Abstract
Background
Arthralgia is a common and debilitating toxicity of aromatase inhibitors (AI) that leads to premature drug discontinuation. We sought to evaluate the clinical and genetic risk factors associated with AI-associated arthralgia (AIAA).
Methods
We performed a cross-sectional study among postmenopausal women with stage 0-III breast cancer who were prescribed a third-generation AI for adjuvant therapy. The primary outcome was patient-reported AIAA occurrence. We extracted and assayed germline DNA for single nucleotide polymorphisms (SNPs) of genes implicated in estrogen and inflammation pathways. Multivariable logistic regression models examined the association between demographic, clinical, and genetic factors and AIAA. Analyses were restricted to White participants.
Results
Among 1049 White participants, 543 (52%) reported AIAA. In multivariable analyses, women who had a college education [Adjusted Odds Ratio (AOR) 1.49, 95% Confidence Interval (CI) 1.00–2.20], had a more recent transition into menopause (<10 years) (5–10 years AOR 1.55, 95% CI 1.09–2.22; <5 years AOR 1.78, 95% CI 1.18–2.67), were within one year of starting AIs (AOR 1.61, 95% CI 1.08–2.40), and those who received chemotherapy (AOR 1.38, 95% CI 1.02–1.88) were significantly more likely to report AIAA. Additionally, SNP rs11648233 (HSD17B2) was significantly associated with higher odds of AIAA (AOR 2.21, 95% CI 1.55–3.16).
Conclusions
Time since menopause and start of AIs, prior chemotherapy, and SNP rs11648233 within the HSD17B2 gene in the estrogen pathway were significantly associated with patient-reported AIAA. These findings suggest that clinical and genetic factors involved in estrogen withdrawal increase the risk of AIAA in postmenopausal breast cancer survivors.
Keywords: Breast neoplasm, Aromatase inhibitor, Genetics, Arthralgia, Postmenopausal, Risk factors
Highlights
-
•
One in two postmenopausal women experience aromatase inhibitor-associated arthralgia.
-
•
Shorter time since starting menopause and aromatase inhibitors are clinical risk factors for AIAA.
-
•
SNP rs11648233 (HSD17B2) in the estrogen pathway may contribute to AIAA.
Introduction
In 2017, approximately 252,710 new breast cancer cases were diagnosed in the United States [1]. The majority of these cases were in postmenopausal women with early stage estrogen-receptor positive tumors for which adjuvant endocrine treatment is aromatase inhibitors (AI) [2]. Arthralgia, or joint pain, is a major source of symptom burden in breast cancer survivors on AI therapy [3,4]. In clinical practice settings, nearly 50% of patients taking AIs report having arthralgia and attribute their arthralgia to AIs [5,6]. Of those who have AI-associated arthralgia (AIAA), 50% had a new onset of arthralgia following the initiation of AI therapy, and 50% reported that their pre-existing pain had worsened since starting an AI [5,6]. Additionally, AIAA leads to premature discontinuation and sub-optimal adherence to AIs [7,8]. Further, both early discontinuation of and non-adherence to AIs have been independently associated with increased overall mortality [9].
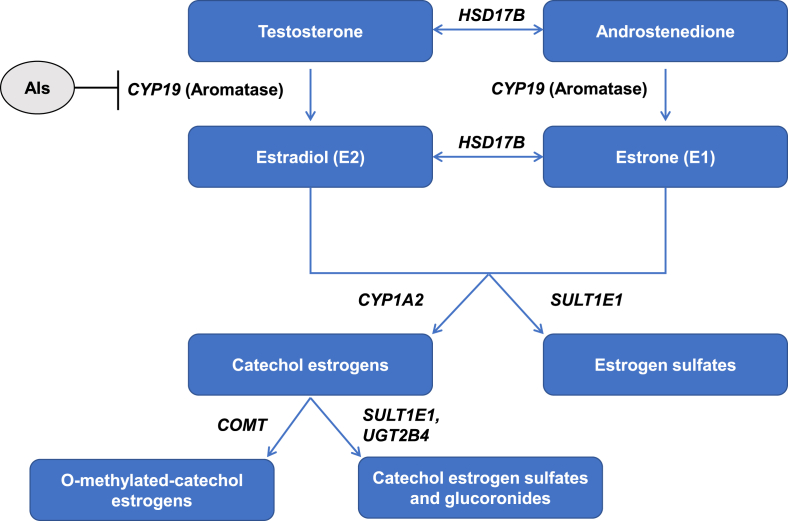
In terms of risk factors for AIAA, shorter time since menopause [5,10], chemotherapy exposure [6,10,11], obesity [11], and a history of menopausal hormone therapy [11] have been shown to be associated with AIAA. These clinical risk factors suggest that rapid estrogen deprivation due to AI therapy may play a pivotal role in the development of AIAA. Estradiol and estrone are metabolized by multiple mechanisms, including sulfation, glucuronidation, reduction, oxidation, and hydroxylation. Previous research has demonstrated that genetic variations in genes involved in estrogen metabolism, particularly HSD17B, CYP1A2, SULT1E1, COMT, and UGT2B4, are associated with estrogen levels, menopause-related symptoms during the natural menopausal transition, and breast cancer susceptibility (Fig. 1) [12,13]. Further, we and others have previously found polymorphisms in CYP19A1, a gene regulating aromatase synthesis, to be associated with patient-reported AIAA symptoms [[14], [15], [16], [17]].
