Abstract
Introduction
The radial artery has become the standard access site for percutaneous coronary intervention (PCI) in stable coronary artery disease and acute coronary syndrome, because of less access site related bleeding complications. Patients with complex coronary lesions are under-represented in randomised trials comparing radial with femoral access with regard to safety and efficacy. The femoral artery is currently the most applied access site in patients with complex coronary lesions, especially when large bore guiding catheters are required. With slender technology, transradial PCI may be increasingly applied in patients with complex coronary lesions when large bore guiding catheters are mandatory and might be a safer alternative as compared with the transfemoral approach.
Methods and analysis
A total of 388 patients undergoing complex PCI will be randomised to radial 7 French access with Terumo Glidesheath Slender (Terumo, Japan) or femoral 7 French access as comparator. The primary outcome is the incidence of the composite end point of clinically relevant access site related bleeding and/or vascular complications requiring intervention. Procedural success and major adverse cardiovascular events up to 1 month will also be compared between both groups.
Ethics and dissemination
Ethical approval for the study was granted by the local Ethics Committee at each recruiting center (‘Medisch Ethische Toetsing Commissie Isala Zwolle’, ‘Commissie voor medische ethiek ZNA’, ‘Comité Medische Ethiek Ziekenhuis Oost-Limburg’, ‘Comité d’éthique CHU-Charleroi-ISPPC’, ‘Commission cantonale d'éthique de la recherche CCER-Republique et Canton de Geneve’, ‘Ethik Kommission de Ärztekammer Nordrhein’ and ‘Riverside Research Ethics Committee’). The trial outcomes will be published in peer-reviewed journals of the concerned literature.
Trial registration number
Keywords: coronary intervention, cardiology, coronary heart disease
Strengths and limitations of this study.
The design as a randomised 1:1 open label study (radial 7 Fr vs femoral 7 Fr) and the vast experience with complex percutaneous coronary intervention (PCI) of the participating centres.
Clinical event committee adjudicated and clinically relevant primary endpoint.
First study assessing extremity dysfunction after complex large bore PCI.
As a limitation, bias could be derived from the unblinded nature of the study for the treating interventional cardiologist.
As a limitation, use of secondary access sites for hybrid approach of chronic total occlusions lesions will influence efficacy outcomes, although it will not influence the primary endpoint.
Background
The radial artery has become the standard access site for percutaneous coronary interventions (PCI), driven not only by lower rates of major bleeding and vascular complications, but also by reduced mortality in patients presenting with acute coronary syndrome (ACS).1–3 This has led the 2018 ESC/EACTS guidelines on myocardial revascularisation to recommend transradial access (TRA) over transfemoral access (TFA) as a class Ia indication in patients with ACS undergoing invasive management.4 In patients with stable coronary artery disease, several small randomised trials comparing radial and femoral access have shown significantly less bleeding in favour of radial access but no mortality benefit.1 5 6 Of note, patients with complex coronary lesions were not included in these trials or not specifically described. PCI of chronic total occlusions (CTO), left main disease, heavily calcified or complex bifurcation lesions often require the use of large-bore guiding catheters (7 Fr or larger inner diameter). Indeed, large-bore guiding catheters provide more back-up and stability in addition to better materials’ compatibility, leading to higher procedural success rates in more complex lesions.7 8 Because of potential radial artery-sheath mismatch, spasms or back-up problems, the femoral artery is still the most applied access site for complex PCI.9 10 In return, TFA with increased sheath size is associated with bleeding and vascular complications and adverse clinical outcome, including myocardial infarction (MI), stroke and death.11 12 The recent availability of modern slender technology, such as the thin-walled radial introducer sheath (Glidesheath Slender, Terumo, Japan), has the potential to expand the use of TRA for complex PCI. As compared with the average outer diameter of a standard sheath, the outer diameter of these slender sheaths has been reduced by approximately 1 Fr while maintaining the inner diameter equivalent. In a prospective single-arm study, it was recently shown that complex transradial (TR) PCI with a 7 Fr Glidesheath Slender is safe and effective.13 Several observational studies have been published describing feasibility of large bore TRA for PCI of CTO’s, left main disease, heavily calcified lesions and complex bifurcations without affecting procedural success rates.8 10 14–17 However, randomised data comparing TRA and TFA for percutaneous treatment of complex coronary lesions are lacking. Therefore, we have designed a randomised study, comparing the safety and efficacy of TRA and TFA for complex PCI using large-bore guiding catheters.
Methods
Study design
The Complex Large-Bore Radial PCI (COLOR) trial is an investigator-initiated international multicentre study with a prospective, randomised controlled design. Participating centres are the Isala Heart Center (Zwolle, the Netherlands), Catharina Hospital (Eindhoven, the Netherlands), Radboud University Medical Center (Nijmegen, The Netherlands), Elisabeth-Krankenhaus (Essen, Germany), NorthWest Clinics (Alkmaar, the Netherlands), Onze Lieve Vrouwe Gasthuis Hospital (Amsterdam, the Netherlands), Centre Hospilatier Universitaire de Charleroi (Charleroi, Belgium), ZNA Middelheim (Antwerpen, Belgium), Hospital Oost-Limburg (Genk, Belgium), Geneva University Hospital (Geneva, Switzerland), VU University Medical Center (Amsterdam, The Netherlands) and Frimley National Health Service (NHS) (Surrey, UK). All centres have been selected based on their high volumes and experience with complex PCI and large bore access. For CTO, each centre has a dedicated programme for an average of 6 years, with 1–3 dedicated CTO operators and an average of 110 procedures per year (spreading from 55 to 200 procedures per year). Eighty-three per cent of CTO procedures are done with dual arterial access, with biradial access in 20%, bifemoral access in 24% and radial/femoral (hybrid) access in the remaining 49% of cases. Large bore access is used in 89% of cases. For non-CTO complex PCI, the participating centres have a dedicated programme for an average of 11 years, performing an average of 245 procedures per year with 3–5 complex PCI operators. Seventy-six per cent of these cases are done with TRA and 24% with TFA. Large bore access is used in 62% of all complex non CTO PCI.
