ABSTRACT
Nuclear factor erythroid 2-related factor 2 (NFE2L2, also known as NRF2) is a transcription factor and master regulator of cellular antioxidant response. Aberrantly high NRF2-dependent transcription is recurrent in human cancer, but conversely NRF2 activity diminishes with age and in neurodegenerative and metabolic disorders. Although NRF2-activating drugs are clinically beneficial, NRF2 inhibitors do not yet exist. Here, we describe use of a gain-of-function genetic screen of the kinome to identify new druggable regulators of NRF2 signaling. We found that the under-studied protein kinase brain-specific kinase 2 (BRSK2) and the related BRSK1 kinases suppress NRF2-dependent transcription and NRF2 protein levels in an activity-dependent manner. Integrated phosphoproteomics and RNAseq studies revealed that BRSK2 drives 5′-AMP-activated protein kinase α2 (AMPK) signaling and suppresses the mTOR pathway. As a result, BRSK2 kinase activation suppresses ribosome-RNA complexes, global protein synthesis and NRF2 protein levels. Collectively, our data illuminate the BRSK2 and BRSK1 kinases, in part by functionally connecting them to NRF2 signaling and mTOR. This signaling axis might prove useful for therapeutically targeting NRF2 in human disease.
This article has an associated First Person interview with the first author of the paper.
KEY WORDS: NRF2, Oxidative stress response, Phosphoproteomics, Functional genomics, Kinase, BRSK2, BRSK1, AMPK, mTOR
Summary: BRSK2 suppresses NRF2 signaling by inhibiting protein synthesis through mTOR downregulation.
INTRODUCTION
The transcription factor nuclear factor erythroid 2-related factor 2 (NFE2L2, hereafter referred to as NRF2) is central to the cellular response to oxidative and electrophilic stress (Itoh et al., 2010; Suzuki and Yamamoto, 2015). When active, NRF2 provides strong cytoprotective functions by upregulating expression of (1) xenobiotic metabolism enzymes, (2) phase II detoxification enzymes, (3) drug efflux pumps and (4) the thioredoxin and glutathione antioxidant systems (Itoh et al., 2010; Kensler et al., 2007). Under homeostatic conditions, the E3 ubiquitin ligase adaptor kelch-like ECH-associated protein 1 (KEAP1) binds ETGE and DLG motifs within NRF2 to promote NRF2 ubiquitylation and proteasomal degradation (Cullinan et al., 2004; Itoh et al., 1999; Tong et al., 2006). Electrophilic attack of reactive cysteines within KEAP1 during oxidative stress suppresses KEAP1-dependent ubiquitylation/degradation of NRF2, resulting in NRF2 stabilization, nuclear translocation and transcriptional activation of genes containing antioxidant response elements (AREs) (Baird et al., 2013; Ichikawa et al., 2009; Kensler et al., 2007; Suzuki et al., 2019). Although KEAP1 and cullin-3 (CUL3) are prominent NRF2 regulators in most tissues and disease models, data describing additional mechanisms are still emerging.
As a key regulator of metabolism and redox balance, it is not surprising that alterations in NRF2 contribute to numerous human diseases. NRF2 expression and transcriptional activity declines with age and is decreased in several neurodegenerative diseases and diabetes (Cuadrado et al., 2018; Tsakiri et al., 2019; Zhang et al., 2015). Conversely, NRF2 transcriptional activity is constitutively active in many cancers, including those of the lung, head/neck, kidney, liver and bladder (Menegon et al., 2016; Rojo de la Vega et al., 2018). Multiple mechanisms promote NRF2 activation in cancer: activating mutations in NRF2; inactivating mutations in KEAP1; NRF2 copy number amplifications; overexpression of KEAP1-binding proteins, which competitively displace NRF2; and various post-translational modifications to KEAP1 and NRF2 (Cloer et al., 2019; Rojo de la Vega et al., 2018). High NRF2 activity promotes tumor growth, metastasis and chemo-/radiation resistance (Homma et al., 2009; Tao et al., 2014). Patients with mutations in KEAP1 or NRF2 leading to NRF2 stabilization have poor survival and are comparatively resistant to many commonly used cytotoxic cancer therapies (Homma et al., 2009; Satoh et al., 2013; Tao et al., 2014). Although there are numerous small molecule activators of NRF2 signaling, there are no approved NRF2 inhibitors (Cuadrado et al., 2018).
Here, we performed a gain-of-function screen of human kinases to identify new regulators of NRF2-dependent transcription. We focused on kinases given their exceptional druggability and because a rigorous, comprehensive annotation of the kinome for NRF2 activity is lacking. Our previous phosphoproteomic analysis of KEAP1 and NRF2 showed that both proteins are phosphorylated at multiple sites. Most of these phosphorylation events are not linked to specific kinases and are of unknown functional importance (Tamir et al., 2016). That said, recent studies have revealed a few kinases that influence NRF2 protein stability, subcellular localization and transcriptional activity. NRF2 is directly phosphorylated by glycogen synthase kinase-3β, resulting in NRF2 ubiquitylation by β-TrCP and subsequent proteasomal degradation (Chowdhry et al., 2012; Cuadrado, 2015). Phosphorylation of NRF2 at serine residues 40 and 550, mediated by protein kinase C (PKC) and 5'-AMP-activated protein kinase α2 (AMPK), respectively, leads to increased NRF2 stability and signaling (Huang et al., 2002; Joo et al., 2016). NRF2 is reported to be a substrate of several MAPKs (e.g. JNK, p38, ERK1/2, ASK1 and TAK1); however, the functional relevance of MAPK-directed phosphorylation is uncertain (Naidu et al., 2009; Shen et al., 2004; Sun et al., 2009). Activation of the PERK kinase during the unfolded protein response (UPR) leads to increased NRF2 nuclear accumulation and cell survival (Cullinan et al., 2003; Del Vecchio et al., 2014). Casein kinase 2 and TAK1 kinases phosphorylate NRF2 to induce NRF2 nuclear localization (Apopa et al., 2008; Shen et al., 2004). There is also evidence that activation of the PI3K/AKT/PKB pathway and PIM kinase signaling induces NRF2- activity and cellular protection (Lim et al., 2008; Nakaso et al., 2003; Warfel et al., 2016). Phosphorylation of KEAP1 or its interacting partners also induces NRF2 stability and signaling. For example, a recent study reports that phosphorylation of KEAP1 by MST1/2 on T51/S53/S55/S80 reduces NRF2 ubiquitylation (Wang et al., 2019). Additionally, proteins containing ETGE-like motifs, such as p62/SQSTM1, bind to KEAP1 and stabilize NRF2 upon phosphorylation by upstream kinases (e.g. mTORC1, TAK1) (Hashimoto et al., 2016; Ichimura et al., 2013; Lau et al., 2010). Overall, these findings suggest a wide array of kinase-directed signaling inputs for NRF2.
Our gain-of-function kinome screen revealed that brain specific kinases 2 and 1 (BRSK2 and BRSK1, also known as SAD-A and SAD-B, respectively) act as negative regulators of NRF2. The BRSKs are understudied members of the AMPK-related family of kinases (Bright et al., 2008). Both BRSK2 and BRSK1 contain an N-terminal kinase domain, followed by an ubiquitin-associated domain (UBA), a proline-rich region (PRR), and a kinase-associated domain (KA1) with an auto-inhibitory sequence (AIS) at the C terminus (Wang et al., 2018; Wu et al., 2015). These kinases are known to function downstream of liver kinase B1 (LKB1) signaling, but are also activated by PAK1, CAMKII and PKA (Bright et al., 2008; Lizcano et al., 2004; Nie et al., 2012). In various model organisms and mammals, BRSK2 and BRSK1 are expressed the most in the brain. BRSK2 is also expressed in pancreas, and BRSK1 is expressed in gonads as well as endocrine tissues (Uhlén et al., 2015; Uhlen et al., 2010). In C. elegans, the BRSK1 homologue SAD-1 is required for neuron polarization and synaptic vesicle transport (Kim et al., 2010; Morrison et al., 2018). In mouse neurons, Brsk2 and Brsk1 promote formation of vesicles, neurite differentiation and synapse formation (Kishi et al., 2005; Lilley et al., 2014). In pancreatic islets, BRSK2 promotes insulin secretion in response to glucose stimulation (Chen et al., 2012; Nie et al., 2018, 2012). To date, BRSK2 and BRSK1 have been implicated in positive regulation of cell cycle progression, endoplasmic reticulum-associated protein degradation (ERAD), neuronal polarity, autophagy and insulin signaling (Li et al., 2012; Muller et al., 2010; Wang et al., 2012, 2013). Specifically, BRSK2 kinase activity is induced by starvation to inhibit mTOR and promote autophagy, similarly to AMPK, and new evidence suggests that BRSK2 leads to activation of PI3K/AKT signaling (Bakula et al., 2017; Chen et al., 2012; Saiyin et al., 2017). Although BRSK2 has not yet been directly linked to NRF2 signaling, some studies show that BRSK2 is protective during ER stress (Wang et al., 2012, 2013). Here we identify BRSK2 as a novel repressor of NRF2 signaling, and mechanistically establish that BRSK2 induces phosphorylation of AMPK substrates, suppresses mTOR and decreases protein translation.
RESULTS
Kinome gain-of-function screen identifies regulators of NRF2 activity
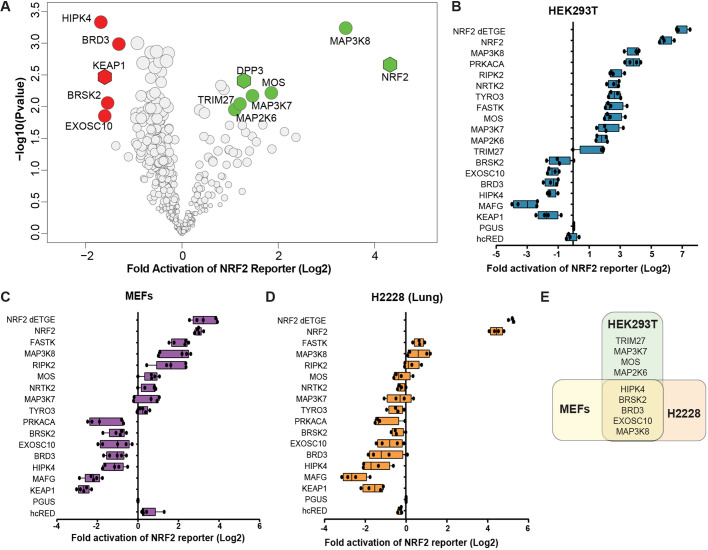
To identify kinase regulators of NRF2 signaling, we employed a gain-of-function arrayed screen where 385 kinases and kinase-associated proteins were overexpressed in HEK293T cells (Table S4). NRF2 transcriptional activity was quantified with the hQR41-firefly luciferase reporter normalized to constitutively expressed Renilla luciferase. The hQR41 reporter contains a NRF2-responsive fragment of the human NQO1 promoter, which is a consensus NRF2 target gene across species and tissue types. Overexpression of the positive controls NRF2 and dipeptyl peptidase 3 (DPP3) activated hQR41-luciferase, whereas KEAP1 expression suppressed the reporter activity (Hast et al., 2013). Kinases that activated hQR41 include MAP3K8, MOS, MAP3K7/TAK1 and MAP2K6, whereas HIPK4, BRD3 and BRSK2 were identified as repressors of NRF2-mediated transcription (Fig. 1A; Table S4). Hits from this primary screen were validated in focused reporter assays across multiple cell lines, including HEK293Ts, MEFs and H2228 lung adenocarcinoma cells (Fig. 1B-E). Several of the validated kinases are confirmed in recent reports. MAP3K7/TAK1 activates NRF2 via phosphorylation of p62/SQSTM to drive degradation of KEAP1 (Hashimoto et al., 2016). Although BRD3 has not been directly tested, other members of the BRD family of proteins negatively regulate NRF2-mediated transcription (Hussong et al., 2014; Michaeloudes et al., 2014). Similarly, the HIPK4-related protein HIPK2 is a direct transcriptional target of NRF2 and a positive regulator of NRF2 cytoprotection (Torrente et al., 2017). Because of its novelty within the NRF2 pathway, we sought to better our understanding of BRSK2 and its role in NRF2 signaling.
