Abstract
Background
Nipple-sparing mastectomy (NSM), followed by immediate reconstruction (IR) of the breast, has become a preferred surgical procedure with good cosmesis results and patient satisfaction. However, nipple-areolar complex (NAC) ischemia and necrosis remain major problems after NSM and IR.
Methods
We retrospectively analyzed patients who underwent NSM and IR at Gangnam Severance Hospital from January 2009 to June 2018. We compared the patient characteristics and complication rate among three different incisions (inframammary fold [IMF], radial, periareolar). Additionally, we identified the risk factors of NAC necrosis.
Results
Data from 290 eligible breasts in 275 patients were analyzed. Patients with IMF incision had relatively lower breast weights. The overall complication rate was the highest with periareolar incision and the lowest with IMF incision (42.6% vs. 18.8%, p < 0.001). The rate of NAC ischemia or necrosis was significantly different among the three incisions (9.7%, 17.0%, and 31.1% in IMF, radial, and periareolar, respectively; p < 0.001). Moreover, surgical treatments were more frequently needed in patients with periareolar incision. Periareolar incision, short distance from the tumor to the nipple base, and large breast weight were independent risk factors of NAC ischemia or necrosis in multivariable analysis.
Conclusions
Compared with IMF incision, periareolar incision was associated with higher incidences of surgical complications and NAC necrosis. Careful consideration is needed when planning NSM in patients with a large breast volume or a tumor close to the nipple.
Keywords: Nipple-sparing mastectomy, Nipple-areolar complex necrosis, Complication, Incision
Highlights
-
•
Periareolar incision shows higher complication rate in nipple-sparing mastectomy.
-
•
Periareolar incision is a risk factor for nipple-areolar complex necrosis.
-
•
Other risk factors include tumor-nipple distance and breast weight.
1. Introduction
Nipple-sparing mastectomy (NSM) is a surgical technique that preserves the patient’s own breast skin and nipple-areolar complex (NAC) while removing the glandular and ductal tissues [1,2]. NSM followed by immediate reconstruction (IR) has gained popularity owing to its superior outcomes in terms of cosmesis and patient satisfaction [1,3,4]. Because the procedure has been proven to be as safe as conventional mastectomy with respect to oncologic aspects, the number of NSM procedures performed with therapeutic and prophylactic intent has been increasing annually [2,[5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]].
In contrast to the acceptable overall complication rate compared with skin-sparing mastectomy or modified radical mastectomy, skin (including NAC) necrosis was significantly higher in NSM based on a meta-analysis study using the Cochrane database [15]. During the surgery, glandular and ductal tissues are maximally resected to reduce the possibility of locoregional recurrence [16], which can injure the blood supply of the breast skin and the NAC [17,18].
NAC necrosis is considered the most problematic complication of NSM, leading to adverse outcomes such as nipple deformity, hypopigmentation in the NAC area, or loss of the NAC. The rate of NAC ischemia or necrosis after NSM ranges from 0% to 48%, with large variations across different studies [2,5,8,[19], [20], [21], [22]]. However, a meta-analysis of recent studies revealed an overall partial NAC necrosis rate of 4.62% and a complete NAC necrosis rate of 2.49%, which are acceptably low [22]. Several studies have identified the type of surgical incision as one of the risk factors of NAC necrosis, with the highest necrosis rate in periareolar incision [8,19,[22], [23], [24], [25], [26]].
In this study, we compared the complications of NSM followed by IR among three incision types from the database of our center. Further, we analyzed the patient characteristics, tumor characteristics, and surgical factors in a multivariable analysis to investigate the risk factors of NAC necrosis.
2. Methods
2.1. Demographics
We retrospectively reviewed the electronic medical records of patients who underwent NSM followed by IR at Gangnam Severance Hospital from January 2009 to June 2018. NSM was mostly performed as a treatment for invasive breast cancer without suspected NAC invasion. The other indications included mastectomy for a phyllodes tumor or for prophylaxis. Patient and tumor characteristics as well as surgical factors, including oncologic surgeon, plastic surgeon, incision type, type of axillary surgery, and reconstruction methods were investigated. Smoking history was gathered from survey-based outpatient and inpatient records. Current and ex-smokers were considered as ‘yes’ and non-smokers were considered as ‘no’. Distance from the nipple base to the tumor [tumor-nipple distance, TND] was estimated using magnetic resonance imaging (MRI), except for patients with neoadjuvant chemotherapy or patients without tumor. Patients who had undergone a prior breast augmentation surgery were excluded.
The protocol of the study was approved by the institutional review board of Gangnam Severance Hospital. The need for informed consent was waived under the approval of the institutional review board, owing to the retrospective design.
