Abstract
Background/aim
The Trento screening program transitioned to digital breast tomosynthesis (DBT) screening based on evidence that DBT improves breast cancer (BC) detection compared to mammography; an evaluation of the transition to DBT is reported in this pilot study.
Methods
Prospective implementation of DBT screening included women aged ≥50 years who attended the Trento program for biennial screening. DBT screening included DBT acquisitions with synthesized 2D-images. A historical cohort of women who attended the program (January 2013–October 2014) and received digital mammography (DM) provided a comparison group. Independent double-reading (with a third arbitrating read for discordance) was used for DBT and DM screening. Screening outcomes included cancer detection rate (CDR/1000 screens), percentage of screens recalled to assessment (recall%), interval cancer rate (ICR/1000 screens) at 2-year follow-up, and screening sensitivity. Rate ratios (RR) and 95% confidence interval (95%CI) examined outcomes for DBT versus DM screening.
Results
From women aged 50–69 years who accepted an invitation to screening (October 2014–October 2016) 46,343 comprised the DBT-screened group: amongst these 402 BCs (includes 50 ductal carcinoma in-situ (DCIS)) were detected (CDR 8.67/1000), whereas 205 BCs (includes 33 DCIS) were detected amongst 37,436 DM screens (CDR 5.48/1000) [RR for CDR:1.58 (1.34–1.87)]. Recall% was lower for DBT (2.55%) than DM (3.21%) [RR:0.79 (0.73–0.86)]. Compared to DM, DBT screening increased CDR for stage I-II BC, for all tumour size and grade categories, and for node-negative BC, but did not increase CDR for DCIS. Estimated ICR for DBT was 1.1/1000 whereas ICR for DM was 1.36/1000 [RR:0.81 (0.55–1.19)]. Screening sensitivity was 88.74% for DBT versus 80.08% for DM [RR:1.11 (0.94–1.31)].
Conclusion
DBT significantly improved early-detection measures but did not significantly reduce ICR (relative to DM screening), suggesting that it could add benefit as well as adding over-detection in population BC screening.
Keywords: Breast cancer, Cancer screening, Digital breast tomosynthesis, Population screening, Interval cancers
Highlights
-
•
Evidence from a prospective population-based evaluation of tomosynthesis (DBT) screening.
-
•
Amongst 46,343 DBT screens 402 cancers (50 DCIS) were detected (CDR 8.67/1000).
-
•
Amongst 37,436 mammography screens 205 cancers (33 DCIS) were detected (CDR 5.48/1000).
-
•
DBT increased detection rates of stage I-II cancer (across tumour size and grade categories).
-
•
Transition to DBT did not increase DCIS detection rates.
-
•
Recall for DBT screening (2.55%) was lower than that for mammography (3.21%).
-
•
Interval cancer rate for DBT (October 1, 1000) did not differ from that for mammography (1.36/1000).
-
•
Screening sensitivity for DBT (88.74%) was higher than that for mammography (80.08%).
1. Introduction
The use of digital breast tomosynthesis (DBT) for breast cancer (BC) screening is not a novel concept, the evidence on this ‘new mammography’ has emerged rapidly since the early European trials evaluated DBT technology for screening [[1], [2], [3], [4], [5], [6]]. Meta-analysis of the prospective studies of DBT shows that DBT increases BC detection (pooled incremental detection 2.4 per 1000 screens) and increases recall (pooled increase in recall 0.5%) [6]. The Trento screening program was one of the first BC screening programs in Europe to evaluate DBT in conjunction with digital mammography (DM), contributing knowledge on the effect of DBT screening on screen-detection measures through two trials [1,3].
Based on the above-described evidence that DBT screening improves BC detection [[1], [2], [3], [4], [5], [6]], with further evidence emerging from studies undertaken in population screening programs in Norway and Italy [7,8], the Trento screening program adopted a policy decision (endorsed by national screening authorities) to transition to DBT screening. A planned transition was commenced as a prospectively implemented pilot aiming to screen a cohort of women with DBT over two screening rounds, to determine the impact of DBT on screening outcomes. In this study, we report results of the first biennial screening round from the Trento DBT pilot, inclusive of initial detection measures and interval cancer rates at follow-up. We compare outcomes of the DBT-screened cohort with a historical cohort of women who attended the same screening service and had DM screening. We hypothesized that the transition to DBT screening would improve screen-detection measures (specifically, that it would increase BC detection) and hence might reduce the frequency of interval BCs at follow-up.
