Abstract
Purpose
This study evaluated the influence of prognostic factors and whole brain radiotherapy (WBRT) on overall survival (OS) of breast cancer (BC) patients with brain metastases (BM).
Methods and materials
Medical records of 730 BC patients diagnosed with BM from 2000 to 2014 at 17 institutions were retrospectively reviewed. OS was calculated from BM diagnosis. Median follow-up duration was 11.9 months (range, 0.1–126.2).
Results
Median OS was 15.0 months (95% CI: 14.0–16.9). Patients with different BC-specific graded prognostic assessment (GPA) scores showed significant differences (p < 0.001) in OS. In multivariate analysis, histologic grade 3 (p = 0.014), presence of extracranial metastasis (p < 0.001), the number of BM (>4; p = 0.002), hormone receptor negativity (p = 0.005), HER2-negativity (p = 0.003), and shorter time interval (<30 months) between BC and BM diagnosis (p = 0.007) were associated with inferior OS. By summing the β-coefficients of variables that were prognostic in multivariate analyses, we developed a prognostic model that stratified patients into low-risk (≤0.673) and high-risk (>0.673) subgroups; the high-risk subgroup had poorer median OS (10.1 months, 95% CI: 7.9–11.9 vs. 21.9 months, 95% CI: 19.5–27.1, p < 0.001). Univariate and multivariate analyses of propensity score-matched patients diagnosed with BM ≥ 30 months after BC diagnosis (n = 389, “late BM”) revealed that WBRT-treated patients showed superior OS compared to non-WBRT-treated patients (p = 0.070 and 0.030, respectively).
Conclusion
Our prognostic model identified high-risk BC patients with BM who might benefit from increased surveillance; if validated, our model could guide treatment selection for such patients. Patients with late BM might benefit from WBRT as initial local treatment.
Keywords: Breast cancer, Brain metastasis, Overall survival, Prognostic model, Whole brain radiotherapy
Abbreviations: BC, breast cancer; BM, brain metastases; CI, confidence interval; CT, computed tomography; Dx, diagnosis; ER, estrogen receptor; FSRT, fractionated stereotactic radiotherapy; GPA, graded prognostic assessment; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; KPS, Karnofsky performance status; KROG, Korean Radiation Oncology Group; MRI, magnetic resonance imaging; Op, operation; OS, overall survival; PET, positron emission tomography; PSM, propensity score matching; PR, progesterone receptor; RTOG, Radiation Therapy Oncology Group; SRS, single-fraction stereotactic radiosurgery; WBRT, whole brain radiotherapy
Highlights
-
•
Large retrospective analysis of patients with brain metastases from breast cancer.
-
•
Our multivariable prognostic model can classify patients with a low survival rate.
-
•
WBRT-treated patients among those with late brain metastases have a superior OS.
1. Introduction
Breast cancer (BC) is the second most common cause of brain metastases (BM) [1]. Up to 30% of metastatic BC patients experience BM during the disease course [2]. In these patients, the prognosis is extremely poor and very heterogeneous by tumor molecular subtype [3]. Because BM can cause severe neurological impairment that limit the patient’s quality of life [4], treatment of BM while improving the patient’s survival/quality of life presents a major clinical challenge [5].
The treatment choice of BM is currently determined by patient and tumor characteristics, including patient performance status, patient preference, comorbidities, size and the number of lesions, anatomical location [6,7]. Surgery, single-fraction stereotactic radiosurgery (SRS), and whole brain radiotherapy (WBRT) are the most common currently-used local treatment modalities. Historically, WBRT or surgical resection (if appropriate) have been performed [5].
WBRT causes neurocognitive deterioration [[8], [9], [10]]. In randomized clinical trials that analyzed the effect of SRS with or without WBRT [8,10], patients receiving additional WBRT showed greater deterioration of neurocognitive function than those treated with SRS alone [8]. Notably, the omission of WBRT did not decrease overall survival (OS), although patients treated with SRS alone had a worse intracranial tumor progression rate [8]. Therefore, SRS is generally considered the treatment of choice for patients with a limited number of BM [11]. However, SRS is associated with certain limitations, such as the number of SRSs that can be performed and the shorter local failure-free survival when compared to WBRT [8,10]. Tumor shrinkage due to WBRT was reported to be associated with better survival and neurocognitive function preservation [12]. Hence, there is an unmet clinical need to develop tools that enable selection of the appropriate patient group for these treatment modalities.
We conducted a multicenter retrospective study to investigate survival outcomes and prognostic factors affecting OS in BC patients with BM. This study also aimed to identify patients that might show a significant survival benefit with WBRT as initial local treatment.
2. Methods and Materials
2.1. Patients
We retrospectively analyzed medical records of BC patients diagnosed with BM between 2000 and 2014 in 17 institutions. We excluded patients with leptomeningeal seeding, history of other types of malignancy before or after the diagnosis of BC, including contralateral BC, or male BC. Finally, 730 patients were included in this study. This study was approved by the Institutional Review Board of each institution.