2.2. Surgical procedure
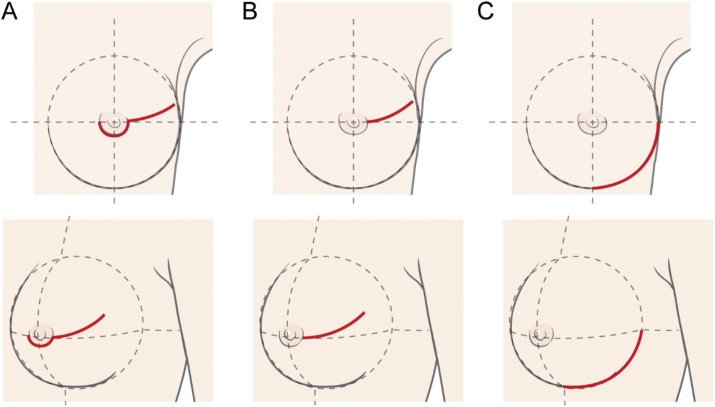
We performed NSM using three types of incision: radial, periareolar, and inframammary fold (IMF) incisions (Fig. 1). Radial incision starts at the lateral side of NAC, extending to the axilla obliquely. This incision could be abutting to the areola but did not include the border of the areola. Periareolar incision was similar to radial incision but included part of the border of either the upper or lower side of the areola, which is usually determined according to the tumor location. Lastly, IMF incision, which includes the lower outer arc of the circle line of ipsilateral breast area, was made along the natural skin crease of the IMF. Thus, IMF incision did not include the NAC.
Fig. 1.
Incisions in nipple-sparing mastectomy (A: periareolar, B: radial, C: inframammary fold).
A skin flap was made along the superficial mammary fascia using electrocautery, with a thickness of approximately 7–15 mm. The range of skin flap was concordant to the breast anatomy, superiorly to 1–2 cm below the clavicle, inferiorly to the upper part of the rectus sheath, medially to the parasternal line, and laterally to the anterior border of the latissimus dorsi. In cases of cancer surgery, the subareolar margin was examined by a pathologist during surgery through frozen biopsy. When cancer invasion was found in the subareolar margin, the NAC was resected and the patient was excluded from this study. On the basis of the patient’s node status, either sentinel lymph node biopsy or axillary lymph node dissection was performed.
After mastectomy, IR of the breast was performed by plastic surgeons. In prosthetic-based reconstructions such as direct-to-implant (DTI) or tissue expander reconstruction, all devices were inserted in the subpectoral plane and covered with an allogenic dermal matrix sling. Before the procedure, the tissue expander was filled to about one-third of the specimen volume to reduce skin tension. In cases of autologous breast reconstruction, deep inferior epigastric artery perforator (DIEP) free flap reconstruction or latissimus dorsi myocutaneous island flap reconstruction was performed. Jackson-Pratt drains were inserted during surgery and removed when the amount of daily drainage became < 30 mL. Second-generation cephalosporins were administered at anesthesia induction and continued until the removal of drains.
2.3. Complications
To evaluate the complications related to incision types, we included postoperative complications that occurred in the early phase until 6 months after surgery in the analysis. We divided the complications into six categories: infection, skin necrosis, nipple necrosis, requirement for implant removal, others, and surgical treatment requirement.
Postoperative infection was defined as any of the following: (i) requirement for escalation or continuation of antibiotics for signs of clinical infection or (ii) confirmation of pathogenic bacteria cultured from the wound site. Skin or NAC necrosis included partial necrosis, which spontaneously healed after conservative care, or complete necrosis, which required surgical treatment. Other complications included hematoma, dehiscence, or minor wound problems. Surgical treatment requirement was defined as the need for an operative procedure to resolve complications which occurred in operation site and were directly related to NSM and IR procedures. Complications related to autologous flap were not included. Surgical treatments for complications included debridement, wound revision, NAC removal, implant removal, and incision & drainage.
2.4. Oncologic outcome
After the surgery, patients were recommended to visit the outpatient clinic every six months. Patients were evaluated for tumor recurrence by mammography, accompanied by either breast sonography or breast MRI. We assessed the local recurrence, which was defined as disease in the ipsilateral chest wall or skin.
2.5. Statistical analysis
The data were analyzed using SPSS version 23.0 (IBM Co., Armonk, NY, USA). The chi-square test or analysis of variance was used to compare the patients’ characteristics. The chi-square test was also used to compare the rates of complications among the incisions. We examined potential risk factors of NAC necrosis in univariable logistic regression model. Unadjusted odds-ratios (OR) and 95% confidence intervals were calculated. The factors that showed statistical significance (p < 0.1) in univariable analysis were entered into the multivariable logistic regression model. Adjusted odds-ratios and 95% confidence intervals were calculated based on the multivariable logistic regression model. Values of p < 0.05 were considered to indicate statistical significance. As year of surgery could affect surgeons’ skill and act as a confounding factor, we fit a series of multivariable model adjusting with or without this factor. Model 1 was adjusted only for year of surgery; Model 2 for risk factors without year of surgery; and Model 3 for risk factors with year of surgery.
The Kaplan-Meier method and the log-rank test were used to compare the local recurrence-free survival among the incisions, which was defined as the time from surgery until the date of local recurrence.
3. Results
3.1. Demographics
A total of 275 patients underwent NSM with IR for 290 breasts. Fifteen (5.5%) patients had bilateral mastectomy. Mastectomy for breast cancer or in situ carcinoma was performed in 283 breasts, whereas mastectomy for phyllodes tumor was performed in 2 breasts. Five cases were prophylactic mastectomies. The characteristics of the patients are described in Table 1.
Table 1.
