Abstract
Background
Persistent pain in breast cancer survivors is common. Psychological and sleep-related factors modulate perception, interpretation and coping with pain and may contribute to the clinical phenotype. The present analysis pursued the hypothesis that breast cancer survivors form subgroups, based on psychological and sleep-related parameters that are relevant to the impact of pain on the patients’ life.
Methods
We analysed 337 women treated for breast cancer, in whom psychological and sleep-related parameters as well as parameters related to pain intensity and interference had been acquired. Data were analysed by using supervised and unsupervised machine-learning techniques (i) to detect patient subgroups based on the pattern of psychological or sleep-related parameters, (ii) to interpret the detected cluster structure and (iii) to relate this data structure to pain interference and impact on life.
Results
Artificial intelligence-based detection of data structure, implemented as self-organizing neuronal maps, identified two different clusters of patients. A smaller cluster (11.5% of the patients) had comparatively lower resilience, more depressive symptoms and lower extraversion than the other patients. In these patients, life-satisfaction, mood, and life in general were comparatively more impeded by persistent pain.
Conclusions
The results support the initial hypothesis that psychological and sleep-related parameter patterns are meaningful for subgrouping patients with respect to how persistent pain after breast cancer treatments interferes with their life. This indicates that management of pain should address more complex features than just pain intensity. Artificial intelligence is a useful tool in the identification of subgroups of patients based on psychological factors.
Keywords: Pain, Quality of life, Breast cancer survivers, Data science, Machine-learning
Highlights
-
•
Pain interference with daily life is relevant for post-surgery breast cancer patients.
-
•
11.5% of 337 patients belonged to a subgroup with high life interference of pain.
-
•
Life interference was not a function of pain intensity.
-
•
Risk factors were lower resilience, more depressive symptoms and lower extraversion.
-
•
Life-satisfaction, mood, and life in general were impeded by persistent pain.
1. Introduction
As treatment of breast cancer becomes more efficacious, the number of survivors increases. Persistent treatment-related pain of moderate to severe intensity is a significant problem in 14%–25% of the patients [1,2]. Persistent pain can be challenging to manage [3]. Psychological factors and pain interact in the experience of pain [4]. Psychological factors modulate both perception and interpretation of pain and coping with pain [4,5]. On the other hand, pain may modulate the patient’s mood, sleep, and social activities [6,7]. The negative roles of anxiety, depressive symptoms, and catastrophising in pain experience are well accepted [4] but also protective psychological factors that may support meaningful life with pain have been acknowledged [5,8]. Psychological resilience refers to a person’s ability to adapt successfully or to reach a positive outcome in case of severe life adversity, such as cancer and persistent pain [9]. In the context of chronic pain, psychological resilience associates inversely with pain interference and depressive symptoms [10,11].
While the intensity of pain has been the traditional outcome in research and therapy, it has become obvious, that this focus reflects the clinical facets of pain only partly. A discrepancy between pain intensity and its interference is common, and recognition of the underlying psychological traits is necessary to provide successful multimodal pain management. The associations between personality and sleep with pain-related outcomes have been studied to some extent [12,13]. In this context, personality traits have been associated with either higher intensity or poorer coping with pain [12,14]. Indeed, the impact of pain on a persons’ life is not a linear consequence of its intensity, but an interplay between the protective and vulnerability factors [4] the patient may have.
As the patients have to cope with the pain related to breast cancer treatment for many years, it is important to know whether the patients represent a psychologically heterogeneous cohort that would allow subgroup segregation with possible relevance regarding treatment strategies. Therefore, the present study aimed at investigating subgroup segregation based on psychological patterns of breast cancer survivors. Advancements in data science [15] facilitate data-driven approaches [16] in this field to insights or hypotheses regarding the intersection between pain, life interference and psychological factors. The goal was to identify clinically meaningful subgroups of patients in the patterns emerging in a number of psychological parameters (mood, personality, resilience, pain-related catastrophizing, and sleep) and to select parameters that enable interpretation of the observed pattern and association of patients to clinically relevant scenarios of persistent pain and its interference with life. Information about the combination of those psychological features that may have an effect on poorer coping with persistent pain following breast cancer treatments, may help clinicians to recognize these patients and to provide them with psychosocial interventions to improve their quality of life.
2. Methods
2.1. Patients and data acquisition
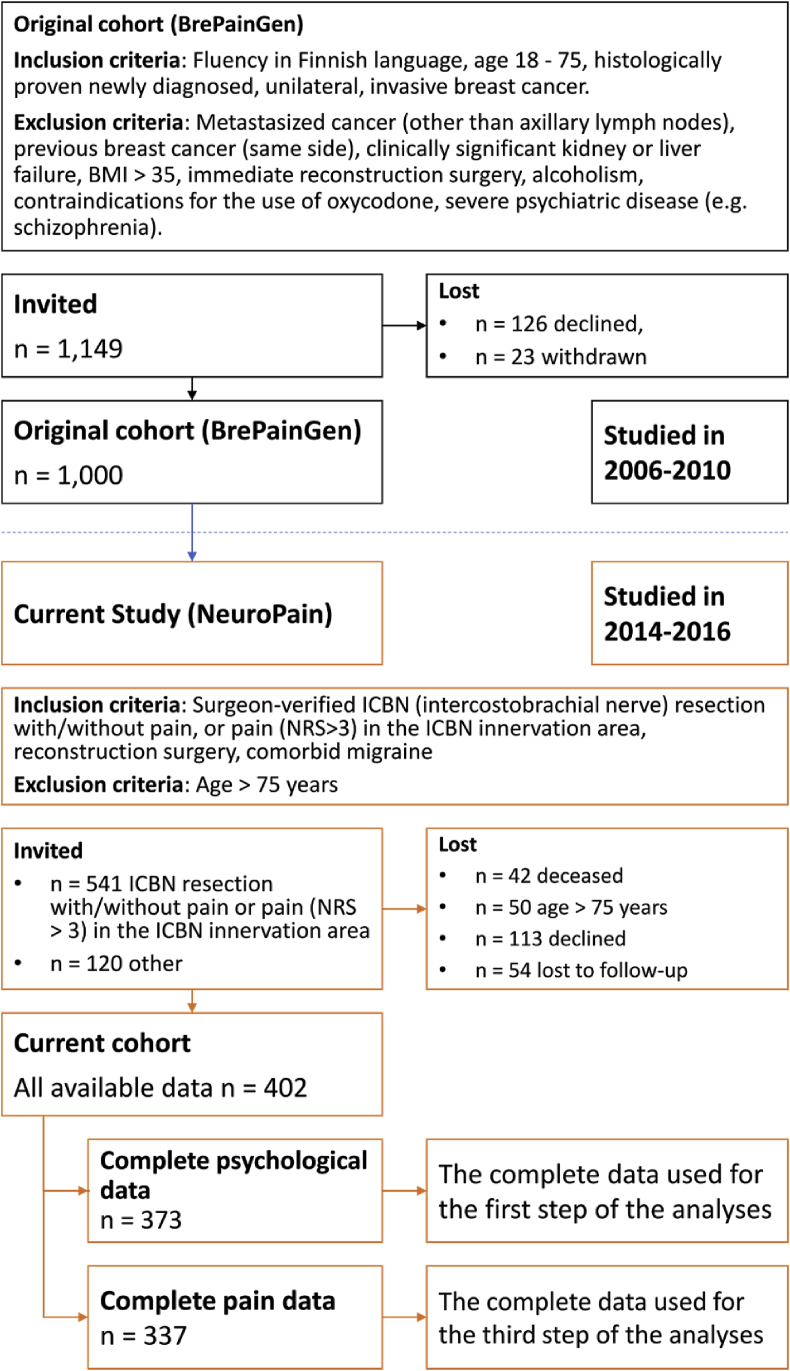
The patient sample consisted of a subgroup of 402 women (“NeuroPain” study, Fig. 1) from a cohort of 1,000 women (the original “BrePainGen” study) (18–75 years) operated for breast cancer at the Helsinki University Hospital (during years 2006–2010). The present cross-sectional data were collected 4–9 years after the patients had undergone the index surgery. The patients answered the questionnaires during the research visit. The study protocols have been described earlier in detail [17,18]. Information about patient recruitment is provided in Fig. 1. The study was approved by the Coordinating Ethics Board of the Helsinki and Uusimaa Hospital District and it was registered in ClinicalTrials.gov (NCT02487524). All patients provided an informed written consent.
