Abstract
Purpose:
Although adoptive cell therapy with chimeric antigen receptor (CAR)-engineered T cells has shown durable clinical efficacy in patients with CD19+ B-cell malignancies, the application of this approach to solid tumors is challenging. The goal of this proof-of-concept study was to investigate whether loading of CD19-CAR T cells (CART19) with anti-HER2 or anti-EGFR bispecific antibodies (BiAb) will target HER2+/EGFR+ CD19- targets and signal the intracellular domain of CAR without engaging antigen-specific CD19 ScFv of CAR T cells.
Methods:
We used CART19 armed with anti-CD3 (OKT3) × anti-HER2 BiAb (HER2Bi) or anti-CD3 (OKT3) × anti-EGFR BiAb (EGFRBi) to evaluate the cytotoxicity directed at HER2 or EGFR expressing cancer cell lines compared with unarmed CART19 measured by short-term 51Cr release assay and long-term real-time cell analysis using xCelligence. We also determined the differences in exhaustion or effector phenotypes and cytokine profiles during the short- and long-term cytotoxicity assays.
Results:
Specific cytotoxicity was exhibited by CART19 armed with HER2Bi or EGFRBi against multiple tumor cell lines. Armed CART19 and armed activated T cells (ATC) showed comparable specific cytotoxicity that ranged between 10 and 90% against breast, pancreatic, ovarian, prostate, and lung cancer cell lines at 10:1 E/T ratio. Serial killing (repeated killing) by HER2Bi-armed CART19 ranged between 80 and 100% at 10:1 E/T ratio against MCF-7 cells up to 19 days (up to 4th round of repeated killing) measured by a real-time cell analysis without CART19 becoming exhausted.
Conclusions:
HER2Bi- or EGFRBi-armed CART19 exhibited specific cytotoxicity against multiple HER2+/EGFR+/CD19− tumor targets in overnight and long-term serial killing assays. CART19 showed improved survival and were resistant to exhaustion after prolonged repeated exposure to tumor cells.
Keywords: Pancreatic cancer, breast cancer, CAR T cells, bispecific antibody, Th1 cytokines
Introduction
Adoptive transfer of chimeric antigen receptor (CAR)-engineered T cells has demonstrated robust and unprecedented clinical efficacy in patients with CD19+ B-cell malignancies (1–4). Recent FDA approval of CAR T cell therapies for the treatment of CD19+ malignancies establishes that the use of CAR T cell-based therapy can be a clinical option for the treatment of other diseases. Substantial efforts are being invested in improving design and manufacturing of engineered CAR T cells to be effective against solid tumors. Similar to infusions of bispecific antibody (BiAb) armed activated T cells (ATC) in solid tumors (5–7), genetically engineered T cells that express CAR may target the tumor cells, release Th1 cytokines, and induce specific endogenous immune responses while avoiding the restriction of major histocompatibility complex (MHC). In this proof-of-concept study, we asked a question whether loading CD19-CAR T cells (CART19) with OKT3 × anti-HER2 bispecific antibody (HER2Bi) or OKT3 × anti-EGFR bispecific antibody (EGFRBi) can redirect the antigen-specific cytotoxicity against HER2 and EGFR expressing solid tumor targets while retaining intracellular signaling of CAR domain. An advantage of bispecific antibody (BiAb) loading approach is that commercial antibodies (Herceptin, cetuximab, etc.) can be easily derivatized into BiAbs with anti-CD3 monoclonal antibody (OKT3) as the TCR-binding partner and the anti-tumor-associated antigen monoclonal antibody as the tumor-binding partner. The BiAb retargeting strategy utilizes the high-affinity humanized monoclonal antibody against target antigen to produce BiAb with OKT3 to redirect the non-MHC restricted, perforin/granzyme-mediated cytotoxicity of target cells.
This study shows that BiAb-armed CART19 cells exhibit robust target-specific killing of multiple cells lines, showed enhanced killing when two antigens were sequentially targeted, proliferated and were serially able to kill target cells up to 3 weeks without engaging the CD19 CAR, secreted Th1 cytokines and chemokines, and did not display exhaustion receptors. Retargeting of CART cells with BiAb provides a new alternative approach for leveraging the “horsepower” of the CART and pre-manufacturing BiAb to improve the T cell killing machine in solid tumors.
Materials and methods
Chimeric antigen receptor T cells (CART) and culture of CART19 cells.