Demographics of overall cases (n = 290).
| Age, years | 46.5 ± 7.9 |
| BMI, kg/m2 | 22.40 ± 2.95 |
| HTN | 19 (6.6%) |
| DM | 6 (2.1%) |
| Smoking | 5 (1.7%) |
| Neoadjuvant CTx | 34 (11.7%) |
| Pre RTx Hx | 21 (7.2%) |
| Pre surgery Hx | 48 (16.6%) |
| Site | |
| Right | 147 (50.7%) |
| Left | 143 (49.3%) |
| TND, cma | 2.81 ± 1.61 |
| Breast weight, g | 387.22 ± 178.80 |
| Axillary surgery | |
| Not done | 13 (4.5%) |
| SLNB | 208 (71.7%) |
| ALND | 69 (23.8%) |
| Oncologic surgeon | |
| A | 235 (81.0%) |
| B | 52 (17.9%) |
| Others | 3 (1.0%) |
| Plastic surgeon | |
| A | 212 (73.1%) |
| B | 78 (26.9%) |
| Reconstruction | |
| DTI | 258 (89.0%) |
| Tissue expander | 19 (6.6%) |
| Autologous | 13 (4.5%) |
| Postmastectomy RTx | 50 (17.2%) |
Data are mean ± standard deviation or n (%).
Missing value: (BMI: body mass index, HTN, hypertension, DM: diabetes mellitus, CTx: chemotherapy, Pre: previous, RTx: radiation therapy, Hx: history, TND: tumor-nipple distance, SLNB: sentinel lymph node biopsy, ALND: axillary lymph node dissection, DTI: direct-to-implant).
3.2. Characteristics according to incision types
The patient characteristics compared according to incision types are presented in Table 2. The BMI was higher in patients with radial incision (23.2 kg/m2 vs. 22.4 kg/m2 [IMF] and 21.7 kg/m2 [periareolar], p = 0.037). Patients with IMF incision had a relatively lighter breast weight than other patients (352.7 g vs. 438.9 g [radial] and 444.7 g [periareolar], p < 0.001). Accordingly, the DTI volume was also smaller in patients with IMF incision (p < 0.001). Breast reconstruction using an autologous flap was mostly performed in patients with periareolar incision. More patients with IMF incision had prior chemotherapy, but the difference was not statistically significant. Periareolar and radial incisions were mostly performed by oncologic surgeon A. The other factors were similar among the incision groups.
Table 2.
Characteristics according to incision types.
| IMF (n = 176) | Radial (n = 53) | Periareolar (n = 61) | P | |
|---|---|---|---|---|
| Age, years | 47.2 ± 7.3 | 45.8 ± 7.9 | 45.1 ± 9.37 | 0.146 |
| BMI, kg/m2 | 22.4 ± 3.1 | 23.2 ± 2.7 | 21.7 ± 2.7 | 0.037 |
| Smoking | 4 (2.3%) | 0 (0.0%) | 1 (1.6%) | 0.825a |
| DM | 4 (2.3%) | 1 (1.9%) | 1 (1.6%) | >0.999a |
| HTN | 8 (4.5%) | 5 (9.4%) | 6 (9.8%) | 0.181a |
| Pre RTx | 13 (7.4%) | 6 (11.3%) | 2 (3.3%) | 0.261a |
| Neoadj. CTx | 26 (14.8%) | 6 (11.3%) | 2 (3.3%) | 0.054 |
| Pre Surgery Hx | 28 (15.9%) | 11 (20.8%) | 9 (14.8%) | 0.648 |
| TNDa | 2.78 ± 1.64 | 2.49 ± 1.52 | 3.12 ± 1.59 | 0.126 |
| Axillary surgery | 0.135 | |||
| Not done | 10 (5.7%) | 2 (3.8%) | 1 (1.6%) | |
| SLNB | 123 (69.9%) | 34 (64.2%) | 51 (83.6%) | |
| ALND | 43 (24.4%) | 17 (32.1%) | 9 (14.8%) | |
| Oncologic surgeon | 0.002a | |||
| A | 133 (75.6%) | 45 (84.9%) | 57 (93.4%) | |
| B | 42 (23.9%) | 7 (13.2%) | 3 (4.9%) | |
| Others | 1 (0.6%) | 1 (1.9%) | 1 (1.6%) | |
| Breast weight | 352.7 ± 160.6 | 438.9 ± 178.0 | 444.7 ± 206.6 | < 0.001 |
| Reconstruction | < 0.001a | |||
| DTI | 164 (93.2%) | 48 (90.6%) | 46 (75.4%) | |
| Tissue expander | 12 (6.8%) | 4 (7.5%) | 3 (4.9%) | |
| Autologous | 0 (0.0%) | 1 (1.9%) | 12 (19.7%) | |
| Implant volume, mlb | 210.6 ± 69.9 | 234.1 ± 67.3 | 260.3 ± 76.2 | < 0.001 |
| Postmastectomy RTx | 31 (17.6%) | 13 (24.5%) | 6 (9.8%) | 0.124 |
Data are mean ± standard deviation or n (%).
Fisher’s exact test.
Missing value: (BMI: body mass index, DM: diabetes mellitus, HTN: hypertension, Neoadj.: neoadjuvant, CTx: chemotherapy, Pre: previous, RTx: radiotherapy, Hx: history, TND: tumor-nipple distance, SLNB: sentinel lymph node biopsy, ALND: axillary lymph node dissection, DTI: direct-to-implant).