Trial organisation
The trial is approved by the appropriate ethics review board at each clinical site. Written informed consent will be obtained from all patients before enrolment. The trial was designed in accordance with the Declaration of Helsinki. All data will be collected in an electronic data capturing system, the electronic case record form Diagnostic REsearch And Management. Diagram BV, Zwolle, the Netherlands will be responsible for overall trial and data management, as well as monitoring of the study. Evaluation of serious adverse events (AEs) is being performed by an independent data safety monitoring board (DSMB). A clinical events committee (CEC) will review and adjudicate all endpoint related AEs.
Objectives
The primary objective of this study is to investigate whether TR PCI is superior to transfemoral (TF) PCI in complex coronary lesions with large-bore guiding catheters with respect to clinically relevant access site related bleeding and/or vascular complications.
As secondary objectives, TR and TF large-bore access will be compared with regard to procedural success, procedural time, fluoroscopy time, contrast use, cross-over rates, major adverse cardiovascular events (MACE) and non-access site-related bleeding or vascular complications for complex PCI.
For exploratory purposes extremity dysfunction and discomfort will be compared between TR and TF treated patients for complex PCI with large-bore guiding catheters.
Inclusion
All patients of 18 years or older, presenting with stable coronary artery disease, unstable angina or non-ST elevation MI and planned for PCI of the following complex coronary lesions: CTO, left main stem, heavily calcified lesions which may require calcium modification techniques (rotational atherectomy or intravascular lithotripsy) and complex bifurcations in whom the operator anticipates that a 7 Fr guiding catheter is indicated, are screened for inclusion. CTO is defined as a lesion exhibiting thrombolysis in myocardial infarction (TIMI) 0–1 flow in a native coronary artery with an occlusion duration of ≥3 months.18 Heavily calcified lesions are characterised by multiple persisting opacifications of the coronary wall visible in more than one projection surrounding the complete lumen of the coronary artery at the site of the lesion.19 Complex bifurcation includes lesions with Medina classification 0.1.1, 1.1.1 or 1.0.1.20 Patients with ST elevation MI or cardiogenic shock will be excluded. Patients with contraindications for femoral or radial access, such as occlusive peripheral artery disease, known severe spasm or known anatomical variants prohibiting radial or femoral access on both sides will be excluded as well. See also figure 1 for graphic representation of study inclusion.
Figure 1.
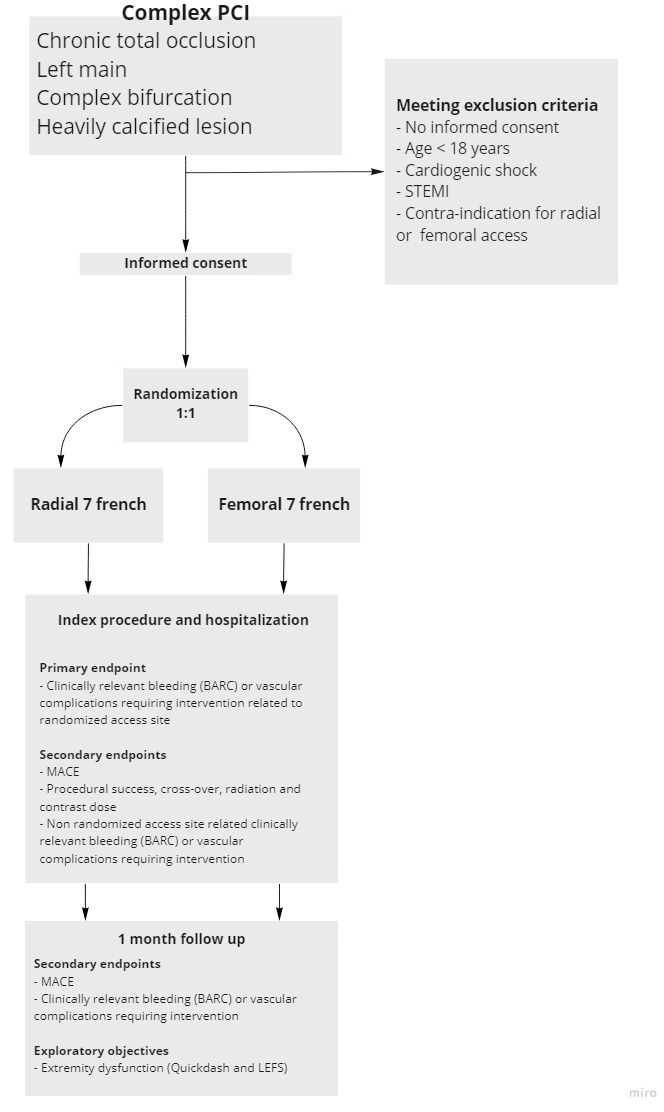
Inclusion flow chart for the COLOR trial. Graphic representation of inclusion for the COLOR trial. BARC, bleeding academic research group; COLOR, Complex Large-Bore Radial PCI; LEFS, Lower Extremity Functional Scale; MACE, major adverse cardiovascular events; PCI, percutaneous coronary intervention; STEMI, ST elevation myocardial infarction.
Randomisation
After providing written informed consent, eligible subjects are randomly assigned to receive one of the two study treatments in a 1:1 ratio. Treatment assignments are performed centrally through a dedicated website as part of the electronic case report form according to a computer-generated random schedule in random permuted blocks with stratification by site.21 There will be no blinding of the randomisation assignment.
Endpoints
Clinically relevant access site-related bleeding or vascular complication requiring intervention of the randomised access site during hospitalisation is defined as primary endpoint. Bleeding will be classified according to the Bleeding Academic Research Consortium (BARC) criteria,22 and considered clinically relevant when the score is ≥2 (CEC adjudicated).23 Severity and type of intervention of vascular complications is specified in the CEC manual (online supplementary file 1).
bmjopen-2020-038042supp001.pdf (85.1KB, pdf)
Secondary safety and efficacy endpoints are:
Procedural success (defined as successful PCI of the target lesion with a residual stenosis of less than 20%, without in-hospital MACE), procedural time, fluoroscopy time, contrast use and crossover rate (crossover is defined as conversion from TF to TR or vice versa; conversion to contralateral TR or TF access site is not considered crossover).