Fig. 1.
Flow-chart of the patient selection from the original 1000 patient cohortAbbreviations: ICBN: InterCostoBrachial Nerve, NRS: Numerical Rating Scale.
2.2. Acquisition of psychological questionnaires
To evaluate the intensity and the interference of pain during the past week, both in the surgical area and other parts of the body, the Brief Pain Inventory (BPI) was used in its long form [19]. The pain intensity variable was formed by calculating the mean of “the worst pain”, “the mildest pain”, “the average pain”, and “pain at the moment” items. The pain interference items of the questionnaire asked the patients to evaluate the overall pain interference and in addition, interference with function, mood, walking, working, relationships, sleeping, and life-satisfaction. Patients completed the BPI separately for any pain and for the pain in the operated area on a Numerical Rating Scale from 0 (not at all) to 10 (the worst possible pain intensity/very much pain interference).
To assess mood, the Hospital Anxiety and Depression Scale (HADS) [20] was used. A sum score of all items (0–21) was calculated. Higher scores indicate higher amounts of mood symptoms. Pain-related catastrophising was assessed using the Pain Catastrophising Scale (PCS) sum score (0–52). A higher value indicates a higher tendency for catastrophic thinking [21]. Symptoms of insomnia were queried using the Insomnia Severity Index (ISI) [22] (scores from 0 to 28). Again, higher scores indicate more severe symptoms. Personality traits were assessed using the Ten Point Personality Inventory (TIPI) [23]. The “Big Five” framework is a hierarchical model of personality traits suggesting that most individual differences in personality can be classified into five domains, i.e., agreeableness, emotional stability, extraversion, openness, and conscientiousness. Scores of the TIPI vary from 0 to 7, with higher scores indicating a stronger profile for the factor in question. Finally, the Temperament and Character Inventory (TCI) [24] was used to measure temperament: Harm avoidance (HA), novelty seeking (NS), reward dependence (RD), and persistence (P) and character: self-directedness (SD), cooperativeness (CO), and self-transcendence (ST)) traits.
2.3. Data analysis
The data analysis was performed using the R software package version 3.5.1 for Linux (http://CRAN.R-project.org/ [25]) on an Intel Core i7® - 7500U notebook computer running on Ubuntu Linux 18.04.1 64-bit. The data set comprised d = 17 psychological and sleep-related parameters (insomnia severity, pain catastrophising, resilience, temperament traits NS, HA, RD, P (see above) and characters SD, CO, and ST, personality traits extraversion, agreeableness, conscientiousness, emotional stability, openness, anxiety, and depression, d = 24 parameters related to pain and its interference with the patient’s life (separately for pain in the treated area and other pains: worst pain intensity, mildest pain, average pain, pain now, pain interference with function, mood, walking, working, to relationships, sleep, and life-satisfaction), which were acquired completely from up to n = 373 patients (Fig. 1).
The data were analysed using machine learning methods, which are increasingly proving to be suitable for knowledge discovery in pain-related data. A summary on the application of this family of methods has recently been presented [16]. The analysis of the present data set was performed in three main steps, comprising (i) the detection of subgroups based on the pattern of psychological or sleep-related parameters, (ii) the psychological interpretation of the detected cluster structure and (iii) the interpretation of the cluster structure from a pain interference and impact perspective. For the first step, unsupervised methods were used to detect subgroups in the data without imposing any pre-classification.
Among the various methods, an algorithm based on artificial intelligence was preferred to classical clustering based on earlier observations [26] that such algorithms occasionally fail to recognize the correct cluster structure. I.e. by imposing a cluster shape instead of identifying true structures in the data, faulty clusters or false cluster associations of individual cases may result. Therefore, emergent self-organizing feature maps (ESOM) were used as an unbiased alternative method for identifying true clusters in high-dimensional data space [[26], [27], [28]], where the imposition of clusters is overcome by addressing the structures without assuming a specific cluster shape. For the second and third steps, supervised methods were applied with the idea of training an artificial intelligence with a randomly drawn fraction of the data in such a way that it is able to assign a patient to the correct cluster in the remaining data not available to the AI during training. In order to accommodate the concept of explainable AI (XAI) [29], an algorithm type was chosen that was designed to create simple tree-based rules that make class assignment transparent [30]. The three main steps of data analysis are described in detail below.
2.3.1. Subgroups detection based on the pattern of psychological or sleep-related parameters
In the first step of the data analysis, for psychological subgroup detection, d = 17 psychological and sleep-related parameters, subsequently also called “features”, were available. For the subsequent projection and clustering as measure of “similarity”, the Euclidian distance was used. Each person’s rating to the pain stimuli (d = 17 dimensions) was treated as a point in a high-dimensional Euclidean vector space (data space). Subgroups of patients were identified using unsupervised machine learning, which was implemented as a self-organizing map of artificial neurons [31] arranged in 50 rows and 80 columns (n = 4,000 units; for the sizing of SOM see Ref. [26]). The high-dimensional data points were projected onto a two-dimensional grid consisting of a network of artificial neurons. Specifically, the map space used was toroidal [32] and thus borderless, i.e. opposite edges are connected to each other. The projection is neighborhood-preserving [33], i.e. points that are adjacent in the high-dimensional data space are also neighbors in the map space. In addition to the input vector from 17-dimensional space, each neuron contains another vector that carries “weights” with the same dimensions as the 17 input dimensions. The weights were initially drawn randomly from the range of data variables. Then they were adapted to the data (learning phase).
