Abstract
Background
Invasive micropapillary carcinoma (IMPC) is a rare histological subtype of breast cancer. The outcome of IMPC remains controversial; we conducted a meta-analysis of propensity score matching (PSM) studies to evaluate the prognostic difference between IMPC and invasive ductal carcinoma (IDC).
Methods
We searched PubMed, EMBASE and the Cochrane library for PSM studies comparing survival data between IMPC and IDC. The summarized odds ratios (ORs) and 95% confidence interval (95% CI) are calculated by STATA software utilizing fixed-effect or random-effect models, depending on the heterogeneity of the eligible studies.
Results
Eight PSM studies including 2102 IMPC patients are included in the meta-analysis. Compared with IDC, IMPC has a similar overall survival (OS) (estimated OR = 0.87, 95% CI: 0.61–1.25), but a shorter relapse free survival (RFS) (estimated OR = 1.31, 95% CI: 1.06–1.61); the shorter RFS might owe to the significantly higher loco-regional recurrence rate of IMPC (estimated OR = 3.60, 95% CI: 1.99–6.52). Funnel plots and Egger’s tests are used to evaluate publication bias and the p value for OS and RFS are 0.036 and 0.564 respectively.
Conclusions
Our results demonstrate that compared with IDC, IMPC exhibits a similar, even favorite OS, but a shorter RFS; and the shorter RFS might owe to the significantly higher loco-regional recurrence rate of IMPC. These results could contribute to the individualized therapy and follow-up strategies for IMPC patients.
Keywords: Invasive micropapillary carcinoma, Breast cancer, Prognosis, PSM study, Meta-analysis
Highlights
-
•
This is the first meta-analysis of PSM studies concerning on prognosis of IMPC, with the largest sample until now.
-
•
Compared with IDC, IMPC exhibits a similar, even favorite OS, but a shorter RFS; and the shorter RFS might owe to the significantly higher loco-regional recurrence rate.
1. Introduction
Breast cancer (BC) is the most common malignancy in the world. Invasive micropapillary carcinoma (IMPC, ICD-O code 8507/3), which was first described by Siriaunkgul in 1993 [1], is a special subtype of invasive breast carcinoma according to WHO classification and accounts for 3–6% of all invasive breast cancers[2]. Compared with invasive ductal carcinomas (IDC), IMPC is characterized by micropapillary and tubuloalveolar arrangements of tumor cell clusters surrounded by empty stromal space in pathology[3]. IMPC shows extremely high proclivities for lympho-vascular invasion (LVI), lymph node (LN) metastasis, thus exhibiting a more aggressive behavior than IDC[4,5].
IMPC is often mixed with other invasive carcinoma component, mostly IDC. In clinical practice, pure IMPC, which is defined as more than 75% IMPC components, is rather rare[6]. Studies of Fu et al. demonstrate that even if IMPC is present as a minor component and mixed with non-micropapillary invasive carcinoma, there is still higher incidence of LVI, LN metastasis, and recurrence [7,8]. As to molecular level, IMPC is reported to show intact expression of E-cadherin and low expression of CD44 [9,10], which may contribute to its propensity of LVI and LN metastasis.
Due to its highly invasive biological behavior, IMPC has been assumed to show an unfavorable prognosis compared with IDC[5,7]. However, previous studies show inconsistency on this issue[[11], [12], [13], [14], [15], [16], [17], [18], [19], [20]]. The major reason may be that most of the early studies included small samples of IMPC patients, and therefore the results could not be appropriately investigated. In 2017, a meta-analysis comparing the prognosis of IMPC with IDC, which included 14 studies and 1888 IMPC patients, demonstrated no significant difference in overall survival (OS), disease-specific survival (DSS) between them [21]. But there were some defects in the meta-analysis: study of Chen [22](comparing IMPC with triple negative breast cancer) was not suitable for quantitive analysis, and mostly the IMPC group had more advanced stage than IDC group in the majority of studies included (10 of 14). Therefore, the controversies still stay.
In recent years, propensity-matching method, or propensity score matching (PSM) method has been widely applied in retrospective analysis[23]. PSM has been supposed to reduce the effect of selection bias and thus to be especially proper for comparison between unbalanced groups. Until now, there are some PSM studies concerning on the prognosis of IMPC. However, the results were still inconsistent. For example, Chen investigated 984 IMPC cases from the SEER database and demonstrated the IMPC even had a better prognosis than IDC[24].
The aim of current study is to analyze existing PSM studies and to make a more comprehensive comparison on prognosis between IMPC and IDC. This study may help clinicians to tailor more appropriate strategies for the therapy and follow-up of IMPC patients.
2. Methods
2.1. Publication selection
We searched three databases (PubMed, EMBASE and the Cochrane library) in September 2019 for all studies concerning on IMPC prognosis. The searching strategy is to search the following words in the title/abstract: breast, invasive micropapillary carcinoma, (invasive carcinoma, micropapillary), prognosis, outcome, survival. Only studies written in English were included.
2.2. Inclusion and exclusion criteria
The inclusion criteria for the present analysis are as follows: (1) studies should include comparison of prognosis between IMPC and IDC; (2) detailed survival data, such as rates of overall survival (OS), breast cancer specific survival (BCSS), relapse free survival (RFS), local-regional recurrence free survival (LRRFS) or distant metastasis-free survival (DMFS), should be provided in the study; (3) when the studied data are duplicated, only the publication with the largest sample size could be included; (4) the PSM method has been used in study for adjusting the baseline characteristics. Publications which meet all the criteria above are included; otherwise, studies are excluded to avoid significant heterogeneity and to reduce bias. Case reports, meeting abstracts, commentary letters, editorials, and reviews are also excluded.
2.3. Definitions of survival data
Loco-regional recurrence (LRR) is defined as the appearance of tumors in these areas: ipsilateral chest wall or breast, ipsilateral axillary, infraclavicular or supraclavicular area, ipsilateral internal mammary area. Otherwise, recurrence was defined as distant metastasis. Overall survival (OS) was measured from the date of surgery or diagnosis to death, while breast cancer specific survival (BCSS) was measured to the death due to breast cancer progression. Relapse-free survival (RFS), LRR-free survival (LRRFS) and distant metastasis-free survival (DMFS) were measured from the date of surgery to LRR and/or distant metastasis, LRR and distant metastasis, respectively.
