Abstract
Objective
Treatment options for HER-2-positive metastatic breast cancer (mBC) patients have expanded markedly since trastuzumab approval in 1998. Several other regimens are now available, including pertuzumab plus trastuzumab plus docetaxel, T-DM1, capecitabine plus lapatinib, and trastuzumab plus lapatinib. This study assesses the cost-effectiveness of four treatment sequences for HER-2-positive mBC according to the Taiwanese National Health Insurance Administration (TNHIA).
Methods
Costs (U.S. Dollars) and effectiveness (quality-adjusted life years) of four treatment sequences for HER-2-positive mBC patients were examined using a Markov model over a lifetime horizon. Transition probabilities, disease progression, and probability of adverse events and survival were derived from clinical trial data. Costs and health utilities were estimated from TNHIA, Taipei Medical University Hospital, and the literature. Deterministic, probabilistic sensitivity analyses and a scenario analysis examined parameter uncertainty and accounted for drug wastage in dosage and cost calculations.
Results
Sequence 3 (1st line: trastuzumab plus docetaxel; 2nd line: T-DM1; 3rd line: trastuzumab plus lapatinib) was the most cost-effective sequence followed by sequence 1 (1st line: pertuzumab plus trastuzumab plus docetaxel; 2nd line: T-DM1; 3rd line: capecitabine plus lapatinib), and sequence 4 (1st line: trastuzumab plus docetaxel; 2nd line: trastuzumab plus lapatinib; 3rd line: trastuzumab plus capecitabine), respectively. The model was sensitive to costs and transition probabilities, but not particularly sensitive to the wastage assumption.
Conclusions
From the perspective of the TNHIA, trastuzumab plus docetaxel as 1st line followed by T-DM1 and trastuzumab plus lapatinib as 2nd and 3rd line represents the most cost-effective strategy among the four sequences considered for treating HER-2-positive mBC patients.
Keywords: Breast cancer, Neoplasm metastasis, Cost-effectiveness analysis, Markov chains, Taiwan
Highlights
-
•
In health systems in which resources are scarce, treatment selection should be carefully considered to preserve efficiency.
-
•
We conducted a CEA of four treatment sequences for HER-2-positive mBC in Taiwan, accounting for intravenous drug wastage.
-
•
Results of such analyses can be useful to policymakers as they decide on coverage of expensive new anticancer treatments.
1. Introduction
In 2018, according to Globocan, breast cancer was still the leading cause of cancer death in women worldwide [1]. In Taiwan, a country with a population of 23 million, the incidence of breast cancer is rising, with one in 120 women suffering from the disease [2]. The standardized incidence was 52.34 per 100,000 person-years and 93.00 per 100,000 person-years in 1997 and 2013 respectively, representing a 1.8-fold increase [2]. Amplification of the human epidermal growth factor receptor HER-2 gene is present in 18%–20% of breast cancers [3]. Chinese breast cancer patients have been reported to have an even higher prevalence of HER-2-positive tumors—around 25.8% [4]. Amplification is the primary mechanism of HER-2 overexpression, and multiple studies have demonstrated an association with a worsened overall survival (OS) rate and a shorter time to relapse [5]. HER-2-directed therapy has drastically increased the survival of metastatic breast cancer (mBC) patients in the last two decades [5]. Since the introduction of the monoclonal antibody trastuzumab in 1998, there have been three subsequent approvals for anti-HER-2 agents for mBC. Lapatinib is a dual tyrosine kinase inhibitor that interferes with the HER-2-neu and EGFR pathways [6]. It received approval in 2007 with the use of capecitabine. In the phase III trial that led to its approval, the addition of lapatinib to capecitabine produced a longer median OS for the combination arm, 75 weeks vs. 64.7 weeks, respectively [7]. Nonetheless, in the Cerebel trial [8], the addition of lapatinib to capecitabine was found to be inferior to the combination of trastuzumab and capecitabine, with longer progression-free survival (PFS) and OS for the trastuzumab-containing arm. In 2012, pertuzumab, an anti-HER-2 humanized monoclonal antibody that hinders receptor dimerization was approved as a first-line treatment for mBC in combination with trastuzumab and docetaxel [9]. This approval occurred after the results of a Phase III trial demonstrated an improvement in OS of 15.7 months induced by in pertuzumab combination with trastuzumab and docetaxel when compared with trastuzumab, docetaxel, and a placebo [9]. Lastly, in 2013, ado-trastuzumab emtansine was approved as a monotherapy for mBC treatment-experienced patients with trastuzumab and taxanes. In the Phase II trial that led to its approval, ado-trastuzumab emtansine was compared with lapatinib and capecitabine, and there was a 5.8-month improvement in survival rates, 29.9 months with ado-trastuzumab vs. 20.9 months with lapatinib, capecitabine [10].
A clinical consensus regarding optimal sequencing has emerged, supported by the American Society of Clinical Oncology [11] and the National Comprehensive Cancer Network [12]. First-line therapy for patients with mBC includes the combination of pertuzumab to trastuzumab and docetaxel, followed by trastuzumab emtansine at progression, and in third-line setting lapatinib in combination with capecitabine is considered the up-front treatment for HER2-positive mBC Nonetheless, the optimal treatment sequence from a pharmacoeconomic standpoint has to be analyzed in each health-payer system to further inform local decision making for practitioners and policymakers. In the current paper, we adopt the perspective of the Taiwanese health payer system.
Taiwan has a unique single-payer healthcare system covering 99% of its population under the government-run National Health Insurance (NHI) scheme [13]. The Taiwanese government enacted its universal health insurance program in 1995 with the goal to improve the efficiency of the country’s existing healthcare system, thus achieving social justice and equity in meeting the healthcare needs of its population [13]. The system is known for its good accessibility, short waiting times, nationwide research database and comprehensive coverage including cancer care [13].