Following training of the neural network, on the top of the SOM grid, the distances between data points were added as a third dimension in the so-called U-matrix [26]. Using a topographical map analogy for colouring, large distances in the high-dimensional space were visualised as white “heights” that resemble snow-covered mountain ranges. They separated green “valleys” and blue “lakes”, where data at small distances in the high-dimensional space were projected. This finally provided a cluster structure [26]. These calculations were performed using our R package “U-matrix” (https://cran.r-project.org/package=Umatrix [34]). As shown previously [26], common clustering algorithms such as k-means, Ward, complete- and average linkage are prone to detect false structures in the data. This was the reason to use the present emergent SOM based method as it has been shown to outperform the mentioned classical clustering algorithms on biomedical and artificial data sets [26]. Following subgroup detection, which resulted in two clusters group differences in all parameters that had been used for subgroup detection in the data space, were statistically analysed by means of Wilcoxon-Mann-Whitney-U tests for group differences were calculated [35,36]. The α level was set at p = 0.05 and the results were interpreted considering the a correction proposed by Bonferroni [37].
2.3.2. Psychological interpretation of the detected cluster structure
In the second step of the data analysis, the psychological meaning of the detected groups was assessed using supervised machine learning. Specifically, to obtain a comprehendible and clinically applicable explanation of the groups, simple decision trees were used implemented as “Fast and Frugal Trees” (FFTs [30]). FFTs [30,38] provide simple decisions trees, usually composed of 1–5 pieces of information. This makes them particularly suitable for biomedical problems as they mimic the processes of making a clinical diagnosis [39]. Rules were first learned on 1,000 data sets obtained by Monte-Carlo random resampling of two thirds of the original data. How many and which psychological parameters were used in each of the 1,000 rule sets was retained. A final feature set was obtained at the most frequent size of the psychological parameters found in the 1,000 rule sets, among the d = 17 psychological and sleep-related candidate features. The relevant features were chosen in decreasing order of the frequency in which the parameters had been used in the rules during the 1,000 runs. Subsequently, the degree at which the features were able to explain this data structure was assessed by training the FFT with 2/3 of the original data and assessing the trees’ performance, quantified as the balanced accuracy, in the remaining 1/3 of the data set. Several further standard measures of classification performance were also calculated (Table 1). The performance in this task was assessed in 1,000 runs using disjoint 2/3 training and 1/3 test data sets resampled without replacement from the original data. To further address potential overfitting, the performance of the decision trees was assessed using negative control data sets, created by random permutation of the psychological parameters across subjects. The calculations were performed using the R package “FFTrees” (https://cran.r-project.org/package=FFTrees [40]).
Table 1.
Performance measures for the correct assignment of subjects to the groups, based on (i) U-matrix clustering or (ii) on the membership to the clinical “low pain intensity but high life activity interference” group, versus the other subjects, i.e., in both cases a two-group assignment task. For group assignment, decision trees built on psychological parameters and implemented as comprehendible Fast and Frugal Trees were used. The performance was measured (i) using the original data, (ii) data sets constructed to provide a negative control by permuting the original psychological parameters. Results represent the medians (and interquartile ranges in parentheses) of the test performance measures from 1,000 model runs using Bootstrap resampling. The parameters correspond to the performance marker set implemented in the R library “caret” (https://cran.r-project.org/package=caret [58]).
| Performance parameter [%] | Psychological parameters |
Pain and interference related parameters |
||
|---|---|---|---|---|
| Original data | Permuted data | Original data | Permuted data | |
| Sensitivity, recall | 92.9 (85.7–100) | 21.4 (0–92.8) | 58.3 (33.3–66.7) | 41.7 (25–66.7) |
| Specificity | 87.2 (83.5–94.5) | 59.6 (41.3–73.4) | 63.6 (54.5–82.8) | 61.6 (37.4–74.7) |
| Positive predictive value, precision | 48.3 (42.4–64.8) | 6.3 (0–20.4) | 16.7 (14.3–20) | 11.5 (6.7–16.3) |
| Negative predictive value | 99 (98.1–100) | 87.7 (81.3–98.9) | 92.4 (90.9–93.9) | 89.5 85.2–92.4) |
| F1 | 63.6 (58.7–71.4) | 29.5 (13.7–40) | 25.5 (21.6–28.6) | 18.5 (11.8–25) |
| Balanced Accuracy | 90.1 (87.1–92.2) | 46.8 (29.4–72.5) | 60.7 56.1–64.3) | 51.3 (41.3–59.2) |
2.3.3. Interpretation of the cluster structure from a pain interference and impact perspective
In the third step of the data analysis, the cluster (subgroup) structure was interpreted in terms of pain and its interference with the patients’ lives. The aim of this step was to find pain-related variables that best explain the identified subgroups. This was done in a similar way to the second step of the analysis, but the focus was shifted from psychological parameters to pain-related parameters. This comprised the set of d = 24 features related to pain intensity and interference (all items from the BPI). Again, parameters most relevant for the psychologically based subgroup structure among patients were selected using FFT followed by computed ABC analysis. Subsequently, the performance of the selected parameters for the assignment of a patient to the correct psychological cluster was assessed by calculating the balanced classification accuracy and other performance measures in 1,000 runs on disjoint training (2/3) and test (1/3) data sets obtained by Monte-Carlo resampling from the original data set.
3. Results
3.1. Participants and descriptive data
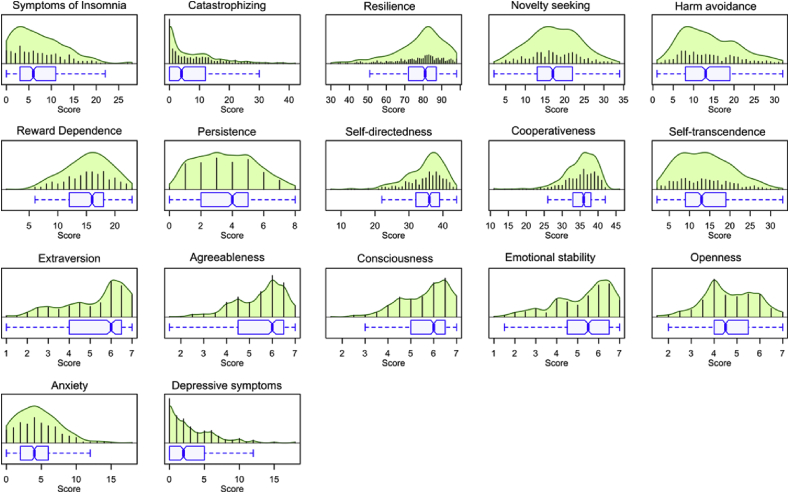
Acquisition of psychological and sleep-related parameters was complete in 373 patients (Fig. 2). Acquisition of parameters related to pain intensity and its interference was completed in 337 (83.8%) patients.
