Abstract
Background
Brain metastases (BM) are a feared progression of breast cancer (BC) with impact on quality of life and survival. Despite improved treatments, it is believed patients suffering from BM are increasing.
Aims
To study potential changes in the number of BM, the possible links between BC subgroup and extent of BM with prognosis. To investigate the interval between primary BC/extra cranial recurrence, and diagnosis of BM in the years 1994–2014.
Patients and methods
Clinical data from 191 patients with BM diagnosed 1994–2014, was retrieved from charts. Primary tumours where re-evaluated histologically.
Results
There was an increase of BM in 5 years cohorts (1994-99 (n = 9); 2000-04 (n = 36); 2005-09 (n = 60); 2010-14 (n = 86)). We found no difference in the time interval from primary BC to BM but an insignificant increase in time from extra cranial relapse to development of BM in the time periods 1994–2004 and 2005–2014 of 15.5 and 25.0 months (p = 0.0612). Survival after BM was 7 months (95% CI 6–10) with a statistically significant difference between HER2 positive and TNBC with an inferior outcome for the latter (p = 0.018) whilst no differences were present when Luminal BC were compared with HER2 positive BC (p = 0.073).
Conclusions
We show an increase of BM over time whilst the time span from primary BC to BM is unchanged supports earlier findings that adjuvant treatments have little preventive function. Time from extra cranial recurrence to BM was prolonged with one year. Patients with TNBC or more advance extent of BM had the shortest survival with BM.
Keywords: Breast cancer, Brain metastases, Incidence, Survival, Blood brain barrier
Highlights
-
•
Increase in number of brain metastases diagnosed from breast cancer over a 21 year period.
-
•
Increased survival in HER2+ breast cancer with brain metastases.
-
•
We confirm that TNBC has a consistently poor prognosis across the time period.
-
•
Despite improvement in overall survival in breast cancer, interval to brain metastasis remains unchanged.
Introduction
Despite introduction of new therapies during past decades, breast cancer (BC) remains the leading cause of death in European women and a main reason for cancer death worldwide [1,2]. Although palliative treatments for metastatic BC are effective in improving survival, clinicians are reporting a perceived increase in patients suffering from brain metastases (BM). In addition to the short survival associated with the condition, the vast majority of patients with BM have severe symptoms that significantly impair daily activities and quality of life such as cognitive dysfunction, severe headache, dizziness, nausea, vertigo, and epileptic seizures. Approximately 10–30% of patients with metastatic BC suffer from BM according to clinical studies [[3], [4], [5]] whereas autopsy studies report more than 30% [[6], [7], [8]].
BC is the most common cause of meningeal carcinomatosis and the second leading cause of solid BM of all malignant tumours. Incidence and survival appears to vary with BC subtype [[9], [10], [11]]. Triple negative BC (TNBC) exhibits the shortest median survival of 5 months, Luminal BC approximately 9 months, HER2+/ER + BC, 16.5 months and HER2+/ER-11.5 months. Survival across all subtypes has a median of 9 months [12].
Previously, BM has been considered a late event in the progression of metastatic disease. Considering longer overall survival (OS) and increased survival in the metastatic setting, the question whether BM is affecting a larger proportion of patients has been raised [2,6]. In addition, more patients receive efficient adjuvant therapies that do not reach the central nervous system due to the blood-brain-barrier, which in turn may lead to an increased number of patients suffering from BM [[13], [14], [15]]. The aim of our study was to investigate if number of patients diagnosed with BM have increased and if the interval between primary BC and diagnosis of BM has changed through a period extending 20 years. In addition, we wanted to investigate if the proportion of patients with BM as first recurrence had changed and explore whether factors such as extent, treatment, and subtype of BC, influence survival and how these factors have changed in the time interval studied.
Material and methods
Patient data
As patients with BM are only treated at Sahlgrenska University Hospital, the hospital digital system of diagnostic codes was used to find all patients with BM from BC in the time span of 1994–2014. Patient selection is summarised in Fig. 1. The charts were then read for a detailed summary of clinical data and patients with other malignancies excluded. For each patient, the following parameters were extracted from the anonymised patients’ charts: 1) age at diagnose of primary BC and recurrence; 2) histopathologic type and grade; 3) ER, PgR, Ki67 proliferation marker, and HER2 status; 4) type of adjuvant treatment; 5) date of systemic relapse, extra-cranial disease, date, radiology used and symptom of BM; 6) localisation of BM (cerebrum, cerebellum, both, meningeal carcinomatosis: in addition to solid metastases or alone), and extent of BM (1–3 or ≥4 BM); 7) local treatment of BM and lines of systemic palliative treatment before and following diagnosis of BM. A patient who was diagnosed with BM within three months from diagnosis of primary or recurrent BC was considered as having simultaneous metastases.
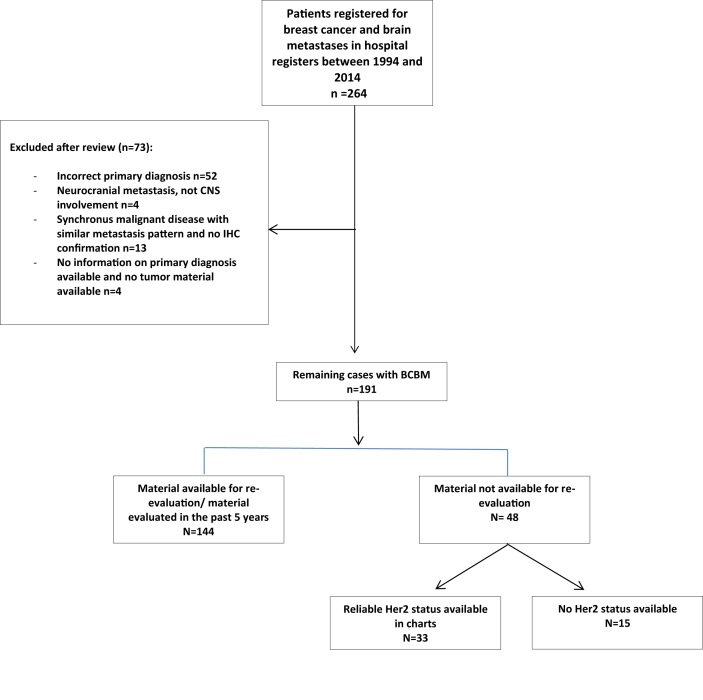
Fig. 1.
Consolidated Standards Of Reporting Trials (CONSORT) diagram showing the selection of patients and number of patients excluded.
BC subgroups were defined as Luminal: HER2 negative tumours expressing oestrogen (ER) and/or progesterone (PgR) disregarding of Ki67 status, TNBC: lacking expression of ER, PgR and HER2, HER2+/ER+: with overexpression of HER2 and ER+ and/or PgR+, and HER2+/ER-with overexpression of HER2 but ER-. Ki67 was available for most of the samples, and all the re-evaluated samples, but as they were from different time periods and the definitions for Luminal A and B have differed this was not used. Also the division into Luminal A and B produced too small groups for statistical analysis. The study has been conducted in accordance with the Declaration of Helsinki and the Sahlgrenska University Hospital Ethical Review Board, Gothenburg, Sweden approved the study. Approval for the chart review was granted from each head of the participating departments. For each institution, a separate biobank application was approved.
Pathology and immunohistochemical (IHC) analysis
Full-face formalin-fixed paraffin-embedded (FFPE) specimens for the 143 available primary BC were collected from participating hospitals in the west-Swedish health care region (Departments Pathology at Sahlgrenska University Hospital in Gothenburg, Södra Älvsborg Hospital in Borås, Norra Älvsborg Hospital in Trollhättan and Halland Hospital in Varberg). The following markers were examined: ER/PR, Ki67, and HercepTest followed by SISH when 2+ or 3 +. ER and PgR was considered positive for >1%. Four μm FFPE sections were subjected to deparaffinisation and rehydration followed by heat-induced epitope retrieval (HIER) Tris/EDTA buffer (pH 9.0) for 20 min at 97 °C using PT Link instrument (PT Link, Dakocytomation, DAKO). The tissue sections were processed using DAKO visualization system (Envision Flex High pH, Link, Ref 8000, DAKO) and DAKO stainer for IHC (Autostainer Plus, Dakocytomation, Denmark) following the manufacturer’s instruction. Peroxidase-catalysed diaminobenzidine was used as the DAB + chromogen, followed by haematoxylin (Envision Flex Hematolxylin, Ref K8018, DAKO) counterstain. The stained slides were rinsed with deionised water followed by the dehydration process in ethanol 70%, ethanol 95%, absolute ethanol, cleared in xylene and added cover glass (Coverslipper, DAKO). The slides were re-evaluated by two independent breast pathologists, blinded to patient outcome. SISH was done using Ventana HER2 Dual ISH Assay on a BenchMark Ultra-system from Roche according to manufacturer’s specifications.