Clinically relevant BARC bleedings or vascular complications (requiring intervention) that are not related to the randomised access (CEC adjudicated).
MACE, defined as composite of death, MI and repeat revascularisation, during hospitalisation and at 1 month (CEC adjudicated).
Index PCI and hospitalisation
Radial access will be performed according to the local protocol, using direct needle technique or venous cannula technique, followed by introduction of a 7 Fr Glidesheath Slender. A standard cocktail of nitroglycerine and verapamil will be given intra-arterially after radial sheath placement. Femoral access will be performed using direct needle technique, followed by introduction of a standard 7 Fr femoral sheath. Use of ultrasound for vascular access will be left to the operator’s discretion. A bolus of unfractionated heparin will be given after sheath placement, adapted to the patient’s body weight. Activated clotting time (ACT) measurements will be performed during the procedure according to local protocol. Additional arterial access will be left to the discretion of the operator, that is, in case of double arterial access for hybrid CTO treatment. In case of randomisation to TRA, a 7 Fr Glidesheath Slender must be inserted in the right or left radial artery. Then, the operator can decide which secondary access site he/she will use and which sheath size is needed for this secondary access. This can be the contralateral radial artery (biradial approach) or the femoral artery. If the patient is randomised to femoral access and needs dual access, a 7 Fr femoral sheath must be placed in the femoral artery (randomised access site) and the operator can decide which second access he/she will use (radial or femoral). Only clinically significant bleeding or vascular complications attributable to the randomised access site will be analysed for the primary endpoint, complications attributable to the secondary access site will be analysed as secondary endpoint. PCI will be performed according to standard procedures with modern drug eluting stents. The applied technique for complex PCI will be left to the discretion of the operator. Patent haemostasis after radial access with the reverse Barbeau test is highly recommended.24 The type of femoral artery haemostasis will be left to the discretion of the treating interventional cardiologist; however, the application of a closure device is advocated. The Visual Analogue Scale will be used to assess post-procedural pain of the access site(s). Before discharge the access site(s) will be checked for bleeding and vascular complications. Radial artery patency will be checked with the reverse Barbeau test.24 Additional ultrasound or Doppler will be performed in those patients with suspected radial or femoral occlusion or the presence of other vascular complications.
Extremity dysfunction
Two validated questionnaires will be used to assess the occurrence of upper and lower extremity dysfunction. Upper extremity function will be measured with the Quick Disabilities of Arm, Shoulder and Hand (QuickDASH) score25 measured at baseline (before PCI) and at 1 months follow-up. Lower extremity function will be measured with the Lower Extremity Functional Scale (LEFS).26 Both questionnaires are valid, reliable and responsive to monitor and assess pain and function of the extremities.
Follow-up
Follow-up will be performed 1 month after index procedure discharge by either phone call or outpatient clinic visit. MACE and access site bleeding or vascular complications will be documented. Extremity function and discomfort will be assessed, using the aforementioned scores. AE’s will be monitored from inclusion to 1-month follow-up and will be assessed by an independent DSMB, composed of two experienced cardiologists and one statistician, reviewing patient safety and study integrity.
Sample size calculation and statistics
Based on a superiority design with a type 1 error of 5% and a power of 80%, assuming the proportion of access site related bleeding or vascular complication to be 3.5% with radial access and 11.3% with femoral access, a total of 352 patients (using a sampling ratio of 1) will be needed.17 Taking into account a 10% rate lost to follow-up, a total of 388 patients will be needed. Data will be analysed according to the intention-to-treat analysis. All statistical tests will be two tailed, and a p<0.05 will be considered statistically significant. All statistical analyses will be performed with SPSS 26. For our primary objective, we will use the Pearson X2 test. The Pearson X2 test will also be used for our secondary objectives with binary outcomes. For our secondary objectives with continuous variables, we will use the Student’s t-test (normally distributed) or the Mann-Whitney U test (non-normally distributed). A prespecified battery of subgroup analyses will be performed as well, including several independent risk factors for clinically significant bleeding and vascular complications. For demographics and baseline characteristics, these subgroups consist of age ≥75 years, female sex, low body weight (body mass index <18.5), hypertension, peripheral arterial disease, left ventricular ejection fraction <30%, severe renal dysfunction (Modification of Diet in Renal Disease <30 mL/1.73 m2) and pre-existent anaemia (haemoglobin <110 g/L).12 27–32 For procedural characteristics, subgroup analyses will be performed for use of secondary access site, ultrasound guided puncture, ACT >150 s right before sheath removal and use of closure device.33–36 In addition, primary and secondary endpoints will be specified for the entire population as well as for each group of complex lesions separately (CTO, left main disease, complex bifurcation and heavy calcification). Statistical analysis will be performed by an independent contract research organisation (Diagram BV, Zwolle, the Netherlands).
