. The present study showed that height loss was significantly higher in the group aged ≥70 years than in the group aged 60–64 years. Additionally, the mean height loss in the group aged 70–74 years was 2.0 cm. Therefore, the risk of vertebral fracture may be particularly higher in the group aged ≥70 years than in the group aged 60 years. Identifying whether height loss is related to other health-related outcomes is important because vertebral fracture and osteoporosis are associated with increased mortality rates [21,22].
The presence of osteoporosis is significantly associated with sarcopenia occurrence [23]. Sarcopenia is an age-related loss of SMM and function, or a disease, or a process of normative aging [24]. Previous studies showed that grip strength and motor function decreased with aging, while ASMI did not show age-related decrease among women aged 40–79 years [14,25]. The present study showed that ASMI calculated by the participant’s tallest recalled height squared were significantly lower in the group aged ≥70 years than in the group aged 60–64 years. Thus, ASMI calculated by the participant’s tallest recalled height squared may be as good an indicator as age.
Previous studies have shown that vertebral fracture with osteoporosis was significantly associated with the decreased walking ability [23,26]. Moreover, odds ratios for height loss and incident vertebral fractures were 13.5, 19.1, and 20.6 in the 2 < loss ≤3 cm, 3 < loss ≤4 cm, and over 4-cm loss groups, respectively [1]. Therefore, we considered that height loss might already occur in osteoporosis and is associated with sarcopenia. The present study showed that height loss was associated with trunk SMM and ASMI calculated by the participant’s tallest recalled height squared. However, there was no association between height loss and sarcopenia for ASMI calculated by the current height. Our study suggested that ASMI should be considered for height loss in women because ASMI calculated by the current measured height squared tend to increase in the over 4-cm height loss group. Additionally, the present study showed that grip strength and gait speed were significantly lower in the ≥3-cm height loss groups, and BMD was significantly lower in the ≥4 cm height loss group than in the nonheight loss group. We considered that low motor functions began at 3–4 cm height loss; moreover, low BMD was already advanced at over 4-cm height loss. This study suggested that height loss might predict the risk of sarcopenia, involving low SMM and motor functions.
From the results of multiple logistic regression analysis, there were significant associations between ≥3-cm height loss and falls; moreover, ≥4-cm height loss was associated with falls. Additionally, ≥4-cm height loss was significantly associated with BMD and the presence of sarcopenia for ASMI calculated by the participant’s tallest recalled height squared. Height loss is determined by vertebral fracture, osteoporosis, and spinal deformities [2]. Two-thirds of patients with vertebral fractures are asymptomatic, which makes the fractures difficult to be detected [4]. Kamimura et al. [27] showed that ≥4-cm height loss was significantly associated with the presence of vertebral fracture. Thus, the presence of ≥4-cm height loss in participants of this study may have unknowingly occurred due to vertebral fracture. Moreover, osteoporosis is significantly associated with sarcopenia occurrence [23]. Prevalence of sarcopenia and lower leg muscle mass among patients with acute vertebral fractures are higher in the osteoporotic vertebral fracture group than in the non-vertebral fracture group [12]. Additionally, Eguchi et al. [13] reported that both appendicular and trunk SMM were significantly lower in the degenerative lumbar group than in the lumbar spinal canal stenosis group, and sarcopenia may be involved in causing spinal deformities. Yoshimura et al. [23] found that there was a significant proportion of patients with coexistent sarcopenia and osteoporosis (so-called ‘osteosarcopenia’). Identifying an individual’s height loss may be used to predict not only osteoporosis but also sarcopenia, involving the low trunk, appendicular SMM, and motor functions.
The association of 3–4 cm and ≥4-cm height loss with a history of falls during the preceding 12 months was also supported by multiple logistic regression analysis, although we adjusted for age, body weight, and BMD. This result is interesting and might represent a significant factor when defining a high risk of falls in community-dwelling elderly women. A previous study reported that patients experiencing a fall over the past 12 months had a 3 times higher risk of multiple falls than patients who reported no falls [28]. Falls are a major cause of injury and death among the elderly [29], and fear of falling decreases the quality of life [30]. Meanwhile, a previous study reported that sarcopenia is a risk factor for falls among community-dwelling older people [15,16]. We considered that height loss might be a predictive index for the risk of falls accompanied by sarcopenia.
This study has several limitations. First, the history of falls and the calculation of height loss relied on the participants’ recollection of their memory and past height. Thus, there is the possibility of recall bias, especially in the elderly. Second, this analysis may differ from the association of degenerative intervertebral disk and scoliosis with height loss. Third, BMD was measured in the forearm, but not in the spine. Thus, this study did not reveal whether the subject had a vertebral fracture. Forth, muscle mass measurement was used by bioelectrical impedance analysis rather than dual-energy X-ray absorptiometry. Lastly, the over 80 years group was relatively smaller than the number of subjects in the other age groups. In the future, prospective studies are required to obtain more accurate data to determine if height loss can predict future deterioration of health in community-dwelling people.