Fig. 2.
Distribution of psychological and activity parameters. The beanplots show the individual observations as small blue lines in a one-dimensional scatter plot. The probability density functions (pdf) of the distributions are shown as green areas. Box and whisker plots of the same data are drawn below the beanplots. They have been constructed using the minimum, quartiles, median (solid black red line within the box), and maximum. The whiskers add 1.5 times the interquartile range (IQR) to the 75th percentile or subtract 1.5 times the IQR from the 25th percentile and are expected to include 99.3% of the data if normally distributed. The notches indicate the confidence interval around the median based on . The figure has been created using library “beanplot” (https://cran.r-project.org/package=beanplot [59]) for the R software package (version 3.5.1 for Linux; http://CRAN.R-project.org/ [25]). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2. Main results
3.2.1. Subgroups of patients based on the pattern of psychological or sleep-related parameters
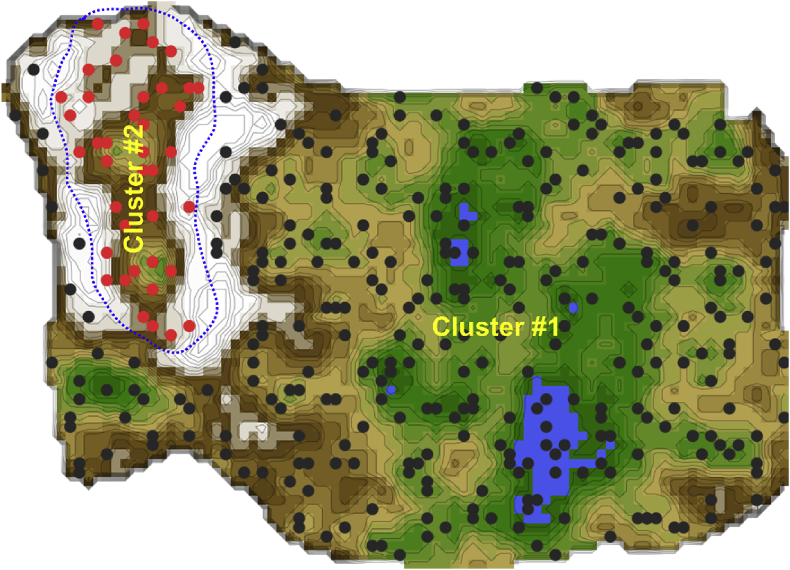
Unsupervised machine learning provided an emergent self-organizing feature map on which a clear two-cluster structure in the data set was seen. That is, the U-matrix visualization resulted in a distinct cluster comprising n = 43 (11.5%) patients, which on the topographic map-analogy was visually separated from the other patients by a “snow-covered mountain ridge” (Fig. 3). Specifically, an 11 %-sized subgroup of data (marked with red dots in the figure) is surrounded on the U-matrix by large high walls indicated with a white color, which corresponds to large distances in the data. This separates this group clearly from the rest of the cohort, marked with black dots in the figure. To show this separation, the cutting line of the U-matrix island was placed in a way that emphasises group separation. This is possible due to the cyclic (toroid) nature of the tiled U-matrix [41]. The red-marked group in itself, however, might contain further subgroups. However, with the small number of data further subgrouping was not pursued and remains subject to further research. For the given data, one can assert that the red and black marked groups are distinct and that the inner variance of the red group is larger than within the black group.
Fig. 3.
Clustering of subjects based on the pattern of psychological and activity related parameters, obtained using unsupervised machine learning implemented as an artificial neuronal network of self-organizing maps. U-matrix visualization of distance-based structures of the serum concentration of d = 17 parameters observed in n = 373 patients. The figure has been obtained using a projection of the data points onto a toroid grid of 4,000 neurons where opposite edges are connected. The dots represent the so-called “best matching units” (BMU), i.e., neurons on the grid that after ESOM learning carried the vector that was most similar to a subjects’ data vector. The U-matrix visualization was coloured as a top view of a topographic map with brown (up to snow-covered) “heights” and green “valleys” with blue “lakes”. Watersheds indicate borderlines between different clusters. Two clusters emerged in this way, separated by the white “mountain ridge” at the left of the U-matrix. BMUs belonging to clusters #1 or #2 are coloured in black or red, respectively. The red-marked group in itself, however, might contain further subgroups. However, with the small number of data, further subgrouping was not pursued and remains subject to further research. For the given data, one can assert that the red and black marked groups are distinct and that the inner variance of the red group is larger than within the black group. The figure has been created using the R software package (version 3.5.1 for Linux; http://CRAN.R-project.org/ [25]). Specifically, the U-matrix was calculated and visualised using our R package “Umatrix” (https://cran.r-project.org/package=Umatrix [34]). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2.2. Psychological interpretation of the detected cluster structure
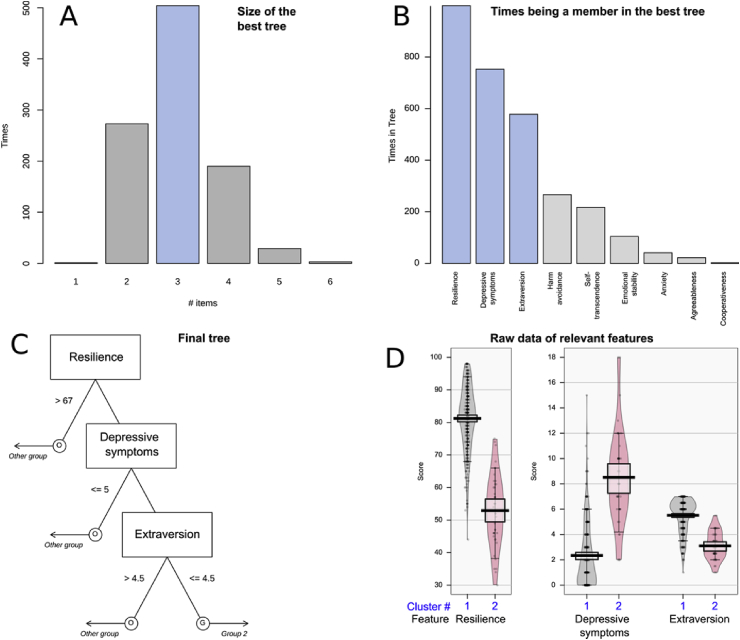
Feature selection implemented as fast and frugal tree (FFT) analysis combined with computed ABC analysis, identified d = 3 parameters to be relevant for patient group segregation, among the d = 17 candidate parameters (Fig. 4). Specifically, the features that were most frequently part of the best performing tree, during the 1,000 runs on randomly drawn 2/3-data subsets, comprised resilience, depressive symptoms and extraversion. Members of the rarer psychological phenotype in cluster #2 had lower resilience, more depressive symptoms and lower extraversion, all with significant differences: resilience: Wilcoxon W = 13,741, p < 2.2 · 10−16, depressive symptoms: W = 1087, p < 2.2 · 10−16, extraversion: W = 12,810, p-value < 2.2 · 10−16.