BM were primarily diagnosed by magnetic resonance imaging (MRI) alone (n = 629). Twenty-six patients were diagnosed via computed tomography (CT) alone, and both imaging modalities (MRI and CT) were used in 68 patients. In five patients, BM were identified by brain positron emission tomography (PET). One patient underwent a biopsy and another patient incidentally was diagnosed with BM, initially thought to be a meningioma, after surgical resection. The median interval from primary BC to BM was 31.2 months (range, 0–178.8).
2.2. Tumor subtype classification
Using immunohistochemistry staining, a tumor showing estrogen receptor (ER) and/or progesterone receptor (PR)-positivity was classified, if human epidermal growth factor receptor 2 (HER2)-negative, as luminal A (i.e. HER2 negative, ER/PR positive), and otherwise, as luminal B (i.e. HER2/ER/PR positive). The HER2-enriched subtype was HER2-positive but, ER/PR-negative. Tumors that were ER/PR/HER2-negative were categorized as triple-negative BC.
2.3. Treatment
The most common first local treatment for BM was WBRT alone (n = 430, 58.9%) followed by fractionated stereotactic radiotherapy (FSRT) or SRS alone (n = 160, 21.9%). Twenty-seven patients (3.7%) underwent surgical tumor resection alone, and others (n = 113, 15.5%) were treated with a combination of modalities (Table 1). The median dose of WBRT was 30Gy in 3Gy per fraction. For FSRT, the median prescribed dose of 36Gy was delivered in six fractions. The dose of SRS was typically prescribed according to the guidelines of each institution. The various cycles of systemic therapies were used before and/or after local treatment. Seventy patients did not receive systemic treatment. Among 319 patients with HER2-positive primary tumors, anti-HER2 treatment was used in 181 patients (56.7%) during the disease course of BM.
Table 1.
Baseline characteristics.
| Characteristics (N = 730) | No. | % | |
|---|---|---|---|
| Age at BM (year) | Median (range) | 50 | 23–85 |
| Histologya | Invasive ductal carcinoma | 690 | 94.5 |
| Invasive lobular carcinoma | 6 | 0.8 | |
| Others | 28 | 3.8 | |
| Histologic gradea | 1–2 | 243 | 33.3 |
| 3 | 335 | 45.9 | |
| Hormone receptor status | Negative | 410 | 56.2 |
| Positive | 320 | 43.8 | |
| HER2 status | Negative | 411 | 56.3 |
| Positive | 319 | 43.7 | |
| Tumor subtype | Luminal A | 194 | 26.6 |
| Luminal B | 126 | 17.3 | |
| HER2-enriched | 193 | 26.4 | |
| Triple-negative | 217 | 29.7 | |
| Primary tumora | Controlled | 542 | 74.2 |
| Uncontrolled | 181 | 24.8 | |
| Extracranial metastasis | Absent | 115 | 15.8 |
| Present | 615 | 84.2 | |
| No. of BM | ≤4 | 390 | 53.4 |
| >4 | 340 | 46.6 | |
| Location of BM | Supratentorial | 240 | 32.9 |
| Infratentorial | 106 | 14.5 | |
| Both | 384 | 52.6 | |
| Systemic therapy firsta | No | 303 | 41.5 |
| Yes | 426 | 58.4 | |
| KPS | <60 | 36 | 4.9 |
| 60 | 157 | 21.5 | |
| 70 | 121 | 16.6 | |
| 80 | 311 | 42.6 | |
| 90 | 59 | 8.1 | |
| 100 | 46 | 6.3 | |
| Initial local treatment of BM | WBRT alone | 430 | 58.9 |
| SRS or FSRT alone | 160 | 21.9 | |
| Op alone | 27 | 3.7 | |
| Op or SRS or FSRT → WBRT | 83 | 11.4 | |
| WBRT → SRS | 5 | 0.7 | |
| Others | 25 | 3.4 |
aAvailable data only; Abbreviation: BM, brain metastasis; HER2, human epidermal growth factor receptor 2; KPS, Karnofsky performance status; WBRT, whole brain radiotherapy; SRS, Single-fraction stereotactic radiosurgery; FSRT, fractionated stereotactic radiotherapy; Op, operation.
2.4. Statistical analysis
OS was defined as the time interval between BM diagnosis and death from any cause. We used the Kaplan-Meier method to estimate OS rate and the log-rank test to compare survival between groups. In univariate and multivariate analyses, Cox proportional hazards models were constructed. For multivariate analysis, variables with p < 0.100 in univariate analyses were used as covariates. For the multivariable prognostic model, the β-coefficients were used for weighting according to the influence of the prognostic factors on OS. We calculated each concordance index of BC-specific graded prognostic assessment (GPA) and our prognostic model to compare the predictability of OS.
For subgroup analysis, patients diagnosed with BM at least 30 months after the diagnosis of primary BC (n = 389) were defined as “late BM” patients. To compare baseline characteristics between groups based on the use of WBRT [WBRT(−), n = 111 vs. WBRT(+), n = 278] as initial local treatment, the chi-squared or Fisher’s exact test was used where appropriate, for categorical data, and Student’s t-test was used for continuous data. Propensity score matching was performed based on histology, HER2 status, tumor subtype, control of primary tumor, the presence of extracranial metastasis, the number of BM, the location of BM, and Karnofsky performance status (KPS). A multivariable logistic regression model generated the propensity score. The WBRT(−) and WBRT(+) groups were matched at a 1:1 ratio using the nearest neighbor method with a caliper of 0.02. The conditional survival rate was the likelihood of an additional 6-month survival, given that the patient has already survived after BM diagnosis for 1, 1.5, 2, and 2.5 years, respectively.