3.3. Complication rate according to incision types
Among 290 breasts, 78 (26.9%) had at least one complication (Table 3). The total complication rate was high, in the order of periareolar, radial, and IMF cases (42.6% vs. 35.8% vs. 18.8%, p < 0.001). The nipple necrosis rate was also the highest in periareolar cases, followed by radial and IMF cases (31.1% vs. 17.0% vs. 9.7%, p < 0.001). Similarly, the rate of surgical treatment requirement was also the highest in periareolar incision, followed by radial and IMF incisions (31.7% vs. 26.0% vs. 14.1%). The other complications were not significantly different among the incision groups.
Table 3.
Complication rate according to incision types.
| Total (%) (n = 290) |
IMF (%) (n = 176) |
Radial (%) (n = 53) |
Periareolar (%) (n = 61) |
P | |
|---|---|---|---|---|---|
| Complication | 78 (26.9) | 33 (18.8) | 19 (35.8) | 26 (42.6) | < 0.001 |
| Infection | 13 (4.5) | 4 (2.3) | 4 (7.5) | 5 (8.2) | 0.057a |
| Skin necrosis | 25 (8.6) | 14 (8.0) | 6 (11.3) | 5 (8.2) | 0.801 |
| Nipple necrosis | 45 (15.5) | 17 (9.7) | 9 (17.0) | 19 (31.1) | < 0.001 |
| (Complete necrosis) | 25 (8.6) | 6 (3.4) | 6 (11.3) | 13 (21.3) | < 0.001 |
| Implant removal | 11 (3.8) | 6 (3.4) | 3 (5.7) | 2 (3.3) | 0.625a |
| Other | 15 (5.2) | 7 (4.0) | 4 (7.5) | 4 (6.6) | 0.454a |
| Surgical treatment | 54 (18.6) | 22 (14.1) | 13 (26.0) | 19 (31.7) | 0.003 |
Fisher’s exact test.
Because there was no autologous reconstruction case with IMF incision compared to about 20% of cases with periareolar incision, we additionally analyzed complication rate after excluding cases with autologous reconstruction. One radial case and 12 periareolar cases were excluded. Main finding was similar with original analysis (supplementary table 1).
3.4. Risk factors of nipple necrosis
As periareolar incision showed significantly high rates of nipple necrosis, we additionally analyzed the risk factors of nipple necrosis. In univariable analysis, the reconstruction method, incision type, breast weight, and implant volume were the risk factors for nipple necrosis (Table 4). Postmastectomy RTx was excluded from analysis because all 12 complications among 50 cases with postmastectomy RTx occurred before starting radiation therapy.
Table 4.
Risk factors of nipple-areola complex necrosis: univariable analysis.
| Risk factor | OR | 95% CI | P |
|---|---|---|---|
| Age | 1.012 | 0.972–1.053 | 0.565 |
| BMI | 1.045 | 0.945–1.156 | 0.392 |
| HTN | 1.496 | 0.473–4.733 | 0.493 |
| DM | 1.091 | 0.124–9.564 | 0.937 |
| Neoadj. CTx | 0.146 | 0.019–1.096 | 0.061 |
| Pre RTx Hx | 0.901 | 0.254–3.194 | 0.871 |
| Pre surgery Hx | 0.744 | 0.296–1.869 | 0.529 |
| Site | |||
| Right | Reference | ||
| Left | 0.980 | 0.519–1.851 | 0.951 |
| Oncologic surgeon: A | 2.695 | 0.922–7.871 | 0.070 |
| TND | 0.814 | 0.647–1.024 | 0.079 |
| Reconstruction | |||
| DTI | Reference | 0.088 | |
| Tissue-expander | 1.120 | 0.311–4.033 | 0.810 |
| Autologous | 3.733 | 1.158–12.032 | 0.027 |
| Incision | |||
| Inframammary | Reference | 0.001 | |
| Radial | 1.913 | 0.798–4.586 | 0.146 |
| Periareolar | 4.231 | 2.024–8.845 | <0.001 |
| Breast weight | 1.003 | 1.001–1.004 | 0.004 |
| Implant volumea | 1.006 | 1.002–1.011 | 0.007 |
| Initial tissue expander volumea | 1.001 | 0.989–1.013 | 0.868 |
Missing value: (OR: odds ratio, CI: confidence interval, BMI: body mass index, HTN, hypertension, DM: diabetes mellitus, Neoadj.: neoadjuvant, CTx: chemotherapy, Pre: previous, RTx: radiotherapy, Hx: history, TND: tumor-nipple distance, SLNB: sentinel lymph node biopsy, ALND: axillary lymph node dissection, DTI: direct-to-implant).
Table 5 shows adjusted ORs and their 95% CIs from multiple logistic regression analyses using three models. Periareolar incision, TND, and breast weight were found to be risk factors for NAC necrosis (Table 5, Model 2), and after adjusting for year of surgery, these factors remained significant (Table 5, Model 3). When compared with IMF incision, periareolar incision increased the risk by 3.628-fold (p = 0.002). Each 1 cm increase in TND decreased the risk by 0.712-fold (p = 0.012). For each 1 g increase in breast weight, the risk of NAC necrosis increased by 1.002-fold (p = 0.014). Implant volume was excluded in multivariable analysis because it was related to breast weight and limited to patients who underwent reconstruction with DTI.
Table 5.
