Abstract
Background
Although randomized controlled clinical trials are optimal to evaluate the effect of an experimental therapy, single-arm trials are required whenever randomization is unethical or not feasible, such as de-escalation studies. We propose using prospectively identified historical controls to place results of single-arm, de-escalation trials into context.
Methods
POSITIVE is a prospective, single-arm study in young women with hormone-receptor-positive early breast cancer to determine if temporarily interrupting adjuvant endocrine therapy in order to become pregnant increases the risk of a breast cancer event. After 272 women enrolled in POSITIVE, we identified a cohort of 1499 SOFT/TEXT patients potentially eligible to enroll in POSITIVE who did not interrupt endocrine therapy. Method I used the SOFT/TEXT cohort to calculate annualized hazard rates by a piecewise exponential model. Method II used the SOFT/TEXT cohort to group-match SOFT/TEXT patients to POSITIVE patients; sample sets of SOFT/TEXT patients were randomly drawn 5000 times to obtain sets having patient, disease, and treatment characteristics more balanced with POSITIVE participants.
Results
Compared with SOFT/TEXT, POSITIVE participants were younger, less likely to be overweight/obese, had fewer positive nodes, and fewer received aromatase inhibitor or chemotherapy. The estimated 3-year breast cancer free interval event rates were 9.5% (95% CI: 7.9%,11.1%) for Method I and 9.4% (95% CI: 7.8%,10.9%) for Method II, compared with 5.8% initially assumed when POSITIVE was designed.
Conclusion
External control datasets should be identified before launching single-arm, de-escalation trials and methods applied during their conduct to provide context for interim monitoring and interpretation of the final analysis.
Highlights
-
•
Prospective identification of external historical controls for single-arm studies.
-
•
Statistical methods for estimating historical control rates for single-arm studies.
-
•
Methods applied for interim monitoring and final analysis of the POSITIVE study.
1. Introduction
A randomized, controlled clinical trial with concurrent control arm is the optimal way to minimize bias when evaluating the effect of an experimental intervention. Sometimes it is infeasible or unethical to carry out a randomized study, and a single-arm trial is conducted. In addition, there is growing interest in studies designed to de-escalate therapy to reduce morbidities such as adverse effects, inconvenience and costs of standard-of-care treatments [1]. Although randomized non-inferiority studies can be conducted, these require large sample sizes, are costly to conduct, are of little interest to highly resourced funders, and are often difficult to interpret due to the arbitrary nature of the non-inferiority margin. Thus, single-arm de-escalation studies are appealing.
One setting for de-escalation, where randomization is not ethical, is to determine whether temporary interruption of adjuvant endocrine therapy for young breast cancer patients who wish to become pregnant is safe [[2], [3], [4]]. POSITIVE (Pregnancy Outcome and Safety of Interrupting Therapy for women with endocrine responsIVE breast cancer; NCT02308085) is a prospective, single-arm, international study designed to evaluate pregnancy outcomes and safety of temporarily interrupting endocrine therapy for young women with estrogen receptor positive (ER+) breast cancer who desire pregnancy. The interim and final analyses are based on assumptions regarding historical control rates of breast cancer events, and the protocol prospectively planned to reassess these assumptions. POSITIVE is led by the International Breast Cancer Study Group (IBCSG), with global participation from the Breast International Group (BIG), and United States National Clinical Trials Network (US NCTN) coordinated by the Alliance for Clinical Trials in Oncology.
In order to place the results of the POSITIVE study into context, we describe two methods that rely only on baseline characteristics of participants to estimate standard-of-care external control breast cancer event rates for the single-arm POSITIVE trial. To facilitate the Data and Safety Monitoring Committee review scheduled for November 2018, 272 POSITIVE participants enrolled prior to August 1, 2018 were included. We use outcome data for patients in the SOFT and TEXT clinical trials [[5], [6], [7], [8]] who did not interrupt their adjuvant endocrine therapy.
2. Materials and methods
2.1. POSITIVE trial
Women between 18 and 42 years of age who desired to become pregnant and had completed 18–30 months of adjuvant endocrine therapy for early-stage ER + breast cancer were eligible to enroll in POSITIVE. An interruption of endocrine therapy for up to 2 years is permitted to allow pregnancy attempt (after a 3-month washout period), delivery, and breastfeeding. Resumption of endocrine therapy to complete 5–10 years of treatment is expected as soon as pregnancy and breastfeeding are finished, or after it is determined that conception is not possible. The accrual goal was 500 patients.
The primary objective is to assess the risk of breast cancer recurrence associated with temporary interruption of endocrine therapy to permit pregnancy. The primary analysis will estimate breast cancer free interval (BCFI), defined as the time from enrollment in the study to the first invasive breast cancer event (local, regional, or distant recurrence or a new invasive contralateral breast cancer), and estimated by Kaplan-Meier method with focus on the 3-year event percentage. The protocol includes three interim monitoring time points to permit early stopping of the trial in case the observed risk of a breast cancer event is higher than anticipated, based on historical control estimates. The primary analysis is planned based on 1600 patient-years of follow-up (approximately 3 years median follow-up).