5. Conclusions
The present cross-sectional analysis clarified the prevalence and height loss associated with falls in community-dwelling older women. In such women, falls and sarcopenia for ASMI calculated by participant’s tallest recalled height squared were significantly associated with height loss. We found that the risk of falls was advanced at 3- to 4-cm height loss and over 4-cm height loss, and sarcopenia started at over 4-cm height loss. Further studies, along with continued longitudinal surveys, will help elucidate the background of falls and sarcopenia, and their relationship with height loss.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
CRediT author statement
Ryoma Asahi: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Satoshi Yuguchi: Data curation, Funding acquisition, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Tomohiko Kamo: Data curation, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Masato Azami: Data curation, Investigation, Methodology, Visualization. Hirofumi Ogihara: Data curation, Investigation, Methodology, Visualization. Satoshi Asano: Data curation, Formal analysis, Resources, Supervision, Validation, Visualization.
Acknowledgments
The work was funded by the 12th Asahi Kasei Research Grant Program from the Japan Osteoporosis Foundation (2019, Ryoma Asahi) and KAKENHI (19K19843) Grant-in-Aid for Young Scientists (2019, Satoshi Yuguchi). The authors wish to thank Satte City, Mrs. Satoko Tejima, Ms. Masami Kuboi, Mrs. Megumi Ikeda, and other members of the public office in Satte City for their assistance in locating and scheduling participants for examinations. Additionally, we are grateful to the participants in our survey. ORCID Ryoma Asahi: 0000-0002-7475-795X. Satoshi Yuguchi: 0000-0001-8668-0315. Tomohiko Kamo: 0000-0003-0490-8393. Masato Azami: 0000-0002-7374-9237. Hirofumi Ogihara: 0000-0001-8335-2279. Satoshi Asano: 0000-0002-8534-5192.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Siminoski K., Jiang G., Adachi J.D., Hanley D.A., Cline G., Ioannidis G. Accuracy of height loss during prospective monitoring for detection of incident vertebral fractures. Osteoporos Int. 2005;16:403–410. doi: 10.1007/s00198-004-1709-z. [DOI] [PubMed] [Google Scholar]
- 2.Briot K., Legrand E., Pouchain D., Monnier S., Roux C. Accuracy of patient-reported height loss and risk factors for height loss among postmenopausal women. CMAJ (Can Med Assoc J) 2010;182:558–562. doi: 10.1503/cmaj.090710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Ross P.D., Lydick E., Davis J.W., Wasnich R.D. Contributions of vertebral fractures to stature loss among elderly Japanese-American women in Hawaii. J Bone Miner Res. 1996;11:408–411. doi: 10.1002/jbmr.5650110315. [DOI] [PubMed] [Google Scholar]
- 4.Cooper C., Melton L.J. Vertebral fractures: how large is the silent epidemic? BMJ. 1992;304:793–794. doi: 10.1136/bmj.304.6830.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ. Tech. Rep. Ser. 1994;843 1-129. [PubMed] [Google Scholar]
- 6.Iihara N., Ohara E., Bando Y., Yoshida T., Ohara M., Kirino Y. Fragility fractures in older people in Japan based on the national health insurance claims database. Biol Pharm Bull. 2019;42:778–785. doi: 10.1248/bpb.b18-00974. [DOI] [PubMed] [Google Scholar]
- 7.Hallberg I., Bachrach-Lindström M., Hammerby S., Toss G., Ek A.C. Health-related quality of life after vertebral or hip fracture: a seven-year follow-up study. BMC Muscoskel Disord. 2009;10:135. doi: 10.1186/1471-2474-10-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melton L.J., 3rd, Atkinson E.J., Cooper C., O’Fallon W.M., Riggs B.L. Vertebral fractures predict subsequent fractures. Osteoporos Int. 1999;10:214–221. doi: 10.1007/s001980050218. [DOI] [PubMed] [Google Scholar]
- 9.Klotzbuecher C.M., Ross P.D., Landsman P.B., Abbott T.A., 3rd, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721–739. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimura N., Muraki S., Oka H., Kawaguchi H., Nakamura K., Akune T. Cohort profile: research on Osteoarthritis/Osteoporosis against Disability study. Int J Epidemiol. 2010;39:988–995. doi: 10.1093/ije/dyp276. [DOI] [PubMed] [Google Scholar]
- 11.Yoshimura N., Kinoshita H., Oka H., Muraki S., Mabuchi A., Kawaguchi H. Cumulative incidence and changes in the prevalence of vertebral fractures in a rural Japanese community: a 10-year follow-up of the Miyama cohort. Arch. Osteoporos. 2006;1:43–49. [Google Scholar]
- 12.Hida T., Shimokata H., Sakai Y., Ito S., Matsui Y., Takemura M. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J. 2016;25:3424–3431. doi: 10.1007/s00586-015-3805-5. [DOI] [PubMed] [Google Scholar]