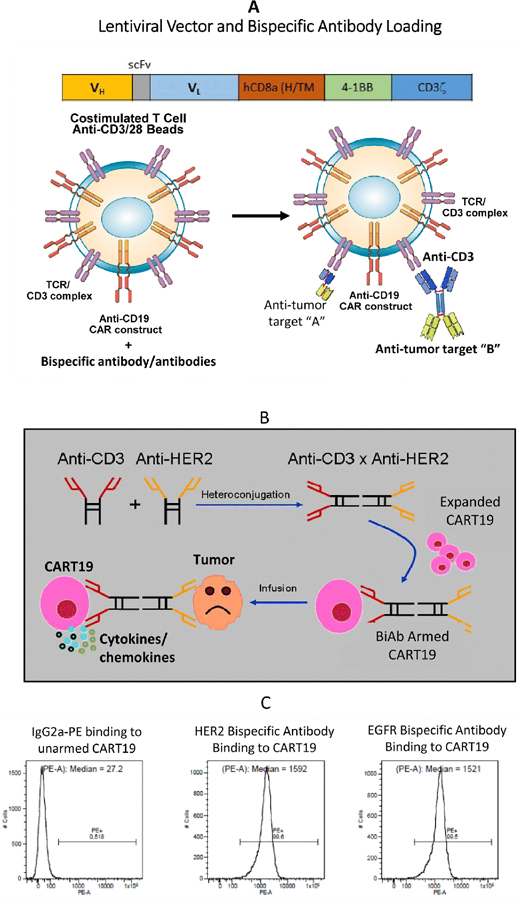
Lentiviral vector (LV) directing expression of anti-CD19 scFv derived from FMC63 murine monoclonal antibody, human CD8α hinge and transmembrane domains, and human 4–1BB and CD3ζ signaling domains (CD19BBz) as described previously (1). CART19 were produced by the laboratory of Dr. Carl H. June (University of Pennsylvania, PA) by transducing T cells with CD19BBz. T cells were stimulated with anti-CD3/CD28 magnetic beads and transduced by LV at a multiplicity of infection (MOI) of 5 and expanded by adding human IL-2 every other day to a final concentration of 50 IU/ml to generate CART19. Schematic representation of the CART19 and arming of transduced cells with BiAbs are shown in Fig. 1A, 1B. The CART19 cells were thawed and expanded in low-dose IL-2 until needed for the various assays.
Figure 1.
A) A basic structure for a CART19 scFv and intracellular signaling domain. The chimeric intracellular signaling molecule includes a human CD8α hinge/ transmembrane domain, and intracellular 4–1BB and CD3z domains. B) Cartoon showing production of the BiAb by chemical heteroconjugation of whole IgG molecules using complementary linkers at the Fc region of the IgG. CART19 cells armed with BiAb can exhibit non-MHC restricted cytotoxicity against target cells and produce cytokines/chemokines. C) Flow cytometry analysis showing binding of BiAb when CART19 cells were armed with either HER2Bi or EGFRBi by detecting OKT3 on CART19 cells.
Activated T cells (ATC) culture.
ATC were produced by soluble OKT3 (20 ng/ml) stimulation of peripheral blood mononuclear cells in RPMI 1640 medium containing 5% human serum and expanded in low-dose IL-2 (100 IU/106 T cells) for 14 days (8, 9) prior to arming for the specific cytotoxicity.
Production of bispecific antibodies.
For the production of the BiAbs, anti-CD3 was linked with anti-TAA monoclonal antibody (i.e. anti-HER2, anti-EGFR or anti-CD20) at the Fc region of the monoclonal IgG. All three BiAbs, anti-CD3 × anti-CD20 (Rituxan, a humanized anti-CD20 IgG1, Genentech Inc., South San Francisco, CA), anti-CD3 × anti-HER2 (Herceptin, a humanized anti-HER2 IgG1, Genentech Inc., South San Francisco, CA) anti-CD3 × anti-EGFR (Erbitux, a humanized anti-epidermal growth factor receptor (EGFR), were prepared by chemical heteroconjugation as previously described (10, 11). Briefly, anti-CD3 (OKT3; Centrocor Ortho-Biotech, Raritan, NJ) was cross-linked with Traut’s reagent (2-iminothiolane HCl; Pierce, Rockford, IL) and anti-TAA was cross-linked with sulphosuccinimidyl 4-(N-maleimidomethyl) cyclohexane-1-carboxylate (Sulpho-SMCC). Cross-linked mAb were desalted on PD-10 columns (Pharmacia, Uppsala, Sweden) to remove unbound cross-linker. The cross-linked OKT3 and anti-TAA were heteroconjugated overnight. The heteroconjugated product was analyzed by non-reducing SDS–PAGE (4–20% gradient; Lonza Inc., Walkersville, MA) and quantified by densitometry using Quantity One software (Bio-Rad Lab., Hercules, CA).
Arming of CART19 and ATC cells.