2.2. SOFT/TEXT trials
Prior to launching POSITIVE, data from the Suppression of Ovarian Function Trial (SOFT) and the Tamoxifen and Exemestane Trial (TEXT) [[5], [6], [7], [8]] were used to estimate the historical control risk of a breast cancer event if adjuvant endocrine therapy had not been interrupted. Based on the entire cohort of 5738 premenopausal women enrolled in SOFT/TEXT between 2003 and 2011, the estimated 3-year BCFI percent was 94.2%, corresponding to a 5.8% 3-year BCFI event percent (2% annual risk of a breast cancer event). This anticipated rate was used to design the interim monitoring plans and primary analysis for the POSITIVE trial. The POSITIVE protocol prespecified that, prior to reporting trial results, SOFT/TEXT data would be used to re-estimate the external control 3-year BCFI event percent based upon characteristics of patients actually enrolled in POSITIVE. Such re-estimation is the subject of this report.
SOFT and TEXT are two international Phase III randomized clinical trials in premenopausal patients with ER + breast cancer led by the IBCSG. In SOFT, patients were randomized to receive either tamoxifen alone, or ovarian function suppression (OFS) with tamoxifen, or OFS with the aromatase inhibitor (AI) exemestane, after completion of adjuvant chemotherapy or following surgery alone. In TEXT, patients were randomized to receive OFS with exemestane or OFS with tamoxifen, after surgery and before starting any adjuvant therapy.
Estimating Historical Control using SOFT/TEXT patients.
First, we identified a cohort of SOFT/TEXT patients with POSITIVE criteria eligibility (“eligible SOFT/TEXT cohort”). Because the median duration of adjuvant endocrine therapy received by the POSITIVE participants prior to their enrollment in the study was 24 months, we identified SOFT/TEXT patients who received at least 24 months of adjuvant endocrine therapy, and were 18–42 years old and still disease-free (no invasive breast cancer recurrence, contralateral breast cancer, or second non-breast malignancy) when they reached 24 months of therapy. Patients who had undergone oophorectomy or hysterectomy by 24 months of endocrine therapy were excluded. Patients with more than 10 positive lymph nodes were excluded to reflect the cancer characteristics of POSITIVE participants. As allowed by the protocol, some SOFT patients had initiated oral endocrine therapy prior to study enrollment, and this was taken into account in assessing eligibility.
Two methods, described below, were used to estimate historical control based on the eligible SOFT/TEXT cohort:
Method I: Direct estimate based on the SOFT/TEXT cohort eligible for POSITIVE. The annualized hazard rate of BCFI event over the first three years since eligibility was calculated using the maximum likelihood estimates from an exponential model. This estimate (reported as percent) is the number of BCFI events occurring within the first three years divided by the total years of follow-up accrued by patients at risk during that interval. The 3-year BCFI event rate was estimated based by Kaplan-Meier method.
Method II: Estimate based on the SOFT/TEXT cohort group-matched to enrolled POSITIVE participants. First, we examined the distribution of patient, disease and treatment characteristics of POSITIVE and eligible SOFT/TEXT cohorts and identified those characteristics that differed (Table 1). The associations of each of these characteristics with BCFI over the 0- to 3-year interval were further examined in the SOFT/TEXT cohort by fitting univariate Cox models (Table 2), and if the factor was unbalanced (i.e., p < 0.20 in the univariate Cox model), then the characteristic was selected for matching. The five identified matching factors were used to divide the POSITIVE participants and the SOFT/TEXT cohort into 72 strata. Factors included age (<35, 35–39, 40–42 years), body mass index (BMI) (unknown/normal, overweight/obese), nodal status (pN0, pN+1–3, pN+4–9), prior AI received (yes, no), and prior chemotherapy received (yes, no). Of note, in some instances the number of patients in a stratum was 0. The SOFT/TEXT cohort was sampled with replacement so that the number of patients allocated to each stratum in the SOFT/TEXT cohort was proportional to the number of participants in the corresponding stratum in POSITIVE. If the matched stratum was empty, then a SOFT/TEXT patient from the stratum nearby was chosen. The random sampling of the SOFT/TEXT cohort was repeated 5000 times. For each sample, the annualized hazard rate of a BCFI event and 3-year BCFI event rate were calculated. The annualized hazard rate of BCFI event was calculated using the maximum likelihood estimate from a piecewise exponential model (for intervals 0–3 years and >3 years) and the 3-year BCFI event rate was calculated based on Kaplan-Meier method. The estimates of the true annualized hazard rate for BCFI and the true 3-year BCFI event rate were calculated by taking the mean of the 5000 estimates. Non-parametric 95% confidence intervals were derived using the 2.5th and 97.5th percentiles of the distribution of the 5000 estimates for each respective parameter.
Table 1.