Typically, cost-effectiveness studies conducted from the Taiwanese NHI perspective have compared mBC treatment regimens per line of therapy, although sequential therapy is the real-life clinical practice most utilized. Recently, Lang H–C et al. simulated the costs and effectiveness associated with the use of trastuzumab therapy in HER-2/neu positive early breast cancer patients, from the Taiwanese NHI perspective [14]. Adjuvant trastuzumab was associated with 1.63 quality-adjusted life years (QALY) compared with no adjuvant trastuzumab treatment [14]. In the base case scenario, the ICER was estimated at US $51,863 per QALY gained, which led the authors to conclude the adjuvant Trastuzumab is a cost-effective treatment option for patients with HER-2 positive breast cancer considering a willingness-to-pay threshold of three-times GDP per capita in Taiwan [14]. Another recent study by Leung HWC et al. estimated the incremental cost-effectiveness ratio of pertuzumab combined with trastuzumab and docetaxel (TDP) compared to trastuzumab combined with docetaxel (TD) in the first-line treatment for HER-2 positive mBC. The authors reported an estimated ICER of NT$18,999,687 (US$593,741) per QALY gained for TDP. The authors concluded that TDP would be cost-effective for HER-2 positive mBC patients under favorable drug cost assumptions only [15]. In the present study, we expand on these studies by conducting a comparative cost-effectiveness analysis of four possible treatment sequences for HER-2-positive mBC in Taiwan and accounting for intravenous drug wastage. Results of such comparative analysis can be more useful to policymakers as they decide on coverage of very expensive new anticancer treatments with marginal survival improvements [[6], [7], [8], [9], [10]].
2. Model overview
We conducted an economic evaluation to simulate the cost and effectiveness associated with first-line THP, followed by T-DM1 and lapatinib/capecitabine for patients newly diagnosed with HER-2 -positive mBC in Taiwan, compared with three other sequencing modalities. A hypothetical cohort of patients with the same characteristics as patients in the CLEOPATRA and EMILIA phase III trials [10,16]. We based sequencing modalities on published phase III trials and assessed the relevance of their use in Taiwan based on international and local hospital treatment protocols. The strategies adopted for this analysis are presented in Table 1.
Table 1.
List of treatment sequences.
| First-line | Second-line | Third-line | Abbreviation | |
|---|---|---|---|---|
| Sequence 1 | Pertuzumab + trastuzumab + docetaxel (THP) [17] | T-DM110 | Capecitabine + lapatinib [7] | THP → TDM1 → Cape/Lapat |
| Sequence 2 | Pertuzumab + trastuzumab + docetaxel (THP) [17] | Trastuzumab + lapatiniba16 | Trastuzumab + capecitabine [18] | THP → Trastuz/Lapat → Trastuz/Cape |
| Sequence 3 | Trastuzumab plus docetaxel [17] | T-DM110 | Trastuzumab + lapatiniba16 | Trastuz/Docet → T-DM1 → Trastuz/Lapat |
| Sequence 4 | Trastuzumab plus docetaxel [17] | Trastuzumab + lapatiniba16 | Trastuzumab + capecitabine [18] | Trastuz/Docet →Trastuz/Lapat → Trastuz/Cape |
Rarely used in the Taiwanese setting; Abbreviation: T-DM1, ado-trastuzumab emtansine.
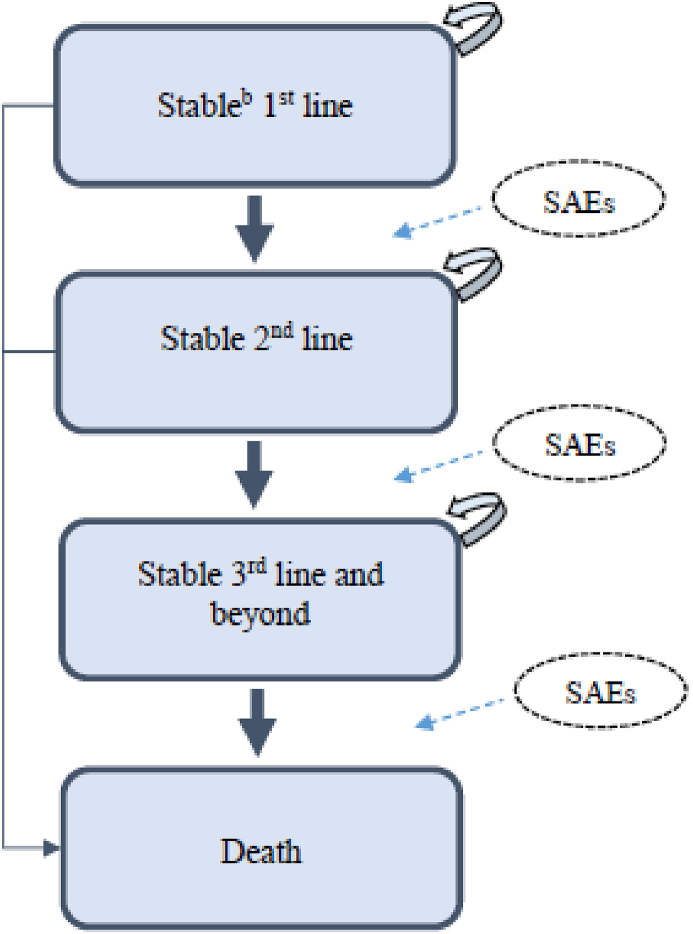
This economic evaluation was carried out according to the Taiwanese National Health Insurance Administration’s (TNHIA) perspective. The Markov model used for the current study is described in detail elsewhere [19]. In short, we utilized a Markov model (weekly cycle length) with four mutually exclusive states, PFS 1st line, PFS 2nd line, PFS 3rd line, and death [19] (Fig. 1 - Adapted from Diaby et al. 201,6 [20]) to project the costs and utilities that patients would accumulate over a lifetime time horizon under the sequencing strategies.
Fig. 1.
Markov model for HER2 metastic breast cancer a a: Adapted from Diaby et al. 201,620; b: Progression-free; SAEs: Serious adverse events.
Patients eligible for treatment initiation entered the model through the stable state (PFS 1st line with no adverse events). Per simulation cycle, patients could remain stable, transition to the next treatment sequence if the disease progressed, grade ¾ adverse events (AEs) occurred, or die, based on transition probabilities. We assumed that patients would receive a 3-month palliative care and ultimately die after treatment sequence failure [21].