Activated T cells were armed with previously optimized (8) concentration (50 ng/106 ATC) of anti-OKT3 × anti-CD20 BiAb (CD20Bi), anti-OKT3 × anti-HER2 BiAb (HER2Bi) or anti-OKT3 × anti-EGFR BiAb (EGFRBi).
Cytotoxicity by 51Cr release assay.
Cancer cell lines from breast (MD-MB-231[MB231], MCF-7, BT-20, SKBR3), pancreas (L3.6. MiaPaCa-2, HCT-8) prostate (PC3), ovarian (SCOV), lung (H292, A549), melanoma (A375), osteosarcoma (HOS) and glioblastoma (U118, U251) were plated in 96-well flat-bottom microtiter plates at 4 × 104 cells/well and allowed to adhere overnight. The cells were labeled with 51Cr at 20 µCi/mL in the labeling media (50% FBS in complete RPMI-1640) for 5 h at 37°C, and washed with complete RPMI-1640 to remove unincorporated isotope (10, 12). Effectors (unarmed CART19 or ATC and armed CART19 or ATC) were then added at 10:1 effector:target (E:T) ratio. Co-cultures were incubated for 18 h and the supernatant was collected for liquid scintillation counting to quantitate the amount of released 51Cr. Percent cytotoxicity was calculated as follows: (experimental cpm – spontaneous cpm) / (maximum cpm – spontaneous cpm) × 100. Means and standard errors were calculated from four to six replicates per sample.
Real-time monitoring of cytotoxicity by xCELLigence system.
In the Real-Time Cell Analysis (RTCA) system, cytotoxicity is measured by cellular impedance readout as Cell Index (CI) to monitor real-time changes in cell number. This is derived from the relative impedance changes corresponding to cellular coverage of the electrode sensors, normalized to baseline impedance values with medium only. Cell attachment was monitored using the RTCA software until the plateau phase was reached, which was usually after approximately 22–24 h before adding effector cells. We used breast (MCF-7) and pancreatic (MiaPaCa-2) cancer cell lines for xCELLigence RTCA as targets and armed or unarmed CART19 or ATC as effector cells. The target cells (10,000– 20,000 cells/well as optimized for each cell line) were plated in 96-well E-Plates followed by adding effectors at 10:1, 2:1 or 1:1 E/T. The target cell impedance signal was monitored for 24–120 h and data were extrapolated as % cytolysis. Untreated targets or effectors without targets served as controls.
Flow cytometry for the exhaustion and activation markers of CART19 cells co-cultured with tumor targets.
We compared exhausted CART19 (with low cytotoxic activity) with armed and unarmed CART19 cells co-cultured with tumor cells for short-term (24 h) and long-term (2 weeks) incubation. Antibodies used for staining include: anti-CD45, -CD3, -CD4, -CD8 -41BB, -ICOS and -OX40 (BD Biosciences San Jose, CA). CART19 cells were collected and washed before staining for 30 min on ice with mixtures of fluorescently conjugated mAbs or isotype-matched controls, washed twice with FACS buffer, and analyzed. Cells were analyzed on a FACScalibur (BD Biosciences), and data were analyzed using FlowJo software (BD Biosciences). The T cell co-stimulation was analyzed on T cells for receptor expression by gating on 41BB/ICOS/OX40 on CD45+/CD3+/CD4+ or CD45+/CD3+/CD8+ T cells and co-inhibitory receptor expression by gating on CD279/PD-1 on CD45+/CD3+/CD4+ or CD45+/CD3+/CD8+ T cells.
Results
Arming of CART19 with HER2Bi or EGFRBi.