The distribution of patient, treatment and disease characteristics for the POSITIVE participants (N = 272), the eligible SOFT/TEXT cohort (N = 1499), and the means of the 5000 random sample sets drawn from the eligible SOFT/TEXT cohort using Method II.
| POSITIVE enrolled (N = 272) |
SOFT/TEXT eligible (N = 1499) |
SOFT/TEXT group matched random samplesa |
|||
|---|---|---|---|---|---|
| N | % | N | % | % | |
| Age (years) | |||||
| <35 | 92 | 33.8 | 286 | 19.1 | 33.9 |
| 35-39 | 110 | 40.4 | 573 | 38.2 | 40.3 |
| 40-42 | 70 | 25.7 | 640 | 42.7 | 25.8 |
| Body mass index (BMI; kg/m2) | |||||
| Normal (<25) | 199 | 73.2 | 871 | 58.1 | 72.9 |
| Overweight (25-<30) | 43 | 15.8 | 337 | 22.5 | 13.2 |
| Obese (≥30) | 21 | 7.7 | 257 | 17.1 | 10.5 |
| Unknown | 9 | 3.3 | 34 | 2.3 | 3.4 |
| Previous pregnancy | |||||
| No | 160 | 58.8 | 387 | 25.8 | 33.0 |
| Yes | 112 | 41.2 | 1112 | 74.2 | 67.0 |
| Previous delivery of a baby | |||||
| No | 200 | 73.5 | 431 | 28.8 | 35.7 |
| Yes | 72 | 26.5 | 1068 | 71.2 | 64.3 |
| No. nodes positive | |||||
| pN0 | 182 | 66.9 | 794 | 53.0 | 66.6 |
| pN+ 1–3 | 75 | 27.6 | 523 | 34.9 | 28.1 |
| pN+ 4–9 | 14 | 5.1 | 175 | 11.7 | 5.3 |
| Unknown | 1 | 0.4 | 7 | 0.5 | |
| Tumor size (path.; cm) | |||||
| ≤2 cm | 169 | 62.1 | 847 | 56.5 | 63.5 |
| >2 cm | 97 | 35.7 | 605 | 40.4 | 33.6 |
| Unknown | 6 | 2.2 | 47 | 3.1 | 3.0 |
| Tumor grade | |||||
| 1 | 47 | 17.3 | 223 | 14.9 | 17.3 |
| 2 | 127 | 46.7 | 770 | 51.4 | 52.5 |
| 3 | 91 | 33.5 | 478 | 31.9 | 28.9 |
| Unknown | 7 | 2.6 | 28 | 1.9 | 1.3 |
| HER2 status | |||||
| Negative | 208 | 76.5 | 1191 | 79.5 | 77.7 |
| Positive | 61 | 22.4 | 256 | 17.1 | 17.6 |
| Unknown | 3 | 1.1 | 52 | 3.5 | 4.7 |
| Estrogen receptor (ER ≥ 10%) | |||||
| Negative | 3 | 1.1 | 42 | 2.8 | 2.1 |
| Positive | 269 | 98.9 | 1457 | 97.2 | 97.9 |
| Progesterone receptor (PgR≥10%) | |||||
| Negative | 30 | 11.0 | 181 | 12.1 | 11.1 |
| Positive | 239 | 87.9 | 1300 | 86.7 | 87.8 |
| Missing | 3 | 1.1 | 18 | 1.2 | 1.1 |
| Prior chemotherapy | |||||
| No | 105 | 38.6 | 359 | 23.9 | 37.5 |
| Yes | 167 | 61.4 | 1140 | 76.1 | 62.5 |
| Prior AI received | |||||
| No | 214 | 78.7 | 893 | 59.6 | 78.5 |
| Yes | 58 | 21.3 | 606 | 40.4 | 21.5 |
| Prior OFS received | |||||
| No | 121 | 44.5 | 315 | 21.0 | 23.4 |
| Yes | 151 | 55.5 | 1184 | 79.0 | 76.6 |
In Method II, 5000 random sample sets of eligible SOFT/TEXT patients were drawn with replacement matching characteristics of the POSITIVE participants. The matched characteristics used are age (<35, 35–39, 40–42), BMI (unknown/normal, overweight/obese), nodal status (pN0, pN+1–3, pN+4–9), prior AI received (yes, no), and prior chemotherapy received (yes, no).
Table 2.
The estimates of 3-year BCFI rates in the eligible SOFT/TEXT cohort (N = 1499) according to unbalanced characteristics (p-values from univariate Cox modelsa).
| 3-year BCFI |
P-value | |
|---|---|---|
| % | ||
| Age (years) | ||
| <35 | 87.7 | 0.12 |
| 35-39 | 90.6 | |
| 40-42 | 91.8 | |
| Body mass index (BMI; kg/m2) | ||
| Normal (<25) | 91.2 | 0.17 |
| Overweight (25-<30) | 87.6 | |
| Obese (≥30) | 91.8 | |
| Previous delivery of a baby | ||
| No | 90.3 | 0.81 |
| Yes | 90.6 | |
| No. nodes positive | ||
| pN0 | 93.4 | <.0001 |
| pN+ 1–3 | 89.1 | |
| pN+ 4–9 | 82.1 | |
| Prior chemotherapy | ||
| No | 95.0 | 0.003 |
| Yes | 89.2 | |
| Prior AI received | ||
| No | 88.4 | 0.002 |
| Yes | 93.7 | |
| Prior OFS received | ||
| No | 89.4 | 0.40 |
| Yes | 90.9 | |
In the Cox models, BCFI was censored at 3 years; the p-values reported are from the univariate Cox model with only the characteristic of interest included.