Fig. 1.
A gain-of-function screen identifies kinases that regulate NRF2-dependent transcription. (A) Volcano plot of kinase overexpression screen of NRF2-driven hQR41-firefly luciferase reporter in HEK293T cells. The experiment was performed in biological triplicate and six technical replicates. NRF2 transcriptional activity was determined by measuring levels of hQR41-firefly luciferase normalized to TK-Renilla luciferase. Data are plotted relative to GFP overexpression control. Significance was measured with Student's t-test including correction for multiple comparison using Benjamini and Hochberg method. Red and green circles indicate candidate kinases that passed an FDR of less than 10%. NRF2, KEAP1and DPP3 serve as positive controls. (B-D) hQR41 reporter validation study for the indicated kinases in HEK293T cells, MEFs and H2228 cells. (E) Venn diagram of validated kinase hits in HEK293T, H2228 and MEFs.
BRSK2 and BRSK1 repress NRF2 signaling
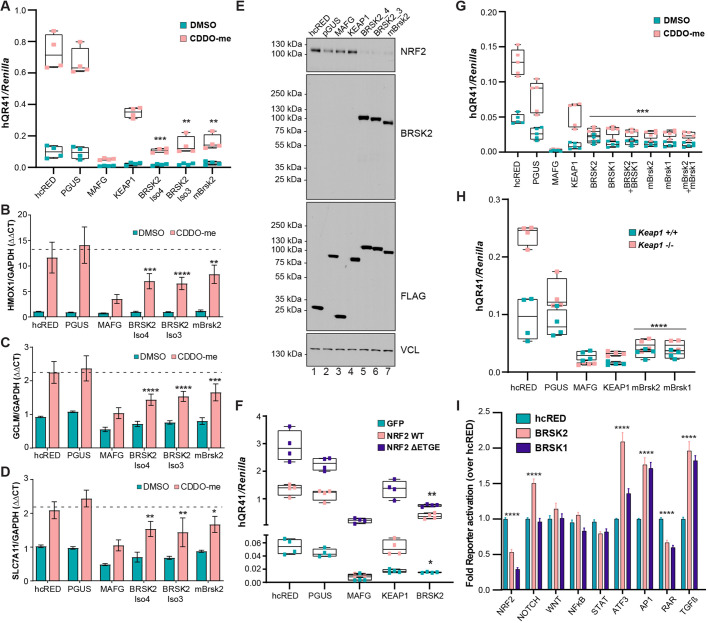
Redox regulation of cysteines within KEAP1 controls KEAP1-catalyzed NRF2 ubiquitylation and degradation. Through reactivity with cysteine 151 in KEAP1, the triterpenoid of oleanolic acid derivative CDDO-methyl (CDDO-me) potently suppresses NRF2 degradation (Suzuki et al., 2019). We first tested whether BRSK2 overexpression suppressed CDDO-me driven NRF2 activation, as quantified by hQR41-luciferase expression in HEK293T cells. We compared murine Brsk2 and two human BRSK2 splice variants, where BRSK2-Isoform4 is missing 20 amino acids compared to BRSK2-Isoform3. KEAP1 and musculoapoeneuorotic factor G (MAFG) served as positive controls for NRF2 inhibitors. Compared with the negative controls [hcRED and glucuronidase-β (pGUS)], all BRSK2 variants suppressed NRF2 transcriptional activity under both vehicle and CDDO-me treated conditions (Fig. 2A). Similarly, murine Brsk2 blocked NRF2-transcriptional activation. To confirm BRSK2 as an inhibitor of endogenous NRF2, we quantified endogenous NRF2 target gene expression by qRT-PCR. Following overexpression of controls or BRSK2 variants in HEK293T cells, we measured changes in NRF2 target genes HMOX1, GCLM and SLC7A11 normalized to GAPDH. BRSK2 variants downregulated NRF2 targets to a similar extent as MAFG in both vehicle and CDDO-me treated conditions (Fig. 2B-D). Because key regulation of NRF2 is post-translational, we tested whether BRSK2 overexpression impacted steady-state NRF2 protein levels. Compared with controls, BRSK2 overexpression decreased NRF2 protein levels in HEK293T cells (Fig. 2E, compare lanes 5-7 with lanes 1 and 2). To explore further a role for KEAP1 in mediating BRSK2 suppression of NRF2, we tested whether BRSK2 overexpression could block a constitutively active mutant of NRF2 that does not bind KEAP1 (NRF2 ΔETGE). BRSK2 repressed both wild-type NRF2 and NRF2 ΔETGE (Fig. 2F).
Fig. 2.
BRSK2 and BRSK1 inhibit NRF2-mediated transcription. (A) hQR41-luciferase assay in HEK293T cells following expression of control (hcRED, pGUS), positive control (MAFG, KEAP1), BRSK2 splice variants (BRSK2iso4, BRISK2 iso3) or mouse Brsk2. Cells were treated with vehicle control or CDDO-me (100 nM, 12 h). (B,D) BRSK2 overexpression inhibits endogenous NRF2 target gene (HMOX1, GCLM and SLC7A11) induction in HEK293T cells (representative of n=3). (E) Western blot of HEK293T cells 24 h after transfection with the indicated expression plasmid. Cells were treated with CDDO-me 12 h before lysis. (F) hQR41-luciferase reporter assay in HEK293T cells expressing the indicated plasmid combinations for 24 h. (G) hQR41-luciferase reporter assay in HEK293T cells expressing the indicated plasmids. Cells were treated with CDDO-me 12 h before lysis. (H) hQR41 reporter assay in wild-type MEFs and KEAP1−/− MEFs expressing the indicated plasmids. (I) HEK293T cells were transiently transfected with the indicated firefly transcriptional reporter, Renilla luciferase, hcRED, BRSK2 or BRSK1. Two-way ANOVA with multiple comparison was performed comparing BRSK2 or BRSK1 to hcRED control. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
BRSK1 is a paralog of BRSK2, sharing ∼68% amino acid sequence similarity and having similar signaling functions. Leveraging the hQR41 reporter assay in HEK293T cells, we overexpressed human or mouse BRSK1, BRSK2 or both. Similarly to BRSK2, BRSK1 expression suppressed NRF2-dependent transcriptional activation (Fig. 2G). We also performed the hQR41 reporter assay in Keap1−/− MEFs, which express high levels of NRF2 (Fig. 2H). Again, both BRSK1 and BRSK2 inhibited NRF2-driven transcription, suggesting that the mechanism of suppression was independent of KEAP1-mediated ubiquitylation. Finally, we evaluated the impact of BRSK1 and BRSK2 on a panel of pathway-specific transcriptional reporters in HEK293T cells (Fig. 2I). BRSK1 and BRSK2 suppressed NRF2 and the retinoic acid receptor (RAR) and activated activator protein-1 (AP1), activating transcription factor 3 (ATF3) and transforming growth factor β (TGFβ) reporters. Neither BRSK family member regulated the WNT/β-catenin, NFκB, or STAT reporters. As such, BRSK1 and BRSK2 have redundant and conserved functions in NRF2 suppression, and do not impact all signaling pathways equally.
Finally, we evaluated the effect of BRSK2 silencing on NRF2 protein expression and NRF2-driven transcription. HEK293T cells were engineered to express dead KRAB-dCas9 nuclease before stable introduction of four scrambled control single-guide RNAs (sgRNAs) or five independent BRSK2-specific sgRNAs. Western blot analysis of the resulting cell lines confirmed efficient sgRNA-mediated CRISPRi silencing of BRSK2 protein and no effect on NRF2 protein levels (Fig. S1A). NRF2 hQR41 reporter assays in these cells did not show a BRSK2-silencing phenotype on NRF2 activity. Because BRSK2 RNA levels are highest in brain and pancreas, we evaluated BRSK2 expression in pancreatic cancer cell lines (Fig. S1C). Two cell lines, PANC1 and MIA PaCa-2, expressed the most BRSK2, of which we used MIA PaCa-2 for CRISPRi silencing. Like HEK293T cells, MIA PaCa-2 cells deficient for BRSK2 expressed comparable levels of NRF2 as control cells (Fig. S1D). These data suggest that, under homeostatic conditions, endogenous levels of BRSK2 do not control NRF2 activity. Further experiments in a BRSK2 null background as opposed to silenced background are needed.
BRSK2 kinase function is required to suppress NRF2 signaling
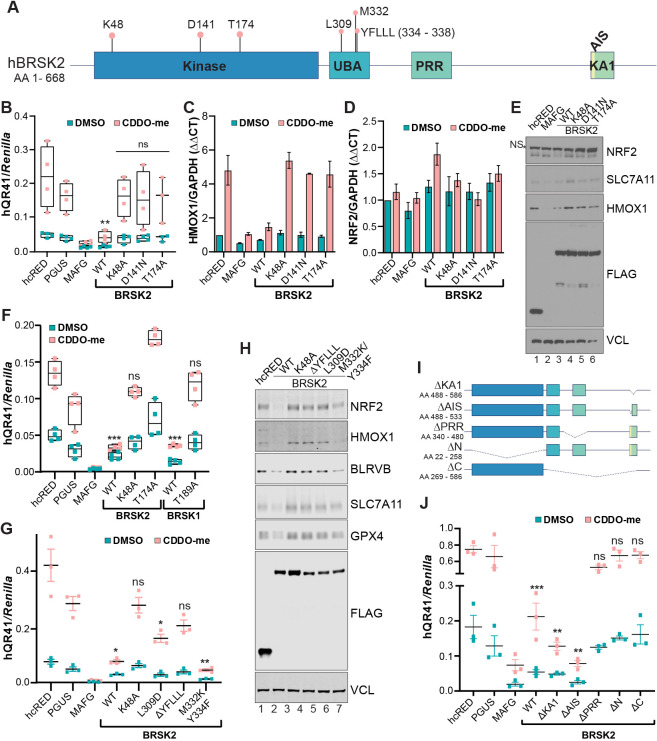
We next determined whether BRSK2-mediated inhibition of NRF2 requires BRSK2 kinase activity. We mutated K48 and D141 in the kinase domain of BRSK2; these residues are required for ATP binding and substrate phosphorylation (Lizcano et al., 2004). We also mutated T174, which is phosphorylated by LKB1, which activates members of the AMPK kinase family (Fig. 3A) (Lizcano et al., 2004). Compared with controls, overexpression of wild-type BRSK2 decreased NRF2-dependent hQR41 luciferase expression whereas kinase-dead variants had no significant effect (Fig. 3B). The kinase dependency of BRSK2-mediated NRF2 downregulation was further confirmed using qRT-PCR of endogenous NRF2 target gene HMOX1, which was repressed by wild-type BRSK2, but not by the kinase-dead mutants (Fig. 3C). qRT-PCR for the NRF2 transcript showed that expression of neither wild-type nor kinase-dead BRSK2 mutants affected NRF2 mRNA levels (Fig. 3D). Finally, we evaluated NRF2 protein levels in HEK293T cells overexpressing BRSK2 or the kinase-dead mutants. As expected, NRF2 protein levels were decreased by overexpression of wild-type, but not kinase-dead, BRSK2 (Fig. 3E). Similarly, kinase-dead mutants of BRSK1 did not impact NRF2 signaling (Fig. 3F).