Risk factors of nipple-areola complex necrosis: multivariable analysis.
| Risk factor | Model1 |
Model2 |
Model3 |
|||
|---|---|---|---|---|---|---|
| OR(95% CI) | P | OR(95% CI) | P | OR(95% CI) | P | |
| Periareolar incision (vs. IMF incision) | 4.231(2.024–8.845) | <0.001 | 3.624(1.594–8.239) | 0.002 | 3.628(1.596–8.250) | 0.002 |
| TND | 0.777(0.613–0.986) | 0.038 | 0.713(0.547–0.929) | 0.012 | 0.712(0.546–0.927) | 0.012 |
| Breast weight | 1.002(1.001–1.004) | 0.01 | 1.002(1.000–1.004) | 0.014 | 1.002(1.000–1.004) | 0.014 |
OR: odds ratio, CI: confidence interval, Neoadj.: neoadjuvant, CTx: chemotherapy, TND: tumor-nipple distance.
Model1: Adjusted for year of surgery.
Model2: Adjusted for Neoadj. CTx, oncologic surgeon, TND, reconstruction, incision, breast weight.
Model3: Adjusted for Neoadj. CTx, oncologic surgeon, TND, reconstruction, incision, breast weight, year of surgery.
3.5. Oncologic outcome
The median follow-up time for each incision was 67 months for periareolar incision (range, 22–98), 54 months for radial incision (range, 9–63), and 34.5 months (range, 0–96) for IMF incision. The local recurrence events were identified in 2 (3.3%), 1 (1.9%), and 6 (3.4%) patients, respectively. The two-year local recurrence-free survival rates were 98.4%, 98.1%, and 97.6%, respectively. No significant difference in local recurrence-free survival was noted among three incisions (log rank p = 0.648, graph not shown).
4. Discussion
Our results showed that the rates of overall complications, NAC necrosis, and subsequent surgical treatment were higher in periareolar cases than in cases of other incisions. In contrast, infection, skin necrosis, and other complications showed no difference according to incision type. Surgical treatments (for skin/nipple necrosis, wound dehiscence, or other reasons) and implant removal were more frequently needed in patients with periareolar incision. Moreover, periareolar incision was associated with higher risk of NAC necrosis in multivariable analysis.
The inferior outcome of periareolar incision in terms of nipple necrosis has been reported in several studies [8,19,[22], [23], [24], [25], [26]]. Daar et al. performed a systematic literature review and meta-analysis including 51 studies with 9975 NSMs, and identified that periareolar incision had the highest NAC necrosis rate (18.10%). On the contrary, IMF incision had a comparably low NAC necrosis rate (6.82%) [22]. In our data, periareolar incision similarly showed a higher overall NAC necrosis rate than IMF incision (31.1% vs 9.7%). The values are lower for complete nipple necrosis, with a rate of 21.3% in periareolar incision cases and 3.4% in IMF incision cases.
Although radial incision showed higher rate of nipple necrosis than IMF incision, it was not a significant risk factor of nipple necrosis in multivariable analysis. In Garwood et al.’s study, necrotic complications were less in surgical incisions which did not cross the NAC tissue or involved less than 30% of the NAC [8]. In Odom et al.’s prospective cohort study, nipple perfusion as represented by fluorescence intensity was not significantly different between IMF and radial lateral incision [27]. From these results, it could be assumed that injury of nipple blood supply and nipple necrosis may be prevented by using incisions which do not involve the border of areola.
In our study, the other factors that increased the risk of NAC necrosis were short TND and large breast weight. TND has been a main concern with respect to oncologic safety in NSM, and several studies have reported the feasibility of NSM in patients with a short TND [[28], [29], [30], [31]]. However, few papers have investigated the correlation between TND and nipple necrosis. Recently, Ito et al. first reported on the relationship between TND and nipple necrosis, showing negative results [32]. In contrast, our study showed, for the first time, that short TND is a significant risk factor of NAC necrosis. We assume that if the tumor is close to the nipple, it is likely that the NAC flap will become thinner, which may disrupt the blood supply. Algaithy et al. reported thin areolar flap (<5 mm) as a risk factor for NAC necrosis [19].
Breast weight or breast volume is a well-known risk factor based on previous studies [33,34]. A large breast volume may result in increased skin tension, longer distance from the source of blood supply to the nipple, and decreased blood flow to the NAC. Further, surgeons could apply excessive tension to the flap during surgery when attempting to acquire sufficient surgical vision. Although implant volume was also a significant risk factor for NAC necrosis in univariable analysis, we did not include this factor in multivariable analysis because it was directly related to the specimen weight. Plastic surgeons at our institution decided the volume based on breast specimen weight (55–80% of breast specimen) [35].
We analyzed the influence of prior treatment, including surgery, neoadjuvant chemotherapy, or RTx, on nipple necrosis and did not find any relevance. Among those factors, several studies have reported conflicting results on the effects of previous RTx on nipple necrosis after NSM [24,[36], [37], [38], [39]]. Progressive fibrosis, depletion of parenchymal and stem cells, and release of bioactive cytokines induced by RTx are believed to cause impaired wound healing [40]. The reason of negative finding on the effect of RTx in our study seems unclear. One possible assumption is that few patients in our study had a smoking history, which was another risk factor for nipple necrosis and could create synergic effects with RTx. Moreover, the radiation dose, duration, and range or the time interval from RTx to surgery need to be considered. To draw a clearer conclusion on the effect of RTx on nipple necrosis in NSM, prospectively designed studies including the effect of RTx are needed to compare against most published studies, which had a retrospective design.