The outcomes of interest were PFS, OS, costs (2018 U S. Dollars), quality-adjusted life years (QALYs), and the incremental cost per QALY-gained ratio. We assessed the cost-effectiveness of the four treatment options using the following willingness-to-pay (WTP) thresholds: US$50,000 per QALY, US$100,000 per QALY, US$150,000 per QALY, and US$200,000 per QALY [[22], [23], [24], [25]]. We considered the net-monetary benefit (NMB) as an additional metric to assess the cost-effectiveness of the competing treatment options [26]. We used TreeAge Pro 2017 (TreeAge Software Inc., Williamstown, MA, USA) to conduct all analyses.
2.1Markov model: clinical input parameters
We obtained equations for the weekly cycle length transitional probabilities from the literature [19]. Diaby et al. [19]. Approximated individual patient data (IPD) from PFS and OS Kaplan–Meier curves of published Phase III trials comparing the treatments under evaluation [17]. Afterward, they fitted five standard parametric distributions to the IPDs: exponential, Weibull, Gompertz, lognormal, and log-logistic. The authors fitted log-logistic models to the reconstructed IPD and derived equations for transition probabilities using the shape and scale parameters of the fitted models [19].
2.2. Markov model: cost input parameters
We identified direct medical costs from the TNHIA perspective (Table 2). Costs were considered for (1) medical visits, (2) acquisition of treatment, (3) computed tomography scans, (4) laboratory tests, (5) echocardiograms, and costs associated with (6) managing AEs (grades 3–4) and (7) end-of-life care. Using the national claim database in Taiwan [27], the cost of palliative/end-of-life care was calculated by summing the total medical costs related to mBC patients starting from their first palliative claim and assigned one time to patients who experienced disease progression after the third-line treatment sequence and to those who discontinued treatment due to AEs. Dosing regimens of HER-2 targeted and chemotherapeutic agents are mostly weight or body surface area (BSA) dependent. In order to calculate the appropriate number of tablets and/or vials per drug and line of therapy, we used the average Taiwanese female population parameter for height (155.9 cm) and weight (58.1 kg) and the calculated average BSA of 1.59 m2 [28]. In our base case analysis, we used full unit prices per tablet or opened vial and incorporated waste in our cost parameters. For example, one vial of Herceptin® contains 440 mg of trastuzumab and has a list price of US$1,852.07. The loading dose recommendations per guideline are 8 mg/kg of body weight [12]. Using the average Taiwanese body weight of 58.1 kg, the total trastuzumab loading dose yielded to 464.8 mg and thus 2 × 440 mg vials of Herceptin® at a price of US$3,704.14. Details of all price and dosage calculations can be found in Appendix 1. All cost data were converted from Taiwanese Dollars (TWD) to U.S. Dollars (USD) using a rate of 1 USD = 31.92 TWD [29] and were inflated using 2018 as the base year.
Table 2.
Model parameters with the ranges for sensitivity analysis.
| Description | Value (range or 95% CI) | PSA (SD) | References | Distribution |
|---|---|---|---|---|
| Medical visit ($) | ||||
| Physician Fees | 8.15 (6.11–10.19) | 1.02 | [30] | Gamma |
| Acquisition cost of treatments ($) | ||||
| Loading dose pertuzumab (840 mg) | 4564.42 (2,282.21–6,846.63) | 1141.11 | Personal communicationa | Gamma |
| Maintenance dose pertuzumab (420 mg) | 2282.21 (1,141.11–3,423.32) | 570.55 | Personal communicationa | Gamma |
| Loading dose trastuzumab (8 mg/kg) | 3704.14 (1,852.07–5,556.21) | 926.04 | [31] | Gamma |
| Maintenance dose trastuzumab (6 mg/kg) | 1852.07 (926.04–2,778.11) | 463.02 | [31] | Gamma |
| Docetaxel (1 mg) | 765.86 (382.93–1,148.79) | 191.47 | [31] | Gamma |
| Pegfilgrastim (6 mg) | 662.68 (331.34–994.02) | 165.67 | [31] | Gamma |
| TDM1 | 4,666.48 (2,333.24–6,999.72) | 1166.62 | Personal communicationa | Gamma |
| Capecitabine (500 mg) | 158.27 (79.14–237.41) | 39.57 | [31] | Gamma |
| Lapatinib (1500 mg daily) | 684.18 (342.09–1,026.27) | 171.05 | [31] | Gamma |
| Management of adverse events (grade 3/4) cost ($) | ||||
| Pertuzumab + trastuzumab + docetaxel | 6628.78 (3,351.33–15,468.774) | 3029.36 | [32] | Gamma |
| Trastuzumab + docetaxel | 4749.98 (2,411.92–11,520.08) | 2277.04 | [32] | Gamma |
| TDM1 | 2347.695 (1,166.94–4,626.34) | 864.85 | [32] | Gamma |
| Lapatinib + capecitabine | 601.43 (297.29–1,160.69) | 215.85 | [32] | Gamma |
| Trastuzumab + lapatinib | 321.7 (158.98–619.85) | 115.23 | [32] | Gamma |
| Trastuzumab + capecitabine | 976.68 (519.18–1,954.28) | 358.77 | [32,33] | Gamma |
| Computed tomography scan ($) | 142.86 (107.14–178.57) | 17.86 | [30] | Gamma |
| Laboratory tests ($) | ||||
| Blood work | 6.27 (4.7–7.84) | 0.78 | [30] | Gamma |
| Echocardiogram | 119.05 (89.29–148.82) | 14.88 | [30] | Gamma |
| Palliative care/end-of-life ($) | 7185.72 (3,592.86–10,778.58) | 1796.43 | 26 | Gamma |
| Utilities | ||||
| Progression-free under treatment | 0.786 (0.485–0.935) | 0.113 | [34] | Beta |
| Treatment response | 0.061 (0.025–0.074) | 0.012 | [34] | Beta |
