ABSTRACT
The enteric nervous system (ENS) is essential for normal gastrointestinal function. Although the embryonic origin of enteric neurons from the neural crest is well established, conflicting evidence exists regarding postnatal enteric neurogenesis. Here, we address this by examining the origin of de novo neurogenesis in the post-embryonic zebrafish ENS. Although new neurons are added during growth and after injury, the larval intestine appears to lack resident neurogenic precursors or classical glia marked by sox10, plp1a, gfap or s100. Rather, lineage tracing with lipophilic dye or inducible Sox10-Cre suggests that post-embryonic enteric neurons arise from trunk neural crest-derived Schwann cell precursors that migrate from the spinal cord into the intestine. Furthermore, the 5-HT4 receptor agonist prucalopride increases enteric neurogenesis in normal development and after injury. Taken together, the results suggest that despite the lack of resident progenitors in the gut, post-embryonic enteric neurogenesis occurs via gut-extrinsic Schwann cell precursors during development and injury, and is promoted by serotonin receptor agonists. The absence of classical glia in the ENS further suggests that neural crest-derived enteric glia might have evolved after the teleost lineage.
This article has an associated 'The people behind the papers' interview.
KEY WORDS: Enteric nervous system, Neural crest, Prucalopride, 5-HT4
Highlighted Article: Cell-labelling and lineage-tracing experiments showed that, although zebrafish larvae lack resident neuronal precursors, enteric neurogenesis occurs from trunk neural crest-derived precursors during development and injury, and is promoted by prucalopride.
INTRODUCTION
The enteric nervous system (ENS) consists of as many neurons as the spinal cord and is responsible for mediating crucial functions of the gastrointestinal tract, including motility, afferent ‘sensing’ and secretion (Furness, 2006). Pathologies involving the ENS range from congenital neurocristopathies, such as Hirschsprung disease (Lake and Heuckeroth, 2013), to acquired conditions, such as oesophageal achalasia (Kraichely and Farrugia, 2006) and diabetic gastroparesis (Farrugia, 2015). Thus, establishing fundamental features of ENS homeostasis and the potential for enteric neuronal regeneration will probably assist in the discovery of novel therapies to treat enteric neuropathies.
As the intestine lengthens during postnatal life (Struijs et al., 2009; Weaver et al., 1991), and is susceptible to injury during episodes of inflammation (Brierley and Linden, 2014) and mechanical stress (Wood, 2011), there is probably a need for continuous ENS neurogenesis throughout life to increase numbers and/or replace lost enteric neurons. Surprisingly, the question of postnatal enteric neurogenesis remains controversial, with conflicting reports arguing that postnatal enteric neurogenesis does not occur (Joseph et al., 2011), occurs after injury (Goto et al., 2013; Katsui et al., 2009; Laranjeira et al., 2011), occurs after exposure to 5-HT4 receptor agonists (Goto et al., 2013; Katsui et al., 2009; Liu et al., 2009; Margolis et al., 2016; Matsuyoshi et al., 2010) or occurs rapidly with a turnover measured in days (Kulkarni et al., 2017).
During development, the ENS is classically described as arising from the vagal neural crest, which emerges from the caudal hindbrain, migrates to and invades the foregut, and then migrates along the rostrocaudal extent of the gut to colonize the entire length of the intestinal tract (Lake and Heuckeroth, 2013). In addition to the vagal neural crest, there is a modest contribution from the sacral neural crest to the hindgut in some species (Burns et al., 2000; Wang et al., 2011). During initial colonization of the intestine, studies in mice have revealed that the spatial and functional organization of the mammalian ENS occurs via clonal expansion of precursors of neuronal and/or glial character, and depends on factors such as isometric growth and lineally unrelated neighbouring cells (Lasrado et al., 2017).
More recently, evidence has arisen for a novel source of enteric neurons originating from neural crest stem cells that remain nascent along peripheral nerves and are often referred to as ‘Schwann cell precursors (SCPs)’ (Furlan and Adameyko, 2018). These progenitors do not express glial markers such as GFAP and S100β, and reside within and migrate along peripheral nerves to give rise to diverse cell types, including parasympathetic and sympathetic neurons (Dyachuk et al., 2014; Espinosa-Medina et al., 2014; Kastriti et al., 2019), neuroendocrine chromaffin cells (Furlan et al., 2017) and melanocytes (Adameyko et al., 2009). SCPs account for 20% of neurons in the colons of mice (Uesaka et al., 2015), approximately half of foregut neurons in chicks (Espinosa-Medina et al., 2017) and all enteric neurons in lampreys (Green et al., 2017), which are basal vertebrates that lack a vagal neural crest. Moreover, these SCPs often arise from the trunk rather than vagal levels. This suggests that the original evolutionary strategy to populate the intestine with neurons occurs via SCPs rather than vagal neural crest cells, and a trunk neural crest contribution has been retained in avian and mammalian species.
Although best studied in chick and mouse, the development of the enteric nervous system is largely conserved across jawed vertebrates, including teleosts like zebrafish (Ganz, 2018; Heanue et al., 2016). As in chick and mouse, zebrafish vagal neural crest cells enter the foregut and migrate rostrocaudally to colonize its entire length. Zebrafish offer several advantages for studying ENS development and maturation. First, they are amenable to live-imaging techniques that allow direct visualization in vivo of cell behaviour within the context of the entire organism. Second, zebrafish have a simplified ENS compared with that of chick and mouse, with two streams of vagal neural crest cells migrating along the left and right sides of the intestine, facilitating imaging studies. Third, a variety of transgenic lines are available to label particular cell types of interest. Finally, zebrafish are amenable to experimental manipulation and are highly accessible to drug treatment.
Here, we take advantage of the ease of imaging and manipulation of the zebrafish model to examine the role of de novo neurogenesis as the ENS transitions from embryonic to larval stages. As expected, we find that new neurons are added as the animal grows, as well as after injury. Surprisingly, we show that these new neurons do not arise from resident progenitors or enteric glia, and indeed that zebrafish appear to lack these cell populations. Rather than originating from enteric precursors in the intestine, we provide evidence that post-embryonic enteric neurons arise from trunk neural crest-derived Schwann cell precursors that migrate into the intestine and differentiate into new neurons. Last, we find that prucalopride, a 5-HT4 receptor (5HT4R) agonist recently approved for use in the USA (www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210166Orig1s000TOC), promotes enteric neurogenesis and is protective in an injury model. Taken together, our results reveal novel roles for Schwann cell precursors in the context of ongoing neurogenesis in the post-embryonic intestine in both normal development and after injury, and suggest that cells with characteristics of classical glia are absent from the ENS and might have evolved after the teleost lineage.
RESULTS
Sox10-expressing cells and enteric glia appear to be absent in the post-embryonic zebrafish intestine
sox10 is an early neural crest marker that is important for the differentiation of nearly every neural crest lineage with the exception of cartilage, which instead uses its paralog sox9 (Martik and Bronner, 2017). sox10 marks early migratory neural crest cells and is retained after differentiation by enteric glia, as well as melanocytes, but lost from enteric neurons (Heanue and Pachnis, 2007). During embryogenesis, vagal neural crest cells expressing sox10 delaminate from the neural tube, invade the foregut and migrate in a generally rostral to caudal fashion along the intestine (Lake and Heuckeroth, 2013; Rao and Gershon, 2018). In chick and murine models, these enteric vagal neural crest cells proliferate as they migrate, and a proportion of the daughter cells cease migration, and differentiate into neurons and glia. As vagal neural crest cells differentiate into enteric neurons, they downregulate sox10 expression and express phox2b and other neuronal differentiation markers (Heanue and Pachnis, 2007); in contrast, enteric glia maintain sox10 expression and upregulate GFAP, S100B, and PLP1 (Gulbransen and Sharkey, 2012; Rao et al., 2015). In zebrafish, enteric vagal neural crest cells complete their colonization of the hindgut by 3 days postfertilization (dpf) (Heanue et al., 2016).
Previous studies have hypothesized that enteric neurogenesis is maintained postnatally, arising either from resident enteric neuronal precursors or enteric glia (Joseph et al., 2011; Kulkarni et al., 2017; Laranjeira et al., 2011). In search of such resident progenitors in the larval zebrafish intestine, we first performed in situ hybridization (ISH) for sox10 and the enteric glial marker plp1a. Surprisingly, although sox10 signal was identified as expected on migrating vagal neural crest cells within the intestine at embryonic stages, it was downregulated in the intestines of 3.5 dpf larvae, corresponding with phox2b expression throughout the gut. Furthermore, plp1a transcripts also were absent in the intestine at both stages, albeit present in other parts of the nervous system (Fig. 1A-D).
