Abstract
Purpose
The 8th edition of the American Joint Committee on Cancer (AJCC) pathological staging system for breast cancer considers biologic factors in addition to the anatomical features included in the previous systems. The purpose of this study was to determine the validity of the 8th AJCC staging system for T1-2N1 breast cancer and to assess the effect of additional chemotherapy and radiotherapy according to the new pathologic stages.
Methods
The cohort included patients from the Surveillance, Epidemiology, and End Results program (2010–2012) who had stage T1-2N1 invasive breast carcinoma and underwent mastectomy. All patients were restaged using the 8th AJCC staging system. The Kaplan–Meier method, Cox proportional hazards regression, and competing risks models were used for data analysis.
Results
We identified 9908 patients including 3022 (30.5%), 3131 (31.6%), 1940 (19.6%), 1194 (12.1%), and 621 (6.3%) were classified with stage IA, IB, IIA, IIB, and IIIA disease, respectively. The 5-year breast cancer-specific survival (BCSS) was 97.3%, 94.3%, 88.3%, 84.0%, and 71.1% for stage IA, IB, IIA, IIB, and IIIA disease, respectively. Higher pathological stage was associated with a significantly higher risk of breast cancer-related death. Chemotherapy was associated with better BCSS regardless of the pathological stage, but radiotherapy was only associated with better BCSS in stage IIIA disease.
Conclusions
The 8th AJCC pathological staging system provides more refined stratification for T1-2N1 breast cancer patients after mastectomy and may meet the needs of the current trend of individualized decision making for chemotherapy and radiotherapy in this patient subset.
Keywords: Breast cancer, Mastectomy, Radiotherapy, Chemotherapy, AJCC
Highlights
-
•
Higher pathological stage was associated with significantly lower BCSS.
-
•
Chemotherapy was associated with better BCSS regardless of the pathological stage.
-
•
Radiotherapy was associated with better BCSS in only stage IIIA disease.
1. Introduction
The traditionally used American Joint Committee on Cancer (AJCC) staging system for breast cancer is based on the anatomic features including primary tumor size (T), the number and location of lymph nodes involved (N), and the distant metastasis status (M) [1]. This anatomy-based TNM breast cancer staging system has been widely used to predict prognosis and to make therapeutic decisions worldwide. However, this system does not account for changes in molecular factors such as estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor-2 (HER2), and histological grade, which have been definitively found to have prognostic and predictive value in breast cancer evaluation and treatment [2]. Studies have indicated that the inclusion of these biological factors in the traditional anatomic staging system would be beneficial [[3], [4], [5], [6], [7], [8]], and accordingly, some changes were made in the 8th AJCC breast cancer staging system [1,2,7,8], which is the first staging system to incorporate biological factors into the TNM staging system. The new prognostic staging system is considered to be a significantly superior tool for predicting survival outcome as compared to the previous one, and its prognostic benefits have been validated in several studies [[9], [10], [11], [12], [13], [14], [15]].
The traditional TNM staging system divided stage T1-2 tumors with one to three positive lymph nodes (T1-2N1) into stage IIA (T1N1) and IIB (T2N1) tumors [16]. However, in T1-2N1 breast cancers, there are significant differences in locoregional recurrence (LRR), distant metastasis (DM), and overall survival (OS) that are caused by various biological factors [17,18]. The modified staging system overcomes this issue by taking into account the high heterogeneity of stage T1-2N1 disease and classifying T1-2N1 tumors into five substages: IA, IB, IIA, IIB, and IIIA (1). This new pathological substaging seems promising, but very few studies have validated these new substages of stage T1-2N1 disease [19]. There is also some controversy about the effect of chemotherapy and postmastectomy radiotherapy (PMRT) in patients with stage T1-2N1 disease [[19], [20], [21], [22], [23], [24], [25], [26], [27]]. To the best of our knowledge, no studies so far have tried to determine the effect of locoregional and systemic treatment in this patient subset. Given these gaps in the literature, in the present study, we have conducted a population-based assessment of the new pathological staging system for T1-2N1 breast cancers in terms of predicting survival, prognosis, and treatment effect, and investigated the effect of additional chemotherapy and PMRT on various pathologic stages in this patient subset.
2. Materials and methods
2.1. Patient selection
Patients were selected from the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute. The SEER program collects data on population-based cancer incidence, demographic and tumor characteristics, treatment, and survival for approximately 28% of the United States population [28]. Patients with stage T1-2N1 invasive breast carcinoma treated with mastectomy between 2010 and 2012 were identified from the program’s database. From among them, the following patients were excluded: those who had no positive pathological diagnosis; those who were treated with non-beam external irradiation; and those for whom data on the number of lymph nodes involved, race/ethnicity, hormone receptor status, and tumor grade were unavailable. Our study was exempt from approval by the Institutional Review Board because patient data are anonymized in the SEER database.
2.2. Baseline patient characteristics
The following baseline patient characteristics were collected in our study: age, race/ethnicity, tumor grade, tumor size, histological features, the number of lymph nodes involved, ER status, PR status, HER2 status, radiotherapy, and chemotherapy. Breast cancer-specific death was defined as death caused by breast cancer. All cases were restaged using the 8th AJCC pathological staging system.
2.3. Statistical analysis
Patient characteristics between treatment arms were compared with the chi-squared test. Breast cancer-specific survival (BCSS) was evaluated using the Kaplan–Meier method and compared with the log-rank test. Cox proportional hazards regression analysis was used to assess the prognostic indicators associated with BCSS. In addition, we also used univariate and multivariable competing risk models to determine the cumulative incidence of breast cancer-related death. Competing risks models in the Cox model framework as proposed by Fine and Gray were also to assess combined effects of the variables on breast cancer-related death. Statistical analyses were conducted using IBM SPSS 22.0 (IBM Corp., Armonk, NY) and Stata/SE version 14 (StataCorp, TX, USA). P values < 0.05 were considered to indicate statistical significance.