| Utility for disease progression under treatment | 0.538 (0.196–0.848) | 0.163 | [34] | Beta |
| Disutility associated with disease progression and adverse events due to treatments | ||||
| Disease progression | 0.248 (0.289–0.087) | 0.0504 | [34] | Nega beta/uniform |
| Pertuzumab + trastuzumab + docetaxel | 0.056 (0.098–0.016) | 0.0201 | [34,35] | Nega beta/uniform |
| Trastuzumab + docetaxel | 0.040 (0.058–0.011) | 0.0117 | [34,35] | Nega beta/uniform |
| TDM1 | 0.009 (0.013–0.002) | 0.0025 | [35] | Nega beta/uniform |
| Lapatinib + capecitabine | 0.018 (0.032–0.004) | 0.007 | [34] | Nega beta/uniform |
| Trastuzumab + lapatinib | 0.017 (0.026–0.004) | 0.006 | [34,35] | Nega beta/uniform |
| Trastuzumab + capecitabine | 0.040 (0.075–0.009) | 0.016 | [34,35] | Nega beta/uniform |
| Shape and scale parameters | ||||
| OS shape (gamma) | ||||
| Pertuzumab + trastuzumab + docetaxel | 0.544 (0.460–0.642) | 0.09264 | [19] | Gamma |
| Trastuzumab + docetaxel | 0.577 (0.501–0.663) | 0.08267 | [19] | Gamma |
| TDM1 | 0.474 (0.414–0.543) | 0.06585 | [19] | Gamma |
| Lapatinib + capecitabine | 0.465 (0.357–0.606) | 0.12,695 | [19] | Gamma |
| Trastuzumab + lapatinib | 0.588 (0.468–0.740) | 0.13,861 | [19] | Gamma |
| Trastuzumab + capecitabine | 0.451 (0.338–0.602) | 0.13,475 | [19] | Gamma |
| OS scale (lambda) | ||||
| Pertuzumab + trastuzumab + docetaxel | 0.019 (0.016–0.023) | 0.00309 | [19] | Gamma |
| Trastuzumab + docetaxel | 0.025 (0.022–0.029) | 0.00333 | [19] | Gamma |
| TDM1 | 0.033 (0.029–0.037) | 0.0038 | [19] | Gamma |
| Lapatinib + capecitabine | 0.016 (0.013–0.020) | 0.00391 | [19] | Gamma |
| Trastuzumab + lapatinib | 0.019 (0.015–0.024) | 0.00427 | [19] | Gamma |
| Trastuzumab + capecitabine | 0.041 (0.033–0.051) | 0.00913 | [19] | Gamma |
| PFS shape (gamma) | ||||
| Pertuzumab + trastuzumab + docetaxel | 0.622 (0.561–0.690) | 0.066 | [19] | Gamma |
| Trastuzumab + docetaxel | 0.555 (0.504–0.612) | 0.05464 | [19] | Gamma |
| TDM1 | 0.611 (0.552–0.675) | 0.06293 | [19] | Gamma |
| Lapatinib + capecitabine | 0.516 (0.423–0.630) | 0.10,569 | [19] | Gamma |
| Trastuzumab + lapatinib | 0.554 (0.481–0.638) | 0.08017 | [19] | Gamma |
| Trastuzumab + capecitabine | 0.508 (0.410–0.630) | 0.11,211 | [19] | Gamma |
| PFS scale (lambda) | ||||
| Pertuzumab + trastuzumab + docetaxel | 0.052 (0.047–0.058) | 0.00597 | [19] | Gamma |
| Trastuzumab + docetaxel | 0.074 (0.067–0.082) | 0.00739 | [19] | Gamma |
| TDM1 | 0.104 (0.093–0.117) | 0.01184 | [19] | Gamma |
| Lapatinib + capecitabine | 0.034 (0.028–0.041) | 0.00669 | [19] | Gamma |
| Trastuzumab + lapatinib | 0.082 (0.070–0.097) | 0.01376 | [19] | Gamma |
| Trastuzumab + capecitabine | 0.115 (0.094–0.142) | 0.02444 | [19] | Gamma |
| Weekly probability of developing adverse events | ||||
| Pertuzumab + trastuzumab + docetaxel | 0.003 (0.001–0.004) | 0.00064 | [17] | Beta |
| Trastuzumab + docetaxel | 0.002 (0.001–0.003) | 0.00049 | [17] | Beta |
| TDM1 | 0.004 (0.002–0.006) | 0.00101 | [10] | Beta |
| Lapatinib + capecitabine | 0.011 (0.005–0.016) | 0.00263 | [36] | Beta |
| Trastuzumab + lapatinib | 0.002 (0.000–0.003) | 0.00049 | [16] | Beta |
| Trastuzumab + capecitabine | 0.006 (0.003–0.009) | 0.00144 | [18] | Beta |
| Discount rate | 0.05 (0–0.1) | – | [37] | Uniform |
The drug prices of pertuzumab and T-DM1 are based on the amount that an individual hospital charges patients (Ko Y. Personal communication. September 24, 2018). Abbreviations: OS, overall survival; PFS, progression-free survival; PSA, probabilistic sensitivity analysis; SD, standard deviation; TDM1, ado-trastuzumab emtansine.
2.3. Markov model: health utility input parameters
Health utilities associated with the baseline health state, progression status, treatment sequence lines, and AEs were derived from Phase III trials and the literature [34,35,38,39]. We assumed that health utilities are associated with breast cancer progression status, therapy lines, and treatment-related AEs. On the basis of our assumptions, we adjusted health utilities based on progression status, therapy lines, and AEs. We also accounted for the utility decrements associated with the progression of the disease and treatment-related AEs.
2.4. Discounting and half-cycle correction
We converted the 5% annual discount rate [37] to the weekly discount rate to determine the net present value. Additionally, a half-cycle correction was applied to costs and health utilities.
2.5. Base case analysis
An efficiency frontier was plotted using the cost-effectiveness pairs (cost; QALYs) of each treatment strategy. The aim was to identify and remove dominated treatment options. The non-dominated treatment sequences were compared pairwise, and the ICERs derived from these comparisons were established using the benchmark WTP and NMB.
2.6. Sensitivity and scenario analyses
A tornado analysis was performed to identify the key drivers of our Markov model for six possible pairwise comparisons among the treatment sequences. We varied each key parameter according to their associated range. Baseline values of each model key parameter were varied between 25% and 50% in the absence of defined data for the range of a parameter (Table 2).