Our study has several limitations related to its retrospective design. First, there is a chronological order in implementing NSM (i.e., in the order of periareolar, radial, and IMF incision). Hence, improvement of surgical skill, such as learning curve, and accumulation of experience could affect the surgical outcomes. For this reason, we made year-adjusted model for NAC necrosis in order to rule out possible bias. Second, potential bias can exist in the selection of incision. Age, breast size, tumor location, or previous scar could affect the choice of surgeons. In the early phase, there was a tendency for selecting IMF incision for small breasts. Although this preference weakened as the cases increased, breast splitting incision could be more frequently considered to patients with macromastia, close TND, previous scar or marked ptosis. Finally, our study does not include patient reported outcomes (PRO) which could be important in that the main purpose of NSM is for patients’ satisfaction. PRO was not available because our center did not conduct the survey routinely.
Nevertheless, our study has some advantages. Because most of our NSM cases were performed by one skilled surgeon (81% of NSMs were performed by Dr. Jeong), the effect of surgical gap among surgeons may be less than in other studies. The difference in surgical skill could affect the results, as reported in Ahn et al.’s study in which one particular oncologic surgeon showed a much higher rate of NAC ischemia or revisions than others (OR 8.335, 95% CI 1.656–41.962, p = 0.0101) [26]. Further, we first revealed TND as a risk factor for NAC necrosis through a multivariable analysis. As only a few studies have analyzed the relation between TND and NAC necrosis, this should be considered in future studies.
In fact, prospective study with randomization would be needed to accurately compare three types of incisions with less bias. However, because the shortcomings of periareolar incision have already been reported in previous studies, and there is patient’s preference, it is difficult to implement randomization in reality. For this reason, Odom et al. failed the randomization in deciding IMF vs lateral incision in their prospective cohort study [27]. When considering these practical limitations, the results of our retrospective study would be a useful reference showing differences between three types of incisions.
5. Conclusion
In our retrospective review of 290 NSM cases, we found that periareolar incision has a higher incidence of overall complications and a higher risk of NAC necrosis than the other incisions. Inframammary incision or radial incision could be a preferred choice with fewer complications. Careful consideration is needed when planning NSM in patients with a large breast volume or a tumor close to the nipple.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
The authors thank Medical Illustration & Design (MID), a part of the Medical Research Support Services of Yonsei University College of Medicine, for all graphic designs related to this work. We would like to thank Editage (www.editage.co.kr) for English language editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2020.06.009.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Moyer H.R., Ghazi B., Daniel J.R., Gasgarth R., Carlson G.W. Nipple-sparing mastectomy: technical aspects and aesthetic outcomes. Ann Plast Surg. 2012;68:446–450. doi: 10.1097/SAP.0b013e3182394bba. [DOI] [PubMed] [Google Scholar]
- 2.de Alcantara Filho P., Capko D., Barry J.M., Morrow M., Pusic A., Sacchini V.S. Nipple-sparing mastectomy for breast cancer and risk-reducing surgery: the Memorial Sloan-Kettering Cancer Center experience. Ann Surg Oncol. 2011;18:3117–3122. doi: 10.1245/s10434-011-1974-y. [DOI] [PubMed] [Google Scholar]
- 3.Psaila A., Pozzi M., Barone Adesi L., Varanese A., Costantini M., Gullo P. Nipple sparing mastectomy with immediate breast reconstruction: a short term analysis of our experience. J Exp Clin Canc Res. 2006;25:309–312. [PubMed] [Google Scholar]
- 4.Didier F., Radice D., Gandini S., Bedolis R., Rotmensz N., Maldifassi A. Does nipple preservation in mastectomy improve satisfaction with cosmetic results, psychological adjustment, body image and sexuality? Breast Canc Res Treat. 2009;118:623–633. doi: 10.1007/s10549-008-0238-4. [DOI] [PubMed] [Google Scholar]
- 5.Sacchini V., Pinotti J.A., Barros A.C., Luini A., Pluchinotta A., Pinotti M. Nipple-sparing mastectomy for breast cancer and risk reduction: oncologic or technical problem? J Am Coll Surg. 2006;203:704–714. doi: 10.1016/j.jamcollsurg.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Smith B.L., Tang R., Rai U., Plichta J.K., Colwell A.S., Gadd M.A. Oncologic safety of nipple-sparing mastectomy in women with breast cancer. J Am Coll Surg. 2017;225:361–365. doi: 10.1016/j.jamcollsurg.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Boneti C., Yuen J., Santiago C., Diaz Z., Robertson Y., Korourian S. Oncologic safety of nipple skin-sparing or total skin-sparing mastectomies with immediate reconstruction. J Am Coll Surg. 2011;212:686–693. doi: 10.1016/j.jamcollsurg.2010.12.039. discussion 93-5. [DOI] [PubMed] [Google Scholar]