Fig. 4.
Selection of psychological and sleep-related features (parameters) that provide relevant information for the membership of a patient in the U-matrix based cluster #2 (see Fig. 3). Relevant parameters were identified using the Fast and Frugal decision Tree (FFT [30]) algorithm. Decision tree building was performed using 1,000 iterations with randomly resampled disjoint training and test data sets. A: Bar graph of the size of the best performing trees during the 1,000 runs of tree building. B: Bar graph displaying how many times the features were included in the best performing trees during the 1,000 runs of tree building on randomly resampled disjoint training and test data. C: The FFT based decision tree was built on the parameters resilience, depressive symptoms and extraversion. The figure shows the trees along with the decision limits as the basis for the assignment to either the U-matrix based cluster #2 (named “Group 2” in the tree) or to the other subjects, i.e., U-matrix based cluster # (named “Other group in the tree). D: Beanplots of the parameters algorithmically selected for the decision tree. Data are shown separately for U-matrix based cluster #1 (grey) or #2 (red). The individual observations are shown as black circles in a one-dimensional scatter plot, surrounded by the probability density function (pdf) of the distributions (coloured areas). Box and whisker plots of the same data are overlaid on the beanplots. They have been constructed using the minimum, quartiles, median (solid black red line within the box), and maximum. The whiskers add 1.5 times the interquartile range (IQR) to the 75th percentile or subtract 1.5 times the IQR from the 25th percentile. The figure has been created using the R software package (version 3.5.1 for Linux; http://CRAN.R-project.org/ [25]), the R package “FFTrees” (https://cran.r-project.org/package=FFTrees [40]), and the R package “yarr” (https://cran.r-project.org/package=yarrr [60]). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In the subsequent classification performance assessments performed in 1,000 runs on disjoint 2/3 training and 1/3 test data randomly drawn from the original data set, tree-based rules assigned membership to cluster #2 at a balanced accuracy of 90.1% (Table 1). By contrast, when using permuted information for training, the group assignment was obtained at approximately 50% balanced accuracy, as expected from the negative control scenario.
3.2.3. Interpretation of the cluster structure from a pain interference and impact perspective
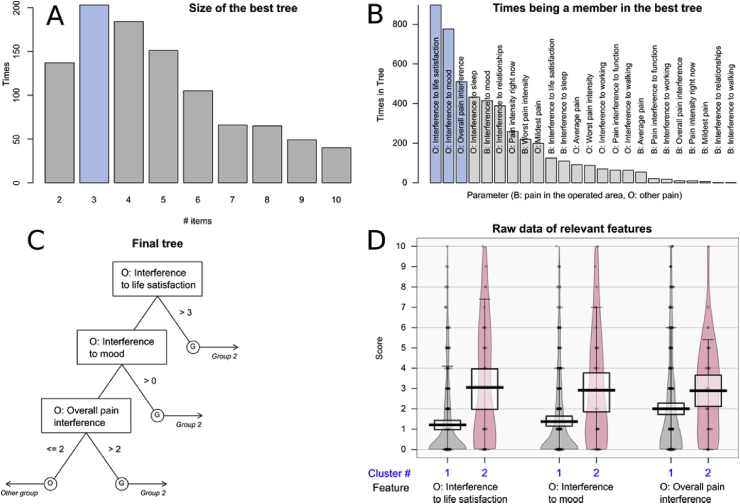
When using the parameters related to pain intensity and its interference for the interpretation of the psychologically-based patient clusters, feature selection implemented as FFT combined with computed ABC analysis identified d = 3 parameters as being relevant, among the d = 24 candidate parameters (Fig. 5). Specifically, the parameters pain interference with life-satisfaction, mood and broadly with life were most frequently part of the best performing tree. Importantly, the selected items referred to other pains, not to the breast cancer surgery-related pain. The impact of pain on these parameters was significantly higher in the subjects belonging to the rare psychological phenotype of cluster #2 (life satisfaction: Wilcoxon tests: W = 3448, p = 3.225 · 10−5, mood: W = 3531.5, p = 0.0001053 and interference: W = 4035, p = 0.005202) (see Fig. 5).
Fig. 5.
Selection of pain intensity and interference related features (parameters) that provide relevant information for the membership of a patient in the U-matrix based cluster #2 (see Fig. 3). Relevant parameters were identified using the Fast and Frugal decision Tree (FFT [30]) algorithm. Decision tree building was performed using 1,000 iterations with randomly resampled disjoint training and test data sets. A: Bar graph of the size of the best performing trees during the 1,000 runs of tree building. B: Bar graph displaying how many times the features were included in the best performing trees during the 1,000 runs of tree building on randomly resampled disjoint training and test data. B: Variables referring to pain in the operated area, O: variables referring to other pains, i.e., without direct relation to the operated area. C: The FFT based decision tree was built on the parameters resilience, depressive symptoms and extraversion. The figure shows the trees along with the decision limits as the basis for the assignment to either the U-matrix based cluster #2 (named “Group 2” in the tree) or to the other subjects, i.e., U-matrix based cluster # (named “Other group in the tree). D: Beanplots of the parameters algorithmically selected for the decision tree. Data are shown separately for U-matrix based cluster #1 (grey) or #2 (red). The individual observations are shown as black circles in a one-dimensional scatter plot, surrounded by the probability density function (pdf) of the distributions (coloured areas). Box and whisker plots of the same data are overlaid on the beanplots. They have been constructed using the minimum, quartiles, median (solid black red line within the box), and maximum. The whiskers add 1.5 times the interquartile range (IQR) to the 75th percentile or subtract 1.5 times the IQR from the 25th percentile. The figure has been created using the R software package (version 3.5.1 for Linux; http://CRAN.R-project.org/ [25]), the R package “FFTrees” (https://cran.r-project.org/package=FFTrees [40]), and the R package “yarr” (https://cran.r-project.org/package=yarrr [60]). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In the subsequent classification performance assessments performed in 1,000 runs on disjoint 2/3 training and 1/3 test data randomly drawn from the original data set, the tree-based rules assigned membership to cluster #2 at a balanced accuracy of 81.2% (Table 1), whereas only approximately 50% were reached with permuted data.