A two-sided p-value <0.05 was considered statistically significant. Statistical analyses were conducted using R project version 3.4.2 (https://www.r-project.org/), and the graph of conditional survival rate was generated by Microsoft Excel 2013 (Redmond, WA).
3. Results
3.1. Patient characteristics
The median duration of follow-up after BM diagnosis was 11.9 months (range, 0.1–126.2). The baseline characteristics of 730 patients are described in Table 1. About half of the patients (n = 335, 45.9%) had a high-grade tumor. Of the total patients, 320 patients (43.8%) were positive for ER and/or PR, and HER2 were positive in 319 patients (43.7%). Primary tumor was controlled in 542 patients (74.2%). Most patients (n = 615, 84.2%) had extracranial metastases. According to the number of BM, 53.4% of patients (n = 390) had ≤4, and 46.6% (n = 340) had >4. There were 384 patients (52.6%) with BM in both tentorial regions. Among patients, 638 patients (87.4%) had neurologic symptoms at BM diagnosis: headache (n = 267); weakness (n = 126); nausea/vomiting (n = 101); dizziness (n = 69); ataxia (n = 59); conscious change (n = 44); visual disturbance (n = 22); seizure (n = 22); and others (n = 16).
3.2. Patients with higher BC-specific GPA scores showed a higher OS than those with lower GPA scores
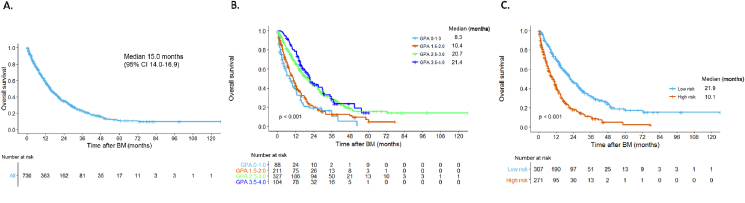
Fig. 1A shows the Kaplan-Meier survival (OS) curves of the patients in this study. The median OS for all patients was 15.0 months [95% confidence interval (CI), 14.0–16.9]. Patients’ OS rate was analyzed using the BC-specific GPA system [13]. The GPA scoring factors included KPS, tumor subtype, and age at BM diagnosis (Table 2). Patients were divided into four groups according to their GPA scores: GPA 0–1.0; 1.5–2.0; 2.5–3.0; and 3.5–4.0, respectively. Median OS rates from the lowest to highest GPA score group were 8.3 (95% CI, 5.4–12.2), 10.4 (95% CI, 8.1–12.4), 20.7 (95% CI, 16.7–24.2), and 21.4 (95% CI, 18.1–30.0) months, respectively (Fig. 1B). These differences were statistically significant (p < 0.001) and the survival curves tended to separate into two groups based on the threshold GPA score of 2 (median OS: GPA 0–2.0, 9.8 months; 95% CI, 7.9–11.8 vs. GPA 2.5–4.0, 20.8 months; 95% CI, 18.0–23.1, p < 0.001).
Fig. 1.
Overall survival curve. (A) Whole cohort, (B) According to breast cancer specific-graded prognostic assessment, (C) According to our prognostic model.
Abbreviation: CI, confidence interval; BM, brain metastasis; GPA, graded prognostic assessment.
Table 2.
Breast cancer specific-graded prognostic assessment (GPA).
| Factors | 0.0 | 0.5 | 1.0 | 1.5 | 2.0 |
|---|---|---|---|---|---|
| KPS | ≤50 | 60 | 70–80 | 90–100 | – |
| Subtype | Triple-negative | – | Luminal A | HER2-enriched | Luminal B |
| Age (year) | ≥60 | <60 | – | – | – |
Abbreviation: KPS, Karnofsky performance status; HER2, human epidermal growth factor receptor 2.
3.3. A novel multivariable prognostic model stratifies BC patients with BM into risk groups with statistically significant differences in OS
We conducted a multivariate analysis of OS using variables with a p-value <0.100 in univariate analyses (Table 3) and found that histologic grade 3 [hazard ratio (HR) = 1.327, 95% CI: 1.059–1.661, p = 0.014], the presence of extracranial metastasis (HR = 2.379, 95% CI: 1.734–3.260, p < 0.001), and the number of BM > 4 (HR = 1.519, 95% CI: 1.165–1.982, p = 0.002) were associated with poorer OS. A longer time interval from primary BC to BM diagnosis (HR = 0.750, 95% CI: 0.608–0.825, p = 0.007), hormone receptor positivity (HR = 0.724, 95% CI: 0.578–0.906, p = 0.005), and HER2 positivity (HR = 0.730, 95% CI: 0.591–0.901, p = 0.003) were significantly associated with superior OS in our multivariate analysis.