Statistical methods
Results are presented descriptively in the form of tables, bar plots, modified boxplots (median and quartiles), and Kaplan-Meier curves. Simple t-tests were used to test differences in lead-times, two proportions z-tests were used to test differences between proportions, and 2-sided log-rank tests were used to test for differences in survival. The patient population was split into two periods for time dependent comparisons, as there were too few patients during the first period for meaningful calculations. Statistical analyses dependent on time and/or outcome were performed with patients classified as Luminal A and Luminal B treated as one subgroup (referred to as Luminal) BC. HER2+ patients were analysed in one group called HER2+ regardless of ER and PgR status.
Results
Clinical characteristics
We retrospectively identified 191 patients (190 female and 1 male) with BM from BC who have been registered with diagnostic codes at Sahlgrenska University Hospital, Gothenburg, Sweden from 1st of January 1994 through 31st December 2014. The initial selection, BC and BM resulted in 264 patients. The population diminished after charts were reviewed as 73 were excluded due to a misclassification, or to the BM being due to other malignancies, most commonly lung cancer (n = 40) or malignant melanoma (n = 8). For detailed patient selection, see the Consolidated Standards Of Reporting Trials (CONSORT) diagram shown in (Fig. 1). All clinical characteristics are summarized and listed for both time periods in Table 1. The median age at diagnosis of primary disease was 51 years (range 25–83). Stage at diagnose of primary BC were as follows; stage I 13%; stage II 37%; stage III 32% and 16% were de novo metastatic at diagnose. Eight (4%) of the patients had BM at the time of diagnosis of BC.
Table 1.
Clinical characteristics of the patients, as well as their treatments over the course of metastatic disease.Left column, all patients, middle column the first time period and the right column is the last time period. studied.
| Both Time Periods |
1994–2004 |
2005–2014 |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All (n = 191) | Luminal (n = 61) | TNBC (n = 57) | HER2+/ER+ (n = 26) | HER2+/ER- (n = 37) | HER2 status missing (n = 10) | All (n = 45) | Luminal (n = 12) | TNBC (n = 11) | HER2+/ER+ (n = 4) | HER2+/ER- (n = 12) | HER2 status missing (n = 6) | All (n = 146) | Luminal (n = 49) | TNBC (n = 46) | HER2+/ER+ (n = 22) | HER2+/ER- (n = 25) | HER2 status missing (n = 4) | |
| Age at diagnosis of BC (years) | ||||||||||||||||||
| Mean (Standard Deviation (SD) | 51(12.6) | 52 (11.1) | 52.1 (13.3) | 47 (11) | 48.7 (12.7) | 59.2 (14.6) | 51.3 (11.3) | 50.4 (11) | 54.8 (9.3) | 50.3 (6.5) | 46.1 (11.1) | 59.4 (13.6) | 51 (12.8) | 52.2 (11.3) | 51.8 (13.8) | 46.4 (11.7) | 49.9 (13.4) | 61 (19.8) |
| Median (min; max) | 50 (25.5;84) | 51 (46.9; 81.2) | 51.8 (25.5; 82.5) | 49.3 (27.6; 68) | 47.2 (28.6; 72.4) | 55 (38.6; 83.7) | 50.3 (29.4; 77.7) | 48.8 (34.3; 76.5) | 52.4 (42.9; 67.6) | 51.2 (42.2; 57) | 47.7 (29.4; 66.1) | 59.7 (38.6; 77.7) | 50.2 (25.5; 83.7) | 52.5 (34.6; 81.3) | 51.3 (25.5; 82.6) | 48.3 (27.6; 68.1) | 46.4 (28.6; 72.4) | 51.9 (47.5; 83.7) |
| Age at diagnosis of BM (years) | ||||||||||||||||||
| Mean (SD) | 56.5 (13) | 59 (11.2) | 55 (13.4) | 52 (11) | 53 (13.3) | 67.8 (13.7) | 55.7 (12) | 56.5 (77.8) | 54.5 (9.1) | 54.5 (8.3) | 49.1 (10.6) | 65.5 (15.5) | 56.4 (13.2) | 59.7 (11.1) | 55 (14.1) | 51.6 (11.6) | 54.9 (14.4) | 72.3 (10.2) |
| Median (min; max) | 56 (28; 84) | 58.5 (38; 84) | 54 (28; 83) | 52 (31; 73) | 51 (30; 80) | 68 (41; 84) | 54 (30; 82) | 56 (47; 71) | 56 (24; 71) | 55.5 (45; 62) | 50 (30; 68) | 65.5 (41; 82) | 56 (28; 84) | 61 (38; 84) | 54 (28; 83) | 52 (31; 73) | 53 (30; 80) | 68 (65; 84) |
| Menopausal Status | ||||||||||||||||||
| Pre-menopausal | 69 (36%) | 24 (39%) | 16 (28%) | 14 (54%) | 14 (38%) | 1 (10%) | 12 (27%) | 5 (42%) | 1 (9%) | 1 (25%) | 4 (33%) | 1 (16.5%) | 57 (39%) | 19 (39%) | 15 (33%) | 13 (59%) | 10 (40%) | 0 |
| Post-menopausal | 79 (41%) | 25 (41%) | 28 (49%) | 7 (27%) | 14 (38%) | 5 (50%) | 16 (36%) | 3 (25%) | 5 (45.5%) | 1 (25%) | 3 (25%) | 4 (67%) | 63 (43%) | 22 (45%) | 23 (50%) | 6 (27%) | 11 (44%) | 1 (25%) |
| Missing | 43 (23%) | 12 (20%) | 13 (23%) | 5 (19%) | 9 (24%) | 4 (40%) | 17 (38%) | 4 (33%) | 5 (45.5%) | 2 (50%) | 5 (42%) | 1 (16.5%) | 26 (18%) | 8 (16%) | 8 (17%) | 3 (14%) | 4 (16%) | 3 (75%) |
| Stage at diagnosis of BC | ||||||||||||||||||
| I | 25 (13%) | 8 (13%) | 5 (9%) | 5 (19%) | 3 (8%) | 2 (20%) | 9 (20%) | 3 (25%) | 2 (18%) | 2 (50%) | 0 | 2 (33%) | 14 (11%) | 5 (10%) | 3 (6.5%) | 3 (14%) | 3 (12%) | |
| II | 71 (37%) | 22 (36%) | 20 (35%) | 12 (46%) | 12 (32%) | 5 (50%) | 18 (40%) | 5 (42%) | 3 (27%) | 2 (50%) | 5 (42%) | 3 (50%) | 53 (36%) | 17 (35%) | 17 (35%) | 10 (45.5%) | 7 (28%) | 2 (50%) |
| III | 60 (32%) | 19 (31%) | 22 (39%) | 7 (27%) | 12 (32%) | 0 | 7 (16%) | 0 | 3 (27%) | 0 | 4 (33%) | 0 | 53 (36%) | 19 (39%) | 19 (41%) | 7 (32%) | 8 (32%) | |
| IV | 31(16%) | 9 (15%) | 10 (17%) | 2 (8%) | 9 (24%) | 3 (30%) | 9 (20%) | 3 (25%) | 3 (27%) | 0 | 2 (17%) | 1 (17%) | 24 (16%) | 6 (12%) | 6 (12%) | 2 (9%) | 7 (28%) | 2 (50%) |