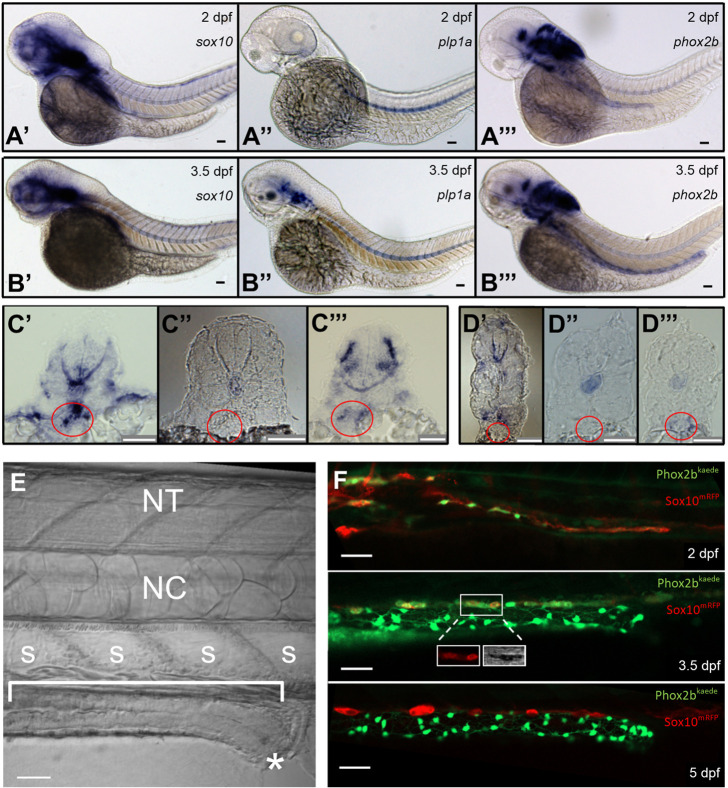
Fig. 1.
Resident neuronal progenitors are absent in the post-embryonic intestine. (A) ISH of enteric neural elements in 2 dpf embryos. sox10 is detected at 2 dpf as a stream in the midgut that does not yet extend to the hindgut (A′), plp1a exhibits no expression (A″) and phox2b has weak expression in the proximal gut (A‴). Expected probe trapping is evident in the notochord. (B) ISH of enteric neural elements in 3.5 dpf larvae. sox10 signal is absent in the intestine, although proximal probe trapping is seen in the nascent swim bladder (B′). plp1a is expressed dorsally but no expression is evident in the intestine (B″). phox2b expression extends throughout the intestine (B‴). (C′-C‴) Proximal cross-sections of 2 dpf embryos stained for sox10 (C′), plp1a (C″) and phox2b (C‴), with the nascent foregut marked by a red circle. (D′-D‴) Distal cross-sections of 3.5 dpf larvae stained for sox10 (D′), plp1a (D″) and phox2b (D‴), with the developing midgut marked by a red circle. (E) Anatomical orientation: fluorescent figures are oriented in this manner unless otherwise stated. The intestine is located ventrally (bracket) and extends anteriorly (left) to posteriorly (right), ending at the anus (*). A row of polygonal somites (S) are arranged dorsal to the intestine. The notochord (NC) and neural tube (NT, not visible in this image) are located dorsally. (F) Live imaging of Phox2b-kaede×Sox10-mRFP fish are consistent with ISH results: a migrating chain of Sox10 cells is observed in the midgut that does not yet extend to the hindgut at 2 dpf, but then Sox10 expression ceases at 3.5 dpf and 5 dpf. A few Sox10-expressing cells, seen dorsolateral to the intestine, are consistent with melanocytes, as supported by visible pigment using the transmitted light photomultiplier tube (TPMT), a detector for transmitted light (inset, 3.5 dpf panel). Scale bars: 50 µm.
We next performed live imaging using the transgenic line Sox10-mRFP (Kucenas et al., 2008) crossed with Tg(-8.3bphox2b:Kaede) (Phox2b-kaede) (Harrison et al., 2014). As expected, Sox10-labelled cells were observed migrating along the intestine at 2 dpf, with only sparse phox2b co-expression in the proximal foregut. In contrast, by 3.5 dpf, Sox10 was no longer expressed within the intestine (although Sox10+ melanocytes were identified dorsolateral to the intestine). By 5 dpf, a conspicuous neuronal plexus expressing Phox2b-kaede had formed but no Sox10 expression was observed in the intestine (Fig. 1F, Movie 1). Together, these results show that from 3.5 dpf onward, Sox10-expressing cells appear to be absent from the zebrafish intestine and confirm our ISH results, suggesting that there are no Sox10-expressing cells resident in the intestine at 3.5 dpf.
Next, we employed an indelible Cre transgenic line, Tg(sox10:GAL4-UAS-Cre;ubi:switch), which permanently labels all Sox10-derived lineages with mCherry (Cavanaugh et al., 2015). Fish were euthanized and fixed at 5 dpf, and then immunostained for the neuronal marker HuC/D and the Cre reporter mCherry. Although this line did not mark all Sox10-derived cells, we consistently found that all Cre-labelled cells colocalized with HuC/D but no Cre-labelled cells were HuC/D− (Fig. 2A), suggesting that (1) all Sox10-derived cells within the intestine have differentiated into enteric neurons by this stage, and (2) there are no non-neuronal Sox10-derived cells (i.e. resident precursors or glia) in the post-embryonic intestine. Of note, at this developmental stage, all Phox2b-kaede-expressing cells colocalized with HuC/D (Fig. S1), indicating that these cells are committed to a neuronal lineage.
Fig. 2.
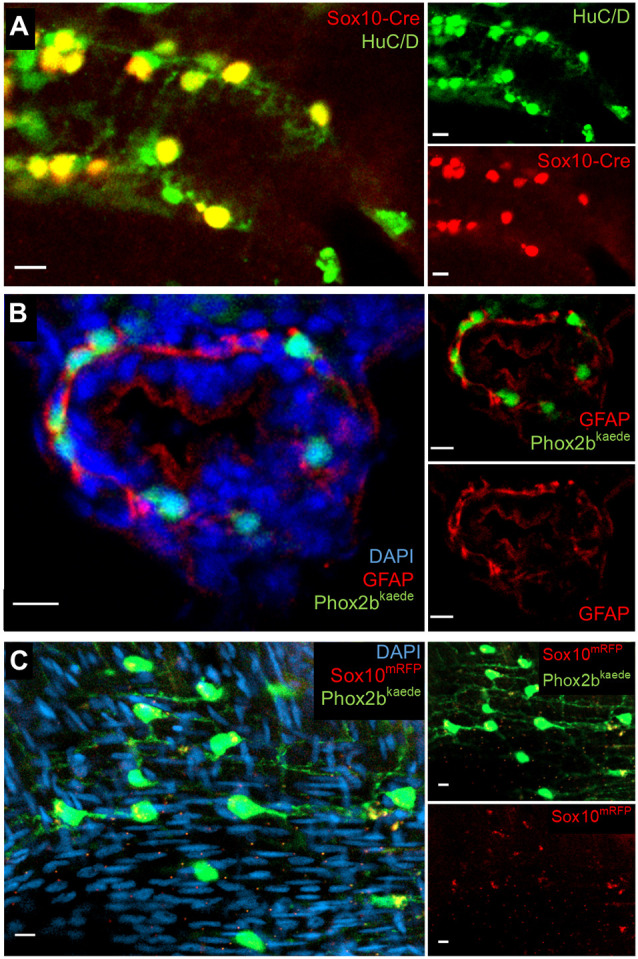
Further assays in larvae and adults support an absence of resident neuronal progenitors and enteric glia in the intestine. (A) Lineage tracing with an indelible Sox10-Cre line suggests enteric neurons are the sole fate of enteric vagal neural crest cells. At 5 dpf, fish were fixed and underwent IHC for the Cre reporter, mCherry and the neuronal marker HuC/D. All Cre-labelled cells colocalized with HuC/D, and no Cre+, HuC/D− cells were observed. (B) IHC with GFAP does not demonstrate convincing enteric glial cell bodies. Phox2b-kaede fish were fixed at 5 dpf, and axially sectioned for IHC for GFAP, a glial marker with cytosolic expression. Imaging of the endogenous Phox2b-kaede signal in concert with the GFAP IHC revealed a fibrillary pattern of GFAP closely associating with enteric neurons and other cells, which probably represents projections from extrinsic glia or nonspecific binding to other fibrillary proteins. (C) Whole-mount imaging of adult zebrafish intestine suggests that enteric glia and resident neuronal progenitors are not detectable by classical criteria later in development. Adult intestine from Phox2b-kaede×Sox10-mRFP fish that underwent optical clearing with RIMS revealed numerous enteric neurons, but no cell bodies expressing Sox10. Extrinsic glial projections are suggested by a fibrillary pattern of Sox10 expression. Scale bars: 10 µm.
An antibody to GFAP has previously been used as a marker to suggest the presence of enteric glia in zebrafish intestine. Therefore, we performed immunohistochemistry (IHC) on 5 dpf Phox2b-kaede larvae sections using an antibody against zebrafish GFAP. As shown previously (Baker et al., 2019; Hagström and Olsson, 2010), we found GFAP immunoreactivity within the intestine; however, GFAP appeared to be associated with cell processes but absent from cell bodies within the intestine (Fig. 2B). These findings probably reflect projections from extrinsic fibres (Fig. S2A) but not resident cells within the intestine. Alternatively, this antibody may be nonspecifically binding other fibrillary proteins, as this expression pattern was not observed in a GFAP-GFP transgenic line (Fig. S2B,C). Furthermore, IHC for S100 (which labels glial nuclei) did not reveal glial cell bodies within the intestine (Fig. S2D,E).