Table 3.
Univariate and multivariate Cox proportional hazards model for overall survival.
| Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|
| p value | HR (95% CI) | p value | HR (95% CI) | β-coefficienta | |
| Age at BM ≥ 50 yr (vs. <50) | 0.377 | 1.083 (0.907–1.293) | |||
| Interval of primary Dx and BM ≥ 30 months (<30) | 0.008 | 0.786 (0.659–0.938) | 0.007 | 0.750 (0.608–0.825) | −0.288 |
| Histology IDC (vs. others) | 0.051 | 1.644 (0.998–2.707) | 0.059 | 2.366 (0.970–5.772) | |
| Histologic grade 3 (vs. 1–2) | 0.004 | 1.351 (1.104–1.653) | 0.014 | 1.327 (1.059–1.661) | 0.283 |
| Hormone receptor status positive (vs. negative) | <0.001 | 0.679 (0.566–0.814) | 0.005 | 0.724 (0.578–0.906) | −0.324 |
| HER2 status positive (vs. negative) | 0.002 | 0.754 (0.631–0.902) | 0.003 | 0.730 (0.591–0.901) | −0.315 |
| Triple-negative (vs. others) | <0.001 | 1.674 (1.384–2.024) | |||
| Primary tumor controlled (vs. uncontrolled) | 0.002 | 0.724 (0.593–0.884) | 0.509 | 0.918 (0.713–1.183) | |
| Extracranial metastasis present (vs. absent) | <0.001 | 2.442 (1.841–3.239) | <0.001 | 2.379 (1.734–3.260) | 0.867 |
| No. of BM > 4 (vs. ≤ 4) | <0.001 | 1.773 (1.483–2.119) | 0.002 | 1.519 (1.165–1.982) | 0.418 |
| Location of BM both (vs. supra- or infra-tentorial) | <0.001 | 1.655 (1.384–1.980) | 0.156 | 1.198 (0.933–1.536) | |
| Systemic therapy first yes (vs. no) | 0.452 | 0.934 (0.782–1.116) | |||
| KPS <70 (vs. ≥70) | <0.001 | 1.411 (1.174–1.696) | 0.124 | 1.194 (0.915–1.507) | |
| Initial WBRT yes (vs. no) | 0.002 | 1.369 (1.125–1.666) | 0.207 | 1.174 (0.915–1.507) | |
Abbreviation: HR, hazard ratio; CI, confidence interval; BM, brain metastasis; Dx, diagnosis; IDC, invasive ductal carcinoma; HER2, human epidermal growth factor receptor 2; KPS, Karnofsky performance status; WBRT, whole brain radiotherapy.
Also known as a log hazard ratio.
We developed a multivariable prognostic model by summing the β-coefficients (detailed in Table 3) of statistically significant variables from our multivariate analysis. Each patient who had available data for these variables (n = 578) was scored according to the presence or absence of prognostic factors. Patients were categorized as low-risk when the prognostic model score was ≤0.673, while the remainder were considered as high-risk patients. There was a significant (p < 0.001) difference in median OS between patients in the low-risk (median OS = 21.9 months; 95% CI: 19.5–27.1) and high-risk (median OS = 10.1 months; 95% CI: 7.9–11.9) subgroups (Fig. 1C).
Among patients with GPA score ≤2 (n = 299), 84 patients were categorized into the low-risk group of our prognostic model. Ninety-nine patient in high GPA score (>2) group (n = 431) were reclassified into the high-risk group. Using 578 patients, the concordance index of BC-specific GPA with a cutoff of 2 was 0.700 (95% CI 0.652–0.749) and for our model was 0.726 (95% CI 0.680–0.773).
3.4. Late BM patients may benefit from WBRT as initial local treatment
Subgroup analyses were conducted to identify the patients who would benefit from WBRT as initial local treatment for BM. The number of patients with late BM by tumor subtype was as follows: 136 (70.1%) luminal A; 80 (63.5%) luminal B; 79 (40.9%) HER2-enriched; and 94 (43.3%) triple-negative BC patients. Among late BM patients, 111 patients did not receive initial WBRT [WBRT(−) group], and 278 patients did [WBRT(+) group] (Table 4). There was a significant difference in histology distribution between the two groups (p = 0.046), but no difference in the proportions of patients with different tumor subtypes (p = 0.179). Primary tumor control was excellent in patients without initial WBRT (85.6% vs. 77.0%, p = 0.020). In patients who underwent initial WBRT, the proportion of patients with >4 BM was higher (58.6% vs. 14.4%, p < 0.001) and these BM tended to be located in both tentorial regions [WBRT(+), 60.4% vs. WBRT(−), 23.4%, p < 0.001]. In order to balance the two groups, propensity score matching was performed using variables with a p-value less than 0.200. Eighty-one patients in each group were successfully matched (Table 4).
Table 4.