3. Results
3.1. Patient characteristics
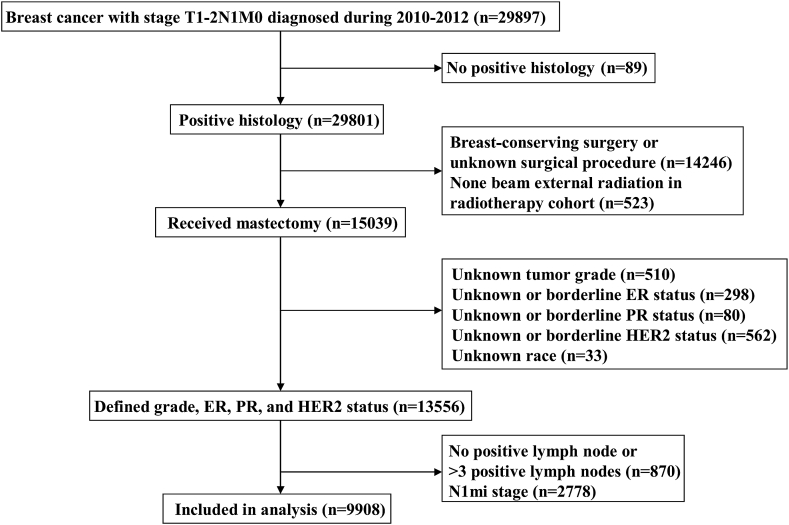
Based on our study criteria, we identified 9908 patients with a median age of 57 years (range, 20–98 years). Fig. 1 depicts the patient selection flowchart in this study. Table 1 lists the patients’ baseline characteristics. The majority of patients had invasive ductal carcinomas (87.0%), and were ER positive (82.5%), PR positive (72.1%), and HER2 negative (82.5%). In addition, two-thirds of the patients were above 65 years (68.5%) and Non-Hispanic White (66.7%), and had T2 stage disease (59.2%). With regard to the pathological stage, 3022 (30.5%), 3131 (31.6%), 1940 (19.6%), 1194 (12.1%), and 621 (6.3%) patients were classified as having stage IA, IB, IIA, IIB, and IIIA disease, respectively.
Fig. 1.
The patient selection flowchart of the study.
Table 1.
Baseline characteristics of the patients included in the study cohort.
| Variables | N (%) | Radiotherapy |
Chemotherapy |
||||
|---|---|---|---|---|---|---|---|
| No (%) | Yes (%) | P | No (%) | Yes (%) | P | ||
| Age (y) | |||||||
| <65 | 6788 (68.5) | 4065 (63.3) | 2723 (78.2) | <0.001 | 1340 (41.9) | 5448 (81.2) | <0.001 |
| ≥65 | 3120 (31.5) | 2361 (36.7) | 759 (21.8) | 1855 (58.1) | 1265 (18.8) | ||
| Race/ethnicity | |||||||
| Non-Hispanic White | 6607 (66.7) | 4344 (67.6) | 2263 (65.0) | 0.010 | 2253 (70.5) | 4354 (64.9) | <0.001 |
| Non-Hispanic Black | 1107 (11.2) | 672 (10.5) | 435 (12.5) | 298 (9.3) | 809 (12.1) | ||
| Hispanic (All Races) | 1265 (12.8) | 820 (12.8) | 445 (12.8) | 371 (11.6) | 894 (13.3) | ||
| Other | 929 (9.4) | 590 (9.2) | 339 (9.7) | 273 (8.5) | 656 (9.8) | ||
| Grade | |||||||
| Well differentiated | 1384 (14.0) | 1021 (15.9) | 363 (10.4) | <0.001 | 695 (21.8) | 689 (10.3) | <0.001 |
| Moderately differentiated | 4524 (45.7) | 2972 (46.2) | 1552 (44.6) | 1583 (49.5) | 2941 (43.8) | ||
| Poorly differentiated/undifferentiated | 4000 (40.4) | 2433 (37.9) | 1567 (45.0) | 917 (28.7) | 3083 (45.9) | ||
| Histological subtype | |||||||
| Infiltrating ductal carcinoma | 8621 (87.0) | 5591 (87.0) | 3030 (87.0) | 0.952 | 2707 (84.7) | 5914 (88.1) | <0.001 |
| Lobular carcinoma | 950 (9.6) | 614 (9.6) | 336 (9.6) | 373 (11.7) | 577 (8.6) | ||
| Other | 337 (3.4) | 221 (3.4) | 116 (3.3) | 115 (3.6) | 222 (3.3) | ||
| Tumor stage | |||||||
| T1 | 4047 (40.8) | 2833 (44.1) | 1214 (34.9) | <0.001 | 1439 (45.0) | 2608 (38.8) | <0.001 |
| T2 | 5861 (59.2) | 3593 (55.9) | 2268 (65.1) | 1756 (55.0) | 4105 (61.2) | ||
| Number of positive lymph nodes | |||||||
| 1 | 5281 (53.3) | 3781 (58.8) | 1500 (43.1) | <0.001 | 1944 (60.8) | 3337 (49.7) | <0.001 |
| 2 | 3027 (30.6) | 1867 (29.1) | 1160 (33.3) | 871 (27.3) | 2156 (32.1) | ||
| 3 | 1600 (16.1) | 778 (12.1) | 822 (23.6) | 380 (11.9) | 1220 (18.2) | ||
| ER status | |||||||
| Negative | 1720 (82.5) | 1070 (83.1) | 650 (81.4) | 0.011 | 345 (10.8) | 1375 (20.5) | <0.001 |
| Positive | 8188 (17.5) | 5356 (16.9) | 2832 (18.6) | 2850 (89.2) | 5338 (79.5) | ||
| PR status | |||||||
| Negative | 2760 (27.9) | 1728 (26.9) | 1032 (29.6) | 0.004 | 643 (20.1) | 2117 (31.5) | <0.001 |
| Positive | 7148 (72.1) | 4698 (73.1) | 2450 (70.4) | 2552 (79.9) | 4596 (68.5) | ||
| HER2 status | |||||||
| Negative | 8172 (82.5) | 5339 (83.1) | 2883 (81.4) | 0.031 | 2852 (89.3) | 5320 (79.2) | <0.001 |
| Positive | 1736 (17.5) | 1087 (16.9) | 649 (18.6) | 343 (10.7) | 1393 (20.8) | ||