Ethics and dissemination
Ethical approval for the study was granted by the local Ethics Committee (‘Medisch Ethische Toetsing Commissie Isala Zwolle’ for all Dutch sites, ‘Commissie voor medische ethiek ZNA’ for ZNA Middelheim, ‘Comité Medische Ethiek Ziekenhuis Oost-Limburg’ for Hospital Oost-Limburg, ‘Comité d’éthique CHU-Charleroi—ISPPC’ for Centre Hospilatier Universitaire de Charleroi, ‘Commission cantonale d'éthique de la recherche CCER—Republique et Canton de Geneve’ for Geneva University Hospital, ‘Ethik Kommission de Ärztekammer Nordrhein’ for Elisabeth-Krankenhaus and ‘Riverside Research Ethics Committee’ for Frimley NHS) after reviewing the protocol, site-specific informed consent forms (local language and English versions, see also (online supplementary file 2), participant education and recruitment materials, other requested documents and any subsequent modifications. Trained research nurses or physicians directly involved in the trial will introduce the trial to eligible patients. Patients will also a receive patient information form (PIF). The research nurse or physician will discuss the trial with patients in light of the information provided in the PIF and will obtain written consent from patients willing to participate in the trial. No reimbursement is provided to study participants. All study-related information will be stored securely at the study site. All participant information will be stored in locked file cabinets in areas with limited access. All reports, data collection, process and administrative forms will be identified by a coded identification-number only to maintain participant confidentiality. All records that contain names or other personal identifiers, such as locator forms and informed consent forms, will be stored separately from study records identified by code number. All local databases will be secured with password-protected access systems. Safety and progress reports to the EC’s will be made at least annually and within 3 months of study termination or completion. These reports will include the total number of participants enrolled and summaries of the DSMB. Any modifications to the protocol which may have impact on the conduct of the study, potential benefit of the patient or may affect patient safety, including changes of study objectives, study design, patient population, sample sizes, study procedures or significant administrative aspects will require a formal amendment to the protocol. Such amendment will have to be approved by the Ethics Committee prior to implementation. The study findings will be disseminated via publication of peer-reviewed manuscripts and presentations at international conferences, as well as through media publications. Results will be published irrespective of whether the findings are positive or negative.
bmjopen-2020-038042supp002.pdf (96.6KB, pdf)
Patient and public involvement
No patient involved.
Discussion
TRA is nowadays the standard for PCI, mainly driven by the lower risk of bleeding and vascular complications compared with TFA, with even a mortality benefit in patients with ACS.2 3 37 38 Randomised data in patients with stable coronary artery disease are limited and more heterogeneous, and show less beneficial effect of radial over femoral access.1 39 40 Moreover, complex coronary lesions are absent or at least not specifically described in most trials supporting current guidelines on myocardial revascularisation. Currently, the femoral artery is still considered the preferred access site for complex PCI by many operators,10 15 41–43 despite the increased risk of bleeding and vascular complications, especially when large bore guiding catheters (≥7 Fr) are required.10 44–47 During CTO-PCI, the use of large-bore guiding catheters has been reported in 60%–70% of cases and is associated with a higher procedural success rate.8 15 Large-bore guiding catheters have better materials' compatibility, especially when using guide extensions and microcatheters. The use of CrossBoss/Stingray (Boston Scientific, Marlborough, Massachusetts, USA) for antegrade dissection/re-entry technique is only possible with large-bore guiding catheters.48 Although registries show increased temporal adoption of TRA for PCI of heavily calcified lesions with use of rotational atherectomy with similar procedural success rates and less bleeding, TFA is still used in a large proportion of these procedures, which often mandate large bore guiding catheters especially for accommodating larger burr sizes.49 50 Application of large-bore guiding catheters for complex PCI of left main and true bifurcations is advocated by experts, though efficacy and safety data are lacking. Limited data show comparable feasibility of TRA versus TFA for left main as well as bifurcation PCI with a tendency towards less bleeding complications.10 51–57
The most important argument to refrain from TR PCI for complex coronary lesions is the limited diameter of the radial artery. Current standard 7 Fr radial sheaths have an outer diameter of 2.97–3.19 mm.58 As such, the percentage of patients with a radial artery smaller than the outer diameter of a 7 Fr sheath ranges between 29% and 67% in men and between 60% and 85% in women.59 This suggests that using a standard 7 Fr sheath for TRA will result in sheath to artery mismatch in a significant proportion of patients, increasing the risk of vascular complications. Radial artery occlusion (RAO) is the most frequent complication after radial access, with increasing RAO rates with increasing sheath size.60 In most instances, RAO will not lead to any clinical sequelae, however, in rare cases RAO may require intervention because of extremity dysfunction or ischemia.61 62 Moreover, RAO prohibits future recannulation of the radial artery, harvesting the radial artery as conduit for CABG or creating a haemodialysis shunt.63 Other arguments to use the femoral artery for complex PCI have been suggested, such as improved back-up with potential higher procedural success rates and shorter procedural time and lower radiation dose. However, this is not supported by observational data showing similar effectiveness, procedural success rates, cross-over rates, radiation dose and contrast use for TRA and TFA.10 15 16 38
Several technologies have been developed to facilitate large bore access through the radial artery.64 A sheathless approach for example was shown to be a feasible alternative for large bore radial access.65 The 7.5 Fr Eaucath sheathless guiding catheter (ASAHI Intecc, Aichi, Japan) has the same inner diameter as a regular 7 Fr guiding catheter, but an outer diameter of 2.49 mm, resulting in a large reduction in outer diameter (approximately 2 Fr) compared with a standard 7 Fr sheath.66 However, PCI with sheathless guiding catheters requires specific experience due to the highly hydrophilic coating, and limited evidence exists regarding the true impact on RAO.67 68 Miniaturisation of TR equipment can also be achieved through a sheath-based approach. Thanks to a reduction in sheath wall thickness (‘slender technology’), thin-walled sheaths have reduced their outer diameter while maintaining the same inner diameter. The 7 Fr Glidesheath Slender (Terumo, Japan) is the first commercially available 7 Fr thin-walled sheath, combining an inner diameter of 2.46 mm, compatible with any 7 Fr guiding catheter, with a reduced outer diameter of 2.79 mm. A recent prospective multicenter study has shown the feasibility and safety of using the 7 Fr Glidesheath Slender for complex TR-PCI in daily practice with a high rate of procedural success and low rate of vascular complications.13
In the literature, several outcome measures have been used to evaluate access site related bleeding complications, such as the TIMI,69 the Global Utilisation of Streptokinase and Tissue plasminogen activator for Occluded coronary arteries (GUSTO)70 or BARC.22 Access site haematoma size has also been used as an outcome measure in studies comparing radial with femoral access. BARC bleeding ≥2 has shown to independently predict 1-year mortality and capture more clinically significant bleeding than TIMI minor/major and GUSTO moderate/severe criteria.22 23 Importantly, haematoma size alone, not meeting criteria for other bleeding outcome measures, has not shown any association with clinically relevant endpoints.71 The current trial will use the BARC bleeding score for the primary outcome measure to detect a clinically relevant difference in bleedings between TRA and TFA for complex PCI, adjudicated by a CEC. Besides bleeding and vascular complications, vascular access may also have a potential effect on extremity function.72 73 Although upper extremity dysfunction is present in a small proportion of patients after TRA, it can lead to important morbidity for the affected patients.72–75 Extremity dysfunction may be more pronounced in patients with large-bore access. In addition, current literature does not provide an insight around prevalence and significance of lower extremity function after TFA.73 Therefore, we will assess the occurrence of extremity dysfunction using the QuickDASH and LEFS questionnaires, which will be valuable information for both patients and doctors.