2. Methods
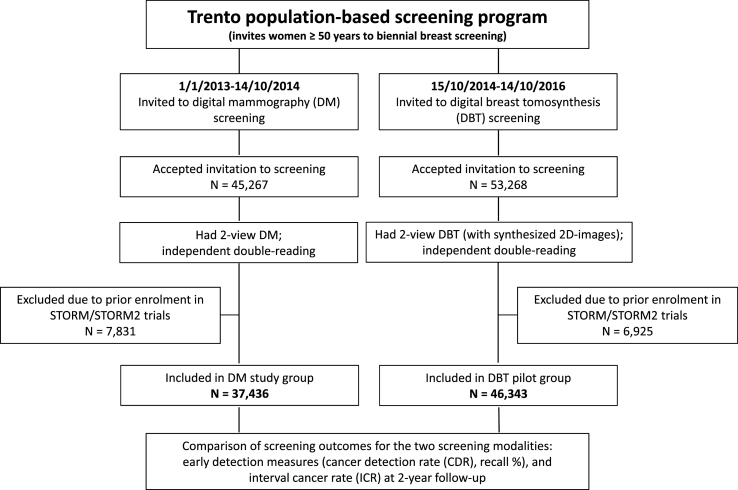
The Trento screening program invites women aged ≥50 years to biennial mammography screening as part of organized population-based BC screening. A prospective pilot evaluation of DBT screening aiming to include women who attend the Trento screening program was commenced in October 2014, with approval of local health and population screening authorities. Women who presented for screening in response to invitation by the population-based program and had DBT screening were eligible to be part of this cohort. Those who had participated in the STORM (‘screening with tomosynthesis or mammography’) trials were not included as they will have received prior DBT plus DM for screening. DBT screening consisted of DBT acquisitions with synthesized 2D-images (reconstructed from the DBT acquisition data). Fig. 1 is a schematic representation of the study.
Fig. 1.
A schematic representation of the Trento DBT (digital breast tomosynthesis) pilot study.
2.1. Cohort screened with DM
A historical cohort of women who attended the Trento screening program from January 01, 2013 to October 14, 2014 and received 37,436 DM screening examinations provided a comparison group from the same population - Fig. 1.
2.2. Screening protocol for DBT and for DM
Medio-lateral oblique and cranio-caudal views were obtained for DBT screens and for DM screens. Screen-reading was based on independent double-reading as practiced in European programs, with a third read arbitrating discordant reads, for DBT and for DM screening. The same group of eight radiologists contributed to screen-reading during the DBT pilot and the prior (historical) DM screening round. All aspects of assessment protocols remained constant throughout the entire timeframe of the study, hence the only difference was that DBT replaced DM as the primary screening modality.
2.3. Outcomes
Primary outcomes (recall for positive screens, screen-detected cancers, and interval cancers) were ascertained based on excision histology in those who received surgery, or based on the completed assessment inclusive of work-up imaging (and histology from needle biopsy where performed) in recalled participants. In addition, all screening participants had follow-up to ascertain interval cancers, as part of the screening program’s quality standards. The following methods were used to ascertain interval cancers for cohorts screened by DBT or DM: all breast cancers identified over two-year follow-up from the screen date via clinical mammography database (2013–2018), Trento province cancer registry, and hospital discharge records (2013–2017).
2.4. Statistical methods
For each screened cohort, the number of screen-detected cancers and the cancer detection rate (CDR) per 1000 screens, and the number and percentage of screens recalled to assessment (recall %) were calculated. We also calculated the positive predictive value (PPV) for recall, and the benign to malignant biopsy percentage. Descriptive statistics of the characteristics of detected cancers (stage, size, histology, grade, and node status) were tabulated for DBT versus DM. The number and rate of interval cancers (ICR) per 1000 screens was based on cancers identified over two-year follow-up from the screen date and was estimated counting all screens, and further calculated in a sensitivity analysis using negative screens as the denominator. Screening sensitivity was calculated as the number of screen-detected cancers from all cancers observed in screened women including interval cancers. The rate ratio (RR) and 95% confidence interval (95%CI) were computed to compare the distribution of outcomes between cohorts screened with DBT versus DM; this approach allows estimation of the magnitude of effect for the comparison between the two groups because the 95%CI presents a range of values on the basis of sample data, in which the population value for the estimated ratio may lie. All analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA) and MedCalc version 19.0.3 (MedCalc Software, Ostend, Belgium).