3. Results
Of 5738 SOFT/TEXT patients, 1499 met the eligibility criteria for POSITIVE. Table 1 shows that the patients enrolled in POSITIVE are younger, less overweight/obese, and had fewer positive lymph nodes than the eligible SOFT/TEXT cohort. Also, as expected, fewer POSITIVE patients had prior delivery/pregnancy and fewer POSITIVE patients received prior AI, chemotherapy and OFS.
Table 2 summarizes 3-year BCFI event percentages and the p-values from the univariate Cox models for the unbalanced factors identified in Table 1. As prior pregnancy is highly correlated with prior delivery, only prior delivery was examined in Table 2. The factors associated with BCFI (with p-value <0.2) are age, BMI, nodal status, whether prior AI was received and whether prior chemotherapy was received. Those factors were used for matching in Method II. The right-hand column in Table 1 summarizes the means of the distributions of the characteristics over the 5000 random sample sets generated from Method II. After matching, as expected, the distributions of the matched characteristics are indeed very similar to those of POSITIVE. Although some unmatched characteristics remain unbalanced, they are both closer to the distribution in POSITIVE and have little influence on BCFI outcome.
Table 3 gives the results obtained from Methods I and II for BCFI. In Method I, based on the eligible SOFT/TEXT cohort with 1499 patients, the annualized hazard rate of BCFI during the first three years is 3.4% per year with 95% CI (2.8%, 4.0%). The 3-year BCFI event rate is 9.5% with 95% CI (7.9%, 11.1%).
Table 3.
BCFI estimates∗ from Methods I and II.
| Annualized hazard rate over the first 3 years | 95% CI | 3-year BCFI event rate | 95% CI | |
|---|---|---|---|---|
| Rate assumed in the original POSITIVE design | 2% | 5.8% | ||
| Method I | 3.4% | (2.8%, 4.0%) | 9.5% | (7.9%, 11.1%) |
| Method IIa | 3.4% | (2.8%, 4.0%) | 9.4% | (7.8%, 10.9%) |
∗There were 168 BCFI events in SOFT/TEXT eligible cohort; among them, 126 occurred within 3 years after time 0.
In Method II, 5000 random sample sets of SOFT/TEXT patients were drawn with replacement matching the characteristics of the POSITIVE participants. The matched characteristics used are age (<35, 35–39, 40–42), BMI (unknown/normal, overweight/obese), nodal status (pN0, pN+1–3, pN+4–9), prior AI received (yes, no), and prior chemotherapy received (yes, no).
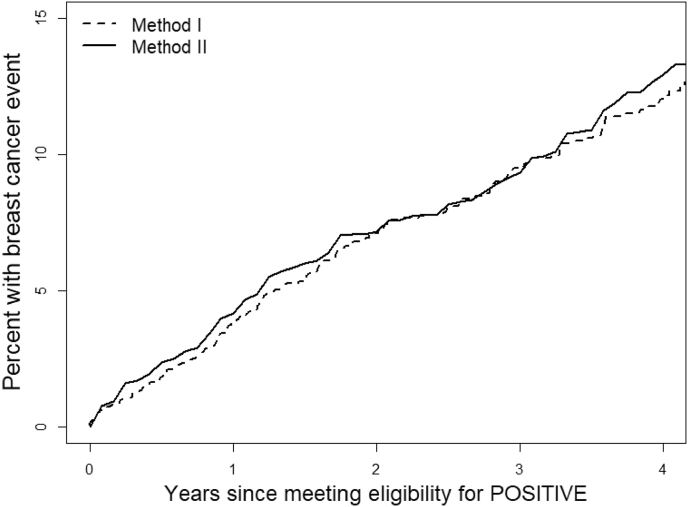
Using Method II, based on the 5000 random sample sets to group-match SOFT/TEXT to POSITIVE patients enrolled, the mean of the annualized risk is 3.4% (95% CI, 2.8%, 4.0%). The mean of 3-year BCFI event rates is 9.4% (95% CI, 7.8%, 10.9%). Fig. 1 shows the Kaplan-Meier curve of the eligible SOFT/TEXT cohort (Method I), and the averages of the Kaplan-Meier estimates from the 5000 random sample sets (Method II). The two curves are very consistent, supporting the finding that the unmatched and group-matched methods give the same result for this analysis.
Fig. 1.
Kaplan-Meier curves of BCFI for the identified SOFT/TEXT cohort (N = 1499; Method I; 3-year event rate: 9.5%) and the average of 5000 random samples (Method II; 3-year event rate: 9.4%).
To visually assess the extent to which the 3-year BCFI estimate is influenced by the selection of covariates used for matching, Table 4 shows results of Method II when covariates used for matching are removed one at a time. The 3-year BCFI estimates range from 8.4% to 10.0%. We note that all these estimates based on SOFT/TEXT are greater than the 5.8% assumed for the design of the POSITIVE trial, and all of the 95% confidence intervals based on the models exclude 5.8%.
Table 4.