Last, to determine whether enteric gliogenesis occurs later in development, we performed whole-mount imaging of refractive index matching solution (RIMS)-cleared adult zebrafish intestine from the Sox10-mRFP×Phox2b-kaede line. Although numerous Phox2b-kaede cells were present within the muscularis, no Sox10 cell bodies were identified, although RFP-positive signal corresponding to cell projections was observed (Fig. 2C). Taken together, these data suggest that vagal neural crest-derived cells within the zebrafish intestine all differentiate into neurons, with an apparent absence of resident glia or progenitors, at least as assayed by the expression of classical glial markers.
Enteric neurogenesis persists in the post-embryonic intestine in normal development and after injury
Given the continued growth of the intestine through adulthood, we hypothesized that enteric neurogenesis persists in post-embryonic stages. To test this, we employed the photoconvertible Phox2b-kaede line that, upon exposure to light, converts from green to red. We photoconverted all kaede-labelled cells within the 4.5 dpf intestine, after the vagal neural crest had completely colonized the intestine, such that all neurons that were initially in the green fluorescent conformation (Fig. 3A) were converted to red (Fig. 3B). At 5 dpf, these fish were re-imaged.
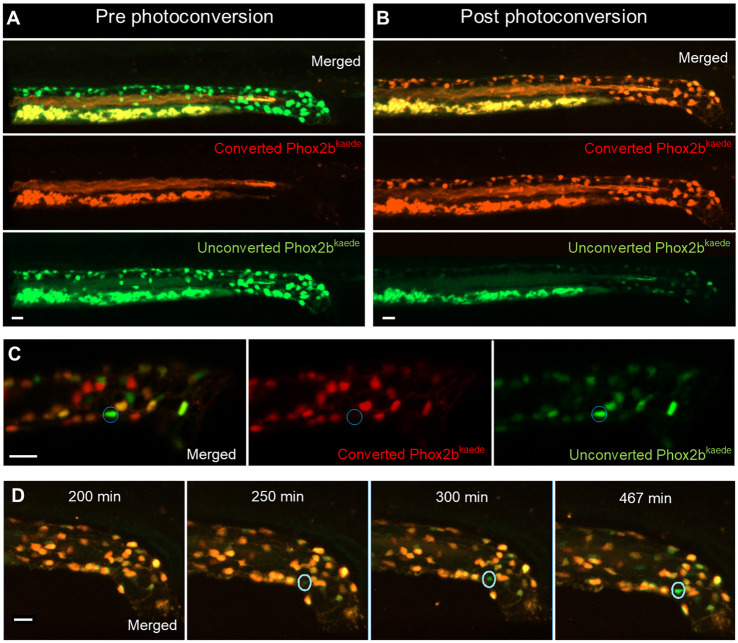
Fig. 3.
Enteric neurogenesis persists in the post-embryonic development despite the apparent absence of resident neuronal precursors. (A,B) 2D projection of z stack from a 4.5 dpf Phox2b-kaede fish demonstrates green fluorescent enteric neurons, but no red fluorescent cells (A). Yolk and intraluminal mucous exhibit expected autofluorescence in both channels. After photoconversion of all Phox2b-kaede neurons in the gut, all enteric neurons fluoresce red, although some retain decreased green fluorescence (B). (C) Live imaging 12 h after photoconversion at 4.5 dpf reveals the appearance of green fluorescent enteric neurons in the intestine with no red fluorescence (a representative neuron is circled), indicating that these neurons did not arise from pre-existing red fluorescent Phox2b-kaede cells. (D) Live 2D projection of a 10 h time-lapse after photoconversion at 4.5 dpf detects the emergence of de novo enteric neurons (circled), as indicated by the gradual appearance of a green-only neuron in a region of the intestine that was originally not occupied by a neuron. Scale bars: 20 µm.
Interestingly, we noted the appearance of Phox2b+ cells that only had green fluorescence (Fig. 3C), suggesting they were newly born enteric neurons that did not arise from pre-existing Phox2b cells. Exposing zebrafish to the thymidine analogue 5-ethynyl-2′-deoxyuridine (EdU) during this same time frame showed that some enteric neurons are marked during this post-embryonic stage, consistent our hypothesis that enteric neurogenesis persists during the post-embryonic stage (Fig. S3). To further validate this, we performed a 10 h live time-lapse imaging experiment after photoconversion of all Phox2b-kaede cells, and captured the emergence of de novo green-only Phox2b-kaede enteric neurons (Fig. 3D, Movie 2).
Next, we examined whether the loss of existing enteric neurons was followed by neurogenesis. Using the Phox2b-kaede line, we conducted two-photon laser ablation of ten Phox2b-kaede cells in the distal hindgut of 4.5 dpf zebrafish (Fig. 4A,B), and immediately photoconverted the remaining cells as described above. Upon re-imaging at 5 dpf, we again detected de novo enteric neurons (Fig. 4C). Time-lapse imaging over 8 h in a 4.5 dpf Phox2b-kaede fish that underwent laser injury of ten distal hindgut enteric neurons, followed by photoconversion of all remaining enteric neurons, revealed an injured neuron involuting and then being replaced by an emerging Phox2b-kaede de novo cell that appeared to actively migrate and extend projections to nearby neurons (Fig. 4D, Movie 3).
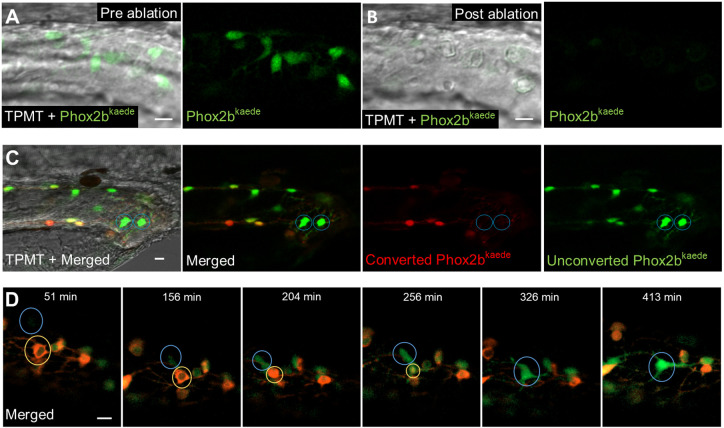
Fig. 4.
De novo enteric neurons replace ablated neurons in a post-embryonic injury model. (A,B) Prior to two-photon laser ablation, Phox2b-kaede enteric neurons are clearly visualized within the hindgut (A). After ablation, these neurons are no longer present in the hindgut, and TPMT reveals the injury site to be restricted to the neuron location (B). (C) At 4.5 dpf, fish underwent laser ablation of ten enteric neurons within the distal hindgut, followed by photoconversion of all remaining enteric neurons within the whole length of the gut. Live imaging was performed 12 h later and detected multiple de novo green fluorescent-only enteric neurons in the hindgut. (D) 8 h time-lapse of a fish at 4.5 dpf that underwent focal injury (but not complete ablation) of enteric neurons followed by pan-gut photoconversion reveals the involution of an injured neuron (yellow circle) that is replaced by a de novo green fluorescent-only enteric neuron. The new neuron (blue circle) initially appears very faintly at the dorsalmost aspect of the intestine but increases in intensity as it migrates to replace the involuted neuron and extends projections to neighbouring neurons. Scale bars: 10 µm.
Lineage tracing supports a trunk neural crest origin of post-embryonic enteric neurogenesis
Given that new Phox2b neurons apparently did not arise from existing neurons and there do not appear to be progenitors/glia in the intestine at this stage, we next investigated the possibility that these cells may arise from extrinsic sources. To explore the possibility that these come from the trunk spinal cord from which some Schwann cell precursors arise, we performed lineage tracing with the lipophilic dye DiIC18(5)-DS, which fluoresces in the far-red wavelength. To this end, we injected dye into the neural tube of Phox2b-kaede embryos at ∼30 h postfertilization (hpf), after the vagal neural crest has completed emigration from the neural tube (Fig. S4A-C). Live imaging of injected fish at 6 dpf revealed numerous dye-labelled enteric neurons: of 30 DiI-injected fish, 15 had Phox2b-kaede enteric neurons that colocalized with the dye (mean: 4.60 dye-labelled enteric neurons per fish, s.d.: 2.29) (Fig. 5A,B). There was no statistically significant difference in the distribution of dye-labelled enteric neurons within the foregut, midgut or hindgut. Given that trunk neural crest cells migrate from the neural tube during this time frame, these findings suggest that trunk, but not vagal, neural crest-derived cells are the source of these new enteric neurons. DiI-labelled cells were also found along peripheral nerves (Fig. S4D), consistent with the possibility that they are Schwann cell precursors.
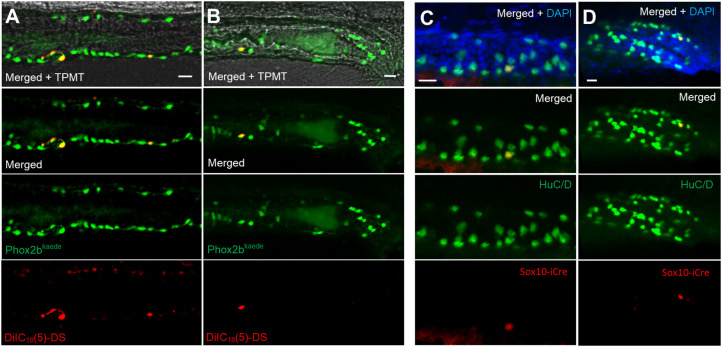
Fig. 5.