| Missing | 4 (2%) | 3 (5%) | 0 | 0 | 1 (3%) | 0 | 2 (4%) | 1 (8%) | 0 | 0 | 1 (8%) | 0 | 2 (1%) | 2 (4%) | 0 | 0 | 0 | |
| Histological Subtype | ||||||||||||||||||
| Ductal Invasive | 156 (82%) | 50 (82%) | 48 (84%) | 19 (73%) | 33 (89%) | 6 (60%) | 36 (80%) | 8 (67%) | 10 (91%) | 4 (100%) | 11 (92%) | 3 (50%) | 120 (82%) | 42 (86%) | 38 (83%) | 15 (68%) | 22 (88%) | 3 (75%) |
| Lobular Invasive | 20 (10%) | 8 (13%) | 4 (7%) | 5 (19%) | 1 (3%) | 2 (20%) | 4 (9%) | 2 (16.5%) | 1 (9%) | 0 | 1 (17%) | 16 (11%) | 6 (12%) | 3 (7%) | 5 (23%) | 1 (4%) | 1 (25%) | |
| Other | 14 (7%) | 2 (3%) | 5 (9%) | 2 (8%) | 3 (8%) | 2 (20%) | 5 (11%) | 2 (16.5%) | 0 | 1 (8%) | 2 (33%) | 9 (6%) | 1 (2%) | 5 (10%) | 2 (9%) | 2 (8%) | 0 | |
| Missing | 1 (1%) | 1 (2%) | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 | 0 | 0 | 0 | ||||||
| Treatment at diagnosis of BC∗ | ||||||||||||||||||
| None | 27 (14%) | 10 (16%) | 5 (9%) | 3 (11.5%) | 4 (11%) | 5 (50%) | 16 (35%) | 6 (50%) | 4 (36%) | 2 (50%) | 1 (8%) | 3 (50%) | 11 (7.5% | 4 (8%) | 1 (2%) | 1 (4.5%) | 3 (12%) | 2 (50%) |
| Anthracyclin Only | 56 (29%) | 8 (13%) | 24 (42%) | 4 (15%) | 18 (49%) | 1 (10%) | 20 (43%) | 1 (8%) | 6 (55%) | 2 (50%) | 10 (84%) | 1 (17%) | 35 (24%) | 7 (14%) | 18 39%) | 2 (9%) | 8 (32%) | 0 |
| Anthracycline and Taxane | 21 (11%) | 0 | 20 (35%) | 0 | 1 (3%) | 0 | 1 (2%) | 0 | 1 (9%) | 0 | 1 (8%) | 0 | 19 (13%) | 0 | 19 (41%) | 0 | 0 | 0 |
| Endocrine Treatment Only | 21 (11%) | 16 (26%) | 0 | 2 (7%) | 1 (3%) | 2 (20%) | 5 (10%) | 3 (25%) | 0 | 0 | 0 | 2 (33%) | 16 (11%) | 13 (27%) | 0 | 2 (9%) | 1 (4%) | 0 |
| Chemotherapy and Endocrine treatment | 33 (17%) | 21 (34%) | 2 (3%) | 9 (35%) | 0 | 1 (10%) | 2 (4%) | 33 (22.5%) | 21 (43%) | 2 (4%) | 9 (41%) | 0 | 1 (25%) | |||||
| HER2 Therapy and Chemotherapy | 11 (6%) | 0 | 0 | 1 (4%) | 10 (27%) | 0 | 0 | 11 (7.5%) | 0 | 0 | 1 (4.5%) | 10 (40%) | 0 | |||||
| HER2-, Chemo- and Endocrine Therapy | 7 (4%) | 0 | 0 | 5 (19%) | 2 (5%)0 | 0 | 0 | 7 (5%) | 0 | 0 | 5 (23%) | 2 (8%) | 0 | |||||
| Missing | 8 (4%) | 0 | 3 (5%) | 1 (4%) | 1 (3%) | 1 (10%) | 0 | 8 (5.5%) | 2 (4%) | 3 (6.5%) | 1 (4.5%) | 1 (4%) | 1 (25%) | |||||
| BM at diagnosis, treatment listed at BM | 9 (4%) | 3 (6%) | 6 (4%) | 2 (4%) | 3 (6.5%) | 1 (4.5%) | 0 | 0 | ||||||||||
| BM at diagnosis of BC | ||||||||||||||||||
| Yes | 8 (4%) | 4 (7%) | 4 (7%) | 1 (4%) | 0 | 0 | 3 (7%) | 2 (17%) | 1 (9%) | 4 (100%) | 6 (4%) | 2 (4%) | 3 (6%) | 1 (4%) | 25 (100%) | 4 (100%) | ||
| No | 183 (96%) | 57 (94%) | 53 (93%) | 25 (96%) | 37 (100%) | 10 (100%) | 42 (93%) | 10 (83%) | 10 (91%) | 12 (100%) | 6 (100%) | 140 (96%) | 47 (96%) | 43 (94% | 21 (96%) | |||
| Brain Metastases prior to other metastases | ||||||||||||||||||
| BM only | 48 (25%) | 10 (16%) | 17 (30%) | 10 (38.5%) | 11 (30%) | 0 | 13 (29%) | 2 (17%) | 4 (36%) | 3 (75%) | 4 (33%) | 0 | 35 (24%) | 8 (16%) | 13 (28%) | 7 (32%) | 7 (28%) | 0 |
| BM after extra cranial mets | 95 (50%) | 33 (54%) | 23 (40%) | 10 (38.5%) | 20 (54%) | 8 (80%) | 20 (44%) | 4 (33%) | 4 (36%) | 0 | 8 (67%) | 4 (67%) | 74 (51%) | 29 (59%) | 19 (41%) | 10 (45.5%) | 12 (48%) | 4 (100%) |
| BM and extra cranial mets simultaneously | 33 (17%) | 12 (20%) | 12 (21%) | 6 (23%) | 2 (5%) | 2 (20%) | 10 (22%) | 4 (33%) | 3 (27%) | 1 (25%) | 0 | 2 (33%) | 24 (16%) | 8 (16%) | 9 (20%) | 5 (22.5%) | 2 (8%) | 0 |
| Extra cranial mets after BM | 15 (8%) | 6 (10%) | 5 (9%) | 0 | 4 (11%) | 0 | 2(4%) | 1 (17%) | 0 | 0 | 0 | 0 | 13 (9%) | 4 (8%) | 5 (11%) | 0 | 4 (16%) | 0 |
| Symtms at diagnosis of BM | ||||||||||||||||||
| None/discovered en passant | 6 (3%) | 1 (2%) | 2 (4%) | 3 (12%) | 0 | 0 | 0 | 6 (4%) | 1 (2%) | 2 (4%) | 3 (13.5%) | 0 | 0 | |||||
| 1-2 symtoms | 168 (88%) | 57 (93%) | 46 (81%) | 22 (85%) | 33 (89%) | 10 (100%) | 40 (89%) | 11 (92%) | 9 (82%) | 4 (100%) | 10 (83%) | 6 (100%) | 128 (88%) | 46 (94%) | 37 (80%) | 18 (82%) | 23 (92%) | 4 (100%) |
| 3 or more symtoms | 17 (9%) | 3 (5%) | 9 (16%) | 1 (4%) | 4 (11%) | 0 | 5 (11%) | 1 (8%) | 2 (18%) | 2 (17%) | 12 (8%) | 2 (4%) | 7 (15%) | 1 (4.5%) | 2 (8%) | |||
| Diagnosis by: | ||||||||||||||||||
| CT Scan | 102 (53%) | 28 (46%) | 34 (60%) | 16 (62%) | 19 (51%) | 7 (70%) | 31 (69%) | 6 (50%) | 8 (73%) | 3 (75%) | 9 (75%) | 5 (83%) | 73 (50%) | 22 (45%) | 26 (56.5%) | 13 (59%) | 10 (40%) | 2 (50%) |
| MRI | 25 (13%) | 9 (15%) | 4 (7%) | 5 (19%) | 4 (11%) | 1 (10%) | 4 (9%) | 2 (17%) | 0 | 1 (25%) | 0 | 1 (17%) | 19 (13%) | 7 (14%) | 4 (9%) | 4 (18%) | 4 (16%) | 0 |
| CT-Scan followed by MRI | 61 (32%) | 24 (39%) | 17 (30%) | 5 (19%) | 14 (37%) | 1 (10%) | 9 (20%) | 4 (33%) | 2 (18%) | 0 | 3 (25% | 0 | 52 (36%) | 20 (41%) | 15 (32.5%) | 5 (23%) | 11 (44%) | 1 (25%) |
| Lumbal puncture | 3 (2%) | 0 | 2 (3%) | 0 | 0 | 1 (10%) | 1 (2%) | 1 (9%) | 0 | 0 | 0 | 2 (1%) | 0 | 1 (2%) | 0 | 0 | 1 (25%) | |
| Location of BM | ||||||||||||||||||
| Meningeal Carcinosis only | 7 (4%) | 3 (5%) | 1 (2%) | 2 (8%) | 0 | 1 (10%) | 1 (2%) | 0 | 1 (9%) | 0 | 0 | 0 | 6 (4%) | 3 (6%) | 0 | 2 (9%) | 0 | 1 (25%) |
| Cerebrum Only | 98 (51%) | 25 (41%) | 38 (67%) | 11 (42%) | 19 (51%) | 5 (50%) | 29 (64%) | 9 (75%) | 7 (64%) | 3 (75%) | 7 (58%) | 3 (50%) | 69 (47%) | 16 (33%) | 31 (67%) | 8 (36%) | 12 (48%) | 2 (50%) |