Fig. 1.
Estrogen synthesis and metabolism.
In addition to the role of estrogen-related pathways in AIAA occurrence, inflammatory pathways may also be involved. In clinical observations, MRI studies have shown that tenosynovial changes and intra-articular fluid retention are associated with AIAA [18,19]. Additionally, we previously found an association between key inflammatory biomarkers and the coexistence of arthralgia, fatigue, and insomnia [20]. Further, single nucleotide polymorphisms (SNPs) in estrogen and inflammation-related genes have been found to be related to AI-associated musculoskeletal adverse effects [[21], [22], [23], [24], [25], [26], [27]].
Taken together, these studies suggest the biological plausibility that genetic variations primarily in estrogen pathways as well as those in inflammatory pathways may be associated with AIAA occurrence. Since AIAA negatively impacts survivor’s quality of life, adherence behavior, and potential survival benefit from AIs due to poor adherence, researchers need to identify both clinical and genetic risk factors to better examine potential contributions to AIAA and inform personalized diagnosis and timely intervention for AIAA. The primary objective of this study was to evaluate the contribution of genetic variants in addition to clinical risk factors associated with patient-reported AIAA occurrence.
2. Materials and Methods
2.1. Study design and patient population
We conducted a cross-sectional study with recruitment from November 2011 through April 2015. Eligible patients were postmenopausal women, 18 years or older, with a history of stage 0-III breast cancer, who had completed primary cancer treatments (surgery, chemotherapy, radiation therapy), and had either currently been using a third-generation AI (anastrozole, letrozole, or exemestane) for adjuvant therapy for at least six months or discontinued an adjuvant AI before the full duration of the prescribed therapy. All patients were required to understand written English and participate in the informed consent process. We excluded women with metastatic (stage IV) breast cancer.
Research assistants recruited patients from two breast cancer clinics, one in an academic tertiary care teaching hospital and the other in a community hospital, within the University of Pennsylvania Health System (Philadelphia, PA, USA). After research assistants obtained written informed consent, each patient completed a self-administered paper survey. Peripheral blood was collected from each patient; individuals who did not want to provide a blood sample were given the option of providing a saliva sample. Samples were banked at −80 °C for genetic and biomarker analysis. The Institutional Review Board of the University of Pennsylvania approved the study protocol.
2.2. Outcome measures
Our primary outcome was patient-reported AIAA occurrence as described previously [5,14]. To determine patients’ perceptions of the impact of AI use on their joint symptoms, we asked participants to attribute their current joint pain to a list of six factors, including arthritis, aging, AIs, weight gain, other medical conditions, and other medications. We also asked patients who had discontinued their AI to select one or more reasons for discontinuation from a list that included joint pain and other symptoms. Patients who attributed their current joint pain symptoms to AI use as well as those who had stopped their AI because of joint pain were classified as having AIAA.
2.3. Demographic and clinical variables
Patients’ self-reported demographic variables included age, race, education status, height, weight, date of last menstrual period (LMP), and reasons for menopause. We obtained clinical variables such as cancer stage, type of chemotherapy, tamoxifen use prior to starting AIs, current AI use, and time since starting AI via chart abstraction. An oncologist (AMD) supervised the chart abstractions and verified the abstracted clinical variables for quality control.
2.4. SNP selection and genotyping
We selected 91 SNPs to include in the analyses based on our previous study [14] and other published studies that identified genes related to estrogen and inflammation pathways [[12], [13], [14],[21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37]]. Participant DNA was extracted from buffy coat specimens using the Qiagen QiaAmp 96 DNA Blood Kit (Valencia, CA). SNPs were genotyped using the SNPlex or the OpenArray platform from Applied Biosystems (Foster City, CA).
2.5. Statistical analysis
We performed data analyses using STATA 12.0 for Windows (STATA Corporation, College Station, TX). Because genetic heterogeneity or population stratification has the potential to lead to either spurious association or reduced power, we carried out population-specific analysis and report here the results restricted to White subjects.
We tested associations between AIAA and demographic and clinical variables using the χ2 test. Demographic and clinical variables with P values of < .10 in the bivariate analyses were carried forward to the multivariable logistic regression models. Next, we examined the association between AIAA and genetic polymorphisms using the χ2 test. Genetic SNPs with P values of < .001 (Bonferroni Adjustment) in the bivariate analyses were carried forward to the multivariable logistic regression models. We performed logistic regression analyses in two steps: 1) Model 1 included only the demographic and clinical variables, and 2) Model 2 added the genetic SNPs to Model 1.