BCFI estimates from Method II eliminating one factor at a time from the Final Modela.
| Annualized hazard rate over the first 3 years | 95% CI | 3-year BCFI event rate | 95% CI | |
|---|---|---|---|---|
| Rate assumed in the original POSITIVE design | 2.0% | 5.8% | ||
| Method I | 3.4% | (2.8%, 4.0%) | 9.5% | (7.9%, 11.1%) |
| Method II: Final Modela | 3.4% | (2.8%, 4.0%) | 9.4% | (7.8%, 10.9%) |
| Method II: eliminating age | 3.2% | (2.6%, 3.8%) | 8.9% | (7.4%, 10.5%) |
| Method II: eliminating BMI | 3.4% | (2.9%, 4.0%) | 9.5% | (8.0%, 11.1%) |
| Method II: eliminating nodal status | 3.6% | (3.0%, 4.2%) | 10.0% | (8.4%, 11.6%) |
| Method II: eliminating prior AI | 3.0% | (2.4%, 3.6%) | 8.4% | (6.9%, 9.9%) |
| Method II: eliminating prior chemotherapy | 3.5% | (2.9%, 4.1%) | 9.7% | (8.2%, 11.3%) |
In the final model, the matched characteristics used are age (<35, 35–39, 40–42), BMI (unknown/normal, overweight/obese), nodal status (pN0, pN+1–3, pN+4–9), prior AI received (yes, no), and prior chemotherapy received (yes, no).
4. Discussion
Although randomized clinical trials are the best methodology for comparing two treatment strategies, in some cases they are unethical or infeasible. In designing the POSITIVE trial, it was not reasonable to randomize women with early breast cancer who desire pregnancy to an arm that would not permit pregnancy attempt. In addition, randomized trials designed to demonstrate non-inferiority of a de-escalated therapy versus standard therapy require substantial numbers of patients, often making them infeasible [9]. Recent single-arm designs assessing de-escalation of therapy, including the APT trial [15] and the low-risk cohorts of the TAILORx and MINDACT trials [16,17], have made this an appealing and clinically valuable design option. However, these single-arm studies are often developed and conducted without pre-specifying historical control datasets that can be used to place the eventual results into clinical context. While estimating “standard-of-care” therapy outcomes for the eligible patient population might be available, estimating historical control outcomes for the cohort of patients actually enrolled is essential to assess the viability of a de-escalation strategy. The methods described in this paper achieve this clinically relevant objective.
Two methods were discussed that estimated the rates of historical controls, using the POSITIVE single-arm breast cancer trial as an example. A cohort of patients from SOFT/TEXT who met POSITIVE eligibility criteria was identified. Method I estimated breast cancer recurrence rates directly using the eligible SOFT/TEXT cohort, while Method II used stratified matching of factors shown to be prognostic. For both methods, only characteristics known at entry into the POSITIVE study were used to estimate the BCFI rates of historical controls. Therefore, in addition to the final analysis and interpretation of definitive results, the described methods have a role for better monitoring event rates during the conduct of the study.
At the later stage of the POSITIVE trial when longer follow-up is available, a traditional Cox proportional hazards modeling or propensity-score Cox proportional hazards modeling [10] controlling for important risk factors, could be performed based on the eligible SOFT/TEXT cohort and POSITIVE patients to examine the trial effect. As the eligible SOFT/TEXT cohort was selected based on eligibility criteria for the POSITIVE trial, the trial effect could be viewed as mainly due to temporarily interrupting endocrine therapy plus any effect, adverse or favorable, of pregnancy.
Other methods can be explored for evaluating the treatment effect in single-arm trials. The use of synthetic control arm was suggested for a single-arm trial where the data from Medidata’s archive of 340 patients with acute myeloid leukemia were mined and matched with the 16 patients from an experimental arm for analysis [11]. Patients were matched on ≥4 of 6 baseline criteria at the individual patient level and then combined to estimate the treatment effect, as well as for other exploratory subgroup analyses. A control arm can also be constructed from historical data by pair-matching the control patient with each patient on the experimental arm via propensity scores [12,13]. Recently, Ventz et al. [14] examined designs with stringent futility early-stopping rules and those that leverage both toxicity and efficacy endpoints, demonstrating that such designs have little impact on power, but enhanced safety.
The results presented in this paper using Methods I and II were based on one historical dataset (SOFT/TEXT) and 272 POSITIVE patients. To assess robustness of historical controls, these methods should be applied using data from multiple sources. In fact, the POSITIVE protocol specifies that the Austrian Breast & Colorectal Cancer Study Group (ABCSG) Trial 12 [18] and ASCO’s CancerLinQ may be candidate datasets.
The annualized hazard rate of BCFI event during the first 3 years and the 3-year BCFI event rate obtained from both Methods I and II were higher than those assumed in the POSITIVE protocol (Table 3). It is reassuring that estimates obtained by the two methods are so similar. These findings are very important for safety monitoring of the POSITIVE trial by the Data and Safety Monitoring Committee, as adjustments to interim monitoring boundaries are appropriate. External control datasets should be identified before launching single-arm, de-escalation trials. Methods, such as those described in this paper, should be applied to provide context for interim monitoring and clinical interpretation of the final results.
Funding
This work was supported by the International Breast Cancer Study Group (IBCSG). The support of IBCSG includes: Frontier Science and Technology Research Foundation, Swiss Group for Clinical Cancer Research (SAKK), Cancer Research Switzerland, Oncosuisse, Cancer League Switzerland, Foundation for Clinical Cancer Research of Eastern Switzerland (OSKK).