| Cerebellum Only | 28 (15%) | 14 (23%) | 7 (12%) | 1 (4%) | 6 (16%) | 0 | 4 (9%) | 1 (8%) | 2 (18%) | 0 | 1 (8%) | 0 | 24 (16%) | 13 (27%) | 5 (11%) | 1 (4.5%) | 5 (20%) | |
| Cerebrum and Cerebellum | 39 (20%) | 12 (20%) | 9 (16%) | 7 (27%) | 8 (22%) | 3 (30%) | 9 (20%) | 1 (8%) | 1 (9%) | 1 (25%) | 4 (33%) | 2 (33%) | 30 (20.5%) | 11 (22%) | 8 (17%) | 6 (27%) | 4 (16%) | 1 (25%) |
| Meninges and Cerebrum | 10 (5%) | 5 8%) | 2 (4%) | 1 (4%) | 1 (3%) | 1 (0%) | 8 (5.5%) | 4 (8%) | 2 (4%) | 1 (4.5%) | 1 (4%) | |||||||
| Meninges and Cerebellum | 3 (2%) | 0 | 0 | 2 (8%) | 1 (3%) | 0 | 2 (4%) | 1 (8%) | 1 (17%) | 3 (2%) | 0 | 0 | 2 (9%) | 1 (4%) | ||||
| All three locations | 6 (3%) | 2 (3%) | 0 | 2 (8%) | 2 (5%) | 0 | 6 (4%) | 2 (4%) | 0 | 2 (9%) | 2 (8%) | |||||||
| Treatment of Extra Cranial Metastases (n = 95) | (n = 23) | (n = 6) | (n = 5) | (n = 8) | (n = 4) | |||||||||||||
| None | 10 (11%) | 2 (5%) | 4 (15%) | 0 | 2 (10%) | 2 (25%) | 4 (17%) | 2 (33%) | 1 (20%) | 1 (12.5%) | 2 (50%) | |||||||
| Chemotherapy | 21 (22%) | 4 (11%) | 14 (52%) | 0 | 3 (15%) | 1 (12.5%) | 2 (9%) | 0 | 3 (60%) | 2 (25%) | ||||||||
| Endocrine Therapy | 5 (5%) | 2 (5% | 0 | 0 | 1 (5%) | 2 (25%) | 1 (4%) | 0 | 0 | 1 (25%) | 1 (25%) | |||||||
| Chemo and Endocrine Therapy | 32 (33%) | 4 (67%) | 4 (15%) | 0 | 1 (5%) | 2 (25%) | 9 39%) | 4 (67%) | 0 | 1 (25%) | 1 (25%) | |||||||
| HER2 and Chemotherapy | 16 (17%) | 0 | 0 | 5 (46%) | 3 (15%) | 1 (12.5%) | 3 (13%) | 0 | 0 | 0 | ||||||||
| HER2, Endocrine and Chemotherapy | 10 (11%) | 1 (3%) | 1 (4%) | 0 | 0 | 0 | ||||||||||||
| Missing | 1 (1%) | 3 (13%) | 1 (20%) | 0 | ||||||||||||||
| Surgery and Radiotherapy Following BM | ||||||||||||||||||
| None | 31 (16%) | 15 (25%) | 6 (11%) | 2 (8%) | 4 (11%) | 4 (40%) | 9 (20%) | 1 (8%) | 3 (27%) | 0 | 1 (8%) | 4 (67%) | 22 (15%) | 14 (29%) | 3 (6.5%) | 2 (9%) | 3 (12%) | 0 |
| Surgical resection | 20 (11%) | 6 (10%) | 7 (12%) | 1 (4%) | 4 (11%) | 2 (20%) | 6 (13%) | 1 (8%) | 2 (18%) | 0 | 2 (17%) | 1 (16.5%) | 14 (10%) | 5 (10%) | 5 (11%) | 1 (4.5%) | 2 (8%) | 1 (25%) |
| Surgical Resection followed by radiotherapy | 50 (26%) | 15 (25%) | 16 (28%) | 6 (23%) | 13 (35%) | 0 | 15 (33%) | 6 (50%) | 3 (27%) | 1 (25%) | 5 (42%) | 0 | 35 (24%) | 9 (18%) | 13 (28%) | 5 (23%) | 8 (32%) | 0 |
| Whole Brain Radiotherapy | 72 (38%) | 21 (34%) | 24 (42%) | 12 (46%) | 11 (30%) | 4 (40%) | 11 (25%) | 4 (33%) | 3 (27%) | 0 | 3 (25%) | 1 (16.5%) | 61 (42%) | 17 (35%) | 21 (46%) | 12 (54.5%) | 8 (32%) | 3 (75%) |
| Stereotactic Radiotherapy | 10 (5%) | 2 (3%) | 1 (2%) | 3 (12%) | 4 (11%) | 0 | 2 (4%) | 0 | 0 | 1 (25%) | 1 (8%) | 0 | 8 (6%) | 2 (4%) | 1 (2%) | 2 (9%) | 3 (12%) | 0 |
| Combinations of radiotherapy | 8 (4%) | 2 (3%) | 3 (5%) | 2 (8%) | 1 (3%) | 0 | 2 (4%) | 0 | 0 | 2 (50%) | 0 | 0 | 6 (4%) | 2 (4%) | 3 (6.5%) | 0 | 1 (4%) | 0 |
| Systemic therapy after BM | ||||||||||||||||||
| None | 73 (38%) | 16 (26%) | 33 (59%) | 3 (11.5%) | 19 (52%) | 2 (20%) | 16 (36%) | 1 (8%) | 7 (64%) | 0 | 6 (50%) | 2 (33%) | 57 (39%) | 15 (31%) | 26 (58%) | 3 (13.5%) | 13 (52%) | |
| Chemotherapy | 33 (17%) | 10 (16%) | 19 (34%) | 1 (3.5%) | 1 (3%) | 2 (20%) | 7 (16%) | 2 (17%) | 4 (36%) | 0 | 1 (8%) | 0 | 26 (18%) | 8 (16%) | 15 (33%) | 1 (4.5%) | 0 | 2 (50%) |
| HER2 Therapy | 6 (3%) | 0 | 1 (2%) | 1 (3.5%) | 4 (3%) | 0 | 2 (4%) | 0 | 0 | 0 | 2 (17%) | 0 | 4 (3%) | 0 | 1 (2%) | 1 (4.5%) | 2 (8%) | |
| Endocrine Therapy | 36 (19%) | 20 (33%) | 2 (4%) | 7 (27%) | 3 (8%) | 4 (40%) | 12 (27%) | 6 (50%) | 0 | 2 (50%) | 1 (8%) | 3 (50%) | 24 (17%) | 14 (29%) | 2 (4%) | 5 (23%) | 2 (8%) | 1 (25%) |
| Chemo and Endocrine Therapy | 19 (10%) | 13 (21%) | 0 | 3 (11.5%) | 1 (3%) | 2 (20%) | 6 (13%) | 3 (25%) | 0 | 2 (50%) | 1 (17%) | 0 | 13 (9%) | 10 (20%) | 0 | 1 (4.5% | 0 | 1 (25%) |
| HER2 and Chemotherapy | 18 (9%) | 1 (2%) | 1 (2%) | 8 (31%) | 8 (22%) | 0 | 2 (4%) | 0 | 0 | 0 | 2 (17%) | 1 (17%) | 16 (11%) | 1 (2%) | 1 (2%) | 8 (36%) | 7 (30%) | |
| HER2, Endocrine and Chemotherapy | 3 (2%) | 0 | 0 | 3 (11.5%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (2%) | 0 | 0 | 3 (14%) | 0 | |
| Missing | 3 (2%) | 2 (1%) | 1 (2%) | 0 | 1 (4%) | |||||||||||||
| Size of BM (mm) | ||||||||||||||||||
| Mean (SD) | 26.1 (12.6) | 25.7 (13.9) | 24.4 (10.3) | 25.3 (14.8) | 31 (11.4) | 19.8 ((10.3) | 27.2 (13.4) | 24.6 (14.2) | 30.3 (12.3) | 25.8 (8.1) | 32.9 (14) | 17.5 (11.3) | 25.8 (12.4) | 26 (13.9) | 23.3 (9.7) | 25.3 (15.9) | 30.2 (10) | 24.3 (8.1) |
| Median (min; max) | 25 (5; 70) | 20 (5; 60) | 22 (10; 50) | 22.5 (15; 65) | 30 (5; 60) | 20 (5; 35) | 30 (5; 60) | 20 (10; 50 | 27.5 (15; 50) | 25 (18; 35) | 32.5 (5; 60) | 15 (5; 35) | 25 (5;70) | 20 (5; 60) | 20 (10; 50) | 22.5 (5; 70) | 30 (5; 52) | 8.1 (15; 30) |
| Number of BM | ||||||||||||||||||
| 0 (Meningeal Carcinosis Only) | 6 (3%) | 2 (3%) | 1 (7%) | 2 (8%) | 0 | 1 (10%) | 1 (2%) | 0 | 1 (9%) | 0 | 0 | 1 (2%) | 5 (3%) | 2 (4%) | 0 | 2 (9%) | 0 | 1 (25%) |
| 1 | 79 (41%) | 26 (43%) | 27 (47%) | 8 (31%) | 14 (38%) | 4 (40%) | 22 (49%) | 7 (58%) | 6 (55%) | 2 (50%) | 5 (42%) | 2 (33%) | 57 (39%) | 19 (39%) | 21 (46%) | 6 (27%) | 9 (36%) | 2 (50%) |
| 2 | 24 (13%) | 6 (10%) | 7 (12%) | 3 (12%) | 8 (22%) | 0 | 4 (9%) | 0 | 1 (9%) | 0 | 3 (25%) | 0 | 20 (14%) | 6 (12%) | 6 (13%) | 3 (14%) | 5 (20%) | |