2.4. Data extraction
The following information is extracted from each eligible publication: author names, publication year, original data source, investigation period, propensity-matching variables, sample size of IMPC and IDC, component of IMPC, TNM stage, median follow-up time and survival data. When the essential prognostic information is not provided in the publications, efforts are made to contact the authors. If the prognostic data are still inaccessible, we extract and transform them from the survival curve[25].
2.5. Statistical analysis
The summarized odds ratios (ORs) and 95% confidence interval (95% CI) are calculated utilizing fixed-effect or random-effect models, depending on the heterogeneity of the eligible studies. Heterogeneity across studies is determined by the χ 2 and Ι2 test methods. Significant heterogeneity is indicated by p < 0.05 and/or Ι2 >50%. When heterogeneity is significant, a random-effect model is used; otherwise, a fixed-effect model is used. Forest plots are generated to show the respective and summarized OR and 95% CI of included studies, and the weights of each publication according to their sample sizes. Funnel plot and Egger’s test method are used to evaluate the potential publication bias. Statistically significant publication bias is determined by Egger’s test as p < 0.05. All statistical analyses were performed with STATA 12.0 software (Stata Corporation, College Station, TX, USA).
3. Results
3.1. Eligible studies
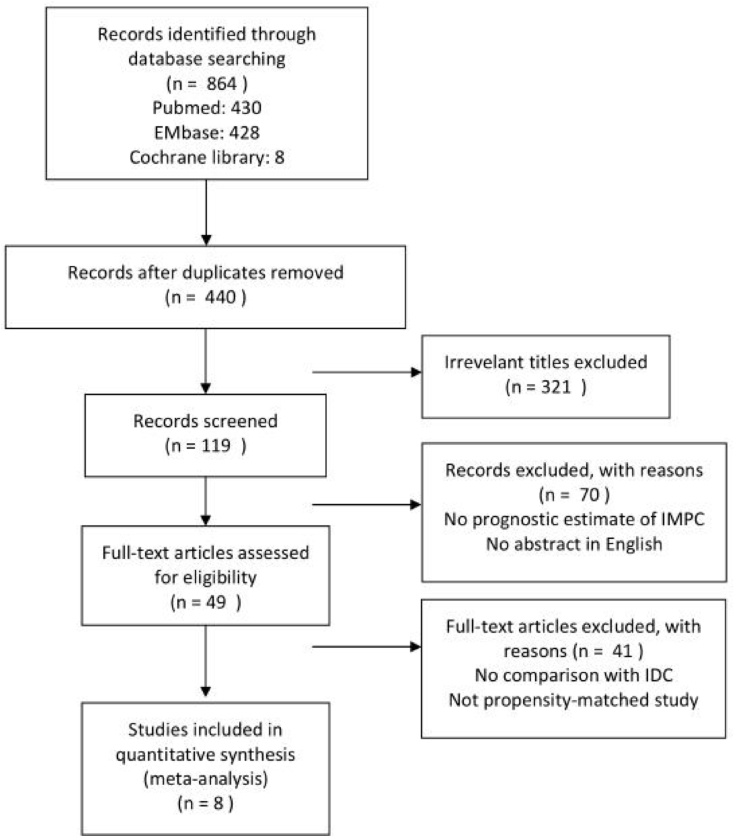
The search of three databases (PubMed, EMBASE and the Cochrane library) yielded 864 publications in all. Four hundred and forty publications remained after removing the duplicates, of which three hundred and twenty one publications were excluded by evaluating the titles. The abstracts of one hundred and nineteen publications were screened, and seventy publications were excluded. Forty nine publications were potential eligible studies which provided prognostic estimate of IMPC and written in English. Forty one studies were excluded for not comparing with IDC or not using PSM method. Finally, eight publications were eligible for inclusion in this meta-analysis [11,15,18,24,[26], [27], [28], [29]]. The PRISMA flow diagram is shown in Fig. 1.
Fig. 1.
The PRISMA flow diagram.
3.2. Study characteristics
Ultimately, eight publications including a total of 2102 IMPC patients, which had compared survival data of IMPC with IDC using PSM method, were included in this meta-analysis. The characteristics and matching variables for PSM of these studies are demonstrated in Table 1. The survival data are shown in Table 2. All of the studies recruited patients with all subtype IDC patients as the control group. IMPC patients in most studies are mixed type. As listed in the table, all of the PSM studies employed node status and age as vital matching variables, and half of them (4 of 8) also employ HR status, HER-2 status as variables. Among the 8 studies, 6 studies used a ratio of 1:1 (IMPC: IDC) for PSM matching, and 2 studies used a ratio of 1:2 (IMPC: IDC).
Table 1.
Characteristics and matching variables of eligible studies.
| Study | Publication year | Original data source | Investigation period | Propensity-matching variables |
|---|---|---|---|---|
| Liu | 2014 | Fudan University Shanghai Cancer Center (FUSCC) | 2005.8–2008.3 | nodal status, age |
| Yu | 2015 | Korean Radiation Oncology Group (KROG) | 1999.1–2011.11 | year of surgery, age, tumor size, node status, surgery method, radiotherapy |
| Yu | 2010 | Samsung Medical Center | 1999.1–2007.12 | year of surgery, age, tumor size, node status, surgery method, radiotherapy |
| Vingiani | 2013 | European Institute of Oncology of Milan | 2000–2009 | year of surgery, age, tumor size and grade, node status, LVI, HR status, HER2 status |
| Hao | 2018 | Fudan University Shanghai Cancer Center (FUSCC) | 2008.1–2012.10 | age, tumor size, nodal status, HR status, HER2 status |
| Chen | 2017 | SEER databse of US National Cancer Institute | 2001.1–2013.12 | year of surgery, age, tumor size and grade, node status, HR status, HER2 status, surgery method, radiotherapy, chemotherapy |
| Hua | 2018 | Beijing Hospital | 2008–2016 | nodal status, age, tumor size |
| Yoon | 2019 | Asan Medical Center | 2007.1–2012.12 | age, tumor size, node status, LVI, HR status, HER2 status, surgery method, radiotherapy, chemotherapy |
Table 2.