Baseline characteristics of patients with late brain metastasis before and after propensity score matching.
| Before PSM |
After PSM |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WBRT(−), N = 111 | WBRT(+), N = 278 | WBRT(−), N = 81 | WBRT(+), N = 81 | |||||||
| Characteristics | No. | % | No. | % | p value | No. | % | No. | % | p value |
| Age at BM (year) | ||||||||||
| Median (range) | 50 | (31–80) | 51 | (28–85) | 0.877 | 49 | (31–74) | 49 | (32–70) | 0.564 |
| Histologya | 0.046 | 0.840 | ||||||||
| IDC | 100 | 90.1 | 264 | 95.0 | 78 | 96.3 | 76 | 93.8 | ||
| ILC | 2 | 1.8 | 1 | 0.4 | 1 | 1.2 | 1 | 1.2 | ||
| Others | 9 | 8.1 | 9 | 3.2 | 2 | 2.5 | 4 | 4.9 | ||
| Histologic gradea | 1.000 | 0.803 | ||||||||
| 1–2 | 48 | 43.2 | 108 | 38.8 | 32 | 39.5 | 33 | 40.7 | ||
| 3 | 49 | 44.1 | 113 | 40.6 | 37 | 45.7 | 33 | 40.7 | ||
| Hormone receptor status | 0.246 | 0.528 | ||||||||
| Negative | 55 | 49.5 | 118 | 42.4 | 34 | 42.0 | 39 | 48.1 | ||
| Positive | 56 | 50.5 | 160 | 57.6 | 47 | 58.0 | 42 | 51.9 | ||
| HER2 status | 0.180 | 0.631 | ||||||||
| Negative | 72 | 64.9 | 158 | 56.8 | 50 | 61.7 | 46 | 56.8 | ||
| Positive | 39 | 35.1 | 120 | 43.2 | 31 | 38.3 | 35 | 43.2 | ||
| Tumor subtype | 0.179 | 0.726 | ||||||||
| Luminal A | 37 | 33.3 | 99 | 35.6 | 31 | 38.3 | 28 | 34.6 | ||
| Luminal B | 19 | 17.1 | 61 | 21.9 | 16 | 19.8 | 14 | 17.3 | ||
| HER2-enriched | 20 | 18.0 | 59 | 21.2 | 15 | 18.5 | 21 | 25.9 | ||
| Triple-negative | 35 | 31.5 | 59 | 21.2 | 19 | 23.5 | 18 | 22.2 | ||
| Primary tumora | 0.020 | 0.585 | ||||||||
| Controlled | 95 | 85.6 | 214 | 77.0 | 70 | 86.4 | 68 | 84.0 | ||
| Uncontrolled | 13 | 11.7 | 62 | 22.3 | 11 | 13.6 | 12 | 14.8 | ||
| Extracranial metastasis | 0.102 | 1.000 | ||||||||
| Absent | 16 | 14.4 | 23 | 8.3 | 7 | 8.6 | 7 | 8.6 | ||
| Present | 95 | 85.6 | 255 | 91.7 | 74 | 91.4 | 74 | 91.4 | ||
| No. of BM | <0.001 | 1.000 | ||||||||
| ≤4 | 95 | 85.6 | 115 | 41.4 | 66 | 81.5 | 65 | 80.2 | ||
| >4 | 16 | 14.4 | 163 | 58.6 | 15 | 18.5 | 16 | 19.8 | ||
| Location of BM | <0.001 | 1.000 | ||||||||
| Supra- or Infra-tentorial | 85 | 76.6 | 110 | 39.6 | 58 | 71.6 | 58 | 71.6 | ||
| Both | 26 | 23.4 | 168 | 60.4 | 23 | 28.4 | 23 | 28.4 | ||
| Systemic therapy firsta | 0.590 | 0.230 | ||||||||
| No | 38 | 34.2 | 107 | 38.5 | 23 | 28.4 | 31 | 38.3 | ||
| Yes | 73 | 65.8 | 170 | 61.2 | 58 | 71.6 | 49 | 60.5 | ||
| KPS | 0.053 | 1.000 | ||||||||
| <70 | 82 | 73.9 | 175 | 62.9 | 60 | 74.1 | 59 | 72.8 | ||
| ≥70 | 29 | 26.1 | 103 | 37.1 | 21 | 25.9 | 22 | 27.2 | ||
Abbreviation: PSM, propensity score matching; BM, brain metastasis; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; HER2, human epidermal growth factor receptor 2; KPS, Karnofsky performance status.
Available data only.
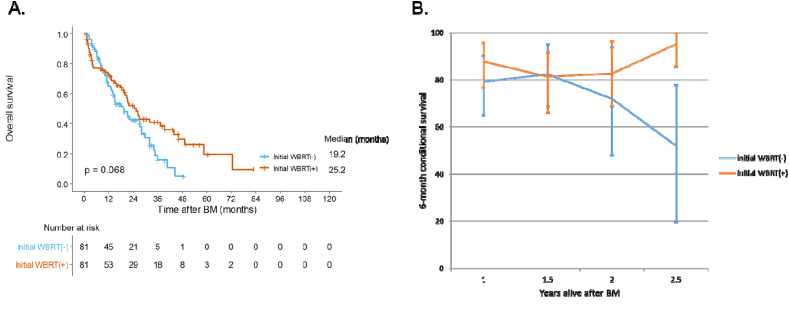
In the matched cohort, WBRT(+) patients had a better OS compared to WBRT(−) patients (median OS, 25.2 vs. 19.2 months, p = 0.068) (Fig. 2A). In terms of conditional survival, as patients receiving WBRT survived longer, the additional 6-month survival rate also increased (Fig. 2B). In multivariate analysis of the matched cohort (Table 5), initial use of WBRT was a favorable prognostic factor (HR = 0.621, 95% CI: 0.404–0.955, p = 0.030). The presence of extracranial metastasis, was associated with poorer OS (HR = 8.683, 95% CI: 2.124–35.498, p = 0.003). In this cohort, patients with low KPS had a poor prognosis (HR = 1.739, 95% CI: 1.103–2.740, p = 0.017), but the number and location of BM did not affect OS (p = 0.267 and 0.113, respectively).