3. Results
3.1. Participant characteristics
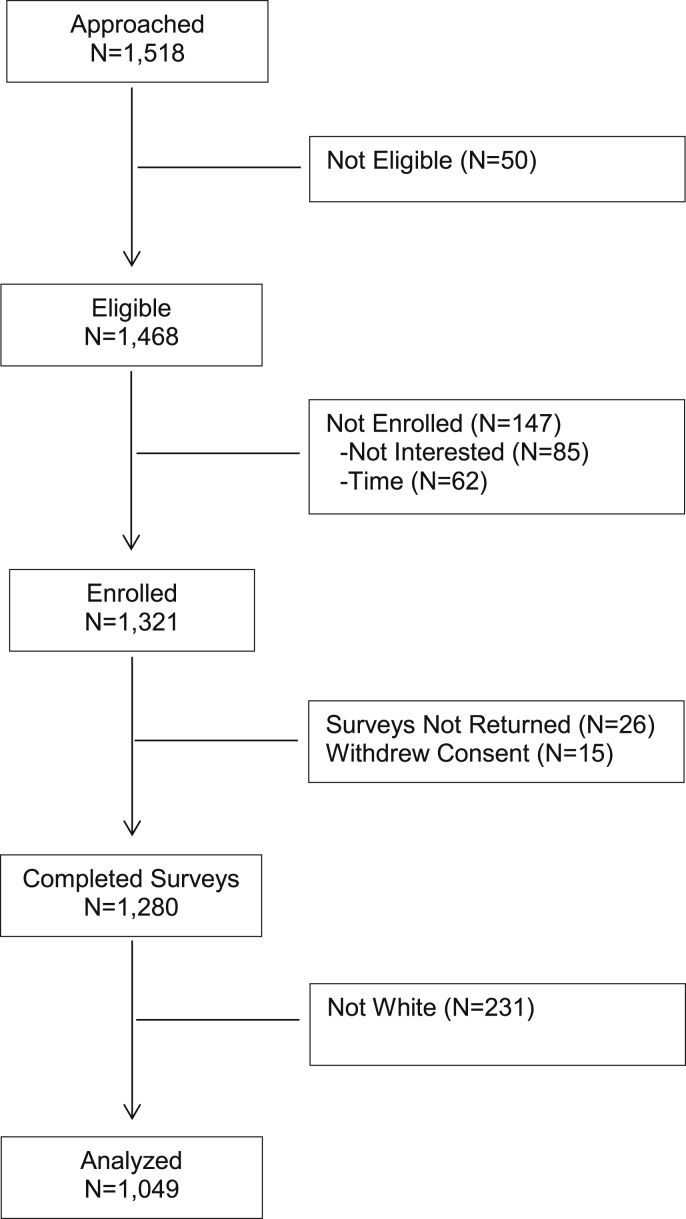
Of 1468 consecutive patients screened and eligible, 1321 (90%) agreed to participate and provided written informed consent. The main reasons for not participating were: lack of time to complete the survey (n = 62, 32%) and lack of desire to participate in research (n = 85, 43%). Additionally, 15 (1%) patients withdrew consent from the study and 26 (2%) did not return their survey after consenting to do so, resulting in the final sample of 1280. For this study, we restricted analysis to the 1049 (82%) White patients who provided evaluable survey data (see Fig. 2 Consort Diagram).
Fig. 2.
Consort diagram of breast cancer survivors enrolled and included in current data analyses.
As shown in Table 1, the mean age was 63 years (standard deviation [SD] 9.8). The majority of patients (83%) reported completing some college or above. Almost two-thirds (58%) of women had gone through natural menopause prior to their diagnosis of breast cancer. Two hundred and eighteen (21%) women were within five years of menopause, and 557 (54%) women were more than ten years from menopause. Overall, 916 (88%) patients were currently taking an AI at the time of enrollment, while 131 (12%) had discontinued AI therapy by the time of the survey. Among those who were taking or had taken an AI, 811 (79%) women were taking anastrozole. At the time of enrollment, 277 (26%) had been on an AI for less than one year, 502 (48%) had taken an AI for one to three years, and 270 (26%) had been on an AI for over three years.
Table 1.
Demographic and clinical characteristics of White postmenopausal breast cancer survivors.
| Characteristics | Overall (N = 1049) N (%) |
AIAA (N = 543) N (% within each category) |
P-value |
|---|---|---|---|
| Age, years (mean ± standard deviation) | 63.0 ± 9.8 | 60.9 ± 9.0 | <0.001 |
| Educational Level | 0.003 | ||
| High school or less | 176 (16.8) | 73 (41.5) | |
| Some college or above | 869 (83.2) | 469 (54.0) | |
| Body mass index, kg/m2 | 0.19 | ||
| <25 | 442 (42.2) | 236 (53.4) | |
| 25 to 30 | 313 (29.8) | 168 (53.7) | |
| >30 | 294 (28.0) | 139 (47.3) | |
| Reasons for menopause | 0.002 | ||
| Natural | 607 (58.2) | 290 (47.8) | |
| Induced | 435 (41.8) | 251 (57.7) | |
| Years since last menstrual period | <0.001 | ||
| >10 | 557 (54.0) | 245 (44.0) | |
| 5 to 10 | 257 (24.9) | 150 (58.4) | |
| <5 | 218 (21.1) | 142 (65.1) | |
| Cancer stage | 0.29 | ||
| 0 and I | 541 (52.1) | 267 (49.4) | |
| II | 373 (36.0) | 202 (54.2) | |
| III | 123 (11.9) | 67 (54.5) | |
| Chemotherapy | <0.001 | ||
| Yes | 558 (53.2) | 318 (57.0) | |
| No | 491 (46.8) | 225 (45.8) | |
| Tamoxifen use prior to starting AIs | 0.14 | ||
| Yes | 260 (24.8) | 145 (55.8) | |
| No | 789 (75.2) | 398 (50.4) | |
| AI type | 0.07 | ||
| Anastrozole (Arimidex) | 811 (78.7) | 421 (51.9) | |
| Exemestane (Aromasin) | 71 (6.9) | 46 (64.8) | |
| Letrozole (Femara) | 149 (14.4) | 72 (48.3) | |
| Years since start on AI | 0.02 | ||
| >3 | 270 (25.7) | 121 (44.8) | |
| 1 to 3 | 502 (47.9) | 264 (52.6) | |
| <1 | 277 (26.4) | 158 (57.0) |
AI, aromatase inhibitor. Note: The bold and italicized text indicates a statistically significant P-value of <0.05.