Lineage tracing demonstrates a trunk neural crest origin of post-embryonic neurons. (A,B) Phox2b-kaede embryos underwent neural tube injections of a far-red lipophilic dye at 30 hpf, after the vagal crest has delaminated from the neural tube. Live images at 6 dpf of the midgut (A) and hindgut (B) demonstrate Phox2b-kaede enteric neurons that colocalize with the dye, indicating their trunk origin. (C,D) Fish from the inducible Sox10-Cre line were exposed to 4-OHT at 3.5 dpf and underwent IHC for the Cre reporter, mCherry and the neuronal marker HuC/D at 5.5 dpf, with Cre-labelled enteric neurons observed in the midgut (C) and hindgut (D). As Cre induction occurring after Sox10 is no longer present within the intestine, these results support a trunk neural crest origin of these enteric neurons. Scale bars: 20 µm.
Next, we performed lineage tracing using transgenic approaches with the inducible Sox10-Cre line, Sox10ERT2×ubi:switch (Mongera et al., 2013) (Fig. S5A). Cells expressing Sox10 during the induction period are permanently labelled with the reporter mCherry (Fig. S5B). Zebrafish were induced at 3.5 dpf (after the vagal crest has completed intestinal colonization and Sox10 expression is no longer observed in the intestine) for a total of 16 h and then fixed at 5.5 dpf (Fig. S5C). These fish then underwent immunostaining using the neuronal marker HuC/D and the Cre reporter mCherry. Although this line labelled only a subset of Sox10-expressing cells, the results revealed enteric neurons that colocalized with the Cre reporter (Fig. 5C,D). Of the 34 induced fish, 11 exhibited Cre-labelled enteric neurons (mean: 1.63 Cre-labelled enteric neurons per fish, s.d.: 0.67). These results confirm that these neurons arose from a neural crest-derived source external to the intestine. Taken together, our two lineage-tracing experiments provide evidence that de novo enteric neurogenesis arises from trunk neural crest-derived progenitors, which are probably Schwann cell precursors that originate from the trunk neural tube and migrate to the intestine.
5HT4R agonism promotes post-embryonic enteric neurogenesis
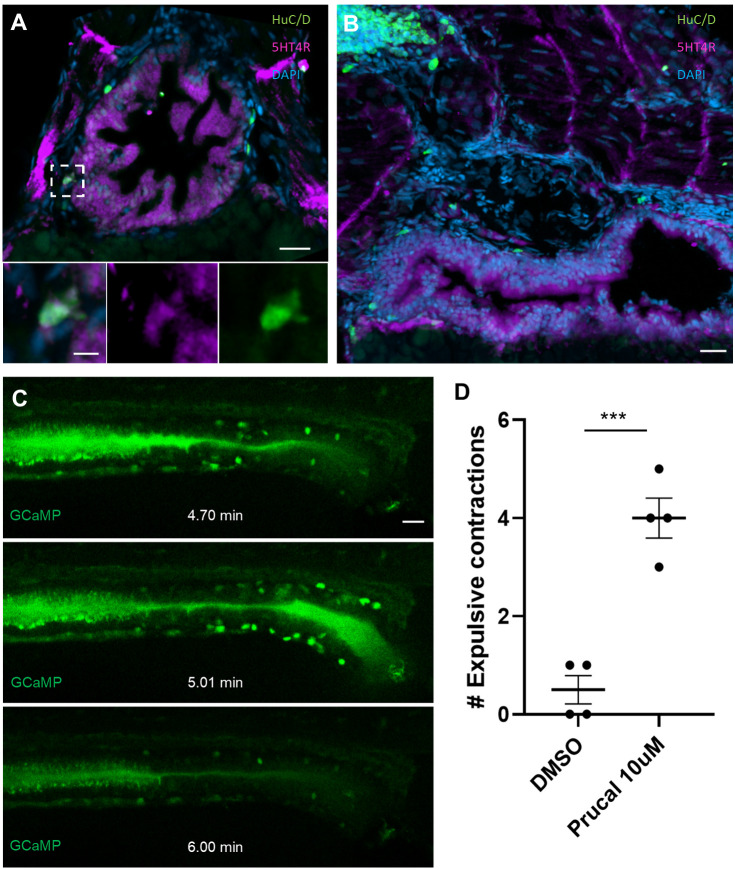
Previous studies in rodents (Liu et al., 2009; Matsuyoshi et al., 2010) have shown that postnatal enteric neurogenesis occurs after exposure to 5HT4R agonists. Recently, the highly specific 5HT4R agonist prucalopride was approved for clinical use in the USA to treat slow-transit constipation as this drug stimulates pro-motility activity of enteric neurons (Wong et al., 2010). IHC for 5HT4R revealed an expression pattern in zebrafish intestine consistent with that described in mammals (Hoffman et al., 2012), with diffuse expression in the mucosa, as well as colocalization with some neurons (Fig. 6A). In addition, we observed 5HT4R+ projections along the dorsoventral axis (Fig. 6B).
Fig. 6.
5HT4R is present in the zebrafish intestine and prucalopride increases intestinal motility. (A,B) Transverse section of 5 dpf IHC for 5HT4R reveals diffuse expression within the intestinal epithelium as well as occasional neurons that colocalize with this label (A). Longitudinal sections demonstrate diffuse expression in the proximal foregut and additionally reveal 5HT4R+ projections spanning the dorsoventral axis (B). (C) Stills from a video of a live HuC-H2B GCaMP6 fish exposed to 10 µM prucalopride at 5 dpf reveal increased intestinal motility, as measured by expulsive contractions of autofluorescent intraluminal mucous into the external environment (0.5 versus 4.0; P=0.0004). Increased GCaMP signal was observed in association with expulsive contractions, suggesting neuronally mediated motility. (D) Fish exposed to prucalopride exhibited significantly more expulsive contractions compared with controls. Data are mean±s.e.m. Scale bars: 20 µm (inset, 5 µm).
Using the zebrafish transgenic line HuC:H2B-GCaMP6 (Freeman et al., 2014), we found that fish exposed to 10 µM prucalopride exhibited significantly increased hindgut contractions resulting in increased intraluminal expulsion at 5 dpf compared with controls (mean: 4 versus 0.5 expulsive contractions; P=0.0004) (Fig. 6C,D, Movies 4,5). Notably, these contractions appeared to be associated with increased GCaMP activity in enteric neurons, suggesting neuronally mediated contractions. The results from this functional assay demonstrate that 5HT4R signalling in zebrafish is active at these drug concentrations.
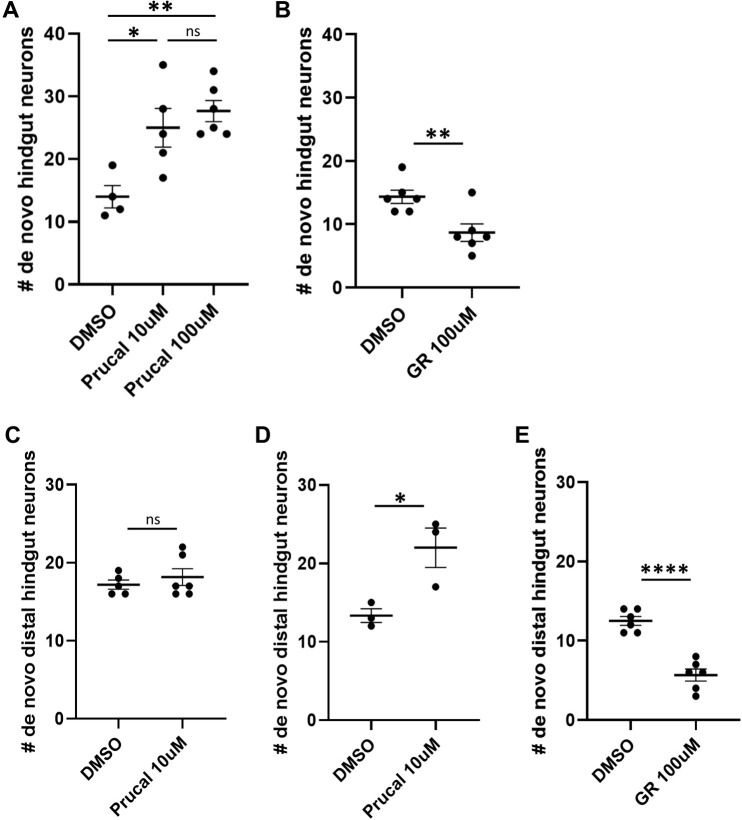
To assess the effects of prucalopride on post-embryonic enteric neurogenesis, we used the Phox2b-kaede line and photoconverted all enteric neurons at 4.5 dpf. Cohorts of these fish were then exposed to 10 µM or 100 μM prucalopride, or dimethyl sulfoxide (DMSO) (Fig. S6A). Live imaging was performed at 5 dpf. The results show that both prucalopride-treated cohorts possessed significantly more de novo enteric neurons (green-only) in the hindgut compared with controls (control, 14; 10 µM, 25; 100 µM, 27.67; P=0.0135 and P=0.0025) (Fig. 7A). As there was no difference in de novo enteric neuron numbers between the 10 µM and 100 µM cohorts, 10 µM of prucalopride was the dose used in subsequent experiments. An analogous experiment was performed with the 5HT4R antagonist GR 113808, which revealed a statistically significant decrease in de novo enteric neuron number (14.33 versus 8.67; P=0.0086) (Fig. 7B).