The POSITIVE trial is sponsored by the IBCSG in non-North American countries and by Alliance for Clinical Trials in Oncology in North America, with collaboration of the Breast International Group (BIG) cooperative groups and US National Clinical Trials Network groups. POSITIVE receives grant support for central trial conduct from Frontier Science & Technology Research Foundation, Southern Switzerland (FSE); BIG and Baillet Latour Fund, Belgium; Pink Ribbon Switzerland; Swiss Cancer League, Switzerland (KLS-3361-02); San Salvatore Foundation, Switzerland; Rising Tide Foundation for Clinical Research, Switzerland (CCR-15-120); Gateway for Cancer Research, USA (G-15-1900); Breast Cancer Research Foundation (BCRF), USA; Swiss Cancer Foundation, Switzerland; Piajoh Fondazione di Famiglia, Switzerland; Gruppo Giovani Pazienti “Anna dai Capelli Corti “, Switzerland; Baärguf, Switzerland; and private donors. Alliance for Clinical Trials in Oncology receives support for trial conduct from the Division of Cancer Prevention (UG1CA189823) of the United States National Cancer Institute, USA.
TEXT and SOFT receive financial support for trial conduct and long-term follow-up from Pfizer, the IBCSG, the US National Institutes of Health, the Breast Cancer Research Foundation (16–185, 17–187, 18–003), Ipsen, TerSera and AstraZeneca. Pfizer and Ipsen provided drug supply. Grant support of participating cooperative groups: IBCSG Statistical and Data Management Center [US NIH CA075362]; Breast Cancer Trials Australia & New Zealand [NHMRC 351161, 510788 and 1105058]; Institute of Cancer Research Clinical Trials and Statistics Unit (ICR-CTSU) on behalf of the National Cancer Research Institute Breast Clinical Studies Group United Kingdom (NCRI-BCSG—ICR-CTSU Partnership) [Cancer Research UK CRUKE/03/022, CRUKE/03/023, A15955; National Institute for Health Research Royal Marsden/Institute of Cancer Research Biomedical Research Center; and National Institute for Health Research/Cambridge Biomedical Research Center; Alliance for Clinical Trials in Oncology [US NIH CA180821]; SWOG [US NIH CA32102]; ECOG-ACRIN Cancer Research Group [US NIH CA21115, CA16116]; NRG Oncology [US NIH grant numbers U10CA180868, U10CA180822, UG1CA189867]; Canadian Cancer Trials Group [US NIH CA077202; and Canadian Cancer Society Research Institute 015469, 021039].
Ethical approval
All three clinical trials discussed in this paper - POSITIVE, SOFT and TEXT - were conducted following Ethics Committees Review and all patients signed committee approved informed consent documents prior to participating in their respective trial.
Conflicts of interest
Zhuoxin Suna, No conflicts
Samuel M. Nimanb, No conflicts
Olivia Paganic, No conflicts
Ann H. Partridged, No conflicts
Hatem A. Azim Jre, honoraria (Roche, Novartis); employment (Innate Pharma)
Fedro A. Peccatorif, paid consultancies from Roche, Ipsen and Astra Zeneca
Monica Ruggerig, No conflicts
Angelo Di Leoh, No conflicts
Marco Colleonii, No conflicts
Richard D. Gelberj, reports grants to institution for salary support from Pfizer, Astra-Zeneca, Merck, GlaxoSmithKline, Celgene, Roche, Ipsen, Ferring
Meredith M. Regank, Research funding to the IBCSG (and/or provision of drug supply for clinical trials) from Novartis, Pfizer, Ipsen, TerSera, Pierre Fabre, Roche, AstraZeneca. Research funding (to institution, DFCI) from Bayer, Bristol-Myers Squibb; consulting or advisory role to Ipsen (institution IBCSG), Bristol-Myers Squibb (uncompensated, but including travel, editorial services).
Acknowledgements
We thank the patients who participated in the POSITIVE, SOFT and TEXT trials, without whom substantial recent progress to improve care for young women with breast cancer would not have been possible. We dedicate this paper to the memory of Professor Aron Goldhirsch (1946–2020), medical oncologist and breast cancer specialist, who died on February 26, 2020, and devoted his life to patient care and clinical research. As scientific co-chair of the International Breast Cancer Study Group (IBCSG) and the Breast International Group (BIG), Prof Goldhirsch played a pivotal role to develop and conduct all three studies that provided data for the analyses presented in this paper.
Footnotes
Presented in part at the 2018 ASCO Annual Meeting, Chicago, IL.
Contributor Information
Zhuoxin Sun, Email: zhuoxin@jimmy.harvard.edu.
Samuel M. Niman, Email: sniman@jimmy.harvard.edu.
Olivia Pagani, Email: opagani@bluewin.ch.
Ann H. Partridge, Email: ahpartridge@partners.org.
Hatem A. Azim, Jr., Email: hatem.azim@gmail.com.
Fedro A. Peccatori, Email: fedro.peccatori@ieo.it.