Fig. 3.
BRSK1 and BRSK2 kinase activity is required for suppression of NRF2-dependent transcription. (A) Scheme of BRSK2 protein, showing residues and domains important for kinase activity. (B) hQR41 reporter assay in HEK293T cells expressing the indicated construct; cells were treated with vehicle or CDDO-me for 12 h prior to luciferase quantification. (C,D) qRT-PCR for HMOX1 and NRF2 in HEK293T cells following overexpression of the indicated constructs (representative of n=3). (E) Western blot analysis of HEK293T cells expressing the indicated plasmids. Cells were treated with CDDO-me for 12 h before lysis. NS, nonspecific. (F,G) hQR41 luciferase assay in HEK293T cells expressing the indicated constructs. (H) Western blot analysis of HEK293T cells expressing the indicated plasmids. Cells were treated with CDDO-me for 12 h before lysis. (I) Scheme showing BRSK2 deletions. (J) hQR41 reporter assay in HEK293T cells expressing the indicated construct; cells were treated with vehicle or CDDO-me for 12 h prior to luciferase quantification. Two-way ANOVA with multiple comparison was performed comparing corresponding hcRED control with treatment groups. *P<0.05, **P<0.01, ***P<0.001; ns, not statistically significant.
Beyond kinase activity, structure–function studies of BRSK2 and the AMPK-like family of kinases have revealed numerous features of functional importance (Bright et al., 2009; Wu et al., 2015). We created a series of point mutations and truncations to determine which domains within BRSK2 are important for NRF2 suppression. Binding of the regulatory UBA and AIS regions of the KA1 domain to the catalytic fold of the kinase domain is thought to hold BRSK2 in an inactive state until phosphorylated by upstream regulators, bound by interaction partners or recruited to the plasma membrane (Wu et al., 2015). As such, mutations in residues of the UBA domain and loss of the AIS or KA1 domain results in an active BRSK2 kinase. We generated and expressed mutant proteins in HEK293T cells followed by hQR41 reporter quantification and NRF2 western blot analysis (Fig. 3G,H). The L309D and ΔYFLLL mutations within the UBA domain did not suppress the hQR41 reporter and NRF2 or its target genes, as shown by western blot analysis (Fig. 3H). Based on previous reports on the role of the BRSK2 UBA domain, it is likely that loss of the YFLLL motif increases auto-inhibition by the AIS/KA1 region (Wu et al., 2015). Mutations of UBA residues that contact the kinase domain (M332K and Y334F) repressed NRF2 activity. We next deleted the kinase domain (ΔN), C terminus (ΔC), PRR (ΔPRR), AIS (ΔAIS) or KA1 (ΔKA1) domains and evaluated the effect on NRF2-mediated transcription via reporter assays (Fig. 3I). Compared with controls, BRSK2 ΔKA1 and ΔAIS inhibited NRF2 activity similarly or more than the BRSK2 wild type, whereas loss of kinase, C-terminal or PRR regions abolished regulation of NRF2 signaling (Fig. 3J).
BRSK2 does not repress NRF2 via the ubiquitin proteasome system
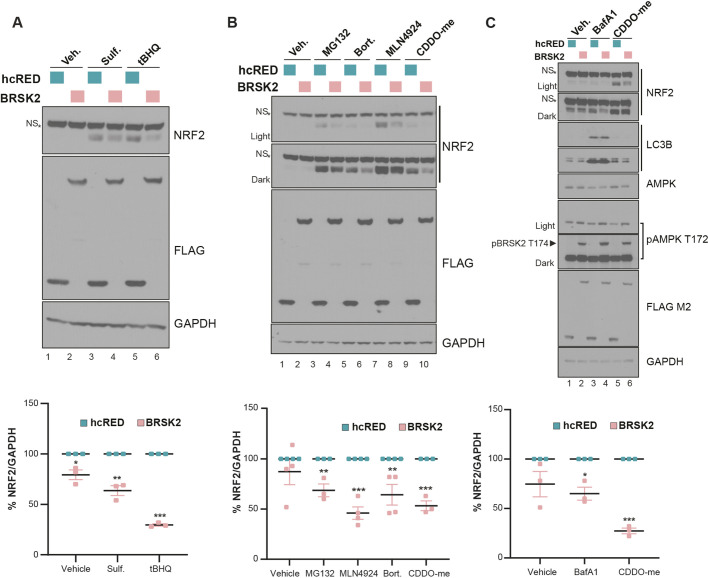
NRF2 is rapidly stabilized by electrophilic compounds that react with cysteines in KEAP1. NRF2 protein levels also accumulate within minutes of chemical inactivation of the ubiquitin proteasome system (UPS). We tested whether BRSK2 expression would suppress NRF2 stabilized by KEAP1-reactive electrophiles or inhibitors of the UPS. First, we treated HEK293T cells with vehicle, sulforaphane or tert-butal hydroquinone (tBHQ) for 6 h after 24 h of BRSK2 expression. Similarly to CDDO-me, these compounds modify cysteine residues on KEAP1 to stabilize NRF2 (Suzuki et al., 2019). Cells overexpressing BRSK2 significantly downregulated NRF2 protein levels compared with the corresponding control (Fig. 4A). Second, we tested the proteasomal inhibitors MG132 and bortezomib as well as the CUL3 neddylation inhibitor MLN4924. NRF2 protein levels were decreased by BRSK2 in all treatment groups compared with control, although to varying degrees (Fig. 4B). Third, we asked whether BRSK2-mediated downregulation of NRF2 involved the autophagy pathway. To evaluate this, we treated BRSK2-overexpressing HEK293T cells with vehicle or bafilomycin A (BafA1) for 12 h. Compared with vehicle, BafA1 treatment did not significantly affect NRF2 in either control or BRSK2-overexpressing conditions (Fig. 4C). LC3B conversion confirmed the efficacy of BafA1 treatment. These data suggest that BRSK2-mediated downregulation of NRF2 is not via the UPS or autophagy. We note that the T172 AMPK phosphorylation-specific antibody recognized BRSK2 at T174; however, BRSK2 overexpression did not impact T172-phosphorylated AMPK.
Fig. 4.
BRSK2-mediated NRF2 downregulation is independent of NRF2 ubiquitylation and degradation. (A) HEK293T cells were transfected with control hcRED or BRSK2 for 24 h before treatment with vehicle (veh), sulforaphane (Sulf., 2 µM) or tert-butylhydroquinone (tBHQ, 50µM) for 4 h. Quantification of biological triplicate experiments is shown in the lower panel, normalized to GAPDH. (B,C) HEK293T cells were transfected with control hcRED or BRSK2 for 24 h before treatment with 10 µM MG132, 40 nM bortezomab (Bort.), 2.5 µM MLN4924 or 200 nM bafilomycin A1 (BafA1). All treatments were for 4 h, except for BafA1, which was 12 h. NS, nonspecific. Two-way ANOVA with multiple comparison was performed comparing hcRED with BRSK2. *P<0.05, **P<0.01, ***P<0.001.
RNAseq and phosphoproteomic characterization of BRSK2 and BRSK1 expression
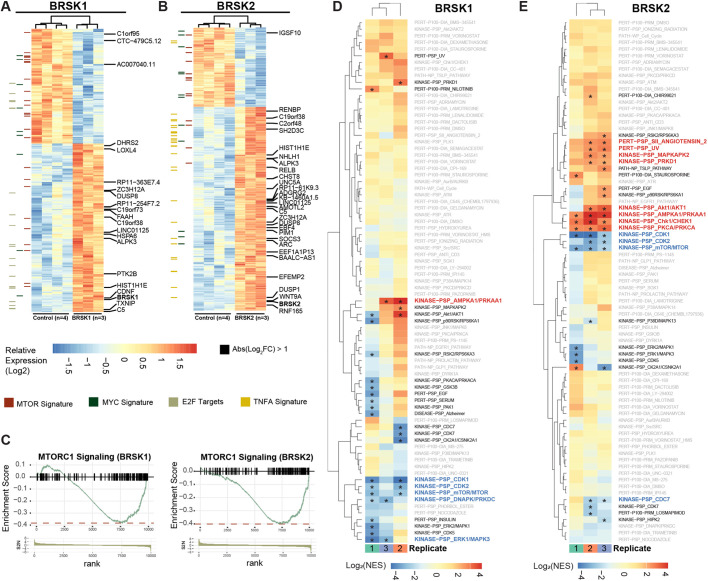
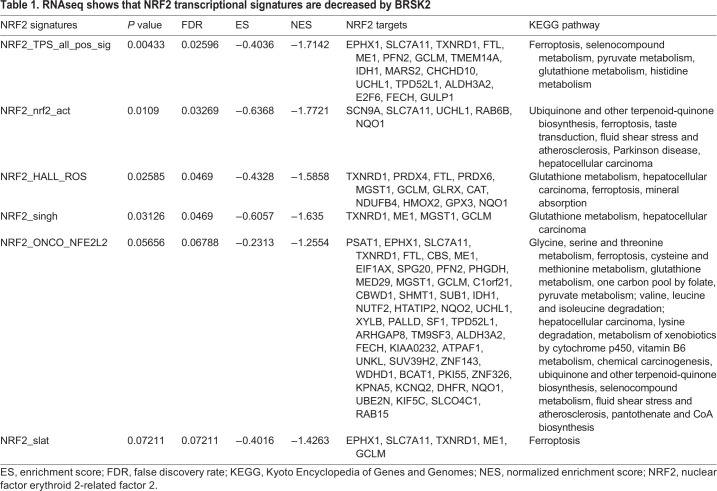
Unbiased comprehensive screening and molecular annotation has improved significantly over the past decade, with newly empowered informatics that distill the resulting large datasets into pathways and biological processes. To understand better the function of BRSK2 and BRSK1 in cells and reveal how BRSK2 suppresses NRF2, we performed RNAseq and global quantitative phosphoproteomic analysis on HEK293T cells expressing BRSK2 and BRSK1, compared with mock-transfected cells or hcRED expression. Hierarchical clustering analysis revealed genes altered by overexpression of BRSK2 and BRSK1, where genes that passed false discovery rate (FDR<5%) and fold change (FC≥2) cutoffs are highlighted (Fig. 5A,B). Compared with control, we observed 723 and 879 differentially expressed genes that passed FDR<5% for BRSK2 and BRSK1, respectively. Based on fold change, differential gene expression due to BRSK2 expression was more robust than for BRSK1. Gene set enrichment analysis (GSEA) using the Hallmark and Oncogenic gene sets in the Molecular Signatures Database (MSigDB) revealed statistically significantly altered signaling pathways (Tables S5–S8). Genes associated with mTOR signaling were robustly downregulated in cells expressing BRSK2 or BRSK1 (Fig. 5C). GSEA for several NRF2 gene signatures revealed downregulation by BRSK2 (Table 1). Close examination by pathway analysis of the downregulated genes revealed enrichment for those involved in pyruvate, glutathione and amino acid metabolism. Interestingly, several of the genes are known players in ferroptosis, a non-canonical and iron-dependent cell death pathway (Table 1). Lastly, we evaluated the correlation of BRSK1 and BRSK2 with NRF2 target gene expression across patient tumor samples from the TCGA PanCan dataset. Although sequencing reads for BRSK2 were too low for PanCan analysis, BRSK1 mRNA expression was negatively correlated with NRF2 target gene expression (Fig. S2). These data suggest that BRSKs negatively regulate NRF2 signaling in vivo.