| 3 | 14 (7%) | 5 (8%) | 3 (5%) | 2 (8%) | 3 (8%) | 1 (10%) | 2 (4%) | 1 (8%) | 0 | 0 | 0 | 1 (17%) | 12 (8%) | 4 (8%) | 3 (6%) | 2 (9%) | 3 (12%) | |
| ≥4 | 68 (36%) | 22 (36%) | 19 (33%) | 11 (42%) | 12 (32%) | 4 (40%) | 16 (36%) | 4 (33%) | 3 (27%) | 2 (50%) | 4 (33%) | 3 (50%) | 52 (36%) | 18 (37%) | 16 (35%) | 9 (41%) | 8 (32%) | 1 (25%) |
| Survival Statistics | ||||||||||||||||||
| Brain Metastases Free Interval (months) | ||||||||||||||||||
| Mean (SD) | 62 (62) | 85.8 (76.1) | 40.5 (44.4) | 60 (37.1) | 52 (57.8) | 95.2 (77.8) | 53.1 (42.5) | 73.1 (52) | 35.2 (28.2) | 50.6 (39.6) | 37.1 (31.5) | 74.1 (47.1) | 65.5 (67) | 89 (81.3) | 41.6 (47) | 61.9 (37.4) | 60 (66.6) | 137.3 (121) |
| Median (min; max) | 43.5 (0–331) | 67 (0; 331) | 28.5 (0; 218.09 | 58.3 (0; 149.6) | 30.9 (9.2; 271.3) | 63.5 (6; 244) | 44 (0;175.7) | 71.2 (0; 175.6) | 22.4 (5; 74.2) | 51.2 (4; 95.8) | 30.9 (12; 128.6) | 61.3 (31; 164) | 43.5 (0; 330) | 64.5 (0; 330) | 30.2 (0; 218.1) | 58.4 (0; 149.6) | 31.4 (9.2; 271.3) | 161.6 (6; 244.3) |
| Survival after Brain Metastases (months) | ||||||||||||||||||
| Mean (SD) | 16 (24.5) | 23.3 (37.7) | 9.4 (9.4) | 17.3 (15) | 16 (19.1) | 7.14 (4.9) | 22.7 (38.5) | 50.6 (64.8) | 4.7 (4.5) | 23.9 (4.5) | 16.8 (12.3) | 6.5 (5.5) | 14 (17.9) | 16.4 (23.5) | 10.2 (9.8) | 16 (12.2) | 15.6 (22) | 8.3 (4) |
| Median (min; max) | 7.9 (0; 212) | 7.7 (0; 212) | 5.6 (0; 52) | 12.9 (2.8; 62.3) | 9.5 (0; 93) | 5.9 (0; 14.3) | 11.5 (0; 212.9) | 20.5 (2.5; 213) | 4.1 (0; 14.9) | 15.1 (2.9; 62.3) | 16.4 (0; 43.04) | 5.2 (0; 14) | 7.2 (0; 111) | 6.4 (0; 111.2) | 6.5 (0; 52) | 12.9 (2.8; 52) | 5.6 (0; 93) | 9.5 (3.8; 11.6) |
| Overall Survival (months) | ||||||||||||||||||
| Mean (SD) | 78.6 (67) | 109 (83.2) | 49.9 (45.8) | 77.3 (39.6) | 68 (59.4) | 102 (78.8) | 75.8 (59.9) | 123.7 (80.7) | 39.8 (31) | 74.5 (37.7 | 53.9 (37.5) | 80.6 (45.5) | 79.5 (69.6) | 105.5 (84.4) | 51.8 (48.1) | 77.9 (40.9) | 75.6 (67.2) | 145.6 (124.3) |
| Median (min; max) | 58 (5; 337) | 81.7 (18.7–337.4) | 37.9 (5.1; 228) | 69.7 (15.6; 178) | 47 (12.3; 276.8) | 69.4 (9.9; 253.9) | 61.6 (5.7; 284.2) | 105.8 (35; 284.2) | 26 (5.7; 89.1) | 83.8 (23; 107.3) | 44.4 (12.9; 156.1) | 66.5 (45.3; 166.7) | 55.4 (5.2; 337.4) | 81.3 (18.7; 337.4) | 38.7; 5.1; 228) | 69 (15.6; 178) | 48.8 (14.4; 276.8) | 173.2 (9.9; 253.8) |
Since most tumours have been re-evaluated using modern techniques some patients have received treatment that was ineffective according to subtype.
∗For the 9 patients that were diagnosed with BM at diagnosis of BC their treatment is listed as treatment after BM.
The median age at diagnosis of BM was 56 years (range 28–84 years). Only 16% were 70 or older when diagnosed with primary BC. Most patients had symptoms at the time of diagnosis (185/191, 97%). In six patients, BM was an incidental discovery, through radiology, PET-scan, or autopsy. Magnetic Resonance Imaging (MRI) increased in usage for diagnosis of BM after 2005. Computerized tomography (CT) was used in 53% of the patients, MRI alone 13%, CT followed by MRI 32%, and 3% through other means such as lumbar puncture or autopsy. The localisation and extent of BM were: cerebrum only in 98 patients (51%), cerebellum only in 28 patients (15%), combined in 39 patients (20%), 1–3 metastases 116 patients (61%), ≥4 metastases 67 patients (35%), and meningeal carcinomatosis alone, 8 patients (4%). A trend showing that it is more common with meningeal carcinomatosis for lobular BC was found. Out of the 28 patients with meningeal carcinomatosis alone or combined with BM, 21% (n = 6) were of lobular subtype compared to 11% (n = 20) in the whole patient population.
Out of the 67 patients with solitary BM, 59 (88%) had surgical resection, whereas 6 out of 37 (16%) with 2–3 metastases had surgery.
Patients were classified into BC subgroups according to the re-evaluated primary tumours (n = 148) and, when not available, data was collected from the original report by the pathologist (n = 43). Out of the primary tumours 61 were Luminal (32%), 57 TNBC (30%), 26 HER2+/ER+ (14%), and 37 HER2+/ER- (19%). Ten patients could not be fully classified due to missing information on HER2 and were excluded from statistical analysis based on BC subgroups, but kept in the tables. Tumours only lacking information regarding Ki67 status were included in analyses, as the Luminal A and B tumours were grouped as Luminal.
Comparisons in clinical characteristics between time periods
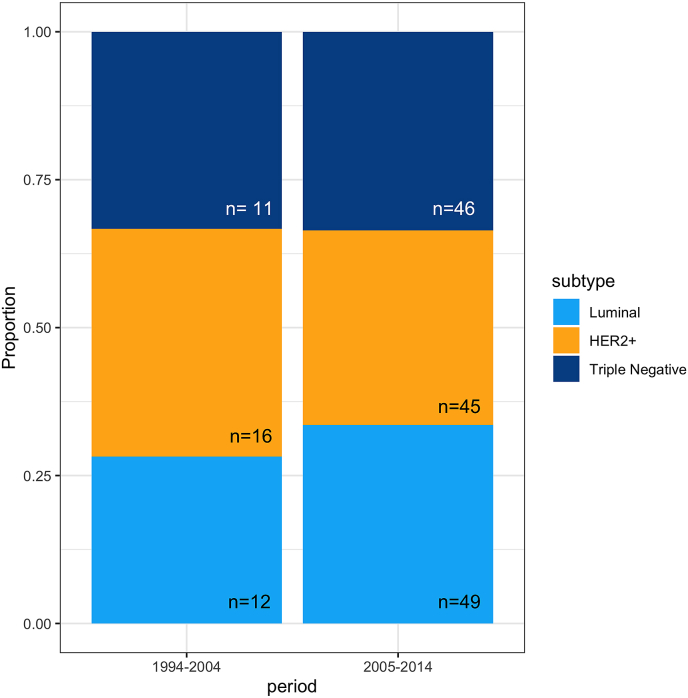
Please refer to table one for complete data on clinical characteristics in the two time periods. When comparing subgroups of BC in patients with BM the proportion of subgroup were equal (Fig. 3, Luminal: p = 0.661, HER2 positive: p = 0.6447, TNBC: p = 1). Also there was no difference in the proportion of patients with metastases solely in the brain between BC subgroups, Luminal: 21%, HER2+: 33%, and TNBC: 28%, nor was there any change in the occurrence of BM as first recurrence. A simple z-test gives the following p-values for difference between BC subtypes: (HER2+ vs Luminal: p = 0.1998; HER2+ vs TNBC: p = 0.738; Luminal vs TNBC: p = 0.453).