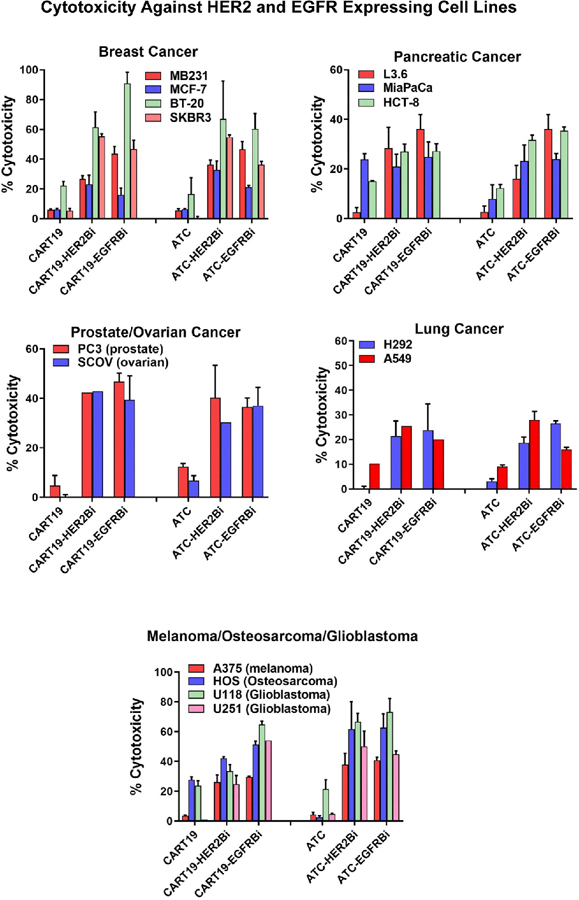
First, we confirmed the HER2Bi or EGFRBi bispecific antibody binding on CART19 by flow cytometry analyses using goat anti-mouse IgG2a-PE (anti-OKT3 antibody). CART19 showed BiAb-positive cells >99% with mean fluorescent intensity (MFI) >1500 (Fig. 1C). Next, we performed the in vitro cytotoxicity studies that showed a robust redirected killing of HER2+ (CD19-) and EGFR+ (CD19-) tumor cells by armed CART19. CART19 either left unarmed or armed with anti-HER2, anti-EGFR or anti-CD20 BiAbs were compared with unarmed and anti-HER2, anti-EGFR or anti-CD20 BiAbs antibodies armed non-CAR T cells (ATC). Specific cytotoxicity of armed or unarmed (without BiAb) CART19 and non-CAR T cells are shown in Fig. 2. Both CART19 and non-CART cells (ATC) demonstrated specific cytotoxicity against the target cells expressing HER2 or EGFR. Specific cytotoxicity by CART19 armed with HER2Bi directed at breast cancer cell lines ranged between 23 and 60% (n = 3) in spite of either low expression of HER2 (MCF-7) or no expression of HER2 (Triple negative [HER2/ER/PR negative cell lines] MB231 and BT-20) in three out of four breast cancer cell lines. CART19 armed with EGFRBi showed mean specific cytotoxicity of 16–90% (n = 3) against breast cancer cell lines tested (MB231, MCF-7, BT-20 and SKBR3). Both, CART19 armed with HER2Bi or EGFRBi showed cytotoxicity against pancreatic (L3.6, MiaPaCa-2 and HTC-8; 20–36%), ovarian (SCOV; 39–42%), prostate (PC3; 42–46%), lung (H292 and A549; 20–25%), melanoma (A375; 26–29%), osteosarcoma (HOS; 42–51%) and glioblastoma (U118 and U251; 25–54%) cancer cell lines (Fig. 2).
Figure 2.
Comparison of specific cytotoxicity by armed and unarmed CART19 cells or armed and unarmed ATC. Effector cells consisted of CART19 or ATC armed with HER2Bi or EGFRBi and unarmed CART19 or ATC and target cells include breast, pancreatic (Top panel), ovarian, prostate, Lung (Middle panel), melanoma, osteosarcoma and glioblastoma cell lines (Bottom left panel). Armed or unarmed CART19 and ATC were plated in triplicate onto HER2 and EGFR expressing cell lines from various cancer types at effector/target (E/T) ratio of 10:1 for 18 h for 51Cr release cytotoxicity show comparable cytotoxicity by CART19 and ATC.
Enhanced cytotoxicity after sequential targeting of HER2 and EGFR by armed CART19.
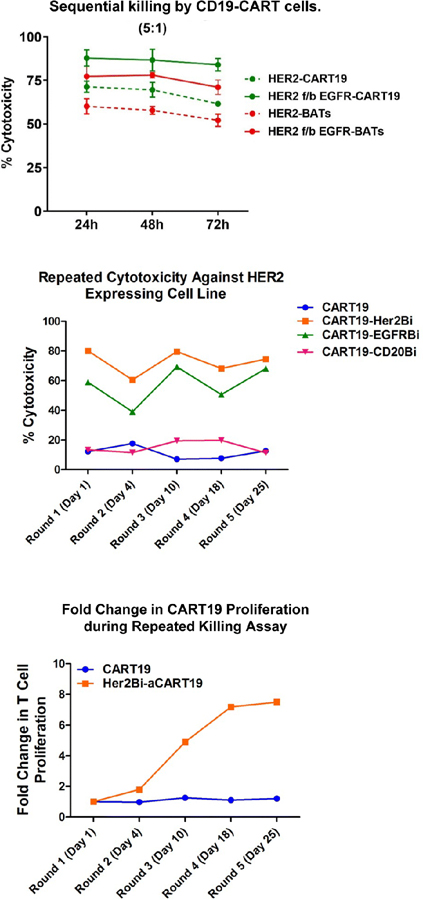
Next, we asked whether sequential targeting by HER2Bi-armed CART19 followed by (f/b) EGFRBi-armed CART19 or vice versa may maintain long-term specific cytotoxicity compared to single targeting by EGFRBi- or HER2Bi-armed CART19 against low EGFR and low HER2-expressing cell lines using the xCELLigence RTCA system. For sequential targeting, HER2Bi-CART19 or HER2-Bispecific antibody-Armed T cells (BATs) were added at 1:1 effector to target (E/T) for 24 h then effector cells were removed, wells were washed f/b adding EGFRBi-CART19 or EGFR-BATs, respectively. Cytotoxicity was monitored by RTCA for 72 h. Sequential targeting of two tumor antigens by CART19 showed significantly enhanced cytotoxicity (87.8%) compared to BATs (76.2%). The single targeting of HER2 by HER2Bi-armed CART19 showed significantly higher cytotoxicity (71.2%) towards MCF7 cells (low HER2-expressing cells) compared to the killing by HER2-BATs (60.0%) at 5:1 E/T (n=3) in the presence of 100 IU/ml IL-2 (Fig. 3, Top panel).