Fig. 5.
RNAseq and phosphoproteomics reveal activation of AMPK and suppression of MTOR following expression of BRSK1 or BRSK2. (A,B) Hierarchical clustering of RNA sequencing analysis of HEK293 T mock-transfected cells and cells transfected with hcRED control, BRSK2 or BRSK1 for 24 h. (C) Differentially expressed genes with FDR<5% were analyzed by GSEA using the Hallmark and Oncogenic gene sets from MSigDB. Enrichment score plots representing genes commonly associated with MTORC1 signaling were decreased by BRSK2 or BRSK1 overexpression based on GSEA. (D,E) Quantitative TMT phosphoproteomic analysis of HEK293T cells expressing hcRED, BRSK1 or BRKS2 for 24 h. TMT ratios from biological triplicate samples were analyzed for enrichment of phosphosites associated with known signaling pathways using the PTMSigDB pipeline (*FDR<10%).
Table 1.
RNAseq shows that NRF2 transcriptional signatures are decreased by BRSK2
Independently of the RNAseq analyses, we performed tandem mass tag (TMT)-based quantitative phosphoproteomics on BRSK-expressing cells, using hcRED as control. Biological triplicate samples were analyzed, revealing ∼10,000 phosphosites in ∼8400 phosphopeptides. Following the RNAseq trend, BRSK2 impacted the phosphoproteome more robustly than did BRSK1 (Fig. S3). Compared with control, at FDR<5%, BRSK2 overexpression induced 307 differentially phosphorylated peptides compared with 189 observed in BRSK1 overexpression (Tables S9, S10). We leveraged PTMSigDB and enrichment analysis to map the observed phosphopeptide changes to annotated signaling pathways (Fig. 5D,E; Fig. S3) (Krug et al., 2019). BRSK2 and BRSK1 positively regulated AMPK and AKT signaling, but negatively impacted the mTOR pathway. BRSK2 also suppressed signaling through the CDK1, CDK2 and CDC7 pathways (Table S11, Fig. S3). However, neither steady-state nor induced levels of NRF2 protein were dramatically altered by AMPK loss-of-function, as observed in AMPK double knockout MEFs compared with controls (Fig. S4) (Laderoute et al., 2006; Li et al., 2019). Overall, this suggests that induction of AMPK signaling activation is due to a redundant signaling axis driven by BRSK2, which also leads to loss of NRF2 protein level.
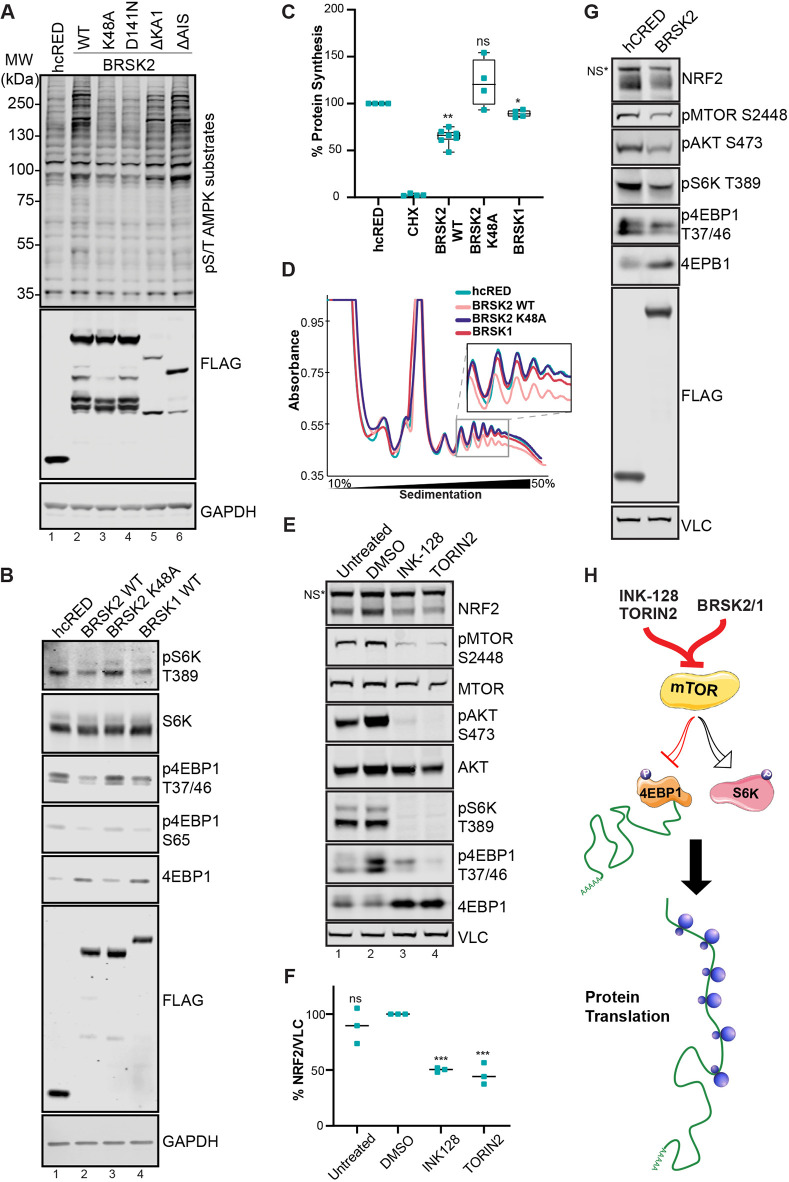
To further validate the activation of AMPK signaling observed in Fig. 5, BRSK2 was expressed in HEK293T cells before western blot analysis for phosphorylation of AMPK substrates [LxRxx(pS/pT)]. We expressed either wild-type, kinase-active (ΔKA1 or ΔAIS) or kinase-dead (K48A or D141N) BRSK2 for 24 h. Overexpression of wild-type and kinase-active BRSK2 (Fig. 6A, lanes 2, 5 and 6) upregulated pS/T AMPK substrate motif compared with control and kinase-dead BRSK2 (Fig. 6A, lanes 1, 3 and 4). We then measured changes in mTOR substrate phosphorylation by evaluating p-S6K T389, p-4EBP1 T37/46 and p-4EBP1 S65 in cells expressing hcRED control, wild-type BRSK2, kinase-dead BRSK2 (K48A) or BRSK1. Expression of BRSK2 or BRSK1 decreased phosphorylation of both S6K and 4EBP1 (Fig. 6B, lanes 2 and 4). Total protein levels of 4EBP1 increased following BRSK2 or BRSK1 expression. Together, these data suggest that BRSK2 and BRSK1 downregulate mTOR signaling while upregulating AMPK substrate phosphorylation.
Fig. 6.
BRSK2 suppresses protein translation. (A) BRSK2 induces AMPK substrate phosphorylation. Western blot analysis of AMPK consensus phosphorylation sites in HEK293T cells transfected with hcRED, BRSK2 or the indicated BRSK2 mutants (representative of n=3). (B) BRSK2 and BRSK1 downregulate mTOR signaling. Western blot analysis of BRSK1 and BRSK2 expression in HEK293T cells for mTOR targets S6K (T389) and 4E-BP1 (T37/46 and S65), which increases 4E-BP1 stability (representative of n=3). (C) BRSK2 and BRSK1 downregulate protein translation. Global protein translation assay performed using 35S-methionine labeling in cells expressing hcRED (control), BRSK2, BRSK2 K48A or BRSK1. (D) BRSK2 reduced polyribosome formation. Polyribosome fraction of HEK293T cells overexpressing hcRED control, BRSK2, BRSK2 K48A or BRSK1 performed on 50-10% sucrose gradient. Experiments represent biological triplicates, unless indicated otherwise. (E) mTOR inhibition reduced NRF2 protein. HEK293T cells were treated with DMSO, INK-128 (100 nM) or TORIN2 (100 nM) for 7 h followed by western blot analysis. (F) Quantification of NRF2 blots using DMSO as control. (G) BRSK2 overexpression recapitulates phenotypes observed with mTOR inhibitors. NS, nonspecific. (H) Signaling model: BRSK2-mediated mTOR downregulation leads to decreased protein translation. Two-way ANOVA with multiple comparison was performed comparing controls to other conditions. *P<0.05, **P<0.01, ***P<0.001; ns, not statistically significant.
Because overexpression of BRSK2 and BRSK1 suppressed mTOR, we quantified the rate of protein translation after expression of BRSK2 and BRSK1 (Fig. 6C). HEK293T cells expressing BRSK2, BRSK1 or control hcRED were pulsed with 35S-methionine before lysis and quantification of nascent polypeptides; cyclohexamide (CHX) served as a negative control. Compared with control and kinase-dead BRSK2, expression of wild-type BRSK2 and BRSK1 downregulated protein translation by 40% and 10%, respectively (Fig. 6C). To further confirm decreased translation, we measured ribosome binding to mRNA in cells expressing BRSKs. Cells transfected with the indicated construct were fractionated in a 10-50% sucrose gradient followed by absorbance measurement for polyribosome tracing. Compared with control, BRSK1 and kinase-dead BRSK2, overexpression of wild-type BRSK2 decreased heavy polyribosome formation on mRNA (Fig. 6D). Last, we tested whether pharmacological inhibition of mTOR impacted NRF2 to recapitulate phenotypes observed by BRSK2 overexpression. HEK293T cells were treated with the mTOR inhibitors INK-128 and TORIN2 before protein extraction and western blot. Both INK-128 and TORIN2 downregulated mTOR signaling as measured by western blot analysis of known phosphorylated substrates S6K (T389), 4E-BP1 (T37/46) and AKT (S473) (Fig. 6E, lanes 3 and 4). Compared with controls (untreated and DMSO), both INK-128 and TORIN2 downregulated NRF2 protein levels, similar to that seen for BRSK2 overexpression (Fig. 6E-G). Overall, these data suggest that either pharmacological inhibition (INK-128 or Torin2) or genetic inhibition (BRSK2 gain-of-function) of mTOR decreased protein translation (Fig. 6H). Therefore, overexpression of catalytically active BRSK2 downregulates mTOR signaling and results in decreased NRF2 protein translation.
DISCUSSION
The NRF2 transcription factor is central to a growing number of human pathologies. The transcriptional program it governs enables life in the presence of oxygen, electrophiles and environmental stressors. As such, aberrant activation or suppression of NRF2 contributes to and causes a number of human diseases, including cancer, inflammation, diabetes and neurodegeneration. Although its relevance and centrality to human health is well established, we have yet to realize the full potential of NRF2-directed therapeutics. In this study, we focus on kinase regulators of NRF2 because the kinome is exceptionally druggable and the mechanistic impact of NRF2 phosphorylation remains largely undefined. We discovered that, in an activity-dependent manner, BRSK kinases suppress NRF2 signaling. We show that BRSK2 induces AMPK substrate phosphorylation and inhibits mTOR, resulting in decreased ribosome loading on mRNAs, which downregulates protein synthesis.