3. Results
There were 53,268 participants who accepted an invitation to screening 15/10/2014–14/10/2016 and had DBT: 46,343 women with an age range of 50–69 years (mean age 57.9 years, SD 5.95) were included in the DBT pilot group (Fig. 1). Amongst these 46,343 DBT screens, 402 BCs including 50 DCIS were detected (CDR 8.67/1000) as shown in Table 1. Amongst 37,436 DM-screened women (mean age 57.9 years, SD 5.87) 205 BCs including 33 DCIS were detected (CDR 5.48/1000); this represents a RR for CDR of 1.58 (95%CI 1.34–1.87). CDR estimates stratified by screening round are shown in Table 1. Recall percentage was lower for DBT screening (2.55%) compared with DM screening (3.21%) [recall RR 0.79; 95%CI 0.73–0.86]; results are also shown stratified by screening round in Table 1 indicating that the lower recall % from DBT was observed predominantly for initial (prevalent) screens.
Table 1.
Cancer detection rate (CDR) and recall to assessment for digital mammography (DM) and digital breast tomosynthesis (DBT), overall and by screening round.
| Digital Mammography (2D) | Digital Breast Tomosynthesis (with synthetic 2D) | Rate Ratio (95% CI)b | |
|---|---|---|---|
| Cancers detected at screening | |||
|
All screening examinations Number of detected BCs [N screens in analysis]; CDR per 1000 screens |
205 [37,436 screens] 5.48/1000 |
402a [46,343 screens] 8.67/1000 |
1.58 (1.34–1.87) |
|
First (prevalent) screening round Number of detected BCs [N screens in analysis]; CDR per 1000 screens |
38 [6,412 screens] 5.93/1000 |
74 [8,569 screens] 8.64/1000 |
1.46 (0.99–2.15) |
|
Repeat (incident) screening round Number of detected BCs [N screens in analysis]; CDR per 1000 screens |
167 [31,024 screens] 5.38/1000 |
328a [37,774 screens] 8.68/1000 |
1.61 (1.34–1.94) |
| Recall for further assessment | |||
|
All screening examinations Number of recalls [N screens in analysis]; recall percentage |
1,201 [37,436 screens] 3.21% |
1,180 [46,343 screens] 2.55% |
0.79 (0.73–0.86) |
|
First (prevalent) screening round Number of recalls [N screens in analysis]; recall percentage |
477 [6,412 screens] 7.44% |
331 [8,569 screens] 3.86% |
0.52 (0.45–0.60) |
|
Repeat (incident) screening round Number of recalls [N screens in analysis]; recall percentage |
724 [31,024 screens] 2.33% |
849 [37,774 screens] 2.25% |
0.96 (0.87–1.06) |
One case was identified during the screening process due to clinical findings rather than a screening abnormality (exclusion of that case from analysis does not alter the estimated rate ratio).
Rate ratio with 95% CI shown in bold indicate statistical significance.
PPV for recall was 34.07% for DBT (402/1,180) whereas PPV for DM was 17.07% (205/1,201) showing that DBT increased the PPV for recall [PPV% ratio 2.00; 95%CI 1.69–2.36]. Benign to malignant biopsy % for DBT was 18.90% (76/402) and that for DM was 13.66% (28/205) [RR 1.38; 95%CI 0.74–2.40].
3.1. Cancer characteristics
Distribution of prognostic characteristics of detected BCs are described in Table 2, showing generally similar distribution of variables (distribution of proportions across categories) for both screening modalities. However, in terms of detection rates, there was evidence that DBT: increased CDR for stage I-II invasive cancer but not for stage 0 (DCIS), increased CDR across most pT categories (but not for Tis in line with stage data), increased CDR of node-negative BC, and also increased CDR across all grades.
Table 2.