To examine the robustness of the mean ICERs (95% confidence intervals) we conducted probabilistic sensitivity analysis (PSA) using 10,000 Monte Carlo simulations and used standard statistical methods to determine the PSA distribution [40]. Cost-effectiveness acceptability curves were developed from these simulations.
We performed a scenario analysis to assess the impact of the “no wastage” assumption on our results. Prices per vial for each intravenous drug were broken down and only the exact amounts, based on body weights and BSA, were incorporated in our cost calculations. For example, as we calculated the trastuzumab loading dose of 464.8 mg based on an average body weight of 58.1 kg, the amount of Herceptin® 440-mg vials needed were 1.056, yielding a total cost of US$1,955.79 (US$1,852.07 per vial × 1.056). More information on the cost inputs factoring into the waste scenario can be found in Appendix 2e.
3. Results
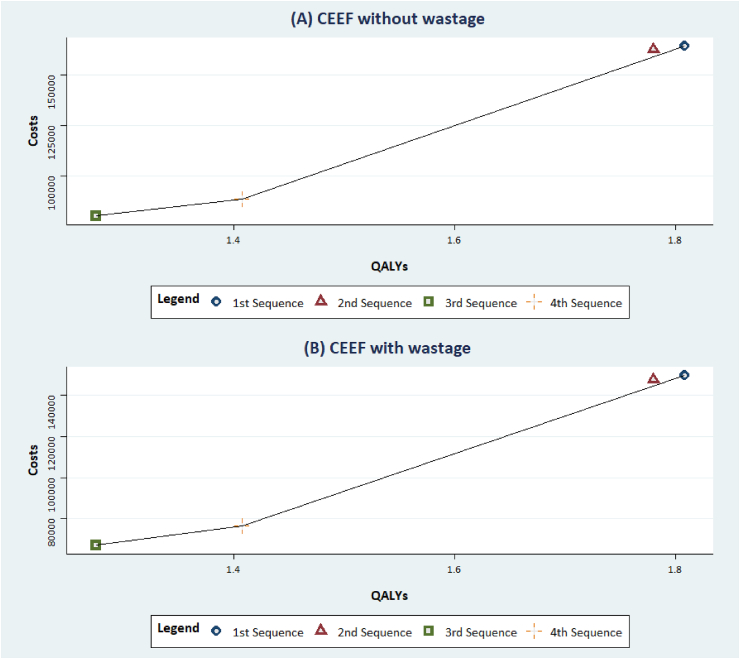
The efficiency frontier scatter plots (no wastage and wastage considerations) show that 2nd sequence (THP → Trastuz/Lapat → Trastuz/Cape) is extendedly dominated, where the ICER for the treatment is higher than a more effective treatment, by other treatments: 1st sequence (THP → T-DM1 → Cape/Lapat), 3rd sequence (Trastuz/Docet → T-DM1 → Trastuz/Lapat), and 4th sequence (Trastuz/Docet → Trastuz/Lapat → Trastuz/Cape) (Fig. 2).
Fig. 2.
Cost-Effectiveness efficiency frontiers without and with wastage considerations. CEEF: Cost-Effectiveness efficiency frontier. (A) CEEF without wastage and (B) CEEF with wastage considerations: The blue circle, red triangle, green square, and the yellow plus sign represent the 1st, 2nd, 3rd and 4th sequence respectively. The black line represents the efficiency frontier. . (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
After excluding the 2nd sequence, we compared the ICERs of other non-dominated treatment sequences and found that 1st sequence (THP → T-DM1 → Cape/Lapat) and 4th sequence (Trastuz/Docet → Trastuz/Lapat → Trastuz/Cape) were not cost-effective compared with 3rd sequence (Trastuz/Docet → T-DM1 → Trastuz/Lapat)—except for a US$200,000 WTP threshold was set at (Table 3). On the basis of the NMB approach, we found that the 3rd sequence returned the greatest benefit, followed by the 1st and 4th sequences, respectively (Table 3).
Table 3.
Base case analysis results (without wastage consideration).
| Treatment sequence | Cost | Incr Cost | QALY | Incr QALY | Incr C/E | NMB |
|||
|---|---|---|---|---|---|---|---|---|---|
| ℷ = 50,000 | ℷ = 100,000 | ℷ = 150,000 | ℷ = 200,000 | ||||||
| All referencing common baseline | |||||||||
| 3rd | 79,958.7 | 1.275 | −16,225.89 | 47,506.92 | 111,239.73 | 174,972.55 | |||
| 4th | 88,392.98 | 8434.28 | 1.407 | 0.132 | 63,887.71 | −1833.42 | 4767.45 | 11,368.31,043 | 17,969.17 |
| 2nd | 162,393 | 82,434.33 | 1.781 | 0.506 | 162,919.8 | −57,135.27 | −31,836.2 | −6537.1525 | 18,761.91 |
| 1st | 164,211.4 | 84,252.69 | 1.808 | 0.534 | 157,888.1 | −137,530.26 | −30,890.4 | −4209.29 | 22,471.84 |
Abbreviations: C/E, cost-effectiveness; Incr, incremental; NMB, net-monetary benefit; QALY, quality-adjusted life years.
3.1. Sensitivity and scenario analyses
The tornado analysis suggests that our results are not sensitive to changes in the parameters of the Markov model. The model was sensitive to costs and transition probabilities.
Although the wastage assumption affected the magnitude of the estimated ICERs, it did not change the ranking of the treatment sequences obtained in the base case analysis (Table 4).
Table 4.
Scenario analysis results with wastage consideration.
| Treatment sequence | Cost | Incr Cost | QALY | Incr QALY | Incr C/E | NMB |
|||
|---|---|---|---|---|---|---|---|---|---|
| ℷ = 50,000 | ℷ = 100,000 | ℷ = 150,000 | ℷ = 200,000 | ||||||
| All referencing common baseline | |||||||||
| 3rd | 67,128.18 | 1.275 | −3395.37 | 60,337.45 | 124,070.26 | 187,803.07 | |||
| 4th | 76,487.72 | 9359.55 | 1.407 | 0.132 | 70,896.37 | −2758.68 | 3842.18 | 10,443.05 | 17,043.91 |
| 2nd | 147,559.6 | 80,431.45 | 1.781 | 0.506 | 158,961.4 | −55,132.39 | −29,833.34 | −4534.28 | 20,764.77 |
| 1st | 149,759 | 82,630.86 | 1.808 | 0.534 | 154,848.9 | −55,949.73 | −29,268.59 | −2587.46 | 24,093.67 |
Abbreviations: C/E, cost-effectiveness; Incr, incremental; NMB, net-monetary benefit; QALY, quality-adjusted life year.