In conclusion, The COLOR trial is the first prospective multicentre randomised trial comparing TRA with TFA using large-bore guiding catheters for complex PCI. Currently, 290 patients are randomised. The results of this trial will provide important insights about the safety and efficacy of large-bore TRA and TFA for complex PCI. If this trial can show that TRA is not only as effective but also safer (less clinically relevant bleeding and vascular complications) in complex large bore PCI, it has a potential impact on daily practice.
Supplementary Material
Footnotes
Twitter: @Jo Dens
TAM and AA contributed equally.
Contributors: MAHvL and AA substantially contributed to conception and design of the study protocol. TAM, AA, KT, MvW, TS, RJvdS, MTD, JFI, PA, JD, PK, SR and MAHvL contributed to acquisition of data. TAM, AA and MAHvL contributed to analysis of data. TAM, AA, MAHvL and NvR contributed to interpretation of data. TAM, AA and MAHvL reviewed the literature, contributed to the design and wrote the draft of the manuscript. TAM, AA, KT, MAHvL, TS, RJvdS, MTD, JFI, PA, JD, PK, SR, JPO, J-HED, VR, ATMG, RSH, NvR and MAHvL contributed to refinement of the study protocol and approved the final manuscript.
Funding: Terumo EMEA (Leuven, Belgium) supported this investigator-initiated study by an unrestricted grant.
Competing interests: MAHvL, AA and and JFI are consultants for Terumo. JFI and TS have received honoraria/speakers fee for Terumo, the other authors have no conflicts of interest to declare.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Ferrante G, Rao SV, Jüni P, et al. Radial versus femoral access for coronary interventions across the entire spectrum of patients with coronary artery disease: a meta-analysis of randomized trials. JACC Cardiovasc Interv 2016;9:1419–34. 10.1016/j.jcin.2016.04.014 [DOI] [PubMed] [Google Scholar]
- 2.Jolly SS, Yusuf S, Cairns J, et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (rival): a randomised, parallel group, multicentre trial. Lancet 2011. [DOI] [PubMed] [Google Scholar]
- 3.Valgimigli M, Frigoli E, Leonardi S, et al. Radial versus femoral access and bivalirudin versus unfractionated heparin in invasively managed patients with acute coronary syndrome (MATRIX): final 1-year results of a multicentre, randomised controlled trial. Lancet 2018;392:835–48. 10.1016/S0140-6736(18)31714-8 [DOI] [PubMed] [Google Scholar]
- 4.Sousa-Uva M, Neumann FJ, Ahlsson A, et al. ESC/EACTS guidelines on myocardial revascularization. Eur J Cardiothorac Surg 2018;2019. [Google Scholar]
- 5.Santas E, Bodí V, Sanchis J, et al. The left radial approach in daily practice. A randomized study comparing femoral and right and left radial approaches. Rev Española Cardiol 2009. [DOI] [PubMed] [Google Scholar]
- 6.Louvard Y, Benamer H, Garot P, et al. Comparison of transradial and transfemoral approaches for coronary angiography and angioplasty in octogenarians (the OCTOPLUS study). Am J Cardiol 2004;94:1177–80. 10.1016/j.amjcard.2004.07.089 [DOI] [PubMed] [Google Scholar]
- 7.Burzotta F, De Vita M, Lefevre T, et al. Radial approach for percutaneous coronary interventions on chronic total occlusions: technical issues and data review. Catheter Cardiovasc Interv 2014;83:47–57. 10.1002/ccd.25118 [DOI] [PubMed] [Google Scholar]
- 8.Tanaka Y, Moriyama N, Ochiai T, et al. Transradial coronary interventions for complex chronic total occlusions. JACC Cardiovasc Interv 2017;10:235–43. 10.1016/j.jcin.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 9.Galassi AR, Tomasello SD, Reifart N, et al. In-Hospital outcomes of percutaneous coronary intervention in patients with chronic total occlusion: insights from the ERCTO (European registry of chronic total occlusion) registry. EuroIntervention 2011;7:472–9. 10.4244/EIJV7I4A77 [DOI] [PubMed] [Google Scholar]
- 10.Chung S, Her S-H, Song PS, et al. Trans-radial versus trans-femoral intervention for the treatment of coronary bifurcations: results from coronary bifurcation stenting registry. J Korean Med Sci 2013;28:388. 10.3346/jkms.2013.28.3.388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smilowitz NR, Kirtane AJ, Guiry M, et al. Practices and complications of vascular closure devices and manual compression in patients undergoing elective transfemoral coronary procedures. 110 American Journal of Cardiology, 2012: 177–82. 10.1016/j.amjcard.2012.02.065 [DOI] [PubMed] [Google Scholar]
- 12.Kinnaird TD, Stabile E, Mintz GS, et al. Incidence, predictors, and prognostic implications of bleeding and blood transfusion following percutaneous coronary interventions. Am J Cardiol 2003;92:930–5. 10.1016/S0002-9149(03)00972-X [DOI] [PubMed] [Google Scholar]
- 13.Aminian A, Iglesias JF, Van Mieghem C, et al. First prospective multicenter experience with the 7 French Glidesheath slender for complex transradial coronary interventions. Catheter Cardiovasc Interv 2017;89:1014–20. 10.1002/ccd.26773 [DOI] [PubMed] [Google Scholar]
- 14.Megaly M, Karatasakis A, Abraham B, et al. Radial versus femoral access in chronic total occlusion percutaneous coronary intervention. Circ Cardiovasc Interv 2019;12:e007778. 10.1161/CIRCINTERVENTIONS.118.007778 [DOI] [PubMed] [Google Scholar]