Survival data of eligible studies.
| Study | Sample size after PSM |
IMPC component | TNM stage | Median follow-up time (months) | Survival data |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| IMPC | IDC | OS | BCSS | RFS | LRRFS | DMFS | ||||
| Liu | 51 | 102 | mixed | I,II,III | 51 | 84.3%vs78.4% (P = 0.606) | ||||
| Yu | 267 | 267 | mixed | I,II,III | 59 | 97.7%vs95.7% (p = 0.67) | 85.5%vs91.5% (p = 0.007) | 92.3%vs95.4% (p = 0.03) | ||
| Yu | 72 | 144 | mixed | I,II,III | 45 | 86.0%vs87.7% (P = 0.18) | 68.2%vs81.4% (P = 0.046) | 84.7%vs94.4% (P = 0.0024) | 78.1%vs79.3% (P = 0.87) | |
| Vingiani | 49 | 98 | pure | I,II,III | 51 | 93.5%vs94.3% (P = 0.80) | 75.5%vs81.6% (P = 0.48) | |||
| Hao | 324 | 324 | mixed | I,II,III | 56.5 | 93.7%vs96.4% (p = 0.752) | 92.0%vs94.1% (p = 0.578) | |||
| Chen | 984 | 984 | mixed | I,II,III | 64 | 91.2%vs82.8% (p < 0.0001) | 95.5%vs89.2% (p < 0.0001) | |||
| Hua | 47 | 93 | mixed | I,II,III | 40 | 88.3%vs74% NS |
66.6%vs63.4% NS | |||
| Yoon | 308 | 308 | mixed | I,II,III | NS | 94.6%vs95.1% (P = 0.335) | 93.4%vs96.0% (P = 0.016) | 95.7%vs98.6% (P = 0.168) | 95.1%vs96.7% (P = 0.017) | |
NS: not shown.
3.3. Survival outcomes of IMPC and IDC
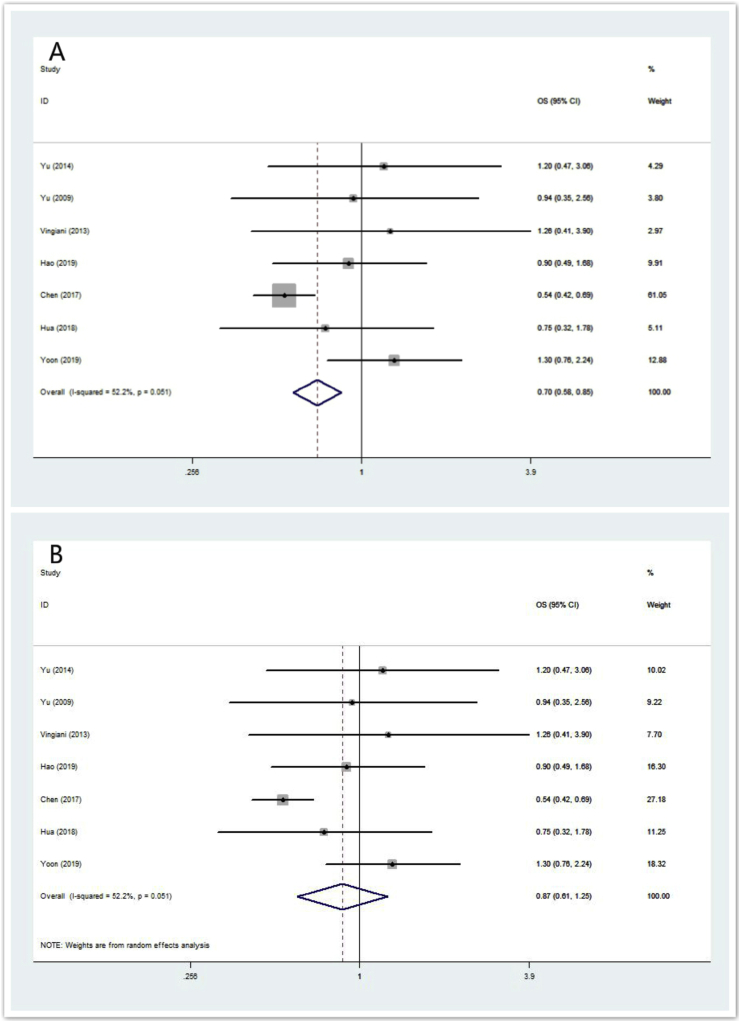
All the eight studies employed age and node status as matching variables. As shown in Table 2, seven of the eight studies provided OS data (n = 2051 IMPC patients). The ORs and 95% CIs for each study and the summarized OR are shown in Fig. 2. The individual OR of the 7 articles ranged from 0.54 to 1.30. The overall summarized estimate OR was 0.70 (95% CI: 0.58–0.85) using a fixed-effect method (Fig. 2A). There was marginal heterogeneity across the studies (Ι2 = 52.2%, χ2 = 12.56, p = 0.051). Using the random-effect method yielded a closer effect estimate (OR = 0.87, 95% CI: 0.61–1.25) between them (Fig. 2B). The summarized OR for OS shows that IMPC at least has a similar overall survival compared with IDC.
Fig. 2.
Forrest plot for OS. (2A) summarized OR using a fixed-effect model, (2B) summarized OR using a random-effect model.