Fig. 2.
(A) Overall survival curve and (B) 6-month conditional survival rate according to initial whole brain radiotherapy in patients with late brain metastasis after propensity score matching.
Error bar means a 95% confidence interval.
Abbreviation: WBRT, whole brain radiotherapy; BM, brain metastasis.
Table 5.
Univariate and multivariate Cox proportional hazards model for overall survival of patients with late brain metastasis after propensity score matching.
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| p value | HR (95% CI) | p value | HR (95% CI) | |
| Age at BM ≥ 50 yr (vs. <50 yr) | 0.329 | 1.217 (0.820–1.807) | ||
| Histology IDC (vs. others) | 0.355 | 1.722 (0.544–5.451) | ||
| Histologic grade 3 (vs. 1–2) | 0.326 | 1.246 (0.803–1.934) | ||
| Hormone receptor status positive (vs. negative) | 0.390 | 0.840 (0.565–1.250) | ||
| HER2 status positive (vs. negative) | 0.440 | 0.854 (0.572–1.275) | ||
| Triple-negative (vs. others) | 0.269 | 1.310 (0.812–2.114) | ||
| Primary tumor controlled (vs. uncontrolled) | 0.323 | 0.762 (0.445–1.306) | ||
| Extracranial metastasis present (vs. absent) | 0.004 | 7.907 (1.941–32.220) | 0.003 | 8.683 (2.124–35.498) |
| No. of BM > 4 (vs. ≤ 4) | 0.010 | 1.884 (1.166–3.044) | 0.267 | 1.384 (0.780–2.455) |
| Location of BM both (vs. supra- or infra-tentorial) | 0.006 | 1.824 (1.192–2.792) | 0.113 | 1.471 (0.912–2.372) |
| Systemic therapy first yes (vs. no) | 0.835 | 0.957 (0.632–1.448) | ||
| KPS <70 (vs. ≥70) | 0.005 | 1.833 (1.198–2.805) | 0.017 | 1.739 (1.103–2.740) |
| Initial WBRT yes (vs. no) | 0.070 | 0.684 (0.454–1.032) | 0.030 | 0.621 (0.404–0.955) |
Abbreviation: HR, hazard ratio; CI, confidence interval; BM, brain metastasis; IDC, invasive ductal carcinoma; HER2, human epidermal growth factor receptor 2; KPS, Karnofsky performance status; WBRT, whole brain radiotherapy.
We additionally conducted subgroup analyses of patients with early BM (<30 months) in order to identify the same effect of WBRT. However, in the propensity score-matched cohort, there was no difference in OS between WBRT(−) and WBRT(+) group (p = 0.180).
4. Discussion
BC patients with BM have a poor prognosis, and the prognosis varies according to the tumor molecular subtype. Also, several systemic and local treatment modalities are available for BM. Therefore, there is an unmet clinical need for tools that guide optimal treatment selection for each patient.
In the current study, the median OS of BC patients with BM was 15.0 months, and OS of patients with the highest BC-specific GPA score group was the best. WBRT was the most commonly used initial local treatment in Korea. The prognostic model we developed in our study revealed that there was a significant difference in survival between low- and high-risk patient subgroups. We also confirmed that WBRT significantly improved the OS of patients with late BM.
Previous studies have delineated several factors that are significantly prognostic in BC patients with BM, including a high-grade tumor, ER/PR-negativity, and the presence of liver and lung metastases [14]. Our results were consistent with these reports as we found that patients with histologic grade 3 or hormone receptor-negative tumors had an unfavorable prognosis. Patients with a small number of BM or without extracranial metastases also tended to survive longer.
A longer interval from primary BC to BM was associated with superior OS. This result can be explained by the fact that many patients in this study with late onset of BM had tumor subtypes with favorable prognostic features, such as luminal A tumor subtype. It was also known that triple-negative or HER2-enriched tumors, which showed worse prognoses, were associated with earlier BM development compared to luminal A tumors [14,15]. However, in our study, the presence of a HER2-positive tumor was a good prognosis factor after developing BM. In a retrospective study, Niikura et al. also reported that BM patients with HER2-positive tumors had longer OS than those with HER2-negative tumors [16]. Anti-HER2 treatments, especially trastuzumab, effectively controlled extracranial metastases and resulted in survival improvement in these patients [[16], [17], [18], [19]].