| Pathological stage | |||||||
| IA | 3022 (30.5) | 2173 (33.8) | 849 (24.4) | <0.001 | 1279 (40.0) | 1743 (26.0) | <0.001 |
| IB | 3131 (31.6) | 1929 (30.0) | 1202 (34.5) | 1006 (31.5) | 2125 (31.7) | ||
| IIA | 1940 (19.6) | 1239 (19.3) | 701 (20.1) | 500 (15.6) | 1440 (21.5) | ||
| IIB | 1194 (12.1) | 727 (11.3) | 467 (13.4) | 288 (9.0) | 906 (13.5) | ||
| IIIA | 621 (6.3) | 358 (5.6) | 263 (7.6) | 122 (3.8) | 499 (7.4) | ||
| Chemotherapy | |||||||
| No | 3195 (32.2) | 2750 (42.8) | 445 (12.8) | <0.001 | – | – | – |
| Yes | 6713 (67.8) | 3676 (57.2) | 3037 (87.2) | – | – | ||
| Radiotherapy | |||||||
| No | – | – | – | – | 2750 (86.1) | 3676 (54.8) | <0.001 |
| Yes | – | – | – | 445 (13.9) | 3037 (45.2) | ||
3.2. Correlation of treatment with patient characteristics
A total of 3482 (35.1%) patients were treated with PMRT. Younger age (P < 0.001), poorly differentiated/undifferentiated tumors (P < 0.001), larger tumor size (P < 0.001), a higher number of positive lymph nodes (P < 0.001), and treatment with chemotherapy (P < 0.001) were associated with a higher likelihood of treatment with PMRT. In addition, patients with higher pathological stages (P < 0.001) were also more likely to receive PMRT (Table 1).
With regard to chemotherapy, 66.8% (n = 6713) of the patients were treated with chemotherapy. Younger age (P < 0.001), poorly differentiated/undifferentiated tumors (P < 0.001), invasive ductal carcinomas (P < 0.001), larger tumor size (P < 0.001), higher number of positive lymph nodes (P < 0.001), absence of ER (P < 0.001), absence of PR (P < 0.001), and treatment with PMRT (P < 0.001) were associated with a higher likelihood of treatment with chemotherapy. In addition, patients with higher pathological stages (P < 0.001) were also more likely to receive chemotherapy (Table 1).
3.3. Survival and prognostic analysis
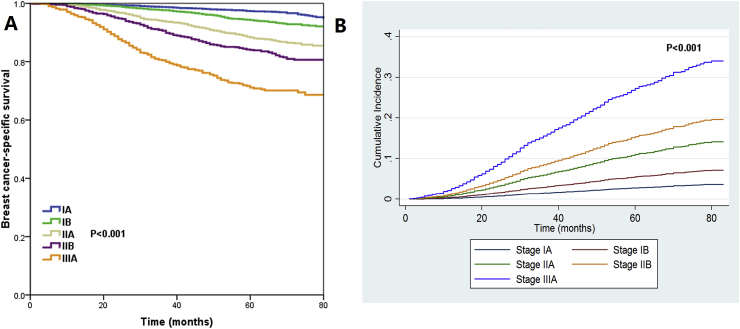
The median follow-up duration was 61 months (range, 0–83 months). A total of 1570 patients died, and breast cancer-related death occurred in 53.9% (n = 846) of these patients. The 5-year BCSS was 91.4%, and was 97.3%, 94.3%, 88.3%, 84.0%, and 71.1% in patients with stage IA, IB, IIA, IIB, and IIIA disease, respectively (P < 0.001) (Fig. 2A). Similar trends regarding the cumulative incidence estimates of breast cancer-related death were observed by prognostic stage (Fig. 2B).
Fig. 2.
Kaplan-Meier curves of breast cancer-specific survival (A) and cumulative incidence estimates of breast cancer-related death (B) stratified by prognostic stage.
According to the results of multivariate prognostic analysis, the new pathological stage was identified as an independent prognostic indicator significantly associated with BCSS (Table 2). Higher pathological stage was associated with lower BCSS. The results of competing risks model in the Cox model framework also showed a higher pathological stage was associated with a higher risk of breast cancer-related death (Table 2). In addition, age, race/ethnicity, and the number of lymph nodes involved were also found to be independent prognostic factors associated with breast cancer-related death using Cox proportional hazards regression model or competing risks model.
Table 2.
Multivariate analysis using Cox proportional hazards regression model and competing risks model to determine the prognostic indicators of breast cancer-specific survival in the patient cohort.