Tumor stage, pT, pN and grading of detected breast cancers for digital mammography (DM) and digital breast tomosynthesis (DBT). Number, percentages and cancer detection rate per 1000 screens.
| Digital Mammography screening |
Digital Breast Tomosynthesis screening |
Rate Ratiob (95% CI) | |||
|---|---|---|---|---|---|
| Number of cancers (%) | CDR/1000 | Number of cancers (%) | CDR/1000 | ||
| Stage distribution (excludes 1 from DBT data with unknown final pTNM category) | |||||
| Stage 0 | 33 (16.10) | 0.88 | 50 (12.47) | 1.08 | 1.22 (0.79–1.90) |
| Stage I | 123 (60.00) | 3.29 | 262 (65.33) | 5.65 | 1.72 (1.38–2.13) |
| Stage II+ | 49 (23.90) | 1.31 | 89 (22.20) | 1.92 | 1.47 (1.04–2.08) |
| Tumour size, pT category distribution (excludes 1 from DBT data with unknown pT category) | |||||
| T0 | - | – | 1 (0.26) | 0.026 | – |
| Tis (in situ cancer) | 33 (16.10) | 0.88 | 50 (12.47) | 1.08 | 1.22 (0.79–1.90) |
| T1a (≤5 mm) | 10 (4.88) | 0.27 | 30 (7.48) | 0.65 | 2.42 (1.18–4.96) |
| T1b (>5 but ≤10 mm) | 55 (26.83) | 1.47 | 120 (29.92) | 2.60 | 1.76 (1.28–2.43) |
| T1c (>10 but ≤20 mm) | 78 (38.05) | 2.08 | 145 (36.16) | 3.13 | 1.50 (1.14–1.98) |
| T2 (>20 but ≤50 mm) | 25 (12.19) | 0.67 | 51 (12.72) | 1.10 | 1.65 (1.02–2.66) |
| T3 (>50 mm) | 4 (1.95) | 0.11 | 4 (0.997) | 0.09 | 0.81 (0.20–3.43) |
| Axillary node status | |||||
| No node metastases | 146 (71.22) | 3.90 | 297 (74.06) | 6.41 | 1.64 (1.35–2.00) |
| Node metastases | 35 (17.07) | 0.93 | 66 (16.46) | 1.42 | 1.52 (1.01–2.29) |
| Nx (unknown) | 24 (11.71) | 0.64 | 38 (9.48) | 0.82 | 1.28 (0.77–2.13) |
| Tumour grade | |||||
| 1 | 48 (23.41) | 1.28 | 118 (29.43) | 2.55 | 1.99 (1.42–2.78) |
| 2 | 117 (57.08) | 3.12 | 205 (51.12) | 4.42 | 1.42 (1.13–1.78) |
| 3 | 40 (19.51) | 1.07 | 78 (19.45) | 1.68 | 1.58 (1.08–2.31) |
| Total | 205 (100) | 5.48 | 401a(100) | 8.65 | 1.58 (1.34–1.87) |
Stage data was incomplete for one case identified during the screening process due to clinical findings rather than a screening abnormality, hence results shown are for 401 cancers.
Rate ratio with 95% CI shown in bold indicate statistical significance.
3.2. Interval cancers and screening sensitivity
At two-year follow-up 51 interval cancers were identified in each of the cohorts as shown in Table 3 (48 in the DM cohort if 3 false-negative assessments are excluded). ICR for DBT was 1.1/1000 screens, for DM 1.36/1000 with a RR of 0.81 (95%CI 0.55–1.19); ICR was 1.13/1000 negative screens for DBT, and 1.32/1000 negative screens for DM with a RR of 0.85 (95%CI 0.57–1.26). Screening sensitivity was 88.74% (402/453) for DBT versus 80.08% for DM (205/256) [RR for sensitivity% RR 1.11; 95%CI 0.94–1.31].
Table 3.
Interval cancer rate (ICR) by screening modality for population-based breast cancer screening.
| Digital Mammography (2D) screening |
Digital Breast Tomosynthesis (with synthetic 2D) |
Rate Ratio (95% CI) | ||
|---|---|---|---|---|
| Number interval cancers [N all screens] | ICR/1000 screens | Number interval cancers [N all screens] | ICR/1000 screens | |
| 51a [37,436] | 1.36 | 51 [46,343] | 1.10 | 0.81 (0.55–1.19) |
| Number interval cancers [N negative screensb] | ICR/1000 negative screens | Number interval cancers [N negative screensb] | ICR/1000 negative screens | |
| 48 [36,235] | 1.32 | 51 [45,163] | 1.13 | 0.85 (0.57–1.26) |
Three false-negative assessments following positive screens included in analysis, however these are not included in the sensitivity analysis for ICR shown in the second row of data which defines interval cancers in terms of a negative screen (see statistical methods).