- 8.Garwood E.R., Moore D., Ewing C., Hwang E.S., Alvarado M., Foster R.D. Total skin-sparing mastectomy: complications and local recurrence rates in 2 cohorts of patients. Ann Surg. 2009;249:26–32. doi: 10.1097/SLA.0b013e31818e41a7. [DOI] [PubMed] [Google Scholar]
- 9.Gerber B., Krause A., Dieterich M., Kundt G., Reimer T. The oncological safety of skin sparing mastectomy with conservation of the nipple-areola complex and autologous reconstruction: an extended follow-up study. Ann Surg. 2009;249:461–468. doi: 10.1097/SLA.0b013e31819a044f. [DOI] [PubMed] [Google Scholar]
- 10.Paepke S., Schmid R., Fleckner S., Paepke D., Niemeyer M., Schmalfeldt B. Subcutaneous mastectomy with conservation of the nipple-areola skin: broadening the indications. Ann Surg. 2009;250:288–292. doi: 10.1097/SLA.0b013e3181b0c7d8. [DOI] [PubMed] [Google Scholar]
- 11.Jakub J.W., Peled A.W., Gray R.J., Greenup R.A., Kiluk J.V., Sacchini V. Oncologic safety of prophylactic nipple-sparing mastectomy in a population with brca mutations: a multi-institutional study. JAMA surgery. 2018;153:123–129. doi: 10.1001/jamasurg.2017.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis R.S., George A., Rusby J.E. Nipple-sparing mastectomy in women at high risk of developing breast cancer. Gland Surg. 2018;7:325–336. doi: 10.21037/gs.2018.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerber B., Krause A., Reimer T., Muller H., Kuchenmeister I., Makovitzky J. Skin-sparing mastectomy with conservation of the nipple-areola complex and autologous reconstruction is an oncologically safe procedure. Ann Surg. 2003;238:120–127. doi: 10.1097/01.SLA.0000077922.38307.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim H.J., Park E.H., Lim W.S., Seo J.Y., Koh B.S., Lee T.J. Nipple areola skin-sparing mastectomy with immediate transverse rectus abdominis musculocutaneous flap reconstruction is an oncologically safe procedure: a single center study. Ann Surg. 2010;251:493–498. doi: 10.1097/SLA.0b013e3181c5dc4e. [DOI] [PubMed] [Google Scholar]
- 15.Mota B.S., Riera R., Ricci M.D., Barrett J., de Castria T.B., Atallah A.N. Nipple- and areola-sparing mastectomy for the treatment of breast cancer. Cochrane Database Syst Rev. 2016;11:CD008932. doi: 10.1002/14651858.CD008932.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petit J.Y., Veronesi U., Rey P., Rotmensz N., Botteri E., Rietjens M. Nipple-sparing mastectomy: risk of nipple-areolar recurrences in a series of 579 cases. Breast Canc Res Treat. 2009;114:97–101. doi: 10.1007/s10549-008-9968-6. [DOI] [PubMed] [Google Scholar]
- 17.van Deventer P.V. The blood supply to the nipple-areola complex of the human mammary gland. Aesthetic Plast Surg. 2004;28:393–398. doi: 10.1007/s00266-003-7113-9. [DOI] [PubMed] [Google Scholar]
- 18.Stirling A.D., Murray C.P., Lee M.A. The arterial supply of the nipple areola complex (NAC) and its relations: an analysis of angiographic CT imaging for breast pedicle design. Surg Radiol Anat. 2017;39:1127–1134. doi: 10.1007/s00276-017-1858-3. [DOI] [PubMed] [Google Scholar]
- 19.Algaithy Z.K., Petit J.Y., Lohsiriwat V., Maisonneuve P., Rey P.C., Baros N. Nipple sparing mastectomy: can we predict the factors predisposing to necrosis? Eur J Surg Oncol : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2012;38:125–129. doi: 10.1016/j.ejso.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Komorowski A.L., Zanini V., Regolo L., Carolei A., Wysocki W.M., Costa A. Necrotic complications after nipple- and areola-sparing mastectomy. World J Surg. 2006;30:1410–1413. doi: 10.1007/s00268-005-0650-4. [DOI] [PubMed] [Google Scholar]
- 21.Crowe J.P., Patrick R.J., Yetman R.J., Djohan R. Nipple-sparing mastectomy update: one hundred forty-nine procedures and clinical outcomes. Arch Surg. 2008;143:1106–1110. doi: 10.1001/archsurg.143.11.1106. discussion 10. [DOI] [PubMed] [Google Scholar]
- 22.Daar D.A., Abdou S.A., Rosario L., Rifkin W.J., Santos P.J., Wirth G.A. Is there A preferred incision location for nipple-sparing mastectomy? A systematic review and meta-analysis. Plast Reconstr Surg. 2019;143(5):906e–919e. doi: 10.1097/PRS.0000000000005502. [DOI] [PubMed] [Google Scholar]
- 23.Carlson G.W., Chu C.K., Moyer H.R., Duggal C., Losken A. Predictors of nipple ischemia after nipple sparing mastectomy. Breast J. 2014;20:69–73. doi: 10.1111/tbj.12208. [DOI] [PubMed] [Google Scholar]
- 24.Colwell A.S., Tessler O., Lin A.M., Liao E., Winograd J., Cetrulo C.L. Breast reconstruction following nipple-sparing mastectomy: predictors of complications, reconstruction outcomes, and 5-year trends. Plast Reconstr Surg. 2014;133:496–506. doi: 10.1097/01.prs.0000438056.67375.75. [DOI] [PubMed] [Google Scholar]