| Variables | Cox proportional hazards regression model |
Competing risks model |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (y) | ||||||
| <65 | 1 | 1 | ||||
| ≥65 | 1.677 | 1.460–1.927 | <0.001 | 1.538 | 1.338–1.770 | <0.001 |
| Race/ethnicity | ||||||
| Non-Hispanic White | 1 | 1 | ||||
| Non-Hispanic Black | 1.122 | 0.925–1.362 | 0.243 | 1.126 | 0.923–1.374 | 0.243 |
| Hispanic (All Races) | 1.011 | 0.821–1.244 | 0.920 | 1.010 | 0.820–1.243 | 0.922 |
| Other | 0.670 | 0.508–0.882 | 0.004 | 0.686 | 0.520–0.906 | 0.008 |
| Histological subtype | ||||||
| Infiltrating ductal carcinoma | 1 | 1 | ||||
| Lobular carcinoma | 0.990 | 0.758–1.292 | 0.938 | 1.010 | 0.773–1.320 | 0.936 |
| Other | 0.812 | 0.557–1.186 | 0.281 | 0.779 | 0.528–1.150 | 0.210 |
| Number of positive lymph nodes | ||||||
| 1 | 1 | 1 | ||||
| 2 | 1.134 | 0.972–1.322 | 0.110 | 1.140 | 0.976–1.329 | 0.098 |
| 3 | 1.262 | 1.052–1.513 | 0.012 | 1.238 | 1.030–1.488 | 0.023 |
| Pathological stage | ||||||
| IA | 1 | 1 | ||||
| IB | 2.003 | 1.553–2.584 | <0.001 | 1.983 | 1.538–2.555 | <0.001 |
| IIA | 4.149 | 3.241–5.310 | <0.001 | 4.105 | 3.206–5.255 | <0.001 |
| IIB | 5.918 | 4.595–7.622 | <0.001 | 5.812 | 4.506–7.495 | <0.001 |
| IIIA | 11.500 | 8.877–14.899 | <0.001 | 11.336 | 8.705–14.761 | <0.001 |
3.4. Effect of chemotherapy and radiotherapy on BCSS according to pathological stage
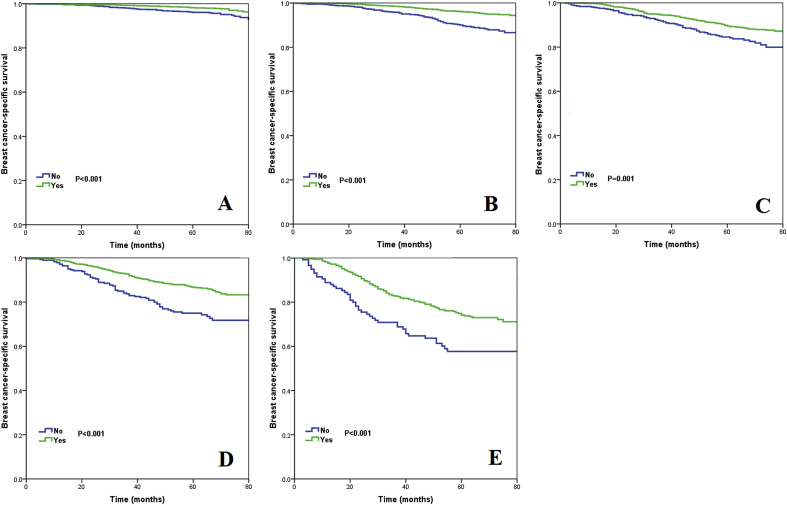
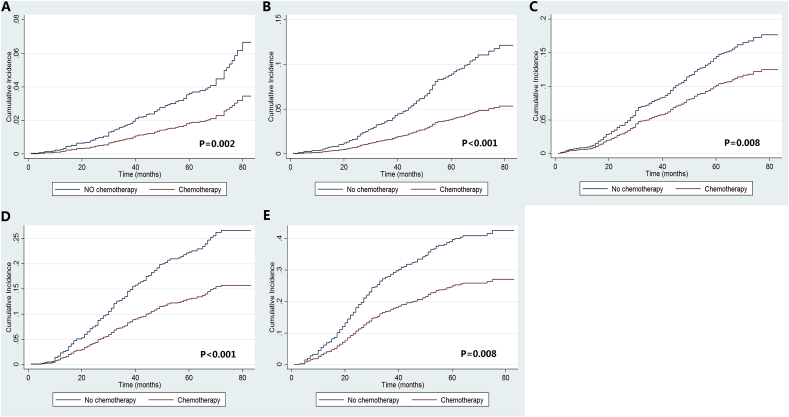
We used five multivariate Cox proportional hazards models to determine the effect of chemotherapy and PMRT on BCSS according to pathological stage after adjustment for age, race/ethnicity, histological grade, and the number of lymph nodes involved (Table 3). The results indicated that treatment with chemotherapy was associated with better BCSS than treatment without chemotherapy, regardless of the pathological stage. The survival curves of patients treated with and without chemotherapy according to pathological stages are shown in Fig. 3A−F. Using competing risks models in the Cox model framework, patients treated with chemotherapy had significantly lower risk of breast cancer-related death in stage IA (P = 0.005), IB (P < 0.001), IIA (P = 0.026), and IIB (P = 0.005) disease compared to those without chemotherapy, and also had borderline effect on breast cancer-related death in stage III disease compared to those without chemotherapy (P = 0.053) (Table 3). The cumulative incidences of breast cancer-related death according to pathological stages are listed in Fig. 4A−F.
Table 3.
Multivariate analysis using Cox proportional hazards regression model and competing risks model to determine the effect of chemotherapy and radiotherapy on breast cancer-specific survival according to pathological stage.
| Variables | Cox proportional hazards regression model |
Competing risks model |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Stage IA | ||||||
| Chemotherapy Yes vs. No | 0.463 | 0.301–0.713 | <0.001 | 0.552 | 0.332–0.820 | 0.005 |
| Radiotherapy Yes vs. No | 0.903 | 0.536–1.520 | 0.701 | 0.763 | 0.571–1.506 | 0.928 |
| Stage IB | ||||||
| Chemotherapy Yes vs. No | 0.476 | 0.342–0.663 | <0.001 | 0.537 | 0.389–0.722 | <0.001 |
| Radiotherapy Yes vs. No | 0.785 | 0.557–1.107 | 0.168 | 0.806 | 0.576–1.128 | 0.209 |
| Stage IIA | ||||||
| Chemotherapy Yes vs. No | 0.619 | 0.467–0.819 | 0.001 | 0.685 | 0.491–0.955 | 0.026 |
| Radiotherapy Yes vs. No | 1.152 | 0.861–1.543 | 0.340 | 1.162 | 0.869–1.559 | 0.315 |
| Stage IIB | ||||||
| Chemotherapy Yes vs. No | 0.546 | 0.399–0.748 | <0.001 | 0.621 | 0.446–0.865 | 0.005 |
| Radiotherapy Yes vs. No | 0.759 | 0.550–1.049 | 0.095 | 0.807 | 0.586–1.105 | 0.179 |
| Stage IIIA | ||||||
| Chemotherapy Yes vs. No | 0.608 | 0.426–0.868 | 0.006 | 0.688 | 0.471–1.004 | 0.053 |
| Radiotherapy Yes vs. No | 0.645 | 0.460–0.905 | 0.011 | 0.625 | 0.445–0.879 | 0.007 |
Fig. 3.