Fig. 3.
Boxplot depicting subgroup (Luminal, HER2+ and TNBC) of breast cancer in patients with brain metastases comparing two periods (1994–2004 and 2005–2014).
No patient received HER2 adjuvant therapy in the first period as it was not introduced at that time in Sweden. In the second time period 28% of HER2+ patients received HER2 blocking therapies in the adjuvant setting. The number of patients receiving no adjuvant treatment at all were 35% in the first time period and 7.5% in the second.
Method of diagnosis of BM differed between time periods with 49% having an MRI in the second time period but only 29% in the first.
Number of BM
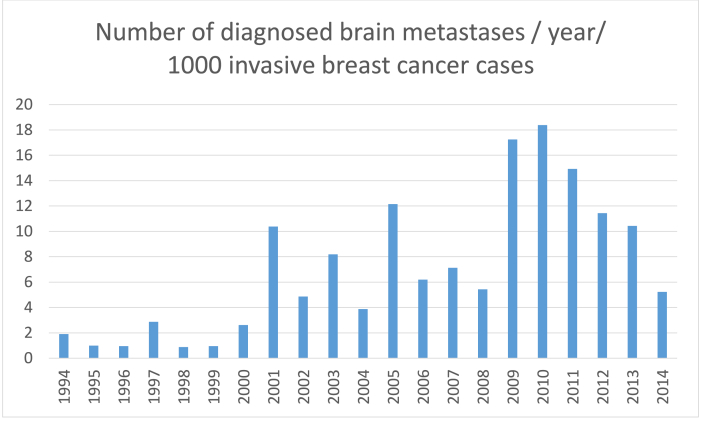
The results show a successive increment in number of patients affected of BM when comparing the four time periods, 1994-99 (n = 9); 2000-04 (n = 36); 2005-09 (n = 60); 2010-14 (n = 86). The results are presented with patients split in two periods: 1994–2004 (n = 45) and 2005–2014 (n = 146) due to the very low number of patients in the first period. Fig. 4 depicts the number of patients diagnosed with BM in each year studied per 1000 patients diagnosed with invasive BC in the same region.
Fig. 4.
Bar diagram depicting the number of brain metastases per 1000 invasive breast cancer cases diagnosed, in the same region studied, between 1994 and 2014.
Time to BM
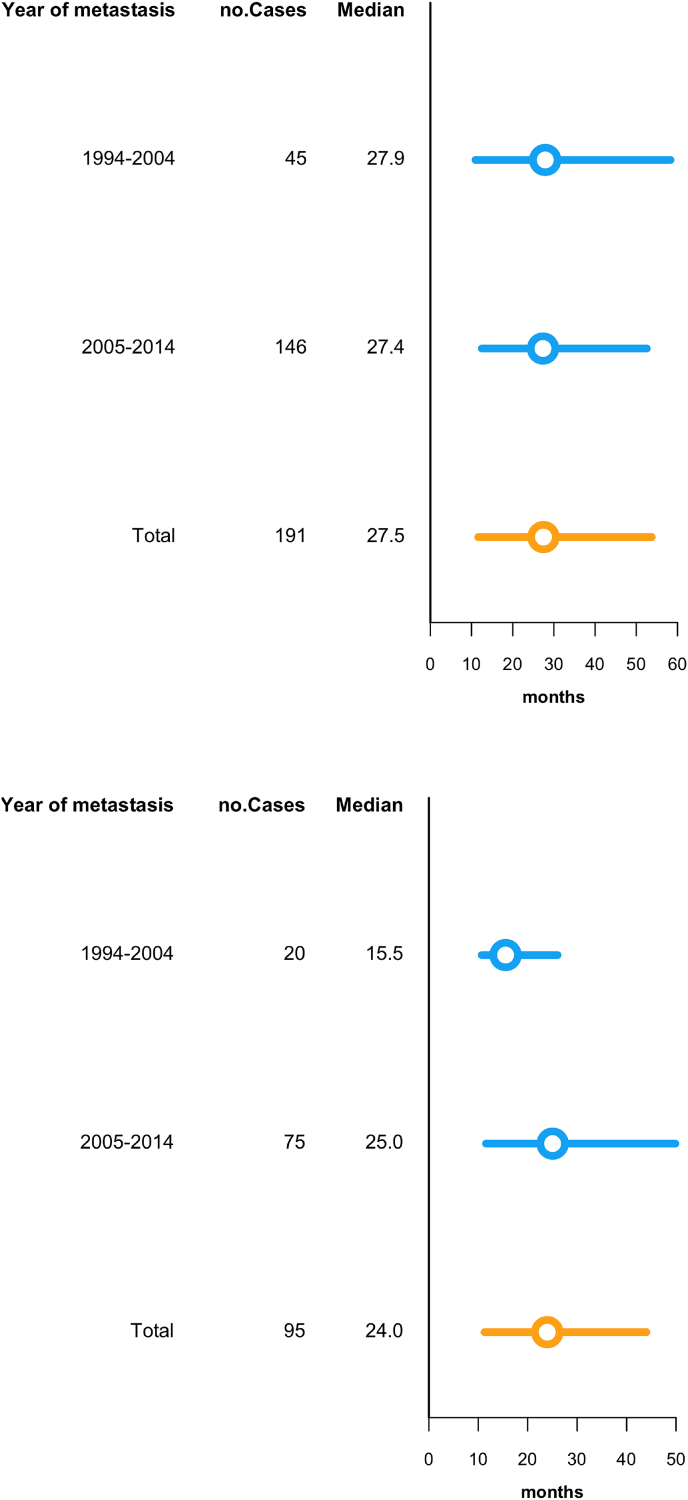
The median time from diagnosis of primary BC to BM (Fig. 2a) was 28 months (95% confidence interval [95% CI] 4.0–5.7) in the whole study period. Median time from diagnosis of metastatic disease to BM was 24 months (ranging from 1 to 191 months). Ninety-five patients in the material had extra cranial disease prior to BM, others developing extra cranial metastases at the same time or after BMs which influenced this interval. Time to BM from metastatic disease was compared in two time periods, 1994–2004 and 2005–2014, and showed no significant difference (Fig. 2b); BM were diagnosed after a median of 28.3 months during the 1st time period compared with 27.9 months during the 2nd time period (p = 0.41). The material was too small to compare BC subgroups in the two periods with respect to time from metastatic disease to BM.
Fig. 2.
a. Modified boxplot depicting time in months from primary diagnosis of breast cancer (n = 191) to brain metastasies comparing two periods (1994–2004 and 2005–2014. The left and right endpoints of the bars represent the first and third quartiles respectively, while the circle denotes the median. b. Modified boxplot depicting time in months from diagnosis of extra cranial metastases (n = 95) to brain metastases comparing two periods (1994–2004 and 2005–2014). The left and right endpoints of the bars represent the first and third quartiles respectively, while the circle denotes the median.
Time to diagnosis of BM after extra cranial relapse
We found an increase, however not statistically significant, in the time from diagnose of extra cranial relapse to development of BM with a median time of 15.5 months for patients 1994–2004 compared with 25 months for patients 2005–2014 (p = 0.0612).
Survival after primary breast cancer
Overall survival for the entire cohort was 4.7 years after diagnosis of BC (95% confidence interval [CI] 4.0–5.7). It varied between subgroups with 6.1 years for Luminal (95% CI 4.7–7.6 years), 4.3 years for HER2+ (95% CI 3.1–5.7), and 3.2 years for TNBC (95% CI 2.3–3.8 years). Patients with TNBC had a statistically shorter breast cancer specific survival both when compared with Luminal BC (p < 0.05) and when compared to HER2+ BC (p = 0.016), whilst no difference was found when Luminal and HER2+ BC were compared (p = 0.137) (Fig. 5).
Fig. 5.
Overall survival as calculated from primary diagnosis of according to subgroup of breast cancer (Luminal, HER2+ and TNBC).