3.2. Demographic and clinical characteristics and patient-reported AIAA
Among the 1049 White patients, 543 (52%) were classified as having AIAA by either reporting joint symptoms attributable to AI or citing arthralgia as a reason for discontinuing their AIs. In unadjusted analyses, higher education, shorter time since menopause, induced menopause, exposure to chemotherapy, and shorter time since starting AI therapy were significantly associated with AAIA occurrence (P < .05) (Table 2).
Table 2.
Demographic, clinical, and genetic factors associated with aromatase inhibitor-associated arthralgia (AIAA).
| Factors | Women with AIAA (N = 543) N (%) | Unadjusted OR |
Model 1 (Demographics & Clinical) |
Model 2 (Model 1 + Genetics) |
|||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | AOR (95% CI) | P-value | AOR (95% CI) | P-value | ||
| Demographic and Clinical | |||||||
| Education | |||||||
| High school or less | 73 (13) | 1 | – | 1 | – | 1 | – |
| Some college or above | 469 (87) | 1.65 (1.19–2.30) | 0.003 | 1.45 (1.03–2.05) | 0.035 | 1.49 (1.00–2.20) | 0.047 |
| Reasons for menopause | |||||||
| Natural | 290 (54) | 1 | – | 1 | – | 1 | – |
| Induced | 251 (46) | 1.49 (1.16–1.91) | 0.002 | 1.26 (0.95–1.66) | 0.11 | 1.20 (0.88–1.64) | 0.24 |
| Years since LMP | |||||||
| >10 years | 245 (46) | 1 | – | 1 | – | 1 | – |
| 5–10 years | 150 (28) | 1.78 (1.32–2.41) | <0.001 | 1.56 (1.13–2.14) | 0.006 | 1.55 (1.09–2.22) | 0.015 |
| <5 years | 142 (26) | 2.38 (1.72–3.29) | <0.001 | 1.88 (1.31–2.70) | 0.001 | 1.78 (1.18–2.67) | 0.006 |
| Chemotherapy | |||||||
| No | 225 (41) | 1 | – | 1 | – | 1 | – |
| Yes | 318 (59) | 1.57 (1.23–2.00) | <0.001 | 1.30 (0.99–1.70) | 0.06 | 1.38 (1.02–1.88) | 0.038 |
| Aromatase inhibitors (AI) | |||||||
| Anastrozole (Arimidex) | 421 (78) | 1 | – | 1 | – | 1 | – |
| Exemestane (Aromasin) | 46 (9) | 1.70 (1.03–2.83) | 0.039 | 1.58 (0.92–2.69) | 0.10 | 1.28 (0.72–2.25) | 0.40 |
| Letrozole (Femara) | 72 (13) | 0.87 (0.61–1.23) | 0.42 | 0.86 (0.59–1.26) | 0.44 | 0.82 (0.53–1.27) | 0.38 |
| Years since start of AI | |||||||
| >3 years | 121 (22) | 1 | – | 1 | – | 1 | – |
| 1–3 years | 264 (49) | 1.36 (1.01–1.84) | 0.040 | 1.32 (0.96–1.82) | 0.09 | 1.26 (0.89–1.78) | 0.20 |
| <1 year | 158 (29) | 1.63 (1.17–2.29) | 0.004 | 1.68 (1.17–2.42) | 0.005 | 1.61 (1.08–2.40) | 0.019 |
| Genetic SNP(s) | |||||||
| rs11648233 | |||||||
| C/C | 67 (15) | 1 | – | - | - | 1 | – |
| C/A or A/A | 372 (85) | 2.03 (1.41–2.91) | <0.001 | - | - | 2.21 (1.55–3.16) | <0.001 |
Abbreviations: AIAA, aromatase inhibitor-associated arthralgia; OR, odds ratio; AOR, adjusted odds ratio; CI, confidence interval; LMP, last menstrual period; SNP, single nucleotide polymorphism. Note: The bold and italicized text indicates a statistically significant P-value of <0.05.