- 13.Eguchi Y., Suzuki M., Yamanaka H., Tamai H., Kobayashi T., Orita S. Associations between sarcopenia and degenerative lumbar scoliosis in older women. Scoliosis Spinal Disord. 2017;12:9. doi: 10.1186/s13013-017-0116-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyakoshi N., Hongo M., Mizutani Y., Shimada Y. Prevalence of sarcopenia in Japanese women with osteopenia and osteoporosis. J Bone Miner Metabol. 2013;31:556–561. doi: 10.1007/s00774-013-0443-z. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X., Huang P., Dou Q., Wang C., Zhang W., Yang Y. Falls among older adults with sarcopenia dwelling in nursing home or community: a meta-analysis. Clin Nutr. 2020;39:33–39. doi: 10.1016/j.clnu.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto H., Tanimura C., Tanishima S., Osaki M., Noma H., Hagino H. Sarcopenia is a risk factor for falling in independently living Japanese older adults: a 2-year prospective cohort study of the GAINA study. Geriatr Gerontol Int. 2017;17:2124–2130. doi: 10.1111/ggi.13047. [DOI] [PubMed] [Google Scholar]
- 17.Nevitt M.C., Cummings S.R., Kidd S., Black D. Risk factors for recurrent nonsyncopal falls. A prospective study. J Am Med Assoc. 1989;261:2663–2668. [PubMed] [Google Scholar]
- 18.Chen L.K., Liu L.K., Woo J., Assantachai P., Auyeung T.W., Bahyah K.S. Sarcopenia in Asia: consensus report of the asian working group for sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 19.Tanimoto Y., Watanabe M., Sun W., Sugiura Y., Tsuda Y., Kimura M. Association between sarcopenia and higher-level functional capacity in daily living in community-dwelling elderly subjects in Japan. Arch Gerontol Geriatr. 2012;55:e9–e13. doi: 10.1016/j.archger.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 20.Kera T., Kawai H., Hirano H., Kojima M., Watanabe Y., Motokawa K. Definition of respiratory sarcopenia with peak expiratory flow rate. J Am Med Dir Assoc. 2019;20:1021–1025. doi: 10.1016/j.jamda.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 21.Nishimura A., Akeda K., Kato K., Asanuma K., Yamada T., Uchida A. Osteoporosis, vertebral fractures and mortality in a Japanese rural community. Mod Rheumatol. 2014;24:840–843. doi: 10.3109/14397595.2013.866921. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki T., Yoshida H. Low bone mineral density at femoral neck is a predictor of increased mortality in elderly Japanese women. Osteoporos Int. 2010;21:71–79. doi: 10.1007/s00198-009-0970-6. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimura N., Muraki S., Oka H., Iidaka T., Kodama R., Kawaguchi H. Is osteoporosis a predictor for future sarcopenia or vice versa? Four-year observations between the second and third ROAD study surveys. Osteoporos Int. 2017;28:189–199. doi: 10.1007/s00198-016-3823-0. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg I.H. Summary comments: epidemiologic and methodologic problems in determining nutritional status of older persons. Am J Clin Nutr. 1989;50:1231–1233. [PubMed] [Google Scholar]
- 25.Shimokata H., Ando F., Yuki A., Otsuka R. Age-related changes in skeletal muscle mass among community-dwelling Japanese: a 12-year longitudinal study. Geriatr Gerontol Int. 2014;14(Suppl 1):85–92. doi: 10.1111/ggi.12219. [DOI] [PubMed] [Google Scholar]
- 26.Horii C., Asai Y., Iidaka T., Muraki S., Oka H., Tsutsui S. Differences in prevalence and associated factors between mild and severe vertebral fractures in Japanese men and women: the third survey of the ROAD study. J Bone Miner Metabol. 2019;37:844–853. doi: 10.1007/s00774-018-0981-5. [DOI] [PubMed] [Google Scholar]
- 27.Kamimura M., Nakamura Y., Sugino N., Uchiyama S., Komatsu M., Ikegami S. Associations of self-reported height loss and kyphosis with vertebral fractures in Japanese women 60 years and older: a cross-sectional survey. Sci Rep. 2016;6:29199. doi: 10.1038/srep29199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guideline for the prevention of falls in older persons. American geriatrics society, British geriatrics society, and American academy of orthopaedic surgeons panel on falls prevention. J Am Geriatr Soc. 2001;49:664–672. [PubMed] [Google Scholar]
- 29.Fife D., Barancik J.I., Chatterjee B.F. Northeastern Ohio Trauma Study: II. Injury rates by age, sex, and cause. Am J Publ Health. 1984;74:473–478. doi: 10.2105/ajph.74.5.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akosile C.O., Anukam G.O., Johnson O.E., Fabunmi A.A., Okoye E.C., Iheukwumere N. Fear of falling and quality of life of apparently-healthy elderly individuals from a Nigerian population. J Cross Cult Gerontol. 2014;29:201–209. doi: 10.1007/s10823-014-9228-7. [DOI] [PubMed] [Google Scholar]