4. Discussion
Patients with pain after breast cancer treatments form a psychologically heterogeneous cohort. Psychological and sleep-related parameters provided a data pattern in which breast cancer survivors segregated into two major subgroups. Patients who formed a small distinct group (cluster #2) from the others had lower resilience, more depressive symptoms and lower extraversion. In these patients, life-satisfaction, mood, and life in general were comparatively more impeded by persistent pain than in the others. This result supports that based on psychological and sleep-related parameter patterns, breast cancer survivors are differently impacted in their lives by persistent pain. This result supports that based on psychological and sleep-related parameter patterns, persistent pain impacts differently the quality of life of breast cancer survivors. In the present cohort, particular severe life interference by pain addressed 11.5% of the patients.
Thus, a first result of the present analysis was the recognition of a pattern in the psychological parameters that clearly separates a subgroup of patients. The three identified variables most relevant for cluster segregation, i.e., resilience, depressive symptoms and extraversion, represent common challenges in (pain) patients, but they may be particularly prominent in cancer survivors. The balance between the identified vulnerability and protective factors defines how a person adopts with her life with pain after breast cancer. Resilience is likely to enable the patients to use better or more effective coping strategies and to better adjust to pain and life after a serious diagnosis [42]. Resilient individuals have been shown to have more positive attributions about live adversities, for example, how challenging or life-restricting pain becomes [8]. Resilience as a protective factor helps to maintain higher quality of life despite adversities. On the other hand, extraversion is a factor related to how well a person works with others or how well social support is received [43]. It may therefore reflect an ability to receive and seek social support after breast cancer. Social support has been associated with better functioning, e.g., in patients with rheumatoid arthritis [44,45]. Similarly, in patients with neurological disorders and impaired motor function, extraversion was an independent predictor of resilience [46]. Hence, a combination of these two traits is likely to reflect a positive affect that contributes to better coping with cancer and pain. Also, it may be hypothesised that less extraverted individuals focus more on their inner experiences and pay more attention to bodily sensations whereas more extraverted individuals focus their attention more outwards. The negative effect of depressive symptoms in coping, life-satisfaction, and social engagement is well established [4,47].
As the identified psychological pattern was highly plausible, based on what is known about the role of psychological factors in coping with cancer and its treatment, and also with pain, the differences between the psychology-based groups were explored for parameters related to pain intensity and interference. Of these, items related to pain interference were found to be more relevant than pain intensity. In the smaller cluster #2, patients reporting higher overall pain interference, and interference with life-satisfaction and mood were overrepresented. In addition, “pain elsewhere” was evaluated as more interfering than pain in the surgical area. A common finding in similar studies is that previous pains are relevant for the experience of a new pain [48], which was the case also in the present patient cohort [18]. It may be hypothesised that the overall pain load is relevant regarding how interfering the patient experiences her pain.
Resilience was the most important factor in the smaller cluster. The results encourage us to concentrate more on the protective factors in breast cancer treated women having pain. For example, acceptance-based therapeutic interventions (ACT) focus particularly on the strengths and finding ways to live a meaningful life despite the pain [49]. There is some evidence to suggest that ACT interventions may improve symptoms including distress, traumatic responses, and pain in cancer patients [50]. These results complement previous knowledge that pain experience and coping with pain are an interplay between protective (e.g. resilience) and vulnerability (e.g. mood) factors [5]. The level of pain intensity is not informative enough to explain this complex and interactive process. This is crucial when planning specific interventions to enhance an individual’s well-being after a serious disease, like cancer [5]. Finally, a role of sleep in pain experience has become obvious in the recent years [13]. In the present analysis, interference with sleep was narrowly rejected by the feature selection procedure. It was the next feature in line after the most relevant features had been identified (Fig. 4). This, however, also implies that its inclusion in the final set feature did not improve the classification performance of the trees further. Nevertheless, the results do not contradict previous findings that have attributed sleep an important role in the impact of persistent pain on the patients’ quality of life.
The present classifier was purposely chosen to be symbolic [51], i.e., a classifier in which the decision how a classification is obtained can be interpreted by a domain expert as a combination of conditions on the features. This is in line with current attempts to make machine-learned algorithms explainable [52]. Typical implementations are hierarchical rules assembled in decision trees, as presently used in the form of so-called fast and frugal trees (FFTs). The latter have the additional simplifying property that each feature is used only for only one decision in the tree. This further enhances the human comprehendibility. Indeed, its makes FFTs particularly suitable for biomedical problems as they mimic the processes of making a clinical diagnosis [39]; however, they also perform well with artificial problems of decision making [53]. FFTs performed well for providing an interpretation of the present smaller cluster, based on the psychological parameters in which the cluster structure had been detected (balanced assignment accuracy of 90.1%). However, their only modest performance in providing an interpretation based on the pain parameters (balanced accuracy 60.7%) raises the question whether alternative classifiers would have performed better. In the present data, this was therefore tested using random forests [54,55], which is a standard classifier that uses hundreds of simple trees, and the class association is made by a majority vote of the single-tree based decisions; however, this did not improve the classification performance (details not shown).
Many psychological variables reported to be associated with pain, such as pain catastrophizing and anxiety, have not been identified in the present analysis as primary descriptors of the cluster or subgroup structure of pain patients. The subgroup detection was carried out using an unsupervised analysis, i.e. without prior assumptions about a possible group structure in the patients. The explanation by means of a supervised algorithm, which was optimized to generate simple, comprehensible rules in the form of a decision tree, focused on most important variables for the detected subgroup structure in this particular patient group. The variables found to provide rules for the interpretation of the cluster structure reflect not only pain-related coping, but also factors that explain better coping in general among breast cancer survivors. In contrast, in patients with active disease, pain catastrophizing has been associated with the pain interference [56]. Therefore, the results cannot be generalised to other patient groups. Another limitation of this study is its cross-sectional design and the rather small group size; in fact, the relevant cluster was only a small fraction of 11.5% of the subjects, which reduced the absolute number of patients from an originally quite large cohort to only n = 43 belonging to the most important subgroup. Nevertheless, the results seem to be clinically relevant and underline that unsupervised and supervised machine learning procedures are well suited for this type of study questions.
5. Conclusions
A cluster structure emerged in the patterns of psychological parameters acquired in patients treated for breast cancer. Patients with low resilience, depressive symptoms and low extraversion formed a separate group in which pain had a significantly more pronounced impact on life-satisfaction, mood, and life in general. Parameters related to the impact of pain were found to be more relevant for this patient subgroup than parameters related to the intensity of pain. This emphasises recent developments in pain research and multimodal pain management that support approaches where the most important parameter that should be recognised or targeted by pain treatments, may not be pain intensity but how interfering the patient experiences her pain. Based on the present observations, a more versatile set of protective factors should be added to the included set of psychological factors, e.g. psychological flexibility and optimism could provide more information about the factors behind a favourable management of breast cancer patients with pain [5]. Finally, the present analysis supports the use of artificial intelligence as an efficient tool in the identification of subgroups of patients, who will require particular attention in pain therapy after cancer treatments. This approach could also be used to create new hypotheses, and to find target variables e.g. for interventional single-case studies [57].