Fig. 7.
Prucalopride promotes enteric neurogenesis in normal development and injury. (A) After photoconversion of all enteric neurons at 4.5 dpf, cohorts of Phox2b-kaede fish were exposed to 10 µM prucalopride (n=5), 100 µM prucalopride (n=6) or DMSO (n=4) for 12 h and then live imaged at 5 dpf. The mean number of de novo hindgut neurons was significantly higher in fish treated with prucalopride (25, 27.7 and 14, respectively; P=0.019 and P=0.0036, respectively). (B) Under a similar design as A, fish were instead exposed to 100 µM GR 113808 (n=6) versus DMSO (n=6) for 12 h and then re-imaged at 5 dpf. The mean number of de novo hindgut neurons was significantly lower in fish treated with GR 113808 than in controls (8.67 versus 14.33; P=0.0086). (C) At 4.5 dpf, Phox2b-kaede fish underwent laser ablation of ten distal hindgut enteric neurons and then photoconversion of all enteric neurons. Cohorts were exposed to 10 µM prucalopride (n=6) or DMSO (n=5) for 12 h and then live imaged at 5 dpf. There was no difference in de novo distal hindgut neurons between these two groups. (D) Under a similar experimental design as C, fish were instead exposed to 10 µM prucalopride (n=3) or DMSO (n=3) at 3.5 dpf for 12 h, and then underwent cell ablation and photoconversion at 4.5 dpf. At 5 dpf, live imaging revealed significantly more distal hindgut neurons in fish pre-treated with prucalopride (22 versus 13.3; P=0.031). (E) Fish were exposed to 100 µM GR 113808 (n=6) or DMSO (n=6) at 3.5 dpf for 12 h, and then underwent cell ablation and photoconversion at 4.5 dpf. At 5 dpf, live imaging revealed significantly fewer distal hindgut neurons in fish pre-treated with GR 113808 than in controls (5.67 versus 12.50; P<0.0001). Data are mean±s.e.m. *P<0.05, **P<0.01,****P<0.0001. ns, not significant.
To assess whether 5HT4R agonism is involved in enteric neurogenesis after injury, we performed two-photon laser ablation of enteric neurons in the hindgut of Phox2b-kaede fish at 4.5 dpf, followed by photoconversion of all enteric neurons within the intestine. Subsequently, one cohort of these fish was treated with 10 µM prucalopride and a control group was treated with DMSO (Fig. S6B). Fish were re-imaged at 5 dpf, and de novo enteric neurons within the distal hindgut were counted; no significant difference was noted between the two groups (17.2 versus 18.2; P=0.47) (Fig. 7C).
We next repeated this experiment but treated one cohort of fish with 10 µM prucalopride for 24 h before laser ablation and photoconversion at 4.5 dpf (Fig. S6C). Compared with controls pre-treated with DMSO, pre-treatment with prucalopride resulted in significantly more (13.3 versus 22; P=0.03) de novo enteric neurons in the distal hindgut after injury (Fig. 7D). An analogous experiment was performed with GR 113808 and revealed a statistically significant decrease in de novo enteric neuron number after injury (12.50 versus 5.67; P<0.0001) (Fig. 7E). These findings suggest that exposure to prucalopride before injury promotes the regeneration of enteric neurons, whereas a short course of treatment after injury has no effect on neurogenesis.
DISCUSSION
In this study, we provide evidence that zebrafish enteric neurogenesis persists in the post-embryonic intestine both during normal development and after ablation of enteric neurons, despite an apparent absence of enteric glia and/or Sox10-derived resident progenitors. Rather, lineage-tracing experiments support the intriguing possibility that trunk crest-derived neural crest stem cells, which are probably Schwann cell precursors that migrate along nerves from the spinal cord to the intestines, are a source of this post-embryonic enteric neurogenesis. Along with the expected pro-motility effect, we also demonstrated that 5HT4R agonism with prucalopride increased post-embryonic neurogenesis in normal development and appeared to promote the regeneration of enteric neurons if the exposure occurred before injury.
Our results are consistent with studies in the basal jawless vertebrate lamprey (Green et al., 2017) and mice (Uesaka et al., 2015), showing that there is an important contribution of Schwann cell precursors to the ENS during development. Moreover, our results suggest that this source persists post-embryonically to support ongoing neurogenesis, reflecting both normal turnover of neurons and regeneration after injury. As lampreys lack a vagal neural crest, enteric neurogenesis from SCPs is probably the basal state for populating the ancestral vertebrate intestine with neurons (Green et al., 2017). With the advent of the vagal neural crest in jawed vertebrates, it became the main embryonic source to populate enteric neurons, whereas SCPs might have been repurposed as a means to supplement additional enteric neurons to accommodate continued growth during post-embryonic development, as well as regeneration after injury.
The simplified zebrafish ENS compared with amniotes, together with the apparent absence of enteric glia, makes it a highly tractable model in which to examine the complex nature of post-embryonic enteric neurogenesis. Importantly, the zebrafish ENS develops in a homologous manner to humans during embryogenesis (Ganz, 2018; Heanue et al., 2016), and the intestine is anatomically and functionally segmented similarly to human small intestine and colon (Wang et al., 2010). Notably, the 5-HT4 receptor arose early in evolution (Hashiguchi and Nishida, 2007; Tierney, 2018), and 82% of disease-related human genes have a zebrafish homologue (Howe et al., 2013); thus, the zebrafish is an ideal system in which to explore fundamental features of post-embryonic enteric neurogenesis.
As SCP-derived enteric neurogenesis is conserved in mammals, our demonstration that this source of enteric neurogenesis may be amenable to pharmacological manipulation deepens the rationale for further exploration into 5HT4R-based therapies for human enteric neuropathies. Other 5HT4R agonists such as mosapride (not available in the USA) and tegaserod (limited use in the USA due to off-target side effects) have previously supported the role of this signalling pathway in enteric neurogenesis (Liu et al., 2009; Matsuyoshi et al., 2010). Our study demonstrates that prucalopride, a highly specific 5HT4R agonist that has recently been approved for use in the USA (Wong et al., 2010), promotes enteric neurogenesis in a wild-type model. This is consistent with the findings of a previous study that showed prucalopride prevented ENS hypoplasia in Ala56-expressing mice, a model in which endogenous neuronal 5-HT is removed excessively rapidly from the synaptic cleft (Margolis et al., 2016). Previous studies suggested 5HT4R agonism mediated its enteric neurogenic effect through a resident progenitor, but the evidence was inconclusive, as a gut-extrinsic source was not assessed (Goto et al., 2013; Katsui et al., 2009; Liu et al., 2009; Matsuyoshi et al., 2010). Indeed, in one of these studies (Liu et al., 2009), neuronal precursors were first detected outside of enteric ganglia and then appeared to migrate within the ganglia, which has led us and others (Uesaka et al., 2015) to hypothesize that these observations are consistent with SCP-derived enteric neurogenesis. Although we found pre-treatment with prucalopride promoted enteric neurogenesis after injury, treatment after injury did not. This might suggest that a period of recovery or a longer treatment duration is required to promote enteric neuronal regeneration, but further investigation is required.
There is conflicting evidence in the literature regarding the presence of enteric glia in zebrafish. Some authors have reported GFAP immunoreactivity in the intestine (Baker et al., 2019; Doodnath et al., 2010; Hagström and Olsson, 2010), leading them to conclude that enteric glia are present. However, the observed immunoreactivity is fibrillary and probably reflects projections from extrinsic glia or other cell types, as no cell bodies are evident. Furthermore, we found that S100, an enteric glial marker with nuclear expression, failed to reveal enteric glia in the zebrafish, consistent with another study (Germanà et al., 2008). However, we cannot exclude the possibility of a vagal crest-derived Sox10-negative resident enteric neuronal progenitor, as suggested by one study (Kulkarni et al., 2017), or that some glia-like cell exists but is undetectable by our methods. On the other hand, our lineage-tracing experiments, using an indelible Sox10-Cre line, dye-labelling and an inducible Sox10-Cre line, support a gut-extrinsic trunk neural crest-derived source of enteric neurogenesis.
Other studies have raised questions about the functional importance of glia in the mammalian ENS. For example, in male mice from which all enteric glia were genetically ablated, no obvious differences were observed in intestinal motility or predilection to enterocolitis (Rao et al., 2017). As zebrafish have a functional intestine despite the apparent absence of enteric glia, when and why enteric glia evolved represents an interesting question for future study. Considering that humans probably possess hundreds of millions of enteric glia (Grubišić and Gulbransen, 2017), clarifying their functional significance carries broad implications in gastroenterology (Gulbransen and Sharkey, 2012) and might be aided by investigating their evolutionary development.