Beyond revealing the BRSK kinases as indirect suppressors of NRF2 translation, this work has several implications. First, signaling through the mTOR pathway has been reported to both promote and inhibit NRF2 in a context-dependent manner (Aramburu et al., 2014; Dayalan Naidu et al., 2017; Shay et al., 2012). Our data argue that mTOR suppression by BRSK2, irrespective of cell type or duration, results in NRF2 protein loss. Specifically, BRSK2 overexpression suppresses NRF2 protein at basal and electrophile-induced conditions. Second, although we connect BRSK2 to NRF2 through suppression of mTOR, it remains unknown whether BRSK2 and BRSK1 act redundantly to drive AMPK substrate phosphorylation. The structural similarity between AMPK and the BRSK kinases supports this possibility. Interestingly, we do not see an impact of AMPK genetic deletion on NRF2 protein levels, suggesting that with respect to NRF2, the AMPK and the BRSK kinases are functionally redundant. Third, the protein–protein interaction networks for the BRSK kinases remain to be fully defined. As such, it remains to be determined which specific BRSK protein interactions and phosphorylation events drive NRF2 suppression. Immunoprecipitation and mass spectrometry experiments for KEAP1, NRF2, BRSK1 and BRSK2 do not reveal a co-complex (The Dark Kinase Knowledgebase: https://darkkinome.org/kinase/BRSK2, 2019). However, false negatives are common in pull-down mass spectrometry experiments, particularly because of the transient nature of kinase–substrate interactions.
The small molecule NRF2 inhibitors halofuginone and brusatol offer further support for a requisite role of protein synthesis as a key point of NRF2 regulation (Harder et al., 2017; Tsuchida et al., 2017). Both halofuginone and brusatol are noted as potent NRF2 inhibitors that sensitize cells to chemotherapy. Halofuginone inhibits prolyl-tRNA synthetase, whereas brusatol acts on peptidyl transferase to suppress protein translation and ultimately decrease proteins with a short half-life, such as NRF2, a phenotype observed with overexpression of BRSK2 (Harder et al., 2017; Tsuchida et al., 2017). This also highlights the underlying role of stress signaling in protein synthesis. Under moderate oxidative stress, mTOR-regulated 5′ cap-dependent translation is inhibited, but can be bypassed via internal ribosomal entry site (IRES)-mediated translation found in the 5′ untranslated region (5′UTR) (Komar and Hatzoglou, 2011). However, under severe stress, translation is inhibited via localization of ribosome–RNA complexes to stress granules to protect nescient polypeptides and translation complex from damage. Although we have not evaluated changes in NRF2 mRNA levels associated with polysomes, NRF2 mRNA does contain an IRES-like region that promotes de novo translation under oxidative stress or treatment with lipoic acid (Lee et al., 2017; Shay et al., 2012). Conversely, NRF2 loss decreases protein translation rate in pancreatic cancer as a result of increased oxidation of proteins in the translational machinery, as well as decreased 4E-BP1 phosphorylation (DeNicola et al., 2012). Because BRSK2 and BRSK1 decrease mTOR activity, which controls 5′ cap-dependent translation, depleted NRF2 protein under low oxidative stress is probably a result of reduced ribosome binding to NRF2 mRNA during BRSK2 overexpression.
Gain-of-function screening and extensive validation confirm BRSK2 as a suppressor of NRF2, yet it is unclear whether BRSK2 loss impacts NRF2 or oxidative stress response signaling. In an acute experiment, we designed and tested a panel of short interfering RNAs (siRNAs) against BRSK2, and observed inconsistent results despite greater than 95% BRSK2 silencing (results not shown). Long-term suppression of BRSK2 expression with stable CRISPRi technology did not impact NRF2 protein levels. Because kinases are catalytic enzymes, it is likely that our loss-of-function approaches failed to eliminate enough BRSK2 activity to impact NRF2. Alternatively, it is possible that our experiments lacked the necessary context, for example the presence of an upstream activating signal. Further studies are needed in neuronal and pancreatic systems to evaluate BRSK loss and NRF2 signal transduction.
BRSK2 and BRSK1 impact cellular signaling pathways beyond NRF2, AMPK and mTOR, as gleaned from the RNAseq and phosphoproteomic experiments. BRSK2 expression activated CHEK1, PKC and AKT1 among other kinase signaling pathways. In a focused screen of various engineered transcriptional reporters, we show that BRSK1 and BRSK2 induced AP-1 and ATF3 stress response signaling, as well at TGFβ signaling. It is possible that some of these responses are coupled indirectly to the suppression of mTOR; further work is needed. Together, these data provide a foundational resource to better interrogate signaling regulated by BRSK2 and BRSK1.
MATERIALS AND METHODS
Cell culture
All cell lines were obtained from American Type Culture Collection (ATCC) and were used within 10 passages after receipt from ATCC to ensure their identities. Cells were cultured in a humidified incubator at 37°C and 5% CO2. Cells were passaged with 0.05% trypsin and 0.53 mM EDTA in sodium bicarbonate (Corning, 25-052-CI), and maintained in the following media supplemented with 10% fetal bovine serum (FBS): HEK293T and MIA PaCa2 in Dulbecco's modified Eagle's medium (DMEM) (Corning, 10-013-CV); H2228 in RPMI-1640 (Corning, 10-040-CV); Keap1+/+ and Keap1−/− mouse embryonic fibroblasts (MEFs), generated as previously described (Cloer et al., 2018; Wakabayashi et al., 2003), and AMPK DKO MEFs (a generous gift from Zhongsheng You, Department of Cell Biology and Physiology, Washington University School of Medicine, 660 South Euclid Ave., St. Louis, MO 63110, USA) (Li et al., 2019); in DMEM/Ham's F-12 50:50 mix supplemented with sodium pyruvate and non-essential amino acids (Corning, 10-092-CV).
Generation of CRISPRi cell lines
Lentivirus for KRAB-dCas9 was generated using PsPax2 and PMD2G packaging vectors (Gilbert et al., 2014). HEK293T and MIA PaCa-2 cells were infected with KRAB-dCas9 lentivirus, and monoclonal lines were generated via single cell sorting following 10 µg/ml blasticidin (GIBCO, A11139-03) selection for 5 passages. Each monoclonal line was cultured in 5 µg/ml blasticidin following sorting. Single guide RNA (sgRNA) vectors were generated by ligating oligonucleotides into AarI (Thermo Fisher Scientific, ER1582) digested VDB783 vector (Table S1). Each sgRNA was lentivirally introduced to the above mentioned cell lines and the cells cultured for 3 passages in 2.5 µg/ml puromycine (Corning, 61-385-RA) and 5 µg/ml blasticidin before further analysis. Stable cell lines were maintained in 1 µg/ml puromycine and 5 µg/ml blasticidin.
Plasmids and reagents
The human kinome ORF library in pDONOR223 was obtained from Addgene (Cambridge, MA, 1000000014) and cloned into a custom pHAGE-CMV-FLAG destination vector using Gateway cloning technology, as previously described (Agajanian et al., 2019). Luciferase reporter plasmids for WNT (BAR), NFκB, AP-1, ATF3, STAT, retinoic acid (RAR) and TGFβ (SMAD) were cloned into transient expression vectors as previously described (Walker et al., 2015; Biechele and Moon, 2008). The NOTCH (CSL) reporter was a gift from Raphael Kopan (Addgene, 41726) (Saxena et al., 2001).
All ORFs were cloned into pHAGE-CMV-FLAG via LR clonase (Thermo Fisher Scientific, 11791-020): pDONOR223.1 BRSK2 (splice isoform 3, BRSK2_Iso3) and Brsk2 were obtained from Harvard PlasmID repository (HsCD00297097 and MmCD00295042). BRSK2 kinase dead (K48A, D141N and T174A) and UBA domain (L309D, M332K/Y334F and ΔYFLLL) mutants were generated using Q5 Hot Start Site Direct Mutagenesis kit (New England BioLabs, E0552S). BRSK2 domain deletion mutations ΔN (Δ kinase), ΔC (Δ C-terminal), ΔPRR (Δ proline-rich region), ΔKA1 (Δ kinase associated domain) and ΔAIS (Δ auto-inhibitory sequence) were generated via Phusion DNA polymerase (New England BioLabs, M0530S), where the PCR product was treated with DpnI (New England BioLabs, R0176S) and purified with Monarch PCR and DNA Purification Kit (New England BioLabs, T1030S) followed by T4 DNA ligase reaction (New England BioLabs, M0202S). BRSK1 wild type and T189A vectors were obtained from MRC PPU Reagents and Services (DU1236 and DU1242) in a pCMV5-HA backbone. Brsk1 was obtained from Origene (MR220008). They were then Gateway converted into pDONOR223.1. Cloning primers are listed in Table S1.
Kinome gain-of-function screen
HEK293T cells were plated in 384-well clear-bottom plates (Corning, 3764), and transfected with a cocktail of 20 ng FLAG-kinase, 4 ng hQR41 (Moehlenkamp and Johnson, 1999) (a generous gift from Jeffery Johnson, University of Wisconsin) and 1 ng HSV-thymidine kinase promoter driven Renilla luciferase (pRL-TK-Renilla, referred to here as Renilla) (Promega, E2241) per well using Lipofectamine 2000 (Life Technologies, 11668-019) in OptiMEM (Gibco, 31985-070). Each kinase was transfected in four technical replicates and biological triplicate using the Promega Dual-Glo Luciferase Assay System as per the manufacturer's protocol (Promega, E2940) (Agajanian et al., 2019). NRF2 transcriptional activity was determined by measuring levels of firefly luciferase and was normalized to Renilla luciferase for well-to-well variability. Fold activation of the reporter was determined compared with GFP-expressing cells. The false discovery rate (FDR) was calculated following adjustment via the Benjamini and Hochberg correction.
Western blotting
All samples were lysed in RIPA (10% glycerol, 50 mM Tris-HCL, 100 mM NaCl, 2 mM EDTA, 0.1% SDS, 1% Nonidet P-40, 0.2% sodium deoxycholate) supplemented with protease inhibitor cocktail (Thermo Fisher Scientific, 78429), phosphatase inhibitor cocktail (Thermo Fisher Scientific, 78426), NEM (Thermo Fisher Scientific, 23030) and benzonase (Sigma, E1014). Lysis was carried out on ice for 30 min and lysates centrifuged at 4°C for 15 min at 21,000×g. Following normalization of protein concentration via BCA (Pierce, 23225), samples were denatured in NuPAGE LDS buffer (Invitrogen, NP0007) with 1 mM dithiothreitol. Samples were treated with the following small molecules to stabilize NRF2: bardoxolone methyl (CDDO-me; S8078, Sigma), tert-butal hydroquinone (tBHQ Sigma, 112941), sulforaphane (Sigma, S6317); MG132 (Calbiochem, 474790), MLN4924 (Calbiochem, 505477001); bortezomib (Selleck Chem, PS-341), TORIN2 (Selleck Chem, S2817), INK-128 (Selleck Chem, S2811). Antibodies used are listed in Table S2.
qRT-PCR and RNA sequencing analysis
RNA was collected using PureLink RNA Mini Kit (Invitrogen, 12183018A) as per the manufacturer's instructions. The extracted RNA was then reverse transcribed to cDNA using iScript cDNA Synthesis Kit (BioRad, 170-8891), which was then used to perform quantitative RT-PCR (qRT-PCR) using SYBR Green (Applied Biosystems, 4385617) for the specified target genes (Table S3). The ddCT values were determined by normalizing to GAPDH and the average of DMSO-treated controls (hcRED and pGUS). Statistical significance was determined via two-way ANOVA with multiple comparisons.
RNA (3 µg) was submitted to Novogene Corp. (Sacramento, CA) for sequencing using the Illumina HiSeq platform, where reads were mapped to reference genome Homo sapiens (GRCh37/hg19). Alignments were done using STAR/HTSeq. Differential expression analysis was performed starting with gene level read count quantification provided by Novogene Corp. Preprocessing, normalization and differential expression analysis were performed according to the analysis pipeline outlined in Law et al. (2016). Marginally detected genes (<5 total read counts across samples) were filtered out in an initial preprocessing step, yielding a unimodal distribution of read counts per million by gene. Data normalization was performed using the trimmed mean of M-values method, as implemented in the calcNormFactors edgeR R-package function (Chen et al., 2014). Differential expression analysis was subsequently performed using the Limma R-packages functions voom and eBayes (Ritchie et al., 2015).