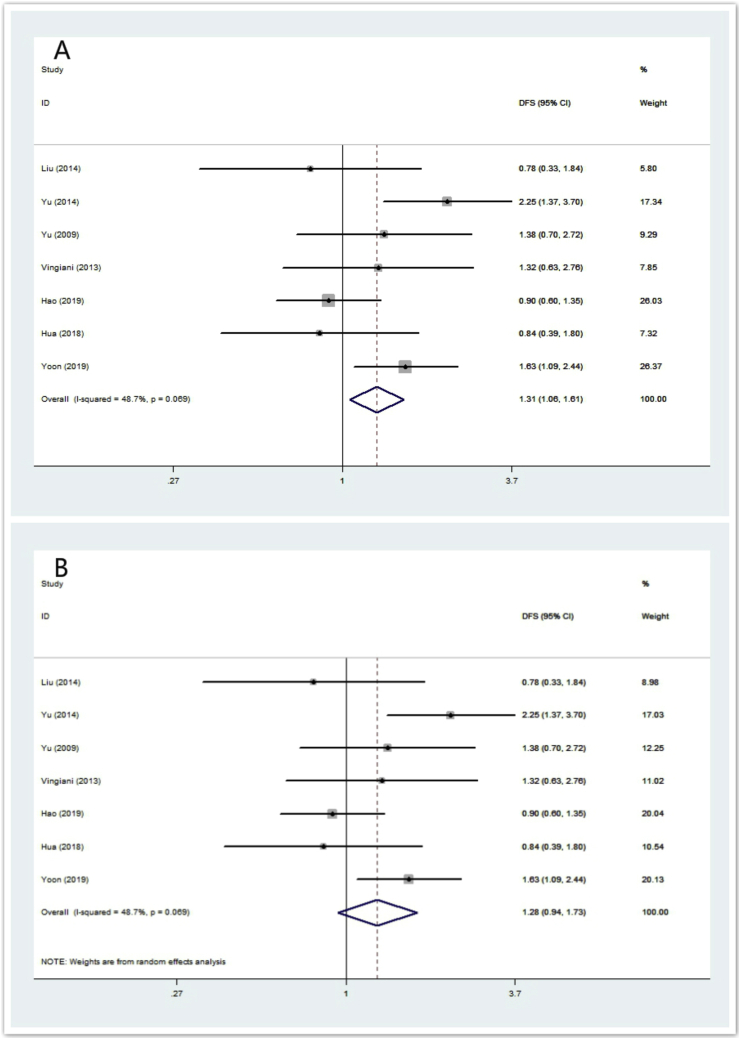
Seven of the eight studies provided RFS data (n = 1118 IMPC patients). The ORs and 95% CIs for each study and the summarized OR are shown in Fig. 3. The individual OR of the 7 articles ranged from 0.78 to 2.25. The overall summarized estimate OR was 1.31 (95% CI: 1.06–1.61) using a fixed-effect method (Fig. 3A), which mean IMPC had a higher recurrence rate than IDC. There was also marginal heterogeneity across the studies (Ι2 = 48.7%, χ2 = 11.70, p = 0.069). Using the random-effect method yielded a closer effect estimate (OR = 1.28, 95% CI: 0.94–1.73) between them (Fig. 3B). The summarized OR for RFS implies that IMPC has a shorter recurrence-free survival.
Fig. 3.
Forrest plot for RFS. (3A) summarized RFS using a fixed-effect model, (3B) summarized RFS using a random-effect model.
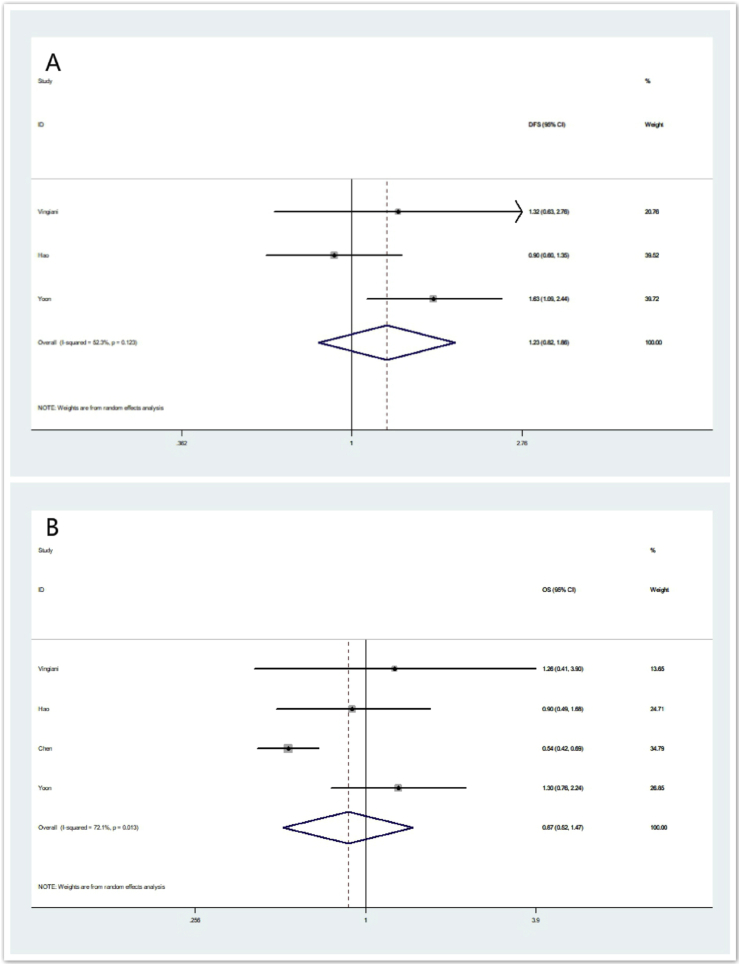
Besides age and node status, four of the eight eligible studies (i.e. Study of Vingiani, Hao, Chen and Yoon) including 1665 IMPC patients also employed HR status and HER-2 status as matching variables. The ORs and 95% CIs for each study and the summarized OR for OS and RFS are shown in Fig. 4. The overall summarized estimate OR for RFS was 1.23 (95% CI: 0.82–1.86) using a random-effect method (Ι2 = 52.3%, χ2 = 4.19, p = 0.123) (Fig. 4A). The overall summarized estimate OR for OS was 0.87 (95% CI: 0.52–1.47) using a random-effect method (Ι2 = 72.1%, χ2 = 10.77, p = 0.013) (Fig. 4B).
Fig. 4.