Our simplified motility assay to assess the effect on the zebrafish intestine of prucalopride is easily accessible without the need for custom-made cameras or image-processing programs, contrasting with other gastrointestinal motility studies in zebrafish (Ganz et al., 2018; Shi et al., 2014). The results demonstrate a functionally significant assay that is intuitively translatable to clinical endpoints.
By applying live-imaging techniques in the zebrafish, we are able to specifically ablate individual enteric neurons with minimal damage to adjacent cells and then capture the regeneration of enteric neurons in real time. Previous studies (Goto et al., 2013; Katsui et al., 2009; Laranjeira et al., 2011; Matsuyoshi et al., 2010) required the broad application of cytotoxic compounds or full-thickness surgical transection, followed by assessment for neurogenesis at later time points. Our approach has the advantage of specifically injuring enteric neurons followed by in vivo time-lapse detection of neurogenesis. Taken together, our results reveal fundamental features of post-embryonic ENS development and regeneration in the zebrafish, pointing toward potential therapeutic strategies to promote Schwann cell precursor-derived enteric neurogenesis in the treatment of enteric neuropathies.
MATERIALS AND METHODS
Transgenic lines
Zebrafish (Danio rerio) were maintained at 28°C, with adults on a 13 h light/11 h dark cycle. All zebrafish work was completed in compliance with the California Institute of Technology Institutional Animal Care and Use Committee. Transgenic lines used in this study were: the photoconvertible Phox2b-kaede line (Harrison et al., 2014); the sox10:GAL4-UAS-Cre (‘indelible Sox10-Cre’) (Cavanaugh et al., 2015) line, which was crossed with the ubi:switch reporter line (Cavanaugh et al., 2015); the cmlc:GFP sox10:ERT2-Cre (‘inducible Sox10-Cre’) (Mongera et al., 2013) line, which was crossed with the ubi:switch reporter line; the sox10-mRFP line (Kucenas et al., 2008); the HuC:GCaMP6 transgenic line (Freeman et al., 2014); and the GFAP-GFP line (Bernardos and Raymond, 2006). All lines were within the AB wild-type background, with the exception of the HucC:GCaMP6 line, which was backcrossed onto the pigmentless ‘casper’ line (White et al., 2008).
ISH and IHC
Embryos and larvae underwent hybridization as previously described (Jowett and Lettice, 1994), with the following changes: samples were stored in ethanol and digestion was performed with 1 mg/ml collagenase IA (Sigma-Aldrich, C9891; 5 min and 12 min for 2 dpf and 3.5 dpf, respectively) before proteinase K digestion (12 min and 14 min for 2 dpf and 3.5 dpf, respectively). All imaging of ISH specimens was performed using a Zeiss Imager M2 with an ApoTome.2 module.
The whole-mount IHC staining of embryos and larvae protocol was adapted from a previous study (Ungos et al., 2003), and was performed by fixation in 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PBS) overnight at 4°C, then washing in 1× PBS, followed by incubation in 0.5× PBS for 30 min. Samples were then placed in blocking solution (2% goat serum, 1% bovine serum albumin, 1% DMSO, 0.1% Triton X-100 and 0.05% Tween 20 in 1× PBS) for 2 h at room temperature. Samples were then incubated in primary antibody diluted in blocking solution overnight at room temperature and washed for 2 h to 3 h in 1× PBS plus 0.1% Triton X-100. Then, samples were incubated overnight in secondary antibody diluted in blocking solution plus DAPI (1:1000; Thermo Fisher Scientific D1306) overnight at room temperature and washed for 2-3 h in 1× PBS plus 0.1% Triton X-100. Samples were then mounted in RIMS (Yang et al., 2014) to achieve optical clearing.
For histological sections, cryosections were collected at 10 µm thickness unless otherwise noted. Blocking and antibody incubation occurred the same as with whole-mount samples, except that antibody incubations occurred at 4°C and samples were mounted with Fluoromount-G (Thermo Fisher Scientific, 00-4958-02).
The primary antibodies used were mouse anti-HuC/D IgG2b (1:200; Thermo Fisher Scientific, A21271), mouse anti-mCherry IgG1 (1:200; Clontech Laboratories, 632543), rabbit anti-GFAP IgG (1:200; GeneTex, GTX 128741), rabbit anti-5HT4 Receptor IgG (1:200; Abcam, ab60359), rabbit anti-S100 IgG (1:200; Dako, GA504) and mouse anti-acetylated tubulin IgG2b (1:500; Sigma-Aldrich, T7541). The secondary antibodies used in this study were goat anti-mouse IgG2b 488, 568 and 647 (1:500; Thermo Fisher Scientific, A21141, A21144 and A21242, respectively), goat anti-mouse IgG1 568 (1:500; Thermo Fisher Scientific, A21124) and goat anti-rabbit IgG 647 (1:500; Thermo Fisher Scientific, A21134). All imaging of IHC specimens was performed on the Zeiss LSM 800 confocal microscope and figures were produced using ImageJ software (National Institutes of Health), except 20 µm sections, which were processed using Imaris (Bitplane).
Adult intestine whole-mount imaging
Adult zebrafish intestine was procured as previously described (Gupta and Mullins, 2010). Intestine was then opened longitudinally, fixed in 4% PFA in 0.1 M phosphate buffer overnight at 4°C, washed in 1× PBS, incubated in DAPI 1:1000 for 2 h at room temperature, washed in 1× PBS and then incubated in RIMS for 2 days at 4°C. The intestine was then mounted onto a slide in RIMS and imaged with the LSM 800 confocal microscope.
Photoconversion
Adapting a protocol described previously (Hatta et al., 2006), we photoconverted all enteric neurons of Phox2b-kaede fish at 4.5 dpf using a Zeiss LSM 800 confocal microscope. Full thickness photoconversion was confirmed by post-conversion imaging through the full z stack in all fish.
Lipophilic dye neural tube fills
The far-red lipophilic dye DiIC18(5)-DS (Thermo Fisher Scientific, D12730) was prepared according to the manufacturer's instructions and injections were performed by adapting a protocol described previously (Gutzman and Sive, 2009). Briefly, 2.3 nl of dye was injected at ∼30 hpf into the anterior neuropore using a glass capillary needle affixed to a Nanoliter 2000 microinjector. Imaging at 6 dpf was performed with a Zeiss LSM 800 confocal microscope.
Two-photon cell ablation
Adapting a protocol described previously (Muto and Kawakami, 2018), we ablated ten enteric neurons within the distal hindgut (i.e. corresponding to the last two somite lengths of hindgut) of Pho2b-kaede fish at 4.5 dpf using a Zeiss LSM 710 confocal microscope with two-photon laser ablation.
Drug/chemical exposure
Prucalopride (MilliporeSigma, SML1371) was prepared at 10 µM and 100 µM in DMSO, and GR 113808 (Sigma-Aldrich, G5918) was prepared at 100 µM in DMSO. Exposure occurred at 4.5 dpf and continued until 5.5 dpf, unless otherwise stated. An aliquot of 4-hydroxytamoxifen (4-OHT) (MilliporeSigma, H7904) was prepared at 20 µM in ethanol and exposure occurred at 3.5 dpf for a total of 16 h. Controls in the prucalopride and 4-OHT experiments were exposed to equal volumes of DMSO or ethanol, respectively.
The thymidine analogue EdU (Thermo Fisher Scientific, C10634) was prepared in DMSO at 10 mM and added to embryo water (final concentration of 500 µM) from 4.5 dpf to 5 dpf. Fish were fixed and sectioned as described above, and the assay was performed following the manufacturer's instructions.
Live-imaging
Live zebrafish larvae were anaesthetized with tricaine and mounted within chamber slides using 1.2% low-melt agarose prepared in embryo water (https://zfin.org/zf_info/zfbook/chapt1/1.5.html)). Additional embryo water was added after solidification of the agarose. All live imaging was performed using a Zeiss LSM 800 confocal microscope with the incubator set at 28°C. For time-lapse experiments, z stacks were collected every 4 min with a duration of 8 h to 10 h. Videos and 2D projections of z stacks were produced using Imaris software. All other live images were produced using ImageJ software.
For the functional assay, a continuous video was collected after placing the mounted larvae in the heated incubator chamber of the microscope for 30 min. Then, a baseline video was collected for 3 min in the z-plane corresponding to the mid-depth of the intestine, followed by addition of prucalopride in DMSO for a final concentration of 10 µM or DMSO alone to the chamber of an individual fish, and then a 15 min video was immediately collected. An ‘expulsive contraction’ was defined as a contraction resulting in the expulsion of autofluorescent intraluminal mucous out of the hindgut and into the external environment. Videos were produced using ImageJ software.
Cell counting and statistics
Cell counting was performed manually using ImageJ software. In non-ablated fish, cell counts were within the hindgut corresponding to the last four somite lengths of hindgut. In the cell ablation experiments, cell counts were within the distal hindgut corresponding to the last two somite lengths of hindgut (i.e. within the field of ablation). Statistics were performed using GraphPad Prism 8 (GraphPad Software) and Student's t-test for two group comparisons and one-way ANOVA for more than two group comparisons, with P<0.05 indicating statistical significance.