- 25.Frey J.D., Salibian A.A., Levine J.P., Karp N.S., Choi M. Incision choices in nipple-sparing mastectomy: a comparative analysis of outcomes and evolution of a clinical algorithm. Plast Reconstr Surg. 2018;142 doi: 10.1097/PRS.0000000000004969. 826e-35e. [DOI] [PubMed] [Google Scholar]
- 26.Ahn S.J., Woo T.Y., Lee D.W., Lew D.H., Song S.Y. Nipple-areolar complex ischemia and necrosis in nipple-sparing mastectomy. Eur J Surg Oncol : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2018;44:1170–1176. doi: 10.1016/j.ejso.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Odom E.B., Parikh R.P., Um G., Kantola S.W., Cyr A.E., Margenthaler J.A. Nipple-sparing mastectomy incisions for cancer extirpation prospective cohort trial: perfusion, complications, and patient outcomes. Plast Reconstr Surg. 2018;142:13–26. doi: 10.1097/PRS.0000000000004498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frey J.D., Salibian A.A., Lee J., Harris K., Axelrod D.M., Guth A.A. Oncologic trends, outcomes, and risk factors for locoregional recurrence: an analysis of tumor-to-nipple distance and critical factors in therapeutic nipple-sparing mastectomy. Plast Reconstr Surg. 2019;143(6):1575–1585. doi: 10.1097/PRS.0000000000005600. [DOI] [PubMed] [Google Scholar]
- 29.Balci F.L., Kara H., Dulgeroglu O., Uras C. Oncologic safety of nipple-sparing mastectomy in patients with short tumor-nipple distance. Breast J. 2019;25(4):612–618. doi: 10.1111/tbj.13289. [DOI] [PubMed] [Google Scholar]
- 30.Ryu J.M., Nam S.J., Kim S.W., Lee S.K., Bae S.Y., Yi H.W. Feasibility of nipple-sparing mastectomy with immediate breast reconstruction in breast cancer patients with tumor-nipple distance less than 2.0 cm. World J Surg. 2016;40:2028–2035. doi: 10.1007/s00268-016-3487-0. [DOI] [PubMed] [Google Scholar]
- 31.Dent B.L., Miller J.A., Eden D.J., Swistel A., Talmor M. Tumor-to-Nipple distance as a predictor of nipple involvement: expanding the inclusion criteria for nipple-sparing mastectomy. Plast Reconstr Surg. 2017;140:1e–8e. doi: 10.1097/PRS.0000000000003414. [DOI] [PubMed] [Google Scholar]
- 32.Ito H., Ueno T., Suga H., Shiraishi T., Isaka H., Imi K. Risk factors for skin flap necrosis in breast cancer patients treated with mastectomy followed by immediate breast reconstruction. World J Surg. 2019;43:846–852. doi: 10.1007/s00268-018-4852-y. [DOI] [PubMed] [Google Scholar]
- 33.Chirappapha P., Petit J.Y., Rietjens M., De Lorenzi F., Garusi C., Martella S. Nipple sparing mastectomy: does breast morphological factor related to necrotic complications? Plast Reconstr Surg Glob Open. 2014;2:e99. doi: 10.1097/GOX.0000000000000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frey J.D., Salibian A.A., Karp N.S., Choi M. The impact of mastectomy weight on reconstructive trends and outcomes in nipple-sparing mastectomy: progressively greater complications with larger breast size. Plast Reconstr Surg. 2018;141:795e–804e. doi: 10.1097/PRS.0000000000004404. [DOI] [PubMed] [Google Scholar]
- 35.Baek W.Y., Byun I.H., Kim Y.S., Lew D.H., Jeong J., Roh T.S. Patient satisfaction with implant based breast reconstruction associated with implant volume and mastectomy specimen weight ratio. Journal of breast cancer. 2017;20:98–103. doi: 10.4048/jbc.2017.20.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alperovich M., Choi M., Frey J.D., Lee Z.H., Levine J.P., Saadeh P.B. Nipple-sparing mastectomy in patients with prior breast irradiation: are patients at higher risk for reconstructive complications? Plast Reconstr Surg. 2014;134 doi: 10.1097/PRS.0000000000000321. 202e-6e. [DOI] [PubMed] [Google Scholar]
- 37.Rawlani V., Fiuk J., Johnson S.A., Buck D.W., 2nd, Hirsch E., Hansen N. The effect of incision choice on outcomes of nipple-sparing mastectomy reconstruction. The Canadian journal of plastic surgery = Journal canadien de chirurgie plastique. 2011;19:129–133. doi: 10.1177/229255031101900410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho J.W., Yoon E.S., You H.J., Kim H.S., Lee B.I., Park S.H. Nipple-areola complex necrosis after nipple-sparing mastectomy with immediate autologous breast reconstruction. Arch Plast Surg. 2015;42:601–607. doi: 10.5999/aps.2015.42.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laporta R., Longo B., Sorotos M., Farcomeni A., Patti C., Mastrangeli M.R. Breast reconstruction following nipple-sparing mastectomy: clinical outcomes and risk factors related complications. Journal of plastic surgery and hand surgery. 2017;51:427–435. doi: 10.1080/2000656X.2017.1303500. [DOI] [PubMed] [Google Scholar]
- 40.Iwahira Y., Nagase T., Nakagami G., Huang L., Ohta Y., Sanada H. Histopathological comparisons of irradiated and non-irradiated breast skin from the same individuals. J Plast Reconstr Aesthetic Surg. 2012;65:1496–1505. doi: 10.1016/j.bjps.2012.05.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.