3.3. Genetic risk factors and patient-reported AIAA
All genotyping failure rates were <1.8%. Genotype distributions satisfied Hardy-Weinberg proportions and were consistent with reported reference SNP frequencies (data not shown). If the frequency for one of the genotypes was <5% of the population, we collapsed the SNPs genotypes into two categories. In unadjusted analyses, we tested the association between AIAA and 91 SNPs in estrogen and inflammation pathways and found 1 SNP (rs11648233) to be associated with higher odds of AIAA occurrence (P < .001 Bonferroni Adjustment) (Table 2).
3.4. Multivariable analyses of demographic, clinical, and genetic risk factors and patient-reported AIAA
As described above in the Methods section and shown in Table 2, Model 1 includes the demographic and clinical variables significantly associated with AIAA in the unadjusted analyses. Model 2 includes demographic and clinical variables plus the genetic SNP. To note, we did not include age in the multivariable models because of the collinearity with time since LMP. After adjusting for all variables in Model 2, having a college education [Adjusted Odds Ratio (AOR) 1.49, 95% Confidence Interval (CI) 1.00–2.20], shorter time since menopause (5–10 years: AOR 1.55, 95% CI 1.09–2.22; <5 years: AOR 1.78, 95% CI 1.18–2.67), exposure to chemotherapy (AOR 1.38, 95% CI 1.02–1.88), and starting AI therapy within the past year (AOR 1.61, 95% CI 1.08–2.40) were statistically significantly associated with higher odds of patient-reported AIAA. Additionally, results from Model 2 found that having at least one A allele in rs11648233 located in the HSD17B2 gene (C/A or A/A genotype, AOR 2.21, 95% CI 1.55–3.16) was significantly associated with higher odds of AIAA occurrence.
4. Discussion
AIs have improved survival for many women with hormone receptor-positive breast cancer, however, AIAA can result in non-adherence to AIs and premature discontinuation of AIs [7,8]. In this study of 1049 White breast cancer survivors, we confirmed previous findings that one in two ambulatory patients who receive AIs report AIAA [5,6] and that AIAA occurrence was inversely related to the length of time since menopause [14]. In addition, we found that having a college education, receipt of chemotherapy, and having started an AI within the past year were significantly associated with AIAA. Additionally, SNP rs11648233 (HSD17B2) was also significantly associated with higher odds of AIAA, suggesting that this gene in the estrogen pathway plays a role in the mechanism of this toxicity.
In this large study of postmenopausal breast cancer survivors, we confirm previous research findings that have identified associations between clinical risk factors and AIAA. We found that prior chemotherapy increased the odds for reporting AIAA, which is in line with others who have found that receipt of chemotherapy is a risk factor for AIAA [6,10,11]. Since younger women treated with chemotherapy may experience premature menopause, which results in more abrupt and a greater magnitude of estrogen withdrawal, it is not surprising for us to confirm that time since menopause is also inversely related to AIAA occurrence [5,14]. We also found that shorter time since starting AI therapy was associated with higher odds of reporting AIAA in our study population, which is consistent with a study by Egawa and colleagues (2016) [10]. These findings emphasize that the host estrogen environment at the time of AI initiation plays an important role in developing AIAA because AIs can cause a larger drop in estrogen levels for those women who have more recently transitioned into menopause.
We identified one SNP in the estrogen metabolism pathway that was associated with AIAA occurrence. Specifically, rs11648233 in the HSD17B2 gene was associated with twice the odds of reporting AIAA. The HSD17B2 enzyme oxidizes estradiol (E2) to the weaker estrone (E1), which has a lower binding affinity for the estrogen receptor, and contributes to lower levels of E2 [38,39]. This reduction of E2 by the HSD17B2 enzyme may help explain a potential mechanism behind its association with AIAA occurrence. Further, HSD17B2 has been shown to be associated with prognostic significance in breast cancer progression and relapse [30,31,36]. In this study, we were not able to identify the same significant SNPs in CYP19A1 as previously reported in our study [14] and in the BIG 1–98 and TEAM trials [16,17].
Previous research from large, prospective trials have provided mixed results showing that emergent musculoskeletal symptoms, like AIAA, may be associated with improved outcomes, such as disease-free survival and breast cancer free interval [17,40,41]. These prospective studies, along with our exploratory findings, contribute to the growing research on how genetic variants in estrogen pathways may impact biological mechanisms related to AIAA occurrence. Given that clinical cancer care is becoming highly personalized based on molecular markers of the host and tumor, increasing research has been dedicated to finding genetic biomarkers to improve risk prediction, diagnosis and cancer treatment-related symptoms [[42], [43], [44]]. Translating genetic risk factors into clinical practice may help clinicians to better identify who is at risk for AIAA and may benefit from early symptom management, potentially leading to better adherence to AI therapy and improved clinical outcomes.