Gene set enrichment analysis (GSEA) was performed based on the above described pre-processed read counts, which were converted to counts per million and log2 transformed (logCPM). From 13,000 genes, the top 10,000 differentially expressed genes were used for GSEA analysis. Genes were ranked according to signal-to-noise ratio as defined by the Broad Institute GSEA software using the R-project fgsea package. Test gene sets (Hallmark and Oncogenic gene sets from theMSigDB, Broad Institute) were downloaded from the MSIG data bank via the msigdbr R-project package (Liberzon et al., 2015; Subramanian et al., 2005). Raw data and processed datasets are available through GEO (GSE139135).
Translation assay and polysome fractionation
Rate of protein translation was measured in HEK293T cells expressing controls or BRSK2 and BRSK1 at 24 h post-transfection using radioactive methionine (35S-Met) labeling. Polysome fractionation was performed as previously described (Graves et al., 2019; Lenarcic et al., 2014).
Phosphoproteomics sample processing and data analysis
Protein from HEK293T cells expressing control, BRSK2 or BRSK1 (1.4 mg) was precipitated in acetone overnight at −20°C. The sample was pelleted and re-suspended in 7 M urea, reduced with 5 mM dithiothreitol and alkylated with 15 mM chloroacetamide. The sample was adjusted with 50 mM ammonium bicarbonate such that the urea concentration was 1 M or less. A standard tryptic digest was performed overnight at 37°C. Solid phase extraction (SPE) was then performed using C18 Prep Sep cartridges (Waters, WAT054960), followed by reconstitution in 0.5% trifluoroacetic acid (TFA). The SPE cartridge was washed with conditioning solution (90% methanol with 0.1% TFA) then equilibrated with 0.1% TFA. The sample was passed slowly (1 drop/s) through the equilibrated cartridge and the cartridge desalted with equilibration solution. The sample was then slowly eluted (1 drop/s) with an elution solution of 50% acetonitrile (ACN) with 0.1% TFA. The sample was then TMT labeled according to kit specifications (Thermo Fisher Scientific, 90110), with the exception that labeling was performed for 6 h instead of 1 h. Following labeling, another SPE was performed, as described above. About 10% of the sample was saved for whole proteome input and 90% was phosphopeptide enriched using Titansphere Phos-TiO Kit (GL Sciences, 5010-21312). Before enrichment, samples were reconstituted in 100 µl of buffer B (75% ACN, 1% TFA, 20% lactic acid – solution B in the kit). The tip was conditioned by centrifugation with 100 µl of buffer A (80% ACN, 1% TFA), followed by conditioning with buffer B (3000×g for 2 min). The sample was then loaded onto the tip and centrifuged twice (1000×g for 5 min). The tip was then washed with 50 µl of buffer B, followed by two washes with 50 µl of buffer A (1000×g for 2 min). Phosphopeptides were eluted with 100 µl of elution solution 1 (20% ACN, 5% NH4OH) then 100 µl of elution solution 2 (20% ACN, 10% NH4OH) (1000×g, 5 min). Following phosphopeptide enrichment, both whole proteome input and phosphopeptides were fractionated utilizing a High pH Reversed-Phase Peptide Fractionation kit (Pierce, 84868), as per the manufacturer's specifications. A final clean-up step was performed using C18 Spin Columns (Pierce, 89870).
Mass spectrometry, data filtering and bioinformatics
Mass spectrometry analysis was carried out as follows: peptides were separated via reverse-phase nano-HPLC using the nanoACQUITY UPLC system (Waters Corporation). Peptides were trapped on a 2 cm column (Pepmap 100, 3 μM particle size, 100 Å pore size; Thermo Fisher Scientific, 164946) and separated on a 25 cm EASYspray analytical column (75 μM internal diameter, 2.0 μm C18 particle size, 100 Å pore size; Thermo Fisher Scientific, ES802) at 45°C. The mobile phases were 0.1% formic acid in water (buffer A) and 0.1% formic acid in ACN (buffer B). A 180 min gradient of 2-30% buffer B was used with a flow rate of 300 nl/min. Mass spectral analysis was performed using an Orbitrap Fusion Lumos mass spectrometer (Thermo Fisher Scientific). The ion source was operated at 2.4 kV and the ion transfer tube was set to 275°C. Full MS scans (350-2000 m/z) were analyzed in the Orbitrap at a resolution of 120,000 and 4e5 AGC target. The MS2 spectra were collected using a 0.7 m/z isolation width and analyzed by the linear ion trap using 1e4 AGC target after HCD fragmentation at 30% collision energy with 50 ms maximum injection time. The MS3 scans (100-500 m/z) were acquired in the Orbitrap at a resolution of 50,000 with a 1e5 AGC, 2 m/z MS2 isolation window, and at 105 ms maximum injection time after HCD fragmentation with a normalized energy of 65%. Precursor ions were selected in 400-2000 m/z mass range with mass exclusion width of 5-18 m/z. Lock mass was 371.10124 m/z (Polysiloxane).
MaxQuant (1.6.6.0) search parameters were as follows: specific tryptic digestion, up to two missed cleavages, a static carbamidomethyl cysteine modification, variable protein N-term acetylation, variable phospho(STY) and methionine oxidation using the human UniProtKB/Swiss-Prot sequence database (downloaded 1 Feb 2017). MaxQuant data was deposited to PRIDE/Proteome Xchange (PXD015884) (Vizcaíno et al., 2014). MaxQuant output files proteinGroups.txt and Phospho(STY)sites.txt were converted using in-house script into GCT format. This GCT file was then rewritten using Morpheus for compatibility with PTM-SEA analysis in R (Krug et al., 2019).
Supplementary Material
Acknowledgements
We would like to thank Drs Michael J. Emanuele and Thomas R. Bonacci for valuable scientific discussion. We thank Drs Kirsten L. Bryant and Channing J. Der for providing expertise and resources to explore mTOR signaling as well as autophagy. Dr Albena Dinkova-Kostova provided invaluable expertise on NRF2 and mTOR signaling. We also thank Dr Zhongsheng You for providing AMPK DKO MEFs.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: T.Y.T., M.B.M.; Methodology: T.Y.T., M.J.A., D.G., T.S.; Software: T.Y.T., D.G., T.P.S., T.S.; Validation: T.Y.T., B.M.B.; Formal analysis: T.Y.T., D.G., T.P.S., R.M.M.; Investigation: T.Y.T., B.M.B., M.J.A., A.E.H., S.J.W., R.M.M.; Resources: T.Y.T., B.M.B., A.E.H., P.F.S., K.M.L., N.J.M.; Data curation: T.Y.T., B.M.B., D.G., T.P.S.; Writing - original draft: T.Y.T., M.B.M.; Writing - review & editing: T.Y.T., B.M.B., M.J.A., B.E.W., M.B.M.; Visualization: T.Y.T.; Supervision: T.Y.T., M.B.M.; Project administration: T.Y.T., N.J.M., M.B.M.; Funding acquisition: B.E.W., M.B.M.
Funding
T.Y.T. and M.J.A. were funded by Howard Hughes Medical Institute (HHMI) Gilliam Fellowships for Advanced Study, the National Institutes of Health (NIH) Initiative for Maximizing Student Diversity Grant (R25-GM055336-16) and NIH T32 Pre-doctoral Training Grants in Pharmacology (T32-GM007040-42). M.J.A. was also funded via NIH/NCI NRSA (F31CA228289) and NCI F99/K00 (1F99CA245724-01). R.M.M. was supported by NIH/NCI NRSA (1F31DE028749-01) and NIH/NIGMS T32 MiBio Training Program (5T32GM119999-03). B.M.B., T.P.S. and K.M.L. were funded by ITCMS T32 Training Grant (T32CA009156). B.M.B. was also supported by NIH/NCI F32 (1F32CA225040-01). This research was supported by American Cancer Society grant to M.B.M. (RSG-14-1657068-01-TBE), NIH/NCI to M.B.M. and B.E.W. (RO1CA216051) and a NIH consortium grant on Illuminating the Druggable Genome (U24-DK116204-01). Deposited in PMC for release after 12 months.
Data availability
Raw data and processed datasets for the RNAseq experiments are available through the Gene Expression Omnibus (GEO) under accession no. GSE139135. MS data has been deposited in PRIDE/Proteome Xchange under accession no. PXD015884.