N negative screens = all screens minus the number of recalled screens.
4. Discussion
The Trento population-based BC screening program is one of the first in Europe to have transitioned from mammography to DBT screening, using a prospective implementation and evaluation – the Trento DBT pilot. We report the effect that the change to DBT screening has had on initial detection measures by comparing the DBT pilot screening outcomes with those from the preceding screening round (DM screening), and importantly we ensure follow-up to identify and report interval BC rates. Our work shows that DBT screening (CDR 8.67/1000) has significantly increased BC detection (versus DM, CDR 5.48/1000) evidenced by the rate ratio of 1.58 (95%CI:1.34–1.87). CDR estimates stratified by screening round indicate that the increased CDR from DBT screening, though observed for both initial and repeat screens, was more evident at repeat screening rounds. The increase in CDR attributed to DBT screening in our pilot study is commensurate with the prospective non-randomised DBT trials that were conducted in Europe [[1], [2], [3], [4], [5]] showing an incremental CDR of 2.4 per 1000 for DBT (versus DM) based on a meta-analysis from Marinovich et al. [6]. Our results also align with those of an interim report from an Italian randomised controlled trial of DBT screening [9].
In population cancer screening, recall is another key outcome measure, and we show that DBT screening had a lower percentage of screens recalled to assessment in the Trento pilot (recall 2.55%) compared with DM (recall of 3.21%), with a RR of 0.79 (0.73–0.86). Recall data stratified by screening round show that this DBT effect was mostly achieved through a reduction in recall for initial screens; it is noteworthy that recall% was generally low for repeat screening rounds for both screening modalities (around 2.3%) hence there was limited scope for DBT to further reduce this low recall percentage. These are reassuring results given the heterogeneous effect DBT screening has had on recall in various screening settings (with increases in recall from DBT reported in some studies) as highlighted in meta-analysis [6]. Further, the PPV for recall was increased (approximately doubled) following the transition to DBT, meaning that the cancer yield for women recalled to assessment was higher for DBT than DM screening. So overall, DBT screening has had a favourable impact on screening parameters.
Examination of the BC characteristics showed somewhat similar distributions of the proportion of variables for both screening modalities, though with a slightly lower proportion of DCIS for DBT (12.47%) than DM (16.10%). When BC characteristics are considered in terms of detection rates per 1000 screens (Table 2), DBT effectively increased CDR for stage I-II invasive cancer, increased CDR for most tumour size categories and grade categories, and increased detection of BCs without (more so than with) node metastases, reflecting a global increase in CDR at DBT screening, with one exception – DBT did not increase CDR for DCIS. This pattern of findings of increased detection from DBT across BC stage and tumour size categories was generally also observed when we examined data by screening round (data available from authors), and was particularly evident in significantly increased CDR for pT1b-c tumours in women having repeat screening rounds. Our results showing increased detection from DBT (for BC stage, tumour size, and grade categories) without an increase in detection of DCIS align with those of a pooled analysis of some of the early studies of DBT screening [10]. Overall, these results from the Trento DBT pilot also suggest that DBT screening has had a favourable impact on initial screening outcome measures.
While there is an accumulating body of evidence on detection measures for DBT screening, there are limited data on its effect on interval cancer rates (ICR), and this is recognised as a key evidence gap for DBT screening [11,12]. The STORM trial, one of the earliest prospective trials of DBT screening, showed little effect of DBT screening on ICR, however, it was a relatively small trial [13]. Bahl et al. [14] recently reported very similar cancer detection and interval cancer rates for DM and DBT in a large retrospective study, although that was in the context of annual screening, and DBT might have less effect on these outcomes in more frequent (annual) screening practice [6,12]. Our pilot study has shown that although DBT screening had a lower ICR (ICR 1.1/1000 screens) than DM screening (ICR 1.36/1000 screens), this was not a statistically significant reduction (RR 0.81 (0.55–1.19). Similarly, although the estimated sensitivity for DBT screening (88.74%) was better than that for DM (80.08%) this was not a significant change with a RR of 1.11 (0.94–1.31). We had hypothesized that DBT would increase CDR and hence would be expected to reduce ICR. Although these effects of DBT were observed in the Trento DBT pilot, the substantial increase in CDR following transition from DM to DBT screening did not lead to a commensurate reduction in ICR. While this raises the possibility of over-diagnosis, it seems likely that our pilot study was not sufficiently large in size to measure an effect on ICR (a post-hoc power analysis suggests that the study was powered for CDR but not so for ICR). It may also be necessary to measure cumulative ICR over several screening rounds if benefit from DBT takes a few years to become evident (ie if the effect of DBT on ICR is modest it may take more than one screening round to be evident). Further, due to length bias in screening (tendency to screen-detect slower growing cancers), relatively slow growing cancers detected with DBT could still become clinically evident after 2 or more screening rounds. However, it may be argued that these cancers could be detected at a subsequent DM screening round (in the absence of DBT) without significantly altering prognosis. Also, while some investigators suggest that ICR reduction is a surrogate for screening effectiveness [11,12], an effect on ICR from using new technology might not be the only indicator of screening benefit; for example, after adjusting for lead time, screen-detection of breast cancer could have benefit due to a more favourable size and node profile at diagnosis [15]. Overall, taking into consideration the significant increase in CDR following the transition to DBT and the lack of statistical significance for the reduction in ICR in our DBT screening pilot, it seems reasonable to suspect that some of the increased detection from DBT is contributing to over-diagnosis in population BC screening.