It is important to acknowledge the limitations of our study. Our cross-sectional study design limits our ability to establish causal relationships between AIAA occurrence and the clinical and genetic risk factors identified in this study. Further, cross-sectional collection of data may miss those participants with intermittent joint symptoms. Second, self-report of AIAA may be subject to recall bias; however, the prevalence of AIAA in this study is similar to prior studies [5,6,14]. Third, despite Bonferroni adjustment, our genetic findings need to be validated in independent cohorts. Lastly, our analyses only included White subjects due to the limited sample size of minority patients in our cohort and may be subject to selection bias. Future research is needed to examine genetic polymorphisms and AIAA in racial/ethnic minority populations.
5. Conclusions
Despite these limitations, our study is the largest to date to evaluate demographic, clinical, and genetic risk factors associated with AIAA among postmenopausal breast cancer survivors. One in two women experience AIAA. Time since starting menopause and AIs, as well as prior chemotherapy, are robust clinical risk factors that can help oncologists and nurses to stratify patients by risk for timely and personalized symptom monitoring and management. Our study also suggests genetic factors, such as SNP rs11648233 in the estrogen pathway, are associated with increased odds of AIAA occurrence. Future validation of these findings in an independent, prospective study with a diverse population and the use of next generation sequencing will help further personalize effective symptom diagnosis and management to reduce AIAA among women with breast cancer.
6. Declarations
Ethics approval and consent to participate: The Institutional Review Board of the University of Pennsylvania approved the study protocol. All participants provided written informed consent to participate in the study.
Availability of data and material: The datasets used in the current study are available from the corresponding author (Jun J. Mao) on reasonable request.
Declaration of competing interest
None.
Acknowledgements
Research related to the development of this paper was supported in part by the National Cancer Institute grants to Dr. Jun J. Mao (R01-CA158243), the University of Pennsylvania Abramson Cancer Center (P30-CA016520), and the Memorial Sloan Kettering Cancer Center (P30-CA008748), as well as the Laurance S. Rockefeller Fund and the Translational and Integrative Medicine Research Fund, both at Memorial Sloan Kettering Cancer Center. The funding sources were not involved in the study design; collection, analysis and interpretation of data; writing of the report; or decision to submit the article for publication. We would like to thank all the breast cancer survivors, physicians, nurse practitioners, and research staff for their support. We would also like to thank the research assistants for their dedication to data collection and management.
Contributor Information
Sally A.D. Romero, Email: romeros1@mskcc.org.
H. Irene Su, Email: hisu@ucsd.edu.
Jaya Satagopan, Email: satagopj@sph.rutgers.edu.
Q. Susan Li, Email: liq2@mskcc.org.
Christina M. Seluzicki, Email: seluzicc@mskcc.org.
Annika Dries, Email: annikamd@stanford.edu.
Angela M. DeMichele, Email: Angela.DeMichele@uphs.upenn.edu.
Jun J. Mao, Email: maoj@mskcc.org.
References
- 1.Cancer A.C.S. American Cancer Society; 2017. Facts and statistics. [Google Scholar]
- 2.Burstein H.J., Prestrud A.A., Seidenfeld J., Anderson H., Buchholz T.A., Davidson N.E. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28:3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chlebowski R.T. Aromatase inhibitor-associated arthralgias. J Clin Oncol. 2009;27:4932–4934. doi: 10.1200/JCO.2009.23.3270. [DOI] [PubMed] [Google Scholar]
- 4.Coleman R.E., Bolten W.W., Lansdown M., Dale S., Jackisch C., Merkel D. Aromatase inhibitor-induced arthralgia: clinical experience and treatment recommendations. Cancer Treat Rev. 2008;34:275–282. doi: 10.1016/j.ctrv.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Mao J.J., Stricker C., Bruner D., Xie S., Bowman M.A., Farrar J.T. Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer. 2009;115:3631–3639. doi: 10.1002/cncr.24419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crew K.D., Greenlee H., Capodice J., Raptis G., Brafman L., Fuentes D. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol. 2007;25:3877–3883. doi: 10.1200/JCO.2007.10.7573. [DOI] [PubMed] [Google Scholar]
- 7.Chim K., Xie S.X., Stricker C.T., Li Q.S., Gross R., Farrar J.T. Joint pain severity predicts premature discontinuation of aromatase inhibitors in breast cancer survivors. BMC Canc. 2013;13:401. doi: 10.1186/1471-2407-13-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hershman D.L., Kushi L.H., Shao T., Buono D., Kershenbaum A., Tsai W.Y. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hershman D.L., Shao T., Kushi L.H., Buono D., Tsai W.Y., Fehrenbacher L. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Canc Res Treat. 2011;126:529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egawa C., Hirokaga K., Takao S., Yamagami K., Miyashita M., Baba M. Risk factors for joint symptoms in postmenopausal Japanese breast cancer patients treated with anastrozole: a prospective multicenter cohort study of patient-reported outcomes. Int J Clin Oncol. 2016;21:262–269. doi: 10.1007/s10147-015-0905-5. [DOI] [PubMed] [Google Scholar]