Figure 3.
Top panel shows representative data targeting MCF-7 (low EGFR and low HER2 expression) with either HER2Bi-armed CART19 cells first followed by (f/b) adding EGFRBi-armed CART19 or EGFRBi-armed CART19 cells first followed by (f/b) adding HER2Bi-armed CART19. Cytotoxicity was monitored by the real-time cell analysis (RTCA), data are presented at 24, 48, 72 h at an E/T of 5:1. The dashed lines represent HER2Bi-armed CART19 or EGFRBi-armed CART19 alone and corresponding color solid lines show the sequential killing of target cells. Middle panel shows five rounds of serial killing data up to 25 days. Cytotoxicity by HER2Bi- or EGFRBi-armed CART19 ranged between 40 and 60% for EGFRBi-armed CART19 and 65 and 80% for HER2Bi-armed CART19. Bottom panel shows the fold expansion of HER2Bi-armed CART19 at days 1, 4, 10, 18 and 25.
Armed CART19 proliferate during serial killing of targets.
Serial killing was set-up for a repeated exposure of armed CART19 to newly plated tumor targets. Basically, after each round of killing, effectors were collected and added to a newly plated tumor targets for the next round of killing. Cytotoxicity was measured over the course of repeated exposures of the unarmed and armed CART19 to SKBR3 at days 1, 4, 10, 18, and 25 (without IL-2). Both, HER2Bi-CART19 and EGFRBi-CART19 showed >70% and >60% cytotoxicity, respectively, at round 5 (day 25) of killing assay (Fig. 3, Middle panel). The Bottom panel (Fig. 3) shows fold change in proliferation of HER2Bi-armed CART19 during repeated killing with continuous exposure to fresh SK-BR-3 targets after each round of killing at days 1, 4, 10, 18, and 25 (without IL-2). These data suggest that intracellular domain containing 4-1BB and CD3z (4–1BBz) of the CAR was capable of ligand-independent signaling that enables CART19 cells for enhanced cytotoxicity and proliferation even after prolonged tumor cell exposure.
Real-time cell analysis during repeat-killing assay.
We first reported serial killing by HER2 BATs (12). We now ask the question of how long will the HER2Bi- or EGFRBi-armed CART19 be able to kill tumor targets in a long-term serial killing assay. The serial killing assay was performed using the same effector-armed CART19 cells that were transferred from one culture to the subsequent culture with no additions except for media and IL-2. We compared the repeat-killing (long-term killing of tumor cells) of MCF-7 by HER2Bi-armed and unarmed CART19 with armed (BAT) and unarmed ATC in a real-time cell analysis (RTCA) using xCelligence. Specific cytotoxicity against MCF-7 at an E/T of 10:1 showed 80–100% killing of target cells withing 24 h the after adding the effector cells up to 3rd killing while the killing by BATs was similar but response was delayed. At 4th round, 80% killing reached at 72–96 h for CART19 but BATs showed only transient killing (40%), thereafter, cytotoxicity mediated by HER2 or EGFR-BATs declined (Fig. 4). Interestingly, at 4th round of killing unarmed ATC and unarmed CART19 showed higher killing of target cells which appears to be due to antigen-specific clonal expansion. This is likely since the CART19 and ATC were repeately primed in vitro with tumor antigens released during long-term repeated co-cultures for 25 days.
Figure 4.