Survival after diagnosis of BM
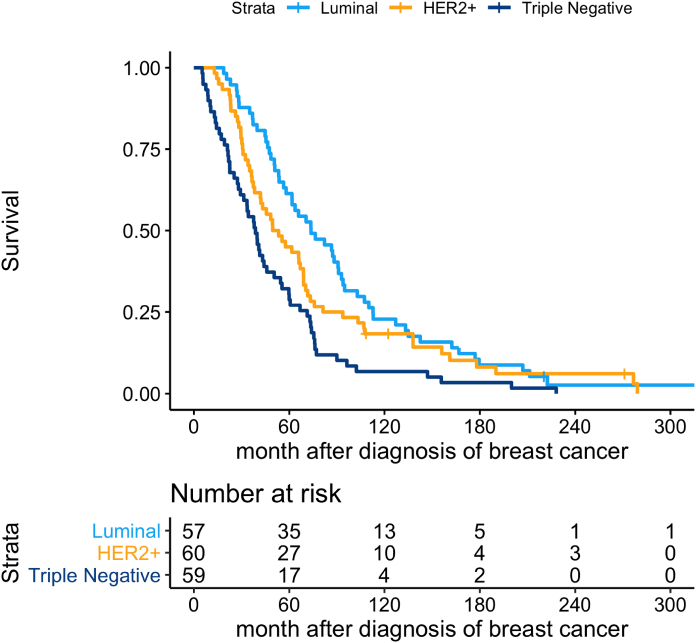
Survival for all patients after BM was 7 months (95% CI 6–10). In the subgroup analysis we found a statistically significant difference between HER2+ and TNBC with an inferior outcome for the latter (p = 0.018) whilst no difference was present when Luminal BC was compared with HER2+ (p = 0.073) (Fig. 6).
Fig. 6.
Overall survival as calculated from diagnosis of brain metastases according to subgroup of breast cancer (Luminal, HER2+ and TNBC).
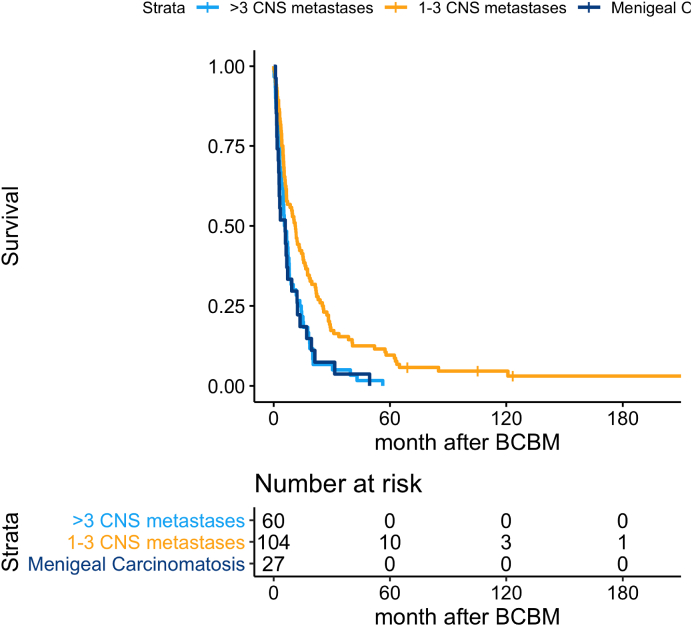
Survival according to extent of BM
Survival after BM was significantly longer when comparing 1–3 BM and meningeal carcinomatosis (p = 0.03), as well as 1–3 BM, and 4 or more BM (p < 0.05) (Fig. 7).
Fig. 7.
Overall survival according to extent of brain metastases; 1–3 metastases versus ≥4 metastases versus meningeal carcinomatosis.
Characteristics in patients exhibiting extended survival
A group of 21 out of the 191 patients was identified exhibiting extended survival after BM, chosen as 3 years or more. The BC subtypes of the 21 patients comprised of 11 Luminal, 9 HER2+, and one TNBC. Four of the patients had metastases at the time of diagnosis of primary BC, three being BM. The median age in the group showing extended survival was 44 years at time of primary BC. In the group five of the patients had BM only, seven had extra cranial metastases at least three months prior to BM, and nine were diagnosed with extra cranial disease simultaneously or after diagnose of BM. Almost all of the patients (17 out of 21 (81%)), had less than three BM. One patient had meningeal carcinomatosis alone. Fourteen of the patients had surgery once or multiple times to remove BM as well as radiotherapy combinations. All patients received systemic targeted therapy where suitable, and the patient with TNBC was subjected to radical surgical excision followed by stereotactic radiotherapy.
Discussion
In this unique population based material consisting of re-evaluated tumour material, we demonstrate an increase in patients diagnosed with BM of BC during a period of 21 years, from 45 in the first time period to 146 in the second. The result is in concordance with the few publications available [[4], [5], [6],13,[16], [17], [18]]. One limitation to the study is the lack of complete data on the number of recurring BC in the same time period. In addition it might be argued that increasing numbers of BM is due to an increase in incidence of BC, however as can be seen in Fig. 4 there is a trend towards a higher number of diagnosed BM despite the increase in diagnosis of BC. Mortality in breast cancer is caused by recurrent disease. BC specific mortality has been stable/decreasing since 2002 from 15.49 to 12.91/100 000 breast cancer patients according to WHO data [19]. Thus, the higher incidence in BC does not fully explain the increased number of patients with BM. Also the higher number of BM is far more apparent than the increased incidence of invasive BC in the same time period as we show in figure four. Most likely, the results are affected in part by the change in access to radiology and improved treatment options resulting in treating physicians more actively screening for BM, and consequently referring patients for consideration by surgeon/oncologist. We do not consider these factors sufficient to account for the large increase in BM. Another aspect is that as the general health in the population is improving, more patients are surviving other afflictions but they are also possibly better equipped to tolerate more therapy in the metastatic setting. The number of patients diagnosed with BM are extracted from the registration codes for any contact with the hospital regarding BC patients with BM at Sahlgrenska University Hospital as it is the only centre in the region to consider these patients for therapy.
There have been major achievements in the treatment of primary BC during the study period ranging from mid-1990s to 2014, such as the introduction of Taxanes, Aromatase Inhibitors, and Trastuzumab. None of the patients in the first time period studied in our material received adjuvant Trastuzumab as it started being used in Sweden after 1999 in the palliative setting and 2005 in the adjuvant setting. The vast majority of the practice-changing trials introducing the new therapies reported statistically significant reductions in unspecified distant metastases but specific data on BM are scarce [[20], [21], [22], [23], [24]]. However, concerning HER2 positive patients, both the HERA-trial as well as the joint survival analyses of NSABP B-31 and NCCTG N983 compared the proportion of patients diagnosed with BM in the groups with standard chemotherapy alone or combined with Trastuzumab [[25], [26], [27]]. Concordant results showed that the addition of one year of Trastuzumab had no preventive effect on the development of BM. Whether Taxanes work in a similar fashion, i.e. efficiently reduce distant metastases but not BM, was investigated in the CNS sub-study of the intergroup BIG 02–98 phase III trial evaluating the addition of docetaxel to standard chemotherapy. This trial reported a similar proportion of patients with BM irrespectively of therapy [14]. Moreover, preclinical models have shown that paclitaxel has a low penetration cross the BBB and is subject to an efficient efflux system of natural toxic compounds [28]. We found no difference in time from primary BC to diagnosis of BM when comparing the time periods. This finding supports that adjuvant treatment does not appear to prevent/delay development of BM.
During the whole study period, the BM-free interval for patients who only had extra cranial metastases at first relapse was 23 months, 15.5 months in the first period and 25 months in the second. The results indicate that local and systemic treatments in the metastatic setting have a positive impact on the prognosis of patients, prolonging the time to development of BM. Most likely this leads to more patients developing BM as a result of not expiring from extra cranial metastases. More treatment options might also select for tumour cells that penetrate the BBB. Concordant results have been presented in a study by Berghoff and colleagues. Patients were included during the years 1996–2010 with a median time from extra cranial disease to BM of 19 months compared to 23 months in our material [29]. Notably, almost half of the patients in our material were diagnosed synchronously with or prior to extra-cranial metastases. We consider this being due to our hospital having access to both radiotherapy and neurosurgery, resulting in referrals of patients with less extensive tumour burden.The corresponding figures in the Austrian study were 20% of the patients diagnosed with BM as the first site.
Survival in the subgroups was significantly longer for Luminal and HER2+ BC compared to TNBC, with no statistical difference between Luminal and HER2+. The survival difference most likely is due to the introduction of HER2 blocking therapies in the second time period. A Japanese material of 1466 patients with BM showed similar comparisons between the subgroups but somewhat longer survival in all BC subgroups [12]. The proportion of patients that received local treatment, surgery, and/or stereotactic radiotherapy are concordant to our group of patients. However, the time period studied is chronologically later (from the year 2000 forwards), and more efficient palliative systemic treatments were hence available.
Other studies show concordant results when comparing the subgroups of BC [[30], [31], [32], [33]]. As expected and demonstrated in previous publications, extent of intracranial disease had a significant effect on survival, with <3 BM granting longer survival [34]. These patients, in general, exhibit a better performance status and tolerability for repeated local treatment with stereotactic RT, surgery, and systemic therapy. There is no difference in the subgroups of the patients with BM in the two time periods, there is however a difference in survival, especially for the HER2+ subgroup. In the adjuvant setting with an intact BBB adjuvant targeted therapies appear to prolong survival whilst not preventing BM, in the palliative setting targeted therapies appear to prolong survival. This in turn increasing the number of patients that live to develop BM. In the metastatic setting, targeted therapies appear to prolong survival, probably due to the heterogenous permeability of the blood tumour barrier.