Supplementary Material
Acknowledgements
We thank the Beckman Institute Biological Imaging Facility of Caltech for technical assistance with microscopy experiments. For generously supplying transgenic fish lines, we thank the Ian Shepherd Lab (Phox2b-kaede), the Jua-Nian Chen Lab (sox10:GAL4-UAS-Cre and ubi:switch) and the Christiane Nüsslein-Volhard Lab (cmlc:GFP-sox10:ERT2-Cre). Special thanks to Megan Martik and Can Li for sharing ISH probes (Sox10, Phox2bb). We also recognize Claire Hu (California Institute of Technology) for assistance with IHC.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: W.N.E.-N., M.E.B.; Methodology: W.N.E.-N., M.E.B.; Validation: W.N.E.-N.; Formal analysis: W.N.E.-N., M.E.B.; Investigation: W.N.E.-N.; Resources: W.N.E.-N., M.E.B.; Data curation: W.N.E.-N., M.E.B.; Writing - original draft: W.N.E.-N.; Writing - review & editing: W.N.E.-N., M.E.B.; Visualization: W.N.E.-N.; Supervision: M.E.B.; Project administration: M.E.B.; Funding acquisition: M.E.B.
Funding
This work was supported by the National Institutes of Health (NIH R35NS111564 to M.E.B. and NIH R01NS108500 to M.E.B.). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at https://dev.biologists.org/lookup/doi/10.1242/dev.186619.supplemental
Peer review history
The peer review history is available online at https://dev.biologists.org/lookup/doi/10.1242/dev.186619.reviewer-comments.pdf
References
- Adameyko I., Lallemend F., Aquino J. B., Pereira J. A., Topilko P., Müller T., Fritz N., Beljajeva A., Mochii M., Liste I. et al. (2009). Schwann cell precursors from nerve innervation are a cellular origin of melanocytes in skin. Cell 139, 366-379. 10.1016/j.cell.2009.07.049 [DOI] [PubMed] [Google Scholar]
- Baker P. A., Meyer M. D., Tsang A. and Uribe R. A. (2019). Immunohistochemical and ultrastructural analysis of the maturing larval zebrafish enteric nervous system reveals the formation of a neuropil pattern. Sci. Rep. 9, 6941 10.1038/s41598-019-43497-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardos R. L. and Raymond P. A. (2006). GFAP transgenic zebrafish. Gene Expr. Patterns 6, 1007-1013. 10.1016/j.modgep.2006.04.006 [DOI] [PubMed] [Google Scholar]
- Brierley S. M. and Linden D. R. (2014). Neuroplasticity and dysfunction after gastrointestinal inflammation. Nat. Rev. Gastroenterol. Hepatol. 11, 611-627. 10.1038/nrgastro.2014.103 [DOI] [PubMed] [Google Scholar]
- Burns A. J., Champeval D. and Le Douarin N. M. (2000). Sacral neural crest cells colonise aganglionic hindgut in vivo but fail to compensate for lack of enteric ganglia. Dev. Biol. 219, 30-43. 10.1006/dbio.1999.9592 [DOI] [PubMed] [Google Scholar]
- Cavanaugh A. M., Huang J. and Chen J.-N. (2015). Two developmentally distinct populations of neural crest cells contribute to the zebrafish heart. Dev. Biol. 404, 103-112. 10.1016/j.ydbio.2015.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doodnath R., Dervan A., Wride M. A. and Puri P. (2010). Zebrafish: an exciting model for investigating the spatio-temporal pattern of enteric nervous system development. Pediatr. Surg. Int. 26, 1217-1221. 10.1007/s00383-010-2746-7 [DOI] [PubMed] [Google Scholar]
- Dyachuk V., Furlan A., Shahidi M. K., Giovenco M., Kaukua N., Konstantinidou C., Pachnis V., Memic F., Marklund U., Müller T. et al. (2014). Parasympathetic neurons originate from nerve-associated peripheral glial progenitors. Science 345, 82-87. 10.1126/science.1253281 [DOI] [PubMed] [Google Scholar]
- Espinosa-Medina I., Outin E., Picard C. A., Chettouh Z., Dymecki S., Consalez G. G., Coppola E. and Brunet J.-F. (2014). Parasympathetic ganglia derive from Schwann cell precursors. Science 345, 87-90. 10.1126/science.1253286 [DOI] [PubMed] [Google Scholar]
- Espinosa-Medina I., Jevans B., Boismoreau F., Chettouh Z., Enomoto H., Müller T., Birchmeier C., Burns A. J. and Brunet J.-F. (2017). Dual origin of enteric neurons in vagal Schwann cell precursors and the sympathetic neural crest. Proc. Natl. Acad. Sci. USA 114, 11980-11985. 10.1073/pnas.1710308114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrugia G. (2015). Histologic changes in diabetic gastroparesis. Gastroenterol. Clin. North Am. 44, 31-38. 10.1016/j.gtc.2014.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman J., Vladimirov N., Kawashima T., Mu Y., Sofroniew N. J., Bennett D. V., Rosen J., Yang C.-T., Looger L. L. and Ahrens M. B. (2014). Mapping brain activity at scale with cluster computing. Nat. Methods 11, 941-950. 10.1038/nmeth.3041 [DOI] [PubMed] [Google Scholar]
- Furlan A. and Adameyko I. (2018). Schwann cell precursor: a neural crest cell in disguise? Dev. Biol. 444 Suppl. 1, S25-S35. 10.1016/j.ydbio.2018.02.008 [DOI] [PubMed] [Google Scholar]
- Furlan A., Dyachuk V., Kastriti M. E., Calvo-Enrique L., Abdo H., Hadjab S., Chontorotzea T., Akkuratova N., Usoskin D., Kamenev D. et al. (2017). Multipotent peripheral glial cells generate neuroendocrine cells of the adrenal medulla. Science 357, eaal3753 10.1126/science.aal3753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J. B. (2006). The Enteric Nervous System, 1st edn.: Blackwell. [Google Scholar]
- Ganz J. (2018). Gut feelings: studying enteric nervous system development, function, and disease in the zebrafish model system. Dev. Dyn. 247, 268-278. 10.1002/dvdy.24597 [DOI] [PubMed] [Google Scholar]
- Ganz J., Baker R. P., Hamilton M. K., Melancon E., Diba P., Eisen J. S. and Parthasarathy R. (2018). Image velocimetry and spectral analysis enable quantitative characterization of larval zebrafish gut motility. Neurogastroenterol. Motil. 30, e13351 10.1111/nmo.13351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germanà A., Marino F., Guerrera M. C., Campo S., de Girolamo P., Montalbano G., Germanà G. P., Ochoa-Erena F. J., Ciriaco E. and Vega J. A. (2008). Expression and distribution of S100 protein in the nervous system of the adult zebrafish (Danio rerio). Microsc. Res. Tech. 71, 248-255. 10.1002/jemt.20544 [DOI] [PubMed] [Google Scholar]
- Goto K., Kato G., Kawahara I., Luo Y., Obata K., Misawa H., Ishikawa T., Kuniyasu H., Nabekura J. and Takaki M. (2013). In vivo imaging of enteric neurogenesis in the deep tissue of mouse small intestine. PLoS ONE 8, e54814 10.1371/journal.pone.0054814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S. A., Uy B. R. and Bronner M. E. (2017). Ancient evolutionary origin of vertebrate enteric neurons from trunk-derived neural crest. Nature 544, 88-91. 10.1038/nature21679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubišić V. and Gulbransen B. D. (2017). Enteric glia: the most alimentary of all glia. J. Physiol. 595, 557-570. 10.1113/JP271021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbransen B. D. and Sharkey K. A. (2012). Novel functional roles for enteric glia in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 9, 625-632. 10.1038/nrgastro.2012.138 [DOI] [PubMed] [Google Scholar]
- Gupta T. and Mullins M. C. (2010). Dissection of organs from the adult zebrafish. J. Vis. Exp. 37, e1717 10.3791/1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzman J. H. and Sive H. (2009). Zebrafish brain ventricle injection. J. Vis. Exp. 26, e1218 10.3791/1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagström C. and Olsson C. (2010). Glial cells revealed by GFAP immunoreactivity in fish gut. Cell Tissue Res. 341, 73-81. 10.1007/s00441-010-0979-3 [DOI] [PubMed] [Google Scholar]
- Harrison C., Wabbersen T. and Shepherd I. T. (2014). In vivo visualization of the development of the enteric nervous system using a Tg(−8.3bphox2b:Kaede) transgenic zebrafish. Genesis 52, 985-990. 10.1002/dvg.22826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiguchi Y. and Nishida M. (2007). Evolution of trace amine–associated receptor (TAAR) gene family in vertebrates: lineage-specific expansions and degradations of a second class of vertebrate chemosensory receptors expressed in the olfactory epithelium. Mol. Biol. Evol. 24, 2099-2107. 10.1093/molbev/msm140 [DOI] [PubMed] [Google Scholar]
- Hatta K., Tsujii H. and Omura T. (2006). Cell tracking using a photoconvertible fluorescent protein. Nat. Protoc. 1, 960-967. 10.1038/nprot.2006.96 [DOI] [PubMed] [Google Scholar]
- Heanue T. A. and Pachnis V. (2007). Enteric nervous system development and Hirschsprung's disease: advances in genetic and stem cell studies. Nat. Rev. Neurosci. 8, 466-479. 10.1038/nrn2137 [DOI] [PubMed] [Google Scholar]
- Heanue T. A., Shepherd I. T. and Burns A. J. (2016). Enteric nervous system development in avian and zebrafish models. Dev. Biol. 417, 129-138. 10.1016/j.ydbio.2016.05.017 [DOI] [PubMed] [Google Scholar]