Specific cytotoxicity by unarmed, HER2Bi-armed CART19, HER2Bi-armed ATC (BAT) and unarmed ATC measured by RTCA using xCelligence over the course of repeated exposures to MCF-7 cells at day 1, 3, 6, and 9 that was monitored up to 10 days (250 h without IL-2) RTCA. Representative data show enhanced cytotoxicity by HER2Bi-armed CART19 compared to HER2Bi-armed ATC (BATs) against MCF-7 cell line in long-term killing assay.
Armed CART19 induce cytokines and chemokines.
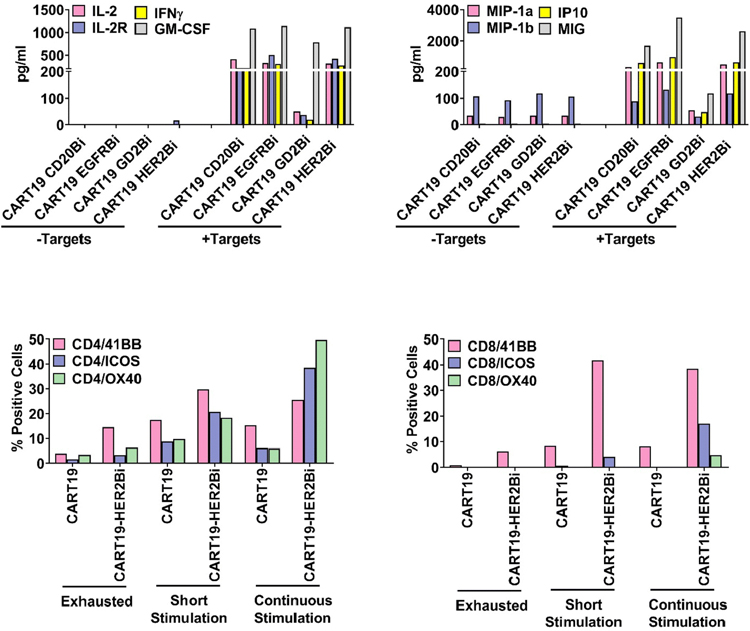
The levels of Th1 cytokines (IFN-γ, IL-2, IL-2R and GM-CSF) and chemokines (MIP-1α, MIP-1β, IP-10 and MIG) were significantly higher in supernatants of co-cultures of HER2Bi-CART19 with tumor cells (SKBR3) and compared to the control HER2Bi-CART19 without tumor cells (Fig. 5, Top Panels).
Figure 5.
Top panel shows the cytokine/chemokine profiles of culture supernatant of HER2Bi-, EGFRBi-, GD2Bi-, CD20Bi-armed CART19 cells cultured with or without their corresponding tumor targets. Data suggest dominant Th1 cytokine (Upper left panel) and chemokine profile (Upper right panel). Lower panels shows activating co-receptors expression on CD4 (Left) and CD8 T cell population (Right) of exhausted (lack of cytotoxicity), short- (24h) or long-term (2 weeks) antigen-exposed unarmed or armed CART19. Data are presented as percentage positive cells within each subset.
Antigenic exposure of unarmed and armed CART19 cells remains activated.
We used three conditions to evaluate the phenotypic changes in HER2Bi-CART19 using a) short 24 h exposure to tumor cells, b) unarmed and armed CART19 were grown with tumor cells for 2 weeks in the presence of IL-2 (continuous antigenic exposure) and, c) exhausted armed and unarmed CART19 (unable to kill tumor cells). Both short and continuous antigenic exposures of HER2Bi-CART19 show increased expression of the co-stimulatory molecule 4–1BB, ICOS, OX40 on CD4+ and CD8+ T cells (Fig. 5, Lower Panel). These phenotyping data indicate that express of co-stimulatory molecules even after long antigenic exposure suggests that the CART19 cells did not undergo T cell exhaustion.
Discussion
Cell-based immunotherapy has recently emerged as a promising approach for achieving robust and long-lasting anti-tumor activity in advanced disease (1–4). A number of CAR- and TCR-based therapies are being evaluated for treatment of solid malignancies (13–16). In this proof-of-concept study, we asked whether arming CART19 cells with target antigen-specific BiAb using the native endogenous TCR will redirect the CART19 to kill multiple cancer types in a non-MHC restricted manner without the need of designing specific transgene construct to generate antigen-specific CAR T cells. Armed CART19 showed a robust redirected killing of EGFR+, CD19- or HER2+, CD19 tumor cell lines. More importantly, armed CART19 not only were able to show enhanced serial killing of target cells but also showed increased proliferation and Th1 cytokine secretion compared to unarmed CART19 during multiple rounds of repeat-killing assay. It is likely that binding of BiAb to CD3ε (via OKT3) on CART19 cells may co-signal the intracellular portion of CAR transgene in a ligand-independent fashion; however, the mechanism of ligand-independent intracellular signaling is the subject of future studies.