Forrest plot for RFS and OS in sub-analysis using age, node status, HR status and HER-2 status as matching variables. (4A) summarized RFS using a random -effect model, (4B) summarized OS using a random-effect model.
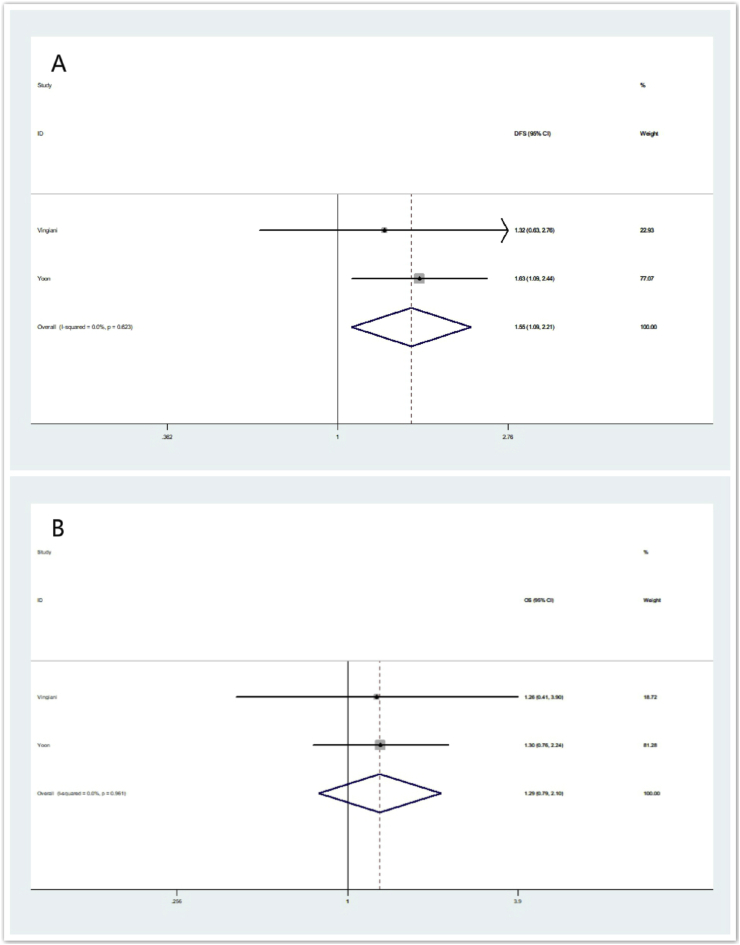
IMPC is characterized by high rates for lympho-vascular invasion (LVI), lymph node (LN) metastasis. In our analysis, two of the eight eligible studies (i.e. Study of Vingiani and Yoon) also employed LVI as matching variables in addition of age, node status, HR status and HER-2 status. For these two studies, the overall summarized estimate OR for OS and RFS are shown in Fig. 5. The overall summarized estimate OR for RFS was 1.55 (95% CI: 1.09–2.21) using a fix-effect method (Ι2 = 0.0%, χ2 = 0.24, p = 0.623) (Fig. 5A). While the summarized estimate OR for OS was 1.29 (95% CI: 0.79–2.10) using a fix-effect method (Ι2 = 0.0%, χ2 = 0.00, p = 0.961) (Fig. 5B).
Fig. 5.
Forrest plot for RFS and OS in sub-analysis using age, node status, HR status, HER-2 status and LVI as matching variables. (5A) summarized RFS using a random -effect model, (5B) summarized OS using a random-effect model.
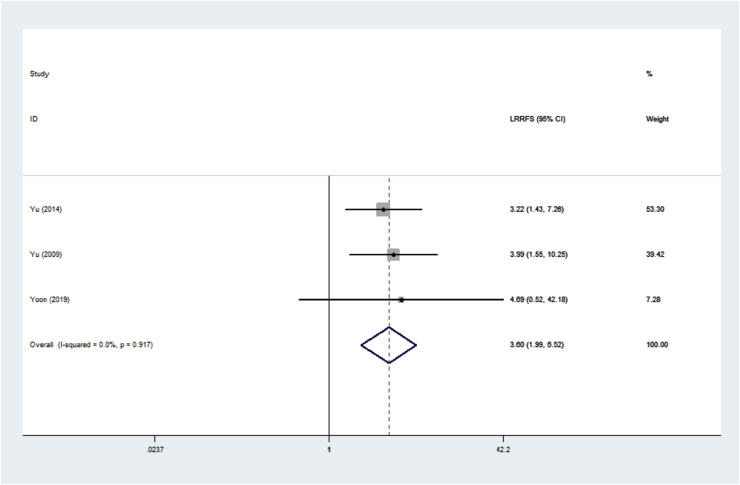
Three studies provided LRRFS data (n = 647 IMPC patients). The ORs and 95% CIs for each study and the summarized OR are shown in Fig. 6. The individual OR of the 3 articles ranged from 3.22 to 4.69. The overall summarized estimate OR was 3.60 (95% CI: 1.99–6.52) using a fixed-effect method (Fig. 6), which mean IMPC had a significantly higher loco-regional recurrence rate than IDC. There was no heterogeneity across the studies (Ι2 = 0.0%, χ2 = 0.17, p = 0.917).
Fig. 6.
Forrest plot for LRRFS.
3.4. Publication bias
Egger’s test are used to detect the publication bias of OS and RFS. The p value for OS and RFS are 0.036 and 0.564 respectively (Supplementary 1&2). We did not do the test for LRRFS because only 3 studies reported the data.
4. Discussion
Breast cancer has been proved to be with high heterogeneity. To date, breast cancers can be classified into 21 distinct histological types according to the WHO. Among them, invasive dutal carcinoma (IDC) is the major histological type, accounting for about 75% all breast cancers. IMPC is a special and rare subtype of invasive breast carcinoma.