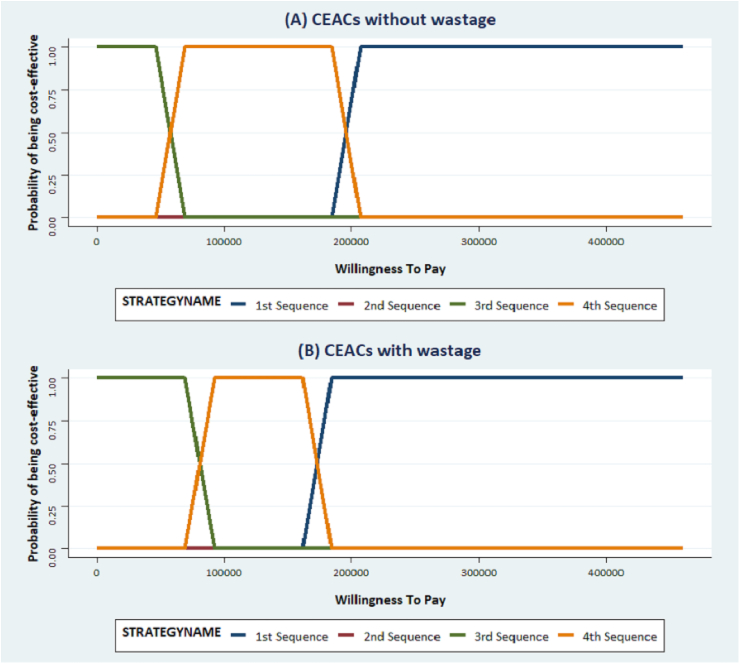
The results of the PSA confirmed the robustness of our results. In all cases (without and with consideration of drug wastage), THP → T-DM1 → Cape/Lapat had a 100% probability of being cost-effective when the WTP was set above US$200,000 (Fig. 3).
Fig. 3.
Cost-effectiveness acceptability curves without and with wastage considerations. . CEACs: Cost-effectiveness Acceptability Curves. Cost-effectiveness acceptability curves without wastage (A) and with wastage considerations (B): The blue, purple, green and orange lines represent the 1st, 2nd, 3rd and 4th sequence respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Sequential HER-2 -directed therapies have led to significant improvements in outcomes for patients with metastatic HER-2 overexpressing breast cancer [5]. Nonetheless, these medications are associated with a high price tag. In this study, we analyzed the ideal sequence from a pharmacoeconomic standpoint for the three FDA approved therapies for the Taiwanese patient population. Sequence 3, first-line trastuzumab plus docetaxel, followed by second-line ado-trastuzumab emtansine, and third-line trastuzumab/lapatinib was the most cost-effective sequence, dominating sequences 1, 2, and 4. Despite its higher clinical efficacy, sequence 1 (pertuzumab, trastuzumab, docetaxel, followed by ado-trastuzumab emtansine, followed by capecitabine and lapatinib) was not considered cost-effective (WTP < US $200,000) based on current drug costs. However, it is important to note that there is an ongoing debate on whether the WTP threshold used in economic evaluations of oncologic and non-oncologic interventions should be the same [[41], [42], [43]]. If a higher WTP threshold were adopted, sequence 1 could also be a cost-effective treatment strategy.
The results found in the current study were robust to variability and uncertainty in the parameter estimates of the Markov model used. Our study reinforces the results of an earlier study conducted using the Taiwanese NHI perspective which also finds that trastuzumab plus docetaxel was the most cost-effective first-line treatment [15]. Our study however also finds that second-line ado-trastuzumab emtansine and third-line trastuzumab/lapatinib is the most cost-effective treatment sequence. Additionally, with the potential market approval of a Trastuzumab biosimilar in Taiwan in 2020, it is likely that treatment sequences incorporating trastuzumab as first-line treatment will continue to remain the most cost-effective treatment option.
The results of the current study are also similar to those reported in the United States by Diaby et al. [19]. The least clinically effective sequence (generating an extra 1.275 QALYs), but most cost-effective, was trastuzumab/docetaxel as a first-line therapy, T-DM1 as a second-line one, and trastuzumab/lapatinib as a third-line one (Trastuz/Docet → T-DM1 → Trastuz/Lapat). In the United Kingdom, the National Institute for Clinical Excellence considers a new technology cost-effective when its incremental cost per QALY gained, compared to the standard of care, is £30,000 or less [44]. The World Health Organization considers interventions that are less than the national income per capita as very cost-effective and a cost of up to three times GDP per capita as cost-effective [45]. However, using these alternate thresholds, sequence 1 (pertuzumab, trastuzumab, docetaxel, followed by ado-trastuzumab emtansine, followed by capecitabine and lapatinib) is still not considered cost-effective in the Taiwanese setting.
As most health economic studies, ours has limitations. Our model did not include the cost of imaging often obtained for restaging and defining progression, which leads to subsequent therapies. Nonetheless, we judged that these costs would be similar across all sequences and would therefore not materially affect our findings. The model also did not include the addition of hormonal therapies, which are often used in combination with HER-2 -directed therapies in patients who are estrogen receptor positive in addition to being HER-2 positive. We used several assumptions to build the Markov model to simulate the costs and effectiveness of the treatment sequences that were evaluated. The impact of these assumptions and inherent uncertainty were tested in several sensitivity and scenario analyses, which showed the robustness of our findings.
Notwithstanding the limitations of this study, it is our belief that its conception and implementation were done in light of international guidelines for the conduct of state of the art economic evaluations [46]. In addition, our analyses accounted for the impact of drug wastage on the cost-effectiveness of the treatment sequences compared.