The prospectively planned pilot evaluation is a strength of our study design, and although we used a historical comparison cohort we ensured this was from the preceding screening round of the same program (with the same screening protocols) and from the same population. A further strength of the study is that the same group of radiologists performed screen-readings during the DBT pilot and the prior DM screening round. A limitation of the study is that there was a slight difference in cancer identification over time as described in the Methods (‘Outcomes’) which could have affected the estimated ICR potentially favouring DBT (compared to DM) due to possible under-ascertainment in 2018. Another limitation is that we did not include a dosimetry report in our study – a preliminary review of radiation data from a small subset of screening examinations indicated that tomosynthesis had roughly double the mean glandular dose per view compared to mammography (similar to a recent report of a tomosynthesis trial in Australia [16]) highlighting the need for a comprehensive dosimetry analysis in future work.
5. Conclusion
Evaluation of the transition from mammography to DBT screening in the Trento screening program has shown that DBT screening was associated with a significant increase in CDR (consistent increase in detection rates of BC across stage and tumour size and grade categories, without an increase in DCIS detection rates) and a reduction in recall percentage. Although this suggests potential screening benefit, and screening sensitivity was improved with DBT screening, there was little effect in terms of a reduction in interval cancer rates. Screening parameters following a second DBT screening round will assist in identifying whether DBT delivers a sustained effect at repeat DBT screening rounds and with longer follow-up.
COI statements
D. Bernardi declares receipt of travel support from Hologic; all other authors do not have COI to declare.
Acknowledgements
This study did not receive funding. N. Houssami receives research support through a National Breast Cancer Foundation (NBCF, Australia) Breast Cancer Research Leadership Fellowship.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2019.09.012
Approvals
This pilot study was approved by local health and population screening authorities as such a separate ethics approval was not required since the data in the study are routinely collected and reported as part of screening quality assurance.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ciatto S., Houssami N., Bernardi D., Caumo F., Pellegrini M., Brunelli S. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 2013 Jun 6;(14):583–589. doi: 10.1016/S1470-2045(13)70134-7. [DOI] [PubMed] [Google Scholar]
- 2.Houssami N., Macaskill P., Bernardi D., Caumo F., Pellegrini M., Brunelli S. Breast screening using 2D-mammography or integrating digital breast tomosynthesis (3D-mammography) for single-reading or double-reading--evidence to guide future screening strategies. European Journal of Cancer. 2014 Jul;50(10):1799–1807. doi: 10.1016/j.ejca.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Bernardi D., Macaskill P., Pellegrini M., Valentini M., Fanto C., Ostillio L. Breast cancer screening with tomosynthesis (3D mammography) with acquired or synthetic 2D mammography compared with 2D mammography alone (STORM-2): a population-based prospective study. Lancet Oncol. 2016 Aug;17(8):1105–1113. doi: 10.1016/S1470-2045(16)30101-2. [DOI] [PubMed] [Google Scholar]