When we categorized patients into four groups according to the BC-specific GPA score [13], OS improved with an increase in patients’ GPA scores. Furthermore, the Kaplan-Meier survival curves of the GPA score groups clustered into two groups were based on the threshold GPA score of 2. Except for the highest GPA score group, the OS of patients in the GPA score groups in our study were better than those (3.4, 7.7, 15.1, and 25.3 months, respectively) reported by Sperduto et al. [13]. This increased median OS should, however, be interpreted with caution, since treatment modalities and supportive care of patients have improved over time. Therefore, a new model is needed to better predict the survival rate.
We explored a prognostic model that utilized the β-coefficients of factors that were prognostic in our multivariable analyses. We found that this model stratified the patients in our study into a high-risk subgroup that a significantly poorer OS compared to the low-risk subgroup. There were shifts in the patients who had a poor prognosis in the BC-specific GPA system into the low-risk group in our prognostic model, vice versa. After calculating the concordance index of each model, we found that our prognostic model tended to predict OS slightly better, although further validation would be required. Hence, our model identified high-risk patients that require more aggressive treatments and close surveillance after initial treatment of BM.
In univariate analysis, the survival of patients receiving WBRT as first local treatment was poor. However, as WBRT is mainly performed in cases with larger intracranial or extracranial tumor burden [11], this result was reasonable. Conversely, our multivariate analysis did not show any significant difference in OS between WBRT(+) and WBRT(−) patients.
In subgroup analysis, late BM patients were found to benefit from WBRT. This is the first study to examine OS differences in BC patients based on the time of onset of BM and receipt of WBRT. Among late BM patients, patients treated with WBRT as the first local treatment had more known risk factors, including a higher number of BM, than those who did not receive this treatment. After propensity score matching between WBRT(−) and WBRT(+) group, OS curves showed marginally significant (p = 0.068) difference between groups, and crossed over each other at around 9 months after BM diagnosis. The conditional survival rate was also not significantly different between the two groups for up to 2 years, although the difference became significant at 2.5 years. To address the caveat that propensity score matching may not have adequately corrected imbalances between the two groups, we performed multivariable analyses and found a significant increase in OS associated with late BM patients receiving WBRT. However, interpretation of these results should be with caution due to the small number of patients.
The “seed and soil” model proposed by Steven Paget [20] hypothesizes that cross-talk between metastatic tumor cells (seed) and each organ’s microenvironment (soil) ultimately underlies metastasis. As previously mentioned, early BM were characterized by aggressive tumor subtypes. When micrometastases occur, they proliferates rapidly to result in a lesion that is either observable by imaging or symptomatic, so an early BM diagnosis is possible. However, late BM may be detected after multiple micrometastases. In other words, because there are micrometastases in many parts of the brain, WBRT would be more helpful rather than SRS/FSRT or surgical resection, which treat only currently-visible lesions.
Despite this being a large multicenter cohort study, the retrospective design of this study has its limitations. Furthermore, external validation of our multivariable prognostic model is required. Additionally, we were unable to analyze data pertaining to the volume of the treated lesions and neurotoxicity, which all have clinical significance. Finally, patients were treated with various types of systemic agents that were not factored into our analyses; so the outcomes of local treatment for BM requires cautious interpretation.
Currently, the RTOG 1119 trial (NCT01622868), a phase II randomized controlled study, compares the efficacy of using lapatinib in WBRT/SRS patients with BM from HER2-positive BC. From this study, we would expect more detailed outcomes of local treatment (WBRT vs. SRS) as well as the efficacy of concurrent use of lapatinib for BM treatment. Furthermore, to overcome the decline of neurocognitive function after WBRT, hippocampal sparing (HS)-WBRT has been proposed as an alternative [21,22]. Indeed, in a prospective study of 24 patients, HS-WBRT was performed for therapeutic or prophylactic purposes, and the dosimetric parameters for the left-sided hippocampus were related to the immediate recall of verbal memory [23]. Therefore, as an initial local treatment, HS-WBRT rather than SRS could be used for patients, especially with late BM.
5. Conclusions
Our prognostic model identified high-risk BC patients who might benefit from increased surveillance for disease progression and more aggressive treatment of their BM; if validated, our model could guide treatment selection for these patients. Our study found that patients with late BM might benefit from WBRT as initial local treatment.
Funding sources
This work was supported by the grants from the Ministry of Science and Information & Communication Technology (NRF #2017R1A2B4002710 & #2017M2A2A7A01018438) to In Ah Kim.
Ethical approval
This study was approved by the institutional review board of each institution. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Because of the retrospective design of the analysis, requirement for obtaining informed consent of participants included in the study was exempted.
Declaration of competing interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2019.10.007.
Contributor Information
Kyubo Kim, Email: kyubokim.ro@gmail.com.