Funding
The work has been supported by the European Union Seventh Framework Programme (FP7/2007–2013) under grant agreement no. 602919 (GLORIA, EK, JL) and no. 602891 (NeuroPain, EK, RS). Additional support was received by the Landesoffensive zur Entwicklung wissenschaftlich-ökonomischer Exzellenz (LOEWE), Zentrum: Translational Medicine and Pharmacology (JL). The funders had no role in method design, data selection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
Conceived and designed the experiments: EK, RS. Conceived and designed the analysis: JL, RS, EK. Analysed the data: JL. Wrote the paper: JL, RS, EK. Provided data: RS, EK. Critical revision of the manuscript for important intellectual content: EK, RS.
Declaration of competing interest
The authors have declared that no competing interests exist.
References
- 1.Meretoja T.J., Leidenius M.H.K., Tasmuth T., Sipila R., Kalso E. Pain at 12 months after surgery for breast cancer. J Am Med Assoc. 2014;311:90–92. doi: 10.1001/jama.2013.278795. [DOI] [PubMed] [Google Scholar]
- 2.Mejdahl M.K., Andersen K.G., Gartner R., Kroman N., Kehlet H. Persistent pain and sensory disturbances after treatment for breast cancer: six year nationwide follow-up study. BMJ. 2013;346:f1865. doi: 10.1136/bmj.f1865. [DOI] [PubMed] [Google Scholar]
- 3.Breivik H., Collett B., Ventafridda V., Cohen R., Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Edwards R.R., Dworkin R.H., Sullivan M.D., Turk D.C., Wasan A.D. The role of psychosocial processes in the development and maintenance of chronic pain. J Pain. 2016;17:T70–T92. doi: 10.1016/j.jpain.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sturgeon J.A., Zautra A.J. Psychological resilience, pain catastrophizing, and positive emotions: perspectives on comprehensive modeling of individual pain adaptation. Curr Pain Headache Rep. 2013;17:317. doi: 10.1007/s11916-012-0317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vlaeyen J.W., Linton S.J. Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain. 2012;153:1144–1147. doi: 10.1016/j.pain.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Vlaeyen J.W., Crombez G., Linton S.J. The fear-avoidance model of pain. Pain. 2016;157:1588–1589. doi: 10.1097/j.pain.0000000000000574. [DOI] [PubMed] [Google Scholar]
- 8.Goubert L., Trompetter H. Towards a science and practice of resilience in the face of pain. Eur J Pain. 2017;21:1301–1315. doi: 10.1002/ejp.1062. [DOI] [PubMed] [Google Scholar]
- 9.Luthar S.S., Cicchetti D., Becker B. The construct of resilience: a critical evaluation and guidelines for future work. Child Dev. 2000;71:543–562. doi: 10.1111/1467-8624.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauer H., Emeny R.T., Baumert J., Ladwig K.H. Resilience moderates the association between chronic pain and depressive symptoms in the elderly. Eur J Pain. 2016;20:1253–1265. doi: 10.1002/ejp.850. [DOI] [PubMed] [Google Scholar]
- 11.Edwards K.A., Alschuler K.A., Ehde D.M., Battalio S.L., Jensen M.P. Changes in resilience predict function in adults with physical disabilities: a longitudinal study. Arch Phys Med Rehabil. 2017;98:329–336. doi: 10.1016/j.apmr.2016.09.123. [DOI] [PubMed] [Google Scholar]
- 12.Asghari A., Nicholas M.K. Personality and pain-related beliefs/coping strategies: a prospective study. Clin J Pain. 2006;22:10–18. doi: 10.1097/01.ajp.0000146218.31780.0b. [DOI] [PubMed] [Google Scholar]
- 13.Finan P.H., Goodin B.R., Smith M.T. The association of sleep and pain: an update and a path forward. J Pain. 2013;14:1539–1552. doi: 10.1016/j.jpain.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strachan E., Poeschla B., Dansie E., Succop A., Chopko L., Afari N. Clinical and evoked pain, personality traits, and emotional states: can familial confounding explain the associations? J Psychosom Res. 2015;78:58–63. doi: 10.1016/j.jpsychores.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wickham H., Grolemund G. O’Reilly Media; 2017. R for data science: import, tidy, transform, visualize, and model data. [Google Scholar]
- 16.Lotsch J., Ultsch A. Machine learning in pain research. Pain. 2017;159:623–630. doi: 10.1097/j.pain.0000000000001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaunisto M.A., Jokela R., Tallgren M., Kambur O., Tikkanen E., Tasmuth T. Pain in 1,000 women treated for breast cancer: a prospective study of pain sensitivity and postoperative pain. Anesthesiology. 2013;119:1410–1421. doi: 10.1097/ALN.0000000000000012. [DOI] [PubMed] [Google Scholar]
- 18.Mustonen L., Aho T., Harno H., Sipilä R., Meretoja T., Kalso E. 2018. What makes surgical nerve injury painful? A 4-9 year follow-up of patients with inter-costobrachial nerve resection in women treated for breast cancer. Pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cleeland C.S., Ryan K.M. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 20.Bjelland I., Dahl A.A., Haug T.T., Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan M.J.L., Bishop S.R., Pivik J. The pain catastrophizing Scale: development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- 22.Morin C.M., Belleville G., Belanger L., Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gosling S.D., Rentfrow P.J., Swann W.B. A very brief measure of the Big-Five personality domains. J Res Pers. 2003;37:504–528. [Google Scholar]
- 24.Cloninger C.R., Svrakic D.M., Przybeck T.R. A psychobiological model of temperament and character. Arch Gen Psychiatr. 1993;50:975–990. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- 25.R Development Core Team . 2008. R: a language and environment for statistical computing. [Google Scholar]
- 26.Ultsch A., Lötsch J. Machine-learned cluster identification in high-dimensional data. J Biomed Inf. 2017;66:95–104. doi: 10.1016/j.jbi.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rimet F., Druart J.-C., Anneville O. Exploring the dynamics of plankton diatom communities in Lake Geneva using emergent self-organizing maps (1974–2007) Ecol Inf. 2009;4:99–110. [Google Scholar]
- 28.Ultsch A., Kämpf D. Proceedings of the European Symposium on Artificial Neural Networks (ESANN 2004) 2004. Knowledge discovery in DNA microarray data of cancer patients with emergent self organizing maps; pp. 501–506. [Google Scholar]