- 4.Skaane P., Bandos A.I., Gullien R., Eben E.B., Ekseth U., Haakenaasen U. Prospective trial comparing full-field digital mammography (FFDM) versus combined FFDM and tomosynthesis in a population-based screening programme using independent double reading with arbitration. Eur Radiol. 2013 Aug;23(8):2061–2071. doi: 10.1007/s00330-013-2820-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zackrisson S., Lång K., Rosso A., Johnson K., Dustler M., Fornvik D. One-view breast tomosynthesis versus two-view mammography in the Malmo Breast Tomosynthesis Screening Trial (MBTST): a prospective, population-based, diagnostic accuracy study. Lancet Oncology. 2018 Nov;19(11):1493–1503. doi: 10.1016/S1470-2045(18)30521-7. [DOI] [PubMed] [Google Scholar]
- 6.Marinovich M.L., Hunter K.E., Macaskill P., Houssami N. Breast cancer screening using tomosynthesis or mammography: a meta-analysis of cancer detection and recall. J Natl Cancer Inst. 2018 Sep 1;110(9):942–949. doi: 10.1093/jnci/djy121. [DOI] [PubMed] [Google Scholar]
- 7.Hofvind S., Hovda T., Holen A.S., Lee C.I., Albertsen J., Bjorndal H. Digital breast tomosynthesis and synthetic 2D mammography versus digital mammography: evaluation in a population-based screening program. Radiology. 2018 Jun;287(3):787–794. doi: 10.1148/radiol.2018171361. [DOI] [PubMed] [Google Scholar]
- 8.Caumo F., Zorzi M., Brunelli S., Romanucci G., Rella R., Cugola L. 2017. Digital breast tomosynthesis with synthesized two-dimensional images versus full-field digital mammography for population screening: outcomes from the Verona screening program. Radiology. [DOI] [PubMed] [Google Scholar]
- 9.Pattacini P., Nitrosi A., Giorgi R.P., Iotti V., Ginocchi V., Ravaioli S. Digital mammography versus digital mammography plus tomosynthesis for breast cancer screening: the reggio emilia tomosynthesis randomized trial. Radiology. 2018 Aug;288(2):375–385. doi: 10.1148/radiol.2018172119. [DOI] [PubMed] [Google Scholar]
- 10.Yun S.J., Ryu C.W., Rhee S.J., Ryu J.K., Oh J.Y. Benefit of adding digital breast tomosynthesis to digital mammography for breast cancer screening focused on cancer characteristics: a meta-analysis. Breast Canc Res Treat. 2017 Aug;164(3):557–569. doi: 10.1007/s10549-017-4298-1. [DOI] [PubMed] [Google Scholar]
- 11.Lauby-Secretan B., Scoccianti C., Loomis D., Benbrahim-Tallaa L., Bouvard V., Bianchini F. Breast-cancer screening -viewpoint of the IARC working group. N Engl J Med. 2015 Jun 11;372(24):2353–2358. doi: 10.1056/NEJMsr1504363. [DOI] [PubMed] [Google Scholar]
- 12.Houssami N., Lång K., Hofvind S., Zackrisson S., Bernardi D., Hunter K. Effectiveness of digital breast tomosynthesis (3D-mammography) in population breast cancer screening: a protocol for a collaborative individual participant data (IPD) meta-analysis. Transl Cancer Res. 2017;6(No 4):869–877. [Google Scholar]
- 13.Houssami N., Bernardi D., Caumo F., Brunelli S., Fanto C., Valentini M. Interval breast cancers in the ’screening with tomosynthesis or standard mammography’ (STORM) population-based trial. Breast. 2018 Apr;38:150–153. doi: 10.1016/j.breast.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Bahl M., Gaffney S., McCarthy A.M., Lowry K.P., Dang P.A., Lehman C.D. Breast cancer characteristics associated with 2D digital mammography versus digital breast tomosynthesis for screening-detected and interval cancers. Radiology. 2018 Apr;287(1):49–57. doi: 10.1148/radiol.2017171148. [DOI] [PubMed] [Google Scholar]
- 15.Allgood P.C., Duffy S.W., Kearins O., O’Sullivan E., Tappenden N., Wallis M.G. Explaining the difference in prognosis between screen-detected and symptomatic breast cancers. BJC (Br J Cancer) 2011 May 24;104(11):1680–1685. doi: 10.1038/bjc.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houssami N, Lockie D, Clemson M, Pridmore V, Taylor D, Marr G. Pilot trial of digital breast tomosynthesis (3D mammography) for population-based screening in BreastScreen Victoria. Med J Aust. 2019 doi: 10.5694/mja2.50320. Published online 26 August 2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.