Monica Ruggeri, Email: monica.ruggeri@ibcsg.org.
Angelo Di Leo, Email: angelo.dileo@uslcentro.toscana.it.
Marco Colleoni, Email: marco.colleoni@ieo.it.
Richard D. Gelber, Email: gelber@jimmy.harvard.edu.
Meredith M. Regan, Email: mregan@jimmy.harvard.edu.
APPENDIX. POSITIVE Trial Steering Committee, International Breast Cancer Study Group (IBCSG) PParticipants and International Networks
POSITIVE Trial Steering Committee: O Pagani (International Chair), A Partridge (North American Chair), H Azim (Translational Research Co-Chair), F Peccatori (Translational Research Co-Chair), K Ribi (Psycho-oncological Companion Study Chair), C Abi-Khattar, F Amant, A Barbro Sætersdal, L Blacher, V Bjelic-Radisic, V Borges, MR Borrego, S Borstnar, F Cardoso, M Colleoni, I Demeestere, A Di Leo, J Eon Lee, T Ferreiro, R Gelber, K Gelmon, A Hiltbrunner, S Hultsch, M Ignatiadis, P Jani, R Kammler, C Kelly, J Kroep, A Mailliez, D Mavroudis, H Moore, M Naughton, S Niman, S Paluch-Shimon, M Rabaglio, M Regan, H Roschitzki, K Ruddy, B Ruepp, M Ruggeri, C Saunders, C Saura, K Scott, H Shaw, C Shimizu, K Smith, T Španić, S Šušnjar, F Symmans, G Viale.
IBCSG Scientific Committee: M Colleoni (Chair), A Di Leo (Co-Chair), S Loi (Co-Chair).
IBCSG Scientific Executive Committee: M Colleoni, A Di Leo, F Boyle, G Jerusalem, S Loi, M Regan, G Viale.
IBCSG Foundation Council: R Stahel (President), S Aebi, F Boyle, A Coates, M Colleoni, A Di Leo, R Gelber, A Goldhirsch✝, G Jerusalem, P Karlsson, I Kössler, M Regan.
IBCSG Coordinating Center, Bern, Switzerland: A Hiltbrunner (Director), G Achille, I Campus, A Carrer-Wagner, D. Celotto, C Comune, M Fournarakou, M Frapolli, A Gasca, R Kammler, R Karagol, L Keglowich, C Maddox, M. Marbot, M. Mathys, M Pardo Contreras, M Nesa, L Nobs, R Pfister, M Rabaglio, H Roschitzki, B Ruepp, M. Ruggeri, E Rugiati, M. Sanchez-Hohl, J Schroeder, P Sicher, S. Troesch, M Wagner, M Weber; M Wenger, I Zenklusen.
IBCSG Statistical Center, Department of Data Sciences, Division of Biostatistics, Dana-Farber Cancer Institute, Boston, MA, USA: M Regan (Director), C Bouzan, R Gelber, S Gelber, H Huang, C Mahoney, S Niman, L Northrop, Z Sun.
IBCSG Data Management Center, Frontier Science & Technology Research Foundation, Amherst, NY, USA: L Blacher (Director), M Blackwell, R Edlund, S Fischer, C Goodwin, P Jani, J Jemison, G Kassab, L Mundy, D Narayanan, V. Palermo, K Scott, H Shaw, R Starkweather, Y Veira, D Weinbaum.
IBCSG Quality of Life Office, Bern, Switzerland: J Bernhard, K Ribi.
CENTRAL LABORATORIES.
IBCSG Central Pathology Office, European Institute of Oncology, Division of Pathology, Milan, Italy: G Viale (Director), S Andrighetto, L. Arrigoni, G Bardeli, E Benini, O Biasi, F Ciocca, P Dell’Orto, L Russo.
Research Laboratory on Human reproduction, Université Libre de Bruxelles, Belgium: I Demeestere (Director), J Dechene.
Medical Oncology Department & Academic Trials Promoting/Breast Cancer Translational Research Laboratory, Institut Jules Bordet, Université Libre de Bruxelles, Belgium: M Ignatiadis, F Rothé, M Maetens.
NETWORKS.
The POSITIVE trial resulted from the collaboration of the Endocrine Working Group of the BIG-NABCG: Co-chairs: A. Goldhirsch✝ (BIG), L. Korde (NABCG).
Breast International Group (BIG): M Piccart-Gebhart, S Hultsch, S Schmitz.
North American Breast Cancer Group (NABCG): L Korde, M Mooney.
Cooperative Groups:
Breast International Group.
Sponsor and lead Group: IBCSG – International Breast Cancer Study Group.
ABCSG - Austrian Breast and Colorectal Cancer Study Group.
BOOG - Dutch Breast Cancer Research Group.
CTI - Cancer Trials Ireland.
GEICAM - Spanish Breast Cancer Group.
HORG - Hellenic Oncology Research Group.
JBCRG Japan Breast Cancer Research Group.
NBCG - Norwegian Breast Cancer Group.
SAKK - Swiss Group for Clinical Cancer Research.
SOLTI - Breast Cancer Research Group.
Coordinating Centers in countries with more than one participating center and no Cooperative Groups involved:
Center Oscar Lambret, Lille (Coordinating Center for French Centers).