IMPC has been presumed to show an unfavorable prognosis compared with IDC for a long time due to its highly invasive biological behavior. However, the early retrospective studies show inconsistency on this issue. The major reason may be that most of the early studies included small samples of IMPC patients, and therefore the results could not be appropriately investigated. On the other hand, in most studies the IMPC group had more advanced stage and smaller sample size than IDC group, and the extremely unbalanced data brought great bias in final analysis. A meta-analysis published in 2017 comparing the prognosis of IMPC with IDC, which included 14 studies and 1888 IMPC patients, tried to draw a conclusion. The result demonstrated that IMPC had a similar OS and DFS compared with IDC. However, due to some evident methodological weaknesses of the eligible studies, the controversies still stay.
In recent years, PSM method has been widely applied in retrospective analysis. PSM can filtrate experimental and control cases of similar characteristics from existing data, so as to reduce the effect of selection bias. As mentioned above, the major difficulty for investigating IMPC prognosis is the small sample of IMPC patients and extremely unbalanced original data between IMPC and IDC. Therefore, PSM studies may be especially proper for investigation of IMPC prognosis. There are some PSM studies in recent years concerning on this issue but the results are not consistent. So we conducted this meta-analysis of PSM studies.
For PSM studies, the first thing is to decide the matching variables. We listed the corresponding matching variables of the 8 eligible PSM studies in Table 1. All the studies employed age and node status as the vital matching variables. Age is a popularly used character in case-control studies for breast cancer prognosis. In the early studies of IMPC prognosis, the IMPC groups usually have more advanced initial stage than IDC groups, due to the high LN metastasis rate of IMPC. For IMPC patients, numerous studies have proved that node status is a significant prognostic factor[30]. Therefore, it is reasonable that all the PSM studies employed node status as a matching variable to balance the two groups. Besides age and node status, half of eligible studies (4/8) including 79.2% IMPC patients (1665 of the total 2102) also employed HR status and HER-2 status as matching variables, since it has been reported that IMPC had a higher HR positive rate [31]. Therefore, in the present meta-analysis, the two groups (IMPC and IDC) are well balanced and comparable in node status and molecular subtypes, thus decreasing the selection bias to a minimum.
For most eligible PSM studies (7 of 8), the component of IMPC is a mixed type. In clinical practice, pure IMPC is rather rare. Most IMPC patients belong to mixed types, majorly mixed with IDC. A recent study by Cemal Kaya et al. demonstrated that micropapillary component ratio did not affect the recurrence and survival rates of IMPC significantly[32]. Therefore we did not distinguish IMPC according to the components.
The quantitive analysis of our study demonstrates an interesting outcome: compared with IDC, IMPC has a similar OS (estimated OR = 0.87, 95% CI: 0.61–1.25), but a shorter RFS (estimated OR = 1.31, 95% CI: 1.06–1.61); the shorter RFS might owe to the significantly higher loco-regional recurrence rate of IMPC (estimated OR = 3.60, 95% CI: 1.99–6.52).
Due to the high proclivities for LVI and LN metastasis, IMPC often exhibits a higher clinical stage than IDC in initial diagnosis. As listed in Table 1, IMPC group has a similar node status as IDC group through the PSM method, and moreover, half of eligible studies (4/8) including 79.2% IMPC patients (1665 of the total 2102) also employ HR status, HER-2 status as matching variables. Therefore, in our study, node status and molecular subtypes have been generally balanced in IMPC group and IDC group. Under the similar circumstances, the results show a similar, even favorite, OS for IMPC. The meta-analysis of the existing data brings two messeages: 1. An early detection and diagnosis is especially important for IMPC, which emphasizes the necessity of regular screening of breast; 2. the comprehensive treatments of IDC are also applicable for IMPC.
IMPC is characterized by high rates for LVI and LN metastasis. Since the presence of LVI has been demonstrated to be an independent predictor of DFS and OS, both in node-negative and node-positive breast cancer patients, we further conducted a quantitive analysis of the two studies employing LVI as a matching variable, besides age, node status, HR status and HER-2 status. The summarized OR implied that IMPC had a similar OS, but a shorter recurrence-free survival, compared with IDC. The results of this well-matched sub-analysis were similar to the gross analysis.
In our study, the results show a shorter RFS for IMPC, which might owe to the significantly higher loco-regional recurrence rate of IMPC (estimated OR = 3.60, 95% CI: 1.99–6.52). Numerous studies have proved that LVI is significantly correlated with loco-regional recurrence of breast cancer, so it is not hard to understand the outcomes, given the high LVI proclivities of IMPC. A previous study based on the SEER database demonstrated that the local-regional treatments (i.e. surgical methods, post-operative radiotherapy) did not influence the OS of IMPC[33]. However, whether the local-regional treatments would influence the loco-regional recurrence of IMPC is still unknown. More prospective researches are needed for this issue to help the clinical decision making.
Among all of the studies included in our analysis, we detect significant statistical publication bias for OS, but not for RFS. The p value for OS and RFS are 0.036 and 0.564 respectively. The publication bias may influence the robustness of the result of OS to some extent. According to the funnel plot, some studies that yielded negative OS results for IMPC might not have been published, thus bringing publication bias. But the publication bias for RFS is not apparent.
Our meta-analysis has some more limitations requiring consideration. First, the number of existing PSM studies is relatively small (only 8 eligible publications) and may contribute to potential publication bias. The survival data of these publications are complete: only scattered studies provided LRRFS and DMFS information. Second, some important factors, such as race, have not been considered in the present meta-analysis. For example, the three eligible studies in our analysis providing the LRRFS data are all based on Asian IMPC patients (from the South Korean), and the circumstance may be not inconsistent in other population. Third, even we applied PSM methods to decrease the selection bias, the small sample size of the publications (4 of 8 studies have a IMPC sample less than 100) limits their power. Future prospective trials are needed to validate our results.
5. Conclusions
The present study is the first meta-analysis of PSM studies to explore the prognosis of IMPC. Our results demonstrate that compared with IDC, IMPC exhibits a similar, even favorite OS, but a shorter RFS; and the shorter RFS might owe to the significantly higher loco-regional recurrence rate of IMPC. These results can contribute to the individualized and tailored therapy for IMPC. However, more prospective or large-scale retrospective studies are needed to further validate our results and figure out the influence of local-regional treatments (i.e. surgical methods, post-operative radiotherapy) on LRRFS of IMPC.