In summary, the most cost-effective treatment sequence in our study is not concordant with the most clinically effective strategy. Given the clinical benefit of pertuzumab in the first-line setting, most practitioners would likely not implement sequence 3 (Trastuz/Docet → T-DM1 → Trastuz/Lapat) if reimbursement for pertuzumab was universal in Taiwan. Nonetheless, given the lack of coverage of pertuzumab by the TNHIA, Trastuz/Docet → T-DM1 → Trastuz/Lapat is the ideal sequence from a pharmacoeconomic standpoint. We need to bring all stakeholders involved in breast cancer treatment decisions together in order to plug this gap.
Author contributions
Conception/Design: Vakaramoko Diaby, Alqhtani Hussain, Sascha van Boemmel-Wegmann, Ching-Yu Wang, Askal Ayalew Ali, Gilberto de Lima Lopes; Collection and/or assembly of data: Yu Ko, Gilberto de Lima Lopes. Data analysis and interpretation: Vakaramoko Diaby, Alqhtani Hussain, Sascha van Boemmel-Wegmann, Ching-Yu Wang, Rajesh Balkrishnan.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgement
This study was not funded.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2019.11.012.
Contributor Information
Vakaramoko Diaby, Email: v.diaby@cop.ufl.edu.
Hussain Alqhtani, Email: hussain.alqhtani@ufl.edu.
Sascha van Boemmel-Wegmann, Email: wegmann@ufl.edu.
Ching-Yu Wang, Email: chingyuwang@ufl.edu.
Askal Ayalew Ali, Email: askal.ali@famu.edu.
Rajesh Balkrishnan, Email: rb9ap@virginia.edu.
Yu Ko, Email: nancyko@tmu.edu.tw.
Sofia Palacio, Email: sofia.palacio@jhsmiami.org.
Gilberto de Lima Lopes, Email: Glopes@med.miami.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Liu F.-C., Lin H.-T., Kuo C.-F., See L.-C., Chiou M.-J., Yu H.-P. Epidemiology and survival outcome of breast cancer in a nationwide study. Oncotarget. 2017;8(10):16939. doi: 10.18632/oncotarget.15207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slamon D.J., Clark G.M., Wong S.G., Levin W.J., Ullrich A., McGuire W.L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 4.Li J., Zhang B.-N., Fan J.-H. A nation-wide multicenter 10-year (1999-2008) retrospective clinical epidemiological study of female breast cancer in China. BMC Canc. 2011;11(1):364. doi: 10.1186/1471-2407-11-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross J.S., Slodkowska E.A., Symmans W.F., Pusztai L., Ravdin P.M., Hortobagyi G.N. The HER-2 receptor and breast cancer: ten years of targeted anti–HER-2 therapy and personalized medicine. The Oncologist. 2009;14(4):320–368. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 6.Higa G.M., Abraham J. Lapatinib in the treatment of breast cancer. Expert Rev Anticancer Ther. 2007;7(9):1183–1192. doi: 10.1586/14737140.7.9.1183. [DOI] [PubMed] [Google Scholar]
- 7.Cameron D., Casey M., Oliva C., Newstat B., Imwalle B., Geyer C.E. Lapatinib plus capecitabine in women with HER-2–positive advanced breast cancer: final survival analysis of a phase III randomized trial. The Oncologist. 2010;15(9):924–934. doi: 10.1634/theoncologist.2009-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pivot X., Manikhas A., Żurawski B. Cerebel (EGF111438): a phase III, randomized, open-label study of lapatinib plus capecitabine versus trastuzumab plus capecitabine in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer. J Clin Oncol. 2015;33(14):1564–1573. doi: 10.1200/JCO.2014.57.1794. [DOI] [PubMed] [Google Scholar]
- 9.Baselga J., Cortés J., Kim S.-B. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verma S., Miles D., Gianni L. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giordano S.H., Temin S., Kirshner J.J. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(19):2078–2099. doi: 10.1200/JCO.2013.54.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network . 2018. NCCN clinical practice guidelines in oncology: breast cancer.https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf Version 3. Published 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu T.-Y., Majeed A., Kuo K.N. An overview of the healthcare system in Taiwan. Lond J Prim Care. 2010;3(2):115–119. doi: 10.1080/17571472.2010.11493315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang H.-C., Chen H.-W., Chiou T.-J., Chan A.L.F. The real-world cost-effectiveness of adjuvant trastuzumab in HER-2/neu-positive early breast cancer in Taiwan. J Med Econ. 2016;19(10):923–927. doi: 10.1080/13696998.2016.1185013. [DOI] [PubMed] [Google Scholar]
- 15.Leung H.W.C., Chan A.L.F., Muo C.-H., Leung J.H. Cost-effectiveness of pertuzumab combined with trastuzumab and docetaxel as a first-line treatment for HER-2 positive metastatic breast cancer. Expert Rev Pharmacoecon Outcomes Res. 2018;18(2):207–213. doi: 10.1080/14737167.2018.1386559. [DOI] [PubMed] [Google Scholar]
- 16.Blackwell K.L., Burstein H.J., Storniolo A.M. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28(7):1124–1130. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 17.Swain S.M., Kim S.B., Cortes J. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14(6):461–471. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Minckwitz G., du Bois A., Schmidt M. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a German breast group 26/breast international group 03-05 study. J Clin Oncol. 2009;27(12):1999–2006. doi: 10.1200/JCO.2008.19.6618. [DOI] [PubMed] [Google Scholar]
- 19.Diaby V., Adunlin G., Ali A.A. Cost-effectiveness analysis of 1st through 3rd line sequential targeted therapy in HER2-positive metastatic breast cancer in the United States. Breast Canc Res Treat. 2016;160(1):187–196. doi: 10.1007/s10549-016-3978-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diaby V., Ali A.A., Adunlin G., Kohn C.G., Montero A.J. Parameterization of a disease progression simulation model for sequentially treated metastatic human epidermal growth factor receptor 2 positive breast cancer patients. Curr Med Res Opin. 2016;32(6):991–996. doi: 10.1185/03007995.2016.1149056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorensen S.V., Goh J.W., Pan F. Incidence-based cost-of-illness model for metastatic breast cancer in the United States. Int J Technol Assess Health Care. 2012;28(1):12–21. doi: 10.1017/S026646231100064X. [DOI] [PubMed] [Google Scholar]
- 22.Cameron D., Ubels J., Norström F. On what basis are medical cost-effectiveness thresholds set? Clashing opinions and an absence of data: a systematic review. Glob Health Action. 2018;11(1) doi: 10.1080/16549716.2018.1447828. 1447828-1447828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martín-Fernández J., Polentinos-Castro E., del Cura-González M.I. Willingness to pay for a quality-adjusted life year: an evaluation of attitudes towards risk and preferences. BMC Health Serv Res. 2014;14 doi: 10.1186/1472-6963-14-287. 287-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neumann P.J., Cohen J.T., Weinstein M.C. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 25.Shiroiwa T., Sung Y.K., Fukuda T., Lang H.C., Bae S.C., Tsutani K. International survey on willingness-to-pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness? Health Econ. 2010;19(4):422–437. doi: 10.1002/hec.1481. [DOI] [PubMed] [Google Scholar]
- 26.Gray A.M., Clarke P.M., Wolstenholme J.L., Wordsworth S. vol. 3. Oxford University Press; 2011. (Applied methods of cost-effectiveness analysis in healthcare). [Google Scholar]
- 27.National Health Insurance Research Database, Taiwan. http://nhird.nhri.org.tw/en/index.htm. Accessed March 1, 2017.