- 11.Sestak I., Cuzick J., Sapunar F., Eastell R., Forbes J.F., Bianco A.R. Risk factors for joint symptoms in patients enrolled in the ATAC trial: a retrospective, exploratory analysis. Lancet Oncol. 2008;9:866–872. doi: 10.1016/S1470-2045(08)70182-7. [DOI] [PubMed] [Google Scholar]
- 12.Rebbeck T.R., Su H.I., Sammel M.D., Lin H., Tran T.V., Gracia C.R. Effect of hormone metabolism genotypes on steroid hormone levels and menopausal symptoms in a prospective population-based cohort of women experiencing the menopausal transition. Menopause. 2010;17:1026–1034. doi: 10.1097/gme.0b013e3181db61a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Low Y.L., Li Y., Humphreys K., Thalamuthu A., Li Y., Darabi H. Multi-variant pathway association analysis reveals the importance of genetic determinants of estrogen metabolism in breast and endometrial cancer susceptibility. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mao J.J., Su H.I., Feng R., Donelson M.L., Aplenc R., Rebbeck T.R. Association of functional polymorphisms in CYP19A1 with aromatase inhibitor associated arthralgia in breast cancer survivors. Breast Cancer Res. 2011;13:R8. doi: 10.1186/bcr2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazzuca F., Botticelli A., Mazzotti E., La Torre M., Borro M., Marchetti L. CYP19A1 genetic polymorphisms rs4646 and osteoporosis in patients treated with aromatase inhibitor-based adjuvant therapy. Eurasian J Med. 2016;48:10–14. doi: 10.5152/eurasianjmed.2015.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fontein D.B.Y., Houtsma D., Nortier J.W.R., Baak-Pablo R.F., Kranenbarg E.M.-K., van der Straaten T.R.J.H.M. Germline variants in the CYP19A1 gene are related to specific adverse events in aromatase inhibitor users: a substudy of Dutch patients in the TEAM trial. Breast Canc Res Treat. 2014;144:599–606. doi: 10.1007/s10549-014-2873-2. [DOI] [PubMed] [Google Scholar]
- 17.Leyland-Jones B., Gray K.P., Abramovitz M., Bouzyk M., Young B., Long B. CYP19A1 polymorphisms and clinical outcomes in postmenopausal women with hormone receptor-positive breast cancer in the BIG 1-98 trial. Breast Canc Res Treat. 2015;151:373–384. doi: 10.1007/s10549-015-3378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lintermans A., Van Asten K., Wildiers H., Laenen A., Paridaens R., Weltens C. A prospective assessment of musculoskeletal toxicity and loss of grip strength in breast cancer patients receiving adjuvant aromatase inhibitors and tamoxifen, and relation with BMI. Breast Canc Res Treat. 2014;146:109–116. doi: 10.1007/s10549-014-2986-7. [DOI] [PubMed] [Google Scholar]
- 19.Morales L., Pans S., Verschueren K., Van Calster B., Paridaens R., Westhovens R. Prospective study to assess short-term intra-articular and tenosynovial changes in the aromatase inhibitor-associated arthralgia syndrome. J Clin Oncol. 2008;26:3147–3152. doi: 10.1200/JCO.2007.15.4005. [DOI] [PubMed] [Google Scholar]
- 20.Bauml J., Chen L., Chen J., Boyer J., Kalos M., Li S.Q. Arthralgia among women taking aromatase inhibitors: is there a shared inflammatory mechanism with co-morbid fatigue and insomnia? Breast Cancer Res. 2015;17:89. doi: 10.1186/s13058-015-0599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu M., Wang L., Bongartz T., Hawse J.R., Markovic S.N., Schaid D.J. Aromatase inhibitors, estrogens and musculoskeletal pain: estrogen-dependent T-cell leukemia 1A (TCL1A) gene-mediated regulation of cytokine expression. Breast Cancer Res. 2012;14:R41. doi: 10.1186/bcr3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Giralt N., Rodriguez-Sanz M., Prieto-Alhambra D., Servitja S., Torres-Del Pliego E., Balcells S. Genetic determinants of aromatase inhibitor-related arthralgia: the B-ABLE cohort study. Breast Canc Res Treat. 2013;140:385–395. doi: 10.1007/s10549-013-2638-3. [DOI] [PubMed] [Google Scholar]
- 23.Lintermans A., Van Asten K., Jongen L., Van Brussel T., Laenen A., Verhaeghe J. Genetic variant in the osteoprotegerin gene is associated with aromatase inhibitor-related musculoskeletal toxicity in breast cancer patients. Eur J Cancer. 2016;56:31–36. doi: 10.1016/j.ejca.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Henry N.L., Skaar T.C., Dantzer J., Li L., Kidwell K., Gersch C. Genetic associations with toxicity-related discontinuation of aromatase inhibitor therapy for breast cancer. Breast Canc Res Treat. 2013;138:807–816. doi: 10.1007/s10549-013-2504-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Servitja S., Martos T., Rodriguez Sanz M., Garcia-Giralt N., Prieto-Alhambra D., Garrigos L. Skeletal adverse effects with aromatase inhibitors in early breast cancer: evidence to date and clinical guidance. Ther Adv Med Oncol. 