In Ah Kim, Email: inah228@snu.ac.kr.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lin N.U., Bellon J.R., Winer E.P. CNS metastases in breast cancer. J Clin Oncol. 2004;22(17):3608–3617. doi: 10.1200/JCO.2004.01.175. [DOI] [PubMed] [Google Scholar]
- 2.Tabouret E., Chinot O., Metellus P., Tallet A., Viens P., Goncalves A. Recent trends in epidemiology of brain metastases: an overview. Anticancer Res. 2012;32(11):4655–4662. [PubMed] [Google Scholar]
- 3.Jeon W., Jang B.S., Jeon S.H. Analysis of survival outcomes based on molecular subtypes in breast cancer brain metastases: a single institutional cohort. Breast J. 2018;24(6):920–926. doi: 10.1111/tbj.13111. [DOI] [PubMed] [Google Scholar]
- 4.Quigley M.R., Fukui O., Chew B., Bhatia S., Karlovits S. The shifting landscape of metastatic breast cancer to the CNS. Neurosurg Rev. 2013;36(3):377–382. doi: 10.1007/s10143-012-0446-6. [DOI] [PubMed] [Google Scholar]
- 5.Witzel I., Oliveira-Ferrer L., Pantel K., Müller V., Wikman H. Breast cancer brain metastases: biology and new clinical perspectives. Breast Cancer Res. 2016;18(1):1–9. doi: 10.1186/s13058-015-0665-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morikawa A., Jhaveri K., Seidman A.D. Clinical trials for breast cancer with brain metastases: challenges and new directions. Curr Breast Canc Rep. 2013;5(4):293–301. doi: 10.1007/s12609-013-0120-1. [DOI] [Google Scholar]
- 7.Tsao M.N., Rades D., Wirth A. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210–225. doi: 10.1016/j.prro.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown P.D., Jaeckle K., Ballman K.V. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases a randomized clinical trial. JAMA, J Am Med Assoc. 2016;316(4):401–409. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta M. The dandelion effect: treat the whole lawn or weed selectively? J Clin Oncol. 2011;29(2):121–124. doi: 10.1200/JCO.2010.33.3294. [DOI] [PubMed] [Google Scholar]
- 10.Chang E., Wefel J., Hess K. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 11.Mainwaring W., Bowers J., Pham N. Stereotactic radiosurgery versus whole brain radiation therapy: a propensity score analysis and predictors of care for patients with brain metastases from breast cancer. Clin Breast Canc. 2018:1–9. doi: 10.1016/j.clbc.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Li J., Bentzen S.M., Renschler M., Mehta M.P. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol. 2007;25(10):1260–1266. doi: 10.1200/JCO.2006.09.2536. [DOI] [PubMed] [Google Scholar]
- 13.Sperduto P.W., Kased N., Roberge D. Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys. 2012;82(5):2111–2117. doi: 10.1016/j.ijrobp.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahn H.K., Park Y.H., Lee S.J. Clinical implication of Time to Brain Metastasis (TTBM) according to breast cancer subtypes. SpringerPlus. 2013;2(1):1–7. doi: 10.1186/2193-1801-2-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heitz F., Harter P., Lueck H.J. Triple-negative and HER2-overexpressing breast cancers exhibit an elevated risk and an earlier occurrence of cerebral metastases. Eur J Cancer. 2009;45(16):2792–2798. doi: 10.1016/j.ejca.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 16.Niikura N., Hayashi N., Masuda N. Treatment outcomes and prognostic factors for patients with brain metastases from breast cancer of each subtype: a multicenter retrospective analysis. Breast Canc Res Treat. 2014;147(1):103–112. doi: 10.1007/s10549-014-3090-8. [DOI] [PubMed] [Google Scholar]
- 17.Bartsch R., Rottenfusser A., Wenzel C. Trastuzumab prolongs overall survival in patients with brain metastases from Her2 positive breast cancer. J Neuro Oncol. 2007;85(3):311–317. doi: 10.1007/s11060-007-9420-5. [DOI] [PubMed] [Google Scholar]
- 18.Church D.N., Modgil R., Guglani S. Extended survival in women with brain metastases from HER2 overexpressing breast cancer. Am J Clin Oncol Canc Clin Trials. 2008;31(3):250–254. doi: 10.1097/COC.0b013e31815a43c4. [DOI] [PubMed] [Google Scholar]
- 19.Yap Y.S., Cornelio G.H., Devi B.C.R. Brain metastases in Asian HER2-positive breast cancer patients: anti-HER2 treatments and their impact on survival. Br J Canc. 2012;107(7):1075–1082. doi: 10.1038/bjc.2012.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paget S. The distribution OF secondary growths IN cancer OF the breast. Lancet. 1889;133(3421):571–573. doi: 10.1016/S0140-6736(00)49915-0. [DOI] [PubMed] [Google Scholar]
- 21.Ghia A., Tome W., Thomas S. Distribution of brain metastases in relation to the Hippocampus: implications for neurocognitive functional preservation. Int J Radiat Oncol Biol Phys. 2007;68(4):971–977. doi: 10.1016/j.ijrobp.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Gondi V., Pugh S.L., Tome W.A. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810–3816. doi: 10.1200/JCO.2014.57.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai P.F., Yang C.C., Chuang C.C. Hippocampal dosimetry correlates with the change in neurocognitive function after hippocampal sparing during whole brain radiotherapy: a prospective study. Radiat Oncol. 2015;10(1) doi: 10.1186/s13014-015-0562-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.