- 15.Bakker EJ, Maeremans J, Zivelonghi C, et al. Fully transradial versus transfemoral approach for percutaneous intervention of coronary chronic total occlusions applying the hybrid algorithm: insights from RECHARGE registry. Circ Cardiovasc Interv 2017;10. 10.1161/CIRCINTERVENTIONS.117.005255 [DOI] [PubMed] [Google Scholar]
- 16.De Maria GL, Burzotta F, Trani C, et al. Trends and outcomes of radial approach in Left-Main bifurcation percutaneous coronary intervention in the drug-eluting stent era: a two-center registry. J Invasive Cardiol 2015;27:125–36. [PubMed] [Google Scholar]
- 17.Rathore S, Hakeem A, Pauriah M, et al. A comparison of the transradial and the transfemoral approach in chronic total occlusion percutaneous coronary intervention. Catheter Cardiovasc Interv 2009;73:883–7. 10.1002/ccd.21922 [DOI] [PubMed] [Google Scholar]
- 18.Stone GW, Reifart NJ, Moussa I, et al. Percutaneous recanalization of chronically occluded coronary arteries: a consensus document: Part II. Circulation 2005;112:2530–7. 10.1161/CIRCULATIONAHA.105.583716 [DOI] [PubMed] [Google Scholar]
- 19.Sianos G, Morel M-A, Kappetein AP, et al. The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention 2005. [PubMed] [Google Scholar]
- 20.Zlotnick DM, Ramanath VS, Brown JR, et al. Classification and treatment of coronary artery bifurcation lesions: putting the Medina classification to the test. Cardiovasc Revasc Med 2012;13:228–33. 10.1016/j.carrev.2012.04.002 [DOI] [PubMed] [Google Scholar]
- 21.Matts JP, Lachin JM. Properties of permuted-block randomization in clinical trials. Control Clin Trials 1988. [DOI] [PubMed] [Google Scholar]
- 22.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research Consortium. Circulation 2011;123:2736–47. 10.1161/CIRCULATIONAHA.110.009449 [DOI] [PubMed] [Google Scholar]
- 23.Vranckx P, White HD, Huang Z, et al. Validation of BARC bleeding criteria in patients with acute coronary syndromes: the tracer trial. J Am Coll Cardiol 2016;67:2135–44. 10.1016/j.jacc.2016.02.056 [DOI] [PubMed] [Google Scholar]
- 24.Wilson SJ, Mitchell A, Gray TJM, et al. Patent haemostasis prevents radial artery occlusion in patients with an acute coronary syndrome. Int J Cardiol 2017;240:78–81. 10.1016/j.ijcard.2017.03.041 [DOI] [PubMed] [Google Scholar]
- 25.Beaton DE, Wright JG, Katz JN, et al. Development of the QuickDASH: comparison of three item-reduction approaches. J Bone Joint Surg Am 2005;87:1038–46. 10.2106/JBJS.D.02060 [DOI] [PubMed] [Google Scholar]
- 26.Binkley J, Stratford P, Lott S, et al. The lower extremity functional scale. Phys Ther 1999. [PubMed] [Google Scholar]
- 27.Numasawa Y, Kohsaka S, Ueda I, et al. Incidence and predictors of bleeding complications after percutaneous coronary intervention. J Cardiol 2017;69:272–9. 10.1016/j.jjcc.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 28.Numasawa Y, Kohsaka S, Miyata H, et al. Impact of body mass index on in-hospital complications in patients undergoing percutaneous coronary intervention in a Japanese real-world multicenter registry. PLoS One 2015;10:e0124399. 10.1371/journal.pone.0124399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh M, Lennon RJ, Darbar D, et al. Effect of peripheral arterial disease in patients undergoing percutaneous coronary intervention with intracoronary stents. Mayo Clin Proc 2004;79:1113–8. 10.1016/S0025-6196(11)62592-5 [DOI] [PubMed] [Google Scholar]
- 30.Ndrepepa G, Groha P, Lahmann AL, et al. Increased bleeding risk during percutaneous coronary interventions by arterial hypertension. Catheter Cardiovasc Interv 2016;88:184–90. 10.1002/ccd.26272 [DOI] [PubMed] [Google Scholar]
- 31.Mamas MA, Anderson SG, O'Kane PD, et al. Impact of left ventricular function in relation to procedural outcomes following percutaneous coronary intervention: insights from the British cardiovascular intervention Society. Eur Heart J 2014;35:3004–12. 10.1093/eurheartj/ehu303 [DOI] [PubMed] [Google Scholar]
- 32.Urban P, Mehran R, Colleran R, et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: a consensus document from the academic research Consortium for high bleeding risk. Eur Heart J 2019;40:2632–53. 10.1093/eurheartj/ehz372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seto AH, Abu-Fadel MS, Sparling JM, et al. Real-time ultrasound guidance facilitates femoral arterial access and reduces vascular complications: FAUST (femoral arterial access with ultrasound trial). JACC Cardiovasc Interv 2010;3:751–8. 10.1016/j.jcin.2010.04.015 [DOI] [PubMed] [Google Scholar]
- 34.Bangalore S, Bhatt DL. Femoral arterial access and closure. Circulation 2011;124 10.1161/CIRCULATIONAHA.111.032235 [DOI] [PubMed] [Google Scholar]
- 35.Kern MJ. Interventional cardiac catheterization handbook. Interv Card Catheter Handb 1977. [Google Scholar]
- 36.Tavris DR, Wang Y, Jacobs S, et al. Bleeding and vascular complications at the femoral access site following percutaneous coronary intervention (PCI): an evaluation of hemostasis strategies. J Invasive Cardiol 2012;24:328–34. [PubMed] [Google Scholar]
- 37.Bernat I, Horak D, Stasek J, et al. ST-segment elevation myocardial infarction treated by radial or femoral approach in a multicenter randomized clinical trial: the STEMI-RADIAL trial. J Am Coll Cardiol 2014. [DOI] [PubMed] [Google Scholar]