- Hoffman J. M., Tyler K., MacEachern S. J., Balemba O. B., Johnson A. C., Brooks E. M., Zhao H., Swain G. M., Moses P. L., Galligan J. J. et al. (2012). Activation of colonic mucosal 5-HT4 receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology 142, 844-854.e4. 10.1053/j.gastro.2011.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe K., Clark M. D., Torroja C. F., Torrance J., Berthelot C., Muffato M., Collins J. E., Humphray S., McLaren K., Matthews L. et al. (2013). The zebrafish reference genome sequence and its relationship to the human genome. Nature 496, 498-503. 10.1038/nature12111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph N. M., He S., Quintana E., Kim Y.-G., Núñez G. and Morrison S. J. (2011). Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut. J. Clin. Invest. 121, 3398-3411. 10.1172/JCI58186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jowett T. and Lettice L. (1994). Whole-mount in situ hybridization on zebrafish embryos using a mixture of digoxigenin- and fluorescein-labelled probes. Trends Genet. 10, 73-74. 10.1016/0168-9525(94)90220-8 [DOI] [PubMed] [Google Scholar]
- Kastriti M. E., Kameneva P., Kamenev D., Dyachuk V., Furlan A., Hampl M., Memic F., Marklund U., Lallemend F., Hadjab S. et al. (2019). Schwann cell precursors generate the majority of chromaffin cells in Zuckerkandl organ and some sympathetic neurons in Paraganglia. Front. Mol. Neurosci. 12, 6 10.3389/fnmol.2019.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsui R., Kuniyasu H., Matsuyoshi H., Fujii H., Nakajima Y. and Takaki M. (2009). The plasticity of the defecation reflex pathway in the enteric nervous system of guinea pigs. J. Smooth Muscle Res. 45, 1-13. 10.1540/jsmr.45.1 [DOI] [PubMed] [Google Scholar]
- Kraichely R. E. and Farrugia G. (2006). Achalasia: physiology and etiopathogenesis. Dis. Esophagus 19, 213-223. 10.1111/j.1442-2050.2006.00569.x [DOI] [PubMed] [Google Scholar]
- Kucenas S., Takada N., Park H.-C., Woodruff E., Broadie K. and Appel B. (2008). CNS-derived glia ensheath peripheral nerves and mediate motor root development. Nat. Neurosci. 11, 143-151. 10.1038/nn2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni S., Micci M.-A., Leser J., Shin C., Tang S.-C., Fu Y.-Y., Liu L., Li Q., Saha M., Li C. et al. (2017). Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc. Natl. Acad. Sci. USA 114, E3709-E3718. 10.1073/pnas.1619406114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake J. I. and Heuckeroth R. O. (2013). Enteric nervous system development: migration, differentiation, and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G1-G24. 10.1152/ajpgi.00452.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laranjeira C., Sandgren K., Kessaris N., Richardson W., Potocnik A., Vanden Berghe P. and Pachnis V. (2011). Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. J. Clin. Invest. 121, 3412-3424. 10.1172/jci58200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasrado R., Boesmans W., Kleinjung J., Pin C., Bell D., Bhaw L., McCallum S., Zong H., Luo L., Clevers H. et al. (2017). Lineage-dependent spatial and functional organization of the mammalian enteric nervous system. Science 356, 722-726. 10.1126/science.aam7511 [DOI] [PubMed] [Google Scholar]
- Liu M.-T., Kuan Y.-H., Wang J., Hen R. and Gershon M. D. (2009). 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J. Neurosci. 29, 9683-9699. 10.1523/jneurosci.1145-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis K. G., Li Z., Stevanovic K., Saurman V., Israelyan N., Anderson G. M., Snyder I., Veenstra-VanderWeele J., Blakely R. D. and Gershon M. D. (2016). Serotonin transporter variant drives preventable gastrointestinal abnormalities in development and function. J. Clin. Invest. 126, 2221-2235. 10.1172/JCI84877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martik M. L. and Bronner M. E. (2017). Regulatory logic underlying diversification of the neural crest. Trends Genet. 33, 715-727. 10.1016/j.tig.2017.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyoshi H., Kuniyasu H., Okumura M., Misawa H., Katsui R., Zhang G.-X., Obata K. and Takaki M. (2010). A 5-HT4-receptor activation-induced neural plasticity enhances in vivo reconstructs of enteric nerve circuit insult. Neurogastroenterol. Motil. 22, 806-813 e226. 10.1111/j.1365-2982.2010.01474.x [DOI] [PubMed] [Google Scholar]
- Mongera A., Singh A. P., Levesque M. P., Chen Y.-Y., Konstantinidis P. and Nüsslein-Volhard C. (2013). Genetic lineage labeling in zebrafish uncovers novel neural crest contributions to the head, including gill pillar cells. Development 140, 916-925. 10.1242/dev.091066 [DOI] [PubMed] [Google Scholar]
- Muto A. and Kawakami K. (2018). Ablation of a neuronal population using a two-photon laser and its assessment using calcium imaging and behavioral recording in zebrafish larvae. J. Vis. Exp. 136, 57485 10.3791/57485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M. and Gershon M. D. (2018). Enteric nervous system development: what could possibly go wrong? Nat. Rev. Neurosci. 19, 552-565. 10.1038/s41583-018-0041-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M., Nelms B. D., Dong L., Salinas-Rios V., Rutlin M., Gershon M. and Corfas G. (2015). Enteric glia express proteolipid protein 1 and are a transcriptionally unique population of glia in the mammalian nervous system. Glia 63, 2040-2057. 10.1002/glia.22876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M., Rastelli D., Dong L., Chiu S., Setlik W., Gershon M. D. and Corfas G. (2017). Enteric glia regulate gastrointestinal motility but are not required for maintenance of the epithelium in mice. Gastroenterology 153, 1068-1081. e7. 10.1053/j.gastro.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Zhang Y., Zhao F., Ruan H., Huang H., Luo L. and Li L. (2014). Acetylcholine serves as a derepressor in Loperamide-induced opioid-induced bowel dysfunction (OIBD) in zebrafish. Sci. Rep. 4, 5602 10.1038/srep05602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struijs M.-C., Diamond I. R., de Silva N. and Wales P. W. (2009). Establishing norms for intestinal length in children. J. Pediatr. Surg. 44, 933-938. 10.1016/j.jpedsurg.2009.01.031 [DOI] [PubMed] [Google Scholar]
- Tierney A. J. (2018). Invertebrate serotonin receptors: a molecular perspective on classification and pharmacology. J. Exp. Biol. 221, jeb184838 10.1242/jeb.184838 [DOI] [PubMed] [Google Scholar]
- Uesaka T., Nagashimada M. and Enomoto H. (2015). Neuronal differentiation in schwann cell lineage underlies postnatal neurogenesis in the enteric nervous system. J. Neurosci. 35, 9879-9888. 10.1523/JNEUROSCI.1239-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungos J. M., Karlstrom R. O. and Raible D. W. (2003). Hedgehog signaling is directly required for the development of zebrafish dorsal root ganglia neurons. Development 130, 5351-5362. 10.1242/dev.00722 [DOI] [PubMed] [Google Scholar]
- Wang Z., Du J., Lam S. H., Mathavan S., Matsudaira P. and Gong Z. (2010). Morphological and molecular evidence for functional organization along the rostrocaudal axis of the adult zebrafish intestine. BMC Genomics 11, 392 10.1186/1471-2164-11-392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Chan A. K. K., Sham M. H., Burns A. J. and Chan W. Y. (2011). Analysis of the sacral neural crest cell contribution to the hindgut enteric nervous system in the mouse embryo. Gastroenterology 141, 992-1002.e6. 10.1053/j.gastro.2011.06.002 [DOI] [PubMed] [Google Scholar]
- Weaver L. T., Austin S. and Cole T. J. (1991). Small intestinal length: a factor essential for gut adaptation. Gut 32, 1321-1323. 10.1136/gut.32.11.1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. M., Sessa A., Burke C., Bowman T., LeBlanc J., Ceol C., Bourque C., Dovey M., Goessling W., Burns C. E. et al. (2008). Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2, 183-189. 10.1016/j.stem.2007.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong B. S., Manabe N. and Camilleri M. (2010). Role of prucalopride, a serotonin (5-HT(4)) receptor agonist, for the treatment of chronic constipation. Clin. Exp. Gastroenterol. 3, 49-56. 10.2147/ceg.s8091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. D. (2011). Enteric nervous system neuropathy: repair and restoration. Curr. Opin. Gastroenterol. 27, 106-111. 10.1097/MOG.0b013e328342a6ea [DOI] [PubMed] [Google Scholar]
- Yang B., Treweek J. B., Kulkarni R. P., Deverman B. E., Chen C.-K., Lubeck E., Shah S., Cai L. and Gradinaru V. (2014). Single-cell phenotyping within transparent intact tissue through whole-body clearing. Cell 158, 945-958. 10.1016/j.cell.2014.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.