Supplementary information
Supplementary information available online at https://jcs.biologists.org/lookup/doi/10.1242/jcs.241356.supplemental
Peer review history
The peer review history is available online at https://jcs.biologists.org/lookup/doi/10.1242/jcs.241356.reviewer-comments.pdf
References
- Agajanian M. J., Walker M. P., Axtman A. D., Ruela-de-Sousa R. R., Serafin D. S., Rabinowitz A. D., Graham D. M., Ryan M. B., Tamir T., Nakamichi Y. et al. (2019). WNT activates the AAK1 kinase to promote clathrin-mediated endocytosis of LRP6 and establish a negative feedback loop. Cell Rep. 26, 79-93.e8. 10.1016/j.celrep.2018.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apopa P. L., He X. and Ma Q. (2008). Phosphorylation of Nrf2 in the transcription activation domain by casein kinase 2 (CK2) is critical for the nuclear translocation and transcription activation function of Nrf2 in IMR-32 neuroblastoma cells. J. Biochem. Mol. Toxicol. 22, 63-76. 10.1002/jbt.20212 [DOI] [PubMed] [Google Scholar]
- Aramburu J., Ortells M. C., Tejedor S., Buxade M. and Lopez-Rodriguez C. (2014). Transcriptional regulation of the stress response by mTOR. Sci. Signal. 7, re2 10.1126/scisignal.2005326 [DOI] [PubMed] [Google Scholar]
- Baird L., Llères D., Swift S. and Dinkova-Kostova A. T. (2013). Regulatory flexibility in the Nrf2-mediated stress response is conferred by conformational cycling of the Keap1-Nrf2 protein complex. Proc. Natl Acad. Sci. USA 110, 15259-15264. 10.1073/pnas.1305687110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakula D., Müller A. J., Zuleger T., Takacs Z., Franz-Wachtel M., Thost A.-K., Brigger D., Tschan M. P., Frickey T., Robenek H. et al. (2017). WIPI3 and WIPI4 β-propellers are scaffolds for LKB1-AMPK-TSC signalling circuits in the control of autophagy. Nat. Commun. 8, 15637 10.1038/ncomms15637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biechele T. L. and Moon R. T. (2008). Assaying beta-catenin/TCF transcription with beta-catenin/TCF transcription-based reporter constructs. Methods Mol. Biol. 468, 99-110. [DOI] [PubMed] [Google Scholar]
- Bright N. J., Carling D. and Thornton C. (2008). Investigating the regulation of brain-specific kinases 1 and 2 by phosphorylation. J. Biol. Chem. 283, 14946-14954. 10.1074/jbc.M710381200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright N. J., Thornton C. and Carling D. (2009). The regulation and function of mammalian AMPK-related kinases. Acta Physiol. (Oxf.) 196, 15-26. 10.1111/j.1748-1716.2009.01971.x [DOI] [PubMed] [Google Scholar]
- Chen X.-Y., Gu X.-T., Saiyin H., Wan B., Zhang Y.-J., Li J., Wang Y.-L., Gao R., Wang Y.-F., Dong W.-P. et al. (2012). Brain-selective Kinase 2 (BRSK2) phosphorylation on PCTAIRE1 negatively regulates glucose-stimulated insulin secretion in pancreatic β-cells. J. Biol. Chem. 287, 30368-30375. 10.1074/jbc.M112.375618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Lun A. T. L. and Smyth G. K. (2014). Differential expression analysis of complex RNA-seq experiments using edgeR. In Statistical Analysis of Next Generation Sequence Data (ed. Datta S. and Nettleton D. S.), pp 51-74. Cham: Springer. [Google Scholar]
- Chowdhry S., Zhang Y., McMahon M., Sutherland C., Cuadrado A. and Hayes J. D. (2012). Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene 32, 3765-3781. 10.1038/onc.2012.388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloer E. W., Siesser P. F., Cousins E. M., Goldfarb D., Mowrey D. D., Harrison J. S., Weir S. J., Dokholyan N. V. and Major M. B. (2018). p62-dependent phase separation of patient-derived KEAP1 mutations and NRF2. Mol. Cell. Biol. 38, e00644-17 10.1128/MCB.00644-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloer E. W., Goldfarb D., Schrank T. P., Weissman B. E. and Major M. B. (2019). NRF2 activation in cancer: from DNA to protein. Cancer Res. 79, 889-898. 10.1158/0008-5472.CAN-18-2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado A. (2015). Structural and functional characterization of Nrf2 degradation by glycogen synthase kinase 3/β-TrCP. Free Radic. Biol. Med. 88, 147-157. 10.1016/j.freeradbiomed.2015.04.029 [DOI] [PubMed] [Google Scholar]
- Cuadrado A., Manda G., Hassan A., Alcaraz M. J., Barbas C., Daiber A., Ghezzi P., León R., López M. G., Oliva B. et al. (2018). Transcription factor NRF2 as a therapeutic target for chronic diseases: a systems medicine approach. Pharmacol. Rev. 70, 348-383. 10.1124/pr.117.014753 [DOI] [PubMed] [Google Scholar]
- Cullinan S. B., Zhang D., Hannink M., Arvisais E., Kaufman R. J. and Diehl J. A. (2003). Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell. Biol. 23, 7198-7209. 10.1128/MCB.23.20.7198-7209.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan S. B., Gordan J. D., Jin J., Harper J. W. and Diehl J. A. (2004). The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol. Cell. Biol. 24, 8477-8486. 10.1128/MCB.24.19.8477-8486.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayalan Naidu S., Dikovskaya D., Gaurilcikaite E., Knatko E. V., Healy Z. R., Mohan H., Koh G., Laurell A., Ball G., Olagnier D. et al. (2017). Transcription factors NRF2 and HSF1 have opposing functions in autophagy. Sci. Rep. 7, 11023 10.1038/s41598-017-11262-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Vecchio C. A., Feng Y., Sokol E. S., Tillman E. J., Sanduja S., Reinhardt F. and Gupta P. B. (2014). De-differentiation confers multidrug resistance via noncanonical PERK-Nrf2 signaling. PLoS Biol. 12, e1001945 10.1371/journal.pbio.1001945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNicola G. M., Karreth F. A., Humpton T. J., Gopinathan A., Wei C., Frese K., Mangal D., Yu K. H., Yeo C. J., Calhoun E. S. et al. (2012). Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 475, 106-109. 10.1038/nature10189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert L. A., Horlbeck M. A., Adamson B., Villalta J. E., Chen Y., Whitehead E. H., Guimaraes C., Panning B., Ploegh H. L., Bassik M. C. et al. (2014). Genome-scale CRISPR-mediated control of gene repression and activation. Cell 159, 647-661. 10.1016/j.cell.2014.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves P. R., Aponte-Collazo L. J., Fennell E. M. J., Graves A. C., Hale A. E., Dicheva N., Herring L. E., Gilbert T. S. K., East M. P., McDonald I. M. et al. (2019). Mitochondrial protease ClpP is a target for the anticancer compounds ONC201 and related analogues. ACS Chem. Biol. 14, 1020-1029. 10.1021/acschembio.9b00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder B., Tian W., La Clair J. J., Tan A.-C., Ooi A., Chapman E. and Zhang D. D. (2017). Brusatol overcomes chemoresistance through inhibition of protein translation. Mol. Carcinog. 56, 1493-1500. 10.1002/mc.22609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K., Simmons A. N., Kajino-Sakamoto R., Tsuji Y. and Ninomiya-Tsuji J. (2016). TAK1 regulates the Nrf2 antioxidant system through modulating p62/SQSTM1. Antioxid Redox Signal. 25, 953-964. 10.1089/ars.2016.6663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hast B. E., Goldfarb D., Mulvaney K. M., Hast M. A., Siesser P. F., Yan F., Hayes D. N. and Major M. B. (2013). Proteomic analysis of ubiquitin ligase KEAP1 reveals associated proteins that inhibit NRF2 ubiquitination. Cancer Res. 73, 2199-2210. 10.1158/0008-5472.CAN-12-4400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma S., Ishii Y., Morishima Y., Yamadori T., Matsuno Y., Haraguchi N., Kikuchi N., Satoh H., Sakamoto T., Hizawa N. et al. (2009). Nrf2 enhances cell proliferation and resistance to anticancer drugs in human lung cancer. Clin. Cancer Res. 15, 3423-3432. 10.1158/1078-0432.CCR-08-2822 [DOI] [PubMed] [Google Scholar]
- Huang H.-C., Nguyen T. and Pickett C. B. (2002). Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J. Biol. Chem. 277, 42769-42774. 10.1074/jbc.M206911200 [DOI] [PubMed] [Google Scholar]
- Hussong M., Börno S. T., Kerick M., Wunderlich A., Franz A., Sültmann H., Timmermann B., Lehrach H., Hirsch-Kauffmann M. and Schweiger M. R. (2014). The bromodomain protein BRD4 regulates the KEAP1/NRF2-dependent oxidative stress response. Cell Death Dis. 5, e1195-e1111. 10.1038/cddis.2014.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa T., Li J., Meyer C. J., Janicki J. S., Hannink M. and Cui T. (2009). Dihydro-CDDO-trifluoroethyl amide (dh404), a novel Nrf2 activator, suppresses oxidative stress in cardiomyocytes. PLoS ONE 4, e8391 10.1371/journal.pone.0008391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura Y., Waguri S., Sou Y.-S., Kageyama S., Hasegawa J., Ishimura R., Saito T., Yang Y., Kouno T., Fukutomi T. et al. (2013). Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol. Cell 51, 618-631. 10.1016/j.molcel.2013.08.003 [DOI] [PubMed] [Google Scholar]
- Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K., Engel J. D. and Yamamoto M. (1999). Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 13, 76-86. 10.1101/gad.13.1.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K., Mimura J. and Yamamoto M. (2010). Discovery of the negative regulator of Nrf2, Keap1: a historical overview. Antioxid Redox Signal. 13, 1665-1678. 10.1089/ars.2010.3222 [DOI] [PubMed] [Google Scholar]
- Joo M. S., Kim W. D., Lee K. Y., Kim J. H., Koo J. H. and Kim S. G. (2016). AMPK facilitates nuclear accumulation of Nrf2 by phosphorylating at serine 550. Mol. Cell. Biol. 36, 1931-1942. 10.1128/MCB.00118-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler T. W., Wakabayashi N. and Biswal S. (2007). Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 47, 89-116. 10.1146/annurev.pharmtox.46.120604.141046 [DOI] [PubMed] [Google Scholar]
- Kim J. S. M., Hung W., Narbonne P., Roy R. and Zhen M. (2010). C. elegans STRADα and SAD cooperatively regulate neuronal polarity and synaptic organization. Development 137, 93-102. 10.1242/dev.041459 [DOI] [PubMed] [Google Scholar]
- Kishi M., Pan Y. A., Crump J. G. and Sanes J. R. (2005). Mammalian SAD kinases are required for neuronal polarization. Science 307, 929-932. 10.1126/science.1107403 [DOI] [PubMed] [Google Scholar]
- Komar A. A. and Hatzoglou M. (2011). Cellular IRES-mediated translation: the war of ITAFs in pathophysiological states. Cell Cycle 10, 229-240. 10.4161/cc.10.2.14472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug K., Mertins P., Zhang B., Hornbeck P., Raju R., Ahmad R., Szucs M., Mundt F., Forestier D., Jane-Valbuena J. et al. (2019). A curated resource for phosphosite-specific signature analysis. Mol. Cell. Proteomics 18, 576-593. 10.1074/mcp.TIR118.000943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laderoute K. R., Amin K., Calaoagan J. M., Knapp M., Le T., Orduna J., Foretz M. and Viollet B. (2006). 5′-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol. Cell. Biol. 26, 5336-5347. 10.1128/MCB.00166-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau A., Wang X.-J., Zhao F., Villeneuve N. F., Wu T., Jiang T., Sun Z., White E. and Zhang D. D. (2010). A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Mol. Cell. Biol. 30, 3275-3285. 10.1128/MCB.00248-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law C. W., Alhamdoosh M., Su S., Dong X., Tian L., Smyth G. K. and Ritchie M. E. (2016). RNA-seq analysis is easy as 1-2-3 with limma, Glimma and edgeR. F1000Res 5, 1408 10.12688/f1000research.9005.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. C., Zhang J., Strom J., Yang D., Dinh T. N., Kappeler K. and Chen Q. M. (2017). G-Quadruplex in the NRF2 mRNA 5′ untranslated region regulates de novo NRF2 protein translation under oxidative stress. Mol. Cell. Biol. 37 10.1128/MCB.00122-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenarcic E. M., Ziehr B., De Leon G., Mitchell D. and Moorman N. J. (2014). Differential role for host translation factors in host and viral protein synthesis during human cytomegalovirus infection. J. Virol. 88, 1473-1483. 10.1128/JVI.02321-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Wan B., Zhou J., Wang Y., Luo T., Gu X., Chen F. and Yu L. (2012). APC/CCdh1 targets Brain-Specific Kinase 2 (BRSK2) for degradation via the ubiquitin-proteasome pathway. PLoS ONE 7, e45932 10.1371/journal.pone.0045932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Lavagnino Z., Lemacon D., Kong L., Ustione A., Ng X., Zhang Y., Wang Y., Zheng B., Piwnica-Worms H. et al. (2019). Ca2+-stimulated AMPK-dependent phosphorylation of Exo1 protects stressed replication forks from aberrant resection. Mol. Cell 74, 1123-1137.e6. 10.1016/j.molcel.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon A., Birger C., Thorvaldsdóttir H., Ghandi M., Mesirov J. P. and Tamayo P. (2015). The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 1, 417-425. 10.1016/j.cels.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley B. N., Krishnaswamy A., Wang Z., Kishi M., Frank E. and Sanes J. R. (2014). SAD kinases control the maturation of nerve terminals in the mammalian peripheral and central nervous systems. Proc. Natl. Acad. Sci. USA 111, 1138-1143. 10.1073/pnas.1321990111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J. H., Kim K.-M., Kim S. W., Hwang O. and Choi H. J. (2008). Bromocriptine activates NQO1 via Nrf2-PI3K/Akt signaling: Novel cytoprotective mechanism against oxidative damage. Pharmacol. Res. 57, 325-331. 10.1016/j.phrs.2008.03.004 [DOI] [PubMed] [Google Scholar]