Funding
This work was sponsored by funds from the National Natural Science Foundation of China (81872152, Xiaoming Xie), the Natural Science Foundation of Guangdong (2018A0303130285, Feng Ye) and Science and Technology Planning Projects of Guangdong (2017A020215197, Feng Ye).
Authors’ contributions
Ping Yu, Peng Liu, Feng Ye conceived the study and wrote the manuscript. Na Li and Hailin Tang took charge of data base reaching, data interpretation and language editting. Feng Ye, Anli Yang and Xinhua Xie peformed all data analysis. All the authors were all involved in approval of the final version.
Consent for publication
Not applicable.
Declaration of competing interest
The authors declare to have no competing interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2020.01.041.
Contributor Information
Feng Ye, Email: yefeng@sysucc.org.cn.
Ping Yu, Email: yuping@sysucc.org.cn.
Na Li, Email: lina@sysucc.org.cn.
Anli Yang, Email: yangal@sysucc.org.cn.
Xinhua Xie, Email: xiexh@sysucc.org.cn.
Hailin Tang, Email: tanghl@sysucc.org.cn.
Peng Liu, Email: liupeng@sysucc.org.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Siriaunkgul S., Tavassoli F.A. Invasive micropapillary carcinoma of the breast. Mod Pathol. 1993;6:660–662. [PubMed] [Google Scholar]
- 2.Andrew M.H., Clare w, Tavassoli F.A., Devilee P. WHO Classification of Tumours series - volume IV. vol. 6. IARC Press; Lyon, France: 2004. Pathology and genetics: tumours of the breast and female genital organs; p. 133. Breast Canc Res. [Google Scholar]
- 3.Middleton L.P., Tressera F., Sobel M.E., Bryant B.R., Alburquerque A., Grases P., Merino M.J. Infiltrating micropapillary carcinoma of the breast. Mod Pathol. 1999;12:499–504. [PubMed] [Google Scholar]
- 4.Kuroda H., Sakamoto G., Ohnisi K., Itoyama S. Clinical and pathologic features of invasive micropapillary carcinoma. Breast Cancer. 2004;11:169–174. doi: 10.1007/BF02968297. [DOI] [PubMed] [Google Scholar]
- 5.De la Cruz C., Moriya T., Endoh M., Watanabe M., Takeyama J., Yang M., Oguma M., Sakamoto K., Suzuki T., Hirakawa H. Invasive micropapillary carcinoma of the breast: clinicopathological and immunohistochemical study. Pathol Int. 2004;54:90–96. doi: 10.1111/j.1440-1827.2004.01590.x. [DOI] [PubMed] [Google Scholar]
- 6.Isabel A., Jo茫o M., Margarida D. Invasive micropapillary carcinoma of the breast: are the pure forms more aggressive than the mixed forms? Breast J. 2003;9:337–338. doi: 10.1046/j.1524-4741.2003.09423.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen L., Fan Y., Lang R.G., Guo X.J., Sun Y.L., Cui L.F., Liu F.F., Wei J., Zhang X.M., Fu L. Breast carcinoma with micropapillary features: clinicopathologic study and long-term follow-up of 100 cases. Int J Surg Pathol. 2008;16:155–163. doi: 10.1177/1066896907307047. [DOI] [PubMed] [Google Scholar]
- 8.Guo X., Chen L., Lang R., Fan Y., Zhang X., Fu L. Invasive micropapillary carcinoma of the breast: association of pathologic features with lymph node metastasis. Am J Clin Pathol. 2006;126:740–746. doi: 10.1309/AXYY-4AJT-MNW6-FRMW. [DOI] [PubMed] [Google Scholar]
- 9.Umeda T., Ishida M., Murata S., Mori T., Kawai Y., Itoi N., Tomida K., Tanaka A., Sakai S., Kitamura M. Immunohistochemical analyses of CD44 variant isoforms in invasive micropapillary carcinoma of the breast: comparison with a concurrent conventional invasive carcinoma of no special type component. Breast Cancer. 2016;23:869–875. doi: 10.1007/s12282-015-0653-4. [DOI] [PubMed] [Google Scholar]
- 10.Simonetti S., Terracciano L., Zlobec I., Kilic E., Stasio L., Quarto M., Pettinato G., Insabato L. Immunophenotyping analysis in invasive micropapillary carcinoma of the breast: role of CD24 and CD44 isoforms expression. Breast. 2012;21:165–170. doi: 10.1016/j.breast.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Yin L., Xiaoyan H., Rui B., Wentao Y., Zhimin S. Similar prognoses for invasive micropapillary breast carcinoma and pure invasive ductal carcinoma: a retrospectively matched cohort study in China. PloS One. 2014;9 doi: 10.1371/journal.pone.0106564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gokce H., Durak M.G., Akin M.M., Canda T., Balci P., Ellidokuz H., Demirkan B., Gorken I.B., Sevinc A.I., Kocdor M.A. Invasive micropapillary carcinoma of the breast: a clinicopathologic study of 103 cases of an unusual and highly aggressive variant of breast carcinoma. Breast J. 2013;19:374–381. doi: 10.1111/tbj.12128. [DOI] [PubMed] [Google Scholar]
- 13.Chen A.C., Paulino A.C., Schwartz M.R., Rodriguez A.A., Bass B.L., Chang J.C., Teh B.S. Population-based comparison of prognostic factors in invasive micropapillary and invasive ductal carcinoma of the breast. Br J Canc. 2014;111:619–622. doi: 10.1038/bjc.2014.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi W.B., Yang L.J., Hu X., Zhou J., Zhang Q., Shao Z.M. Clinico-pathological features and prognosis of invasive micropapillary carcinoma compared to invasive ductal carcinoma: a population-based study from China. PloS One. 2014;9 doi: 10.1371/journal.pone.0101390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vingiani A., Maisonneuve P., Dell’orto P., Farante G., Rotmensz N., Lissidini G., Del Castillo A., Renne G., Luini A., Colleoni M. The clinical relevance of micropapillary carcinoma of the breast: a case-control study. Histopathology. 2013;63:217–224. doi: 10.1111/his.12147. [DOI] [PubMed] [Google Scholar]