We were unable to find a subgroup, age, or other predictor that correlated to extent of BM. We did find a larger number of patients with lobular BC in the group suffering from meningeal carcinomatosis. Concordant results are published by other researchers with 25–35% of patients with lobular BC developing meningeal carcinomatosis compared to 10–15% in an unselected material of BC patients [[35], [36], [37], [38]]. We found no difference in BM as a sole metastatic location or as first occurrence between time periods.
Notably, 10% of the patients survived more than 3 years and we found this group not to be comprised solely of patients with limited advanced disease. Out of those 19 patients, nine were HER2+, nine Luminal, and only one was TNBC. This latter patient had surgery of the BM, and had no extra cranial disease. This implies that targeted therapy available for HER2+ and Luminal patients is an important key to prolonging survival after BM as has been hypothesised by other studies [39]. A recent published trial (EMBRACA) showed an effect on the BM progression-free survival, raising hope that efficient targeted therapies may be available also for the TNBC in the near future [40]. The role of immune therapy on BM in BC is so far not fully elucidated.
In summary, in this material consisting of mainly re-evaluated breast cancer tumours, in a number that well represents the population in the region studied, we show that there is a substantial increase of patients diagnosed with BM, and that advances in adjuvant treatment do not appear to prevent intracranial metastases. However, overall survival, as well as survival after BM, has improved with an additional year and in 10% of the patients (mainly patients with HER2+ and Luminal BC subtypes) we found extensive survival times.
Declaration of competing interest
The authors declare no existing conflicts of interest.
Acknowledgment/funding
This study was supported by grants from the Swedish Breast Cancer Association and the Swedish Government under the LUA-agreement (Sahlgrenska University Hospital, Gothenburg), Sweden.
References
- 1.Eurostat . 2013. Causes of death - standardised death rate per 100 000 inhabitants 2013. [Google Scholar]
- 2.Jemal A. Global cancer statistics. CA A Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Barnholtz-Sloan J.S. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the metropolitan detroit cancer surveillance system. J Clin Oncol. 2004;22(14):2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 4.Smedby K.E. Brain metastases admissions in Sweden between 1987 and 2006. Br J Canc. 2009;101(11):1919–1924. doi: 10.1038/sj.bjc.6605373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frisk G. Incidence and time trends of brain metastases admissions among breast cancer patients in Sweden. Br J Canc. 2012;106(11):1850–1853. doi: 10.1038/bjc.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsukada Y. Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer. 1983;52(12):2349–2354. doi: 10.1002/1097-0142(19831215)52:12<2349::aid-cncr2820521231>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 7.Cho S.Y., Choi H.Y. Causes of death and metastatic patterns in patients with mammary cancer. Ten-year autopsy study. Am J Clin Pathol. 1980;73(2):232–234. doi: 10.1093/ajcp/73.2.232. [DOI] [PubMed] [Google Scholar]
- 8.DiStefano A. The natural history of breast cancer patients with brain metastases. Cancer. 1979;44(5):1913–1918. doi: 10.1002/1097-0142(197911)44:5<1913::aid-cncr2820440554>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 9.Crozier J.A. Breast cancer brain metastases: molecular subtype, treatment and survival. Breast Dis. 2016;36(4):133–141. doi: 10.3233/BD-160237. [DOI] [PubMed] [Google Scholar]
- 10.Kennecke H. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28(20):3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 11.Lassman A.B., DeAngelis L.M. Brain metastases. Neurol Clin. 2003;21(1):1–23. doi: 10.1016/s0733-8619(02)00035-x. [vii] [DOI] [PubMed] [Google Scholar]
- 12.Niikura N. Treatment outcomes and prognostic factors for patients with brain metastases from breast cancer of each subtype: a multicenter retrospective analysis. Breast Canc Res Treat. 2014;147(1):103–112. doi: 10.1007/s10549-014-3090-8. [DOI] [PubMed] [Google Scholar]
- 13.Brufsky A.M. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Canc Res. 2011;17(14):4834–4843. doi: 10.1158/1078-0432.CCR-10-2962. [DOI] [PubMed] [Google Scholar]
- 14.Pestalozzi B.C. Is risk of central nervous system (CNS) relapse related to adjuvant taxane treatment in node-positive breast cancer? Results of the CNS substudy in the intergroup Phase III BIG 02-98 Trial. Ann Oncol. 2008;19(11):1837–1841. doi: 10.1093/annonc/mdn385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olson E.M. Incidence and risk of central nervous system metastases as site of first recurrence in patients with HER2-positive breast cancer treated with adjuvant trastuzumab. Ann Oncol. 2013;24(6):1526–1533. doi: 10.1093/annonc/mdt036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cagney D.N. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro Oncol. 2017;19(11):1511–1521. doi: 10.1093/neuonc/nox077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin J. Incidence, pattern and prognosis of brain metastases in patients with metastatic triple negative breast cancer. BMC Canc. 2018;18:446. doi: 10.1186/s12885-018-4371-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schouten L.J. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94(10):2698–2705. doi: 10.1002/cncr.10541. [DOI] [PubMed] [Google Scholar]
- 19.Carioli G. Trends and predictions to 2020 in breast cancer mortality in Europe. Breast. 2017;36:89–95. doi: 10.1016/j.breast.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Roche H. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNCLCC PACS 01 Trial. J Clin Oncol. 2006;24(36):5664–5671. doi: 10.1200/JCO.2006.07.3916. [DOI] [PubMed] [Google Scholar]
- 21.Martin M. Randomized phase 3 trial of fluorouracil, epirubicin, and cyclophosphamide alone or followed by Paclitaxel for early breast cancer. J Natl Cancer Inst. 2008;100(11):805–814. doi: 10.1093/jnci/djn151. [DOI] [PubMed] [Google Scholar]
- 22.Forbes J.F. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9(1):45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 23.Coates A.S. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol. 2007;25(5):486–492. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 24.Coombes R.C. Survival and safety of exemestane versus tamoxifen after 2-3 years’ tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet. 2007;369(9561):559–570. doi: 10.1016/S0140-6736(07)60200-1. [DOI] [PubMed] [Google Scholar]
- 25.Gianni L. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol. 2011;12(3):236–244. doi: 10.1016/S1470-2045(11)70033-X. [DOI] [PubMed] [Google Scholar]
- 26.Cameron D. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389(10075):1195–1205. doi: 10.1016/S0140-6736(16)32616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez E.A. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32(33):3744–3752. doi: 10.1200/JCO.2014.55.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pardridge W.M. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2(1):3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berghoff A. Brain metastases free survival differs between breast cancer subtypes. Br J Canc. 2012;106(3):440–446. doi: 10.1038/bjc.2011.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nieder C. Prognostic and predictive factors in patients with brain metastases from solid tumors: a review of published nomograms. Crit Rev Oncol Hematol. 2018;126:13–18. doi: 10.1016/j.critrevonc.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Huang Z. A nomogram for predicting survival in patients with breast cancer brain metastasis. Oncol Lett. 2018;15(5):7090–7096. doi: 10.3892/ol.2018.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bachmann C. CNS metastases in breast cancer patients: prognostic implications of tumor subtype. Med Oncol. 2015;32(1):400. doi: 10.1007/s12032-014-0400-2. [DOI] [PubMed] [Google Scholar]
- 33.Witzel I. Treatment and outcomes of patients in the brain metastases in breast cancer network registry. Eur J Canc. 2018;102:1–9. doi: 10.1016/j.ejca.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Rostami R. Brain metastasis in breast cancer: a comprehensive literature review. J Neuro Oncol. 2016;127(3):407–414. doi: 10.1007/s11060-016-2075-3. [DOI] [PubMed] [Google Scholar]
- 35.Niwińska A., Rudnicka H., Murawska M. Breast cancer leptomeningeal metastasis: propensity of breast cancer subtypes for leptomeninges and the analysis of factors influencing survival. Med Oncol. 2013;30(1) doi: 10.1007/s12032-012-0408-4. 408-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frank G.A. [WHO classification of tumors of the breast, 2012] Arkh Patol. 2013;75(2):53–63. [PubMed] [Google Scholar]
- 37.Gauthier H. Survival of breast cancer patients with meningeal carcinomatosis. Ann Oncol. 2010;21(11):2183–2187. doi: 10.1093/annonc/mdq232. [DOI] [PubMed] [Google Scholar]
- 38.Le Rhun E. 479 Primary breast cancer phenotype associated with propensity for leptomeningeal metastases. Europ J Canc Suppl. 2010;8(3):199. [Google Scholar]
- 39.Lin N.U. vol. 7. 2013. p. 307. (Breast cancer brain metastases: new directions in systemic therapy. Ecancermedicalscience). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Litton J.K. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379(8):753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]