Kaplan-Meier curves for assessment of the effect of chemotherapy on breast cancer-specific survival stratified by prognostic stage (A, stage IA; B, stage IB; C, stage IIA; D, stage IIB; E, stage IIIA).
Fig. 4.
Cumulative incidence estimates of the effect of chemotherapy on breast cancer-related death stratified by prognostic stage (A, stage IA; B, stage IB; C, stage IIA; D, stage IIB; E, stage IIIA).
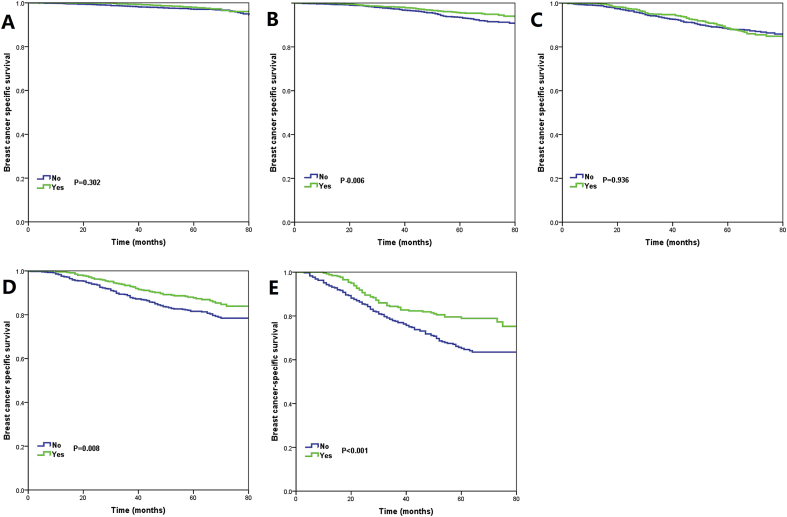
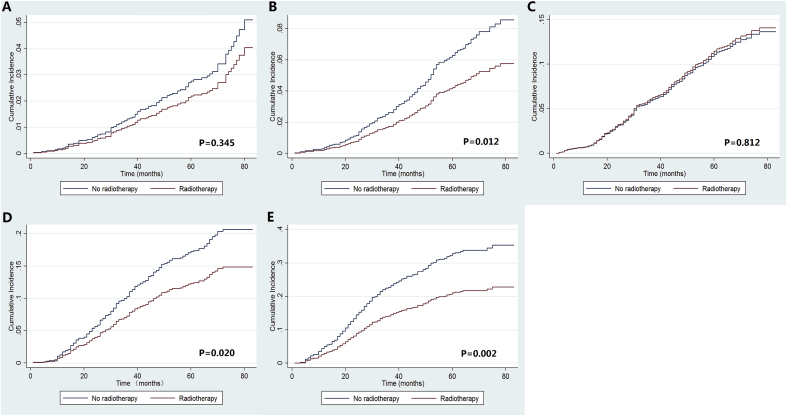
In the Kaplan–Meier analyses, patients treated with PMRT had better BCSS in stage IB (P = 0.006), IIB (P = 0.008), and IIIA (P < 0.001) disease (Fig. 5A–F). Using the multivariable Cox proportional hazards models, we only found that treatment with PMRT was associated with better BCSS than treatment without PMRT in stage IIIA disease (hazards ratio [HR] = 0.645, 95% confidence interval [CI] = 0.460–0.905, P = 0.011), the 5-year BCSS was 78.9% and 65.2% in patients with and without PMRT, respectively (P = 0.001) (Fig. 5F). However, in patients with stage IA (P = 0.707), IB (P = 0.168), IIA (P = 0.340), and IIB (P = 0.095) disease, BCSS was comparable between those treated with and without PMRT in multivariable prognostic analysis. Similar trends were observed using univariate and multivariable competing risk models (Table 3 and Fig. 6A–F).
Fig. 5.
Kaplan-Meier curves for assessment of the effect of radiotherapy on breast cancer-specific survival stratified by prognostic stage (A, stage IA; B, stage IB; C, stage IIA; D, stage IIB; E, stage IIIA).
Fig. 6.
Cumulative incidence estimates of the effect of radiotherapy on breast cancer-related death stratified by prognostic stage (A, stage IA; B, stage IB; C, stage IIA; D, stage IIB; E, stage IIIA).
4. Discussion
In this study, we validated the value of the 8th edition of AJCC pathological staging system in stage T1-2N1 breast cancer after mastectomy, and further determined whether the new pathological staging system could affect the treatment decision making of chemotherapy and PMRT in this patients subset. Our results showed that the new pathological staging system could better reflect the prognosis of patients, and chemotherapy could improve BCSS in all substages, whereas PMRT could only associated with better BCSS in patients with stage IIIA disease. Our study was the first to determine the effect of the selection in locoregional and systemic treatment using the new pathological staging system.