- 16.Li G., Yang S., Yao J., Wang Z., Yao G., Liu M., Ye C. Invasive micropapillary carcinoma of the breast had poor clinical characteristics but showed no difference in prognosis compared with invasive ductal carcinoma. World J Surg Oncol. 2016;14:207. doi: 10.1186/s12957-016-0960-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuba S., Ohtani H., Yamaguchi J., Hayashi H., Uga T., Kanematsu T. Incomplete inside-out growth pattern in invasive breast carcinoma: association with lymph vessel invasion and recurrence-free survival. Virchows Arch. 2011;458:159–169. doi: 10.1007/s00428-010-1033-2. [DOI] [PubMed] [Google Scholar]
- 18.Jeong Il Y., Doo Ho C., Won P., Seung Jae H., Eun Yoon C., Young Hyuk L., Jin Suk A., Jung Hyun Y., Suk Jin N. Differences in prognostic factors and patterns of failure between invasive micropapillary carcinoma and invasive ductal carcinoma of the breast: matched case control study. 2010;19:231–237. doi: 10.1016/j.breast.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Zekioglu O., Erhan Y., Ciris M., Bayramoglu H., Ozdemir N. Invasive micropapillary carcinoma of the breast: high incidence of lymph node metastasis with extranodal extension and its immunohistochemical profile compared with invasive ductal carcinoma. Histopathology. 2004;44:18–23. doi: 10.1111/j.1365-2559.2004.01757.x. [DOI] [PubMed] [Google Scholar]
- 20.Shen-li T., Ji-qiao Y., Zheng-gui D., Qiu-wen T., Qing L. Clinicopathologic study of invasive micropapillary carcinoma of the breast. Oncotarget. 2017;8:42455–42465. doi: 10.18632/oncotarget.16405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y., Zhang N., Yang Q. The prognosis of invasive micropapillary carcinoma compared with invasive ductal carcinoma in the breast: a meta-analysis. BMC Canc. 2017;17:839. doi: 10.1186/s12885-017-3855-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H-l, Ding A. Comparison of invasive micropapillary and triple negative invasive ductal carcinoma of the breast. Breast. 2015;24 doi: 10.1016/j.breast.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Reeve B.B., Smith A.W., Arora N.K., Hays R.D. Reducing bias in cancer research: application of propensity score matching. Health Care Financ Rev. 2008;29:69. [PMC free article] [PubMed] [Google Scholar]
- 24.Chen H., Wu K., Wang M., Wang F., Zhang M., Zhang P. 2017. Invasive micropapillary carcinoma of the breast has a better long-term survival than invasive ductal carcinoma of the breast in spite of its aggressive clinical presentations: a comparison based on large population database and case-control analysis. Cancer Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tierney J.F., Stewart L.A., Ghersi D., Burdett S., Sydes M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hua B., Lu X., Xiao W.Z., Yang X., He S.R., Wang Z. [Comparison of prognosis between invasive micropapillary carcinoma and invasive ductal carcinoma of breast: a single center. retrospective case-control study] 2018;56:56–60. doi: 10.3760/cma.j.issn.0529-5815.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Yoon G.Y., Cha J.H., Kim H.H., Shin H.J., Chae E.Y., Choi W.J. Comparison of invasive micropapillary and invasive ductal carcinoma of the breast: a matched cohort study. Acta Radiol. 2019;60:1405–1413. doi: 10.1177/0284185119834689. [DOI] [PubMed] [Google Scholar]
- 28.Hao S., Zhao Y.Y., Peng J.J., Ren F., Yang W.T., Yu K.D., Shao Z.M. Invasive micropapillary carcinoma of the breast had no difference in prognosis compared with invasive ductal carcinoma: a propensity-matched analysis. Sci Rep. 2019;9:286. doi: 10.1038/s41598-018-36362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu J.I., Choi D.H., Huh S.J., Cho E.Y., Kim K., Chie E.K., Ha S.W., Park I.A., Ahn S.J., Lee J.S. Differences in prognostic factors and failure patterns between invasive micropapillary carcinoma and carcinoma with micropapillary component versus invasive ductal carcinoma of the breast: retrospective multicenter case-control study (KROG 13-06) Clin Breast Canc. 2015;15 doi: 10.1016/j.clbc.2015.01.008. 353-361 e351-352. [DOI] [PubMed] [Google Scholar]
- 30.Li W., Han Y., Wang C., Guo X., Shen B., Liu F., Jiang C., Li Y., Yang Y., Lang R. vol. 31. 2018. (Precise pathologic diagnosis and individualized treatment improve the outcomes of invasive micropapillary carcinoma of the breast: a 12-year prospective clinical study). [DOI] [PubMed] [Google Scholar]
- 31.Lewis G.D., Xing Y., Haque W., Patel T., Schwartz M.R., Chen A.C., Farach A., Hatch S.S., Butler E.B., Chang J.C., Teh B.S. The impact of molecular status on survival outcomes for invasive micropapillary carcinoma of the breast. Breast J. 2019;25:1171–1176. doi: 10.1111/tbj.13432. [DOI] [PubMed] [Google Scholar]
- 32.Kaya C., Ucak R., Bozkurt E., Omeroglu S., Kartal K., Yazici P., Idiz U.O., Mihmanli M. The impact of micropapillary component ratio on the prognosis of patients with invasive micropapillary breast carcinoma. J Invest Surg. 2018:1–9. doi: 10.1080/08941939.2018.1474302. [DOI] [PubMed] [Google Scholar]
- 33.Wu S.G., Zhang W.W., Sun J.Y., Li F.Y., Chen Y.X., He Z.Y. Postoperative radiotherapy for invasive micropapillary carcinoma of the breast: an analysis of Surveillance, Epidemiology, and End Results database. Canc Manag Res. 2017;9:453–459. doi: 10.2147/CMAR.S141338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.