- Lizcano J. M., Göransson O., Toth R., Deak M., Morrice N. A., Boudeau J., Hawley S. A., Udd L., Mäkelä T. P., Hardie D. G. et al. (2004). LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 23, 833-843. 10.1038/sj.emboj.7600110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegon S., Columbano A. and Giordano S. (2016). The dual roles of NRF2 in cancer. Trends Mol. Med. 22, 578-593. 10.1016/j.molmed.2016.05.002 [DOI] [PubMed] [Google Scholar]
- Michaeloudes C., Mercado N., Clarke C., Bhavsar P. K., Adcock I. M., Barnes P. J. and Chung K. F. (2014). Bromodomain and extraterminal proteins suppress NF-E2-related factor 2-mediated antioxidant gene expression. J. Immunol. 192, 4913-4920. 10.4049/jimmunol.1301984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehlenkamp J. D. and Johnson J. A. (1999). Activation of antioxidant/electrophile-responsive elements in IMR-32 human neuroblastoma cells. Arch. Biochem. Biophys. 363, 98-106. 10.1006/abbi.1998.1046 [DOI] [PubMed] [Google Scholar]
- Morrison L. M., Edwards S. L., Manning L., Stec N., Richmond J. E. and Miller K. G. (2018). Sentryn and SAD kinase link the guided transport and capture of dense core vesicles in Caenorhabditis elegans. Genetics 210, 925-946. 10.1534/genetics.118.300847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M., Lutter D. and Puschel A. W. (2010). Persistence of the cell-cycle checkpoint kinase Wee1 in SadA- and SadB-deficient neurons disrupts neuronal polarity. J. Cell Sci. 123, 286-294. 10.1242/jcs.058230 [DOI] [PubMed] [Google Scholar]
- Naidu S., Vijayan V., Santoso S., Kietzmann T. and Immenschuh S. (2009). Inhibition and genetic deficiency of p38 MAPK up-regulates heme oxygenase-1 gene expression via Nrf2. J. Immunol. 182, 7048-7057. 10.4049/jimmunol.0900006 [DOI] [PubMed] [Google Scholar]
- Nakaso K., Yano H., Fukuhara Y., Takeshima T., Wada-Isoe K. and Nakashima K. (2003). PI3K is a key molecule in the Nrf2-mediated regulation of antioxidative proteins by hemin in human neuroblastoma cells. FEBS Lett. 546, 181-184. 10.1016/S0014-5793(03)00517-9 [DOI] [PubMed] [Google Scholar]
- Nie J., Sun C., Faruque O., Ye G., Li J., Liang Q., Chang Z., Yang W., Han X. and Shi Y. (2012). Synapses of amphids defective (SAD-A) kinase promotes glucose-stimulated insulin secretion through activation of p21-activated kinase (PAK1) in pancreatic β-Cells. J. Biol. Chem. 287, 26435-26444. 10.1074/jbc.M112.378372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie J., Sun C., Chang Z., Musi N. and Shi Y. (2018). SAD-A promotes glucose-stimulated insulin secretion through phosphorylation and inhibition of GDIα in male islet β cells. Endocrinology 159, 3036-3047. 10.1210/en.2017-03243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie M. E., Phipson B., Wu D., Hu Y., Law C. W., Shi W. and Smyth G. K. (2015). limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 10.1093/nar/gkv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo de la Vega M., Chapman E. and Zhang D. D. (2018). NRF2 and the hallmarks of cancer. Cancer Cell 34, 21-43. 10.1016/j.ccell.2018.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiyin H., Na N., Han X., Fang Y., Wu Y., Lou W. and Yang X. (2017). BRSK2 induced by nutrient deprivation promotes Akt activity in pancreatic cancer via downregulation of mTOR activity. Oncotarget 8, 44669-44681. 10.18632/oncotarget.17965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H., Moriguchi T., Takai J., Ebina M. and Yamamoto M. (2013). Nrf2 prevents initiation but accelerates progression through the Kras signaling pathway during lung carcinogenesis. Cancer Res. 73, 4158-4168. 10.1158/0008-5472.CAN-12-4499 [DOI] [PubMed] [Google Scholar]
- Saxena M. T., Schroeter E. H., Mumm J. S. and Kopan R. (2001). Murine notch homologs (N1-4) undergo presenilin-dependent proteolysis. J. Biol. Chem. 276, 40268-40273. 10.1074/jbc.M107234200 [DOI] [PubMed] [Google Scholar]
- Shay K. P., Michels A. J., Li W., Kong A.-N. T. and Hagen T. M. (2012). Cap-independent Nrf2 translation is part of a lipoic acid-stimulated detoxification stress response. Biochim. Biophys. Acta 1823, 1102-1109. 10.1016/j.bbamcr.2012.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen G., Hebbar V., Nair S., Xu C., Li W., Lin W., Keum Y.-S., Han J., Gallo M. A. and Kong A.-N. T. (2004). Regulation of Nrf2 transactivation domain activity. The differential effects of mitogen-activated protein kinase cascades and synergistic stimulatory effect of Raf and CREB-binding protein. J. Biol. Chem. 279, 23052-23060. 10.1074/jbc.M401368200 [DOI] [PubMed] [Google Scholar]
- Subramanian A., Tamayo P., Mootha V. K., Mukherjee S., Ebert B. L., Gillette M. A., Paulovich A., Pomeroy S. L., Golub T. R., Lander E. S. et al. (2005). Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545-15550. 10.1073/pnas.0506580102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z., Huang Z. and Zhang D. D. (2009). Phosphorylation of Nrf2 at multiple sites by MAP kinases has a limited contribution in modulating the Nrf2-dependent antioxidant response. PLoS ONE 4, e6588 10.1371/journal.pone.0006588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T. and Yamamoto M. (2015). Molecular basis of the Keap1–Nrf2 system. Free Radic. Biol. Med. 88, 93-100. 10.1016/j.freeradbiomed.2015.06.006 [DOI] [PubMed] [Google Scholar]
- Suzuki T., Muramatsu A., Saito R., Iso T., Shibata T., Kuwata K., Kawaguchi S.-I., Iwawaki T., Adachi S., Suda H. et al. (2019). Molecular mechanism of cellular oxidative stress sensing by Keap1. Cell Rep. 28, 746-758.e4. 10.1016/j.celrep.2019.06.047 [DOI] [PubMed] [Google Scholar]
- Tamir T. Y., Mulvaney K. M. and Major M. B. (2016). Dissecting the Keap1/Nrf2 pathway through proteomics. Curr. Opin. Toxicol. 1, 118-124. 10.1016/j.cotox.2016.10.007 [DOI] [Google Scholar]
- Tao S., Wang S., Moghaddam S. J., Ooi A., Chapman E., Wong P. K. and Zhang D. D. (2014). Oncogenic KRAS confers chemoresistance by upregulating NRF2. Cancer Res. 74, 7430-7441. 10.1158/0008-5472.CAN-14-1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong K. I., Katoh Y., Kusunoki H., Itoh K., Tanaka T. and Yamamoto M. (2006). Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model. Mol. Cell. Biol. 26, 2887-2900. 10.1128/MCB.26.8.2887-2900.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrente L., Sanchez C., Moreno R., Chowdhry S., Cabello P., Isono K., Koseki H., Honda T., Hayes J. D., Dinkova-Kostova A. T. et al. (2017). Crosstalk between NRF2 and HIPK2 shapes cytoprotective responses. Oncogene 36, 6204-6212. 10.1038/onc.2017.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiri E. N., Gumeni S., Iliaki K. K., Benaki D., Vougas K., Sykiotis G. P., Gorgoulis V. G., Mikros E., Scorrano L. and Trougakos I. P. (2019). Hyperactivation of Nrf2 increases stress tolerance at the cost of aging acceleration due to metabolic deregulation. Aging Cell 18, e12845 10.1111/acel.12845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida K., Tsujita T., Hayashi M., Ojima A., Keleku-Lukwete N., Katsuoka F., Otsuki A., Kikuchi H., Oshima Y., Suzuki M. et al. (2017). Halofuginone enhances the chemo-sensitivity of cancer cells by suppressing NRF2 accumulation. Free Radic. Biol. Med. 103, 236-247. 10.1016/j.freeradbiomed.2016.12.041 [DOI] [PubMed] [Google Scholar]
- Uhlen M., Oksvold P., Fagerberg L., Lundberg E., Jonasson K., Forsberg M., Zwahlen M., Kampf C., Wester K., Hober S. et al. (2010). Towards a knowledge-based human protein atlas. Nat. Biotechnol. 28, 1248-1250. 10.1038/nbt1210-1248 [DOI] [PubMed] [Google Scholar]
- Uhlén M., Fagerberg L., Hallström B. M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson A., Kampf C., Sjöstedt E., Asplund A. et al. (2015). Proteomics. Tissue-based map of the human proteome. Science 347, 1260419 10.1126/science.1260419 [DOI] [PubMed] [Google Scholar]
- Vizcaíno J. A., Deutsch E. W., Wang R., Csordas A., Reisinger F., Ríos D., Dianes J. A., Sun Z., Farrah T., Bandeira N. et al. (2014). ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat. Biotechnol. 32, 223-226. 10.1038/nbt.2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi N., Itoh K., Wakabayashi J., Motohashi H., Noda S., Takahashi S., Imakado S., Kotsuji T., Otsuka F., Roop D. R. et al. (2003). Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat. Genet. 35, 238-245. 10.1038/ng1248 [DOI] [PubMed] [Google Scholar]
- Walker M. P., Stopford C. M., Cederlund M., Fang F., Jahn C., Rabinowitz A. D., Goldfarb D., Graham D. M., Yan F., Deal A. M. et al. (2015). FOXP1 potentiates Wnt/β-catenin signaling in diffuse large B cell lymphoma. Sci. Signal. 8, ra12 10.1126/scisignal.2005654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wan B., Li D., Zhou J., Li R., Bai M., Chen F. and Yu L. (2012). BRSK2 is regulated by ER stress in protein level and involved in ER stress-induced apoptosis. Biochem. Biophys. Res. Commun. 423, 813-818. 10.1016/j.bbrc.2012.06.046 [DOI] [PubMed] [Google Scholar]
- Wang Y., Wan B., Zhou J., Li R. and Yu L. (2013). BRSK2 is a valosin-containing protein (VCP)-interacting protein that affects VCP functioning in endoplasmic reticulum-associated degradation. Biotechnol. Lett. 35, 1983-1989. 10.1007/s10529-013-1295-2 [DOI] [PubMed] [Google Scholar]
- Wang Y.-L., Wang J., Chen X., Wang Z.-X. and Wu J.-W. (2018). Crystal structure of the kinase and UBA domains of SNRK reveals a distinct UBA binding mode in the AMPK family. Biochem. Biophys. Res. Commun. 495, 1-6. 10.1016/j.bbrc.2017.10.105 [DOI] [PubMed] [Google Scholar]
- Wang P., Geng J., Gao J., Zhao H., Li J., Shi Y., Yang B., Xiao C., Linghu Y., Sun X. et al. (2019). Macrophage achieves self-protection against oxidative stress-induced ageing through the Mst-Nrf2 axis. Nat. Commun. 10, 755 10.1038/s41467-019-08680-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfel N. A., Sainz A. G., Song J. H. and Kraft A. S. (2016). PIM kinase inhibitors kill hypoxic tumor cells by reducing Nrf2 signaling and increasing reactive oxygen species. Mol. Cancer Ther. 15, 1637-1647. 10.1158/1535-7163.MCT-15-1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.-X., Cheng Y.-S., Wang J., Chen L., Ding M. and Wu J.-W. (2015). Structural insight into the mechanism of synergistic autoinhibition of SAD kinases. Nat. Commun. 6, 8953 10.1038/ncomms9953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Davies K. J. A. and Forman H. J. (2015). Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 88, 314-336. 10.1016/j.freeradbiomed.2015.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.