The 8th edition AJCC pathological staging system for the first time incorporated breast cancer biologic factors into the traditionally anatomic TNM staging system. The initial model for establishing pathological staging system in the 8th edition of AJCC staging system were using 305,519 patients information from National Cancer Database between 2010 and 2012, which caused more than 35% of patients downstaged or upstaged from the 7th AJCC staging system [29]. The optimal effect for predicting prognosis in 8th AJCC staging system compared to 7th AJCC staging system has been validated in several studies [[9], [10], [11], [12], [13], [14], [15]]. However, limited studied focused on patients with stage T1-2N1 disease, a specific staging presented with greater heterogeneity. A study using Chinese cohort from Sun et al. (n = 1823) found that the 8th edition of the AJCC staging system had significant differences in LRR, DM, disease free survival (DFS), and OS, and it had better prognostic accuracy compared to the 7th edition of the AJCC staging system [19]. However, there were no significant difference in LRR, DM, DFS, and OS between stage IIB and IIIA disease, and there were also comparable LRR, DM, and DFS between stage IB and IIA disease [19]. The limited number of included patients was the main reason for the results by Sun et al. (IA, IB, IIA, IIB, and IIA were 588, 530, 348, 299, and 88 patients, respectively) [19]. In our study with a larger cohort (n = 9908), the BCSS improved in the lower stages and worsened as the stage increased. Our study suggested that the 8th pathological staging system would be the most accurate predictor of risk stratification in patients with stage T1-2N1 disease. The new AJCC staging system better reflects the heterogeneity of stage T1-2N1 breast cancer and helps guide more detailed individualized treatment and prognosis assessment in the current clinical practice.
We noted that the patients with stage IIIA disease (T2N1, histological grade III, ER-, PR-, HER2-) showed the worst BCSS compared with other substages, with a 5-year BCSS rate of 71.1%, which suggested that triple negative breast carcinoma should be regarded as an upstaging biologic factor [5]. Although only 6.3% of patients were in stage IIIA disease, the risk of breast cancer related death in stage IIIA disease was about seven times and double time compared to patients in stage I and II diseases, respectively. While anatomic TNM staging provides a more straightforward and easily applied system for classification of breast cancer, the newly developed pathological staging system emphasized the equally of tumor burden and tumor biologic factors in the era of personalized treatment of breast cancer [30]. This finding indicates that systemic and locoregional treatment might be changed in several anatomic stage groups. Our study supported by the recommendation from the updated guidelines by American Society of Clinical Oncology which showed that the recommendation of PMRT in stage T1-2N1 breast cancer should be based on an assessment of the individual recurrence risk using tumor and biologic characteristics [31].
As generally, stage T1-2N1 breast cancer is considered to have an intermediate risk of lororegional and distant recurrence, with much controversy regarding the adjuvant treatment. The incorporation of biologic factors into the pathological prognostic staging system could better guide personalized care under more accurate prognosis prediction. However, the adjuvant treatment of breast cancer including chemotherapy and PMRT remains mainly based on the tumor size and regional nodal status in the current National Comprehensive Cancer Network (NCCN) guidelines [32]. However, the anatomic staging system might not be enough for predicting prognosis in treatment decision making [6,33]. To the best of our knowledge, there is currently no study to determine the effect of adjuvant treatment based on newly staging system. The administration of chemotherapy was significantly improved BCSS regardless of prognostic stages in our study. Although the treatment guideline from European Society for Medical Oncology does not recommend adjuvant chemotherapy for T1-2N1 patients with luminal-A subtype [34]. The guidelines from the NCCN recommend adjuvant chemotherapy for patients with lymph nodes involvement [32]. It should be noted that the new staging system is better able to determine the survival outcome of patients, and the prognosis reflected the standardized treatment based on the patient tumor and biologic characteristics [29,35]. Patients with lower stage does not mean that the patients need de-escalation of primary treatment, but rather reflects that the patient has better biological characteristics or more effective response to treatment.
The effect of PMRT in stage T1-2N1 breast cancer is vigorously debated. Although the meta-analysis from Early Breast Cancer Trialists’ Collaborative Group provides a high-level evidence to guide decision-making of PMRT [36]. A higher rate of LRR in the non-PMRT in above meta-analysis [36], stage migration with sentinel node biopsy, and possible adverse events of PMRT may influence the decision-making of PMRT in this patient subset [25]. The secondary analyses of two prospective studies have indicated the administration of PMRT was related to better locoregional control (10-years LRR 2–2.5% vs. 6.5–9.0%). However, there were comparable survival outcomes between the treatment arms [23,24]. Therefore, in the modern era of individualized treatment, most stage T1-2N1 patients may not need additional PMRT [26]. However, there were no decisive tools to predict the benefit of PMRT in this patient subset in the current clinical practice.
Due to the limitation of the SEER database, we were unable to obtain the data of LRR. According to the data form 1823 patients (17.2% of patients treated with PMRT) by Sun et al., the 5-year LRR was less than 5% in stage IA and IB diseases, approximately 7%, 12%, and 16% in patients with stage IIA, IIB, and IIIA disease, respectively [19]. However, they were not analysis the effect of PMRT by different pathological stages. In this study, we further assessed whether the new AJCC staging system could guide the optimal administration of PMRT. Our study found that PMRT could only improve BCSS in patients with stage IIIA, while PMRT was not associated with better BCSS in stage IA, IB, IIA, and IIB disease compared to those without PMRT. Our study indicated that the pathological prognostic stage system incorporated the anatomic tumor burden and tumor biologic factors might provide accurate predictor of effect of PMRT in stage T1-2N1 breast cancer.
Several limitations in the present study should be acknowledged. First, the intrinsic bias in retrospective designs should not be neglected. Second, the data of chemotherapy and radiotherapy receipt are known to be under-reported in the SEER program. Third, the information on anti-Her2 treatment and hormone therapy was also not recorded in the SEER database, which might impact the prognostic assessment. Moreover, the sequencing of chemotherapy and radiotherapy, information on locoregional and distant recurrence were not collect in the SEER database. However, the primary strength of our study was the first to determine the effect of additional systemic therapy and PMRT in various new pathologic stages in stage T1-2N1 breast cancer after mastectomy.
In conclusion, the 8th AJCC prognostic staging system has the advantage of providing more refined stratification of T1-2N1 breast cancer after mastectomy, and meets the needs of the current trend of individualized decision making with regard to chemotherapy and radiotherapy in this patient subset. Future prospective studies must be conducted in different populations to validate our results.