IEO - European Institute of Oncology, Milan (Coordinating Center for Italian Centers).
SMC - Samsung Medical Center, Seoul (Coordinating Centers for South Korean Centers).
UZ Leuven (Coordinating Center for Belgian Centers).
North American Breast Cancer Group.
Sponsor and lead Group: Alliance for Clinical Trials in Oncology.
SWOG Cancer Research Network.
ECOG-ACRIN Cancer Research Group.
NRG Oncology.
CCTG Canadian Cancer Trial Group.
References
- 1.Curigliano G., Burstein H.J., P Winer E., Gnant M., Dubsky P., Loibl S. Panel members of the st. Gallen international expert consensus on the primary therapy of early breast cancer 2017. De-escalating and escalating treatments for early-stage breast cancer. Ann Oncol. 2017;28(8):1700–1712. doi: 10.1093/annonc/mdx308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pagani O., Ruggeri M., Manunta S., Saunders C., Peccatori F., Cardoso F. Pregnancy after breast cancer: are young patients willing to participate in clinical studies? Breast. 2015;24(3):201–207. doi: 10.1016/j.breast.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg S.M., Gelber S., Gelber R.D., Krop E., Korde L.A., Pagani O. Oncology physicians’ perspectives on practices and barriers to fertility preservation and the feasibility of a prospective study of pregnancy after breast cancer. J Adolesc Young Adult Oncol. 2017;6(3):429–434. doi: 10.1089/jayao.2017.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruggeri M., Pagan E., Bagnardi V., Bianco N., Gallerani E., Giordano M. Fertility concerns, preservation strategies and quality of life in young women with breast cancer: baseline results from an ongoing prospective cohort study in selected European Centers. Breast. 2019;47:85–92. doi: 10.1016/j.breast.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Pagani O., Regan M.M., Walley B.A., Fleming G.F., Colleoni M., Láng I. TEXT and SOFT Investigators and the International Breast Cancer Study Group. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371(2):107–118. doi: 10.1056/NEJMoa1404037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francis P.A., Regan M.M., Fleming G.F., Láng I., Ciruelos E., Bellet M. SOFT investigators and the international breast cancer study group. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372(5):436–446. doi: 10.1056/NEJMoa1412379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regan M.M., Francis P.A., Pagani O., Fleming G.F., Viale G., Colleoni M. Absolute benefit of adjuvant endocrine therapies for premenopausal women with hormone receptor–positive, human epidermal growth factor receptor 2–negative early breast cancer: TEXT and SOFT trials. J Clin Oncol. 2016;34(19):2221–2231. doi: 10.1200/JCO.2015.64.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francis P.A., Pagani O., Fleming G.F., Walley B.A., Colleoni M., Láng I. SOFT and TEXT Investigators and the International Breast Cancer Study Group. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018;379(2):122–137. doi: 10.1056/NEJMoa1803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Regan M.M., Barry W.T. Accelerating innovation for patients: trial designs and results supporting treatment de-escalation and escalation. Breast. 2017;34(Suppl 1):S10–S12. doi: 10.1016/j.breast.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 10.Rosenbaum P.R., Rubin D.B. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 11.Barry D.A., Elashoff M., Blotner S., Davi R., Beineke P., Chandler M. Creating a synthetic control arm from previous clinical trials: Application to establishing early end points as indicators of overall survival in acute myeloid leukemia (AML) J Clin Oncol. 2017;35(15) 7021-7021. [Google Scholar]
- 12.Ho D.E., Imai K., King G., Stuart E.A. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Pol Anal. 2007;15(3):199–236. [Google Scholar]
- 13.Hansen B.B., Klopfer S.O. Optimal full matching and related designs via network flows. J Comput Graph Stat. 2006;15(3):609–627. [Google Scholar]
- 14.Ventz S., Trippa L., Schoenfeld J.D. Lessons learned from de-escalation trials in favorable risk HPV-associated squamous cell head and neck cancer – a perspective on future trial designs. Clin Canc Res. 2019;25(24):7281–7286. doi: 10.1158/1078-0432.CCR-19-0945. [DOI] [PubMed] [Google Scholar]
- 15.Tolaney S.M., Barry W.T., Dang C.T., Yardley D.A., Marcom P.K., Albain K.S. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;372:134–141. doi: 10.1056/NEJMoa1406281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sparano J.A., Gray R.J., Makower D.F., Pritchard K.I., Albain K.S., Hayes D.F. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015;373:2005–2014. doi: 10.1056/NEJMoa1510764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cardoso F., van’t Veer L.J., Bogaerts J., Slaets L., Viale G., Delaloge S. MINDACT Investigators. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;375:717–729. doi: 10.1056/NEJMoa1602253. [DOI] [PubMed] [Google Scholar]
- 18.Gnant M., Mlineritsch B., Stoeger H., Luschin-Ebengreuth G., Knauer M., Moik M. Austrian Breast and Colorectal Cancer Study Group Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozole plus ovarian function suppression in premenopausal early breast cancer: final analysis of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Ann Oncol. 2015;26(2):313–320. doi: 10.1093/annonc/mdu544. [DOI] [PubMed] [Google Scholar]