The application of CAR T cells to treat solid malignancies has been limited due to a number of reasons. One major reason is the lack of exclusiveness of tumor antigens that cause on-target off-tumor toxicity through their recognition of healthy cells that express the target antigen (10–13). The occurrence of cytokine release syndrome or “cytokine storm” has limited the absolute dose of CAR-T and does not allow for repeated infusions. A number of approaches to decrease the toxicity profiles of CAR-T infusions are being developed. “Suicide” genes or an inducible “caspase 9” transgene has been introduced into the CAR-T to avoid the development of CRS from infusions r of CAR T cells (17). Further efforts lead to use an alternative approach of transient expression of CAR mRNA that required repeated infusions of CAR T cells (18). However, the repeated infusions of the CAR-T cells in one patient resulted in anaphylaxis and cardiac arrest within minutes of completing the 3rd infusion, authors suggest that this is most likely through IgE antibodies specific to the murine CAR (15). These results indicate that the potential immunogenicity of CARs derived from murine antibodies may be a safety issue for mRNA CARs. On the other hand, BiAb-loaded CART cells are self-limiting as with each cell division the amount of BiAb becomes half and approximately after 6–7 cell divisions BiAb will be lost from the CART cells. In other words, the amount of BiAb decreases in subsequent daughter generations eventually extinguishing the binding.
In our clinical studies, adoptively transferred BiAb-loaded T cells have not shown any persistent grade 3 or 4 toxicities or dose limiting toxicities in multiple clinical trials (5–7). We showed that arming with HER2Bi was able to target HER2-expressing tumor cell lines to redirect the non-MHC restricted, perforin/granzyme-mediated cytotoxicity of ATC (12) to both high and low HER2-expressing targets in vitro and as well as HER2 0–2+ and HER2–3+ patients in phase I clinical trial (5, 7). Our data show that BATs infusions were safe and induced endogenous immune responses that persisted up to 4 months (5). Our working hypothesis is that BiAb-armed CART cells infusions will provide tumor killing due to their ability to proliferate, remain activated and modify tumor microenvironment (TME) by releasing Th1 cytokines and chemokines. Armed CART19 mediated tumor lysis and release of Th1 cytokines/chemokines may create “conducive” environment for in situ immunization through the recruitment and activation of endogenous immune cells that may result in antigen/epitope spreading seen in our vaccinate and boost protocol using multiple infusions of HER2 BATs to “vaccinate” the patient and a boost of immune ATC grown from a second apheresis that was given after high dose chemotherapy and stem cell transplantation (7). In vaccinate and boost protocol, there was clear evidence that HER2 peptide antigen-specific TCR clones would respond by producing cytoplasmic IFN-γ with a specific reactive TCR (7). These changes may induce the development of long-term tumor specific memory T cells as well as a shift in tumor microenvironment.
The ability to target multiple antigens with a single, standardized immune receptor using exogenous BiAbs targeting approach represents a flexible platform for tumor eradication without the necessity to re-engineer or develop unique CAR for each target. Universal immune receptors are the part of this evolution, arming CAR T cells with 2–3 BiAbs each with unique antigen offers the potential to circumvent the limitations that accompany CAR T cell therapy.
In addition, this approach is self-limiting as the amount of BiAb on the BiAb-armed CART19 will become half with every cell division, and BiAb will be lost after multiple division during rounds of repeated killing and, therefore, the cytotoxicity activity will be turned off avoiding CRS and off-target toxicity. This strategy allows for multiple infusions of twice weekly or once per week for 4–8 weeks. In summary, BiAb-loaded CART19 show enhanced specific killing of multiple cells lines targeting single tumor-associated antigen, produced Th1 cytokines/chemokines and showed far superior cytotoxicity when two antigens were sequentially targeted. In a serial killing assay, armed CART19 proliferated, remain activated and repeatedly killed target cells up to 3 weeks in a long-term killing assay. Further investigation is warranted to understand the mechanism of ligand-independent signaling in scFv deleted CAR T cells.
Acknowledgments
Funding: This study was primarily supported by funding from in part by R01 CA 182526, P30CA022453 (Microscopy, Imaging, and Cytometry Resources Core) and startup funds from the University of Virginia Cancer Center.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Originality Disclosure: The data presented in this manuscript are original and have not been published elsewhere except in the form of abstracts and poster presentations at symposia and meetings.