Declaration of competing interest
The authors have no conflicts of interest to disclose.
Acknowledgments
This work was partly supported by the National Natural Science Foundation of China (No. 81872459), the Commission Young and Middle-aged Talents Training Project of Fujian Health, China Commission (No. 2019-ZQNB-25), the Science and Technology Planning Projects of Xiamen Science & Technology Bureau, China (No. 3502Z20174070), and the Natural Science Foundation of Guangdong Province, China (No. 2018A030313666, 2017A030310422).
Contributor Information
Yong-Xiong Chen, Email: yxchen1962@xmu.edu.cn.
Zhen-Yu He, Email: hezhy@sysucc.org.cn.
References
- 1.Cserni G., Chmielik E., Cserni B., Tot T. The new TNM-based staging of breast cancer. Virchows Arch. 2018;472:697–703. doi: 10.1007/s00428-018-2301-9. [DOI] [PubMed] [Google Scholar]
- 2.Giuliano A.E., Edge S.B., Hortobagyi G.N. Eighth edition of the AJCC cancer staging manual: breast cancer. Ann Surg Oncol. 2018;25:1783–1785. doi: 10.1245/s10434-018-6486-6. [DOI] [PubMed] [Google Scholar]
- 3.Veronesi U., Zurrida S., Viale G., Galimberti V., Arnone P., Nolè F. Rethinking TNM: a breast cancer classification to guide to treatment and facilitate research. Breast J. 2009;15:291–295. doi: 10.1111/j.1524-4741.2009.00719.x. [DOI] [PubMed] [Google Scholar]
- 4.Yi M., Mittendorf E.A., Cormier J.N., Buchholz T.A., Bilimoria K., Sahin A.A. Novel staging system for predicting disease-specific survival in patients with breast cancer treated with surgery as the first intervention: time to modify the current American Joint Committee on Cancer staging system. J Clin Oncol. 2011;29:4654–4661. doi: 10.1200/JCO.2011.38.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagaria S.P., Ray P.S., Sim M.S., Ye X., Shamonki J.M., Cui X. Personalizing breast cancer staging by the inclusion of ER, PR, and HER2. JAMA Surg. 2014;149:125–129. doi: 10.1001/jamasurg.2013.3181. [DOI] [PubMed] [Google Scholar]
- 6.Park Y.H., Lee S.J., Cho E.Y., Choi Y.L., Lee J.E., Nam S.J. Clinical relevance of TNM staging system according to breast cancer subtypes. Ann Oncol. 2011;22:1554–1560. doi: 10.1093/annonc/mdq617. [DOI] [PubMed] [Google Scholar]
- 7.Amin M.B., Greene F.L., Edge S.B., Compton C.C., Gershenwald J.E., Brookland R.K. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA A Cancer J Clin. 2017;67:93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 8.Giuliano A.E., Connolly J.L., Edge S.B., Mittendorf E.A., Rugo H.S., Solin L.J. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA A Cancer J Clin. 2017;67:290–303. doi: 10.3322/caac.21393. [DOI] [PubMed] [Google Scholar]
- 9.Ibis K., Ozkurt S., Kucucuk S., Yavuz E., Saip P. Comparison of pathological prognostic stage and anatomic stage groups according to the updated version of the American Joint committee on cancer (AJCC) breast cancer staging 8th edition. Med Sci Mon Int Med J Exp Clin Res. 2018;24:3637–3643. doi: 10.12659/MSM.911022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S.B., Sohn G., Kim J., Chung I.Y., Lee J.W., Kim H.J. A retrospective prognostic evaluation analysis using the 8th edition of the American Joint Committee on Cancer staging system for breast cancer. Breast Canc Res Treat. 2018;169:257–266. doi: 10.1007/s10549-018-4682-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss A., Chavez-MacGregor M., Lichtensztajn D.Y., Yi M., Tadros A., Hortobagyi G.N. Validation study of the American Joint committee on cancer eighth edition prognostic stage compared with the anatomic stage in breast cancer. JAMA Oncol. 2018;4:203–209. doi: 10.1001/jamaoncol.2017.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M., Chen H., Wu K., Ding A., Zhang M., Zhang P. Evaluation of the prognostic stage in the 8th edition of the American Joint Committee on Cancer in locally advanced breast cancer: an analysis based on SEER 18 database. Breast. 2018;37:56–63. doi: 10.1016/j.breast.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Plichta J.K., Ren Y., Thomas S.M., Greenup R.A., Fayanju O.M., Rosenberger L.H. Implications for breast cancer restaging based on the 8th edition AJCC staging manual. Ann Surg. 2020;271:169–176. doi: 10.1097/SLA.0000000000003071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim I., Choi H.J., Ryu J.M., Lee S.K., Yu J.H., Kim S.W. Prognostic validation of the American Joint committee on cancer 8th staging system in 24,014 Korean patients with breast cancer. J Breast Cancer. 2018;21:173–181. doi: 10.4048/jbc.2018.21.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss A., Chavez-MacGregor M., Lichtensztajn D.Y., Yi M., Tadros A., Hortobagyi G.N. Validation study of the American Joint committee on cancer eighth edition prognostic stage compared with the anatomic stage in breast cancer. JAMA Oncol. 2018;4:203–209. doi: 10.1001/jamaoncol.2017.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hortobagyi G.N., Edge S.B., Giuliano A. New and important changes in the TNM staging system for breast cancer. Am Soc Clin Oncol Educ Book. 2018;38:457–467. doi: 10.1200/EDBK_201313. [DOI] [PubMed] [Google Scholar]