- 28.Ministry of Health and Welfare, Department of Statistics, Taiwan. https://dep.mohw.gov.tw/DOS/cp-1720-7366-113.htmlAccessed March 1, 2017.
- 29.Central Bank of the Republic of China, Taiwan. https://www.cbc.gov.tw/mp2.html. Accessed May 12, 2018.
- 30.National Health Insurance Administration, Ministry of Health and Welfare, Taiwan. Fee Schedule for Medical Services of National Health Insurance. https://www.nhi.gov.tw/Content_List.aspx?n=58ED9C8D8417D00B&topn=D39E2B72B0BDFA15. Accessed March 1, 2017.
- 31.National Health Insurance Administration, Ministry of Health and Welfare, Taiwan. Health Insurance Drug Inquiries. https://www.nhi.gov.tw/QueryN/Query1.aspx?n=FC660C5B07007373&sms=36A0BB334ECB4011&topn=3185A4DF68749BA9&upn=80567D1327F69CB9. Accessed March 1 2017.
- 32.Niraula S., Amir E., Vera-Badillo F., Seruga B., Ocana A., Tannock I.F. Risk of incremental toxicities and associated costs of new anticancer drugs: a meta-analysis. J Clin Oncol. 2014;32(32):3634–3642. doi: 10.1200/JCO.2014.55.8437. [DOI] [PubMed] [Google Scholar]
- 33.Garrison L.P., Jr., Lubeck D., Lalla D., Paton V., Dueck A., Perez E.A. Cost-effectiveness analysis of trastuzumab in the adjuvant setting for treatment of HER2-positive breast cancer. Cancer. 2007;110(3):489–498. doi: 10.1002/cncr.22806. [DOI] [PubMed] [Google Scholar]
- 34.Lloyd A., Nafees B., Narewska J., Dewilde S., Watkins J. Health state utilities for metastatic breast cancer. Br J Canc. 2006;95(6):683–690. doi: 10.1038/sj.bjc.6603326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Attard C.L., Brown S., Alloul K., Moore M.J. Cost-effectiveness of folfirinox for first-line treatment of metastatic pancreatic cancer. Curr Oncol. 2014;21(1) doi: 10.3747/co.21.1327. e41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geyer C.E., Forster J., Lindquist D. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 37.International Society for Pharmacoeconomics and Outcomes Research . 2006. Guidelines of methodological standards for pharmacoeconomic evaluations in Taiwan.https://tools.ispor.org/PEguidelines/source/2006_PEG_EN_2009.pdf Published. [Google Scholar]
- 38.Doyle S., Lloyd A., Walker M. Health state utility scores in advanced non-small cell lung cancer. Lung Cancer. 2008;62(3):374–380. doi: 10.1016/j.lungcan.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 39.Nafees B., Stafford M., Gavriel S., Bhalla S., Watkins J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6:84. doi: 10.1186/1477-7525-6-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Briggs A., Sculpher M., Claxton K. OUP Oxford; 2006. Decision modelling for health economic evaluation. [Google Scholar]
- 41.Lang H.C. Willingness to pay for lung cancer treatment. Value Health : The Journal of the International Society for Pharmacoeconomics and Outcomes Research. 2010;13(6):743–749. doi: 10.1111/j.1524-4733.2010.00743.x. [DOI] [PubMed] [Google Scholar]
- 42.Oh D.Y., Crawford B., Kim S.B. Evaluation of the willingness-to-pay for cancer treatment in Korean metastatic breast cancer patients: a multicenter, cross-sectional study. Asia Pac J Clin Oncol. 2012;8(3):282–291. doi: 10.1111/j.1743-7563.2012.01546.x. [DOI] [PubMed] [Google Scholar]
- 43.Chino F., Peppercorn J.M., Rushing C. Going for broke: a longitudinal study of patient-reported financial sacrifice in cancer care. J Oncol Pract. 2018;14(9):e533–e546. doi: 10.1200/JOP.18.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Claxton K., Martin S., Soares M. Methods for the estimation of the national Institute for health and care excellence cost-effectiveness threshold. Health Technol Assess. 2015;19(14):1–503. doi: 10.3310/hta19140. v-vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bertram M.Y., Lauer J.A., De Joncheere K. Cost-effectiveness thresholds: pros and cons. Bull World Health Organ. 2016;94(12):925–930. doi: 10.2471/BLT.15.164418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eddy D.M., Hollingworth W., Caro J.J., Tsevat J., McDonald K.M., Wong J.B. Model transparency and validation: a report of the ISPOR-SMDM modeling good research practices task force-7. Med Decis Mak: an International Journal of the Society for Medical Decision Making. 2012;32(5):733–743. doi: 10.1177/0272989X12454579. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.