2015;7:291–296. doi: 10.1177/1758834015598536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J., Lu K., Song Y., Zhao S., Ma W., Xuan Q. RANKL and OPG polymorphisms are associated with aromatase inhibitor-related musculoskeletal adverse events in Chinese han breast cancer patients. PLoS One. 2015;10 doi: 10.1371/journal.pone.0133964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ingle J.N., Schaid D.J., Goss P.E., Liu M., Mushiroda T., Chapman J.A. Genome-wide associations and functional genomic studies of musculoskeletal adverse events in women receiving aromatase inhibitors. J Clin Oncol. 2010;28:4674–4682. doi: 10.1200/JCO.2010.28.5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho M.F., Bongartz T., Liu M., Kalari K.R., Goss P.E., Shepherd L.E. Estrogen, SNP-dependent chemokine expression and selective estrogen receptor modulator regulation. Mol Endocrinol. 2016;30:382–398. doi: 10.1210/me.2015-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guillemette C., Bélanger A., Lépine J. Metabolic inactivation of estrogens in breast tissue by UDP-glucuronosyltransferase enzymes: an overview. Breast Cancer Res. 2004;6:246–254. doi: 10.1186/bcr936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gunnarsson C., Hellqvist E., Stal O. 17beta-Hydroxysteroid dehydrogenases involved in local oestrogen synthesis have prognostic significance in breast cancer. Br J Canc. 2005;92:547–552. doi: 10.1038/sj.bjc.6602375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilborn E., Stal O., Jansson A. Estrogen and androgen-converting enzymes 17beta-hydroxysteroid dehydrogenase and their involvement in cancer: with a special focus on 17beta-hydroxysteroid dehydrogenase type 1, 2, and breast cancer. Oncotarget. 2017;8:30552–30562. doi: 10.18632/oncotarget.15547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raftogianis R., Creveling C., Weinshilboum R., Weisz J. Estrogen metabolism by conjugation. J Natl Cancer Inst Monogr. 2000:113–124. doi: 10.1093/oxfordjournals.jncimonographs.a024234. [DOI] [PubMed] [Google Scholar]
- 33.Simonsson M., Veerla S., Markkula A., Rose C., Ingvar C., Jernstrom H. CYP1A2--a novel genetic marker for early aromatase inhibitor response in the treatment of breast cancer patients. BMC Canc. 2016;16:256. doi: 10.1186/s12885-016-2284-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sowers M.R., Jannausch M.L., McConnell D.S., Kardia S.R., Randolph J.F., Jr. Endogenous estradiol and its association with estrogen receptor gene polymorphisms. Am J Med. 2006;119:S16–S22. doi: 10.1016/j.amjmed.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Sowers M.R., Wilson A.L., Karvonen-Gutierrez C.A., Kardia S.R. Sex steroid hormone pathway genes and health-related measures in women of 4 races/ethnicities: the Study of Women’s Health across the Nation (SWAN) Am J Med. 2006;119:S103–S110. doi: 10.1016/j.amjmed.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Wu L., Einstein M., Geissler W.M., Chan H.K., Elliston K.O., Andersson S. Expression cloning and characterization of human 17 beta-hydroxysteroid dehydrogenase type 2, a microsomal enzyme possessing 20 alpha-hydroxysteroid dehydrogenase activity. J Biol Chem. 1993;268:12964–12969. [PubMed] [Google Scholar]
- 37.Gervasini G., Jara C., Olier C., Romero N., Martinez R., Carrillo J.A. Polymorphisms in ABCB1 and CYP19A1 genes affect anastrozole plasma concentrations and clinical outcomes in postmenopausal breast cancer patients. Br J Clin Pharmacol. 2017;83:562–571. doi: 10.1111/bcp.13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen C., Huang H., Wu C.H. Protein bioinformatics databases and resources. Methods Mol Biol. 2017;1558:3–39. doi: 10.1007/978-1-4939-6783-4_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The UniProt Consortium UniProt: the universal protein knowledgebase. Nucleic Acids Res. 2017;45:D158–D169. doi: 10.1093/nar/gkw1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huober J., Cole B.F., Rabaglio M., Giobbie-Hurder A., Wu J., Ejlertsen B. Symptoms of endocrine treatment and outcome in the BIG 1-98 study. Breast Canc Res Treat. 2014;143:159–169. doi: 10.1007/s10549-013-2792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stearns V., Chapman J.A., Ma C.X., Ellis M.J., Ingle J.N., Pritchard K.I. Treatment-associated musculoskeletal and vasomotor symptoms and relapse-free survival in the NCIC CTG MA.27 adjuvant breast cancer aromatase inhibitor trial. J Clin Oncol. 2015;33:265–271. doi: 10.1200/JCO.2014.57.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diatchenko L., Slade G.D., Nackley A.G., Bhalang K., Sigurdsson A., Belfer I. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14:135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 43.Edwards R.R. Genetic predictors of acute and chronic pain. Curr Rheumatol Rep. 2006;8:411–417. doi: 10.1007/s11926-006-0034-2. [DOI] [PubMed] [Google Scholar]
- 44.Torkamani A, Wineinger NE, Topol EJ. The personal and clinical utility of polygenic risk scores. Nat Rev Genet. 2018 Sep;19(9):581–590. doi: 10.1038/s41576-018-0018-x. [DOI] [PubMed] [Google Scholar]