- 38.Romagnoli E, Biondi-Zoccai G, Sciahbasi A, et al. Radial versus femoral randomized investigation in ST-segment elevation acute coronary syndrome: the RIFLE-STEACS (radial versus femoral randomized investigation in ST-elevation acute coronary syndrome) study. J Am Coll Cardiol 2012;60:2481–9. 10.1016/j.jacc.2012.06.017 [DOI] [PubMed] [Google Scholar]
- 39.Diehl D, de Ribamar Costa J, Costa R, et al. Propensity-score comparison of patients with stable coronary artery disease undergoing percutaneous coronary intervention by radial versus femoral approach. J Am Coll Cardiol 2016. [Google Scholar]
- 40.Rao SV, Hess CN, Barham B, et al. A registry-based randomized trial comparing radial and femoral approaches in women undergoing percutaneous coronary intervention: the SAFE-PCI for women (study of access site for enhancement of PCI for women) trial. JACC Cardiovasc Interv 2014;7:857–67. 10.1016/j.jcin.2014.04.007 [DOI] [PubMed] [Google Scholar]
- 41.Koifman E, Gaglia MA, Escarcega RO, et al. Comparison of transradial and transfemoral access in patients undergoing percutaneous coronary intervention for complex coronary lesions. Catheter Cardiovasc Interv 2017;89:640–6. 10.1002/ccd.26669 [DOI] [PubMed] [Google Scholar]
- 42.Alaswad K, Menon RV, Christopoulos G, et al. Transradial approach for coronary chronic total occlusion interventions: insights from a contemporary multicenter registry. Catheter Cardiovasc Interv 2015;85:1123–9. 10.1002/ccd.25827 [DOI] [PubMed] [Google Scholar]
- 43.Watt J, Austin D, Mackay D, et al. Radial versus femoral access for rotational atherectomy: a UK observational study of 8622 patients. Circ Cardiovasc Interv 2017;10. 10.1161/CIRCINTERVENTIONS.117.005311 [DOI] [PubMed] [Google Scholar]
- 44.Doyle BJ, Ting HH, Bell MR, et al. Major femoral bleeding complications after percutaneous coronary intervention: incidence, predictors, and impact on long-term survival among 17,901 patients treated at the Mayo clinic from 1994 to 2005. JACC Cardiovasc Interv 2008;1:202–9. 10.1016/j.jcin.2007.12.006 [DOI] [PubMed] [Google Scholar]
- 45.Goel PK, Jatain S, Khanna R, et al. Left main PCI: an observational analysis from large single-centre experience. Indian Heart J 2016;68:36–42. 10.1016/j.ihj.2015.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gorol J, Tajstra M, Hudzik B, et al. Comparison of outcomes in patients undergoing rotational atherectomy after unsuccessful coronary angioplasty versus elective rotational atherectomy. Postepy Kardiol Interwencyjnej 2018;14:128–34. 10.5114/aic.2018.76403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kinnaird T, Anderson R, Ossei-Gerning N, et al. Vascular access site and outcomes among 26,807 chronic total coronary occlusion angioplasty cases from the British cardiovascular interventions Society national database. JACC Cardiovasc Interv 2017;10:635–44. 10.1016/j.jcin.2016.11.055 [DOI] [PubMed] [Google Scholar]
- 48.Maeremans J, Palmers P-J, Dens J. Initial experience and feasibility of the new low-profile stingray catheter as part of the antegrade dissection and re-entry revascularization strategy for coronary chronic total occlusions. Am J Case Rep 2017;18:104–9. 10.12659/AJCR.902178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kinnaird T, Cockburn J, Gallagher S, et al. Temporal changes in radial access use, associates and outcomes in patients undergoing PCI using rotational atherectomy between 2007 and 2014: results from the British cardiovascular intervention Society national database. Am Heart J 2018;198:46–54. 10.1016/j.ahj.2018.01.001 [DOI] [PubMed] [Google Scholar]
- 50.Yin W-H, Tseng C-K, Tsao T-P, et al. Transradial versus transfemoral rotablation for heavily calcified coronary lesions in contemporary drug-eluting stent era. J Geriatr Cardiol 2015;12:489–96. 10.11909/j.issn.1671-5411.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang Y-J, Kandzari DE, Gao Z, et al. Transradial versus transfemoral method of percutaneous coronary revascularization for unprotected left main coronary artery disease: comparison of procedural and late-term outcomes. JACC Cardiovasc Interv 2010;3:1035–42. 10.1016/j.jcin.2010.09.003 [DOI] [PubMed] [Google Scholar]
- 52.Kinnaird T, Anderson R, Gallagher S, et al. Access Site and outcomes for unprotected left main stem percutaneous coronary intervention: an analysis of the British cardiovascular intervention society database. JACC Cardiovasc Interv 2018;11:2480–91. 10.1016/j.jcin.2018.09.035 [DOI] [PubMed] [Google Scholar]
- 53.Ziakas A, Klinke P, Mildenberger R, et al. Comparison of the radial and femoral approaches in left main PCI: a retrospective study. J Invasive Cardiol 2004;16:129–32. [PubMed] [Google Scholar]
- 54.Gao Z, Xu B, Yang Y, et al. Transradial versus transfemoral method of two-stent implantation for true bifurcation lesions: comparison of immediate and long-term outcomes. J Interv Cardiol 2014;27:99–107. 10.1111/joic.12095 [DOI] [PubMed] [Google Scholar]
- 55.Hsueh S-K, Hsieh Y-K, Wu C-J, et al. Immediate results of percutaneous coronary intervention for unprotected left main coronary artery stenoses: transradial versus transfemoral approach. Chang Gung Med J 2008;31:190–200. [PubMed] [Google Scholar]