- 17.Park H.J., Shin K.H., Kim J.H., Ahn S.D., Kim J.Y., Park W. Incorporating risk factors to identify the indication of post-mastectomy radiotherapy in N1 breast cancer treated with optimal systemic therapy: a multicenter analysis in Korea (KROG 14-23) Cancer Res Treat. 2017;49:739–747. doi: 10.4143/crt.2016.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaššák F., Rossier C., Picardi C., Bernier J. Postmastectomy radiotherapy in T1-2 patients with one to three positive lymph nodes - past, present and future. Breast. 2019;48:73–81. doi: 10.1016/j.breast.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Sun G.Y., Wang S.L., Tang Y., Yang Y., Fang H., Wang J.Y. [The 8th edition of the American Joint Committee on Cancer staging system provide improved prognostic accuracy in T1-2N1M0 postmastectomy breast cancer patients] Zhonghua Zhongliu Zazhi. 2019;41:615–623. doi: 10.3760/cma.j.issn.0253-3766.2019.08.011. [In Chinese] [DOI] [PubMed] [Google Scholar]
- 20.Chang J.S., Lee J., Kim K.H., Sohn J.H., Kim S.I., Park B.W. Do recent advances in diagnostic and therapeutic procedures negate the benefit of postmastectomy radiotherapy in N1 patients with a low risk of locoregional recurrence? Medicine (Baltim) 2015;94(33):e1259. doi: 10.1097/MD.0000000000001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu C., Xu H., Chen X., Tong Z., Liu X., Jia Y. Irradiation after surgery for breast cancer patients with primary tumours and one to three positive axillary lymph nodes: yes or no? Curr Oncol. 2013;20:e585–e592. doi: 10.3747/co.20.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bazan J.G., Majithia L., Quick A.M., Wobb J.L., Terando A.M., Agnese D.M. Heterogeneity in outcomes of pathologic T1-2N1 breast cancer after mastectomy: looking beyond locoregional failure rates. Ann Surg Oncol. 2018;25(8):2288–2295. doi: 10.1245/s10434-018-6565-8. [DOI] [PubMed] [Google Scholar]
- 23.Tam M.M., Wu S.P., Perez C., Gerber N.K. The effect of post-mastectomy radiation in women with one to three positive nodes enrolled on the control arm of BCIRG-005 at ten year follow-up. Radiother Oncol. 2017;123:10–14. doi: 10.1016/j.radonc.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Zeidan Y.H., Habib J.G., Ameye L., Paesmans M., de Azambuja E., Gelber R.D. Postmastectomy radiation therapy in women with T1-T2 tumors and 1 to 3 positive lymph nodes: analysis of the breast international Group 02-98 trial. Int J Radiat Oncol Biol Phys. 2018;101:316–324. doi: 10.1016/j.ijrobp.2018.01.105. [DOI] [PubMed] [Google Scholar]
- 25.Ishikawa T., Kaise H., Yamada K., Hosonaga M., Chishima T., Narui K. Objection to postoperative radiation therapy in breast cancer with one to three lymph nodes involvements. Breast Cancer. 2017;24:496–501. doi: 10.1007/s12282-016-0749-5. [DOI] [PubMed] [Google Scholar]
- 26.Muhsen S., Moo T.A., Patil S., Stempel M., Powell S., Morrow M. Most breast cancer patients with T1-2 tumors and one to three positive lymph nodes do not need postmastectomy radiotherapy. Ann Surg Oncol. 2018;25:1912–1920. doi: 10.1245/s10434-018-6422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huo D., Hou N., Jaskowiak N., Winchester D.J., Winchester D.P., Yao K. Use of postmastectomy radiotherapy and survival rates for breast cancer patients with T1-T2 and one to three positive lymph nodes. Ann Surg Oncol. 2015;22:4295–4304. doi: 10.1245/s10434-015-4528-x. [DOI] [PubMed] [Google Scholar]
- 28.Surveillance, Epidemiology, and End results (SEER) program. Natl Can Inst. 2016 www.seer.cancer.gov SEER∗Stat Database: Incidence - SEER 18 Regs Custom Data (with additional treatment fields), Nov 2018 Sub (1975-2016 varying) - Linked To County Attributes - Total U.S., 1969-2017 Counties. DCCPS, Surveillance Research Program, released April 2019, based on the November 2018 submission. [Google Scholar]
- 29.AJCC cancer staging manual. Springer International Publishing; New York: 2018. [Google Scholar]
- 30.Jackisch C., Lammers P., Jacobs I. Evolving landscape of human epidermal growth factor receptor 2-positive breast cancer treatment and the future of biosimilars. Breast. 2017;32:199–216. doi: 10.1016/j.breast.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Recht A., Comen E.A., Fine R.E., Fleming G.F., Hardenbergh P.H., Ho A.Y. Postmastectomy radiotherapy: an American society of clinical oncology, American society for radiation oncology, and society of surgical oncology focused guideline update. J Clin Oncol. 2016;34:4431–4442. doi: 10.1200/JCO.2016.69.1188. [DOI] [PubMed] [Google Scholar]
- 32.NCCN. NCCN clinical Practice guidelines in oncology V.2.2019. Breast Cancer. 2019. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf Available online at:
- 33.Cleator S., Heller W., Coombes R.C. Triple-negative breast cancer: therapeutic options. Lancet Oncol. 2007;8:235–244. doi: 10.1016/S1470-2045(07)70074-8. [DOI] [PubMed] [Google Scholar]
- 34.Cardoso F., Kyriakides S., Ohno S., Penault-Llorca F., Poortmans P., Rubio I.T. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:1674. doi: 10.1093/annonc/mdz189. [DOI] [PubMed] [Google Scholar]
- 35.Mittendorf E.A., Chavez-MacGregor M., Vila J., Yi M., Lichtensztajn D.Y., Clarke C.A. Bioscore: a staging system for breast cancer patients that reflects the prognostic significance of underlying tumor biology. Ann Surg Oncol. 2017;24:3502–3509. doi: 10.1245/s10434-017-6009-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.EBCTCG (Early Breast Cancer Trialists’ Collaborative Group) McGale P., Taylor C., Correa C., Cutter D., Duane F. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]