- 29.Arrieta A.B., Díaz-Rodríguez N., Ser J.D., Bennetot A., Tabik S., Barbado A. 2019. Explainable artificial intelligence (XAI): Concepts, Taxonomies, Opportunities and challenges toward responsible AI. [Google Scholar]
- 30.Gigerenzer G., Todd P.M. Oxford University Press; New York, NY, US: 1999. Fast and frugal heuristics: the adaptive toolbox. Simple heuristics that make us smart; pp. 3–34. [Google Scholar]
- 31.Kohonen T. Self-organized formation of topologically correct feature maps. Biol Cybern. 1982;43:59–69. [Google Scholar]
- 32.Ultsch A. Maps for visualization of high-dimensional data spaces. WSOM. 2003:225–230. [Google Scholar]
- 33.Heskes T. Energy functions for self-organizing maps. In: Oja E., Kaski S., editors. Kohonen Maps. Elsevier; Amsterdam: 1999. [Google Scholar]
- 34.Lötsch J., Lerch F., Djaldetti R., Tegeder I., Ultsch A. Identification of disease-distinct complex biomarker patterns by means of unsupervised machine-learning using an interactive R toolbox (Umatrix) BMC Big Data Analytics. 2018;3 doi: 10.1186/s41044-018-0032-1. [DOI] [Google Scholar]
- 35.Wilcoxon F. Individual comparisons by ranking methods. Biometrics. 1945;1:80–83. [Google Scholar]
- 36.Mann H.B., Whitney D.R. On a test of whether one of two random variables is stochastically larger than the other. Ann Math Stat. 1947;18:50–60. [Google Scholar]
- 37.Bonferroni C.E. Pubblicazioni del R Istituto Superiore di Scienze Economiche e Commerciali di Firenze. vol. 8. 1936. Teoria statistica delle classi e calcolo delle probabilita; pp. 3–62. [Google Scholar]
- 38.Martignon L., Katsikopoulos K.V., Woike J.K. Categorization with limited resources: a family of simple heuristics. J Math Psychol. 2008;52:352–361. [Google Scholar]
- 39.Marewski J.N., Gigerenzer G. Heuristic decision making in medicine. Dialogues Clin Neurosci. 2012;14:77–89. doi: 10.31887/DCNS.2012.14.1/jmarewski. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phillips N., Neth H., Woike J., Gaissmaer W. 2018. FFTrees: generate, Visualise, and evaluate fast-and-frugal decision trees. R package version 1.4.0. [Google Scholar]
- 41.Ultsch A. The U-Matrix as Visualization for Projections of high-dimensional data. In: Locarek-Junge H, editor. Proc 11th IFCS Biennial Conference2003.
- 42.Wright L.J., Zautra A.J., Going S. Adaptation to early knee osteoarthritis: the role of risk, resilience, and disease severity on pain and physical functioning. Ann Behav Med. 2008;36:70–80. doi: 10.1007/s12160-008-9048-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirsh J.B., Deyoung C.G., Peterson J.B. Metatraits of the Big Five differentially predict engagement and restraint of behavior. J Pers. 2009;77:1085–1102. doi: 10.1111/j.1467-6494.2009.00575.x. [DOI] [PubMed] [Google Scholar]
- 44.Evers A.W., Kraaimaat F.W., Geenen R., Jacobs J.W., Bijlsma J.W. Pain coping and social support as predictors of long-term functional disability and pain in early rheumatoid arthritis. Behav Res Ther. 2003;41:1295–1310. doi: 10.1016/s0005-7967(03)00036-6. [DOI] [PubMed] [Google Scholar]
- 45.Duffy K.A., Helzer E.G., Hoyle R.H., Fukukura Helzer J., Chartrand T.L. Pessimistic expectations and poorer experiences: the role of (low) extraversion in anticipated and experienced enjoyment of social interaction. PloS One. 2018;13 doi: 10.1371/journal.pone.0199146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jalilianhasanpour R., Williams B., Gilman I., Burke M.J., Glass S., Fricchione G.L. Resilience linked to personality dimensions, alexithymia and affective symptoms in motor functional neurological disorders. J Psychosom Res. 2018;107:55–61. doi: 10.1016/j.jpsychores.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gureje O. Treating chronic pain in the context of comorbid depression. Pain. 2008;134:3–4. doi: 10.1016/j.pain.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 48.Gerbershagen H.J., Pogatzki-Zahn E., Aduckathil S., Peelen L.M., Kappen T.H., van Wijck A.J. Procedure-specific risk factor analysis for the development of severe postoperative pain. Anesthesiology. 2014;120:1237–1245. doi: 10.1097/ALN.0000000000000108. [DOI] [PubMed] [Google Scholar]
- 49.Wicksell R.K., Olsson G.L. Predicting and preventing chronic postsurgical pain and disability. Anesthesiology. 2010;113:1260–1261. doi: 10.1097/ALN.0b013e3181da89f8. [DOI] [PubMed] [Google Scholar]
- 50.Fashler S.R., Weinrib A.Z., Azam M.A., Katz J. The use of acceptance and commitment therapy in oncology settings: a narrative review. Psychol Rep. 2018;121:229–252. doi: 10.1177/0033294117726061. [DOI] [PubMed] [Google Scholar]
- 51.Newell A., Simon H.A. Computer science as empirical inquiry: symbols and search. Commun ACM. 1976;19:113–126. [Google Scholar]
- 52.Molnar C. Lulu Press; Morrisville, NC, USA: 2019. Interpretable machine learning: a guide for making black box models explainable. [Google Scholar]
- 53.Gigerenzer G., Czerlinski J., Martignon L. How good are fast and frugal heuristics? In: Shanteau J., Mellers B.A., Schum D.A., editors. Decision science and technology: reflections on the contributions of ward edwards. Springer US; Boston, MA: 1999. pp. 81–103. [Google Scholar]
- 54.Ho T.K. vol. 1. IEEE Computer Society; 1995. Random decision forests; p. 278. (Proceedings of the third international conference on document analysis and recognition (volume 1)). [Google Scholar]
- 55.Breiman L. Random forests. Mach Learn. 2001;45:5–32. [Google Scholar]
- 56.Exposito-Vizcaino S., Sanchez-Rodriguez E., Miro J. The role of physical, cognitive and social factors in pain interference with activities of daily living among individuals with chronic cancer pain. Eur J Canc Care. 2019 doi: 10.1111/ecc.13203. [DOI] [PubMed] [Google Scholar]
- 57.Tate R.L., Perdices M., Rosenkoetter U., Shadish W., Vohra S., Barlow D.H. The single-case reporting guideline in BEhavioural interventions (SCRIBE) 2016 statement. Neuropsychol Rehabil. 2017;27:1–15. doi: 10.1080/09602011.2016.1190533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuhn M. 2018. Caret: classification and Regression training. [Google Scholar]
- 59.Kampstra P. Beanplot: a boxplot alternative for visual comparison of distributions. J Stat Software. 2008;28 Code Snippet 1. [Google Scholar]
- 60.Phillips N. 2017. Yarrr: a Companion to the e-Book "YaRrr!: the Pirate’s Guide to R. [Google Scholar]