Conflict of Interest: LGL is co-founder of Transtarget Inc. and serves on the SAB for Rapa Therapeutics, CHJ is a co-founder of Tmunity Therapeutics, Inc. and AT is co-founder of Nova Immune Platform LLC, JS and ETB have no conflict of interest.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Porter DL, Levine BL, Kalos M, Bagg A, and June CH (2011) Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 365, 725–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porter DL, Kalos M, Zheng Z, Levine B, and June C (2011) Chimeric Antigen Receptor Therapy for B-cell Malignancies. J Cancer 2, 331–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guedan S, Ruella M, and June CH Emerging Cellular Therapies for Cancer. Annu Rev Immunol. 2019. April 26;37:145–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter D, Rheingold S, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. New England Journal of Medicine. 2013;368:1509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lum LG, Thakur A, Al-Kadhimi Z, Colvin GA, Cummings FJ, Legare RD, Dizon DS, Kouttab N, Maizel A, Colaiace W, Liu Q, and Rathore R (2015) Targeted T-cell Therapy in Stage IV Breast Cancer: A Phase I Clinical Trial. Clin Cancer Res 21, 2305–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaishampayan U, Thakur A, Rathore R, Kouttab N, and Lum LG (2015) Phase I Study of Anti-CD3 × Anti-Her2 Bispecific Antibody in Metastatic Castrate Resistant Prostate Cancer Patients. Prostate Cancer 2015, 285193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thakur A, Rathore R, Kondadasula SV, Uberti JP, Ratanatharathorn V, and Lum LG (2018) Immune T cells can transfer and boost anti-breast cancer immunity. Oncoimmunology 7, e1500672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ueda M, Joshi ID, Dan M, Uberti JP, Chou TH, Sensenbrenner LL, Lum LG. Preclinical studies for adoptive immunotherapy in bone marrow transplantation. Generation of anti-CD3 activated cytotoxic T cells from normal donors and autologous bone marrow transplant candidates. Transplantation. 1993. August;56(2):351–6. [PubMed] [Google Scholar]
- 9.Uberti JP, Joshi I, Ueda M, Martilotti F, Sensenbrenner LL, Lum LG. Preclinical studies using immobilized OKT3 to activate human T cells for adoptive immunotherapy: optimal conditions for the proliferation and induction of non-MHC-restricted cytotoxicity. Clin Immunol Immunopathol. 1994. March;70(3):234–40. [DOI] [PubMed] [Google Scholar]
- 10.Sen M, et al. , Use of anti-CD3 × anti-HER2/neu bispecific antibody for redirecting cytotoxicity of activated T cells toward HER2/neu+ tumors. J Hematother Stem Cell Res, 2001. 10(2): p. 247–60. [DOI] [PubMed] [Google Scholar]
- 11.Reusch Ursula, Sundaram Magesh, Davol Pamela A., Olson Sarah D., Davis James B., Demel Kurt, Nissim Julie, Rathore Ritesh, Liu Paul Y. and Lum Lawrence G. Anti-CD3 × Anti-Epidermal Growth Factor Receptor (EGFR) Bispecific Antibody Redirects T-Cell Cytolytic Activity to EGFR-Positive Cancers In vitro and in an Animal Model. Clin Cancer Res January 1 2006. 12 (1) 183–190; DOI: 10.1158/1078-0432.CCR-05-1855 [DOI] [PubMed] [Google Scholar]
- 12.Grabert RC, Cousens LP, Smith JA, Olson S, Gall J, Young WB, Davol PA, and Lum LG (2006) Human T cells armed with Her2/neu bispecific antibodies divide, are cytotoxic, and secrete cytokines with repeated stimulation. Clin Cancer Res 12, 569–576 [DOI] [PubMed] [Google Scholar]
- 13.Lamers CH, Sleijfer S, van Steenbergen S, van Elzakker P, van Krimpen B, Groot C, et al. Treatment of Metastatic Renal Cell Carcinoma With CAIX CAR-engineered T cells: Clinical Evaluation and Management of On-target Toxicity. Mol Ther. 2013;14:904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamers CH, van Steenbergen-Langeveld S, van Brakel M, Groot-van Ruijven CM, van Elzakker PM, van Krimpen B, Sleijfer S, Debets R. T cell receptor-engineered T cells to treat solid tumors: T cell processing toward optimal T cell fitness. Hum Gene Ther Methods. 2014. December;25(6):345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe K, Kuramitsu S, Posey AD Jr., and June CH (2018) Expanding the Therapeutic Window for CAR T Cell Therapy in Solid Tumors: The Knowns and Unknowns of CAR T Cell Biology. Front Immunol 9, 2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–e2. [DOI] [PubMed] [Google Scholar]
- 17.Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beatty Gregory L et al. “Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies.” Cancer immunology research vol. 2; 2 (2014): 112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]