- 56.Chung S, Yang JH, Choi S-H, et al. Transradial versus transfemoral intervention for the treatment of left main coronary bifurcations: results from the COBIS (coronary bifurcation stenting) II registry. J Invasive Cardiol 2015;27:35–40. [PubMed] [Google Scholar]
- 57.Williams PD, Eichhöfer J, Mamas MA, et al. Transradial intervention via large-bore guide catheters: a study of coronary bifurcation disease treatment using the crush technique. J Invasive Cardiol 2013;25:455–9. [PubMed] [Google Scholar]
- 58.Bernat I, Aminian A, Pancholy S, et al. Best practices for the prevention of radial artery occlusion after transradial diagnostic angiography and intervention: an international consensus paper. 12 JACC: Cardiovascular Interventions, 2019: 2235–46. 10.1016/j.jcin.2019.07.043 [DOI] [PubMed] [Google Scholar]
- 59.Saito S, Ikei H, Hosokawa G, et al. Influence of the ratio between radial artery inner diameter and sheath outer diameter on radial artery flow after transradial coronary intervention. Catheter Cardiovasc Interv 1999;46:173–8. [DOI] [PubMed] [Google Scholar]
- 60.Kotowycz MA, Dzavík V, Dẑavík V. Radial artery patency after transradial catheterization. Circ Cardiovasc Interv 2012;5:127–33. 10.1161/CIRCINTERVENTIONS.111.965871 [DOI] [PubMed] [Google Scholar]
- 61.Rademakers LM, Laarman GJ. Critical hand ischaemia after transradial cardiac catheterisation: an uncommon complication of a common procedure. Neth Heart J 2012;20:372–5. 10.1007/s12471-012-0276-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ayan M, Smer A, Azzouz M, et al. Hand ischemia after transradial coronary angiography: resulting in right ring finger amputation. Cardiovasc Revasc Med 2015;16:367–9. 10.1016/j.carrev.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 63.Amin H. Prevention of radial artery occlusion: it's the right thing to do. EuroIntervention 2015;11:731–3. 10.4244/EIJV11I7A148 [DOI] [PubMed] [Google Scholar]
- 64.Kiemeneij F, Yoshimachi F, Matsukage T, et al. Focus on maximal miniaturisation of transradial coronary access materials and techniques by the slender Club Japan and Europe: an overview and classification. EuroIntervention 2015;10:1178–86. 10.4244/EIJY14M09_09 [DOI] [PubMed] [Google Scholar]
- 65.Mamas MA, Fath-Ordoubadi F, Fraser DG. Atraumatic complex transradial intervention using large bore sheathless guide catheter. Catheter Cardiovasc Interv 2008;72:357–64. 10.1002/ccd.21637 [DOI] [PubMed] [Google Scholar]
- 66.Fraser D, Mamas MA. Transradial Sheathless approach for PCI. Curr Cardiol Rep 2015;17:47. 10.1007/s11886-015-0597-5 [DOI] [PubMed] [Google Scholar]
- 67.Horie K, Tada N, Isawa T, et al. A randomised comparison of incidence of radial artery occlusion and symptomatic radial artery spasm associated with elective transradial coronary intervention using 6.5 Fr SheathLess Eaucath guiding catheter vs. 6.0 Fr Glidesheath slender. 13 EuroIntervention, 2018: 2018–25. 10.4244/EIJ-D-17-00239 [DOI] [PubMed] [Google Scholar]
- 68.Mohsen A, Alqasrawi M, Shantha GPS, et al. Comparison of radial artery occlusion following Transradial access for percutaneous coronary intervention using Sheath-based versus Sheathless technique. Sci Rep 2018;8 10.1038/s41598-018-30462-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chesebro JH, Knatterud G, Roberts R, et al. Thrombolysis in myocardial infarction (TIMI) trial, phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase. clinical findings through hospital discharge. Circulation 1987;76:142–54. 10.1161/01.CIR.76.1.142 [DOI] [PubMed] [Google Scholar]
- 70.GUSTO investigators An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993;329:673–82. 10.1056/NEJM199309023291001 [DOI] [PubMed] [Google Scholar]
- 71.White HD, Aylward PE, Gallo R, et al. Hematomas of at least 5 cm and outcomes in patients undergoing elective percutaneous coronary intervention: insights from the safety and efficacy of enoxaparin in PCI patients, an internationaL randomized evaluation (steeple) trial. Am Heart J 2010;159:110–6. 10.1016/j.ahj.2009.10.034 [DOI] [PubMed] [Google Scholar]
- 72.van Leeuwen MAH, Hollander MR, van der Heijden DJ, et al. The ACRA anatomy study (assessment of disability after coronary procedures using radial access): a comprehensive anatomic and functional assessment of the vasculature of the hand and relation to outcome after Transradial catheterization. Circ Cardiovasc Interv 2017;10. 10.1161/CIRCINTERVENTIONS.117.005753 [DOI] [PubMed] [Google Scholar]
- 73.Ul Haq MA, Rashid M, Kwok CS, et al. Hand dysfunction after transradial artery catheterization for coronary procedures. World J Cardiol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ijsselmuiden A, Zwaan E, Kofflard M, et al. TCT-639 Upper extremity function after transradial PCI:preliminary long term results of the ARCUS trial. J Am Coll Cardiol 2017. [Google Scholar]
- 75.Zwaan EM, Koopman AGMM, Holtzer CAJ, et al. Revealing the impact of local access-site complications and upper extremity dysfunction post transradial percutaneous coronary procedures. Neth Heart J 2015;23:514–24. 10.1007/s12471-015-0747-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-038042supp001.pdf (85.1KB, pdf)
bmjopen-2020-038042supp002.pdf (96.6KB, pdf)


